Abstract
We report a fatal case of SARS-CoV-2 and Mycobacterium tuberculosis coinfection in an infant, Botswana’s first paediatric COVID-19-associated fatality. The patient, a 3-month-old HIV-unexposed boy, presented with fever and respiratory distress in the setting of failure to thrive. Both the patient and his mother tested positive for rifampin-sensitive M. tuberculosis (Xpert MTB/Rif) and SARS-CoV-2 (real time-PCR). Initially stable on supplemental oxygen and antitubercular therapy, the patient experienced precipitous clinical decline 5 days after presentation and subsequently died. Autopsy identified evidence of disseminated tuberculosis (TB) as well as histopathological findings similar to those described in recent reports of SARS-CoV-2 infections, including diffuse microthrombosis. TB remains a serious public health threat in hyperendemic regions like sub-Saharan Africa, and is often diagnosed late in infants. In addition to raising the question of additive/synergistic pathophysiology and/or immune reconstitution, this case of coinfection also highlights the importance of leveraging the COVID-19 pandemic response to strengthen efforts for TB prevention, screening and detection.
Keywords: TB and other respiratory infections, preventative paediatrics, infant health, failure to thrive, childhood nutrition (paediatrics)
Background
Tuberculosis (TB), caused by Mycobacterium tuberculosis infection, remains the deadliest infectious disease globally, and sub-Saharan Africa bears a high proportion of the global TB burden.1 Botswana has an annual incidence of approximately 300 new TB cases per 100 000 people, over half of which occur among people living with HIV.2 TB case fatality is highest in infants, who are more likely than older children and adults to experience severe and disseminated infections.3
Superimposed on hyperendemic TB and HIV in sub-Saharan Africa is the evolving COVID-19 pandemic. The first cases of COVID-19 in Botswana were detected in March of 2020 and community transmission was confirmed in August of 2020. As of 5 November 2020, Botswana had registered 6642 cases (2805 cases per 1 million population) while neighbouring South Africa had registered 728 836 cases (12 236 cases per 1 million population).4 5 As of the same date, Botswana had registered 964 cases of COVID-19 in children 13 years of age and younger.6
Reports of SARS-CoV-2 and TB coinfection are emerging, but data from the paediatric population are limited. A large cohort study of adults from South Africa with COVID-19 identified HIV and current TB as independent risk factors for mortality.7 One international cohort identified an infant with disseminated TB who responded well to antitubercular therapy (ATT).8
Case presentation
We report the case of a 3-month-old male infant who presented with fever and respiratory distress in the setting of failure to thrive. He was born via spontaneous vaginal delivery at full term to a 27-year-old gravida 1, para 1 mother with no prenatal or perinatal complications. During the third trimester of pregnancy, the infant’s mother tested negative for HIV but reported that she began coughing, losing weight and experiencing intermittent fever at that time. She attributed the symptoms to stress and did not seek medical attention for it. The patient weighed 4 kg at birth which was appropriate for gestational age. The patient and his mother resided alone in one room, part of a multiresidential complex in a periurban area, with shared water, kitchen and ablution facilities. There were no household members or visitors known to have TB.
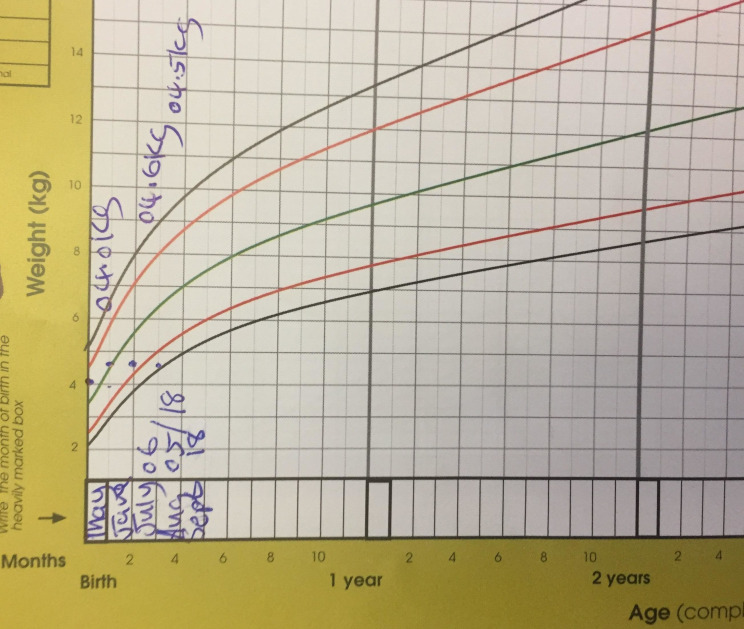
At about 6 weeks of age, the patient received hepatitis B and BCG vaccination without adverse reaction and at 2 months, he received the pentavalent diphtheria/pertussis/tetanus—hepatitis B—Haemophilus influenzae-type B, pneumococcal conjugate, oral polio and rotavirus vaccines. The patient was exclusively breast fed and his mother reported adequate suck and latch but poor weight gain was recorded at three subsequent routine clinic visits. By 3 months of age, his weight was recorded to be only 4.5 kg, plotting below the −3 SD of the age-specific and sex-specific weight-for-height nomogram, indicating failure to thrive (figure 1).
Figure 1.
Growth chart demonstrating a weight-for-age above the 0 SD line at birth, then with essentially no weight gain over months 1–3, and approaching the −3 SD line by 3 months of age; failure to gain weight appropriately was likely an early sign of tuberculosis in this patient.
At 3 months of age, the patient was noted by his mother to have cough, intermittent subjective fever and difficulty breathing. About 2 weeks later, his mother brought him to the clinic for medical attention. On presentation to care, the infant displayed signs of respiratory distress including nasal flaring, tachypnoea and hypoxaemia. There was poor air entry on auscultation and bibasilar crackles were noted, with left-sided predominance. The liver was noted to be 3 cm below the costal margin. There was no reported vomiting, diarrhoea, feeding difficulty, rash, palpable lymphadenopathy or splenomegaly.
On arrival at the referral hospital, a chest radiograph was obtained revealing significant bilateral infiltrates, perihilar opacities, consolidations predominantly on the left side and bilateral hyperinflation (figure 2). A gastric lavage was performed on the day of admission and nucleic acid amplification via Xpert MTB/Rif confirmed rifampin-sensitive M. tuberculosis by the third day of hospitalisation. Nasopharyngeal and oropharyngeal samples tested positive for SARS-CoV-2 on real-time PCR by the end of the first day of hospitalisation.
Figure 2.
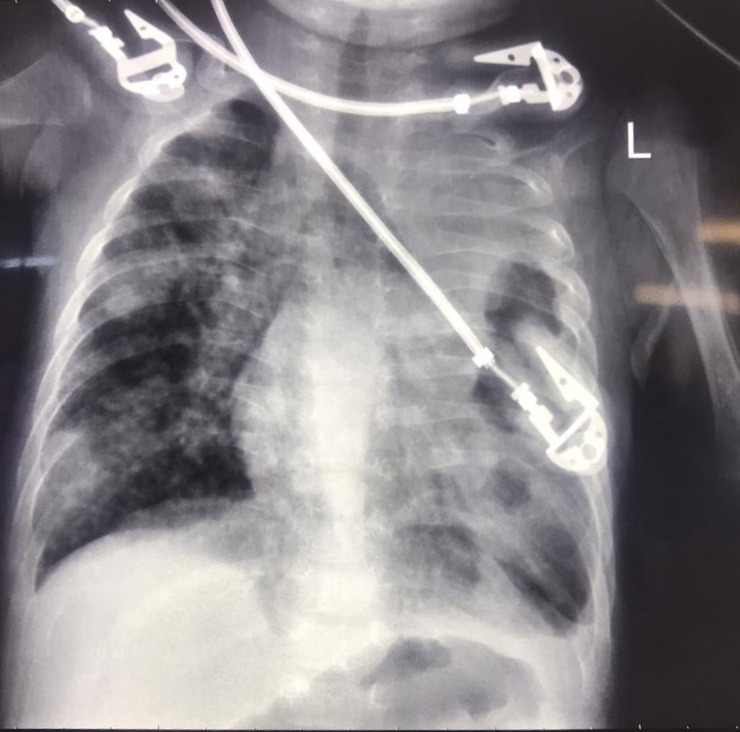
Chest radiograph obtained on admission displaying significant bilateral infiltrates, perihilar opacities, areas of consolidation predominantly on left side and bilateral hyperinflation.
Laboratory evaluation revealed the presence of mild thrombocytopaenia (platelet count=93×109/L) but no significant leucocytosis (white cell count=11.5×109/L). Haemoglobin was low normal for age (106 g/L; mean corpuscular volume=69.6 fL). Electrolytes were within normal limits but liver function tests revealed elevated aspartate aminotransferase (127 U/L), elevated gamma glutamyl transferase (151 IU/L) and hypoalbuminaemia (24.6 g/L).
The infant’s mother, who had experienced persistent cough but no other notable symptoms since giving birth, also tested positive for both rifampin-sensitive M. tuberculosis on sputum sample and SARS-CoV-2 via nasopharyngeal/oropharyngeal sample. HIV-1/2 antigen/antibody combination immunoassay test performed on the patient’s mother was negative.
On admission, the patient received supplemental oxygen with satisfactory improvement to his respiratory distress. He was initiated on empirical antibiotic therapy with ampicillin and gentamicin for possible community-acquired pneumonia at that time. Expressed breast milk feeding was augmented with infant formula. On day 4 of admission, the patient was started on rifampicin, isoniazid, pyrazinamide and ethambutol, as per Botswana National Tuberculosis Programme Guidelines. The patient continued to have mild tachypnoea and subcostal retractions, but no hypoxia when receiving 2 L of supplemental oxygen.
On day 5 of hospitalisation, the patient was noted to be febrile and tachycardic, but was well perfused and without features of meningitis. There was no evidence of progressive respiratory failure at that time. Within hours, he experienced cardiac arrest and later died.
The autopsy revealed signs of severe malnutrition, including macrovesicular steatosis, as well as evidence of disseminated TB, with numerous necrotising granulomatous lesions in the lungs, liver, spleen and mediastinal lymph nodes. Of note, diffuse platelet–fibrin microthrombi were also identified in the lungs and myocardium (figure 3). Additional findings in the lung included proliferative diffuse alveolar damage, alveolar haemorrhage and lymphocytic interstitial inflammatory infiltrate. Mild interstitial oedema and mononuclear inflammatory infiltrates were also noted in the myocardium.
Figure 3.
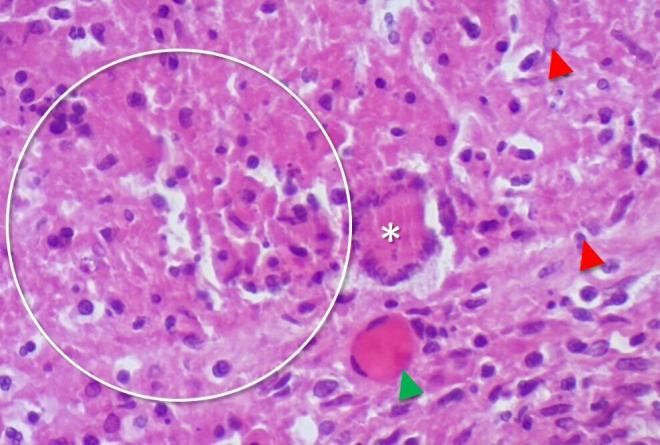
H&E stained section of the described patient’s lung parenchyma (40× magnification). The circle demonstrates necrotising granulomatous inflammation. There are also epithelioid histiocytes (red arrowhead), a Langhan’s-type giant cell (*) and a fibrin–platelet thrombus (green arrowhead).
Global health problem list
TB remains a deadly threat to public health in hyperendemic regions like Botswana, particularly among young children who are at higher risk of experiencing severe disease. There is often a delay in diagnosing TB in young children because of non-specific signs.
The COVID-19 pandemic poses a double risk to TB-endemic regions: directly through its additional illness burden, and indirectly as COVID-19 control efforts threaten to interrupt and overshadow ongoing TB screening and prevention efforts.
Little is known about outcomes of SARS-CoV-2 and M. tuberculosis coinfection, particularly among infants.
Global health problem analysis
TB incidence has declined over the past two decades in sub-Saharan Africa, due in part to enhanced TB prevention and control efforts as well as improved access to highly active antiretroviral therapy for people living with HIV.2 Still, TB remains a major threat to public health in Botswana, accounting for 13% of all adult deaths.9 Children are more likely than adults to develop severe or disseminated TB, yet prompt diagnosis is often elusive because of non-specific symptoms and diagnostic challenges.3
In Botswana, approximately half of children diagnosed with TB are reported to have concurrent failure to thrive (including undernutrition and malnutrition) and one study found that 6% of children with failure to thrive had concurrent TB.10 Failure to thrive is thus a critical red flag for young children in endemic areas and should elicit prompt TB screening. Earlier recognition of failure to thrive may have led to an earlier diagnosis in the case of the infant described. Additionally, although symptom screening in pregnancy may lack sensitivity for TB diagnosis in endemic regions,11 the importance of perinatal maternal health maintenance cannot be overemphasised. Intermittent fever, weight loss and non-resolving cough in this patient’s mother should have elicited a TB work-up prenatally. Diagnosis of TB is often based on a constellation of clinical findings and empirical ATT may be warranted even in the absence of positive diagnostic testing. Infants born to mothers with TB should start 6 months of isoniazid preventive therapy and defer BCG vaccination if asymptomatic or be treated with ATT if symptomatic.
Control of TB in endemic regions relies on primary, secondary and tertiary prevention efforts. These include active case finding, contact tracing, infection prevention in healthcare settings, BCG vaccination, isoniazid prophylaxis and strict adherence to appropriate ATT. Since early 2020, healthcare systems in sub-Saharan Africa have had to pivot swiftly to control the COVID-19 epidemic, often leveraging existing diagnostic and surveillance platforms built for TB prevention and control.12 13 Enormous efforts have been expended on COVID-19 screening and testing, contact tracing, isolation, quarantine and case management. Clinical services and public health infrastructure have been stretched thin trying to respond to the COVID-19 pandemic. However, as countries like Botswana rise to this challenge, it is important to seek ways to leverage COVID-19 mitigation efforts to help address endemic illnesses like TB and ensure that TB control efforts are not neglected.14 Quality improvement and assurance measures should continue to be employed to ensure that existing systems for TB management are maintained even as they are used to assist in the fight against COVID-19.
TB and COVID-19 share important characteristics which make them conducive to cross-beneficial control efforts. Both are primarily respiratory illnesses which can be transmitted in the air. Prevention of transmission in healthcare settings relies on good airborne infection control measures and contact tracing is essential for detection of clusters and, in the case of TB, prompt initiation of prophylaxis. In Botswana, where patients with TB are generally well accommodated in the community but misconceptions still exist regarding TB transmission, community engagement has been necessary to prevent TB-associated stigma and will likely be critical in addressing COVID-19-associated stigma.15
This case of SARS-CoV-2 and M. tuberculosis coinfection in an infant highlights the still emerging nature of this pandemic, particularly as it interfaces with infections endemic to regions in the global south. Some have suggested that, in patients with TB, SARS-CoV-2 detection is simply an incidental finding,16 while others speculate that there could be pathophysiological synergy.17 The precipitous nature of the patient’s clinical decline was not well explained by TB alone; the lack of signs of progressive respiratory failure in the hours leading up to his death is inconsistent with pulmonary TB as the primary cause of death. This, along with the presence of diffuse microthrombosis noted on this patient’s autopsy, suggests possible synergistic or additive pathological effect. Microthrombosis has been reported in a subset of SARS-CoV-2 infections and was thus attributed to the viral infection in this patient.18 Immunomodulation may have played a role; immune reconstitution inflammation syndrome (IRIS) has been described in HIV-negative patients with TB, and IRIS has already been described in a patient with SARS-CoV-2 infection.19 More research investigating the dynamics of SARS-CoV-2 and M. tuberculosis coinfection is needed.
Patient’s perspective.
Dictated by patient’s mother:
My son was a beautiful baby. He smiled a lot. Even when he got sick, he still smiled. When he died, I was very sad and I still am so very sad. But it’s because of my son that I found out I also had Corona and TB and I am now on treatment for that. So I am grateful to my son because without him, I might not be alive.
Learning points.
Clinicians practising in tuberculosis (TB)-endemic areas should be vigilant about using growth parameters and symptom screening of household members as important clues for early TB detection in young children. They should screen for TB symptoms among household contacts, especially mothers, and proactively test for TB in infants with acute respiratory distress.
Public health practitioners should leverage the COVID-19 response to strengthen ongoing public health efforts aimed at endemic diseases, including contact tracing household members of patients diagnosed with TB; healthcare facilities should use newly blazed trails of infection control policies to develop more robust approaches to control TB and other common illnesses.
More research is needed to better characterise the emerging clinical entity of SARS-CoV-2 and TB coinfection, including in children.
Acknowledgments
The authors extend their gratitude and thanks to: Sir Ketumile Masire Teaching Hospital management and staff, Tlamelo Daman, Thabo Dambe, Oteng Ketshoseng, Mosepele Mosepele, Mogomotsi Matshaba, Mohamed Zaakir Patel, Tonya Arscott-Mills, Andrew Steenhoff.
Footnotes
Twitter: @unamulale
Contributors: The following people made a contribution to the article: UKM—writing/editing/caring for the patient. JS—writing/editing/obtaining consent. TK—writing/editing/performed autopsy/ pathology report. LTK—writing/editing/performed autopsy/pathology report/overall guidance.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: None declared.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Lawn SD, Churchyard G. Epidemiology of HIV-associated tuberculosis. Curr Opin HIV AIDS 2009;4:325–33. 10.1097/COH.0b013e32832c7d61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.World Health Organisation . Tuberculosis country profiles: Botswana, 2018. Available: https://www.who.int/tb/country/data/profiles/en/ [Accessed 7 Sep 2020].
- 3.Vanden Driessche K, Persson A, Marais BJ, et al. Immune vulnerability of infants to tuberculosis. Clin Dev Immunol 2013;2013:1–16. 10.1155/2013/781320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Worldometer . Worldometer coronavirus Info: Botswana. Available: https://www.worldometers.info/coronavirus/country/botswana/ [Accessed 5 Nov 2020].
- 5.South African online portal . COVID-19 corona virus South African resource portal. Available: https://sacoronavirus.co.za/ [Accessed 5 Nov 2020].
- 6.Unpublished official data collected by the National Health Lab and the National Emergency Operation Center (NEOC) . Botswana National health laboratory statistics, verbal communication. Statistics communicated by Dr Matshaba and Dr Matsheke of NEOC. [Accessed 05 Nov 2020].
- 7.Boulle A, Davies M-A, Hussey H, et al. Risk factors for COVID-19 death in a population cohort study from the Western Cape Province, South Africa. Clin Infect Dis 2020. 10.1093/cid/ciaa1198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tadolini M, Codecasa LR, García-García J-M. Active tuberculosis, sequelae and COVID-19 co-infection: first cohort of 49 cases. Eur Respir J 2020;56. [Epub ahead of print: 09 Jul 2020]. 10.1183/13993003.01398-2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ministry of health, Botswana . Botswana national TB care policy guidelines. Botswana: Gaborone, 2018. [Google Scholar]
- 10.Arscott-Mills T, Ho-Foster A, Lowenstein M, et al. Yield of screening for TB and HIV among children failing to thrive in Botswana. J Trop Pediatr 2014;60:27–32. 10.1093/tropej/fmt072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kosgei RJ, Szkwarko D, Callens S, et al. Screening for tuberculosis in pregnancy: do we need more than a symptom screen? experience from Western Kenya. Public Health Action 2013;3:294–8. 10.5588/pha.13.0073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rakotosamimanana N, Randrianirina F, Randremanana R, et al. Genexpert for the diagnosis of COVID-19 in LMICs. Lancet Glob Health 2020;8:e1457–8. 10.1016/S2214-109X(20)30428-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.World Health Organisation . Nigeria: integrating COVID-19 screening into TB surveillance activities. Available: https://www.who.int/news-room/feature-stories/detail/nigeria-integrating-covid-19-screening-into-tb-surveillance-activities [Accessed 5 Nov 2020].
- 14.Senghore M, Savi MK, Gnangnon B, et al. Leveraging Africa's preparedness towards the next phase of the COVID-19 pandemic. Lancet Glob Health 2020;8:e884–5. 10.1016/S2214-109X(20)30234-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Musuka G, Teveredzi V, Busang L, et al. Community attitudes on tuberculosis in Botswana: an opportunity for improving the National tuberculosis programme outcomes, 2011. BMC Res Notes 2018;11:499. 10.1186/s13104-018-3585-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Khurana AK, Aggarwal D. The (in)significance of TB and COVID-19 co-infection. Eur Respir J 2020;56. 10.1183/13993003.02105-2020. [Epub ahead of print: 20 Aug 2020]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kumar DR, Bhattacharya DB, Meena DV, et al. COVID-19 and TB co-infection - 'Finishing touch'' in perfect recipe to 'severity' or 'death'. J Infect 2020;81:e39–40. 10.1016/j.jinf.2020.06.062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mohanty SK, Satapathy A, Naidu MM, et al. Severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) and coronavirus disease 19 (COVID-19) - anatomic pathology perspective on current knowledge. Diagn Pathol 2020;15:103. 10.1186/s13000-020-01017-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mertens J, Laghrib Y, Kenyon C. A case of steroid-responsive, COVID-19 immune reconstitution inflammatory syndrome following the use of granulocyte colony-stimulating factor. Open Forum Infect Dis 2020;7:ofaa326. 10.1093/ofid/ofaa326 [DOI] [PMC free article] [PubMed] [Google Scholar]