Abstract
Thiazide and thiazide-like diuretics are widely used for the management of hypercalciuria among stone-forming patients. Although the effects of different thiazides should be relatively similar in terms of prevention of stone recurrence, their potency and side effects may differ. However, there is scarce data concerning the metabolic and bone effects of these agents among recurrent nephrolithiasis patients with hypercalciuria. The aim of this update article was to compare our experience in the use of thiazide and thiazide- like diuretics with that of the current literature, concerning their anticalciuric properties and consequent reduction of recurrent stone formation. Their impact on bone mass and potential side effects were also discussed.
Keywords: Diuretics, Hydrochlorothiazide, Hypercalciuria, Indapamide, Kidney Stones, Urolithiasis.
Resumo
Diuréticos tiazídicos e tiazídicos-like são amplamente usados para o tratamento da hipercalciúria em pacientes com formação de cálculos. Embora os efeitos dos diferentes tiazídicos devam ser relativamente semelhantes em termos de prevenção da recorrência do cálculo, sua potência e efeitos colaterais podem ser diferentes. No entanto, há poucos dados sobre os efeitos metabólicos e ósseos desses agentes em pacientes com nefrolitíase recorrente com hipercalciúria. O objetivo deste artigo de atualização foi comparar nossa experiência quanto ao uso de tiazídicos e tiazídicos-like com a publicada na literatura atual, no que diz respeito às suas propriedades anticalciúricas e consequente redução da formação de cálculos recorrentes. Discutimos também seu impacto na massa óssea e potenciais efeitos colaterais.
Descritores: Diurético, Hidroclorotiazida, Hipercalciúria, Indapamida, Cálculos Renais, Urolitíase.
Introduction
Kidney stone recurrence can be either symptomatic through multiple episodes of renal colic or manifested as new stone formation of previous stone growth. Idiopathic hypercalciuria represents the most frequent metabolic disorder found in stone formers, affecting approximately 50% of all patients1, and thiazide and thiazide-like diuretics are widely used for both treatment and prevention of recurrence. Besides lowering the total urinary calcium excretion due to augmented distal calcium reabsorption, increased proximal tubule reabsorption of sodium and calcium (driven by volume contraction) can provide further benefits because of the diminished delivery of calcium to the distal medullar interstitium. These effects, associated to reduced urinary pH and calcium phosphate supersaturation, may contribute to a potential decrease in Randall’s plaque formation2.
Although the effects of different thiazides should be relatively similar in terms of prevention of stone recurrence or treatment of hypertension, Reilly et al3 have stressed about evidence-based distinctions with respect to the use and dosage of hydrochlorothiazide (HCTZ) compared to the thiazide-like diuretics such as chlorthalidone (CTD) or indapamide (IDP). Their potency and side effects also differ, and IDP can be distinguished from HCTZ for not containing the benzothiadiazine core. The risk of hyponatremia, hypokalemia, hyperuricemia, and dyslipidemia seems to be lower with IDP, but this still remains controversial since the differences have not been proven4.
A recent highly debated potential side effect seen after the long-term use of thiazide or thiazide-like diuretics is their association with a higher risk of skin cancer, especially cutaneous and lip squamous cell carcinoma, due to their photosensitizing properties5 - 14.
Osteopenia has been often recognized among kidney stone patients presenting with long lasting idiopathic hypercalciuria, but whether bone resorption plays a primary or a secondary role in the pathogenesis of hypercalciuria still remains unclear15. Thiazide and thiazide-like diuretics, by their ability to decrease urinary calcium excretion, could be particularly useful for ameliorating bone mass or even decreasing bone resorption in hypercalciuric patients with osteopenia16. In an experimental model of hypercalciuric rats with nephrolithiasis, the use of thiazides not only reduced urinary calcium excretion, but also ameliorated trabecular bone quality17. Previous studies conducted in the general population have shown protective effects of thiazides against hip fracture, that may disappear soon after its discontinuation18.
However, the majority of studies comparing different types of thiazides have focused on the effect of lowering blood pressure and/or their adverse effects in the hypertensive population. Therefore, there is scarce data concerning the metabolic and bone effects of different thiazides among recurrent nephrolithiasis patients with hypercalciuria.
The aim of this update article was to compile data about the use of thiazides and their potential side effects in hypercalciuric stone formers and also provide some personal experience of the authors in this field.
Discussion
Metabolic and Clinical Aspects of Thiazide and Thiazide-Like Diuretics Use in Nephrolithiasis
In order to compare our experience in the use of thiazide and thiazide-like diuretics with that of the current literature regarding metabolic aspects and bone effects of these drugs, we performed a retrospective analysis of the medical records of recurrent kidney stone outpatients with idiopathic hypercalciuria followed up at the Universidade Federal de São Paulo (UNIFESP), who had previously been on a regular therapy with HCTZ (25 mg/day) or IDP (2.5 mg/day) for at least one year (unpublished data). Patients with evidence of heart, liver or endocrine diseases, postmenopausal females, and those with abnormal renal function were excluded, but not those with hypertension. For the purpose of the present analysis, we just selected patients who had available data concerning serum and urinary biochemistry before starting the use of these drugs and after around three months on treatment. Data regarding dual energy X-ray absorptiometry (DEXA) before and after 1 year on treatment were retrieved from a total of 28 patients (35±11 years old), 17 men and 11 women, with a mean BMI (body mass index) of 25.9±3.2 for IDP (n=11) and 27.9±3.6 kg/m2 for HCTZ (n=17) groups, respectively. The initial values of mean blood pressure did not differ significantly between the group taking HCTZ compared with the one taking IDP (99.2 ± 10.0 mmHg and 102.7 ± 14 mmHg, respectively), but the achieved values after three months were significantly lower for HCTZ versus IDP (88.5 ± 12.0 vs. 96.0 ± 13.0 mmHg, p <0.001). Regarding side effects, one patient reported both sexual dysfunction and dizziness with HCTZ, suggesting hypotension, and was lost to follow-up. No other severe side effect had been reported in the medical records during this short period of time. Urinary and blood results are shown in Table 1. There was a significant and similar reduction of urinary calcium after treatment with either HCTZ or IDP and a significant increase in mean urinary sodium only in those receiving IDP. Urinary uric acid and citrate did not differ between groups. The expected, comparable, and significant reduction of urinary calcium excretion observed after the first three months on treatment was in accordance with previous reports in stone formers19 , 20. In the present series, the employed dose of HCTZ (25 mg/d) was lower compared to previous studies which claimed that indapamide 2.5 mg/d corresponds to HCTZ 50 mg/d in terms of control of urinary calcium levels21 , 22. Importantly, as shown in Table 2, most of the randomized clinical trials (RCTs) conducted in the past employing thiazides focused on prevention of new stone formation as the main outcome, with a marked reduction on the risk of recurrence in most of them23 - 29 with the exception of three who conducted less than 2 years of follow-up30 - 32. Nevertheless, all but two of these studies (assessing trichlormethiazide and indapamide)27 , 28, had been designed for stone forming patients who were not selected on the basis of urinary calcium excretion and employed only high doses of thiazides (50 to 100 mg). Curiously, the stone incidence has been lowered even among normocalciuric patients23 - 26, what suggests that the less calcium in the urine the better, even when within the normal range. In summary, meaningful data concerning dose-response effects upon calciuria is still lacking. Nowadays, lower doses of thiazides are often employed aiming to reduce metabolic adverse effects, but their efficacy as such is not well established3 , 21 , 33. In order to clarify these unsolved questions, a 3-year prospective double-blind ongoing trial was initiated in 2017 with the purpose of assessing the efficacy of standard and low dose HCTZ in the recurrence prevention of calcium stone formation34.
Table 1. Serum and urinary parameters before (pre) and after (post) treatment.
| Indapamide | Hydrochlorothiazide | |||
|---|---|---|---|---|
| Serum | Pre | Post | Pre | Post |
| Calcium (mg/dL) | 9.3 ± 0.5 | 9.6 ± 0.9 | 10.0 ± 0.1 | 10.0 ± 0.1 |
| Potassium (mEq/L) | 4.5 ± 0.3 | 4.0 ± 0.4* | 4.3 ± 0.4 | 4.0 ± 0.3 |
| Uric Acid (mg/dL) | 5.3 ± 0.8 | 5.9 ± 1.8 | 4.6 ± 1.3 | 6.3 ± 2.6* |
| Cholesterol (mg/dL) | 194 ± 45 | 200 ± 52 | 185 ± 34 | 200 ± 24 |
| Triglycerides (mg/dL) | 115 ± 68 | 117 ± 71 | 117 ± 99 | 116 ± 96 |
| Glucose (mg/dL) | 91 ± 12 | 95 ± 21 | 76 ± 9.3 | 83 ± 10.8 |
| Urine | ||||
| Calcium (mg/kg/24h) | 4.4 ± 0.9 | 3.0 ± 0.9* | 5.0 ± 1.3 | 3.3 ± 0.7* |
| Sodium (mEq/24h) | 223 ± 67 | 271 ± 77* | 195 ± 90 | 221 ± 123 |
| Uric Acid (mg/24h) | 612 ± 181 | 716 ± 231 | 574 ± 405 | 512 ± 392 |
| Citrate (mg/24h) | 510 ± 370 | 470 ± 288 | 439 ± 187 | 461 ± 161 |
Data are reported as mean ± SD;
p<0.05 (versus pre).
Table 2. Randomized clinical trials of thiazide and thiazide-like diuretics in stone formers.
| Author, year | Drug | Dose | Selection for Hypercalciuria | Number treated/placebo | RR for Recurrence | Followup (years) |
|---|---|---|---|---|---|---|
| Brocks, 1981 | Bendroflumethiazide | 2.5 mg TID | No | 33/29 | NS | 1.6 |
| Scholz, 1982 | HCTZ | 25 mg BID | No | 25/26 | NS | 1 |
| Mortensen, 1986 | Bendroflumethiazide + KCl | 2.5 mg TID | No | 12/10 | NS | 2 |
| Laerum, 1984 | HCTZ | 25 mg BID | No | 25/25 | 0.39 | 3 |
| Wilson, 1984 | HCTZ | 100 mg daily | No | 23/21 | 0.48 | 2,8 |
| Robertson, 1985 | Bendroflumethiazide | 2.5 mg TID | No | 13/9 | 0.38 | 3-5 |
| Ettinger, 1988 | Chlorthalidone | 25/50 mg | No | 19 (25mg), 23 (50mg) /31 (placebo) | 0.23 | 3 |
| Ohkawa, 1992 | Triclormetiazida | 4 mg | Yes | 82/93 | 0.42 | 2,1-2,2 |
| Borghi, 1993 | Indapamida | 2.5 mg dia | Yes | 43/14 | 0.21 | 3 |
| Fernandes-Rodrigues, 2006 | HCTZ | 50 mg dia | No | 50/50 | 0.56 | 3 |
HCTZ: hydrochlorothiazide; KCl: potassium chloride; BID: twice daily; TID: three times daily; RR: relative risk.
Notwithstanding the evidence of lower stone recurrence on thiazides compared to placebo, it is worth mentioning that most of the aforementioned studies had been conducted along the 1980s and 1990s. That said, the way to evaluate stone recurrence was through kidney/ureter/bladder (KUB) X-ray, intravenous pyelogram (IVP), or US every 6 to 12 months. Therefore, some of the flaws of such RCTs in the preceding era of computed tomography (CT) scans, relied on the difficulties regarding the identification of new calculi formation or growth of pre-existing ones in KUB X-ray alone as follow-up image.
Systemic and Metabolic Adverse Effects of Thiazide and Thiazide-Like Diuretics
Concerning metabolic side effects, a handful of clinical studies have disclosed them for stone formers using such drugs. In the present series, we observed that mean serum potassium was significantly lower versus baseline levels (although within the normal range) in the group taking IDP, which was not observed with HCTZ (Table 1). Although these findings may be ascribed to a higher baseline level of serum potassium in the former group, such data contrast with the one in the literature revealing less hypokalemia for IDP than HCTZ3. Conversely, the mean serum levels of uric acid were significantly higher for HCTZ than IDP, in agreement with other investigators35. Mean serum glucose and lipid levels did not differ after treatment nor between both drugs. This is in line with data from Singh et al36 which showed that stone formers who received thiazide therapy solely for kidney stone prophylaxis were not at increased risk for subsequent diabetes mellitus (DM). Lately, there have been several studies reporting associations with the long-term use of thiazide and thiazide-like diuretics with the risk of non-melanoma skin cancers5 - 14, as shown in Table 3. Association between use of HCTZ and malignant melanoma is less elucidated, but recent data have suggested increased risk (cumulative and dose-dependent) of nodular melanoma and lentigo melanoma11, what still warrants further investigation. A more recent meta-analysis of observational studies evaluating the association between the use of thiazides and the risk of skin cancers suggested that such use may be associated with an increased risk of skin cancers especially squamous cell carcinomas which reinforces the several studies mentioned above37. Of note, it is important to mention that many other antihypertensive drugs are also considered photosensitizers including not only thiazides, but e.g. loop diuretics, potassium-sparing agents, and alpha-2 receptor agonists, which may act as co-carcinogens under ultraviolet radiation (UVR) exposure12. Anyway, a note of caution concerning special care for sun exposure in thiazide users should be given. The short duration of follow-up in our retrospective analysis does not allow us any comment about such associations.
Table 3. Clinical studies about the use of thiazides and the risk of skin cancers.
| Author, year | Study design | Cancer type | Drug | Results |
|---|---|---|---|---|
| Jensen, 2008 | Case-control | SCC | HCTZ + amiloride (>5 years) | IRR 1.79 (1.45-2.21) |
| Ruiter, 2010 | Cohort | BCC | Thiazides | HR 1.0 (0.95-1.05) |
| Friedman, 2012 | Case-control | SCC Lip cancer | HCTZ | OR 4.22 (2.82-6.31) |
| De Vries, 2012 | Case-control | SCC | Thiazides | OR 1.66 (1.16-2.37) |
| Robinson, 2013 | Case-control | SCC | Thiazides | OR 1.3 (0.7-2.4) |
| Schmidt, 2015 | Case-control | SCC | Thiazides + potassium sparing agents | OR 2.68 (2.24-3.21) |
| Pottergard, 2017 | Caso-controle | SCC CA lábio | HCTZ ≥ 25.000 mg | OR 3.9 (3.0-4.9) |
| Thiazides | ||||
| Nardone, 2017 | Coorte | BCC/SCC/MM | Thiazides | BCC: OR 2.11 1.60-2.79) |
| SCC: OR 4.11 (2.66-6.35) | ||||
| MM: OR 1.82 (1.01-3.82) | ||||
| Pedersen, 2018 | Case-control | NMSC | HCTZ >50.000mg | BCC: OR 1.29 (1.23-1.35) |
| (BCC/SCC) | (cumulative dose) | SCC: OR 3.98 (3.68-4.31) | ||
| Su, 2018 | Cohort | SCC | Tiazídicos Thiazides | HR 1.09 (0.99-1.19) |
SCC: squamous cell carcinoma; IRR: incidence rate ratio; OR: odds ratio (confidence interval); NMSC: nonmelanoma skin cancer; BCC: basal cell carcinoma; HR: hazard ratio; HCTZ: hydrochlorothiazide; MM: malignant melanoma.
Bone Effects of Thiazide and Thiazide-Like Diuretics
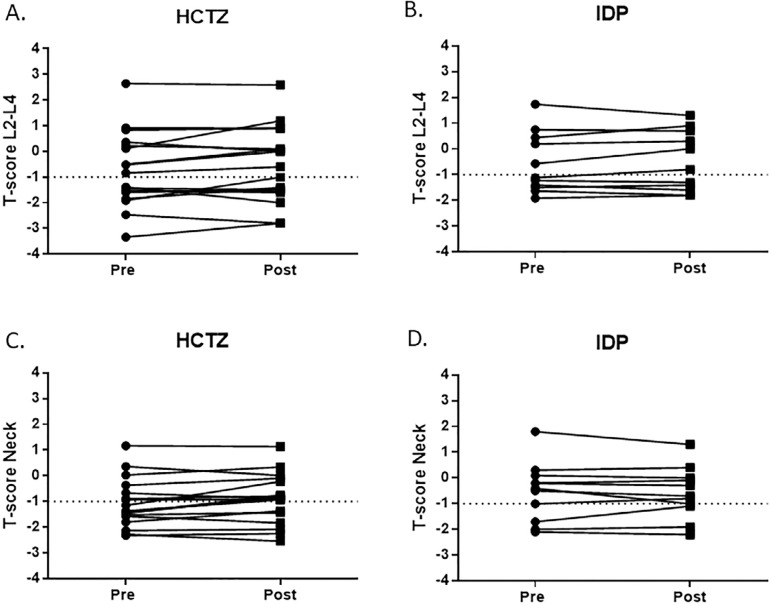
In the present study, the results of bone mineral density (BMD) obtained after one year on treatment showed that patients using HCTZ presented a slight, but not significant improvement (data not shown in tables) of the mean value of T-score in L2-L4 (-1.04 ± 0.36 versus -1.63 ± 0.28) and a significant increase in the femur neck (-1.22 ± 0.22 versus -1.55 ± 0.20, p < 0.05). The increases in mean T-score with IDP were less evident and not significant at both L2-L4 (-0.565 ± 1.2 versus -0.49 ± 1.2) and femur neck sites (-0.53 ? 1.1 versus -0.6 ? 1.0). However, there has been a wide variation of the individual BMD values pre- and post-treatment, as shown in Figure 1, with 11 out of 17 patients taking HCTZ presenting increases in T-score from 2.3 to 80.0% and 6 out of 11 taking IDP presenting increases in T-score from 5.0 to 42.0%. Both HCTZ and indapamide might decrease the risk of fractures by reducing urinary calcium excretion and enhancing calcium balance38 , 39. However, such effect of thiazide and thiazide-like diuretics on bone could also extend beyond its anti-calciuric actions since in vitro they may potentially stimulate osteoblastic bone formation enhancing osteocalcin production and expression of thiazide-sensitive sodium chloride co-transporter, which leads to osteoblastic proliferation40. Moreover, an inhibition of osteoclastic bone resorption was also observed and can be important to sustain the bone mass16. For all these reasons, thiazide therapy approach to improve bone mass and strength in hypercalciuric patients is recommended.
Figure 1. T-score individual values obtained by bone densitometry at lumbar spine (L2-L4) and neck sites in hypercalciuric stone formers before (pre) and after (post) one year on hydrochlorothiazide (HCTZ) or indapamide (IDP) therapy.
Conclusion
Kidney stone treatment and prevention of recurrence are clinically important. Thiazide and thiazide-like diuretics have been widely used for the management of idiopathic hypercalciuria. However, there is a lack of RCT studies including hypercalciuric patients aiming to compare potency and side effects, employing different doses and classes of these agents, with a long-term follow-up. In our personal experience, HCTZ (25 mg daily) and indapamide (2.5 mg daily) were well tolerated as initial dose therapy and provided reduction of urinary calcium excretion with no severe side effects, with a promising protective effect on bone mass.
Acknowledgements
This study was supported by Fundação Oswaldo Ramos - Hospital do Rim (HRIM) - Universidade Federal de São Paulo (UNIFESP) and Conselho Nacional de Desenvolvimento Científco e Tecnológico (CNPQ - Grant 309045/2018-5, IPH). The study was approved by the Ethics Advisory Committee of Universidade Federal de São Paulo - Nr. 3.817.316 (Plataforma Brasil).
References
- 1.Coe FL, Worcester EM, Evan AP. Idiopathic hypercalciuria and formation of calcium renal stones. Nat Rev Nephrol. 2016 Sep;12(9):519–533. doi: 10.1038/nrneph.2016.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bergsland KJ, Worcester EM, Coe FL. Role of proximal tubule in the hypocalciuric response to thiazide of patients with idiopathic hypercalciuria. Am J Physiol Renal Physiol. 2013;305(4):592–599. doi: 10.1152/ajprenal.00116.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Reilly RF, Peixoto AJ, Desir GV. The evidence-based use of thiazide diuretics in hypertension and nephrolithiasis. Clin J Am Soc Nephrol. 2010 Oct;5(10):1893–1903. doi: 10.2215/CJN.04670510. [DOI] [PubMed] [Google Scholar]
- 4.Huen SC, Goldfarb DS. Adverse metabolic side effects of thiazides implications for patients with calcium nephrolithiasis. J Urol. 2007 Apr;177(4):1238–1243. doi: 10.1016/j.juro.2006.11.040. [DOI] [PubMed] [Google Scholar]
- 5.Jensen AO, Thomsen HF, Engebjerg MC, Olesen AB, Sorensen HT, Karagas MR. Use of photosensitising diuretics and risk of skin cancer a population-based case-control study. Br J Cancer. 2008 Nov;99(9):1522–1528. doi: 10.1038/sj.bjc.6604686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ruiter R, Visser LE, Eijgelsheim M, Rodenburg EM, Hofman A, Coebergh JW. High-ceiling diuretics are associated with an increased risk of basal cell carcinoma in a population-based follow-up study. Eur J Cancer. 2010 Sep;46(13):2467–2472. doi: 10.1016/j.ejca.2010.04.024. [DOI] [PubMed] [Google Scholar]
- 7.Pedersen SA, Gaist D, Schmidt SAJ, Hölmich LR, Friis S, Pottegard A. Hydrochlorothiazide use and risk of nonmelanoma skin cancer: a nationwide case-control study from Denmark. J Am Acad Dermatol. (e9) 2018;78(4):673–681. doi: 10.1016/j.jaad.2017.11.042. [DOI] [PubMed] [Google Scholar]
- 8.Pottegard A, Hallas J, Olesen M, Svendsen MT, Habel LA, Friedman GD. Hydrochlorothiazide use is strongly associated with risk of lip cancer. J Inter Med. 2017 Oct;282(4):322–331. doi: 10.1111/joim.12629. [DOI] [PubMed] [Google Scholar]
- 9.Robinson SN, Zens MS, Perry AE, Spencer SK, Duell EJ, Karagas MR. Photosensitizing agents and the risk of non-melanoma skin cancer a population-based case-control study. J Invest Dermatol. 2013 Aug;133(8):1950–1955. doi: 10.1038/jid.2013.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Vries E, Trakatelli M, Kalabalikis D, Ferrandiz L, Ruiz-de-Casas A, Moreno-Ramirez D. Known and potential new risk factors for skin cancer in European populations: a multicentre case-control study. Br J Dermatol. 2012 Aug;167(Suppl 2):1–13. doi: 10.1111/j.1365-2133.2012.11081.x. [DOI] [PubMed] [Google Scholar]
- 11.Nardone B, Majewski S, Kim AS, Kiguradze T, Martinez-Escala EM, Friedland R. Melanoma and non-melanoma skin cancer associated with angiotensin-converting-enzyme inhibitors, angiotensin-receptor blockers and thiazides a matched cohort study. Drug Saf. 2017 Mar;40(3):249–255. doi: 10.1007/s40264-016-0487-9. [DOI] [PubMed] [Google Scholar]
- 12.Schmidt SAJ, Schmidt M, Mehnert F, Lemeshow S, Sorensen HT. Use of antihypertensive drugs and risk of skin cancer. J Eur Acad Dermatol Venereol. 2015 Aug;29(8):1545–1554. doi: 10.1111/jdv.12921. [DOI] [PubMed] [Google Scholar]
- 13.Friedman GD, Asgari MM, Warton EM, Chan J, Habel LA. Antihypertensive drugs and lip cancer in non-Hispanic whites. Arch Inter Med. 2012 Sep;172(16):1246–1251. doi: 10.1001/archinternmed.2012.2754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Su KA, Habel LA, Achacoso NS, Friedman GD, Asgari MM. Photosensitizing antihypertensive drug use and risk of cutaneous squamous cell carcinoma. Br J Dermatol. 2018 Nov;179(5):1088–1094. doi: 10.1111/bjd.16713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Heilberg IP, Weisinger JR. Bone disease in idiopathic hypercalciuria. Curr Opin Nephrol Hypertens. 2006 Jul;15(4):394–402. doi: 10.1097/01.mnh.0000232880.58340.0c. [DOI] [PubMed] [Google Scholar]
- 16.Sakhaee K, Maalouf NM, Kumar R, Pasch A, Moe OW. Nephrolithiasis-associated bone disease pathogenesis and treatment options. Kidney Int. 2011 Feb;79(4):393–403. doi: 10.1038/ki.2010.473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bushinsky DA, Asplin JR. Thiazides reduce brushite, but not calcium oxalate, supersaturation, and stone formation in genetic hypercalciuric stone-forming rats. J Am Soc Nephrol. 2005;16(2):417–424. doi: 10.1681/ASN.2004070543. [DOI] [PubMed] [Google Scholar]
- 18.Schoofs MW, Van Der Klift M, Hofman A, Laet CE, Herings RM, Stijnen T. Thiazide diuretics and the risk for hip fracture. Ann Intern Med. 2003 Sep;139(6):476–482. doi: 10.7326/0003-4819-139-6-200309160-00010. [DOI] [PubMed] [Google Scholar]
- 19.Maschio G, Angelo A, Fabris A, Tessitore N, Sartori L, Morbiato F. Long-term effects of low-dose thiazide and amiloride administration in recurrent renal stone formers. Contrib Nephrol. 1985;49:108–117. doi: 10.1159/000411903. [DOI] [PubMed] [Google Scholar]
- 20.Borghi L, Elia G, Trapassi MR, Melloni E, Amato F, Barbarese F. Acute effect of indapamide on urine calcium excretion in nephrolithiasis and human essential hypertension. Pharmacology. 1988;36(5):348–355. doi: 10.1159/000138405. [DOI] [PubMed] [Google Scholar]
- 21.Vigen R, Weideman RA, Reilly RF. Thiazides diuretics in the treatment of nephrolithiasis are we using them in an evidence-based fashion?. Int Urol. Nephrol. 2011 Sep;43(3):813–819. doi: 10.1007/s11255-010-9824-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Martins MC, Meyers AM, Whalley NA, Margolius LP, Buys ME. Indapamide (Natrilix) the agent of choice in the treatment of recurrent renal calculi associated with idiopathic hypercalciuria. Br J Urol. 1996 Aug;78(2):176–180. doi: 10.1046/j.1464-410x.1996.00633.x. [DOI] [PubMed] [Google Scholar]
- 23.Laerum E, Larsen S. Thiazide prophylaxis of urolithiasis A double-blind study in general practice. Acta Med Scand. 1984;215(4):383–389. [PubMed] [Google Scholar]
- 24.Wilson DR, Strauss AL, Manuel MA. Comparison of medical treatments for the prevention of recurrent calcium nephrolithiasis. Urol Res. 1984;12:39–40. [Google Scholar]
- 25.Robertson WG, Peacock M, Selby PL, Williams RE, Clark P, Chisholm GD. A multicentre trial to evaluate three treatments for recurrent idiopathic calcium stone disease - a preliminary report. In: Schwille PO, Smith LH, Robertson WG, Vahlensieck W, editors. Urolithiasis Relat Clin Res. New York: Plenum Press; 1985. pp. 545–548. [Google Scholar]
- 26.Ettinger B, Citron JT, Livermore B, Dolman LI. Chlorthalidone reduces calcium oxalate calculus recurrence but magnesium hydroxide does not. J Urol. 1988 Apr;139(4):679–684. doi: 10.1016/s0022-5347(17)42599-7. [DOI] [PubMed] [Google Scholar]
- 27.Ohkawa M, Tokunaga S, Nakashima T, Orito M, Hisazumi H. Thiazide treatment for calcium urolithiasis in patients with idiopathic hypercalciuria. Br J Urol. 1992 Jun;69(6):571–576. doi: 10.1111/j.1464-410x.1992.tb15624.x. [DOI] [PubMed] [Google Scholar]
- 28.Borghi L, Meschi T, Guerra A, Novarini A. Randomized prospective study of a nonthiazide diuretic, indapamide, in preventing calcium stone recurrences. J Cardiovasc Pharmacol. 1993;22(Suppl 6):S78–S86. [PubMed] [Google Scholar]
- 29.Fernández-Rodríguez A, Arrabal-Martín M, García-Ruiz MJ, Arrabal-Polo MA, Pichardo-Pichardo S, Zuluaga-Gómez A. The role of thiazides in the prophylaxis of recurrent calcium lithiasis. Actas Urol Esp. 2006 Mar;30(3):305–309. doi: 10.1016/s0210-4806(06)73444-1. [DOI] [PubMed] [Google Scholar]
- 30.Brocks P, Dahl C, Wolf H, Transbol I. Do thiazides prevent recurrent idiopathic renal calcium stones? Lancet. 1981;2(8238):124–125. doi: 10.1016/s0140-6736(81)90302-0. [DOI] [PubMed] [Google Scholar]
- 31.Scholz D, Schwille PO, Sigel A. Double-blind study with thiazide in recurrent calcium lithiasis. J Urol. 1982 Nov;128(5):903–907. doi: 10.1016/s0022-5347(17)53269-3. [DOI] [PubMed] [Google Scholar]
- 32.Mortensen JT, Schultz A, Ostergaard AH. Thiazides in the prophylatic treatment of recurrent idiopathic kidney stones. Int Urol Nephrol. 1986;18(3):265–269. doi: 10.1007/BF02082712. [DOI] [PubMed] [Google Scholar]
- 33.Alexander RT, McArthur E, Jandoc R, Welk B, Fuster DG, Garg AX. Thiazide diuretic dose and risk of kidney stones in older adults: a retrospective cohort study. Can J Kidney Heal Dis. 2018 Jul;5:205435811878748–205435811878748. doi: 10.1177/2054358118787480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dhayat NA, Faller N, Bonny O, Mohebbi N, Ritter A, Pellegrini L. Efficacy of standard and low dose hydrochlorothiazide in the recurrence prevention of calcium nephrolithiasis (NOSTONE trial): protocol for a randomized double-blind placebo-controlled trial. BMC Nephrol. 2018;19(1):349–349. doi: 10.1186/s12882-018-1144-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gurwitz JH, Kalish SC, Bohn RL, Glynn RJ, Monane M, Mogun H. Thiazide diuretics and the initiation of anti-gout therapy. J Clin Epidemiol. 1997 Aug;50(8):953–959. doi: 10.1016/s0895-4356(97)00101-7. [DOI] [PubMed] [Google Scholar]
- 36.Singh P, Knoedler JJ, Krambeck AE, Lieske JC, Bergstralh EJ, Rule AD. Thiazide diuretic prophylaxis for kidney stones and the risk of diabetes mellitus. J Urol. 2014 Dec;192(6):1700–1704. doi: 10.1016/j.juro.2014.06.078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Shin D, Lee ES, Kim J, Guerra L, Naik D, Prida X. Association between the use of thiazide diuretics and the risk of skin cancers: a meta-analysis of observational studies. J Clin Med Res. 2019 Apr;11(4):247–255. doi: 10.14740/jocmr3744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pak CYC, Heller HJ, Pearle MS, Odvina CV, Poindexter JR, Peterson RD. Prevention of stone formation and bone loss in absorptive hypercalciuria by combined dietary and pharmacological interventions. J Urol. 2003 Feb;169(2):465–469. doi: 10.1097/01.ju.0000047341.55340.19. [DOI] [PubMed] [Google Scholar]
- 39.Lalande A, Roux C, Graulet AM, Schiavi P, Vernejoul MC. The diuretic indapamide increases bone mass and decreases bone resorption in spontaneously hypertensive rats supplemented with sodium. J Bone Miner Res. 1998 Sep;13(9):1444–1450. doi: 10.1359/jbmr.1998.13.9.1444. [DOI] [PubMed] [Google Scholar]
- 40.Lalande A, Roux S, Denne MA, Stanley ER, Schiavi P, Guez D. Indapamide, a thiazide-like diuretic, decreases bone resorption in vitro. J Bone Miner Res. 2001 Feb;16(2):361–370. doi: 10.1359/jbmr.2001.16.2.361. [DOI] [PubMed] [Google Scholar]