Abstract
Objective
To investigate the efficacy, safety, immunogenicity and pharmacokinetics of biosimilar adalimumab (ADL) PF-06410293 (ADL-PF; adalimumab-afzb) versus EU-sourced reference ADL (ADL-EU) in patients with active rheumatoid arthritis (RA) on longer-term treatment and after being switched from ADL-EU to ADL-PF.
Methods
In this multinational, double-blind study, patients with active RA were initially randomised to ADL-PF or ADL-EU for 26 weeks (treatment period (TP) 1). At the start of TP2 (weeks 26–52), patients in the ADL-EU arm were blindly re-randomised 1:1 to remain on ADL-EU (ADL-EU/ADL-EU; n=135) or switched to ADL-PF (ADL-EU/ADL-PF; n=134); patients receiving ADL-PF continued blinded treatment (ADL-PF/ADL-PF; n=283).
Results
The American College of Rheumatology 20% improvement (ACR20) response rates were comparable between treatment groups at all visits during TP2. At week 52, ACR20 response rates were 82.7% (ADL-PF/ADL-PF), 79.3% (ADL-EU/ADL-EU) and 84.3% (ADL-EU/ADL-PF). Other measures of deep response (ACR50/70, ACR/EULAR-defined remission, EULAR good response, and Disease Activity Score in 28 Joints Based on High-Sensitivity C-Reactive Protein <2.6) and Health Assessment Questionnaire−Disability Index were maintained over TP2 and comparable between groups. Treatment-emergent adverse events were reported in 43.5% (ADL-PF/ADL-PF), 44.4% (ADL-EU/ADL-EU) and 38.3% (ADL-EU/ADL-PF) of patients; there were no clinically meaningful differences in the safety profiles between groups. The percentage of patients who were antidrug antibody positive was comparable overall among ADL-PF/ADL-PF (47.3%), ADL-EU/ADL-EU (54.1%) and ADL-EU/ADL-PF (45.9%).
Conclusions
The similar efficacy, safety, immunogenicity and pharmacokinetics of ADL-PF and ADL-EU, maintained up to week 52, were unaffected by blinded treatment switch from ADL-EU to ADL-PF at week 26.
Trial registration number
ClinicalTrials.gov identifier: NCT02480153; EudraCT number: 2014-000352-29.
Keywords: adalimumab, arthritis, rheumatoid, biosimilar pharmaceuticals
Key messages.
What is already known about this subject?
Treatment period 1 (weeks 0–26) of a randomised controlled trial in patients with active rheumatoid arthritis demonstrated similar efficacy, safety, immunogenicity and pharmacological profiles of biosimilar adalimumab PF-06410293 (ADL-PF) and reference ADL sourced from the European Union (ADL-EU).
What does this study add?
These results from treatment period 2 (weeks 26–52) of the same comparative study demonstrate that comparable efficacy, safety and immunogenicity between ADL-PF and ADL-EU was maintained up to week 52 and was unaffected by a blinded treatment switch from ADL-EU to ADL-PF at week 26.
How might this impact on clinical practice?
These findings provide long-term clinical data that complement the existing evidence for biosimilarity of PF-06410293 to reference ADL and continue to support the use of PF-06410293 as an ADL biosimilar in clinical practice.
Introduction
Adalimumab (ADL) is a recombinant, fully human IgG1 monoclonal antibody that binds specifically to tumour necrosis factor alpha (TNFα) and interferes with its binding to cell surface TNF receptors.1 Binding of ADL leads to a subsequent reduction of disease symptoms and inhibition of radiographic progression in a majority of patients.2–4 Biologic original disease-modifying antirheumatic drug (boDMARD) therapies, including ADL, have greatly expanded effective treatment options for patients with active rheumatoid arthritis (RA) and other inflammatory and autoimmune diseases.5 Patient access to boDMARDs has historically been limited because of costs.6
Biosimilars are biopharmaceuticals that are highly similar to an already licensed biologic product (known as the reference or originator product), notwithstanding minor differences in clinically inactive components and for which there are no clinically meaningful differences in purity, potency and safety between the two products.7 8 PF-06410293 (ADL-PF; adalimumab-afzb; Pfizer Inc, New York, New York, USA and Pfizer Europe MA EEIG, Bruxelles, Belgium) was recently approved by the US Food and Drug Administration and the European Medicines Agency as an ADL biosimilar DMARD (bsDMARD) for the treatment of all indications of reference ADL, the boDMARD (Humira; AbbVie Inc, North Chicago, Illinois, USA; and AbbVie Deutschland GmbH Co. KG, Ludwigshafen, Germany), except for paediatric Crohn’s disease, hidradenitis suppurativa and uveitis in the USA, as these have orphan status.9 10
Biosimilarity is established based on the totality of the evidence from comparative analytical and non-clinical testing, a clinical bioequivalence study and a comparative clinical study (ie, pharmacokinetics (PK), pharmacodynamics (PD), efficacy, safety and immunogenicity) conducted in a patient population that is sufficiently sensitive to detect differences between the biosimilar product and reference product, should they exist.7 8 Therapeutic equivalence of ADL-PF and reference ADL sourced from the European Union (ADL-EU; AbbVie Deutschland GmbH Co. KG, Ludwigshafen, Germany) was demonstrated based on the primary efficacy endpoint of American College of Rheumatology (ACR) 20% improvement (ACR20) at week 12 in a randomised controlled trial of patients with RA.11 In addition, the safety, immunogenicity, PK and PD of ADL-PF and ADL-EU were demonstrated to be similar.11 The design of this randomised controlled trial also allowed for assessment of the impact of patients switching from ADL-EU to ADL-PF after 26 weeks. Here, we report the efficacy, safety, immunogenicity, PK and PD of ADL-PF in comparison with ADL-EU in patients with active RA on longer-term treatment, and following a treatment switch from ADL-EU to ADL-PF in a subset of patients.
Methods
The study population and design have been described in detail in a previous publication and are briefly summarised here.11
Study population
Adults (aged ≥18 years) with a diagnosis of RA ≥4 months, based on the 2010 ACR/EULAR criteria,12 were eligible for inclusion in this study. Patients must have been treated with methotrexate (MTX) for ≥12 weeks and been on a stable dose of 10–25 mg/week for ≥4 weeks before the first dose of study drug. In geographical regions where 6 mg/week was a recommended initial dose by local guidance or standard of care, patients could receive a lower dose (6–25 mg/week).
Study design and treatments
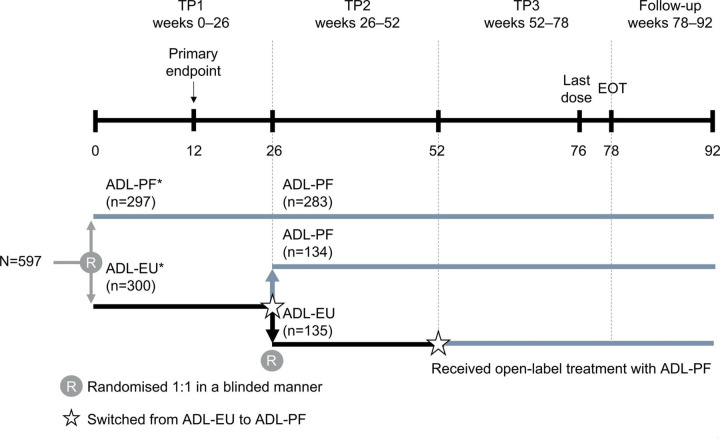
This multinational, two-armed, randomised, double-blind, parallel-group study in patients with active RA despite MTX was conducted in 173 centres across 24 countries.11 In treatment period (TP) 1 (weeks 0–26), patients were randomised 1:1 (stratified by geographical regions (North America and Western Europe, Japan, Republic of Korea and Taiwan, Latin America and the Rest of World)) to receive either ADL-PF or ADL-EU for 26 weeks while continuing MTX (figure 1). Patients who had demonstrated a ≥20% improvement from baseline in tender joint count (68 joints assessed) and swollen joint count (66 joints assessed) at the end of TP1 were eligible to continue into TP2 (weeks 26–52). Patients who failed to achieve a ≥20% improvement in both tender and swollen joint counts by the end of TP1 (week 26) were discontinued from their study treatment and documented as withdrawn because of inadequate response to study treatment, unless the investigator did not consider the week-26 assessment to be representative of the patient’s response to TNF inhibition.
Figure 1.
Study design. *ADL-PF or ADL-EU (40 mg) was administered subcutaneously every 2 weeks. ADL-EU, reference adalimumab sourced from the European Union; ADL-PF, PF-06410293; EOT, end of treatment; TP, treatment period. Adapted from ‘A comparative clinical study of PF-06410293, a candidate adalimumab biosimilar, and adalimumab reference product (Humira) in the treatment of active rheumatoid arthritis’ by Fleischmann RM et al. 11
The study was also designed to assess the safety and efficacy of switching from ADL-EU to ADL-PF, which is clinically relevant as bsDMARDs have become more accessible in many countries. Before dosing at week 26 (beginning of TP2), patients in the ADL-EU arm were blindly re-randomised 1:1 to remain on ADL-EU or switched to ADL-PF, while patients receiving ADL-PF continued treatment in a blinded manner. At week 52 (beginning of TP3), all patients who remained on ADL-EU were switched to ADL-PF. ADL-PF or ADL-EU were administered as a subcutaneous injection (40 mg every other week) throughout the study, in addition to a stable background dose of oral or intramuscular MTX (10–25 mg/week; lower doses of 6 mg/week were allowed if indicated in local guidance or standards of care, or in patients who were intolerant to MTX) and a stable background dose of oral folic (≥1 mg/day on ≥5 days/week)/folinic acid (≥5 mg once/week) for ≥21 days before the first dose of study drug. Patients could be treated with additional concomitant therapies: low-dose oral corticosteroids (≤10 mg prednisone or equivalent per day); one non-steroidal anti-inflammatory drug; and non-opioid and low-potency opioid analgesics (tramadol, codeine, hydrocodone, oxycodone and propoxyphene hydrochloride or propoxyphene napsylate).
This study was conducted in compliance with the ethical principles originating in, or derived from, the Declaration of Helsinki and in compliance with all International Conference on Harmonisation Good Clinical Practice Guidelines. The study protocol, all amendments and informed consent documentation were reviewed and approved by the Institutional Review Boards and/or Independent Ethics Committees. In addition, all local regulatory requirements were followed; in particular, those affording greater protection of the safety of trial participants. All patients provided informed consent before undergoing any screening procedures. The study was sponsored by Pfizer and registered on ClinicalTrials.gov identifier: NCT02480153 and EudraCT number: 2014-000352-29.
Objectives and endpoint assessments
As reported previously,11 the primary efficacy endpoint was the proportion of patients achieving ACR20 at week 12. Therapeutic equivalence between ADL-PF and ADL-EU in the primary endpoint was demonstrated using prespecified symmetric and asymmetric margins.11 Secondary efficacy endpoints in TP2 were assessed at weeks 26, 30, 36, 44 and 52 and included ACR20/50/70; EULAR response; Disease Activity Score (DAS) 28-4 (C-reactive protein (CRP))<2.6; and ACR/EULAR-defined remission, which provided categorical and continuous measures of efficacy.
Safety endpoints included the type, incidence, severity, timing, seriousness and relatedness of adverse events (AEs), and laboratory abnormalities. AEs were graded on a scale of 1–5 in accordance with the National Cancer Institute Common Terminology Criteria for Adverse Events (NCI CTCAE; V.4.03). Prespecified treatment-emergent AE (TEAE) categories of special interest were injection-site reactions (ISRs), anaphylaxis/angioedema/urticaria, and opportunistic infections including tuberculosis (TB). Additional prespecified TEAE categories of interest included blood and lymphatic events, cardiovascular events, demyelinating conditions, gastric/hepatic events, hypersensitivity, infections and infestations, neoplasms and other. Other safety measures included vital signs, physical examinations, TB monitoring and ECGs.
Immunogenicity endpoints were the incidence and titres of antidrug antibodies (ADAs) and neutralising antibodies (NAbs) evaluated using a tiered approach of screening, confirmation and titre determination. Serum samples were analysed using a validated electrochemiluminescent immunoassay for ADA against ADL-PF or ADL-EU. An acid-dissociation step was used in the ADA assay to dissociate the binding between the drug (ADL-EU or ADL-PF) and the target (endogenous TNFα). The validation parameters of drug tolerance at the low positive control (250 ng/mL) and high positive control (8000 ng/mL) were up to 6.4 and 50 µg/mL, respectively, in the assay against ADL-EU, and were 6.4 and 150 µg/mL, respectively, in the assay against ADL-PF. A minimum required dilution (MRD; ie, by how much the sample must be diluted to avoid matrix effects) of 1:75 was used to determine ADA-negative and ADA-positive cut-offs, such that samples with ADA titres of log10(75) or <1.88 were considered ADA-negative, and those with ADA titres ≥1.88 were considered ADA-positive. ADA-positive samples were tested for NAb using a validated cell-based assay with ADL-PF or ADL-EU as a reagent. An MRD of 1:5 was used to determine NAb-negative and NAb-positive cut-offs, such that samples with NAb titres of log10(5) or <0.70 were considered NAb-negative, and those with NAb titres ≥0.70 were considered NAb-positive. PK serum samples were analysed for ADL-PF and ADL-EU using a validated analytical assay QPS, LLC (Newark, Delaware, USA) with a lower limit of quantification of 250 ng/mL. The PD endpoint was serum high-sensitivity CRP (hs-CRP) concentration.
Statistical methods
All data from TP2 were summarised descriptively based on the observed results. No imputation was applied to missing data during TP2.
The secondary efficacy endpoints were presented for each visit by the three treatment groups in the intent-to-treat (ITT) population, which was defined as all patients who were enrolled into TP2. The three treatment groups corresponded to the treatment sequence (TP1/TP2) patients received during the study and comprised: (1) ADL-PF/ADL-PF: patients who were randomised to receive ADL-PF in TP1 and enrolled in TP2; (2) ADL-EU/ADL-EU: patients who were randomised to receive ADL-EU in TP1 and randomised to continue receiving ADL-EU in TP2; and (3) ADL-EU/ADL-PF: patients who were randomised to receive ADL-EU in TP1 and randomised to receive ADL-PF in TP2.
Safety and immunogenicity analyses used the safety population, which was defined as all patients who were randomised and received ≥1 dose of study treatment in TP1, and received ≥1 dose of study drug in TP2. For the safety analysis, the incidence of AEs—including all-causality TEAEs, treatment-related AEs, serious AEs (SAEs), AEs of special interest and AEs leading to discontinuation of study treatment—and the severity of AEs (graded according to the NCI CTCAE terminology) were summarised by treatment group. For the immunogenicity analysis, the percentages of patients who tested positive for ADAs and the percentages of patients who tested positive for NAbs (among those who were confirmed ADA-positive) in TP2 were summarised by visit and by treatment group.
PK analysis was performed using the PK population, which was defined as all patients who were treated with ADL-PF or ADL-EU in TP2 who provided ≥1 post-drug concentration measurement. For the PK analysis, drug concentration–time data were summarised according to visit and treatment group. Serum drug concentrations were also summarised by ADA and NAb status. PD analysis using hs-CRP concentration over time was summarised by treatment group.
Results
Patient disposition and demographics
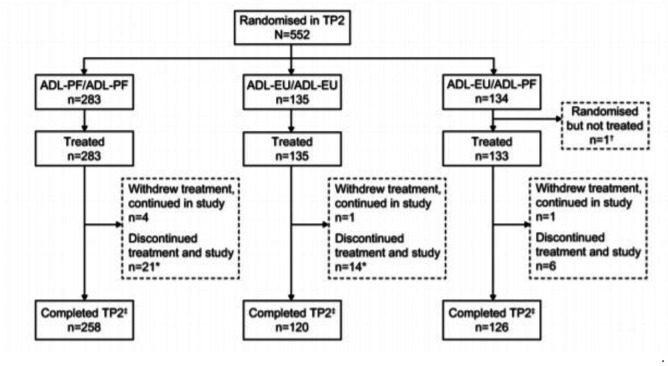
At week 26, 552 patients entered TP2 (ADL-PF/ADL-PF, n=283; ADL-EU/ADL-EU, n=135; ADL-EU/ADL-PF, n=134) (figure 2). One patient in the ADL-EU/ADL-PF group entered TP2 but was not treated. Eight (1.3%) patients (ADL-PF, n=2 (0.7%); ADL-EU, n=6 (2.0%)) discontinued from treatment during TP1 because of insufficient clinical response. In total, 258 out of 283 (91.2%) patients in the ADL-PF/ADL-PF, 120 out of 135 patients (88.9%) in the ADL-EU/ADL-EU and 126 out of 134 (94.0%) patients in the ADL-EU/ADL-PF group completed TP2. Median duration of study treatment was 50.1 weeks for all treatment groups.
Figure 2.
Patient disposition (ITT population). *One patient each in the ADL-PF/ADL-PF and ADL-EU/ADL-EU groups withdrew from treatment because of adverse events and completed the follow-up according to the study protocol, but were incorrectly recorded as ‘discontinued from study’ because of adverse events. †One patient in the ADL-EU/ADL-PF group was re-randomised but never received study treatment in TP2. This patient’s final dose of study treatment was in TP1 and so the patient should not have been re-randomised into TP2. ‡Patients did not discontinue from treatment or study during TP2. ADL-EU, reference adalimumab sourced from the European Union; ADL-PF, PF-06410293; ITT, intent-to-treat; TP, treatment period.
There were no notable differences at baseline in demographic or clinical characteristics between the treatment groups (table 1). The majority of the patients who entered TP2 were female (432/552, 78.3%) and white (479/552, 86.8%). Patients had a mean age of 52.4 years, and the mean RA disease duration was 6.9 years. Thirteen patients (2.4%) had previously been treated with ≤2 doses of one biologic drug (not ADL). The baseline mean MTX dose was 15.2 mg/week (table 1).
Table 1.
Patient demographic and clinical characteristics at baseline in TP2 (ITT population)
| ADL-PF/ADL-PF (n=283) |
ADL-EU/ADL-EU (n=135) |
ADL-EU/ADL-PF (n=134) |
|
| Demographics | |||
| Gender, n (%) | |||
| Female | 229 (80.9) | 108 (80.0) | 95 (70.9) |
| Male | 54 (19.1) | 27 (20.0) | 39 (29.1) |
| Age, mean (SD), years | 51.3 (13.7) | 53.6 (12.1) | 53.4 (13.4) |
| Weight, mean (SD), kg | 74.6 (17.7) | 76.2 (20.4) | 75.7 (18.7) |
| BMI, mean (SD), kg/m2 | 27.5 (6.2) | 28.4 (7.4) | 27.5 (6.4) |
| Race, n (%) | |||
| White | 250 (88.3) | 113 (83.7) | 116 (86.6) |
| Black | 6 (2.1) | 7 (5.2) | 2 (1.5) |
| Asian | 14 (4.9) | 8 (5.9) | 6 (4.5) |
| Other | 13 (4.6) | 7 (5.2) | 10 (7.5) |
| Clinical characteristics | |||
| RA duration, mean (SD), years | 6.9 (7.3) | 7.1 (6.6) | 6.6 (7.0) |
| Swollen joint count (66), mean (SD) | 15.1 (7.7) | 17.1 (9.6) | 17.0 (10.3) |
| Tender joint count (68), mean (SD) | 23.7 (11.9) | 26.8 (14.7) | 25.5 (15.0) |
| hs-CRP, mg/L, mean (SD) | 21.2 (22.5) | 22.0 (24.5) | 22.3 (25.9) |
| DAS28–4(CRP), mean (SD) | 5.9 (0.9) | 6.1 (0.8) | 6.0 (1.0) |
| HAQ-DI, mean (SD) | 1.5 (0.6) | 1.6 (0.7) | 1.7 (0.6) |
| Prior use of one biologic*, n (%) | 8 (2.8) | 4 (3.0) | 1 (0.7) |
| Number of prior and current non-biologic DMARDs (in addition to MTX), mean (SD) | 1.5 (0.9) | 1.5 (0.9) | 1.5 (0.8) |
| MTX dose, mean (SD), mg/week | 15.2 (4.4) | 15.7 (4.7) | 14.7 (4.0) |
| Corticosteroid use, n (%) | 155 (54.8) | 77 (57.0) | 80 (59.7) |
*Includes the use of no more than two doses of one non-adalimumab biologic drug.
ADL-EU, reference adalimumab sourced from the European Union; ADL-PF, PF-06410293; BMI, body mass index; CRP, C-reactive protein; DAS28-4(CRP), Disease Activity Score-28: 4 components using CRP; DMARD, disease-modifying antirheumatic drug; HAQ-DI, Health Assessment Questionnaire–Disability Index; hs-CRP, high-sensitivity CRP; ITT, intent-to-treat; MTX, methotrexate; RA, rheumatoid arthritis; TP2, treatment period 2.
Efficacy
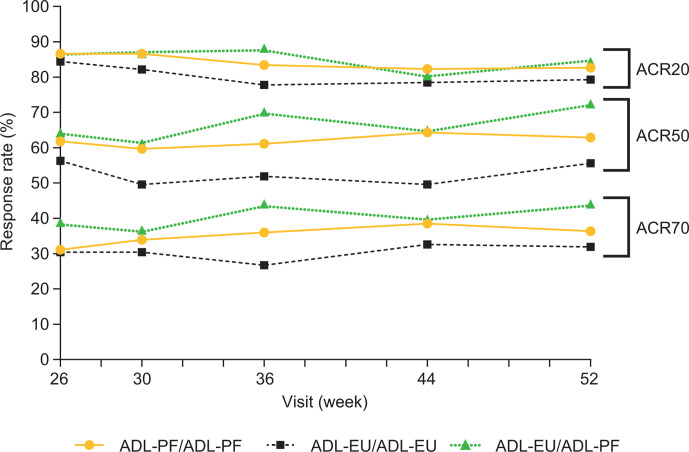
Observed ACR20/50/70 response rates were comparable between treatment groups at all visits during TP2 (figure 3). At week 26 pre-dose, ACR20 response rates were 86.6%, 84.4% and 86.6%, and at week 52 were 82.7%, 79.3% and 84.3%, respectively, for patients in the ADL-PF/ADL-PF, ADL-EU/ADL-EU and ADL-EU/ADL-PF groups. Other measures of deep response (ACR/EULAR-defined remission, EULAR good response and DAS28-4(CRP) <2.6) and individual ACR parameters, such as the Health Assessment Questionnaire–Disability Index, were maintained over TP2 and were comparable between all treatment groups (online supplemental figures 1–4).
Figure 3.
ACR20 response rates by visit (ITT population). ACR20/50/70, American College of Rheumatology 20%/50%/70% improvement; ADL-EU, reference adalimumab sourced from the European Union; ADL-PF, PF-06410293; ITT, intent-to-treat.
rmdopen-2021-001578supp001.pdf (3.9MB, pdf)
Safety
Overall, 243, 112 and 100 all-causality TEAEs were reported by 123 out of 283 (43.5%), 60 out of 135 (44.4%) and 51 out of 133 (38.3%) patients in the ADL-PF/ADL-PF, ADL-EU/ADL-EU and ADL-EU/ADL-PF groups, respectively (table 2). Infections and infestations (17.3%, 17.0% and 21.1%); investigations (7.8%, 9.6% and 7.5%), including increased blood creatinine (0.4%, 2.2% and 0%), increased alanine aminotransferase (1.8%, 3.0% and 3.0%) and increased aspartate aminotransferase (1.1%, 3.0% and 0.8%); and musculoskeletal and connective tissue disorders (7.4%, 9.6% and 7.5%), including RA (1.4%, 1.5% and 2.3%), were the most frequently reported classes of TEAEs for patients in the ADL-PF/ADL-PF, ADL-EU/ADL-EU and ADL-EU/ADL-PF groups, respectively (online supplemental table 1).
Table 2.
Summary of all-causality TEAEs and prespecified TEAEs of special interest (safety population)
| Patients with all-causality TEAEs | ADL-PF/ADL-PF (n=283) |
ADL-EU/ADL-EU (n=135) |
ADL-EU/ADL-PF (n=133) |
| Number of TEAEs | 243 | 112 | 100 |
| Patients with ≥1 TEAE, n (%) | 123 (43.5) | 60 (44.4) | 51 (38.3) |
| Patients with SAEs, n (%) | 4 (1.4) | 6 (4.4) | 3 (2.3) |
| Patients with TEAEs leading to treatment discontinuation, n (%) | 6 (2.1) | 8 (5.9) | 2 (1.5) |
| Patients with TEAEs leading to study discontinuation, n (%) | 5 (1.8) | 8 (5.9) | 1 (0.8) |
| Patients with TEAEs grade ≥3, n (%) | 7 (2.5) | 7 (5.2) | 4 (3.0) |
| Deaths, n | 0 | 0 | 0 |
| Patients with all-causality TEAEs of special interest | |||
| Category and PT, n (%) | |||
| Infections and infestations | 49 (17.3) | 23 (17.0) | 28 (21.1) |
| Blood and lymphatic system disorders | 12 (4.2) | 9 (6.7) | 4 (3.0) |
| Hypersensitivity | 10 (3.5) | 6 (4.4) | 5 (3.8) |
| Cardiac disorders | 9 (3.2) | 4 (3.0) | 3 (2.3) |
| Other | 9 (3.2) | 3 (2.2) | 2 (1.5) |
| Gastric/hepatic events | 8 (2.8) | 6 (4.4) | 4 (3.0) |
| Neoplasms | 4 (1.4)* | 1 (0.7)† | 0 |
| Opportunistic infections‡ | 3 (1.1) | 4 (3.0) | 1 (0.8) |
| Latent TB | 2 (0.7) | 2 (1.5) | 1 (0.8) |
| Herpes zoster | 1 (0.4) | 0 | 0 |
| Bronchopulmonary aspergillosis | 0 | 1 (0.7) | 0 |
| Gastroenteritis salmonella | 0 | 1 (0.7) | 0 |
| Injection-site reaction | 1 (0.4) | 0 | 1 (0.8) |
| Anaphylaxis/angioedema/urticaria | 0 | 0 | 0 |
| Demyelinating conditions | 0 | 0 | 0 |
*A total of seven events of neoplasms were reported in four patients in the ADL-PF/ADL-PF group, including one event each of seborrheic keratosis, kidney angiomyolipoma and melanocytic nevus, and two events each of basal cell carcinoma and squamous cell carcinoma. None of the events were considered to be serious.
†A total of one event of neoplasms (non-Hodgkin diffuse large B-cell lymphoma) was reported in one patient in the ADL-EU/ADL-EU group. The event of lymphoma was considered to be serious.
‡Opportunistic infections were predefined in the study as including latent TB.
ADL-EU, reference adalimumab sourced from the European Union; ADL-PF, PF-06410293; PT, preferred term; SAE, serious adverse event; TB, tuberculosis; TEAE, treatment-emergent adverse event.
There were no clinically meaningful differences between treatment groups in the incidences of reported TEAEs of special interest, including ISRs (0.4%, 0% and 0.8% for ADL-PF/ADL-PF, ADL-EU/ADL-EU and ADL-EU/ADL-PF, respectively), anaphylaxis/angioedema/urticaria (0%, 0% and 0%) or opportunistic infections (1.1%, 3.0% and 0.8%) (table 2). Serious AEs were reported in 4 out of 283 (1.4%), 6 out of 135 (4.4%) and 3 out of 133 (2.3%) patients in the ADL-PF/ADL-PF, ADL-EU/ADL-EU and ADL-EU/ADL-PF groups, respectively (table 2 and online supplemental table 2). Treatment-related SAEs were reported in 2 (0.7%), 2 (1.5%) and 0 patients in the ADL-PF/ADL-PF, ADL-EU/ADL-EU and ADL-EU/ADL-PF groups, respectively. No deaths were reported during TP2.
All-causality TEAEs of grade 3 or higher were reported by 7 (2.5%), 7 (5.2%) and 4 (3.0%) patients in the ADL-PF/ADL-PF, ADL-EU/ADL-EU and ADL-EU/ADL-PF groups, respectively (table 2 and online supplemental table 3). The number of patients who permanently discontinued from treatment or the study, or temporarily discontinued from treatment because of TEAEs in TP2, was comparable between treatment groups (table 2).
In the ADL-PF/ADL-PF, ADL-EU/ADL-EU and ADL-EU/ADL-PF groups, respectively, 7 out of 148 (4.7%), 3 out of 80 (3.8%) and 3 out of 66 (4.5%) patients who tested ADA-positive (during TP1 or TP2) reported TEAEs of hypersensitivity on or after the date of testing positive for ADA. In the total population of patients who tested ADA-positive during TP1 or TP2, none reported serious or grade ≥3 TEAEs of hypersensitivity on or after the date of testing positive for ADA, or TEAEs of hypersensitivity on or after the date of testing positive for ADA that resulted in permanent or temporary discontinuation of treatment or study. Among patients who developed their first post-dose ADA-positive test during TP2, none reported TEAEs of hypersensitivity in TP2 on or after the date of first testing ADA-positive. Among the patients who newly developed ADAs in TP2, none tested positive for NAbs. Therefore, no new efficacy or safety analyses were performed for the NAb-positive patient subgroup in TP2.
Immunogenicity
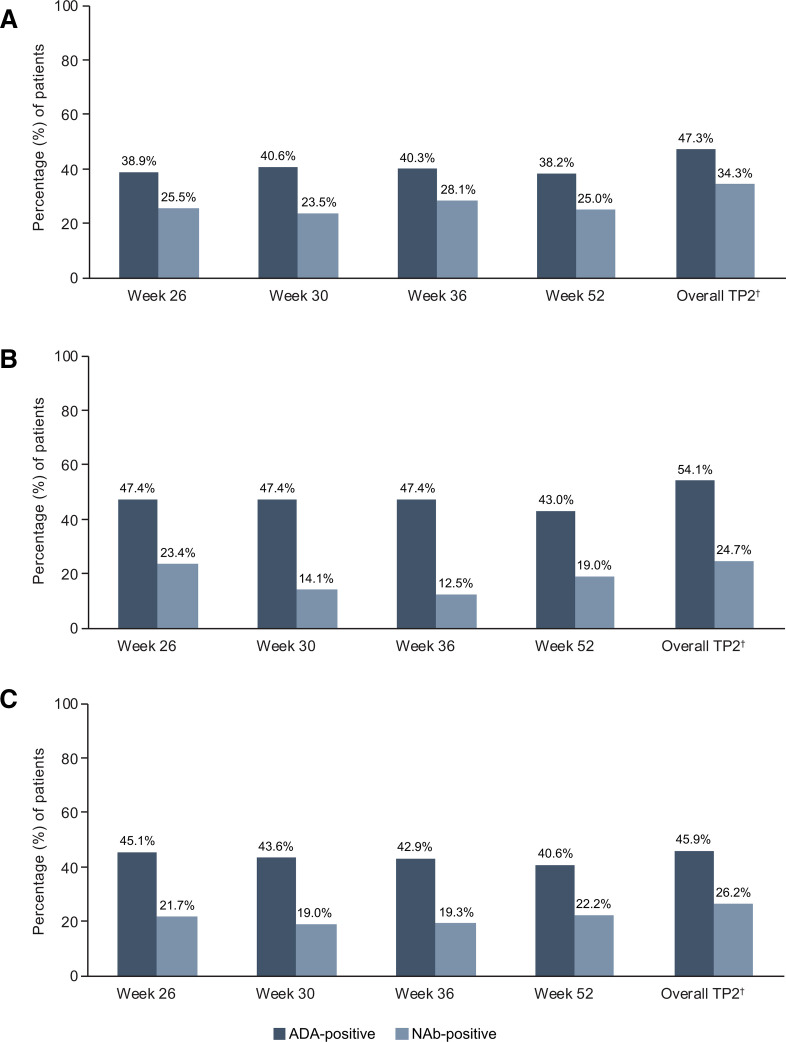
Overall, incidences of ADA through to week 52 were comparable between ADL-PF/ADL-PF (47.3%), ADL-EU/ADL-EU (54.1%) and ADL-EU/ADL-PF (45.9%) treatment groups (figure 4). Specifically, for patients in the ADL-EU/ADL-PF group compared with patients in the ADL-EU/ADL-EU group, the increase in ADA incidence over TP2 was 0.8% (from 45.1% to 45.9%) vs 6.7% (from 47.4% to 54.1%), respectively. Of the ADA-positive patients, 34.3% (ADL-PF/ADL-PF), 24.7% (ADL-EU/ADL-EU) and 26.2% (ADL-EU/ADL-PF) tested positive for NAbs.
Figure 4.
ADA and NAb incidence by study visit (safety population) in patients treated with (A) ADL-PF/ADL-PF, (B) ADL-EU/ADL-EU and (C) ADL-EU/ADL-PF*. (A) ADA and NAb incidence by study visit (safety population) in patients treated with ADL-PF/ADL-PF*. (B) ADA and NAb incidence by study visit (safety population) in patients treated with ADL-EU/ADL-EU*. (C) ADA and NAb incidence by study visit (safety population) in patients treated with ADL-EU/ADL-PF*. *Not done: Samples were not collected or were collected but not analysed. †Overall TP2 includes data from weeks 30, 36, 52, EOT/ET, follow-up and unplanned visits in TP2. Week 26 was not included in overall TP2 because ADA samples were obtained before dosing and therefore represented TP1 before entering TP2. ADA, antidrug antibody; ADL-EU, reference adalimumab sourced from the European Union; ADL-PF, PF-06410293; EOT, end of treatment; ET, early termination; NAb, neutralising antibody; TP2, treatment period 2.
A total of 292 (53.0%; 292/551) patients, including 157 (55.5%; 157/283), 66 (48.9%; 66/135) and 69 (51.9%; 69/133) in the ADL-PF/ADL-PF, ADL-EU/ADL-EU and ADL-EU/ADL-PF groups, respectively, tested ADA-negative before entry into TP2. Of these previously ADA-negative patients, 35 (12.0%) developed their first post-dose ADA-positive test during TP2. For this subset of patients, the rate of first ADA-positive test in TP2 was numerically higher for the ADL-PF/ADL-PF (14.0%; 22/157) and ADL-EU/ADL-EU (16.7%; 11/66) groups compared with the ADL-EU/ADL-PF group (2.9%; 2/69). Among the patients who developed their first post-dose ADA-positive test during TP2, the majority had ADA onset at week 30, including 10 (3.5%) and 6 (4.4%) patients in the ADL-PF/ADL-PF and ADL-EU/ADL-EU groups, respectively. No patient who first tested positive for ADA in TP2 tested positive for NAbs in TP2.
For ADA-positive patients, the maximal ADA titres in TP2 were evenly distributed across all four quartiles within each treatment group, and the maximal titre distribution was generally similar between ADL-PF/ADL-PF, ADL-EU/ADL-EU and ADL-EU/ADL-PF at each visit. Similar results were observed for NAb titres in NAb-positive patients.
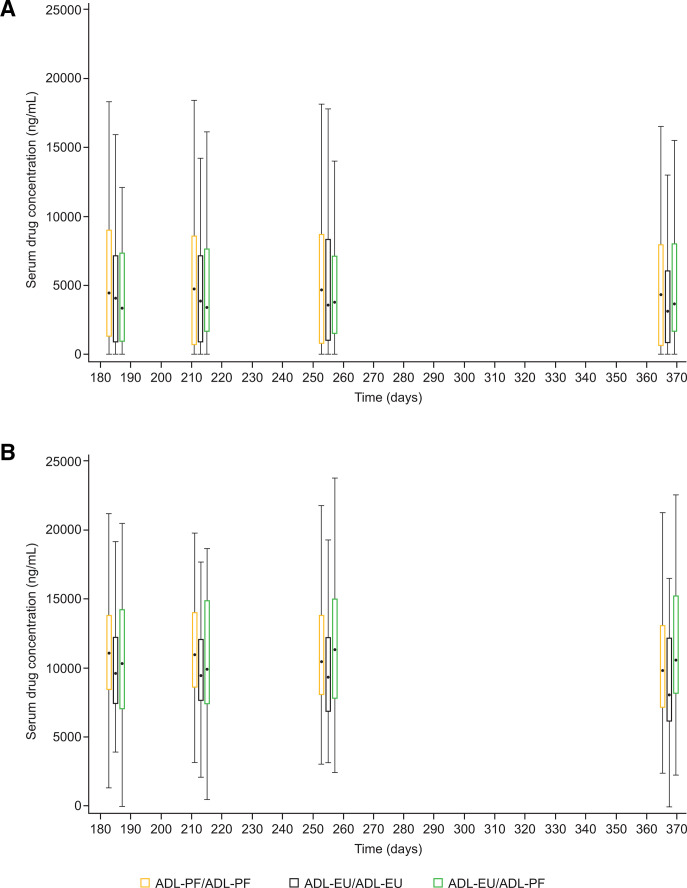
Pharmacokinetics
The mean serum drug concentrations at week 52 were numerically slightly higher for patients in the ADL-PF/ADL-PF (7491 ng/mL) and ADL-EU/ADL-PF (8157 ng/mL) groups as compared with patients in the ADL-EU/ADL-EU (6252 ng/mL) group. As expected, mean serum drug trough concentrations for ADA-positive patients were lower compared with ADA-negative patients in all treatment groups (figure 5). At week 52, mean serum drug trough concentrations for ADA-positive and ADA-negative patients, respectively, were 4855 and 10 400 ng/mL for ADL-PF/ADL-PF, 4273 and 9102 ng/mL for ADL-EU/ADL-EU, and 4991 and 11 430 ng/mL for ADL-EU/ADL-PF. Mean serum drug trough concentrations for ADA-positive/NAb-positive patients were lower compared with patients who were ADA-positive/NAb-negative (online supplemental table 4). At week 52, mean serum drug trough concentrations were 1076 (ADL-PF/ADL-PF), 1054 (ADL-EU/ADL-EU) and 1598 (ADL-EU/ADL-PF) ng/mL for ADA-positive/NAb-positive patients, and were 6603 (ADL-PF/ADL-PF), 5268 (ADL-EU/ADL-EU) and 6423 (ADL-EU/ADL-PF) ng/mL for ADA-positive/NAb-negative patients.
Figure 5.
Box plots for serum drug concentrations at prespecified times post-dose for ADL-PF and ADL-EU in (A) ADA-positive patients and (B) ADA-negative patients (PK population)*. (A) Box plots for serum drug concentrations at prespecified times post-dose for ADL-PF and ADL-EU in ADA-positive patients†. (B) Box plots for serum drug concentrations at prespecified times post-dose for ADL-PF and ADL-EU in ADA-negative patients. *Summary statistics were calculated by setting concentration values below the LLOQ to 0 (LLOQ=250 ng/mL). Box plots provide medians and 25%/75% quartiles with whiskers to the last point within 1.5 times the IQR. Black dots represent median values. Unplanned, EOT/ET and follow-up readings were excluded from the presentation. †An ADA-positive patient was defined as having ≥1 post-dose sample that tested positive during TP1 or TP2. ADA, anti-drug antibody; ADL-EU, reference adalimumab sourced from the European Union; ADL-PF, PF-06410293; EOT, end of treatment; ET, early termination; LLOQ, lower limit of quantification; PK, pharmacokinetic; TP, treatment period.
Pharmacodynamics
Mean hs-CRP concentrations at week 26 pre-dose were 9.9, 10.8 and 6.5 mg/L in the ADL-PF/ADL-PF, ADL-EU/ADL-EU and ADL-EU/ADL-PF groups, respectively, and at week 52 were 9.6, 10.3 and 9.9 mg/L, respectively. The mean change from study baseline in hs-CRP concentrations were −11.3, –11.2 and −15.8 mg/L at week 26 pre-dose, and −10.6, –11.8 and −12.8 mg/L at week 52 in the ADL-PF/ADL-PF, ADL-EU/ADL-EU and ADL-EU/ADL-PF groups, respectively.
Discussion
First, this study provides long-term (52 weeks) evidence for the efficacy, safety and immunogenicity of ADL-PF in combination with MTX in patients with active RA. Second, consistent with the results from TP1, comparable clinical responses were observed among patients who continued to receive ADL-PF or ADL-EU, or, importantly, who switched from ADL-EU to ADL-PF. Comparable ACR20 rates were observed between all treatment groups at all visits during TP2. Other clinical measures—including those of deep response such as ACR/EULAR-defined remission, ACR50, ACR70, EULAR good response and DAS28-4(CRP) <2.6—were maintained during TP2 in all treatment groups. There was no apparent effect on efficacy, either positive or negative, in patients who switched from ADL-EU to ADL-PF. Consistent with previous findings with TNFα inhibitors and results from TP1, mean hs-CRP concentrations decreased to a comparable extent in all treatment groups during TP2 with respect to study baseline.
Safety profiles in TP2 were comparable in all treatment groups. Prespecified TEAEs of special interest, including infections, latent TB, neoplasms, ISRs and hypersensitivity, were comparable in all treatment groups. Comparable ADA and NAb incidence and titres were observed during TP2 for all treatment groups. The majority of patients who developed ADAs did not report TEAEs of hypersensitivity. There was no evidence for increased immunogenicity in patients who switched from ADL-EU to ADL-PF. In each of the three treatment groups, mean serum drug trough concentrations for ADA-positive patients were lower compared with those for ADA-negative patients.
Analyses of efficacy by antibody status during TP2 of this study were not performed. However, it was previously demonstrated that antibody status may impact efficacy response during TP1 of the study.11 Specifically, Fleischmann et al reported numerically higher ACR20 response rates at week 12 for ADA-negative patients (ADL-PF, 70.9%; ADL-EU, 77.2%) compared with ADA-positive patients (ADL-PF, 63.7%; ADL-EU, 65.7%).11 ACR20 response rates for NAb-negative patients (ADL-PF, 70.9%; ADL-EU, 74.0%) were also numerically higher than for NAb-positive patients (ADL-PF, 50.0%; ADL-EU, 64.0%).11 Although such analyses were not performed in the current study, we would anticipate the same potential impact of antibody status on efficacy during TP2.
A limitation of the study is the absence of a control group including patients maintained on ADL-EU throughout the study. The majority of patients enrolled in TP2 had achieved ACR20 by end of treatment (week 26) in TP1. However, baseline demographic and RA disease characteristics of patients who entered TP2 were similar to the overall population at study entry. Another limitation of the study is the concomitant administration of MTX. Co-administration of MTX and TNF inhibitors (TNFis), such as ADL, attenuates the ADAs produced in response to TNFis in patients with inflammatory diseases.13–16 In the current study, all patients received concomitant MTX and ADL (ADL-PF or ADL-EU), and the incidence of ADA was similar between treatment arms. However, it is possible that co-administration of MTX and ADL in the current study decreased the sensitivity of detecting differences in ADA response between the treatment groups. Furthermore, no formal statistical testing was performed in TP2 because the study was not powered to compare the three treatment groups. Therefore, all study results were summarised using descriptive statistics.
In conclusion, results from TP2 demonstrated that comparable efficacy, safety, immunogenicity, PK and PD between ADL-PF and ADL-EU were maintained up to week 52. Furthermore, efficacy, safety, immunogenicity and pharmacologic profiles were unaffected by a switch from ADL-EU to ADL-PF at week 26. The latter finding—that no loss of efficacy was observed with switching from ADL-EU to ADL-PF—is relevant and consistent with increasing literature, suggesting that reported loss of efficacy, after switching from a reference biologic to a biosimilar in clinical practice, may be attributable to the nocebo effect.17–19
Acknowledgments
The authors thank K. Lea Sewell, formerly of Pfizer, for valuable contributions to the PF-06410293 clinical development programme. Medical writing support was provided by Jacqui Oliver, PhD, and Elyse Smith, PhD, of Engage Scientific Solutions and was funded by Pfizer.
Footnotes
Contributors: DFA, AEB, CC and IV contributed to conception or design of the study; RA and RMF contributed to the acquisition of data; and WZ contributed to data analysis. All authors participated in the interpretation of the data, contributed to the drafting or revision of the manuscript, read and gave final approval of the submitted manuscript, were involved in the decision to submit the manuscript for publication and accept accountability for all aspects of the work.
Funding: This study was sponsored by Pfizer.
Competing interests: DFA, AEB and IV are full-time employees of, and declare shareholdings, stock holdings and/or stock options from, Pfizer. CC was a full-time employee of Pfizer at the time of data generation and manuscript development and declares shareholdings, stock holdings and/or stock options from Pfizer. WZ is a full-time employee of Pfizer and declares shareholdings, stock holdings and/or stock options from Abbott, AbbVie and Pfizer. RMF has received research grants/support and consulting fees from Pfizer. RA has received research grants/support and consulting fees from AbbVie, Bristol-Myers Squibb, Gilead, Lilly, Novartis, Pfizer and UCB.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data availability statement
Data are available upon reasonable request. Upon request, and subject to certain criteria, conditions and exceptions (see https://www.pfizer.com/science/clinical-trials/trial-data-and-resultsformoreinformation), Pfizer will provide access to individual deidentified participant data from Pfizer-sponsored global interventional clinical studies conducted for medicines, vaccines and medical devices (1) for indications that have been approved in the USA and/or EU or (2) in programmes that have been terminated (ie, development for all indications has been discontinued). Pfizer will also consider requests for the protocol, data dictionary and statistical analysis plan. Data may be requested from Pfizer trials 24 months after study completion. The deidentified participant data will be made available to researchers whose proposals meet the research criteria and other conditions, and for which an exception does not apply, via a secure portal. To gain access, data requestors must enter into a data access agreement with Pfizer.
References
- 1. AbbVie. Humira (adalimumab) prescribing information, 2002. Available: http://www.rxabbvie.com/pdf/humira.pdf [Accessed 26 Feb 2020].
- 2. Breedveld FC, Weisman MH, Kavanaugh AF, et al. The premier study: a multicenter, randomized, double-blind clinical trial of combination therapy with adalimumab plus methotrexate versus methotrexate alone or adalimumab alone in patients with early, aggressive rheumatoid arthritis who had not had previous methotrexate treatment. Arthritis Rheum 2006;54:26–37. 10.1002/art.21519 [DOI] [PubMed] [Google Scholar]
- 3. Keystone EC, Kavanaugh AF, Sharp JT, et al. Radiographic, clinical, and functional outcomes of treatment with adalimumab (a human anti-tumor necrosis factor monoclonal antibody) in patients with active rheumatoid arthritis receiving concomitant methotrexate therapy: a randomized, placebo-controlled, 52-week trial. Arthritis Rheum 2004;50:1400–11. 10.1002/art.20217 [DOI] [PubMed] [Google Scholar]
- 4. Weinblatt ME, Keystone EC, Furst DE, et al. Adalimumab, a fully human anti-tumor necrosis factor alpha monoclonal antibody, for the treatment of rheumatoid arthritis in patients taking concomitant methotrexate: the armada trial. Arthritis Rheum 2003;48:35–45. 10.1002/art.10697 [DOI] [PubMed] [Google Scholar]
- 5. Curtis JR, Singh JA. Use of biologics in rheumatoid arthritis: current and emerging paradigms of care. Clin Ther 2011;33:679–707. 10.1016/j.clinthera.2011.05.044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Putrik P, Ramiro S, Kvien TK, et al. Inequities in access to biologic and synthetic DMARDs across 46 European countries. Ann Rheum Dis 2014;73:198–206. 10.1136/annrheumdis-2012-202603 [DOI] [PubMed] [Google Scholar]
- 7. European Medicines Agency . Guideline on similar biological medicinal products containing monoclonal antibodies – non-clinical and clinical issues, 2012. Available: https://www.ema.europa.eu/en/documents/scientific-guideline/guideline-similar-biological-medicinal-products-containing-monoclonal-antibodies-non-clinical_en.pdf [Accessed 26 Feb 2020].
- 8. US Food and Drug Administration . Scientific considerations in demonstrating Biosimilarity to a reference product: guidance for industry, 2015. https://www.fda.gov/media/82647/download. (Accessed 26 February 2020). [Google Scholar]
- 9. Pfizer Inc . Abrilada™ (adalimumab-afzb) prescribing information, 2019. Available: http://labeling.pfizer.com/ShowLabeling.aspx?id=12780 [Accessed 26 Feb 2020].
- 10. European Medicines Agency . Amsparity: summary of product characteristics, 2020. Available: https://www.ema.europa.eu/en/documents/product-information/amsparity-epar-product-information_en.pdf [Accessed 26 Feb 2020].
- 11. Fleischmann RM, Alten R, Pileckyte M, et al. A comparative clinical study of PF-06410293, a candidate adalimumab biosimilar, and adalimumab reference product (Humira®) in the treatment of active rheumatoid arthritis. Arthritis Res Ther 2018;20:178. 10.1186/s13075-018-1676-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Aletaha D, Neogi T, Silman AJ. 2010 rheumatoid arthritis classification criteria: an American College of Rheumatology/European League Against Rheumatism collaborative initiative. Arthritis Rheum 2010;62:2569–81. [DOI] [PubMed] [Google Scholar]
- 13. Garcês S, Demengeot J, Benito-Garcia E. The immunogenicity of anti-TNF therapy in immune-mediated inflammatory diseases: a systematic review of the literature with a meta-analysis. Ann Rheum Dis 2013;72:1947–55. 10.1136/annrheumdis-2012-202220 [DOI] [PubMed] [Google Scholar]
- 14. Krieckaert CLM, Bartelds GM, Lems WF, et al. The effect of immunomodulators on the immunogenicity of TNF-blocking therapeutic monoclonal antibodies: a review. Arthritis Res Ther 2010;12:217. 10.1186/ar3147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Schaeverbeke T, Truchetet M-E, Kostine M, et al. Immunogenicity of biologic agents in rheumatoid arthritis patients: lessons for clinical practice. Rheumatology 2016;55:210–20. 10.1093/rheumatology/kev277 [DOI] [PubMed] [Google Scholar]
- 16. Maini RN, Breedveld FC, Kalden JR, et al. Therapeutic efficacy of multiple intravenous infusions of anti-tumor necrosis factor alpha monoclonal antibody combined with low-dose weekly methotrexate in rheumatoid arthritis. Arthritis Rheum 1998;41:1552–63. [DOI] [PubMed] [Google Scholar]
- 17. Alten R, Batko B, Hala T, et al. Randomised, double-blind, phase III study comparing the infliximab biosimilar, PF-06438179/GP1111, with reference infliximab: efficacy, safety and immunogenicity from week 30 to week 54. RMD Open 2019;5:e000876. 10.1136/rmdopen-2018-000876 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Kristensen LE, Alten R, Puig L, et al. Non-Pharmacological effects in switching medication: the nocebo effect in switching from originator to biosimilar agent. BioDrugs 2018;32:397–404. 10.1007/s40259-018-0306-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Rezk MF, Pieper B. To see or NOsee: the debate on the nocebo effect and optimizing the use of biosimilars. Adv Ther 2018;35:749–53. 10.1007/s12325-018-0719-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
rmdopen-2021-001578supp001.pdf (3.9MB, pdf)
Data Availability Statement
Data are available upon reasonable request. Upon request, and subject to certain criteria, conditions and exceptions (see https://www.pfizer.com/science/clinical-trials/trial-data-and-resultsformoreinformation), Pfizer will provide access to individual deidentified participant data from Pfizer-sponsored global interventional clinical studies conducted for medicines, vaccines and medical devices (1) for indications that have been approved in the USA and/or EU or (2) in programmes that have been terminated (ie, development for all indications has been discontinued). Pfizer will also consider requests for the protocol, data dictionary and statistical analysis plan. Data may be requested from Pfizer trials 24 months after study completion. The deidentified participant data will be made available to researchers whose proposals meet the research criteria and other conditions, and for which an exception does not apply, via a secure portal. To gain access, data requestors must enter into a data access agreement with Pfizer.