Abstract
Accurate distance measurements between proton and nitrogen can provide detailed information on the structures and dynamics of various molecules. The combination of broadband phase-modulated (PM) pulse and rotational-echo saturation-pulse double-resonance (RESPDOR) sequence at fast magic-angle spinning (MAS) has enabled the measurement of multiple 1H-14N distances with high accuracy. However, complications may arise when applying this sequence to systems with multiple inequivalent 14N nuclei, especially a single 1H sitting close to multiple 14N atoms. Due to its broadband characteristics, the PM pulse saturates all 14N atoms; hence, the single 1H simultaneously experiences the RESPDOR effect from multiple 1H-14N couplings. Consequently, no reliable H-N distances are obtained. To overcome the problem, selective 14N saturation is desired, but it is difficult because 14N is an integer quadrupolar nucleus. Alternatively, 14N overtone (OT) NMR spectroscopy can be employed owing to its narrow bandwidth for selectivity. Moreover, owing to the sole presence of two energy levels (m = ± 1), the 14N OT spin dynamics behaves similarly to that of spin-1/2. This allows the interchangeability between RESPDOR and rotational-echo double-resonance (REDOR) since their principles are the same except the degree of 14N OT population transfer; saturation for the former whereas inversion for the latter. As the ideal saturation/inversion is impractical due to the slow and orientation-dependent effective nutation of 14N OT, the working condition is usually an intermediate between REDOR and RESPDOR. The degree of 14N OT population transfer can be determined from the results of protons with short distances to 14N and then can be used to obtain long-distance determination of other protons to the same 14N site. Herein, we combine the 14N OT and REDOR/RESPDOR to explore the feasibility of selective 1H-14N distance measurements. Experimental demonstrations on simple biological compounds of L-tyrosine.HCl, N-acetyl-L-alanine, and L-alanyl-L-alanine were performed at 14.1 T and MAS frequency of 62.5 kHz. The former two consist of a single 14N site, whereas the latter consists of two 14N sites. The experimental optimizations and reliable fittings by the universal curves are described. The extracted 1H-14N distances by OT-REDOR are in good agreement with those determined by PM-RESPDOR and diffraction techniques.
Keywords: 1H-14N distances, 14N overtone spectroscopy, PM-S-RESPDOR, REDOR, fast MAS frequency
Introduction
H-N distance is of importance for deeper insights into the structures and dynamics of chemical and biological systems due to the ubiquity of both proton and nitrogen. Such distance can be obtained by solid-state nuclear magnetic resonance (ssNMR) through the determination of H-N dipolar coupling, which is inversely proportional to the cube of the H-N distance. There are a few reasons why ssNMR has advantages for the H-N measurement over traditional diffraction techniques. First, ssNMR spectroscopy is applicable to various systems no matter their states, i.e., lacking long-range order or even being a disorder, which are intractable by diffraction techniques. Second, it enables the precise location of the H-atom positions, which is poorly determined by X-ray diffraction (XRD) or electron diffraction (Guzmán-Afonso et al., 2019).
Despite such advantages, the H-N distance measurement by NMR has difficulties due to some unfavorable nuclear characteristics of these two elements. For nitrogen, it has two stable and NMR active isotopes, 15N and 14N. The former is preferred in ssNMR because it is a spin-1/2 nucleus; thus, it is easy to manipulate and to obtains high resolution. Many methods have been designed to measure 1H-15N distances (Hohwy et al., 2000; Zhao et al., 2001; Schnell and Saalwächter, 2002; Fu, 2003; Chevelkov et al., 2009; Hou et al., 2011; Schanda et al., 2011; Paluch et al., 2013; Hou et al., 2014; Nishiyama et al., 2016). Nevertheless, the main drawback of 15N isotope is its insensitivity owing to the low natural abundance (0.4%). It makes the measurements lengthy for sufficient signal-to-noise ratio (S/N); otherwise, 1) the isotopic labeling is needed, which is not always simple and cost-effective or 2) dynamic nuclear polarization experiments are required (Zhao et al., 2018). However, the 1H-15N experiments on that work only allowed the determination of the shortest 1H-15N distance due to the dipolar truncation effect. On the other hand, 14N isotope benefits from the high natural abundance (99.6%), but it suffers from the severe quadrupolar broadening and complicated spin dynamics because 14N is an integer quadrupolar nucleus (spin I = 1). For protons, the intense 1H–1H homonuclear dipolar couplings in the solid state cause 1H line broadening and shorten the 1H coherence time. Consequently, these unfavorable characteristics of both N isotopes and H nucleus make H-N distance measurement by NMR challenging.
The development of fast magic-angle spinning (MAS, ν R ≥ 60 kHz) with proton detection has made 14N NMR spectroscopy a routinely used method, overcoming the difficulty associated with quadrupolar interaction (Cavadini et al., 2006; Gan et al., 2007; Cavadini, 2010; Nishiyama et al., 2011; Brown, 2014; Pandey and Nishiyama, 2015; Shen et al., 2015; Pandey et al., 2016; Carnevale et al., 2017; Hung et al., 2019; Jarvis et al., 2019; Rankin et al., 2019; Wijesekara et al., 2020). Furthermore, under fast MAS conditions, the strong 1H–1H dipolar network is largely suppressed (Nishiyama, 2016). These two advantages potentially facilitate the 1H-14N distance measurement. Recently, our group have introduced a combination of phase-modulated (PM) pulse (Nimerovsky et al., 2014; Makrinich et al., 2017; Makrinich et al., 2018), SR42 1 recoupling (Brinkmann and Kentgens, 2006), and rotational-echo saturation-pulse double-resonance (RESPDOR) (Gan, 2006; Chen et al., 2010a; Chen et al., 2010b; Lu et al., 2011) (PM-S-RESPDOR) that can extract multiple 1H-14N distances with high accuracy at fast MAS of 70 kHz (Duong et al., 2019). Such success mainly comes from the robustness that universal fraction curves can be obtained for the distance measurement under the saturation by the PM pulse for a wide range of 14N quadrupolar coupling constant (C Q) and 1H-14N dipolar coupling. This broadband characteristics of PM pulse is useful when we work on systems containing a single 14N site, as shown in the previous study. However, complications may arise for systems where multiple 14N sites are present, as shown below.
For example, a 5-spin system, as shown in Figure 1, consists of two N and three H atoms. We assume that the 14N and 1H NMR peaks are well resolved for simplicity. The first difficulty associated with this system is the ambiguity of 1H-14N distance measurement. For instance, we can determine the distance of H3-N by PM-S-RESPDOR sequence but cannot know whether such distance is between H3 and N1 or H3 and N2. The second difficulty relates to the complex spin dynamics of H2 nucleus, which is close to both N1 and N2 nuclei. As PM pulse is broadband, it completely saturates both 14N1 and 14N2 nuclei; thus, the PM-S-RESPDOR sequence will give the H2-N fraction curve experiencing the combined effects of H2-N1 and H2-N2 pairs. The H2-N distance from the fraction curve would be shorter than those extracted from H2-N1 or H2-N2 pair; or in other words, no reliable distance is yielded. A solution to overcome this cumulative contribution is to selectively saturate each N nucleus, which can be achieved in the manner of Delays Alternating with Nutation for Tailored Excitation (DANTE) (Vitzthum et al., 2011; Vitzthum et al., 2012; Lu et al., 2013; Pourpoint et al., 2014). This approach can be our future work.
FIGURE 1.

The 5-spin system with two N and three H atoms complicating the 1H-14N distance measurements by PM-S-RESPDOR.
An alternative approach is the 14N overtone (OT) NMR spectroscopy, where the forbidden transitions |Δm| = 2 are weakly allowed (m is the energy level) (Bloom and LeGros, 1986; Tycko and Opella, 1987; Jayanthi and Ramanathan, 2011; O’Dell and Ratcliffe, 2011; Nishiyama et al., 2013; O’Dell and Brinkmann, 2013; O’Dell et al., 2013; Haies et al., 2015a; Haies et al., 2015b; Shen et al., 2017; Concistré et al., 2018; Gan et al., 2018; Pandey and Nishiyama, 2018). Because it is twice the fundamental frequency, 14N OT frequency is more available to commercial MAS probes since many probes are not designed to tune to 14N Larmor frequency. Importantly, the 14N overtone excitation can achieve band-selective observation of 14N (Pandey and Nishiyama, 2018). The narrow bandwidth results from the slow effective 14N OT nutation, which is proportional to C Q/ν 0, where ν 0 is the 14N Larmor frequency. Besides the selectivity, 14N OT spectra are free from the first-order quadrupolar interaction because of the symmetric transitions, m = −1 ↔ m = +1. Therefore, 14N OT NMR is much narrower than the single-quantum 14N spectra and robust to the misadjustment of the magic angle. Moreover, since the transitions are only between two energy levels involved in OT (m = ±1), the spin dynamics of 14N OT behaves similarly to that of spin-1/2. Hence, for a 1H-14N OT system, the working conditions under RESPDOR can also be described by rotational-echo double-resonance (REDOR) (Gullion and Schaefer, 1989; Gullion, 2007) depending on whether the population transfer is saturation (RESPDOR) or inversion (REDOR) (Nimerovsky et al., 2017). Since the ideal saturation or inversion by continuous-wave (CW) is impractical, the working condition is an intermediate between REDOR and RESPDOR regimes. In this work, we combine 14N OT and REDOR sequence (1H-14N OT-REDOR) to explore its feasibility for distance measurements. This sequence is firstly demonstrated using two model biological compounds of L-tyrosine.HCl (Tyr) and N-acetyl-L-alanine (AcAla) and then applied to a more complex dipeptide system of L-alanyl-L-alanine (AlaAla) that involves two inequivalent nitrogen sites in a single molecule.
Pulse Sequence and the Universal Expression
Figure 2 depicts the 1H-14N OT-REDOR sequence. It is identical to the conventional S-REDOR sequence (Chen et al., 2010b), where SR42 1 recoupling (lasting for τ mix) is used to recover the 1H-14N dipolar coupling and CW (lasting for τ CW) is used to saturate/invert the 14N OT populations between the two energy levels. We note that since SR42 1 is not γ-encoded, the interval between the two SR4 blocks should be rotor-synchronized to avoid the spatial modulation of the recoupled 1H-14N dipolar couplings. For distance measurement, we measure two signals, S 0 and S’, acquired without and with CW pulse, respectively, for obtaining the fraction curve ΔS/S 0 = (S 0–S′)/S 0 as a function of τ mix.
FIGURE 2.

1H-14N OT-REDOR sequence. On 1H channel, SR42 1 recoupling is used to reintroduce the 1H-14N dipolar couplings. On 14N OT channel, CW pulse is used to perturb 14N OT populations. For the fraction curve, two sets of data (S 0 and S′) are acquired by employing OT-REDOR sequence without and with CW pulse (within the two brackets), respectively.
The fraction curve excludes the signal attenuation from T 2 relaxation, making it dependent solely on the dipolar coupling constant as for the case of REDOR with complete inversion. However, for 14N OT, complete inversion is difficult to achieve. Subsequently, the distance is extracted by fitting the fraction curve to either the numerically exact or universal curves. For the numerically exact curve, the simulation is extremely difficult as it must work in the laboratory frame without high-field approximation, leading to time-consuming calculations (O’Dell and Brinkmann, 2013). Even if this condition is met, various parameters must be known; for instance, the 14N C Q and the 14N OT effective nutation fields, which are not straightforwardly determined. For the universal curve, it has shown to be an almost identical match to the numerically exact 1H-14N PM-S-RESPDOR curve, allowing simple distance extractions (Duong et al., 2019). In addition, the condition and knowledge required by the numerically exact 1H-14N OT-REDOR curve above are not necessary for the universal curve. Indeed, semiquantitative evaluation for 14N OT transitions only requires the fitting parameter f and the 1H-14N dipolar coupling (shown below). Thus, for objective fitting, we use the universal curve approach, which is derived by following the original work of Gan or later analysis of Chen and coworkers (Gan, 2006; Chen et al., 2010a). The derivation starts with fundamental 14N transitions for verification and then applies to 14N OT.
A general expression for any spin and type of experiment is
| (1) |
where P i = 1/(2I + 1) is the population of spin state m = i under high-temperature approximation, W ij is the population transfer probability from m = i to m = j spin state, and REDOR(|Δm|) presents the normalized dipolar-dephased signal intensity for classical REDOR. The general expression helps to derive the universal curves mentioned in Figure 3. It is worth noting that the natural abundance of a specific isotope should also be considered in Eq. (1). However, the natural abundance of 14N isotope is 99.6%, very close to 100%; hence, we can safely neglect it.
FIGURE 3.
The population transfers and their probabilities (W ij) for ZQ (Δm = 0), SQ (|Δm| = 1), and DQ (|Δm| = 2) transitions of (A) 14N and (B) 14N OT to derive the universal curves.
For 14N (I = 1) spin, under the Zeeman interaction with the external magnetic field, there are three energy levels of m = 0 and ±1. We assume the population for each level is 1/3. The population transfers among the energy levels are categorized into zero- (ZQ or Δm = 0), single- (SQ or |Δm| = 1), and double-quantum (DQ or |Δm| = 2) transitions, which determine the RESPDOR effect. Both energy levels m = +1 and m = −1 are involved in all three ZQ, SQ, and DQ transitions, as shown in Figure 3A. Under the ideal saturation of 14N spin, W ±1j for these transfers are equal; hence, each transition has W ±1j of 1/3. On the other hand, the energy level m = 0 is only involved in ZQ and SQ transitions, but there are two SQ transitions of (m = 0 → m = −1) and (m = 0 → m = +1). Hence, for m = 0, W 0j of ZQ, SQ, and DQ transitions are 1/3, 2/3, and 0/3, respectively. Taken together, under the complete saturation of 14N spin, ΣP i W ij for ZQ, SQ, and DQ transitions for m = (−1, 0, 1) are 1/3·3/3, 1/3·4/3, and 1/3·2/3, respectively (see Figure 3A). Replacing these P i and W ij in Eq. (1), the universal expression for 14N is given by
| (2) |
where J ±1/4 denotes the ±1/4-order Bessel functions of the first kind and b 1H–14N/(2π) is the 1H-14N dipolar coupling constant while τ mix is the total mixing time of SR42 1 recoupling sequence. Eq. (2) is identical to the universal curve for 1H-14N RESPDOR in the literature (Gan, 2006; Chen et al., 2010b), verifying our analysis.
Next, we consider the case of 14N OT. Again, three energy levels are present with the population P i of 1/3 for each level. The energy level m = 0 is not involved in OT transitions; thus, it is blurred in Figure 3B. P i of m = 0 remains at ZQ transition, meaning that W 0j is 1 for j = 0 and 0 for j ≠ 0. Conversely, both energy levels m = ±1 are involved in the saturation/inversion of ZQ and DQ transitions. However, owing to the slow and orientation-dependent effective nutation of 14N OT, the complete saturation/inversion is difficult. Considering this incompletion, we assume that the DQ W ±1j for m = ±1 are f with 0 ≤ f ≤ 1, in which f = 0.5 corresponds to complete saturation while f = 1.0 corresponds to complete inversion. Although we mentioned that the working condition for 1H-14N OT-REDOR is between REDOR and RESPDOR regimes in the Introduction section, this does not mean that f should be between 0.5 and 1.0. Indeed, if the complete saturation is not achieved, parameter f could be smaller than 0.5. With the introduction of f, W ±1j for ZQ and DQ transitions are 1–f and f, respectively. Combining P i of each transition for each level and under incomplete saturation/inversion of 14N OT, Figure 3B shows that ΣP i W ij for ZQ, SQ, and DQ transitions are 1/3·(1 + 2(1–f)), 0, and 1/3·2f, respectively. The universal expression for REDOR/RESPDOR on 14N OT is given by
| (3) |
From Eq. (3), the coefficient for REDOR (|Δm| = 2) is proportional to f, affecting the slope of the fraction curve. However, since f uniformly affects the other elements in the equation, the universal curves derived from Eq. (3) would reach the maximum at the same τ mix no matter f value. It is worth noting that the introduction of f makes the fitting among universal curves and experimental fraction curve better, but it makes the extracted b 1H–14N inaccurate. Particularly, when the fraction curve has not reached the maximum ΔS/S 0, universal curves generated by different combinations of f and b 1H–14N can reproduce the very similar fraction curve, thus giving ambiguous results. To avoid this situation, our fitting strategy consists of two steps. The first is to determine f, which is possible only when the fraction curve of the shortest H-N distance must show the maximum. Under this condition, the fitting parameter f is determined as the ratio of the experimental and theoretical maxima ΔS/S 0 (2/3 = 0.67). That precisely known f leads to the unambiguous determination of b 1H–14N. For longer H-N distance, the REDOR curve may possibly not show the maximum while the oscillation is damped, making the fitting difficult. Under this situation, accurate distance determinations are still possible by the second step. It is to use this observed f from the first step for measuring longer 1H-14N distances of the same 14N site. This strategy is a disadvantage of OT-REDOR compared to PM-S-RESPDOR. The latter does not require the prior knowledge of the fitting parameter f owing to the complete saturation of all 14N crystallites by the PM pulse, thus enabling the reliable fitting even when the maximum is not observed.
It is of practical use to clarify the differences between the universal expressions for 1H-14N RESPDOR and 1H-14N OT-REDOR. This can be done by comparing the universal curves resulting from Eqs. 2, 3 under the same b 1H–14N. Figure 4 compares the three universal curves, one from Eq. (2) and two from Eq. (3) with f = 0.5 (complete saturation) and f = 1.0 (complete inversion). These two f values are chosen because they correspond to ideal RESPDOR (f = 0.5) and REDOR (f = 1.0) conditions and our working condition is an intermediate between these two, as mentioned in the Introduction. The two curves from Eq. (3) are identical except for the intensity (a factor of 2), which is in agreement with the discussion above (see Figure 4). A notable difference between the universal curves of 1H-14N OT and that of 1H-14N is that the dephasing rate of the former curves is about two times faster than that of the latter (0.77 ms compared to 1.60 ms, respectively). This is because, for 1H-14N OT, the REDOR effect is determined by the DQ transitions, whereas, for 1H-14N, the RESPDOR effect is determined by both the SQ and DQ transitions. Such a faster dephasing rate associated with the multiple quantum transitions has been known in the literature (Pruski et al., 1999). This potentially allows 1H-14N OT-REDOR to probe long 1H-14N distance better than 1H-14N RESPDOR as it is less affected by the poor sensitivity and uncertainty of ΔS/S 0 at long τ mix.
FIGURE 4.

Comparison between the universal curves of 1H-14N RESPDOR (black) and 1H-14N OT-REDOR (grey) with f = 0.5 (complete saturation) and 1.0 (complete inversion) derived from Eqs 2,3, respectively, under the same 1H-14N dipolar coupling of 2.0 kHz. The optimum τ mix of both curves are shown and highlighted by the dashed lines.
Results and Discussions
In this section, we firstly explore the feasibility of 1H-14N OT-REDOR on two model biological compounds of Tyr and AcAla. These two compounds only consist of a single 14N site and were well characterized by 1H-14N PM-S-RESPDOR in the previous study. Upon the feasibility test, we apply this technique to a more complex dipeptide system of AlaAla where two inequivalent 14N sites are present.
L-Tyrosine.HCl
In order to obtain an efficient 1H-14N OT-REDOR fraction curve, experimental optimizations are required. Such optimizations require the knowledge of 14N OT resonance frequency as it significantly affects the sensitivity of OT experiments due to the narrow bandwidth. In this current work, the 14N OT frequency is indirectly determined by the two-dimensional (2D) 1H-{14N OT} D- or T-HMQC (sequences in Supplementary Figures S1A,B respectively) at the second OT spinning sideband (n = -2 SSB) for the highest S/N. Such n value depends on the sense of rotation with respect to the magnetic field (Gan et al., 2018). For our configuration, the magnetic field is toward the top of the magnet and the spinning rotation is clockwise looking from the top. Taking benefits of optimum S/N, all the remaining experiments were also performed at the second 14N OT SSB (n = −2). Figure 5 clearly indicates the 14N OT frequency and its correlations to both proton sites of NH 3 and CH by the D-HMQC experiment. The smaller correlation of N to CH under τ mix of 0.51 ms is explained due to the longer 1H-14N distance compared to the directly bonded H-N distance of the NH3 group. This 2D spectrum is in agreement with the 1H-{14N} D-HMQC spectrum in the previous study (Duong et al., 2019).
FIGURE 5.

The 2D 1H-{14N OT} D-HMQC experiment of Tyr at the magnetic field (B 0) of 14.1 T and ν R of 62.5 kHz. The experiment was performed at the second 14N OT SSB (n = -2) for the highest S/N. Further details are given in the Experiments section.
After the 14N OT frequency has been determined, the next parameter for optimization is τ CW so that as many as possible 14N crystallites can be saturated/inverted. The 14N OT rf-field was 120 kHz, the highest technically possible value. It was calibrated by the use of the Bloch–Siegert shift of the proton approach (Hung et al., 2020). However, it is noted that the 14N OT nutation frequency is scaled on C Q, the magnetic field, the powder distribution, making it much weaker than 14N OT rf-field. Figure 6 shows the signal fraction ΔS/S 0 of NH 3 and CH at a fixed τ mix of 1.15 ms (or 18 loops of SR42 1 recoupling blocks) under varying τ CW values.
FIGURE 6.

Tyr: the signal fraction ΔS/S 0 of NH 3 (black squares) and CH (red circles) as a function of τ CW. Experiments were performed at τ mix of 1.15 ms. The optimum τ CW is shown and highlighted by the dashed line. Further experimental details are given in the Experiments section.
Once the parameters were optimized, we performed the 1H-14N OT-REDOR experiment on Tyr. Figure 7A shows the one-dimensional (1D) 1H spin echo (S 0), the dephased (S′), and the difference (S 0–S’) spectra, in which the former two were extracted from the 1H-14N OT-REDOR experiment with τ mix of 0.51 ms, whereas the latter is calculated from the former two. The NH 3 and CH sites are evidently assigned in Figure 7A and are located in the molecular structure of Tyr (Figure 7B). The experimental fraction curves (black circles) of NH 3 and CH are shown in Figures 7C,D, respectively. There are two important points to mention for the fraction curve of NH 3 in Figure 7C. First, the oscillation is observed and the maximum ΔS/S 0 is reached at τ mix of 0.6 ms. In our previous study, the 1H-14N PM-S-RESPDOR fraction curve shows the maximum at τ mix of ∼1.4 ms (Duong et al., 2019), which is about two times slower than that of 1H-14N OT-REDOR fraction curve. This result is in qualitative agreement with the analysis in Pulse Sequence and the Universal Expression and Figure 4. Second, the fraction curve in Figure 7C shows the experimental maximum ΔS/S 0 of 0.55, smaller than the theoretical maximum of 0.67 by the universal curve in Eq. (3) for complete inversion. Thus, the fitting parameter f of 0.55/0.67∼0.82 is required for the reliable fittings. In addition, the value of 0.82 is close to f = 1 in the case of complete inversion, revealing the dominance of the inversion process under the application of 14N OT CW pulse. Once f is determined, the only unknown remaining parameter is b 1H–14N. Moreover, according to the fitting strategy in Pulse Sequence and the Universal Expression, this fitting parameter f can also be used in Figure 7D. This is because f only depends on 14N C Q, τ CW, and 14N OT nutation frequency (which are the same as fraction curves in Figures 7C,D are from the single experiment) and thus should remain the same for other 1H-14N pairs from the same 14N site. The 1H-14N dipolar couplings, thus distances, can be extracted by fitting the scaled universal curves (red solid lines) to the experimental fraction curves (black circles) presented in Figures 7C,D. Although the fraction curve in Figure 7C shows the oscillation up to τ mix of ∼1.8 ms, the fitting by the universal curves is only up to τ mix of ∼1.0 ms, owing to the poor agreement between the experimental and universal curves for NH3 at τ mix > 1.0 ms (Supplementary Figure S2A). The deviation is mainly caused by the fact that each crystallite experiences different 14N OT saturation/inversion extent depending on its relative orientation between quadrupolar tensor to the rotor-fixed frame, whereas, for the universal approach, the behaviors of the entire crystallites are considered uniform. The root-mean-square deviation (RMSD) in Figures 7C,D was calculated for the best fit of 1H-14N dipolar couplings. It is noted that, for NH 3 (Figure 7C), a scaling factor P2(cos(θ)) (θ, the angle between H-N and C-N, is 109.5°) is used for the dynamic average of the N-H dipolar coupling due to the threefold rotation. The 1H-14N distances by OT-REDOR are shown in Table 1 along with those by PM-S-RESPDOR and neutron diffraction (ND). The distances are in good agreement with each other, which demonstrates the feasibility of OT-REDOR for obtaining accurate 1H-14N distances. It is worth noting that the longer distances by ssNMR than those from neutron result from the different vibrational averages of the internuclear distances of the two techniques (Ishii et al., 1997).
FIGURE 7.
(A) The 1D 1H spin echo (S 0), the dephased (S′), and the difference (S 0–S′) spectra of Tyr. The former two were extracted from 1H-14N OT-REDOR with τ mix of 0.51 ms, whereas the latter is calculated from the former two. (B) The molecular structure of Tyr where NH 3 and CH are presented. (C,D) The fitting of experimental 1H-14N OT-REDOR fraction curves (black circles) by the universal curves (red lines) for NH 3 at 7.6 ppm in (C) and CH at 4.4 ppm in (D). The fitting parameter f for universal curves is 0.82. The RMSD analyses (inset) were calculated for the best fitting 1H-14N dipolar couplings.
TABLE 1.
1H-14N distances measured by OT-REDOR, PM-S-RESPDOR, and ND on Tyr.
| OT-REDOR | PM-S-RESPDOR | ND | |
|---|---|---|---|
| NH 3 | 1.05 ± 0.03 Å | 1.16 Å | 1.01 Å |
| CH | 2.21 ± 0.10 Å | 2.24 Å | 2.10 Å |
N-Acetyl-L-alanine
To further demonstrate the feasibility of OT-REDOR for a system with a larger 14N C Q, we apply it to AcAla. A similar experimental procedure as described for Tyr was applied, including the 1) determination of 14N OT resonance frequency, 2) optimization of τ CW, and 3) implementation of OT-REDOR. These experiments were all performed at the second 14N OT SSB for the highest S/N. For step 1, the 1H-{14N OT} T-HMQC was performed (Supplementary Figure S3). Again, for the efficient OT-REDOR fraction curve, τ CW must be optimized. For step 2, such optimization for NH (black squares) at τ mix of 0.19 ms (or three loops of SR42 1 recoupling blocks) and CH (red circles) at τ mix of 0.96 ms (or 15 loops of SR42 1 recoupling blocks) under identical τ CW range is shown in Figure 8. The reason for different τ mix is due to the large difference between 1H-14N distances for these proton sites. The optimum τ CW of 0.192 ms for 14 NH in AcAla is shorter than τ CW of 0.464 ms for 14 NH3 in Tyr. This result is expected since the 14N site of NH has a larger quadrupolar interaction, thus resulting in a larger 14N OT nutation field and shorter pulse length for efficient saturation/inversion.
FIGURE 8.

AcAla: the signal fraction ΔS/S 0 of NH (black squares) at τ mix of 0.19 ms and CH (red circles) at τ mix of 0.96 ms as a function of τ CW. The optimum τ CW is shown and highlighted by the dashed line. Further experimental details are given in the Experiments section.
For step 3, these parameters were used for the OT-REDOR experiment on AcAla. Figure 9A shows the one-dimensional (1D) 1H spin echo (S 0), the dephased (S′), and the difference (S 0–S′) spectra. The former two were extracted from the 1H-14N OT-REDOR experiment with τ mix of 0.26 ms, whereas the latter results from the difference of the former two. For readability, the intensity of the difference spectrum is triple, showing the NH and CH sites which experience the REDOR effect. These two sites are also located in the molecular structure of AcAla (Figure 9B). Figures 9C,D show the fittings between the universal curves (red lines) and the experimental fraction curves (black circles) for NH and CH, respectively. For reliable fittings, the fitting parameter f must be known. From Figure 9C, the experimental maximum ΔS/S 0 intensity of 0.33 results in f of 0.33/0.67 = 0.50. While Tyr shows the dominance of inversion (f = 0.82), AcAla experiences the saturation of overall magnetization (f = 0.50). The difference may arise from the large frequency linewidth (up to 8.6 kHz in Supplementary Figure S3) of 14N OT spectrum of NH of AcAla relative to the weak 14N OT nutation frequency. Since both fraction curves in Figures 9C,D were obtained from the single experiment where 14N C Q, τ CW, and 14N OT nutation frequency are the same, the identical f value can be used in Figure 9D. The extracted 1H-14N distances are summarized in Table 2 along with those from PM-S-RESPDOR and XRD. The distances are in agreement with each other. We note that the deviation between the distance of N–H by XRD and those by OT-REDOR and PM-S-RESPDOR is due to the poor capability of XRD to locate H position, resulting from the limited scattering power of hydrogen and the vibrational effect mentioned in L-Tyrosine.HCl. In short, the applicability of OT-REDOR on Tyr and AcAla for obtaining accurate 1H-14N distances has been validated.
FIGURE 9.
(A) The 1D 1H spin echo (S 0), the dephased (S′), and the difference (S 0–S′) spectra of AcAla. These spectra result from the 1H-14N OT-REDOR experiment with τ mix of 0.26 ms. For readability, the intensity of the difference spectrum is triple. (B) The molecular structure of AcAla where NH and CH are located. (C,D) The fitting of experimental 1H-14N OT-REDOR fraction curves (black circles) by the universal curves (red lines) for NH at 7.9 ppm in (C) and CH at 3.9 ppm in (D). The fitting parameter f for universal curves is 0.50. The RMSD analyses (inset) were calculated for the best fitting 1H-14N dipolar couplings.
TABLE 2.
1H-14N distances measured by OT-REDOR, PM-S-RESPDOR, and XRD on AcAla.
| OT-REDOR | PM-S-RESPDOR | XRD | |
|---|---|---|---|
| NH | 1.07 ± 0.06 Å | 1.06 Å | 0.78 Å |
| CH | 2.21 ± 0.12 Å | 2.11 Å | 2.09 Å |
L-Alanyl-L-alanine
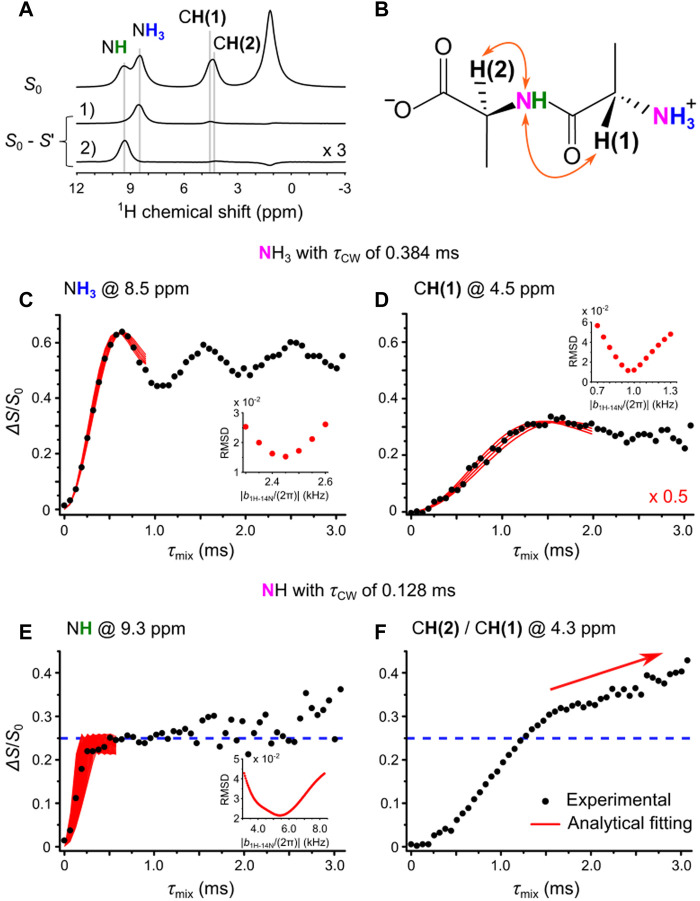
In the previous section, OT-REDOR experiments have been successfully applied to Tyr and AcAla, both containing a single 14N site. As the main usage of OT-REDOR is for systems where multiple 14N sites are present, here, we apply this sequence to AlaAla. Besides the 1D 1H spin echo (S 0) at the top, Figure 10A also shows the two difference (S 0–S’) spectra where 1) NH 3 and 2) NH sites are saturated/inverted during 1H-14N OT-REDOR experiments with τ mix of 0.51 ms and 0.19 ms, respectively. The NH, NH 3, CH(1), and CH(2) sites are unambiguously assigned (Figure 10A) and located in the molecular structure of AlaAla (Figure 10B). This compound consists of two 14N sites of NH3 and NH; hence, it is similar to the combination of 14N sites of Tyr and AcAla.
FIGURE 10.
(A) The 1D 1H spin echo and the two difference spectra where 1) NH 3 and 2) NH sites are saturated/inverted during 1H-14N OT-REDOR experiments with τ mix of 0.51 ms and 0.19 ms, respectively. For readability, the intensity of spectrum 2) is triple. (B) The NH, NH 3, CH(1), and CH(2) sites, assigned in (A), are located in the molecular structure of AlaAla. (C–F) The fitting of experimental 1H-14N OT-REDOR fraction curves (black circles) by the universal curves (red lines) for NH 3 at 8.5 ppm in (C) and CH(1) at 4.5 ppm in (D) when 14 NH3 OT was saturated/inverted by CW pulse with τ CW of 0.384 ms and NH at 9.3 ppm in (E) when 14 NH OT was saturated/inverted by CW pulse with τ CW of 0.128 ms. The fitting parameter f for universal curves is (C,D) 0.94 and (E) 0.37. For (D), the universal curves are halved, highlighted by a factor of 0.5 in red. For (E), the maximum signal fraction ΔS/S 0 of 0.25 for NH is presented by the horizontal dash line. For (F), the signal fraction ΔS/S 0 of CH(2)/CH(1) at 4.3 ppm with 14 NH OT irradiation is over this line and is still increasing with τ mix (highlighted by the arrow). The RMSD analyses (inset) were calculated for the best fitting 1H-14N dipolar couplings. The two arrows show the proximity of both CH(2)/CH(1) to 14 NH site.
Similar experimental procedures were applied. D-HMQC experiments were performed again at the second 14N OT SSB (n = -2) for the highest S/N to determine 14N OT frequencies (Supplementary Figures S4A,B ). Then, τ CW for 14 NH3 and 14 NH of AlaAla were optimized, which were 0.384 and 0.128 ms, respectively (Supplementary Figures S4C,D). These optimized values, similar to those of Tyr and AcAla, were used to obtain 1H-14N OT-REDOR fraction curves.
We discuss first the case where 14 NH3 is saturated/inverted by CW pulse with τ CW of 0.384 ms. The experimental fraction curves of NH 3 and CH(1) (black circles) are shown in Figures 10C,D, respectively. For NH 3, the experimental curve shows the oscillation and the maximum ΔS/S 0 is 0.63 (see Figure 10C). Thus, the fitting parameter f of 0.63/0.67∼0.94 for universal curves is required for reliable fittings. This indicates that an almost complete inversion of 14N OT is achieved (f = 1), similar to the case of NH3 of Tyr. Another similarity to Tyr is that significant deviation is observed, especially at long τ mix for the full-scale fitting between the fraction curve of Figure 10C and the universal curves (see Supplementary Figure S2B). For CH(1), the fraction curve reaches the plateau ΔS/S 0 of 0.35 at τ mix of 1.50 ms (Supplementary Figure S5). The reason for the lower signal fraction ΔS/S 0 is the overlapping of 1H signals of CH(1) and CH(2) sites, in which only CH(1) is close to 14 NH3. For a good match with the experimental fraction curve, we need to halve the universal curves in Figure 10D. The reason for using a factor of 0.5 is explained in the Supplementary Eq. S3. Without scaling, the obtained 1H-14N distance by NMR is in poor agreement with that reported from XRD (see Supplementary Figure S5). The fitting of the scaled universal curves to the 1H-14N OT-REDOR fraction curves gives the 1H-14N distances of 1.05 ± 0.03 Å for NH3 (after a modulation factor of P2(cos(θ)) as described in L-Tyrosine.HCl and Pulse Sequence and the Universal Expression and 2.09 ± 0.05 Å for CH(1) (after halving the universal curves). Both measured distances are in good agreement with those reported from XRD (see Table 3).
TABLE 3.
1H-14N distances measured by OT-REDOR and XRD on AlaAla.
| Saturation/inversion on 14N(H3) | Saturation/inversion on 14N(H) | ||
|---|---|---|---|
| NH3 | CH(1) | NH | |
| OT-REDOR | 1.05 ± 0.03 Å | 2.09 ± 0.05 Å | 1.19 ± 0.20 Å |
| XRD | 0.93 Å | 1.97 Å | 0.96 Å |
We then consider the case where 14 NH is saturated/inverted by CW pulse with τ CW of 0.128 ms. The fraction curves of NH and CH(2)/CH(1) (black circles) are shown in Figures 10E,F, respectively. For NH in Figure 10E, the experimental curve shows the plateau ΔS/S 0 of 0.25 at τ mix of 0.50 ms and then large fluctuations of ΔS/S 0 at τ mix larger than 1.50 ms. The origin of such fluctuations may be due to t 1 noise from spinning frequency fluctuation (Nishiyama et al., 2020). As the maximum ΔS/S 0 is smaller than the theoretical maximum of 0.67 of the universal curve, a fitting parameter f of 0.25/0.67∼0.37 is required. Based on the fittings of the universal curves (red solid lines) to the 1H-14N OT-REDOR fraction curve, the 1H-14N distance is measured to be 1.19 ± 0.20 Å. This value is in excellent agreement with 1H-15N distance by inverse cross-polarization with variable contact (CPVC) (Nishiyama et al., 2016) (Supplementary Figure S6) and in close agreement with the distance of 0.96 Å by XRD (see Table 3). An advantage of 1H-14N OT-REDOR compared to 1H-15N inverse CPVC is that multiple H-N distances can be simultaneously determined by the former, whereas only directly bonded distance is determined by the latter due to the dipolar truncation effect. Indeed, 1H-15N inverse CPVC only provides the distance of directly bonded H-N for the NH site (see Supplementary Figure S6). For CH(2)/CH(1) fraction curve, its fraction signal ΔS/S 0 is larger than 0.25 and continues to grow at long τ mix (see Figure 10F). Although this curve is the combination of two curves because there are two CH groups that are close to 14 NH site and their 1H chemical shifts are overlapped, ΔS/S 0 is larger than 0.25 may result from the intermolecular couplings. Because of this complexity, we did not fit this with the universal curves.
In conclusion, for AlaAla, the 1H-14N OT-REDOR experiment can be used for the accurate measurement of 1H-14N distances for the bonded H–N distances of each nitrogen. However, extracting distances for nonbonded H-N pairs is still difficult, especially when the chemical shifts of these 1H sites are overlapped, as shown in Figure 10F. Such problem will be solved by the multidimensional NMR experiments, for example, with an addition of the 13C dimension.
Conclusion
In summary, we have presented the feasibility of 1H-14N OT-REDOR with proton detection at fast MAS to extract 1H-14N distances for Tyr, AcAla, and AlaAla. Owing to the selective characteristics of 14N OT spectroscopy, this sequence is useful for systems with multiple 14N sites. Other advantages of 14N OT are the availability of commercial MAS probes, the robustness of misadjustment of the magic angle, and the fast dephasing rate. The final advantage is that it allows probing longer 1H-14N distances better than 1H-14N RESPDOR experiment. For efficient 1H-14N OT-REDOR fraction curve, the 14N OT resonance frequency, in this work, must be determined with D- or T-HMQC experiments and the CW pulse length must be optimized. For reliable 1H-14N distances, the fitting parameter f is a prerequisite; otherwise, distances cannot be accurately determined. The knowledge of f value also enables the evaluation of saturation/inversion degree of 14N OT by the CW pulse. For Tyr and AcAla compounds, the extracted distances from OT-REDOR are in good agreement with PM-S-RESPDOR and the diffraction techniques. For AlaAla, the extracted 1H-14N distances from directly bonded N–H well agree with those reported from XRD and 1H-15N inverse CPVC. However, this is not the case for nonbonded N–H pairs since distance deviations from those reported by XRD are observed. The reason for such deviation is the overlapping of 1H signals. This issue can be overcome by performing multidimensional NMR experiments. In conclusion, we believe that the 1H-14N OT-REDOR has the potential of selectively measuring 1H-14N distances on systems containing multiple 14N sites, giving deep insights into structural studies of biological, chemical, and pharmaceutical compounds. It is worth noting that 14N selective saturation can also be achieved in the manner of DANTE. It is promising to perform 1H-14N DANTE-RESPDOR experiments in future studies.
Experiments
L-tyrosine.HCl (Tyr), N-acetyl-L-alanine (AcAla), and L-alanyl-L-alanine were purchased from Sigma-Aldrich and used as received. The samples were separately packed into 1.0 mm zirconia rotors and then inserted into 1 mm 1H/X double-resonance probe. The rotors were spun at a MAS frequency of 62.5 kHz, except for 1H-15N inverse CPVC at 70 kHz.
All NMR experiments were recorded at a room temperature of 25 °C on JNM-ECZ600R (JEOL RESONANCE Inc.) at 14.1 T solid-state NMR spectrometers. The 1H and 14N OT Larmor frequencies are 600.0 and 86.8 MHz, respectively. For the highest S/N, the 14N OT frequency was set at the second SSB (n = −2). The 14N and 14N OT shifts are referenced to CH3NO2, whose 14N and 14N OT shifts are equal to 0 ppm or 0 kHz. The 1H rf-field was 328 kHz for π/2 and π pulses and 140 kHz for the SR42 1 recoupling sequence. The 14N OT rf-field was 120 kHz.
For Tyr, the 2D 1H-{14N} D-HMQC spectrum in Figure 5 was recorded using the sequence shown in Supplementary Figure S1A with 8 scans, 32 t 1 points, and rotor-synchronized t 1 increment of 16.0 µs. τ p, τ mix, and recycling delay (RD) were 200 µs, 512 µs, and 4 s, respectively. The experimental time was about 0.6 h. The States-TPPI method was employed for quadrature detection along the indirect dimension. For Figure 6, τ CW was optimized within the range from 16 µs to 656 µs with a step of 32 µs; the 14N OT frequency was −1.73 ppt (parts per thousand), the τmix was fixed at 1152 µs, the number of scans (NS) was 18, and RD was 5.0 s. The experimental time was 1.1 h. For Figure 7, the 1H-14N OT-REDOR was performed at τ CW of 464 µs, 14N OT frequency of −1.73 ppt, NS of 72, RD of 6.5 s, and τ mix from 0 to 3456 µs with a step of 64 µs. The experimental time was 14.3 h.
For AcAla, the τ CW optimization in Figure 8 was implemented within the range from 32 µs to 256 µs with a step of 16 µs; the 14N OT frequency was −1.267 ppt, NS was 18 and RD was 10.0 s, and τ mix was fixed at 192 µs for NH at 7.9 ppm and 960 µs for CH at 3.9 ppm. The experimental times for both experiments were 1.5 h. For Figure 9, the 1H-14N OT-REDOR was performed at τ CW of 192 µs, 14N OT frequency of −1.267 ppt, NS of 108, RD of 10 s, and τ mix from 0 to 2048 µs with a step of 64 µs. The experimental time was 19.8 h.
For AlaAla, the 1H-14N OT-REDOR experiments in Figure 10 were performed at NS of 144, RD of 2.5 s, τ mix from 0 to 3072 µs with a step of 64 µs, and τ CW, 14N OT frequencies were of 384 µs, −1.72 ppt and 128 µs, −1.26 ppt for 14 NH3 and 14 NH, respectively. The experimental times for both experiments were 9.8 h.
NMR data are available upon request.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material; further inquiries can be directed to the corresponding author.
Author Contributions
ND was responsible for methodology, NMR measurements, and analysis; ZG was responsible for conceptualization, and methodology; YN was responsible for conceptualization, methodology, and supervision. All authors were responsible for writing the manuscript.
Funding
This work was supported by JSPS KAKENHI Grant Number 20K05483 to YN. ZG acknowledges the support from the US National Science Foundation through NSF/DMR-1644779 and the National High Magnetic Field Laboratory at Florida.
Conflict of Interest
YN is employed by JEOL RESONANCE Inc.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The handling editor declared a past co-authorship with one of the authors YN.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmolb.2021.645347/full#supplementary-material.
References
- Bloom M., LeGros M. A. (1986). Direct detection of two-quantum coherence. Can. J. Phys. 64, 1522–1528. 10.1139/p86-271 [DOI] [Google Scholar]
- Brinkmann A, Kentgens A. P. (2006). Proton-selective 17O-1H distance measurements in fast magic-angle-spinning solid-state NMR spectroscopy for the determination of hydrogen bond lengths. J. Am. Chem. Soc. 128, 14758–14759. 10.1021/ja065415k [DOI] [PubMed] [Google Scholar]
- Brown S. P. (2014). Nitrogen-proton correlation experiments of organic solids at natural isotopic abundance. eMagRes. 3, 243–254. 10.1002/9780470034590.emrstm1323 [DOI] [Google Scholar]
- Carnevale D., Ji X., Bodenhausen G. (2017). Double cross polarization for the indirect detection of nitrogen-14 nuclei in magic angle spinning NMR spectroscopy. J. Chem. Phys. 147, 184201. 10.1063/1.5000689 [DOI] [PubMed] [Google Scholar]
- Cavadini S. (2010). Indirect detection of nitrogen-14 in solid-state NMR spectroscopy. Prog. Nucl. Magn. Reson. Spectrosc. 56, 46–77. 10.1016/j.pnmrs.2009.08.001 [DOI] [PubMed] [Google Scholar]
- Cavadini S., Antonijevic S., Lupulescu A., Bodenhausen G. (2006). Indirect detection of nitrogen-14 in solids via protons by nuclear magnetic resonance spectroscopy. J. Magn. Reson. 182, 168–172. 10.1016/j.jmr.2006.06.003 [DOI] [PubMed] [Google Scholar]
- Chen L., Lu X., Wang Q., Lafon O., Trébosc J., Deng F., et al. (2010a). Distance measurement between a spin-1/2 and a half-integer quadrupolar nuclei by solid-state NMR using exact analytical expressions. J. Magn. Reson. 206, 269–273. 10.1016/j.jmr.2010.07.009 [DOI] [PubMed] [Google Scholar]
- Chen L., Wang Q., Hu B., Lafon O., Trébosc J., Deng F., et al. (2010b). Measurement of hetero-nuclear distances using a symmetry-based pulse sequence in solid-state NMR. Phys. Chem. Chem. Phys. 12, 9395–9405. 10.1039/b926546e [DOI] [PubMed] [Google Scholar]
- Chevelkov V., Fink U., Reif B. (2009). Accurate determination of order parameters from 1H,15N dipolar couplings in MAS solid-state NMR experiments. J. Am. Chem. Soc. 131, 14018–14022. 10.1021/ja902649u [DOI] [PubMed] [Google Scholar]
- Concistré M., Kuprov I., Haies I. M., Williams P. T. F., Carravetta M. (2018). 14N overtone NMR under MAS: signal enhancement using cross-polarization methods. J. Magn. Reson. 298, 1–5. 10.1016/j.jmr.2018.10.017 [DOI] [PubMed] [Google Scholar]
- Duong N. T., Rossi F., Makrinich M., Goldbourt A., Chierotti M. R., Gobetto R., et al. (2019). Accurate 1H-14N distance measurements by phase-modulated RESPDOR at ultra-fast MAS. J. Magn. Reson. 308, 106559. 10.1016/j.jmr.2019.07.046 [DOI] [PubMed] [Google Scholar]
- Fu R. (2003). Measurement of 15N-1H bond lengths by rotational-echo double-resonance NMR spectroscopy. Chem. Phys. Lett. 376, 62–67. 10.1016/S0009-2614(03)00951-5 [DOI] [Google Scholar]
- Gan Z. (2006). Measuring multiple carbon-nitrogen distances in natural abundant solids using R-RESPDOR NMR. Chem Commun (Camb). 7, 4712–4714. 10.1039/b611447d [DOI] [PubMed] [Google Scholar]
- Gan Z., Amoureux J. P., Trébosc J. (2007). Proton-detected 14N MAS NMR using homonuclear decoupled rotary resonance. Chem. Phys. Lett. 435, 163–169. 10.1016/j.cplett.2006.12.066 [DOI] [Google Scholar]
- Gan Z., Hung I., Nishiyama Y., Amoureux J. P., Lafon O., Nagashima H., et al. (2018). 14N overtone nuclear magnetic resonance of rotating solids. J. Chem. Phys. 149, 064201. 10.1063/1.5044653 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gullion T. (2007). Rotational-Echo, Double-Resonance NMR. MRI. 1, 713–718. 10.1007/1-4020-3910-7_89 [DOI] [PubMed] [Google Scholar]
- Gullion T., Schaefer J. (1989). Rotational-echo double-resonance NMR. J. Magn. Reson. Imaging. 81, 196–200. 10.1016/0022-2364(89)90280-1 [DOI] [PubMed] [Google Scholar]
- Guzmán-Afonso C., Hong Y. L., Colaux H., Iijima H., Saitow A., Fukumura T., et al. (2019). Understanding hydrogen-bonding structures of molecular crystals via electron and NMR nanocrystallography. Nat. Commun. 10, 3537. 10.1038/s41467-019-11469-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haies IM, Jarvis JA, Bentley H, Heinmaa I, Kuprov I, Williamson PT, et al. (2015a). (14)N overtone NMR under MAS: signal enhancement using symmetry-based sequences and novel simulation strategies. Phys. Chem. Chem. Phys. 17, 6577–87. 10.1039/C4CP03994G [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haies I. M., Jarvis J. A., Brown L. J., Kuprov I., Williamson P. T., Carravetta M. (2015b). (14)N overtone transition in double rotation solid-state NMR. Phys. Chem. Chem. Phys. 17, 23748–23753. 10.1039/C5CP03266K [DOI] [PubMed] [Google Scholar]
- Hohwy M., Jaroniec C. P., Reif B., Rienstra C. M., Griffin R. G. (2000). Local structure and relaxation in solid-state NMR: accurate measurement of amide N−H bond lengths and H−N−H bond angles. J. Am. Chem. Soc. 122, 3218–3219. 10.1021/ja9913737 [DOI] [Google Scholar]
- Hou G., Byeon I. J. L., Ahn J., Gronenborn A. M., Polenova T. (2011). 1H-13C/1H-15N heteronuclear dipolar recoupling by R-symmetry sequences under fast magic angle spinning for dynamics analysis of biological and organic solids. J. Am. Chem. Soc. 133, 18646–18655. 10.1021/ja203771a [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hou G., Lu X., Vega A. J., Polenova T. (2014). Accurate measurement of heteronuclear dipolar couplings by phase-alternating R-symmetry (PARS) sequences in magic angle spinning NMR spectroscopy. J. Chem. Phys. 141, 104202. 10.1063/1.4894226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hung I., Gor’kov P., Gan Z. (2019). Efficient and sideband-free 1H-detected 14N magic-angle spinning NMR. J. Chem. Phys. 151, 154202. 10.1063/1.5126599 [DOI] [PubMed] [Google Scholar]
- Hung I., Gor'kov P., Gan Z. (2020). Using the heteronuclear Bloch-Siegert shift of protons for B1 calibration of insensitive nuclei not present in the sample. J. Magn. Reson. 310, 106636. 10.1016/j.jmr.2019.106636 [DOI] [PubMed] [Google Scholar]
- Ishii Y., Terao T., Hayashi S. (1997). Theory and simulation of vibrational effects on structural measurements by solid-state nuclear magnetic resonance. J. Chem. Phys. 107, 2760–2774. 10.1063/1.474633 [DOI] [Google Scholar]
- Jarvis J. A., Concistre M., Haies I. M., Bounds R. W., Kuprov I., Carravetta M., et al. (2019). Quantitative analysis of 14N quadrupolar coupling using 1H detected 14N solid-state NMR. Phys. Chem. Chem. Phys. 21, 5941–5949. 10.1039/c8cp06276e [DOI] [PubMed] [Google Scholar]
- Jayanthi S., Ramanathan K. V. (2011). Excitation and correlation of 14N overtone transitions and measurement of heteronuclear dipolar coupling using DAPT. Chem. Phys. Lett. 502, 121–125. 10.1016/j.cplett.2010.12.007 [DOI] [Google Scholar]
- Lu X., Lafon O., Trébosc J., Amoureux J. P. (2011). Detailed analysis of the S-RESPDOR solid-state NMR method for inter-nuclear distance measurement between spin-1/2 and quadrupolar nuclei. J. Magn. Reson. 215, 34–49. 10.1016/j.jmr.2011.12.009 [DOI] [PubMed] [Google Scholar]
- Lu X., Trébosc J., Lafon O., Carnevale D., Ulzega S., Bodenhausen G., et al. (2013). Broadband excitation in solid-state NMR using interleaved DANTE pulse trains with N pulses per rotor period. J. Magn. Reson. 236, 105–116. 10.1016/j.jmr.2013.09.003 [DOI] [PubMed] [Google Scholar]
- Makrinich M., Gupta R., Polenova T., Goldbourt A. (2017). Saturation capability of short phase modulated pulses facilitates the measurement of longitudinal relaxation times of quadrupolar nuclei. Solid State Nucl. Magn. Reson. 84, 196–203. 10.1016/j.ssnmr.2017.04.003 [DOI] [PubMed] [Google Scholar]
- Makrinich M., Nimerovsky E., Goldbourt A. (2018). Pushing the limit of NMR-based distance measurements – retrieving dipolar couplings to spins with extensively large quadrupolar frequencies. Solid State Nucl. Magn. Reson. 92, 19–24. 10.1016/j.ssnmr.2018.04.001 [DOI] [PubMed] [Google Scholar]
- Nimerovsky E., Gupta R., Yehl J., Li M., Polenova T., Goldbourt A. (2014). Phase-modulated LA-REDOR: A robust, accurate and efficient solid-state NMR technique for distance measurements between a spin-1/2 and a quadrupole spin. J. Magn. Reson. 244, 107–113. 10.1016/j.jmr.2014.03.003 [DOI] [PubMed] [Google Scholar]
- Nimerovsky E, Makrinich M, Goldbourt A. (2017). Analysis of large-anisotropy-spin recoupling pulses for distance measurement under magic-angle spinning NMR shows the superiority and robustness of a phase modulated saturation pulse. J. Chem. Phys. 146, 124202. 10.1063/1.4978472 [DOI] [PubMed] [Google Scholar]
- Nishiyama Y. (2016). Fast magic-angle sample spinning solid-state NMR at 60-100kHz for natural abundance samples. Solid State Nucl. Magn. Reson. 78, 24–36. 10.1016/j.ssnmr.2016.06.002 [DOI] [PubMed] [Google Scholar]
- Nishiyama Y., Agarwal V., Zhang R. (2020). t 1-Noise Suppression by γ-Free Recoupling Sequences in Solid-State NMR for Structural Characterization of Fully Protonated Molecules at Fast MAS. J. Phys. Chem. C. 128, 26332–26343. 10.1021/acs.jpcc.0c08828 [DOI] [Google Scholar]
- Nishiyama Y, Endo Y, Nemoto T, Utsumi H, Yamauchi K, Hioka K, et al. (2011). Very fast magic angle spinning (1)H-(14)N 2D solid-state NMR: sub-micro-liter sample data collection in a few minutes. J. Magn. Reson. 208, 44–48. 10.1016/j.jmr.2010.10.001 [DOI] [PubMed] [Google Scholar]
- Nishiyama Y., Malon M., Gan Z., Endo Y., Nemoto T. (2013). Proton-nitrogen-14 overtone two-dimensional correlation NMR spectroscopy of solid-sample at very fast magic angle sample spinning. J. Magn. Reson. 230, 160–164. 10.1016/j.jmr.2013.02.015 [DOI] [PubMed] [Google Scholar]
- Nishiyama Y., Malon M., Potrzebowski M. J., Paluch P., Amoureux J. P. (2016). Accurate NMR determination of C-H or N-H distances for unlabeled molecules. Solid State Nucl. Magn. Reson. 73, 15–21. 10.1016/j.ssnmr.2015.06.005 [DOI] [PubMed] [Google Scholar]
- O’Dell L. A., Brinkmann A. (2013). 14N overtone NMR spectra under magic angle spinning: Experiments and numerically exact simulations. J. Chem. Phys. 138, 064201. 10.1063/1.4775592 [DOI] [PubMed] [Google Scholar]
- O’Dell L. A., He R., Pandohee J. (2013). Identifying H–N proximities in solid-state NMR using 14N overtone irradiation under fast MAS. CrystEngComm. 15, 8657–8667. 10.1039/c3ce40967h [DOI] [Google Scholar]
- O’Dell L. A., Ratcliffe C. I. (2011). 14N magic angle spinning overtone NMR spectra. J. Chem. Phys. 514, 168–173. 10.1016/j.cplett.2011.08.030 [DOI] [Google Scholar]
- Paluch P., Pawlak T., Amoureux J. P., Potrzebowski M. J. (2013). Simple and accurate determination of X-H distances under ultra-fast MAS NMR. J. Magn. Reson. 233, 56–63. 10.1016/j.jmr.2013.05.005 [DOI] [PubMed] [Google Scholar]
- Pandey M. K., Amoureux J. P., Asakura T., Nishiyama Y. (2016). Sensitivity enhanced (14)N/(14)N correlations to probe inter-beta-sheet interactions using fast magic angle spinning solid-state NMR in biological solids. Phys. Chem. Chem. Phys. 18, 22583–22589. 10.1039/c6cp03848d [DOI] [PubMed] [Google Scholar]
- Pandey M. K., Nishiyama Y. (2015). Proton-detected 3D 14N/14N/1H isotropic shift correlation experiment mediated through 1H-1H RFDR mixing on a natural abundant sample under ultrafast MAS. J. Magn. Reson. 258, 96–101. 10.1016/j.jmr.2015.06.012 [DOI] [PubMed] [Google Scholar]
- Pandey M. K., Nishiyama Y. (2018). A one-dimensional solid-state NMR approach for 14NH/14NH overtone correlation through 1H/1H mixing under fast MAS. Phys. Chem. Chem. Phys. 20, 25849–25853. 10.1039/c8cp05000g [DOI] [PubMed] [Google Scholar]
- Pourpoint F., Trébosc J., Bonhomme C., Durupthy O., Steunou N., Lafon O., et al. (2014). Quantitative analysis of the proximities of OH ligands and vanadium sites in a polyoxovanadate cluster using frequency-selective 1H-51V solid-state NMR spectroscopy. J. Phys. Chem. C. 118, 18580–18588. 10.1021/jp505194q [DOI] [Google Scholar]
- Pruski M., Bailly A., Lang D. P., Amoureux J. P., Fernandez C. (1999). Studies of heteronuclear dipolar interactions between spin-1/2 and quadrupolar nuclei by using REDOR during multiple quantum evolution. Chem. Phys. Lett. 307, 35–40. 10.1016/S0009-2614(99)00490-X [DOI] [Google Scholar]
- Rankin A. G. M., Trébosc J., Paluch P., Lafon O., Amoureux J. P. (2019). Evaluation of excitation schemes for indirect detection of 14N via solid-state HMQC NMR experiments. J Magn Reson. 303, 28–41. 10.1016/j.jmr.2019.04.004 [DOI] [PubMed] [Google Scholar]
- Schanda P., Meier B. H., Ernst M. (2011). Accurate measurement of one-bond H-X heteronuclear dipolar couplings in MAS solid-state NMR. J. Magn. Reson. 210, 246–259. 10.1016/j.jmr.2011.03.015 [DOI] [PubMed] [Google Scholar]
- Schnell I., Saalwächter K. (2002). (15)N-(1)H bond length determination in natural abundance by inverse detection in fast-MAS solid-state NMR spectroscopy. J. Am. Chem. Soc. 124, 10938–10939. 10.1021/ja026657x [DOI] [PubMed] [Google Scholar]
- Shen M., Chen Q., Hu B. (2017). Composite pulses in directly and indirectly detected 14N MAS overtone spectroscopy. Solid State Nucl. Magn. Reson. 84, 132–136. 10.1016/j.ssnmr.2017.01.007 [DOI] [PubMed] [Google Scholar]
- Shen M., Trébosc J., O'Dell L. A., Lafon O., Pourpoint F., Hu B., et al. (2015). Comparison of various NMR methods for the indirect detection of nitrogen-14 nuclei via protons in solids. J. Magn. Reson. 258, 86–95. 10.1016/j.jmr.2015.06.008 [DOI] [PubMed] [Google Scholar]
- Tycko R., Opella S. J. (1987). Overtone NMR spectroscopy. J. Chem. Phys. 86, 1761. 10.1063/1.452176 [DOI] [Google Scholar]
- Vitzthum V., Caporini M. A., Ulzega S., Bodenhausen G. (2011). Broadband excitation and indirect detection of nitrogen-14 in rotating solids using Delays Alternating with Nutation (DANTE). J. Magn. Reson. 212, 234–239. 10.1016/j.jmr.2011.06.013 [DOI] [PubMed] [Google Scholar]
- Vitzthum V., Caporini M. A., Ulzega S., Trébosc J., Lafon O., Amoureux J. P., et al. (2012). Uniform broadband excitation of crystallites in rotating solids using interleaved sequences of delays alternating with nutation. J. Magn. Reson. 223, 228–236. 10.1016/j.jmr.2012.05.024 [DOI] [PubMed] [Google Scholar]
- Wijesekara A. V., Venkatesh A., Lampkin B. J., VanVeller B., Lubach J. W., Nagapudi K., et al. (2020). Fast acquisition of proton‐detected HETCOR solid‐state NMR spectra of quadrupolar nuclei and rapid measurement of NH bond lengths by frequency selective HMQC and RESPDOR pulse sequences. Chemist. 26, 7881–7888. 10.1002/chem.202000390 [DOI] [PubMed] [Google Scholar]
- Zhao L., Hanrahan M. P., Chakravarty P., Dipasquale A. G., Sirois L. E., Nagapudi K., et al. (2018). Characterization of pharmaceutical cocrystals and salts by dynamic nuclear polarization-enhanced solid-state NMR spectroscopy. Cryst. Growth Des. 18, 2588–2601. 10.1021/acs.cgd.8b00203 [DOI] [Google Scholar]
- Zhao X., Sudmeier J. L., Bachovchin W. W., Levitt M. H. (2001). Measurement of NH bond lengths by fast magic-angle spinning solid-state NMR spectroscopy: A new method for the quantification of hydrogen bonds. J. Am. Chem. Soc. 123, 11097–11098. 10.1021/ja016328p [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material; further inquiries can be directed to the corresponding author.