Abstract
We present a HIV-infected patient who developed severe anaemia due to chronic parvovirus B19 infection and subsequently had an unplanned pregnancy. This is in the context of poor adherence to antiretroviral therapy and significant immunosuppression; there was a delay in diagnosis of chronic parvovirus infection due to attribution of anaemia to HIV. She received immunoglobulin therapy and effective antiretroviral therapy, with reduction in parvovirus load and improvement in anaemia. She was counselled regarding the need for monitoring in pregnancy due to risk of intrauterine infection. We review the literature of management of chronic parvovirus infection in the immunosuppressed and the consequences of intrauterine infection.
Keywords: haematology (incl blood transfusion), HIV / AIDS, infectious diseases, materno-fetal medicine
Background
Parvovirus B19 is a single-stranded DNA virus that can present with a range of clinical syndromes, including erythema infectiosum (fifth disease) in childhood, rash, arthralgia and transient aplastic crises.1 2 It is a common infectious pathogen, with seroprevalence ranging from 2%–15% in early childhood to 50%–85% in adults.3 4 Women of childbearing age have an annual seroconversion rate of 1.5%.5 6 Persistent parvovirus infection in immunocompromised individuals is due to inadequate neutralising antibody production. Parvovirus B19 replicates in human erythroid progenitor cells and can cause hypoplasia or aplasia of erythroid and erythroid precursors.2 7 Chronic suppression of erythropoiesis in persistent parvovirus infection is associated with severe anaemia requiring red cell transfusion.2 7 Predisposing conditions include haematologic malignancies, organ transplantation, chemotherapy and immunodeficiency including HIV.3
Case presentation
A 25-year-old woman diagnosed at age 15 with perinatally acquired HIV infection; at diagnosis, CD4 count 70 (5%) cells/uL with a plasma HIV viral load (VL) of 10 990 c/mL. She was hepatitis B and C negative. She had a background of chronic iron deficiency anaemia (baseline haemoglobin (Hb) 80–90 g/L, mean corpuscular volume 65–75 fL, ferritin 6 ug/L) due to poor nutrition, menorrhagia, poor adherence to iron supplementation, exacerbated by uncontrolled HIV infection. She commenced boosted protease inhibitor-based antiretroviral therapy (ART) but struggled with adherence due to unstable living circumstances and depression and anxiety during adolescence and early adulthood. She achieved sustained viral suppression during her first pregnancy at the age 20 on darunavir, ritonavir, emtricitabine and tenofovir, with a maximal rise in CD4 to 280 cells/uL, CD4:8 0.35. However postdelivery of her healthy HIV-negative child again struggled with sustained adherence to ART.
She presented to her local hospital at age 24 with acute fatigue, shortness of breath and syncope; Hb 42 g/L (white cells 6.6×109/L, lymphocytes 0.3×109/L, platelets 690×109/L). Anaemia was attributed to poor diet, menorrhagia and chronic HIV infection with extensive oral candida and shingles. There was no history of gastrointestinal bleeding. HIV VL was 27 851 c/mL, CD4 count of 28 cells/uL (4%). She was transfused with five units of packed red cells; post-transfusion Hb was 112 g/L. She commenced dolutegravir (DTG), emtricitabine and tenofovir alafenamide (F/TAF) with cotrimoxazole prophylaxis, acyclovir and fluconazole. HIV genotyping did not demonstrate HIV-1-associated drug resistance mutations.
She represented to her local hospital 1 month later with syncope and shortness of breath, Hb 45 g/L, requiring a further two units of packed red cells.
Investigations
Two weeks later she attended her HIV outpatient service with Hb 65 g/L, reticulocyte count 5.1×19/L (12–96), 0.2% (0.3%–2%), with no evidence of haemolysis (serum haptoglobin 1.46 (0.52–2.24) g/L, lactate dehydrogenase 170 (125–243) U/L). Folate was low (2.0 ug/L), and ferritin was slightly elevated (380 ng/mL). Hb electrophoresis was normal. Direct antibody testing; no red cell auto-antibodies were present. CD4 23 cells/uL, HIV VL 52 800 c/mL. Plasma parvovirus B19 DNA was >300 billion IU/mL.
Treatment
She received intravenous immunoglobulin (IVIG) totalling 2 g/kg divided over 4 days with three packed red blood cell units. Her Hb stabilised (Hb 95–105 g/L) and reticulocyte count improved (reticulocyte count 180×109/L, 4.8%). She was compliant with antiretroviral therapy and parvovirus viraemia reduced to 2.5 million after 6 weeks. She did not require further IVIG.
Three months later (HIV VL 304 c/mL on DTG and F/TAF, CD4 149 cells/uL (figure 1)), she presented with an unplanned pregnancy, estimated 10-week gestation by ultrasound.
Figure 1.
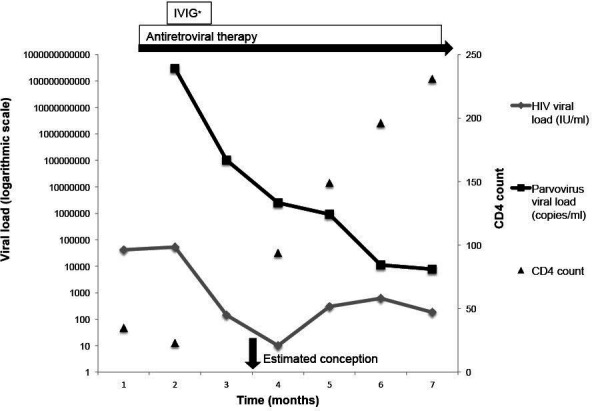
Viral loads and CD4 counts over time. *One course of IVIG given at 500 mg/kg/day for 4 days. IVIG, intravenous immunoglobulin.
Outcome and follow-up
She was counselled that conceiving on DTG was potentially associated with a slightly higher risk of neural tube defects compared with other ART exposure at conception,8 but as postneural tube closure, it was recommended to continue with the current regimen, switching F/TAF to tenofovir desproxil fumarate due to lack of data with F/TAF in pregnancy.9 10
Parvovirus viraemia was 921 000 DNA IU/mL (figure 1), and she was counselled that there was a risk of in utero infection given ongoing viraemia and immunosuppression as well as fetal loss. The need for close monitoring in pregnancy was discussed. She subsequently elected for a first trimester termination of pregnancy due to unplanned pregnancy and family complexities. In 18 months of follow-up, she has maintained an HIV plasma VL <200 c/mL with immune reconstitution to a CD4 count 559 cells/uL, CD4:8 0.6, and parvovirus 670 DNA IU/mL.
Discussion
Severe chronic parvovirus infection causing anaemia has been reported in immunosuppressed patients. We will consider current evidence for management strategies of chronic parvovirus infection in people living with HIV and maternal and fetal implications.
Most case reports of severe parvovirus infection in advanced HIV occurred before widespread availability of ART, with few recent publications for IVIG use patients with HIV in this setting. Our patient presented with severe anaemia and reticulocytopenia, without evidence of haemolysis, and reduced total lymphocyte count in the setting of advanced HIV, suggesting pure red cell aplasia due to parvovirus infection. Initiation of ART and immune reconstitution is integral to sustained remission of chronic parvovirus infection in HIV.11 12 There is no specific antiviral therapy for parvovirus B19. Management strategies include reduction of immunosuppression and the use of immunoglobulin. Seroprevalence in adults is between 50% and 85%, and IVIG contains a large amount of parvovirus B19 IgG.3 13 Published case reports indicate that IVIG can temporarily reduce parvovirus viraemia and anaemia, though dosing regimens are uncertain.14–16 Guidance exists for treatment of symptomatic parvovirus B19 in solid organ transplant recipients with IVIG.13 When considering patients with other causes of immunosuppression, the literature indicates that at least 2 g/kg of IVIG per treatment course is required, as doses <1 g/kg were often unsuccessful.16 Most treatment courses for solid organ transplant prescribe IVIG 400 mg/kg over 5 days, though shorter treatment courses with higher daily doses have been used.13 Shorter courses with daily doses >1 g/kg have been associated with adverse effects including nephrotoxicity and pulmonary oedema.16
The incidence of primary parvovirus B19 infection during pregnancy has been estimated as 1%–2%, though can exceed 10% in epidemic periods.17–19 Transplacental transmission occurs in approximately one-third of infected immunocompetent pregnant women.6 18 20–22 There are no estimates for fetal parvovirus infection in immunocompromised mothers.
This case raises maternal and fetal considerations in management and the risk of maternal–fetal transmission of HIV and parvovirus. There was a potential risk of in utero HIV transmission due to detectable HIV VL at conception and during the first trimester, and suppressed CD4 count, though mitigated by subsequent adherence to medication. The risk of parvovirus transmission was difficult to estimate; it is unclear if fetal parvovirus transmission is related to the peak or duration of viraemia. The risk of transplacental parvovirus transmission increases in later gestations of pregnancy.6 However, the risk to the fetus is higher if infected at earlier gestations. The risk of fetal anaemia, hydrops fetalis and fetal loss due to parvovirus infection is higher when women are infected in the first half of pregnancy, with the risk of hydrops fetalis estimated to range from 3% to 17%.23–25 The risk of fetal loss is estimated at 11% before 20 weeks gestation and <1% after 20 weeks gestation.24–26 For our patient, the peak of parvovirus viraemia occurred prior to conception through the fetus was exposed for a longer period. Viral kinetics in immunocompetent individuals suggest slow clearance of parvovirus B19 viraemia after acute infection, even following parvovirus IgM and IgG production.27 She had good clinical response to ART and IVIG with stabilisation of Hb and CD4 suggesting resolution of acute infection, though slow clearance of parvovirus B19.
Fetal parvovirus infection resulting in destruction of erythroid progenitor cells and fetal anaemia is a postulated mechanism for hydrops fetalis.28 However, viral myocarditis and heart failure are other possible mechanisms.29 Fetal anaemia can be assessed by measuring middle cerebral artery peak systolic velocity, though is most accurately measured from fetal blood sampling, which is associated with a 1%–2% fetal loss rate and risks vertical transmission if the mother was not on suppressive ART.30 31 A large survey of obstetricians suggested that intrauterine transfusion reduced the risk of death of the foetus in cases of severe hydrops fetalis secondary to intrauterine parvovirus B19 infection.22 32
The evidence for IVIG to treat acute parvovirus infection in pregnancy is limited, having only been described in one case report.33 It is not currently recommended as routine therapy.
Our case demonstrates the importance of effective ART and immune reconstitution in managing chronic parvovirus infection in patients with HIV. Our patient has remained adherent to ART with immune reconstitution, reduction in parvovirus VL and resolution of anaemia. Effective ART is fundamental in optimising maternal and fetal health and reducing risk of mother-to-child transmission.
Learning points.
Consider parvovirus infection as a cause of acute anaemia in patients with HIV who are not on effective antiretroviral therapy (ART).
Effective ART and immune reconstitution are important in managing chronic parvovirus infection in patients with HIV.
Intravenous immunoglobulin can temporarily reduce parvovirus viraemia in immunosuppressed patients, though evidence for this treatment is mainly in solid organ transplant recipients.
Parvovirus B19 infection in pregnancy can be associated with complications including intrauterine fetal death, miscarriage and nonimmune hydrops fetalis.
Footnotes
Contributors: CF and PR conceptualised the article. HH was the primary author. All authors participated in the editing process. All authors provided substantial contributions to the final manuscript.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: None declared.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.de Jong EP, de Haan TR, Kroes ACM, et al. Parvovirus B19 infection in pregnancy. J Clin Virol 2006;36:1–7. 10.1016/j.jcv.2006.01.004 [DOI] [PubMed] [Google Scholar]
- 2.Young NS, Brown KE. Parvovirus B19. N Engl J Med 2004;350:586–97. 10.1056/NEJMra030840 [DOI] [PubMed] [Google Scholar]
- 3.Heegaard ED, Brown KE. Human parvovirus B19. Clin Microbiol Rev 2002;15:485–505. 10.1128/CMR.15.3.485-505.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kelly HA, Siebert D, Hammond R, et al. The age-specific prevalence of human parvovirus immunity in Victoria, Australia compared with other parts of the world. Epidemiol Infect 2000;124:449–57. 10.1017/S0950268899003817 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Koch WC, Adler SP. Human parvovirus B19 infections in women of childbearing age and within families. Pediatr Infect Dis J 1989;8:83–7. [PubMed] [Google Scholar]
- 6.Prospective study of human parvovirus (B19) infection in pregnancy. public health laboratory service Working Party on fifth disease. BMJ 1990;300:1166–70. 10.1136/bmj.300.6733.1166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Frickhofen N, Abkowitz JL, Safford M, et al. Persistent B19 parvovirus infection in patients infected with human immunodeficiency virus type 1 (HIV-1): a treatable cause of anemia in AIDS. Ann Intern Med 1990;113:926–33. 10.7326/0003-4819-113-12-926 [DOI] [PubMed] [Google Scholar]
- 8.Zash R, Holmes L, Diseko M, et al. Neural-Tube defects and antiretroviral treatment regimens in Botswana. N Engl J Med 2019;381:827–40. 10.1056/NEJMoa1905230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.British HIV Association . British HIV association guidelines for the management of HIV in pregnancy and postpartum 2018, 2018. Available: https://www.bhiva.org/pregnancy-guidelines [DOI] [PubMed]
- 10.Foster C, Fidler S, Lyall E, et al. Careful consideration when responding to new data: dolutegravir and pregnancy. J Virus Erad 2018;4:208. 10.1016/S2055-6640(20)30262-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ware AJ, Moore T. Resolution of chronic parvovirus B19-induced anemia, by use of highly active antiretroviral therapy, in a patient with acquired immunodeficiency syndrome. Clin Infect Dis 2001;32:e122–3. 10.1086/319590 [DOI] [PubMed] [Google Scholar]
- 12.Mylonakis E, Dickinson BP, Mileno MD, et al. Persistent parvovirus B19 related anemia of seven years' duration in an HIV-infected patient: complete remission associated with highly active antiretroviral therapy. Am J Hematol 1999;60:164–6. [DOI] [PubMed] [Google Scholar]
- 13.Eid AJ, Posfay-Barbe KM, ASTIDCo P, AST Infectious Diseases Community of Practice . Parvovirus B19 in solid organ transplant recipients. Am J Transplant 2009;9 Suppl 4:S147–50. 10.1111/j.1600-6143.2009.02905.x [DOI] [PubMed] [Google Scholar]
- 14.Gottlieb F, Deutsch J. Red cell aplasia responsive to immunoglobulin therapy as initial manifestation of human immunodeficiency virus infection. Am J Med 1992;92:331–3. 10.1016/0002-9343(92)90085-P [DOI] [PubMed] [Google Scholar]
- 15.Koduri PR. Parvovirus B19-related anemia in HIV-infected patients. AIDS Patient Care STDS 2000;14:7–11. 10.1089/108729100318082 [DOI] [PubMed] [Google Scholar]
- 16.Crabol Y, Terrier B, Rozenberg F, et al. Intravenous immunoglobulin therapy for pure red cell aplasia related to human parvovirus B19 infection: a retrospective study of 10 patients and review of the literature. Clin Infect Dis 2013;56:968–77. 10.1093/cid/cis1046 [DOI] [PubMed] [Google Scholar]
- 17.Jensen IP, Thorsen P, Jeune B, et al. An epidemic of parvovirus B19 in a population of 3,596 pregnant women: a study of sociodemographic and medical risk factors. BJOG 2000;107:637–43. 10.1111/j.1471-0528.2000.tb13306.x [DOI] [PubMed] [Google Scholar]
- 18.van Gessel PH, Gaytant MA, Vossen ACTM, et al. Incidence of parvovirus B19 infection among an unselected population of pregnant women in the Netherlands: a prospective study. Eur J Obstet Gynecol Reprod Biol 2006;128:46–9. 10.1016/j.ejogrb.2005.11.042 [DOI] [PubMed] [Google Scholar]
- 19.Valeur-Jensen AK, Pedersen CB, Westergaard T, et al. Risk factors for parvovirus B19 infection in pregnancy. JAMA 1999;281:1099–105. 10.1001/jama.281.12.1099 [DOI] [PubMed] [Google Scholar]
- 20.Harger JH, Adler SP, Koch WC, et al. Prospective evaluation of 618 pregnant women exposed to parvovirus B19: risks and symptoms. Obstet Gynecol 1998;91:413–20. 10.1016/S0029-7844(97)00701-1 [DOI] [PubMed] [Google Scholar]
- 21.Gratacós E, Torres PJ, Vidal J, et al. The incidence of human parvovirus B19 infection during pregnancy and its impact on perinatal outcome. J Infect Dis 1995;171:1360–3. 10.1093/infdis/171.5.1360 [DOI] [PubMed] [Google Scholar]
- 22.Rodis JF, Borgida AF, Wilson M, et al. Management of parvovirus infection in pregnancy and outcomes of hydrops: a survey of members of the Society of perinatal obstetricians. Am J Obstet Gynecol 1998;179:985–8. 10.1016/S0002-9378(98)70203-0 [DOI] [PubMed] [Google Scholar]
- 23.Bascietto F, Liberati M, Murgano D, et al. Outcome of fetuses with congenital parvovirus B19 infection: systematic review and meta-analysis. Ultrasound Obstet Gynecol 2018;52:569–76. 10.1002/uog.19092 [DOI] [PubMed] [Google Scholar]
- 24.Enders M, Weidner A, Zoellner I, et al. Fetal morbidity and mortality after acute human parvovirus B19 infection in pregnancy: prospective evaluation of 1018 cases. Prenat Diagn 2004;24:513–8. 10.1002/pd.940 [DOI] [PubMed] [Google Scholar]
- 25.Miller E, Fairley CK, Cohen BJ, et al. Immediate and long term outcome of human parvovirus B19 infection in pregnancy. Br J Obstet Gynaecol 1998;105:174–8. 10.1111/j.1471-0528.1998.tb10048.x [DOI] [PubMed] [Google Scholar]
- 26.Sarfraz AA, Samuelsen SO, Bruu A-L, et al. Maternal human parvovirus B19 infection and the risk of fetal death and low birthweight: a case-control study within 35 940 pregnant women. BJOG 2009;116:1492–8. 10.1111/j.1471-0528.2009.02211.x [DOI] [PubMed] [Google Scholar]
- 27.Lindblom A, Isa A, Norbeck O, et al. Slow clearance of human parvovirus B19 viremia following acute infection. Clin Infect Dis 2005;41:1201–3. 10.1086/444503 [DOI] [PubMed] [Google Scholar]
- 28.Morey AL, Keeling JW, Porter HJ, et al. Clinical and histopathological features of parvovirus B19 infection in the human fetus. Br J Obstet Gynaecol 1992;99:566–74. 10.1111/j.1471-0528.1992.tb13822.x [DOI] [PubMed] [Google Scholar]
- 29.Nigro G, Bastianon V, Colloridi V, et al. Human parvovirus B19 infection in infancy associated with acute and chronic lymphocytic myocarditis and high cytokine levels: report of 3 cases and review. Clin Infect Dis 2000;31:65–9. 10.1086/313929 [DOI] [PubMed] [Google Scholar]
- 30.Cosmi E, Mari G, Delle Chiaie L, et al. Noninvasive diagnosis by Doppler ultrasonography of fetal anemia resulting from parvovirus infection. Am J Obstet Gynecol 2002;187:1290–3. 10.1067/mob.2002.128024 [DOI] [PubMed] [Google Scholar]
- 31.Tongsong T, Wanapirak C, Piyamongkol W, et al. Second-Trimester cordocentesis and the risk of small for gestational age and preterm birth. Obstet Gynecol 2014;124:919–25. 10.1097/AOG.0000000000000502 [DOI] [PubMed] [Google Scholar]
- 32.Fairley CK, Smoleniec JS, Caul OE, et al. Observational study of effect of intrauterine transfusions on outcome of fetal hydrops after parvovirus B19 infection. Lancet 1995;346:1335–7. 10.1016/S0140-6736(95)92346-2 [DOI] [PubMed] [Google Scholar]
- 33.Selbing A, Josefsson A, Dahle LO, et al. Parvovirus B19 infection during pregnancy treated with high-dose intravenous gammaglobulin. Lancet 1995;345:660–1. 10.1016/S0140-6736(95)90569-3 [DOI] [PubMed] [Google Scholar]


