Abstract
Toxic and essential elements are widely distributed in the Earth’s crust and individuals may be exposed to several of them. Indeed, exposure to toxic elements such as mercury (Hg) can be a potential health risk factor of health, mainly by ingestion of fish containing methylmercury (MeHg). On the other hand, essential elements such as manganese (Mn) play an important role in physiological process in human body. However, Mn overexposure may cause toxic effects. In this respect, the neurotoxic effects of MeHg and Mn on the developing brain are well recognized. Therefore, in this critical review, we address the effects of MeHg and Mn on cell signaling pathways which may contribute to molecular mechanisms involved in MeHg- and Mn-induced neurotoxicity.
Keywords: Mercury, Methylmercury, Manganese, Heavy metal, Signaling pathways, Neurotoxicology
1. Introduction
Exposure to heavy metals and metalloids such as arsenic, lead, cadmium and mercury (Hg) can lead to the development of diseases (1, 2). Yet, some metals are considered “essential elements”. These elements, such as manganese (Mn), selenium and cupper, play important roles in the regulation of critical enzyme systems and are essential for several physiological process (3, 4). However, when essential elements are present at higher concentrations, they may disrupt normal biological functions and induce cellular stress responses, thus contributing to development of diseases (5, 6).
Regarding toxic elements, Hg is ranked 3rd in the list of Hazardous Substance Priority List established by the US ATSDR in 2019. This hazardous pollutant occurs in different chemical forms: elemental Hg (Hg°), inorganic Hg compounds (Hg2+), and organic Hg compounds, as methylmercury (MeHg). In aquatic environmental, the Hg2+ can be biomethylated by aquatic sulfate-reducing bacteria, generating MeHg, which has a substantial biomagnification potential and accumulates along the food chain. Consequently, fish intake is the major source of MeHg exposure in humans (7, 8). Several studies have reported that MeHg exposure may lead to neurological alterations, including cognitive and motor dysfunction, and decreases in memory and learning (9, 10).
The essential element Mn is required to several biological process, including brain and skeletal development, immune response and others (11, 12). Moreover, it acts as an important cofactor for numerous enzymes, participating in synthesis and metabolism of amino acids, proteins, lipids as well as enzymes to defense of the organisms against oxidative stress. The major source of exposure to Mn is through the diet (11). In addition, inhalation of high levels of airborne Mn aerosols in occupational activities represents an important source of exposure to workers. It is well documented that Mn exposure may lead to its excessive accumulation in the central nervous system (CNS), resulting in manganism (13). Furthermore, chronic alterations of Mn levels in the brain may trigger the development of neurodegenerative diseases, such as Alzheimer’s disease (AD) and Parkinson’s disease (PD) (14, 15).
Currently, the cumulative evidence suggests the involvement of Mn and MeHg in adverse neurological toxic effects. Interestingly, co-exposure to MeHg and Mn causes more pronounced toxic effects than single metal exposure. It was reported that co-exposure to these metals in Caenorhabditis elegans (C. elegans) caused developmental delays in worms, increased enzymes associated with the antioxidant system and cholinergic degeneration (16). Therefore, both metals could be involved in development of neurotoxicity. However, the molecular mechanisms responsible for metal-induced neurotoxicity cannot be explained by a single process. Rather, several changes in cell signaling pathways involved in the regulation of the cells in the brain contribute to those effects. In this narrative review, we summarize the current knowledge regarding the molecular mechanisms involved in MeHg and Mn neurotoxicity.
2. Methylmercury: new insights in neurotoxicity
Methylmercury (MeHg) is a naturally occurring potent neurotoxin that is produced by microorganisms in water sediment through transformation of inorganic mercury. Human exposure to MeHg is predominantly from consumption of fish that bioaccumulate MeHg from lower tropical organisms in the water. The relatively high level of mercury in top tropical fish poses a potential health risk for people whose regular protein source are marine food, especially developing children and childbearing women. Acute MeHg exposure at high levels causes permanent neuronal damage, while chronic MeHg exposure at developmental stage has long-term impacts on neurobehavioral functions. MeHg-induced neurotoxicity involves multiple mechanisms including oxidative stress, repression of protein translation, disruption of calcium homeostasis and mitochondrial energetics, and post-translational modification of proteins (17). Accumulating evidence shows that these mechanisms are involved in several important cellular processes and functions, culminating in neuronal toxicity (18–20).
Low-level MeHg exposure at critical developmental stage has profound impact on neurobehavior functions and neuronal cell integrity. Gestational exposure to MeHg has been shown to induce cerebellar synaptic and neuritic remodeling during the perinatal period through modulation of the TrkA pathway and Arc expression (18). Emerging evidence utilizing highly tractable animal models illustrates that cellular morphology and organismal behavioral patterns were altered by low levels of MeHg (21, 22).
By comparing neuronal morphology of animals with various long-term MeHg exposure regimes, a chronic MeHg exposure model with C. elegans was recently established for evaluation of dopaminergic (DAergic) neurodegeneration (23). A novel type of neuronal homeostasis was demonstrated in C. elegans showing that proteotoxic stress promoted neuronal cell removal of spatially organized proteins and organelles destined for degradation (24). Studies with the C. elegans model demonstrated that the removal process was inhibited by MeHg (21, 25). The stress inducible protein 1 (STI-1) is a co-chaperone that assists protein folding in the intermediate stage of the chaperone-assisted protein folding pathway (Hsp70/Hsp40/Hsp90) by transferring client proteins from the early complex to the intermediate complex. Further analysis showed that STI-1 plays a critical modulatory role in MeHg-induced disruption of the regulated removal process (21).
MeHg has long been recognized as a disruptor of protein homeostasis and oxidative balance. Altered functions of membrane transporters and receptors following MeHg exposure are one of underlying mechanisms for neurobehavioral damages. For example, rats with developmental MeHg exposure were sensitive to the effects of the dopamine agonist, d-amphetamine, and the disruption of dopamine neurotransmission by MeHg contributes to the baseline-dependent inhibition of behavior by d-amphetamine (26). Novel mechanistic insights were also derived from studies showing that the cytotoxic effects of MeHg in motor neurons were mediated by the activation of AMPA receptors (27). Furthermore, shifted glutamatergic excitotoxicity by MeHg may contribute neurodegeneration and loss of motor function, given that MeHg induces hyperexcitability in lumbar spinal motor neurons by increasing activity of Ca2+-permeable AMPA receptors (28).
In addition to the classical roles of antioxidant pathways in MeHg-induced cytotoxicity, recent studies suggest that the antioxidant signaling network involves several important cellular domains including differentiation and protein degradation (29, 30). In a Drosophila model, muscle development defect following MeHg exposure could be rescued by neuron-specific upregulation of one of the central transcriptional factors invoked for antioxidant defense against MeHg toxicity, cap-n-collar C (CncC), the homolog of nuclear factor erythroid 2–related factor 2 (Nrf2) (29). Surprisingly, MeHg-induced lethality could be potentiated by muscle-specific CncC upregulation, while neuron-specific upregulation of CncC was protective (29).
Neuronal NADPH diaphorase (NADPH-d) acts as a cosubstrate for neuronal nitric oxide synthase, which produces the gaseous neuromodulator nitric oxide (NO). The astrocytic activity of NADPH-d and cell numbers was reduced in the visual cortex of rats with chronic MeHg exposure. However, it seemed that the morphology of NADPH-d neurons was spared from the toxic effects of MeHg. A plausible hypothesis was proposed that the negative effect of chronic MeHg poisoning on both the synthesis and transport of NADPH-d in afferent pathways to the visual cortex contributes to the decrease in astrocytic NADPH-d reactivity (19). In addition, MeHg exposure induced degradation of astrocytic hypoxia-inducible factor-1α (HIF-1α) via generation of reactive oxygen species (ROS). Overexpression of HIF-1α attenuated MeHg-induced cytotoxicity (30). Reactive sulfur species (RSS) contain mobilized sulfur that readily captures xenobiotic electrophiles, forming sulfur adducts. The enzyme necessary for RSS synthesis, cystathionine γ-lyase (CSE), plays a protective role in MeHg-induced motor impairment. CSE-deficient mice were susceptible to toxic effects of MeHg, which can be rescued by restoration of RSS with supplementation of sodium tetrasulfide (31).
It has been recently also recognized that the toxicity of MeHg is modulated by bacteria (32). It was shown that the intestinal microbiota played a significant role in MeHg-induced impairment of locomotor activity. (33). Additionally, metabolomics profiling showed that MeHg induce changes of intestinal microbial composition as well as BDNF level, suggesting a potential link between gut microbiota and MeHg-induced neurotoxicity (20).
3. Manganese: new insights in neurotoxicity
The acute or chronic exposure to Mn may affect important cell signaling pathways that regulate cell survival, differentiation, and apoptosis. Indeed, several factors interplay to form the cascade of events involved in Mn neurotoxicity, such as oxidative stress, neuroinflammation, transporter dysregulation, mitochondrial dysfunction and protein misfolding (34, 35). Moreover, Mn-induced neurotoxicity shares pathways associated with the development of neurodegenerative diseases (14).
Overexposure to Mn may be associated with changes in protein aggregation such as Aβ and Tau that are AD hallmark. Recently, Wang et al. reported that rats showed increased Aβ1–40 and Tau production in brain after Mn exposure. The authors also reported that the levels of NLRP3 (nucleotide binding and oligomerization domain–like receptor family pyrin domain–containing 3)inflammasome and inflammatory factors such as IL-1β, IL-18 were higher in the brain of Mn exposed rats compared to the control, suggesting that Mn promoted the activation of NLRP3 inflammasome, and that the expression of inflammatory factors was upregulated in cerebral tissues (36). Peng et al. (2020) demonstrated that Mn can activate inflammatory pathways such as NF-κB, causing inflammation by increasing the phosphorylation of p65 and IkB-α as shown by increased expression of p-p65 and p-IκB-α in Mn-treated BV2 cells and in the basal ganglia of Mn-exposed rats. In addition, the expression of NLRP3 and cleaved caspase 1 (Cleaved CASP1) was significantly increased after Mn treatment in BV2 cells and in the basal ganglia of rats (37). The effects of Mn exposure on NLRP3 inflammasome and neuroinflammation appear to be associated with mitochondrial dysfunction. Indeed, a recent study has shown that Mn exposure decreased the abundance of mitochondrial fusion protein 2 and Mfn2 degradation. Mn-induced mitochondrial dysfunction stimulated cell-to-cell transfer of the inflammasome adaptor protein ASC (apoptosis associated speck-like protein containing a CARD) through exosomes, further spreading inflammasome activation (38).
The NF-κB signaling pathway has an important role in inflammatory processes. Under physiological conditions, NF-κB is present in the cytosol and bound to IκB-α, its inhibitory protein. The activation of IκB-α leads to phosphorylation and degradation of IκBα, leading to phosphorylation of NF-κB and its translocation from the cytosol to the nucleus where NF-κB activates the transcription of target genes such as TNF-α, IL-1β, and IL-6 (39). In fact, previous studies showed that Mn activated NF-κB signaling, changing the inflammatory gene expression (40). In agreement, it was reported that Mn stimulated NF-κB signaling pathways in the hippocampus and striatum of rats, increasing pro-inflammatory cytokines TNF-α, IL-1β, and IL-6 levels (41). An in vitro study showed that Mn activated the NF-κB pathway in BV2 microglia, further propagating inflammatory cytokine production, concomitant with increased phosphorylation of P65 protein and mRNA expression in BV2 microglia (42). Collectively, these studies support that NF-κB signaling activation is involved in the Mn-induced neuroinflammatory process.
Phosphatidylinositol 3 kinase (PI3K) is a phosphatidylinositol kinase that contributes to oxidative stress and regulation of cell differentiation, growth and apoptosis (43). The downstream mediator of PI3K is serine-threonine protein kinase (Akt) which acts as antiapoptotic activity by preventing the release of cytochrome C from mitochondria and inactivating fork head box transcription factors (FOX). In addition, Akt can activate or inhibit its downstream target proteins (NF-κB, caspase-9, Bad, FoxO3a) (43). It has been reported that Mn exposure activated PI3K/Akt signaling pathway in rat hippocampus, leading to inhibition of the transcription function of apoptotic genes such as Bcl-2 and caspase-3, and the activation of Bax, suggesting that apoptosis may underlie the cognitive dysfunction in these animals (44). Moreover, Cheng et al. (2018) demonstrated that mRNA levels of Akt-1 and FoxO3a were decreased after Mn exposure. However, Akt protein levels were increased, indicating that chronic Mn exposure activated the PI3K/Akt pathway via phosphorylation of Akt, in turn, inhibiting the transcription function of apoptotic genes and leading to cell survival (45). Using C. elegans model, Peres et al. (2018) showed that worms with loss of Akt (akt-1 and akt-2) was associated with higher resistance to Mn compared to wild-type worms, suggesting that Akt may serve as a potential therapeutic target for Mn neurotoxicity (46).
4. Conclusions
Growing evidence suggests that MeHg and Mn overexposure leads to toxic effects, particularly in the brain, contributing to neurodegenerative diseases. Exposure to heavy metals such as MeHg and Mn is an important public health concern. Overall, the findings summarized in this review (Figure 1) suggest that the understanding of how toxic and sub-toxic levels of Me Hg and Mn may stimulate several critical cells signaling pathways that are involved in variety of biological processes and illness states may be used as a strategy to treat or prevent neurotoxic effects induced by these metals. In addition, research using “omic” tools such as proteomic, transcriptomic and bioinformatic analyses are necessary and afford new directions in toxicological research.
Figure 1:
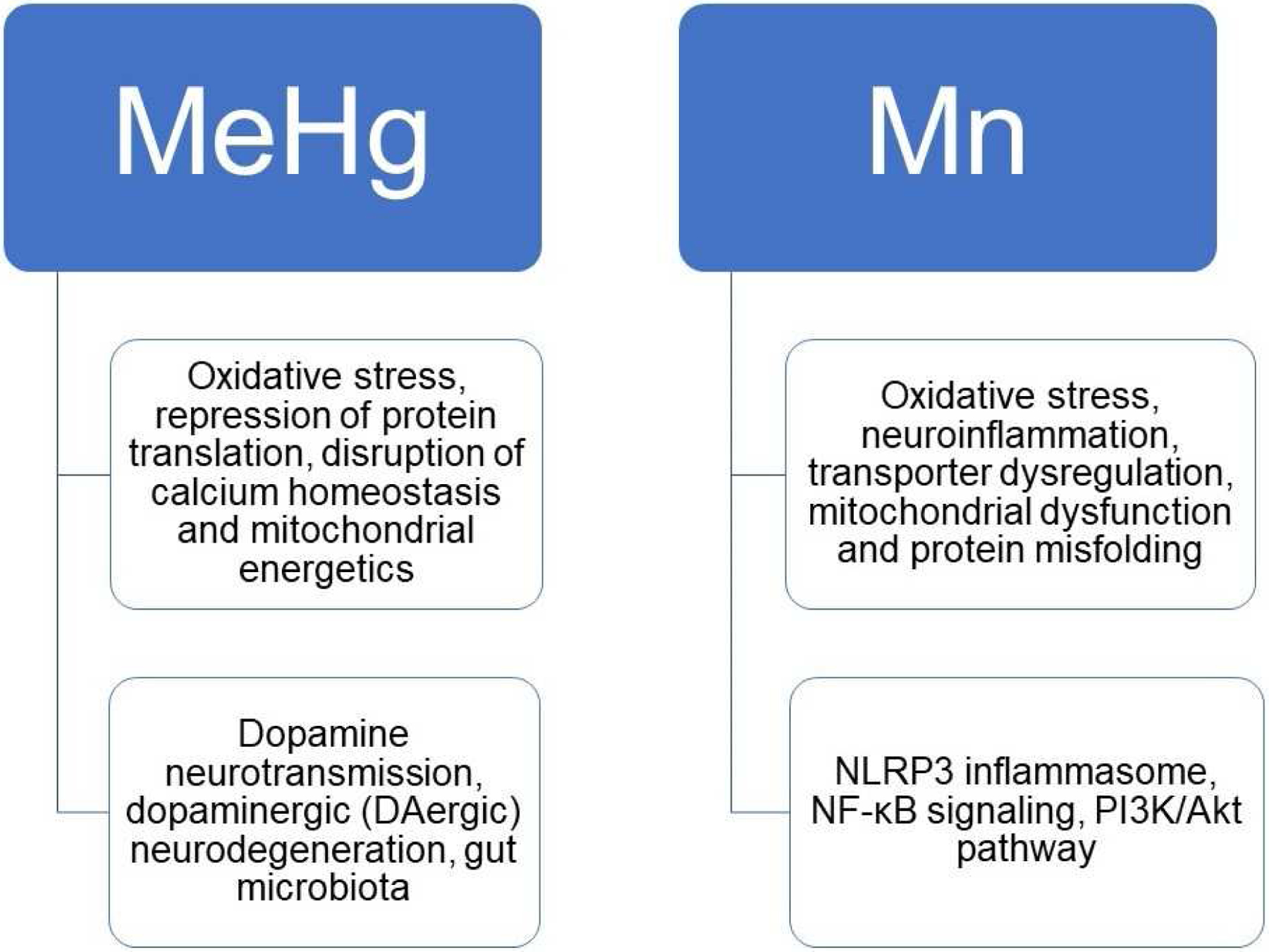
An overview of the classic and new mechanisms underlying MeHg and Mn induced toxicity.
Acknowledgement:
This work was supported by the National Institute of Environmental Health Sciences to MA [NIEHS R01ES007331 and R01ES10563]
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declaration of interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- 1.Kaur I, Behl T, Aleya L, Rahman MH, Kumar A, Arora S, et al. Role of metallic pollutants in neurodegeneration: effects of aluminum, lead, mercury, and arsenic in mediating brain impairment events and autism spectrum disorder. Environ Sci Pollut Res Int. 2021. [DOI] [PubMed] [Google Scholar]
- 2.Martins AC, Urbano MR, Almeida Lopes ACB, Carvalho MFH, Buzzo ML, Docea AO, et al. Blood cadmium levels and sources of exposure in an adult urban population in southern Brazil. Environ Res. 2020;187:109618. [DOI] [PubMed] [Google Scholar]
- 3.Gerardo B, Cabral Pinto M, Nogueira J, Pinto P, Almeida A, Pinto E, et al. Associations between Trace Elements and Cognitive Decline: An Exploratory 5-Year Follow-Up Study of an Elderly Cohort. Int J Environ Res Public Health. 2020;17(17). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Meplan C, Hughes DJ. The Role of Selenium in Health and Disease: Emerging and Recurring Trends. Nutrients. 2020;12(4). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ajsuvakova OP, Tinkov AA, Willkommen D, Skalnaya AA, Danilov AB, Pilipovich AA, et al. Assessment of copper, iron, zinc and manganese status and speciation in patients with Parkinson’s disease: A pilot study. J Trace Elem Med Biol. 2020;59:126423. [DOI] [PubMed] [Google Scholar]
- 6.Martins AC Jr., Morcillo P, Ijomone OM, Venkataramani V, Harrison FE, Lee E, et al. New Insights on the Role of Manganese in Alzheimer’s Disease and Parkinson’s Disease. Int J Environ Res Public Health. 2019;16(19). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Antunes Dos Santos A, Ferrer B, Marques Goncalves F, Tsatsakis AM, Renieri EA, Skalny AV, et al. Oxidative Stress in Methylmercury-Induced Cell Toxicity. Toxics. 2018;6(3). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.da Cunha Martins A Jr., Carneiro MFH, Grotto D, Adeyemi JA, Barbosa F Jr. Arsenic, cadmium, and mercury-induced hypertension: mechanisms and epidemiological findings. J Toxicol Environ Health B Crit Rev. 2018;21(2):61–82. [DOI] [PubMed] [Google Scholar]
- 9.Raposo RDS, Pinto DV, Moreira R, Dias RP, Fontes Ribeiro CA, Oria RB, et al. Methylmercury Impact on Adult Neurogenesis: Is the Worst Yet to Come From Recent Brazilian Environmental Disasters? Front Aging Neurosci. 2020;12:591601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fujimura M, Usuki F, Unoki T. Decreased plasma thiol antioxidant capacity precedes neurological signs in a rat methylmercury intoxication model. Food Chem Toxicol. 2020;146:111810. [DOI] [PubMed] [Google Scholar]
- 11.Martins AC, Krum BN, Queiros L, Tinkov AA, Skalny AV, Bowman AB, et al. Manganese in the Diet: Bioaccessibility, Adequate Intake, and Neurotoxicological Effects. J Agric Food Chem. 2020. [DOI] [PubMed] [Google Scholar]
- 12.Horning KJ, Caito SW, Tipps KG, Bowman AB, Aschner M. Manganese Is Essential for Neuronal Health. Annu Rev Nutr. 2015;35:71–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Miah MR, Ijomone OM, Okoh COA, Ijomone OK, Akingbade GT, Ke T, et al. The effects of manganese overexposure on brain health. Neurochem Int. 2020;135:104688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Martins AC Jr., Gubert P, Villas Boas GR, Meirelles Paes M, Santamaria A, Lee E, et al. Manganese-induced neurodegenerative diseases and possible therapeutic approaches. Expert Rev Neurother. 2020;20(11):1109–21. [DOI] [PMC free article] [PubMed] [Google Scholar]; •This is an important review article that discusses Mn neurotoxicity and possible therapeutic approaches
- 15.Balachandran RC, Mukhopadhyay S, McBride D, Veevers J, Harrison FE, Aschner M, et al. Brain Manganese and the Balance between Essential Roles and Neurotoxicity. J Biol Chem. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schetinger MRC, Peres TV, Arantes LP, Carvalho F, Dressler V, Heidrich G, et al. Combined exposure to methylmercury and manganese during L1 larval stage causes motor dysfunction, cholinergic and monoaminergic up-regulation and oxidative stress in L4 Caenorhabditis elegans. Toxicology. 2019;411:154–62. [DOI] [PMC free article] [PubMed] [Google Scholar]; •This is an important review article that discusses combined Mn and MeHg neurotoxicity. It shows that the relative mRNA content of ace-2, cat-1, sod-3, sod-4 and ctl-3 was increased at the highest concentration of the interaction (50 mM Mn + 50 μM MeHg), establishing that combined exposure to metals was more toxic to the worms than when exposed to a single metal.
- 17.Ke T, Gonçalves FM, Gonçalves CL, dos Santos AA, Rocha JB, Farina M, et al. Post-translational modifications in MeHg-induced neurotoxicity. 2019;1865(8):2068–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fujimura M, Usuki F. Pregnant rats exposed to low-level methylmercury exhibit cerebellar synaptic and neuritic remodeling during the perinatal period. Arch Toxicol. 2020;94(4):1335–47. [DOI] [PubMed] [Google Scholar]
- 19.Freire MAM, Lima RR, Nascimento PC, Gomes-Leal W, Pereira A Jr. Effects of methylmercury on the pattern of NADPH diaphorase expression and astrocytic activation in the rat. Ecotoxicology and environmental safety. 2020;201:110799. [DOI] [PubMed] [Google Scholar]
- 20.Lin X, Zhao J, Zhang W, He L, Wang L, Chang D, et al. Acute oral methylmercury exposure perturbs the gut microbiome and alters gut-brain axis related metabolites in rats. Ecotoxicology and environmental safety. 2020;190:110130. [DOI] [PubMed] [Google Scholar]; • This is an interesting article that describing the involvement of gut microbiome in neurotoxicity induced by methylmercury exposure
- 21.Ke T, Santamaria A, Rocha JBT, Tinkov A, Bornhorst J, Bowman AB, et al. Cephalic Neuronal Vesicle Formation is Developmentally Dependent and Modified by Methylmercury and sti-1 in Caenorhabditis elegans. Neurochem Res. 2020;45(12):2939–48. [DOI] [PubMed] [Google Scholar]
- 22.Zhu J W CD, Gao XS, Zhu JS, Wang L, Cao SY, Wu Q, Qiao SL, Zhang Z, Li L Comparative effects of mercury chloride and methylmercury exposure on early neurodevelopment in zebrafish larvae. RSC Adv. 2019(9):10766–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ke T, Tsatsakis A, Santamaria A, Antunes Soare FA, Tinkov AA, Docea AO, et al. Chronic exposure to methylmercury induces puncta formation in cephalic dopaminergic neurons in Caenorhabditis elegans. Neurotoxicology. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Melentijevic I, Toth ML, Arnold ML, Guasp RJ, Harinath G, Nguyen KC, et al. C. elegans neurons jettison protein aggregates and mitochondria under neurotoxic stress. Nature. 2017;542(7641):367–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ke T, Tsatsakis A, Santamaría A, Soare FAA, Tinkov AA, Docea AO, et al. Chronic exposure to methylmercury induces puncta formation in cephalic dopaminergic neurons in Caenorhabditis elegans. 2020. [DOI] [PMC free article] [PubMed]; • This article shows that CEP dopaminergic neurons are a sensitive target of MeHg. The article describes a chronic MeHg exposure model, and describes the propensity of MeHg was able to induce bright mCherry puncta formation in the CEP dopaminergic neurons of C. elegans in a dose- and time-dependent manner.
- 26.Kendricks DR, Boomhower SR, Newland MC. Methylmercury, attention, and memory: baseline-dependent effects of adult d-amphetamine and marginal effects of adolescent methylmercury. Neurotoxicology. 2020;80:130–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Colón-Rodríguez A, Colón-Carrión NM, Atchison WD. AMPA receptor contribution to methylmercury-mediated alteration of intracellular Ca(2+) concentration in human induced pluripotent stem cell motor neurons. Neurotoxicology. 2020;81:116–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sceniak MP, Spitsbergen JB, Sabo SL, Yuan Y, Atchison WD. Acute neurotoxicant exposure induces hyperexcitability in mouse lumbar spinal motor neurons. J Neurophysiol. 2020;123(4):1448–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gunderson JT, Peppriell AE, Vorojeikina D, Rand MD. Tissue-specific Nrf2 signaling protects against methylmercury toxicity in Drosophila neuromuscular development. Arch Toxicol. 2020;94(12):4007–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chang J, Yang B, Zhou Y, Yin C, Liu T, Qian H, et al. Acute Methylmercury Exposure and the Hypoxia-Inducible Factor-1α Signaling Pathway under Normoxic Conditions in the Rat Brain and Astrocytes in Vitro. Environ Health Perspect. 2019;127(12):127006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Akiyama M, Unoki T, Yoshida E, Ding Y, Yamakawa H, Shinkai Y, et al. Repression of mercury accumulation and adverse effects of methylmercury exposure is mediated by cystathionine γ-lyase to produce reactive sulfur species in mouse brain. Toxicol Lett. 2020;330:128–33. [DOI] [PubMed] [Google Scholar]
- 32.Ke T, Aschner MJN. Bacteria affect Caenorhabditis elegans responses to MeHg toxicity. 2019;75:129–35. [DOI] [PMC free article] [PubMed] [Google Scholar]; •• This is an interesting article that reported the involvement of bacteria in MeHg toxicity
- 33.Zhu J, Tang L, Qiao S, Wang L, Feng Y, Wang L, et al. Low-dose methylmercury exposure impairs the locomotor activity of zebrafish: Role of intestinal inositol metabolism. Environ Res. 2020;190:110020. [DOI] [PubMed] [Google Scholar]
- 34.Ijomone OM, Aluko OM, Okoh COA, Martins AC Jr., Aschner M. Role for calcium signaling in manganese neurotoxicity. J Trace Elem Med Biol. 2019;56:146–55. [DOI] [PubMed] [Google Scholar]
- 35.Harischandra DS, Ghaisas S, Zenitsky G, Jin H, Kanthasamy A, Anantharam V, et al. Manganese-Induced Neurotoxicity: New Insights Into the Triad of Protein Misfolding, Mitochondrial Impairment, and Neuroinflammation. Front Neurosci. 2019;13:654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wang H, Yang F, Xin R, Cui D, He J, Zhang S, et al. The gut microbiota attenuate neuroinflammation in manganese exposure by inhibiting cerebral NLRP3 inflammasome. Biomed Pharmacother. 2020;129:110449. [DOI] [PubMed] [Google Scholar]
- 37.Peng D, Li J, Deng Y, Zhu X, Zhao L, Zhang Y, et al. Sodium para-aminosalicylic acid inhibits manganese-induced NLRP3 inflammasome-dependent pyroptosis by inhibiting NF-kappaB pathway activation and oxidative stress. J Neuroinflammation. 2020;17(1):343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sarkar S, Rokad D, Malovic E, Luo J, Harischandra DS, Jin H, et al. Manganese activates NLRP3 inflammasome signaling and propagates exosomal release of ASC in microglial cells. Sci Signal. 2019;12(563). [DOI] [PMC free article] [PubMed] [Google Scholar]; ••This is an important article describing the involvement of NLRP3 inflammasome signaling in neurotoxicity induced by Mn
- 39.Mattson MP, Meffert MK. Roles for NF-kappaB in nerve cell survival, plasticity, and disease. Cell Death Differ. 2006;13(5):852–60. [DOI] [PubMed] [Google Scholar]
- 40.Popichak KA, Afzali MF, Kirkley KS, Tjalkens RB. Glial-neuronal signaling mechanisms underlying the neuroinflammatory effects of manganese. J Neuroinflammation. 2018;15(1):324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nkpaa KW, Onyeso GI, Kponee KZ. Rutin abrogates manganese-Induced striatal and hippocampal toxicity via inhibition of iron depletion, oxidative stress, inflammation and suppressing the NF-kappaB signaling pathway. J Trace Elem Med Biol. 2019;53:8–15. [DOI] [PubMed] [Google Scholar]
- 42.Li J, Deng Y, Peng D, Zhao L, Fang Y, Zhu X, et al. Sodium P-aminosalicylic Acid Attenuates Manganese-Induced Neuroinflammation in BV2 Microglia by Modulating NF-kappaB Pathway. Biol Trace Elem Res. 2021. [DOI] [PubMed] [Google Scholar]
- 43.Vanhaesebroeck B, Whitehead MA, Pineiro R. Molecules in medicine mini-review: isoforms of PI3K in biology and disease. J Mol Med (Berl). 2016;94(1):5–11. [DOI] [PubMed] [Google Scholar]
- 44.Bryan MR, Uhouse MA, Nordham KD, Joshi P, Rose DIR, O’Brien MT, et al. Phosphatidylinositol 3 kinase (PI3K) modulates manganese homeostasis and manganese-induced cell signaling in a murine striatal cell line. Neurotoxicology. 2018;64:185–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Cheng H, Xia B, Su C, Chen K, Chen X, Chen P, et al. PI3K/Akt signaling pathway and Hsp70 activate in hippocampus of rats with chronic manganese sulfate exposure. J Trace Elem Med Biol. 2018;50:332–8. [DOI] [PubMed] [Google Scholar]
- 46.Peres TV, Arantes LP, Miah MR, Bornhorst J, Schwerdtle T, Bowman AB, et al. Role of Caenorhabditis elegans AKT-1/2 and SGK-1 in Manganese Toxicity. Neurotox Res. 2018;34(3):584–96. [DOI] [PMC free article] [PubMed] [Google Scholar]; •• This article that reported that AKT-1/2 and SGK-1 play a role in C. elegans response to Mn intoxication.


