Abstract
Fresh mechanically deboned meat (MDM) is usually claimed as high-quality ingredient on dry pet food recipes and this aspect may positively influence consumer choice. It is important to determine the scientifically sustainability of this claim and to assess the microbiological safety of MDM inclusion in dry pet food. Objectives were: 1) to evaluate the effect of inclusion of MDM in dry dog food on fatty acid profile and in vivo and in vitro digestibility, proposing a new system (DaisyII Incubator) to measure the in vitro digestibility for dogs; 2) to compare palatability of dry dog food containing MDM with dry dog food in which meat by-products (MBP) are the only animal protein sources; 3) to determine, whether or not, the inclusion of that ingredient changes the microbiology and the storage quality. Results indicated that MDM product was characterized by significant higher nutritional value in terms of fatty acids profile, in vitro digestibility (HV-IVD method) and lower palatability than the MBP product. Microbiological risk assessment showed no microbiological hazards for either product. After 6-months storage, the total mesophilic bacterial count ranged between 1.77 and 2.09 log CFU/g feed, while polyamine values were higher in the MDM (0.37 g/kg) than in the MBP (0.27 g/kg). The DaisyII Incubator was found to be a valid instrument for studying in vitro digestibility also for dogs, providing data simply, quickly, with less variability and costs than in vivo trials. In conclusion, MDM inclusion in dry dog food is microbiologically safe and it can improve its nutritional quality, at the expense of a reduced palatability. The higher polyamine levels fount in MDM-enriched petfood after 6-months storage, however, may represent a possible hazard, and further studies are still warranted.
1. Introduction
The majority of pet owners consider their pets to be family members (63% of pet owners in the United States and more than 71% in Italy), [1, 2]. Humanization of dogs and cats has directed the owners’ preferences for pet food claimed with “human‐grade” ingredients, mainly characterized by the replacement of processed animal proteins with raw animal protein sources (“fresh mechanically deboned meat”, MDM), and usually listed as the first ingredient on pet food labels. This trend in mainly driven by the erroneous belief that these products are safer, more palatable, more digestible and respond to human regulation [1]. Despite the “human-grade” claim have no definition in any animal feed regulations, and there aren’t supporting evidences of MDM inclusion on nutritional traits so far, the pet food industry is keen to increasingly include fresh and unprocessed chicken meat in dry pet food products.
In parallel, an unjustified demonization of the use of meat by-products in pet food has aroused [1].
Consumer uncertainty about what passes as “by-products” may lead pet owners to perceive them as poor-quality ingredients [3]. Processed animal proteins are, in fact, widely used in the pet food industry and provide an excellent source of protein, energy, and minerals [4, 5], however, misperceptions about their origin and content make fresh meat the more desirable ingredient.
Animal by-products are defined in article 3 of Regulation (EC) 1069/2009 as “entire bodies or parts of animals, products of animal origin or other products obtained from animals that are not intended for human consumption” [6]. Animal by-products can fall into three categories and category 3 material includes, among others, processed animal proteins usually purchased by manufacturers for pet food production. In details, “processed animal protein” means meat and bone meal, meat meal, bone meal, blood meal, dried plasma and other blood products, hydrolyzed protein, hoof meal, horn meal, poultry offal meal, feather meal, dry greaves, fishmeal, dicalcium phosphate, gelatin and any other similar products including mixtures, feeding stuffs, feed additives and premixtures, containing these products.
Fresh mechanically deboned meat, on the other hand, refers to meat that has not undergone any treatment except maintaining the cold chain. Treatments such as cooking, drying, freezing, hydrolysis or addition of preservatives exclude the component from being called “fresh” [7]. It is obtained by forcing pureed or ground pork, turkey or chicken meat, under high pressure through a sieve or similar device, to separate the bone from the edible meat tissue. These characteristics, together with the fact that MDM is usually listed as the first ingredient on pet food labels (due to the high- water content), could influence consumer choice among pet food products.
Including meat by-products or MDM in pet food can also have nutritional and technological implications. For example, rendering conditions, as well as thermal treatment and the source and handling of raw materials, can greatly influence nutritional and microbiological traits and digestibility of the protein meals [4, 8].
Most dry foods are produced by extrusion. Correct extrusion conditions favor higher retention of amino acids, high protein and starch digestibility, less lipid oxidation, and higher retention of vitamins [9]. In addition, the extrusion process denatures undesirable enzymes such as anti-nutritional factors (trypsin inhibitors, hemagglutinins, tannins, and phytates) and sterilizes the finished product [9–12]. Although overcooking can diminish the nutritional quality of foods, the relatively high moisture content, moderate temperatures, and short cooking duration all help to maintain the nutritional quality of extruded foods [9–12].
As regards microbiological issues, there have been several recalls of commercial pet foods and treats in the United States because of contamination with Salmonella spp., Escherichia coli, and other foodborne pathogens [13]. Contamination not only poses the risk that pets ingesting these food items can become clinically ill or may become carriers of the pathogens but it also represents a public health concern for pet owners who handle food products and interact with their pets [14, 15].
Finally, the use of animals in research elicits a diverse range of attitudes and emotions, with some people demanding the abolition of research on animals and others expressing strong support. Typically, opponents of animal research cite besides animal welfare and suffering also the uselessness of digestibility and palatability trials [16].
Given the above, the trend is to prefer the use of in vitro enzymatic analysis [17] over in vivo studies, which are more costly, laborious, and require animals anyway. In farm animal nutritional studies, in vitro digestibility is largely estimated using a DaisyII Incubator (Ankom Technology Co., Fairport, NY, USA); this closed-system fermentation apparatus has been previously used in digestibility studies in ruminants [18, 19] and monogastric animals [20, 21]. Initially developed for multiple analysis of feeds, the incubator reduces labor demands, improves precision, and could offer an alternative system to traditional in vitro methods for pet food digestibility studies.
There are few studies to date that evaluate the effect of raw or fresh ingredients on pet food nutritional profile, conservation quality, palatability, and digestibility. The objectives of the present study were: (a) to evaluate the effect of inclusion of MDM in dry dog food on fatty acid profile and in vivo and in vitro digestibility, proposing a new system (DaisyII Incubator) to measure the in vitro digestibility for dogs; (b) to compare palatability of dry dog food containing MDM with dry dog food in which meat by-products (MBP) are the only animal protein sources; (c) to determine whether or not there were differences in MDM/MBP pet food microbiology and conservation quality.
2. Materials and methods
This article does not contain any studies involving animals performed by any of the authors.
2.1. Diet formulation and extrusion parameters
Two isoenergetic and isonitrogenous extruded diets were formulated according to the National Research Council (NRC) guidelines for adult dogs [22], and prepared by a manufacturer located in Italy and authorized for pet food production according to EU legislation. One diet was formulated using mechanically separated chicken meat (MDM) as the first ingredient, while the other diet was formulated with processed animal protein (MBP) as the first ingredient.
Tables 1 and 2 present the mean chemical composition, inorganic mineral, vitamin, and amino acid content of the two feed materials and of the two diets, respectively. Nutrients determination was performed according to AOAC INTERNATIONAL standards (2018) [23].
Table 1. Chemical composition (g/kg dry matter), mineral content (g/kg dry matter), vitamin content and amino acid content (g/100 g dry matter) of the two types of feed materials (FM).
| Items | MDM | MBP |
|---|---|---|
| DM (g/kg FM) | 328 | 951 |
| Crude protein | 360 | 683 |
| Ether extract | 364 | 129 |
| Nitrogen free extract | 11 | 2 |
| Cellulose | 30 | 12 |
| Ash | 235 | 174 |
| Ca | 71 | 49 |
| P | 40 | 27 |
| Mg | 1.9 | 1.9 |
| Zn | 0.09 | 0.11 |
| Fe | 0.03 | 0.13 |
| Vitamin B1 (mg/kg) | 0.70 | 1.2 |
| Vitamin B2 (mg/100g) | n.d.1 | 0.59 |
| Vitamin B12 (μg/kg) | n.d. | 0.04 |
| Aspartic acid | 2.4 | 5.3 |
| Threonine | 1.0 | 2.5 |
| Serine | 1.0 | 2.7 |
| Glutamic acid | 3.8 | 8.7 |
| Proline | 3.0 | 4.0 |
| Glycine | 5.5 | 6.7 |
| Alanine | 3.1 | 4.5 |
| Valine | 1.4 | 3.0 |
| Methionine | 0.24 | 1.3 |
| Isoleucine | 1.0 | 2.4 |
| Leucine | 1.8 | 4.4 |
| Tyrosine | 0.55 | 1.8 |
| Phenylalanine | 1.0 | 2.5 |
| Lysine | 1.6 | 4.3 |
| Histidine | 0.49 | 1.4 |
| Arginine | 2.6 | 5.1 |
| Cysteine | 0.12 | 0.43 |
| Tryptophan | n.d. | 0.33 |
n.d. = not detected.
Table 2. Chemical composition (g/kg, as-fed basis), mineral content (g/kg, as-fed basis), vitamin content and amino acid content (g/100 g, as-fed basis) of the two diets (MDM = Mechanically Deboned Meat diet and MBP = Meat By-Product diet; mean±SD).
| Items | MDM1 | MBP2 |
|---|---|---|
| DM | 940.5±0.5 | 930.1±0.3 |
| Crude protein | 263.1±0.2 | 264.7±1.0 |
| Ether extract | 176.8±0.3 | 187.0±2.7 |
| Nitrogen free extract | 401.4±0.8 | 373.8±4.8 |
| Cellulose | 26.6±0.9 | 24.1±0.6 |
| Ash | 72.6±0.9 | 80.6±1.5 |
| Ca | 15.6±0.8 | 18.4±4.7 |
| P | 10.7±0.4 | 12.4±3.0 |
| Mg | 1.41±0.07 | 1.41±0.34 |
| Zn | 0.28±0.01 | 0.27±0.01 |
| Fe | 0.29±0.01 | 0.26±0.03 |
| Vitamin B1 (mg/kg) | 12.1±0.1 | 10.1±0.2 |
| Vitamin B2 (mg/100g) | 1.47±0.01 | 1.28±0.02 |
| Vitamin B12 (μg/kg) | 0.11±0.01 | 0.09±0.00 |
| Vitamin A (U.I/kg) | 30267±896 | 31000±854 |
| Vitamin D3 (U.I/kg) | 2153±693 | 2280±324 |
| Vitamin E (mg/kg) | 194±17 | 189±7 |
| Aspartic acid | 2.30±0.03 | 2.29±0.03 |
| Threonine | 1.07±0.02 | 1.08±0.02 |
| Serine | 1.22±0.02 | 1.16±0.02 |
| Glutamic acid | 3.62±0.05 | 3.50±0.07 |
| Proline | 1.64±0.09 | 1.56±0.04 |
| Glycine | 2.32±0.04 | 2.45±0.05 |
| Alanine | 1.74±0.02 | 1.78±0.05 |
| Valine | 1.30±0.01 | 1.36±0.04 |
| Methionine | 0.67±0.02 | 0.54±0.02 |
| Isoleucine | 1.04±0.06 | 1.04±0.06 |
| Leucine | 2.11±0.01 | 1.99±0.03 |
| Tyrosine | 0.82±0.03 | 0.66±0.07 |
| Phenylalanine | 1.14±0.02 | 1.15±0.01 |
| Lysine | 1.50±0.06 | 1.60±0.04 |
| Histidine | 0.56±0.02 | 0.56±0.01 |
| Arginine | 1.80±0.03 | 1.84±0.04 |
| Cysteine | 0.26±0.01 | 0.24±0.02 |
| Tryptophan | 0.15±0.01 | 0.13±0.00 |
| Metabolisable Energy (kJ/Kg) | 16789 | 16998 |
1MDM ingredients: mechanically separated chicken meat, processed animal protein, rice, potato protein concentrate, animal fat, maize, beet pulp, brewer’s yeast, hydrolyzed animal protein, Spirulina platensis, Yucca schidigera, hydrolyzed cartilage, hydrolyzed crustaceans, methyl sulfonyl methane, Echinacea root, oregano, garlic, vitamin and mineral mix.
2MBP ingredients: processed animal protein, rice, potato protein concentrate, animal fat, maize, beet pulp, brewer’s yeast, hydrolyzed animal protein, Spirulina platensis, Yucca schidigera, hydrolyzed cartilage, hydrolyzed crustaceans, methyl sulfonyl methane, Echinacea root, oregano, garlic, vitamin and mineral mix.
Specific extrusion parameters used by the manufacturer during petfood production are reported in Table 3.
Table 3. Production of MDM (Mechanically Deboned Meat) diet and MBP (Meat By-Products) diet and relative processing temperature, modified by [24–26].
| Phase | Duration (min) | MDM Temperature (°C) | MBP Temperature (°C) |
|---|---|---|---|
| Preparation of the main ingredient mixture | - | Room temperature | Room temperature |
| Pre-grinding storage | - | Room temperature | Room temperature |
| Grinding | 60 | 40° | 40° |
| Grinding mixture storage | 180 | Room temperature | Room temperature |
| Pre-conditioning | 1 | From 25° to 60° | From 25° to 60° |
| Extrusion | 0.5 | From 85° to 110° | From 70° to 110° |
| Drying | 30 | From 85° to 160° | From 50° to 95° |
2.2. Fatty acid analysis
To determine fatty acid composition of the diets, lipid extraction and gas chromatography (GC) were performed according to Peiretti and Meineri [27]. All analyses were performed in triplicate.
2.3. Microbiological analyses and shelf-life
Microbiological analysis was conducted to determine the microbiological quality of ingredients, intermediate feed, throughout the production stages (where possible contamination may occur), and feed at the end of production and at 6 months of storage under controlled conditions in samples from a box of the same batch. All microbiological methods were ISO [28] or taken from the literature: ISO 6579:2002 for Salmonella also referred to pet food and ISO 4833-1991/AFNOR V08-054 for the total mesophilic bacterial count. All media were Oxoid (Basingtoke, UK), except for Chromocult® (Merck, Germany). Microbial counts are expressed as a logarithm (log) of colony-forming units (cfu) per gram of sample. Quantitative analysis was carried out in duplicate.
Briefly the methods:
Total mesophilic bacterial count: plate count agar at 30°C for 72 h;
Enterobacteriaceae and E. coli counts: violet bile glucose agar and Chromocult® (Merck, Germany), respectively. Plates were incubated at 37° C for 24–48 h;
Salmonella count: 50 g were suspended in buffered peptone water for pre-enrichment at 37°C for 18 h, Rappaport-Vassiliadis medium with soya (RVS broth) at 41.5°C for 24 h and Muller-Kauffmann tetrathionate/novobiocin broth (MKTTn broth) at 37°C for 24 h for selective enrichment, then plated the third day on xylose lysine dextrose agar (XLD) and brilliant green agar (BGA) incubated at 37°C for 24 h; phenotipical identification was carried out on Kligler iron agar and genotypical identification was performed according to Rahn et al. [29];
sulfide reductase clostridium: SPS medium at 37° for 48 h under strict anaerobic conditions [30].
Biogenic amines were determined according to Paulsen et al. [31] on a high-performance liquid chromatography visible UV detector (HPLC UV/Vis). Peroxide determination was performed according to the EU official method [32]. Analyses were performed in triplicate.
2.4. Palatability trial
To evaluate the palatability of MDM and MBP, the two-bowl trial method was used [33]. This trial was prospectively approved by Ethical committee of the University of Turin. Briefly: MDM and MBP were placed in their respective bowls and presented simultaneously to a panel that judged food palatability according to three sensory characteristics: aroma, texture, and macronutrient profile. The panel was composed of 40 adult dogs of any breed and size randomly divided by sex and individually caged at feeding time; for the remainder of the day they were housed in kennels in groups of 20. The trial lasted 24 h during which each dog was presented once, for 30 minutes, with the two bowls and had to choose from which to eat first. The bowls contained 500 g of MDM or MBP. The quantities of each product consumed (intake) and left (outtake) were recorded for each dog. At the end of the trial, the quantities of MDM and MBP consumed by the whole panel were summed to determine which of the two diets was consumed more. Data are expressed as the total amount of food consumed for each diet, intake ratio (IR), preference, and food selected as a first choice. Water was supplied ad libitum.
2.5. In vivo digestibility trial
The digestibility protocol followed the guidelines published in the official method of the Association of American Feed Control Officials [34]. The trial was prospectively approved by Ethical committee of the University of Turin. The in vivo digestibility (Vd) was tested in 10 Beagles (5 males and 5 females) weighing between 8 and 16 kg. All dogs had been part of a trained panel. The animals were individually housed during the trial. In order to test the digestibility of MDM and MBP, the same dogs were employed for both trials but with different schedules according to the official guidelines [6]. Each Vd trial consisted of 3 days of adjustment to the new diet, followed by a 4-day collection period during which the weight of food offered and refused, and feces were recorded daily. A total of 500 g/day was offered as fed, while water was supplied ad libitum. The food was presented in the morning for 30 min. The total individual daily feces were weighed and then kept at -18°C pending analysis. The feces samples of each dog were dried; the 4-day cumulative samples were pooled from the daily samples. Apparent digestibility is expressed as g/kg. The total tract apparent digestibility coefficients of DM were calculated for each diet according to Villaverde et al. [35] using the formula:
| (1) |
Housing and treatment protocols (Registration Number 612.623.82) adhered to European norms for animal welfare [34, 36]. The facility was maintained according to regulations [37].
2.6. In vitro digestibility trials
In vitro digestibility was estimated using a DaisyII Incubator (Ankom Technology, Macedon, NY, USA). The incubator has four digestion jars which rotate at constant and uniform temperature inside a temperature-controlled chamber. Each jar is filled with an enzymatic solution and can hold up to 23 filter bags with samples and one blank.
Filter bags (F57, Ankom Technology Corp.) were pre-rinsed in pure acetone (99%) in order to remove surfactants, which might block the bag pores and inhibit the enzyme activity. Bags were air-dried and numbered using a solvent resistant marker.
The extruded diets, oven-dried and grounded through a 1 mm screen, were weighed (0.5 ± 0.01 g) in triplicate into the filter bags. The heat-sealed bags were put in the jars with the enzyme and buffer solution, and then digested. Two digestibility techniques, differing in phosphate buffer, type and amount of enzymes were used to simulate the digestion. The first solution (HV-IVD), as proposed by Hervera et al. [38], and the second (BG-IVD), as proposed by Biagi et al. [39] were prepared and used as described in Table 4. The enzymes were pepsin (P7125, Sigma Aldrich), pancreatin (P1500, Sigma Aldrich), and bile salt. The phosphate buffer of the HV-IVD solution contained the acid (KH2PO4) and its conjugate base (K2HPO4), [40]. For the BG-IVD solution, the phosphate buffer was prepared as described by Martillotti et al. [23]. During the digestion time, the jars were slightly agitated and kept at constant temperature (39°C). At the end of incubation, the bags were removed from jars and rinsed thoroughly with warm tap water (39°C) with little agitation. Samples were rinsed until the water was clear. Before oven dry over-night at 65–70°C, the sample bags of the HV-IVD method were rinsed in ethanol and acetone by complete immersion for about 5 minutes, respectively. Later, bags were weighted and the final weight was recorded to calculate in vitro digestibility (IVD) as follows:
| (2) |
Table 4. In vitro digestibility using the DaisyII Incubator and according to the method described in Hervera et al. [38], (HV-IVD) and in Biagi et al. [39], (BG-IVD).
| Step | HV-IVD | BG-IVD |
|---|---|---|
| 0.5 ± 0.01 g of sample1 | 0.5 ± 0.01 g of sample | |
| 1 Gastric digestion | 1200 ml phosphate buffer (0.1M, pH 6) 480 ml HCl (0.2M) 480 mg pepsin 24 ml cloramphenicol solution (0.5g/100 ml ethanol) pH 2, 39°C, 2 h |
1440 ml of pepsin-lipase-HCl solution (HCl 0.075N; pepsin 2g/L; gastric lipase 1g/L) 39°C, 2 h |
| 2 Post-gastric digestion | 480 ml phosphate buffer (0.2 M, pH 6.8) 240 ml NaOH (0.6 M) 4.8 g pancreatin pH 6.8, 39°C, 4 h |
1440 ml -pancreatin-bile salt-phosphate buffer solution (10g/L pancreatin 25 g/L; bile salt) pH 7.5, 39°C, 4 h |
| 3 Collection of undigested fraction | F57 washed, twiced with ethanol (96%) and twice with acetone (99%), dried overnight at 70°C, analysed for CP, EE, DM, OM | F57 washed, dried overnight at 65°C, analysed for CP, EE, DM, OM |
1 Quantities for each jar and twenty-four F57 filter bags (twenty-three replicate samples and one blank).
2.7. Statistical analysis
Fatty acid content and data from the digestibility and palatability trials were analyzed using SPSS software (version 11.5.1 for Windows, SPSS Inc., USA) by one-way ANOVA with diet as the main factor; storage quality parameters were analyzed by ANOVA for multifactorial analysis of variance for the two main factors (diet and conservation time) to identify differences. Time effect (month 0 and 6), diet effect (MDM vs. MBP) and the time×diet interaction were considered to be statistically significant at P <0.05.
3. Results
3.1. Fatty acid profile
Table 5 presents the fatty acid composition of the two diets. MDM was richer than MBP in polyunsaturated fatty acids (PUFAs), whereas MBP was higher in saturated fatty acids (SFAs) than MDM, because MDM had higher values for linoleic acid and lower values for some SFAs (capric, lauric, myristic, and palmitic acid). MDM also had higher values for other SFAs (pentadecanoic and margaric acid) and MUFAs (heptadecanoic and elaicid acid).
Table 5. Fatty acid profile (mean±SD; g/100 g of total fatty acid) of the two diets (MDM = Mechanically Deboned Meat; MBP = Meat By-Products) and the significance between them.
| Compound | MDM | MBP | P |
|---|---|---|---|
| Capric acid (C10:0) | n.d.1 | 0.02±0.03 | - |
| Lauric acid (C12:0) | 0.12±0.01 | 0.42±0.01 | 0.000 |
| Myristic acid (C14:0) | 1.16±0.02 | 1.21±0.01 | 0.025 |
| Myristoleic acid (C14:1) | 0.22±0.03 | 0.23±0.01 | 0.692 |
| Pentadecanoic acid (C15:0) | 0.16±0.01 | 0.15±0.00 | 0.016 |
| Palmitic acid (C16:0) | 20.69±0.03 | 20.78±0.03 | 0.020 |
| Palmitoleic acid (C16:1n7) | 4.85±0.03 | 4.80±0.03 | 0.123 |
| Margaric acid (C17:0) | 0.27±0.01 | 0.24±0.00 | 0.001 |
| Heptadecanoic acid (C17:1) | 0.24±0.01 | 0.22±0.01 | 0.013 |
| Stearic acid (C18:0) | 6.24±0.10 | 6.12±0.04 | 0.108 |
| Oleic acid (C18:1n9) | 40.64±0.15 | 40.58±0.11 | 0.592 |
| Elaidic acid (C18:1n9, trans) | 0.54±0.02 | 0.41±0.01 | 0.000 |
| Linoleic acid (C18:2, cis-cis) | 22.60±0.14 | 22.23±0.02 | 0.012 |
| Linoelaidic acid (C18:2, trans-trans) | 0.13±0.03 | 0.11±0.10 | 0.706 |
| α-Linolenic acid (C18:3n3) | 1.96±0.02 | 1.33±1.15 | 0.403 |
| Arachidic acid (C20:0) | 0.15±0.01 | 0.15±0.01 | 1.000 |
| Behenic acid (C22:0) | n.d. | 0.02±0.03 | - |
| Eicosenoic acid (C20:1) | 0.49±0.04 | 0.61±0.10 | 0.374 |
| Lignoceric acid C24:0) | 0.13±0.02 | 0.14±0.01 | 0.275 |
| SFA2 | 28.91±0.09 | 29.23±0.02 | 0.004 |
| MUFA3 | 46.45±0.10 | 46.44±0.02 | 0.878 |
| PUFA4 | 24.55±0.16 | 24.25±0.05 | 0.036 |
1 n.d. = not detected.
2SFA = saturated fatty acid.
3MUFA = monounsaturated fatty acid.
4PUFA = polyunsaturated fatty acid.
3.2. Microbiological profile and shelf-life
Microbiology results are shown in Table 6 (ingredients) and Table 7 (after extrusion, after drying, and final feed formula at time 0 and after 6 months of storage under controlled conditions). All ingredients had a variable and high microbiological count for mesophilic bacteria and Enterobacteriaceae (range from 1.48 log CFU/g to 5.73 log CFU/g). Clostridium spores and E. coli were not detected in any samples.
Table 6. Mean counts (mean±SD, log CFU/g) of the main microbial groups detected in the ingredients.
| Ingredient | Total Mesophilic Bacterial Count | Enterobacteriaceae | E. coli | Clostridium Sulfite Reductase | Salmonella1 |
|---|---|---|---|---|---|
| Chicken meat | 5.28±2.45 | 3.20±1.84 | 1.95±0.62 | n.d.2 | n.d. |
| Meat by-products | 5.23±2.32 | 2.97±1.75 | n.d. | n.d. | Positive |
| Rice | 5.73±2.49 | 3.11±1.84 | n.d. | n.d. | n.d. |
| Potatoes | 5.43±0.01 | n.d. | n.d. | n.d. | n.d. |
| Chicken Fat | 1.48±0.84 | n.d. | n.d. | n.d. | n.d. |
| Maize | 4.59±1.83 | 2.58±1.32 | n.d. | n.d. | n.d. |
| Beet Pulp | 4.20±1.70 | 2.51±1.19 | n.d. | n.d. | n.d. |
| HAP3 | 3.15±2.05 | 3.70±1.35 | n.d. | n.d. | n.d. |
| Spiruline | 3.66±1.62 | n.d. | n.d. | n.d. | n.d. |
1 Determined on 50 g.
2 n.d. = not detected.
3 HAP = hydrolyzed animal protein.
Table 7. Mean counts (mean±SD log CFU/g) of the main microbial groups) in the two diets (MDM = Mechanically Deboned Chicken Meat diet and MBP = Meat By-Products diet) at different phases.
| Diet | Phase1 | Total Mesophilic Bacterial Count | Enterobacteriaceae | E. coli | Clostridium Sulfite Reductase | Salmonella2 |
|---|---|---|---|---|---|---|
| MDM | 1 | 2.08±1.19 | n.d.3 | n.d. | n.d. | n.d. |
| MDM | 2 | 2.15±1.05 | n.d. | n.d. | n.d. | n.d. |
| MDM | 3 | 2.08±0.75 | n.d. | n.d. | n.d. | n.d. |
| MDM | 4 | 2.10±1.19 | n.d. | n.d. | n.d. | n.d. |
| MBP | 1 | 1.85±0.62 | n.d. | n.d. | n.d. | n.d. |
| MBP | 2 | 1.48±0.90 | n.d. | n.d. | n.d. | n.d. |
| MBP | 3 | 1.70±0.92 | n.d. | n.d. | n.d. | n.d. |
| MBP | 4 | 1.78±0.92 | n.d. | n.d. | n.d. | n.d. |
1 Phase 1: after extrusion, Phase 2: after drying, Phase 3: at start of conservation period, Phase 4: after 6 months of conservation.
2 Determined on 50 g.
3 n.d. = not detected.
Salmonella spp. was detected in meat by-product before extrusion, but never after extrusion. Biomolecular analysis confirmed the identification after culture of Salmonella enterica. After 6 months of storage under controlled conditions, the microbiological profile was confirmed: the total mesophilic bacterial count ranged between 1.77 log CFU/g and 2.09 log CFU/g feed. Enterobacteriaceae, Clostidium, and E. coli were under the detection level and Salmonella was never detected.
The microbiological risk assessment showed no microbiological hazards for the use of MDM or MBP.
Table 8 reports the polyamine content of the two types of meat, with lower content of putrescine, spermidine, and spermine in MDM than MBP, that had cadaverine, histamine, and tyramine. Table 9 shows that among the polyamines evaluated, only phenylethylamine, histamine and tyramine contents were greater in MDM than MBP; putrescine, phenylethylamine, histamine, tyramine, and peroxide value were significantly increased, while spermine was decreased after 6 months of storage in both diets.
Table 8. Natural polyamines (mean±SD, mg/kg, as-fed basis) of the two types of meat (fresh and meal).
| Compound | Mechanically Deboned Meat | Meat By-Products |
|---|---|---|
| Putrescine | 1.5±0.1 | 78.6±96.7 |
| Cadaverine | n.d.1 | 164±214 |
| Tryptamine | n.d. | n.d. |
| Phenylethylamine | n.d. | 6.3±0.2 |
| Spermidine | 17.2±0.5 | 35.0±35.7 |
| Spermine | 32.7±7.5 | 60.1±75.5 |
| Histamine | n.d. | 14.1±24.5 |
| Tyramine | n.d. | 61.2±69.7 |
1 n.d. = not detected.
Table 9. Polyamine (mean±SD mg/kg, as-fed basis) and peroxide content (meq/kg, as-fed basis) of the two diets (MDM = Mechanically Deboned Chicken Meat diet and MBP = Meat By-Products diet) at the start and the end of the conservation period.
| Diet | MDM | MBP | Diet | Time | Diet x Time | ||
|---|---|---|---|---|---|---|---|
| Time (month) | 0 | 6 | 0 | 6 | P | P | P |
| Putrescine | 56.9±2.3 | 94.5±14.2 | 58.3±3.7 | 74.2±11.2 | 0.137 | 0.001 | 0.092 |
| Cadaverine | 88.4±3.9 | 112.9±17.1 | 89.8±6.3 | 87.4±13.3 | 0.187 | 0.306 | 0.139 |
| Tryptamine | n.d.1 | 15.7±2.5 | n.d. | n.d. | - | - | - |
| Phenylethylamine | 4.3±0.5 | 7.1±1.3 | 3.9±1.0 | 4.2±1.0 | 0.032 | 0.047 | 0.088 |
| Spermidine | 18.1±2.5 | 19.6±3.0 | 25.4±2.8 | 18.6±2.9 | 0.055 | 0.171 | 0.055 |
| Spermine | 7.3±2.1 | 3.5±0.6 | 13.8±5.4 | 2.1±0.5 | 0.101 | 0.013 | 0.034 |
| Histamine | 10.3±0.6 | 28.5±4.4 | 7.7±0.3 | 16.7±2.6 | 0.002 | 0.001 | 0.126 |
| Tyramine | 52.9±2.6 | 93.7±14.3 | 36.5±3.6 | 67.3±10.3 | 0.003 | 0.001 | 0.310 |
| Peroxide value | 1.9±0.2 | 6.0±0.8 | 2.5±0.4 | 6.0±0.8 | 0.213 | 0.001 | 0.709 |
1 n.d. = not detected.
3.3. Palatability
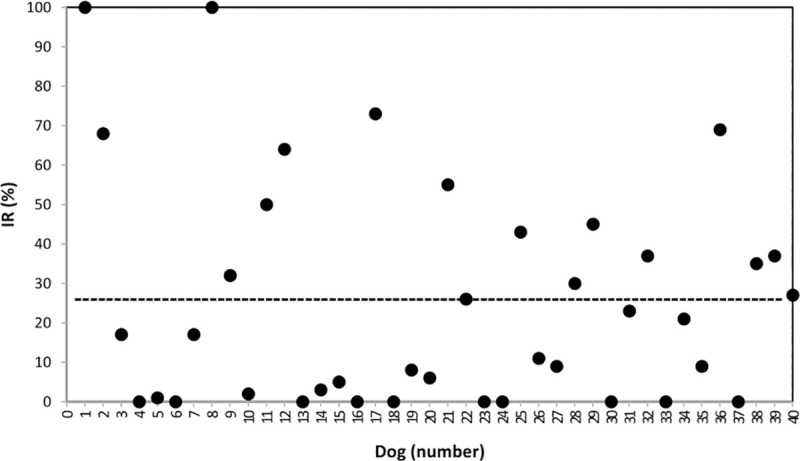
From statistical point of view, the palatability trial showed higher (P<0.001) intake of MBP than MDM (Fig 1) with a mean intake value of 212.0 g vs. 83.5 g, respectively. The MDM diet alone was consumed by a single subject and was preferred by only 4 out of the 40 dogs with a percentage consumption of 27.1% and a percentage first choice of 33%. MBP was appreciated by all the remaining dogs and in 6 subjects exclusively, with a percentage consumption of 72.9% and a percentage first choice of 67%. To eliminate the difference related to breed and food intake, the Intake Ratio of the MDM diet was corrected for total intake per subject and a mean value of 25.6 was found (Fig 2).
Fig 1. Summarized food intake of the two diets [MDM = Mechanically Deboned Chicken Meat (black columns) and MBP = Meat By-Products (white columns)] for each dog (P<0.001).
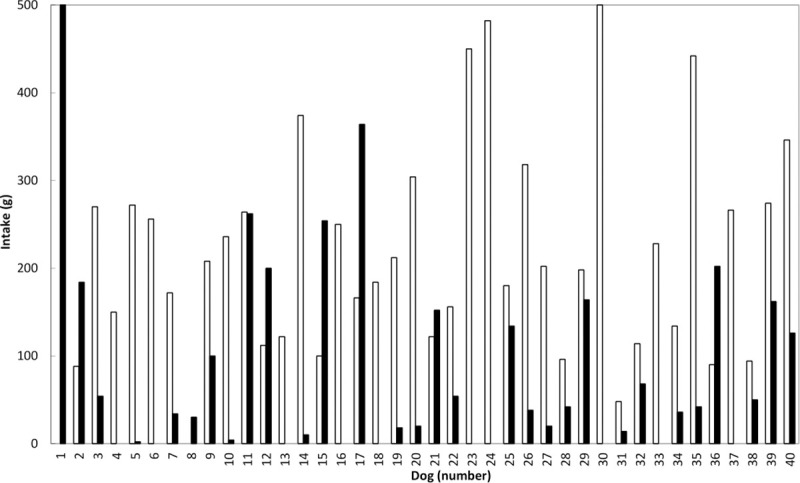
Fig 2. Intake Ratio [IR% = MDM/(MDM + MBP) x 100] of the MDM diet (based on Mechanically Deboned Chicken Meat) as compared with the MBP diet (based on Meat By-Products) for each dog (———Mean value).
3.4. In vivo and in vitro digestibility
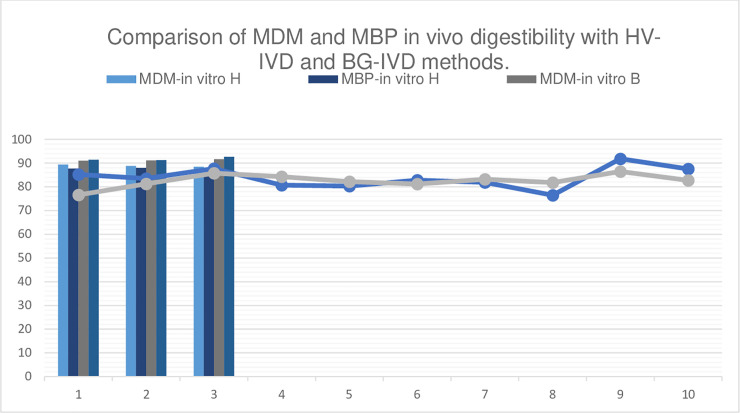
Table 10 presents the results of the in vivo and in vitro trials of diet digestibility. The in vivo method revealed no significant differences in digestibility between the two feeds. As measured by the in vitro method, HV-IVD revealed a significant difference between diets, whereas BG-IVD did not. It can be observed, however, that, as compared with the in vivo digestibility value, the in vitro method slightly overestimated the digestibility coefficients for both diets (Table 10 and Fig 3).
Table 10. In vitro dry matter digestibility (g/kg) according to the Hervera et al. method (HV-IVD), [38] and according to the Biagi et al. method (BG-IVD), [39] and in vivo dry matter digestibility (in vivo) of the two diets (MDM = Mechanically Deboned Chicken Meat diet and MBP = Meat By-Products diet) and the significance between them.
| Digestibility method | MDM | MBP | P |
|---|---|---|---|
| In vivo | 838.0±44.2 | 825.4±27.6 | 0.453 |
| HV-IVD | 888.8±0.5 | 877.9±0.1 | 0.021 |
| BG-IVD | 912.5±0.3 | 917.5±0.8 | 0.360 |
Fig 3. Comparison of MDM and MBP in vivo digestibility with HV-IVD and BG-IVD methods.
4. Discussion
4.1. Fatty acid profile
The MDM diet had better nutritional quality with higher content of PUFAs and lower content of SFAs than the MBP diet, with possible positive implications for some categories of animals (young and old dogs or animals with special needs such as hunting dogs). The PUFA n-6/PUFA n-3 ratio was 11.5 and 16.7 for the MDM and the MBP diet, respectively. These ratios are within the range reported by Kearns et al. [41], who studied the effect of diets formulated to contain PUFA n-6/PUFA n-3 ratios of 5 or 25 on oxidative status and immune response of young and old dogs. They found that a diet with a PUFA n-6/PUFA n-3 ratio of 5 had a positive effect on the immune response of young or geriatric dogs. Hall et al. [42] compared three diets with a PUFA n-6/PUFA n-3 ratio of 1.4, 18, and 40, respectively, fed to geriatric dogs and concluded that the dose of PUFA n-3 administered determined the plasma PUFA n-3 composition, independent of the PUFA n-6/PUFA n-3 ratio. This difference for fatty acid composition could be addressed to the preservation of fat naturally occurring in fresh meat while in MDM diet, the rendering process remove the natural fat and a fat with more standard composition is then added to the final product.
4.2. Microbiological profile and shelf-life
The microbiology of pet food has been associated with certain zoonoses [43–45]. Our results clearly show high microbiological counts for all the ingredients. If we compare our data with the few published data, this is not surprising given the origin of the food [46, 47]. Due to the combination of pressure and temperature, the extrusion process reduced the bacterial count by between 3 and 4 -log in both formulations. This result is in agreement with previous studies [30, 44, 45].
The total mesophilic bacterial count was lower than previously reported [30, 43]. While this parameter is relatively high for a final product with a long shelf-life, in our opinion and as stated by other authors the very low water activity (Aw of 0.40) can easily counteract bacterial growth [30]. The same behavior was noted for Enterobacteriaceae: their counts decreased from 3.70 log CFU/g to below the detection limit (1 log CFU/g) in both formulations. These results evidence a better situation for this batch than reported by other authors [29, 43]. Also, as seen for other dry pet food formulations, the E. coli count was consistently under the detection limit [30].
Salmonella, a highly common zoonotic pathogen responsible for causing infection in pets and owners [43–45], was detected only in untreated raw ingredients, in agreement with Van Bree et al. [47]. Sampling at three different time points in the production chain, where contamination could be expected, gave negative results. Our results show that, for this batch, extrusion treatment was safe, as reported elsewhere in the literature [30, 44, 45]
Furthermore, after 6 months storage there was a slight increase in microbiological parameters in both formulas, as observed by other authors [30]. Our results show that MDM appears more susceptible to degradation than MBP. Natural polyamines are organic compounds that originate from amino acids as a result of decarboxylation. This process can result from bacterial activity and can occur during food processing or storage. The most common monoamines, (histamine, tyramine, and tryptamine) and polyamines (putrescine and cadaverine) are generated, respectively, from the amino acids histidine, tyrosine, tryptophan, ornithine, and lysine; whereas, spermidine and spermine derive from putrescine. Polyamines are found in many protein foods and their amount is an important indicator of the degree of freshness and storage of products [48].
Studies on changes in polyamine content in meat products are inconsistent, however. Changes in polyamine content during meat storage result from bacterial activity. Ruiz-Capillas and Jiménez-Colmenero [49] reported that the polyamine level in minced meat products increased due to muscle fiber disintegration and increased microbial contamination. Putrescine levels in fresh meat are usually low, often near the limit of detection of the analytical procedures used [50]. The only amines present at significant levels in fresh meat are spermidine and spermine [50]. This is in agreement with the results obtained in our study (Table 8).
Regarding the storage quality of the product, after 6 months of storage, the polyamine levels were higher for MDM than MBP (Table 9). Polyamine synthesis requires the availability of amino acid precursors that may be present in the food product. As shown in Table 4, MDM contained more tyrosine and tryptophan than MBP, which are two of the amino acid precursors of tyramine and tryptamine, respectively. Some amines (e.g., tyramine, putrescine, and cadaverine) can form during the meat preservation [51]. Paulsen et al. [31] analyzed 55 samples of canned pet food and found that 75% of the levels of the biogenic amines varied between 5.4 and 21.9 mg/kg, depending on the type of amine, while it ranged from below the detection limit to 133 mg/kg for cadaverine, histamine, phenylethylamine, putrescine, and tyramine. They concluded that amine concentrations in non-fish components are lower than in fish components.
4.3. Palatability
The palatability trial showed a significant increase of MBP intake (72.9%) and difference in intake between the MBP and the MDM diet could have been due to several different factors. First, the composition of the product: the MBP diet was fatter (200 g/kg DM) than the MDM (188 g/kg DM). In fact, dogs prefer fat-rich diets [42] to high-protein or carbohydrate diets. Another factor is the drying process following extrusion (which reduced the humidity to 6% in MDM and to 7% in MBP); for MDM product, the minimum temperature was higher than that used for MBP (85°C vs. 50°C) due to the higher water content of the extruded product. Moreover, MDM diet showed higher polyamines content after 6 months of storage. This aspect, in literature, is described to be responsible for a palatability reduction [42, 52, 53].
4.4. Digestibility
The in vitro digestibility was high for both diets; results were higher than those reported by Brambillasca et al. [54] for two dry food measured in vivo (83.3% and 70.5%). This could be explained by the positive effect of extrusion that allows gelatinization of starches and increases digestibility in comparison with the pelleting process [55]. However, Biagi et al. [39] reported an average of 70.4% for the digestibility of extruded diets for dogs, which contrasts with the average reported in the literature (82.2%) and reported in their article.
Moreover, our data show different results obtained from the two methods and use of the DaisyII Incubator. According to HV-IVD, digestibility was higher for the MDM diet than the MBP diet, (888.8 vs. 877.9 g/kg). Since the chemical composition of the MDM diet had less ether extract—though cellulose content was higher (26.6 vs. 24.1 g/kg), it contained higher quantities of starch (274.7 vs. 248.3 g/kg). Increasing the fiber level in dog diet decreases its digestibility [54], which is negatively related (r = - 0.86) to apparent digestibility of extruded food [55]. BG-IVD did not reveal significant differences between MDM and MBP (912.5 g/kg vs. 917.5 g/kg). In vitro values were always higher than in vivo digestibility and less variable, particularly with the BG-IVD method. The standard deviation ranged between 0.1 and 0.8 g/kg in vitro and 27.6 and 44.2 g/kg in vivo. Both in vitro methods used in this study were carried out in three steps using different types and amounts of enzyme: a higher amount of enzymes was used for BG-IVD than HV-IVD. Regmi et al. [56] found in pigs that in vitro digestibility was greater with longer digestion time, while the amount of enzymes was irrelevant for the digestibility.
Previous studies have shown that the ANKOM method produces digestibility values comparable to traditional procedures for many foods [57–62]. The in vitro method proposed by Hervera et al. [38] and utilized in our study yielded values closer to in vivo results, in line with Hervera et al. [63, 64] who showed the highest accuracy approach of in vivo crude protein apparent digestibility (r = 0.81) and in vivo digestible energy (r2 = 0.94), respectively.
5. Conclusions
Our results indicate that MDM and MBP are both a high-quality source of animal protein for commercial dry pet food. MDM diet demonstrated a higher nutritional value in terms of fatty acids profile (with higher content of PUFAs and lower content of SFAs) and in vitro digestibility (exclusively by HV-IVD method). Microbiological risk assessment revealed no microbiological hazards for the use of either MDM or MBP diet. After 6-months storage, the total mesophilic bacterial count ranged between 1.77 and 2.09 log CFU/g feed, while polyamine values were higher in the MDM (0.37 g/kg) than in the MBP (0.27 g/kg). Simultaneously, MDM diet demonstrated lower palatability compare to MBP diet, maybe related to the higher polyamine values. Furthermore, the DaisyII Incubator was found to be a valid instrument for studying in vitro digestibility also for dogs, providing data simply, quickly, with less variability and costs than in vivo trials. It could represent the future for digestibility studies in pet food. In conclusion, MDM inclusion in dry dog food is microbiologically safe and it can improve its nutritional quality, at the expense of a reduced palatability. The higher polyamine concentrations found in MDM-enriched petfood after 6-months storage, however, may represent a possible risk. Indeed, threshold levels of biogenic amines in petfood have not been established so far, and further studies investigating their possible hazard in companion animals are still warranted.
Acknowledgments
The authors would like to thank Kenneth Adolf Britsch for linguistic revision of the manuscript and Chiara Bianchi for her technical support.
Abbreviations
- MDM
fresh mechanically deboned meat
- MBP
meat by-products
- HV-IVD method
in vitro digestibility, as proposed by Hervera et al., (2007)
- BG-IVD
in vitro digestibility, as proposed by Biagi et al. (2016)
Data Availability
All relevant data are within the manuscript.
Funding Statement
This research was funded by Regione Piemonte - Italy (Grant No. Det. Dirig. N.132MONGE). SPA provided support for this study in the form of salary for EP. The specific roles of the authors are articulated in the ‘author contributions’ section. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.American Veterinary Medical Association. U.S. pet ownership & demographics sourcebook. AVMA: Schaumburg, IL, 2012. [Google Scholar]
- 2.Russo N, Vergnano D, Bergero D, Prola L. Small pilot survey on parents’ perception of the relationship between children and pets. Vet Sci 2017; 4: 52. 10.3390/vetsci4040052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Laflamme D, Izquierdo O; Eirmann L, Binder S. Myths and misperceptions about ingredients used in commercial pet foods. Vet Clin North Am Small Anim Pract. 2014; 44: 689–698. 10.1016/j.cvsm.2014.03.002 [DOI] [PubMed] [Google Scholar]
- 4.Thompson A. Ingredients: where pet food starts. Top Companion Anim Med. 2008; 23:127–32. 10.1053/j.tcam.2008.04.004 [DOI] [PubMed] [Google Scholar]
- 5.Meeker DL, Hamilton CR. An overview of the rendering industry. In: Essential Rendering. Kirby Lithographic Company, Inc.: Arlington, VA, USA. 2006; pp. 1–16. [Google Scholar]
- 6.Regulation (EC) No 1069/2009 of the European Parliament and of the Council of 21 October 2009 on the laying down health rules as regards animal by-products and derived products not intended for human consumption and repealing
- 7.Regulation (EC) No 767/2009 of the European Parliament and of the Council of 13 July 2009 on the placing on the market and use of feed, amending European Parliament and Council Regulation (EC) No 1831/2003 and repealing Council Directive 79/373/EEC, Commission Directive 80/511/EEC, Council Directives 82/471/EEC, 83/228/EEC, 93/74/EEC, 93/113/EC and 96/25/EC and Commission Decision 2004/217/EC. Off J Eur Union. 2009; 229: 181–208 [Google Scholar]
- 8.Wang X, Parsons CM. Effect of raw material source, processing systems, and processing temperatures on amino acid digestibility of meat and bone meals. Poult Sci. 1998; 77: 834–841. 10.1093/ps/77.6.834 [DOI] [PubMed] [Google Scholar]
- 9.Singh S, Wakeling L, Gamlath S. Retention of essential amino acids during extrusion of protein and reducing sugars. J Agric Food Chem. 2007; 55: 8779–8786. 10.1021/jf071769z [DOI] [PubMed] [Google Scholar]
- 10.Alam MS, Kaur J, Khaira H, Gupta K. Extrusion and Extruded Products: Changes in Quality Attributes as Affected by Extrusion Process Parameters: A Review. Crit Rev Food Sci Nutr. 2016; 56:445–75. 10.1080/10408398.2013.779568 . [DOI] [PubMed] [Google Scholar]
- 11.Offiah V, Kontogiorgos V, Falade KO. Extrusion processing of raw food materials and by-products: A review. Crit Rev Food Sci Nutr. 2019; 59:2979–2998. 10.1080/10408398.2018.1480007 Epub 2018 Jul 12. . [DOI] [PubMed] [Google Scholar]
- 12.Leiva A, Molina A, Redondo-Solano M, Artavia G, Rojas-Bogantes L, Granados-Chinchilla F. Pet Food Quality Assurance and Safety and Quality Assurance Survey within the Costa Rican Pet Food Industry. Animals (Basel). 2019. November 15; 9:980. 10.3390/ani9110980 ; PMCID: PMC6912492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Food and Drug Administration. Recalls & Withdrawals, FDA, 2015. Available online: https://www.fda.gov/animalveterinary/safetyhealth/recallswithdrawals/. Accessed on 30 October 2018. [Google Scholar]
- 14.Weese JS, Rousseau J, Arroyo L. Bacteriological evaluation of commercial canine and feline raw diets. Can Vet J. 2005; 46: 513–516. [PMC free article] [PubMed] [Google Scholar]
- 15.KuKanich KS. Update on Salmonella spp. contamination of pet food, treats, and nutritional products and safe feeding recommendations. J Am Vet Med Ass. 2011; 238: 1430–1434. [DOI] [PubMed] [Google Scholar]
- 16.Ormandy EH, Schuppli CA. Public attitudes toward animal research: A review. Animals. 2014; 4: 391–408. 10.3390/ani4030391 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kowalski Z.M.; Ludwin J.; Górka P.; Rinne M.; Weisbjerg M.R.; Jagusiak W. The use of cellulase and filter bag technique to predict digestibility of forages. Anim. Feed Sci. Technol. 2014, 198, 49–56. [Google Scholar]
- 18.Ludwin J, Kowalski ZM, Eridbjrth MR. The use of the in vitro filter bag method for predicting digestibility of forages. J Anim Feed Sci. 2005; 14: 571–574. [Google Scholar]
- 19.Damiran D, DelCurto T, Bohnert DW, Findholt SL. Comparison of techniques and grinding size to estimate digestibility of forage based ruminant diets. Anim Feed Sci Technol. 2008; 141: 15–35. [Google Scholar]
- 20.Lattimer JM, Cooper SR, Freeman DW, Lalman DL. Effect of yeast culture on in vitro fermentation of a high concentrate or high fiber diet using equine fecal inoculum in a DaisyII incubator. J Anim Sci. 2007; 85: 2484–2491. 10.2527/jas.2006-655 [DOI] [PubMed] [Google Scholar]
- 21.Earing JE, Cassill BD, Hayes SH, Vanzant ES, Lawrence LM. Comparison of in vitro digestibility estimates using the DaisyII incubator with in vivo digestibility estimates in horses. J Anim Sci. 2010; 88: 3954–3963. 10.2527/jas.2010-2989 [DOI] [PubMed] [Google Scholar]
- 22.National Research Council. 2006. Nutrient Requirements of Dogs and Cats. Washington, DC: The National Academies Press. 10.17226/10668. [DOI] [Google Scholar]
- 23.AOAC INTERNATIONAL Guidelines for Laboratories Performing Microbiological and Chemical Analyses of Food, Dietary Supplements, and Pharmaceuticals. Online version; 2018. ISBN: 0-935584-88-9 [Google Scholar]
- 24.Serrano X. The extrusion-cooking process in animal feeding. Nutritional implications. In: Feed manufacturing in Southern Europe: New challenges; Morand-Fehr P., Ed.; CIHEAM: Zaragoza, Spain. 1997; pp. 107–114. [Google Scholar]
- 25.Hauck B, Rokey G, Smith O, Herbster J, Sunderland R. Extrusion cooking systems. In: Feed Manufacturing Technology. American Feed Industry Assotiation Inc.: Arlington, VA, USA; 1994. pp.131–139. [Google Scholar]
- 26.Bazolli RS, Vasconcellos RS, De-Oliveira LD, Sá FC, Pereira GT, Carciofi AC. Effect of the particle size of maize, rice, and sorghum in extruded diets for dogs on starch gelatinization, digestibility, and the fecal concentration of fermentation products. J Anim Sci. 2015; 93: 2956–2966. 10.2527/jas.2014-8409 [DOI] [PubMed] [Google Scholar]
- 27.Peiretti PG, Meineri G. Effects on growth performance, carcass characteristics, and the fat and meat fatty acid profile of rabbits fed diets with chia (Salvia hispanica L.) seed supplements. Meat Sci. 2008; 80: 1116–1121. 10.1016/j.meatsci.2008.05.003 [DOI] [PubMed] [Google Scholar]
- 28.International Organization for Standardization. Microbiology of food and animal feeding stuffs. Horizontal methods for the detection and enumeration of Enterobacteriaceae. Part 2: colony-count method. Enumeration. EN ISO 21528–2. International Organization for Standardization, Geneva. 2004. [Google Scholar]
- 29.Rahn K, De Grandis SA, Clarke RC, Galán JE, Ginocchio C, Curtiss R, Gyles CL. Amplification of an invA sequence of Salmonella Typhimurium by polymerase chain reaction as a specific method of detection of Salmonella. Mol Cell Probe. 1992; 6: 271–279. 10.1016/0890-8508(92)90002-f [DOI] [PubMed] [Google Scholar]
- 30.Adelantado C, López S, Inglada R, Vilaseca L, Calvo MA. Viability of micro-organism involved in outbreaks of bacterial food borne diseases in dry extruded pet food. Biotechnol. 2008; 7: 725–731. [Google Scholar]
- 31.Paulsen P, Taub N, Dicakova Z, Bauer F. On the occurrence of biogenic amines in pet food for cats and dogs. Wien Tierarzt Monatsschr. 2000; 87: 236–240. [Google Scholar]
- 32.European Commission. Commission Regulation (EC) No 2016/1784 of 8 October 2016 the olive oil regulation, Annex III. Off J Eur Union. 2016; 273: 1–9. [Google Scholar]
- 33.Araujo JA, Studzinski CM, Larson BT, Milgram NW. Comparison of the cognitive palatability assessment protocol and the two-pan test for use in assessing palatability of two similar foods in dogs. Am J Vet Res. 2004; 65: 1490–1496. 10.2460/ajvr.2004.65.1490 [DOI] [PubMed] [Google Scholar]
- 34.Association of American Feed Control Officials. In: Official Publication, AAFCO: Champaign, IL; 2013. [Google Scholar]
- 35.Villaverde C, Manzanilla EG, Molina J, Larsen JA. Effect of enzyme supplements on macronutrient digestibility by healthy adult dogs. J Nutr Sci. 2017; 6: 1–4. 10.1017/jns.2017.10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Act Animals. Act of May 19, 2011, constituting an integral framework for regulations on captive animals and related topics (Animals Act). The Minister of Security and Justice. Official Diary of the Kingdom of the Netherlands. Gazette: 2011; 345:1–63. [Google Scholar]
- 37.European Commission. Commission Regulation (EC) No 2010/63/EU of 22 September 2010 on the protection of animals used for scientific purposes. Off J Eur Union. 2010; 276: 33–52. [Google Scholar]
- 38.Hervera M, Baucells MD, Blanch F, Castrillo C. Prediction of digestible energy content of extruded dog food by in vitro analyses. J Anim Physiol Anim Nutr. 2007; 91: 205–209. 10.1111/j.1439-0396.2007.00693.x [DOI] [PubMed] [Google Scholar]
- 39.Biagi G, Cipollini I, Grandi M, Pinna C, Vecchiato CG, Zaghini G. A new in vitro method to evaluate digestibility of commercial diets for dogs. Ital J Anim Sci. 2016; 15: 617–62 [Google Scholar]
- 40.Boisen S, Fernández, JA. Prediction of the total tract digestibility of energy in feedstuffs and pig diets by in vitro analyses. Anim Feed Sci Technol. 1997; 68: 277–286. [Google Scholar]
- 41.Kearns RJ, Hayek MG, Turek JJ, Meydani M, Burr JR, Greene RJ, Marshall CA, Adams SM, Borgert RC, Reinhart GA. Effect of age, breed and dietary omega-6 (n-6):omega-3 (n-3) fatty acid ratio on immune function, eicosanoid production, and lipid peroxidation in young and aged dogs. Vet Immunol Immunopathol. 1999; 69: 165–183. 10.1016/s0165-2427(99)00052-5 [DOI] [PubMed] [Google Scholar]
- 42.Hall JA, Picton RA, Skinner MM, Jewell DE, Wander RC. The (n-3) fatty acid dose, independent of the (n-6) to (n-3) fatty acid ratio, affects the plasma fatty acid profile of normal dogs. J Nutr. 2006; 136: 2338–2344. 10.1093/jn/136.9.2338 [DOI] [PubMed] [Google Scholar]
- 43.Damborg P, Broens EM, Chomel BB, Guenther S, Pasmans F, Wagenaar JA, Weese JS, Wieler LH, Windahl U, Vanrompay D, Guardiabassi L. Bacterial zoonoses transmitted by household pets: state-of the-art and future perspectives for targeted research and policy actions. J Comp Pathol. 2016; 155: S27–S40. 10.1016/j.jcpa.2015.03.004 [DOI] [PubMed] [Google Scholar]
- 44.Lambertini E, Buchanan RL, Ford RM, Baker RC, Pradhan AK. Quantitative assessment of human and pet exposure to salmonella associated with dry pet foods. Int J Food Microbiol. 2016; 216: 79–90. 10.1016/j.ijfoodmicro.2015.09.005 [DOI] [PubMed] [Google Scholar]
- 45.Lambertini E, Buchanan RL, Narrod C, Pradhan AK. Transmission of bacterial zoonotic pathogens between pets and humans: The role of pet food. Crit Rev Food Sci. Nutr. 2016; 56: 364–418. 10.1080/10408398.2014.902356 [DOI] [PubMed] [Google Scholar]
- 46.Wodjat E, Kwiatek K, Zasadny R. Microbial quality of pet food in Poland. Pol J Vet. Sci. 2004; 7: 207–209. [PubMed] [Google Scholar]
- 47.Van Bree FPJ, Bokken GCAM, Mineur R, Franssen F, Opsteegh M, Van Der Giessen JWB, Lipman LJA, Overgaauw PAM. Zoonotic bacteria and parasites found in raw meat-based diets for cats and dogs. Vet Rec. 2018; 10: 1–7. 10.1136/vr.104535 [DOI] [PubMed] [Google Scholar]
- 48.Atiya Ali M, Poortvliet E, Strömberg R, Yngve A. Polyamines in foods: development of a food database. Food Nutr Res. 2011; 55:1–15. 10.3402/fnr.v55i0.5572 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ruiz-Capillas C, Jiménez-Colmenero F. Biogenic amines in meat and meat products. Crit Rev Food Sci Nutr. 2004; 44: 489–499. 10.1080/10408690490489341 [DOI] [PubMed] [Google Scholar]
- 50.Kalac P. Biologically active polyamines in beef, pork and meat products: A review. Meat Sci. 2006; 73: 1–11. 10.1016/j.meatsci.2005.11.001 [DOI] [PubMed] [Google Scholar]
- 51.Galgano F, Favati F, Bonadio M, Lorusso V, Romano P. Role of biogenic amines as index of freshness in beef meat packed with different biopolymeric materials. Food Res Int. 2009; 42: 1147–1152. [Google Scholar]
- 52.Murakami FY, de Lima DC, Menezes Souza CM, Kaele GB, Oliveira SGD, Félix AP. Digestibility and palatability of isolated porcine protein in dogs. Ital J Anim Sci. 2018: 1–7. [Google Scholar]
- 53.Hall JA, Vondran JC, Vanchina, MA, Jewell DE. When fed foods with similar palatability, healthy adult dogs and cats choose different macronutrient compositions. J Exp Biol. 2018, 10.1242/jeb.173450 [DOI] [PubMed] [Google Scholar]
- 54.Brambillasca S, Purtscher F, Britos A, Repetto JL, Cajarville C. Digestibility, fecal characteristics, and plasma glucose and urea in dogs fed a commercial dog food once or three times daily. Can Vet J. 2010; 51: 190–194. [PMC free article] [PubMed] [Google Scholar]
- 55.Gröner T, Pfeffer E. Estimation of digestible energy in dry extruded dog foods. J Anim Physiol Anim Nutr. 1997; 77: 207–213. [Google Scholar]
- 56.Regmi PR; Ferguson NS, Zijlstra RT. In vitro techniques to predict apparent total tract energy digestibility of wheat in grower pigs. J Anim Sci. 2009; 87: 3620–3629. 10.2527/jas.2008-1739 [DOI] [PubMed] [Google Scholar]
- 57.Holden LA. Comparison of methods of in vitro dry matter digestibility for ten feeds. J Dairy Sci. 1999; 82: 1791–1794. 10.3168/jds.S0022-0302(99)75409-3 [DOI] [PubMed] [Google Scholar]
- 58.Mabjeesh SJ, Cohen M, Arieli A. In vitro methods for measuring the dry matter digestibility of ruminant feedstuffs: comparison of methods and inoculum source. J Dairy Sci. 2000; 83: 2289–2294. 10.3168/jds.S0022-0302(00)75115-0 [DOI] [PubMed] [Google Scholar]
- 59.Wilman D, Adesogan A. A comparison of filter bag methods with conventional tube methods of determining the in vitro digestibility of forages. Anim Feed Sci Technol. 2000; 84: 33–47. [Google Scholar]
- 60.Adesogan AT. Effect of bag type on the apparent digestibility of feeds in ANKOM DaisyII incubators. Anim Feed Sci Technol. 2005; 119: 333–344. [Google Scholar]
- 61.Castrillo C, Vicente F, Guada JA. The effect of crude fibre on apparent digestibility and digestible energy content of extruded dog foods. J Anim Physiol Anim Nutr. 2001; 85: 231–236. 10.1046/j.1439-0396.2001.00329.x [DOI] [PubMed] [Google Scholar]
- 62.Kawauchi IM, Sakomura NK, Vasconcellos RS, De-Oliveira LD, Gomes MOS, Loureiro BA, Carciofi AC. Digestibility and metabolizable energy of maize gluten feed for dogs as measured by two different techniques. Anim Feed Sci Technol. 2011; 169: 96–103. [Google Scholar]
- 63.Hervera M, Baucells MD, González G, Pérez E, Castrillo C. Prediction of digestible protein content of dry extruded dog foods: comparison of methods. J Anim Physiol Anim Nutr. 2009; 93: 366–372. 10.1111/j.1439-0396.2008.00870.x [DOI] [PubMed] [Google Scholar]
- 64.Hervera M, Baucells MD. Torre C, Buj A, Castrillo C. Prediction of digestible energy value of extruded dog food: comparison of methods. J Anim Physiol Anim Nutr. 2008; 92: 253–259. 10.1111/j.1439-0396.2007.00740.x [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All relevant data are within the manuscript.