Abstract
Asteroid wasting events and mass mortality have occurred for over a century. We currently lack a fundamental understanding of the microbial ecology of asteroid disease, with disease investigations hindered by sparse information about the microorganisms associated with grossly normal specimens. We surveilled viruses and protists associated with grossly normal specimens of three asteroid species (Patiriella regularis, Stichaster australis, Coscinasterias muricata) on the North Island / Te Ika-a-Māui, Aotearoa New Zealand, using metagenomes prepared from virus and ribosome-sized material. We discovered several densovirus-like genome fragments in our RNA and DNA metagenomic libraries. Subsequent survey of their prevalence within populations by quantitative PCR (qPCR) demonstrated their occurrence in only a few (13%) specimens (n = 36). Survey of large and small subunit rRNAs in metagenomes revealed the presence of a mesomycete (most closely matching Ichthyosporea sp.). Survey of large subunit prevalence and load by qPCR revealed that it is widely detectable (80%) and present predominately in body wall tissues across all 3 species of asteroid. Our results raise interesting questions about the roles of these microbiome constituents in host ecology and pathogenesis under changing ocean conditions.
Introduction
Recent and renewed interest in echinoderm microbiome ecology has revealed the paucity in understanding of the roles of the microbial community in host biology and ecology; particularly with respect to negative impacts such as mass mortality. Asteroid mass mortality due to a condition termed “sea star wasting disease” (also known as “asteroid idiopathic wasting syndrome”) has occurred in the northeast Pacific starting in 2013 [1], and in Port Phillip Bay, Australia and Shandong Province, China in 2014 [2]. Indeed, wasting has been observed for over a century [3]. Microbiological investigation of wasting asteroids initially indicated the presence of the Asteroid ambidensovirus 1 [4] (known at the time as Sea Star associated Densovirus or SSaDV; [1]), and that wasted asteroids were inhabited by a suite of cultivable copiotrophic (i.e. bacteria that rapidly consume abundant organic matter) bacteria [5–8]. Recent work suggested that sea star wasting disease is not distinctly associated with any virus [9, 10], but instead results from organic matter enrichment which fuels microbial respiration by copiotrophic prokaryotic taxa, leading to oxygen depletion in the diffusive boundary layer around asteroid tissues [6]. Sea star wasting disease may also be associated with elevated temperature [11–15] [but note inverse relationship in ref [16]]. Hence, firm microbial associations with sea star wasting remain elusive, similar to other echinoderm diseases (reviewed in [17]), and the condition may not be due to an infectious agent [6]. Previous work has highlighted distinct microbiome associations with echinoderms [18], building on previous microscopic and cultivation-based studies [19–21]. These surveys suggest that echinoderms may harbor an underexplored diversity of microorganisms. Environmental perturbation under future climate scenarios may shift the relationship between these microorganisms and their hosts [22]. Hence, there is value in surveying the diversity and prevalence of microorganisms associated with grossly normal specimens, which may then inform future marine disease event investigations, when and if they occur.
The study of viral diversity associated with metazoan hosts has been approached by two methods. First, RNA and ssDNA viral genomes have been recovered from deeply sequenced host transcriptomes [23, 24]. This approach provides key information about expressed host genes in addition to a wealth of viral diversity, including deeply-branching viral genotypes across a wide range of invertebrate hosts [24]. A second approach enriches for viruses by physical size and capsid-induced protection from nucleases [25]. Here, viral metagenomes are typically prepared using a homogenization-size exclusion-nuclease approach, where tissues are normally ‘cleaned’ (washed) of putative epibionts [26]. Viral metagenomes prepared using this approach have potential to yield more information than viruses alone, since only a tiny fraction (typically < 5%) of metavirome sequence space is annotated as viruses [27] and the remaining sequence space is believed to mostly reflect host RNAs. Ribosomes, which are typically 25–30 nm in diameter, are also liberated from cells during homogenization, pass through the filters typically used in metavirome preparation, and transcript RNAs may be protected from nucleases used to digest co-extracted nucleic acids. Thus, ribosomal RNAs are well represented in viral metagenomes and may include protistan, bacterial and archaeal components of the host-associated microbiome. Comparison of non-viral sequences in viral metagenomes against rRNA databases can therefore yield useful information about microbiome composition.
The goal of the present study was to identify viruses and protists in common Aotearoa New Zealand asteroids by surveying virus- and ribosome-sized RNAs, and use this information to guide survey of microbial prevalence within and between populations and between tissue types. We discovered several densovirus genome fragments in two species of asteroid, but these were only detected at low prevalence within the populations studied by quantitative PCR. We also discovered fungal, mycetozoan and mesomycetozoan constituents of the asteroid microbiome. A mesomycetozoan similar to a fish pathogen was prevalent in all asteroids tested, and bore highest loads in body wall samples, suggesting it may be a common constituent of the asteroid microbiome.
Materials and methods
Sample collection
Asteroid samples (n = 77 individuals across 3 species) were collected for metagenomic investigation of viral diversity and viral prevalence at several locations on the North Island / Te Ika-a-Māui, Aotearoa New Zealand, in January and February 2018 (Table 1). All collections were made in public waters and no permits were required. No endangered or protected species were collected in this study. Piha is an exposed, high energy dissipative beach. Stichaster australis specimens were collected from rock outcroppings at low tide approx. 100 m away from the closest human settlement and 500 m from the outflow of Piha stream. Specimens of Coscinasterias muricata from Ti Point were collected at 3 – 5m by SCUBA diver in a channel between Whangeteau Habour and Little Omaha Bay on an ebbing tide. Whangeteau Harbor does not host aquaculture facilities, and experienced a bivalve mass mortality event in 2009 [28]. Specimens of Patiriella regularis were collected at low tide from a rock ledge at Matheson’s Bay (Te Kohuroa). Specimens of P. regularis from Scorching Bay, which is an embayment on the Miramar Peninsula / Te Motu Kairangi within the outflow of Wellington Harbour / Te Whanganui-a-Tara were collected by hand at low tide. All specimens collected were grossly normal, and no sea star wasting disease-like lesions were noted in populations at any site. Specimens were immediately placed into individual plastic bags, which were transported to the laboratory for dissection in a cooler (Fig 1). The taxonomic identity and arm length of individuals was recorded for each specimen. Coelomic fluid was withdrawn from individuals using a 5 mL syringe fitted with a sterile 25G needle. Body wall tissues were removed by sterile (5 mm) biopsy punch. Gonads and pyloric caeca were dissected from coelomic cavities by first creating an incision into the coelomic cavity using clean disposable razor blades, then using sterilized forceps to remove small (~ 2–4 mm) sections of these tissues. All tissue and coelomic fluid samples were preserved in RNALater (Qiagen) at a ratio of 2:1 (vol:vol) and refrigerated for 4–7 d. The specimens were then transported to the laboratory at Cornell University, where they were frozen at -80°C prior to further processing, which occurred within 4 months of collection. No samples were collected from public conservation lands or marine reserves, hence no permits were needed in support of this study.
Table 1. Sampling locations, species and morphological characteristics of asteroids collected as part of this study.
| Location | Latitude | Longitude | Date | Species | n | RL (cm) | RL SE |
|---|---|---|---|---|---|---|---|
| Piha, Auckland / Tāmaki | 36.9597 S | 174.4628 E | 1/22/2018 | Stichaster australis | 19 | 13.58 | 0.56 |
| Ti Point, Northland / Te Tai Tokerau | 36.3178 S | 174.6178 E | 1/27/2018 | Coscinasterias muricata | 17 | 7.42 | 1.10 |
| Matheson’s Bay, Northland / Te Tai Tokerau | 36.3011 S | 171.8011 E | 1/27/2018 | Patiriella regularis | 20 | 2.81 | 0.17 |
| Coscinasterias muricata | 1 | 10.00 | |||||
| Scorching Bay, Wellington / Te Whanganui-a-Tara | 41.3078 S | 174.8325 E | 2/15/2018 | Patiriella regularis | 20 | 1.89 | 0.11 |
Samples collected at Ti Point were collected subtidally by SCUBA Diver, while those collected elsewhere were collected intertidally. RL = Ray length, SE = Standard Error.
Fig 1. Sampled specimens of Stichaster australis (A-B), Coscinasterias muricata (C-D) and Patiriella regularis (E-F).
Viral metagenomes were prepared from body wall (b) samples collected by biopsy punch. Additional specimens of gonad (g) and pyloric caeca (p) were collected for quantification of viral genotypes and the mesomycetozoan.
Metavirome preparation
Three body wall biopsy samples from each species were selected for viral metagenomics (one each from Stichaster australis, Coscinasterias muricata and Patiriella regularis; Table 2). For each sample, the biopsy punch was removed from RNALater and subject to the workflow detailed in [26] with modifications by Ng et al [29] and Hewson et al. [30]. Briefly, the sample was homogenized by bead beating (Zymo Bead Beater tubes) in 1 mL of 0.02 μm-filtered 1 X PBS. The sample was filtered through a 0.2 μm PES syringe filter. The filtrate was treated with DNAse I (5 U; Thermo Fisher Scientific), RNAse One (50 U; Promega) and Benzonase (250 U; Sigma-Aldrich) for 3 h at 37°C in an attempt to remove co-extracted host nucleic acids. Enzyme activity was halted by treatment with 50 μM EDTA. RNA was extracted from the resulting purified viral fraction using the Zymo Mini RNA isolation kit, and subsequently amplified using the TransPlex WTA2 (Sigma Aldrich) kit. We did not standardize template quantity (mass) of extracted RNA, but used 2 μl extracted RNA in each amplification reaction. Amplicons were quantified using PicoGreen and submitted for sequencing on a Illumina MiSeq (2 x 250 bp paired-end) platform after TruSeq PCR-free library preparation at the Cornell Biotechnology Resource Center. Sequences have been deposited in the NCBI under BioProject PRJNA636826, Short Read Archive accessions SRR11923915- SRR11923917, and contig sequences archived under accessions MW080663-MW080666
Table 2. Library characteristics prepared from asteroids in Northland and Auckland region, January 2018.
| Species | Date | Total Reads | Assembled Reads | Total Contigs | Viral Contigs |
|---|---|---|---|---|---|
| Coscinasterias muricata | 1/27/2018 | 3,867,602 | 981,140 | 27,032 | 2 |
| Stichaster australis | 1/22/2018 | 1,673,102 | 681,086 | 2,170 | 0 |
| Patiriella regularis | 1/27/2018 | 1,635,372 | 301,574 | 6,332 | 2 |
Bioinformatic processing
Sequence libraries were initially trimmed for adapters and quality (ambiguous bases <2) using the CLC Genomics Workbench 4.0. Each of the 3 metaviromes were assembled separately using the CLC Genomic Workbench 4.0 native algorithm using a minimum overlap of 0.5 and similarity of 0.8. The resulting contig spectra was aligned against several boutique databases of RNA viruses as described elsewhere [30]. These boutique databases comprised viral genomes and proteins assembled from NCBI (accessed October 2018), including: All RNA viral genomes by tBLASTx (search term “RNA Virus”); Mononegavirus proteins (search term “mononegaviruses”) by BLASTx; Picornavirus RNA-dependent RNA polymerase (RdRp) proteins (search term “RdRp AND picornavirus”) by BLASTx; invertebrate RNA viral proteins (search term “invertebrate AND RNA viruses”) by BLASTx; Flavivirus proteins (search term “flavivirus”) by BLASTx; Coronavirus proteins (search term “coronavirus”) by BLASTx; And nodavirus proteins (search term “nodavirus”) [9, 30]. Because RNA viral metagenomes also capture ssDNA viruses [10], we also searched contig spectra by tBLASTx against a boutique database of densoviral genomes (complete genomes from NCBI using keyword “densovirus”). Sequence matches against any of these databases at an E-value <10−20 were further aligned against the non-redundant (nr) library at NCBI by BLASTx, and contigs discarded if they matched known bacterial or eukaryl proteins at a higher percentage and E-value than viruses. Uncertain amplification biases and variation in template RNA quantity preclude quantitative interpretation of metagenome constituents. Hence, analyses of metagenomes focused on detection of constituents and subsequent quantitative PCR of selected contigs.
Quantitative PCR (qPCR) of densovirus genome fragments
To examine the prevalence and viral load of asteroid densoviruses, we selected two (Coscinasterias muriticata contig 17 and Patiriella regularis contig 15838) that represented the most complete genome fragments for these two species. TaqMan Primer/Probe sets were designed around two contiguous sequences matching the nonstructural (Patiriella regularis contig 15838) and structural (Coscinasterias muricata contig 17) proteins of these densovirus-like genome fragmrnts and validated them against oligonucleotide standards (Table 3). DNA was extracted from 36 biopsy punch body wall samples (10 Stichaster australis, 3 Coscinasterias muricata, 13 Patiriella regularis from near Auckland and 10 Patiriella regularis from Scorching Bay, Wellington) using the Zymo Tissue & Insect Kit. DNA was then subject to quantitative PCR (qPCR) in an Applied Biosystems StepOne Real-Time PCR machine. Each qPCR reaction comprised 1 X SSO Probes SuperMix (BioRad), and 200 pmol of each primer and probe (Table 2). Reactions were subject to a 10 minute incubation step at 50°C, followed by a 3 minute denaturation step at 94°C. Following hot start activation, reactions were subject to 50 cycles of heating to 94°C and annealing at 58°C, where fluorescence was measured at the conclusion of each thermal cycle. Reactions were run in duplicate against an 8-fold dilution (covering 10 to 108 copies reaction-1). A positive detection of the virus was considered when both duplicates were within 1 Ct, and were considered “detected but not quantifiable” (DNQ) when one replicate generated a positive Ct but the other replicate failed to yield an amplicon.
Table 3. Primers and probes used in this study to examine the prevalence and load of densovirus and mesomycetozoan-like contiguous sequences.
| Target | Primer Name | Sequence (5’ - 3’) |
|---|---|---|
| Patiriella regularis contig 15838 | NZ1DV_F | AGTTGTTACTTGGGGCTTGT |
| NZ1DV_R | CCGTGCTCAGTACTTTGTCG | |
| NZ1DV_Pr | [FAM]CAGCACCAGATGTTGCAGCTGTTGA[TAM] | |
| NZ1DV_Std | AGTTGTTACTTGGGGCTTGTATAATAATACTGCTACAGCACCAGATGTTGCAGCTGTTGATCAAGTTAATGCACGACAAAGTACTGAGCACGG | |
| Coscinasterias muricata contig 17 | NZ3DV_F | ATCTTCAATGCACTCGGAGC |
| NZ3DV_R | AGTAACGCCATGGATCTCGA | |
| NZ3DV_Pr | [FAM]AGTGTCACAGAACGCGCTTGTGGA[TAM] | |
| NZ3DV_Std | ATCTTCAATGCACTCGGAGCCAGTGTCACAGAACGCGCTTGTGGAACTACAAGCACAATCAGAATTCGAGATCCATGGCGTTACT | |
| Stichaster australis contig 929 | NZ2Iso_F | GCTAGGGTTCTATGGCTGGT |
| NZ2Iso_R | GCTCCCCAGGATTTTCAAGG | |
| NZ2Iso_Pr | [FAM]CGAGTCCGGTGCGTCCTCGA[TAM] | |
| NZ2Iso_Std | GCTAGGGTTCTATGGCTGGTAGAGCTCGGCACTTCTGCCGAGTCCGGTGCGTCCTCGACGGCCCTTGAAAATCCTGGGGAGC |
Investigation of eukaryote 18S and 28S rRNAs in metaviromes
Contiguous sequences generated from viral metagenomes (described above) were queried against the Silva database (version r132) of 16S/18S and 23S/28S rRNAs [31] by BLASTn and contigs matching at E<10−10 to 18S or 28S rRNAs were considered for further analysis. Matches meeting this criterion were then queried against the non-redundant database at NCBI. Matches to asteroid 18S and 28S rRNAs were removed from further consideration, as were matches to other metazoan rRNAs. The resulting contig spectra were aligned against close matches from NCBI using the CLC Sequence Viewer 8.0 (Qiagen).
Investigation of mesomycetozoan tissue and species specificity
Quantitative PCR (qPCR) primers were designed around the 28S rRNA sequence matching Ichthyosporea sp. (Stichaster contig 929) and used to amplify body wall DNA extracts from 20 Stichaster australis, 6 Coscinasterias muricata, and 10 Patiriella regularis. Additionally, for each of the 20 Stichaster australis, samples of pyloric caeca and gonad were also examined for the presence and abundance of this sequence.
Results and discussion
Viruses associated with asteroid tissues
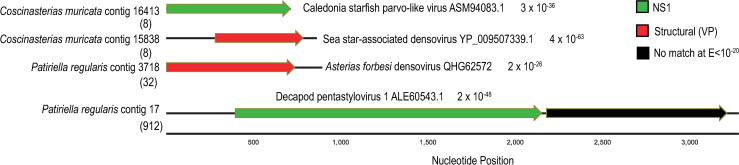
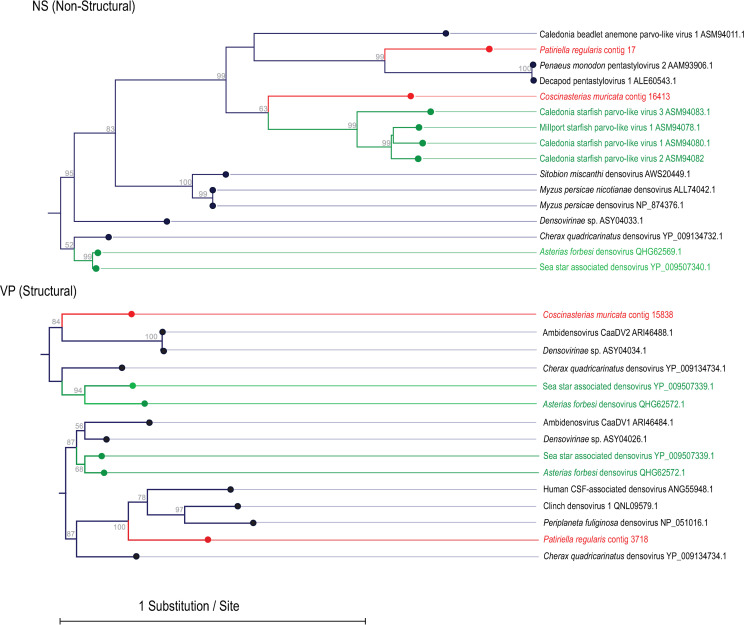
Metaviromes prepared from asteroid body wall samples contained between 1.6–3.9 million paired-end reads (Table 2). Assembly of these resulted in 2,170 to 27,032 contigs, where contigs recruited 18–41% of total reads. No RNA viruses were detected by alignment. However, alignment against densoviral genomes resulted in 2 contigs matching to the nonstructural gene 1 (NS1) and 2 contigs matching structural (VP) genes at E < 10−15 (Fig 2). Three of these contigs–two from Coscinasterias muricata and one from Patiriella regularis- overlapped with ambidensovirus peptide sequences recovered from species of starfish collected worldwide. A further contig from Patiriella regularis matched a decapod penstyldensoviruses. Phylogenetic analyses based on NS1 revealed that Coscinasterias muricata contig 16413 was most similar to densoviruses recovered from Asterias rubens in Scotland [32] (Fig 3). Phylogenetic analyses based on structural genes of the remaining viral contigs (Coscinasterias muricata contig 15838 and Patiriella regularis contig 3718) suggested that these were most similar to ambidensoviruses from molluscs [33, 34], insects [35], a crustacean [36], and human spinal fluid [37]. Quantitative PCR (qPCR) of the densovirus-like Coscinasterias muricata contig 15838 yielded only 3 DNQ results; two in Coscinasterias muricata (of 3 total surveyed) from Ti Point; and one Stichaster australis from Piha. qRT-PCR of Patiriella contig 17 yielded two DNQ results, both from Patiriella regularis collected at Matheson’s Bay. In no sample did we consistently detect the presence of either contig between replicate amplifications. This may be interpreted as indicating their very low copy number (<10) in DNA extracts.
Fig 2. Contig map of densovirus-like genome fragments recovered from Coscinasterias muricata and Patiriella regularis viral metagenomes.
The colors of arrows indicate densoviral gene, and the best match (by BLASTx against the non-redundant database at NCBI) along with e-value is indicated adjacent to each ORF. The black lines running through ORFs indicate total contig length. The numbers in brackets below each contig are the number of reads recruiting to the contig from the origin library.
Fig 3. Phylogenetic representation of Patiriella regularis and Coscinasterias muricata-associated densoviral genome fragments.
The trees are based upon 170 amino acid (Non-Structural), 211 amino acid (Structural; middle), and 104 amino acid (Structural; bottom) alignments performed using the CLC Sequence Viewer version 8.0. The trees were constructed with neighbor joining and Jukes-Cantor distance, where bootstrap values (1000 reps) are indicated above nodes. Red labels indicate sequences obtained in this study, while green labels indicate sequences obtained from asteroids in other studies.
The observation of densoviruses in these species was not surprising, since their recovery in other asteroids [1, 5, 10, 38] and urchins [39] suggests they may be a common constituent of echinoderm microbiomes. Parvoviruses form persistent infections in hosts [38, 40], and are highly prevalent and persistent in asteroid populations [41]. They are also widely endogenized in host genomes [42]. None of the densovirus-like contigs discovered in this survey represented complete genomes, so it is possible that these also represent endogenized densoviruses.
The pathology of densoviruses and significance in wasting diseases or other conditions is unclear. The copy number of Asteroid ambidensovirus-1 (SSaDV) and related densoviruses is elevated in wasting-affected Pycnopodia helianthoides [5]. However, histopathology [43] and other investigations [6, 9, 10, 41] have failed to clinically connect densoviruses (or viruses in general) to sea star wasting disease. Densoviruses, like all parvoviruses, replicate in somatic cells. Infection in arthropods leads to respiratory impairment [44] and triggering of apoptosis [45], and has been linked to elevated mortality in crustacea [36, 46]. The discovery of a penstyldensovirus genome fragment in Patiriella regularis raises interesting questions about its role in host ecology. In penaeid shrimp, persistent infection by penstyldensoviruses delays mortality from white spot syndrome virus [47], suggesting densoviruses in general may play both detrimental and beneficial roles in host ecology. None of the asteroids sampled in this survey were grossly abnormal, and the low prevalence of the Patiriella regularis penstyldensovirus genome fragment in asteroid populations at our collection sites may indicate that these infections represent sub-clinical, or perhaps persistent infections which are unrelated to wasting or mass mortality.
Protists associated with asteroids
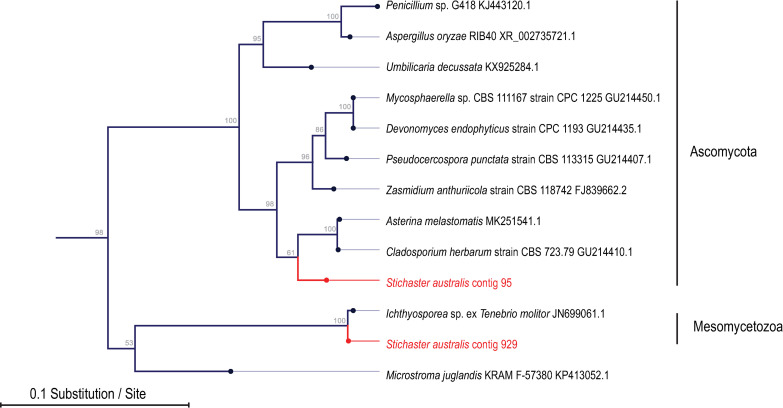
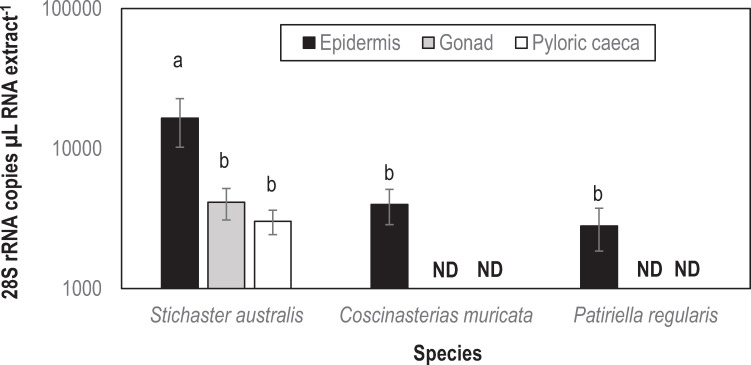
A total of 15 contigs matched 18S and 28S rRNAs based on alignment. Of these, nine were fungal (five were Ascomycetes, four were Basidiomycetes), two were mycetozoan and one was mesomycetozoan (Fig 4; S1–S4 Figs). The mesomycetozoan contiguous sequence (Stichaster contig 929) was most similar to a fish pathogen Ichthyosporea sp. ex Tenebrio molitor (Fig 4). The abundance of this contiguous sequence was significantly higher in the body wall of Stichaster australis than in either Coscinasterias muricata (p = 0.019, Student’s t-test, df = 4) or Patiriella regularis (p = 0.018, Student’s t-test, df = 4) (Fig 5). The abundance in epidermal tissues was also significantly higher in Stichaster australis than in either gonads or pyloric caeca (p = 0.013 and p = 0.006, respectively, Student’s t-test, df = 8). The contiguous sequence was detected in any quantity in 80–85% of all samples tested with no pattern with tissue specificity or species.
Fig 4. Phylogenetic representation of asteroid-associated 28S rRNA sequences in purified virus metagenomes.
The tree was constructed by neighbor joining and based on an 849 nucleotide alignment of eukaryotic 28S rRNA. Shown are close matches by BLAST against the non-redundant database.
Fig 5. Mesomycetozoan 28S rRNA copies as determined by qPCR in asteroid tissues.
a,b denotes significant difference (p < 0.025, Student’s t-test with Bonferroni correction for 2 comparisons).
The association of microbial eukaryotes, especially fungi and fungi-like protists, with echinoderms is not extensively documented in previous surveys. Hewson et al [30] reported the detection of totiviruses, which are fungal viruses, in several Holothuroidea. Similarly, Nerva et al [47] reported the mycovirome of fungi isolated from Holothuria polii. These reports suggest that fungi may be common constitutents of the sea cucumber microbiome. Wei et al [48] reported the cultivation of a symbiotic fungi most similar to Penicillium from an asteroid in China. Labyrinthulids have also been cultivated from the surface of wasting asteroids in the northeast Pacific [49]. However, their role in wasting pathology is unknown. There has been a body of work examining anti-fungal properties of asteroid extracts [50–53], suggesting that fungi discovered in this survey may be adapted to the chemical environment of their host.
Mesomycetozoa are of interest since they represent the closest unicellular ancestor to multicellular animals [54]. They represent parasites of vertebrates [55–57] of which several, including Ichthyosporea spp. are aquatic. Aquatic mesomycetozoans infect fish and amphibians [56–59] and cause dermal disease. Mesomycetozoa may also form symbioses with their hosts (e.g. the mealworm Tenebrio molitor; [60] and other taxa [61, 62] (reviewed in [63]). Our observation of an Icthyosporea-like rRNA in Stichaster is the first report of this group in Asteroidea. The observations of greater load in epidermal tissues than internal organs suggests they may also form dermal infections, and their widespread occurrence in asteroid populations from the North Island / Te Ika-a-Māui of Aotearoa New Zealand suggests that mesomycetozoans are non-specific and broadly prevelant. Because we did not observe gross disease signs in any specimen, it is unlikely that this microorganism is a pathogen, but rather, they may represent a normal constituent of the host microbiome. Since this taxon was found primarily in epidermal tissues, it is also possible that it was incidentally acquired from the environment.
Conclusions
To the best of our knowledge, this is the first investigation of viruses and mesomycetozoa associated with asteroids in Aotearoa New Zealand. Discovery of these taxa suggests an undiscovered bank of potential parasites or symbionts inhabiting echinoderms, and demands further investigation into their ecological roles. Our work demonstrates the value in unbiased surveys of microbiome constituents (i.e. microbial surveillance) which may inform future disease investigations by providing a picture of grossly normal microbiome constituents. Furthermore, this study broadens understanding of densovirus host range, and provides further evidence for their association with asteroid taxa that do not currently experience sea star wasting disease.
Supporting information
The tree was constructed by neighbor joining and based on an 689 nucleotide alignment of eukaryotic 18S rRNAs. Shown are close matches by BLAST against the non-redundant database.
(EPS)
The tree was constructed by neighbor joining and based on an 368 nucleotide alignment of eukaryotic 28S rRNAs. Shown are close matches by BLAST against the non-redundant database.
(EPS)
The tree was constructed by neighbor joining and based on a 481 nucleotide alignment of eukaryotic 28S rRNAs. Shown are close matches by BLAST against the non-redundant database.
(EPS)
The tree was constructed by neighbor joining and based on a 506 nucleotide alignment of eukaryotic 28S rRNAs. Shown are close matches by BLAST against the non-redundant database.
(EPS)
Acknowledgments
The authors thank Richard Taylor for sample collection at Ti Point, and Christopher DeRito, Elliot Jackson and Kalia Bistolas for assistance with laboratory analyses.
Data Availability
Sequences have been deposited in the NCBI under BioProject PRJNA636826, Short Read Archive accessions SRR11923915- SRR11923917, and contig sequences archived under accessions MW080663-MW080666.
Funding Statement
This work was supported by the United States National Science Foundation grants OCE- 1737127 and OCE- 1537111 to IH. The funders had no role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Hewson I, Button JB, Gudenkauf BM, Miner B, Newton AL, Gaydos JK, et al. Densovirus associated with sea-star wasting disease and mass mortality. Proc Nat Acad Sci USA. 2014;111:17276–83. 10.1073/pnas.1416625111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hewson I, Sullivan B, Jackson EW, Xu Q, Long H, Lin C, et al. Perspective: Something old, something new? Review of wasting and other mortality in Asteroidea (Echinodermata). Front Mar Sci. 2019;6(406). 10.3389/fmars.2019.00406 [DOI] [Google Scholar]
- 3.Mead AD. Twenty-eighth annual report of the commissioners of inland fisheries, made to the General Assembly at its January session, 1898. Providence: 1898. [Google Scholar]
- 4.Walker PJ, Siddell SG, Lefkowitz EJ, Mushegian AR, Dempsey DM, Dutilh BE, et al. Changes to virus taxonomy and the International Code of Virus Classification and Nomenclature ratified by the International Committee on Taxonomy of Viruses (2019). Archiv Virol. 2019;164(9):2417–29. 10.1007/s00705-019-04306-w [DOI] [PubMed] [Google Scholar]
- 5.Hewson I, Bistolas KSI, Quijano Carde EM, Button JB, Foster PJ, Flanzenbaum JM, et al. Investigating the complex association between viral ecology, environment and Northeast Pacific Sea Star Wasting. Front Mar Sci. 2018; 5:77 10.3389/fmars.2018.00077. [DOI] [Google Scholar]
- 6.Aquino CA, Besemer RM, DeRito CM, Kocian J, Porter IR, Raimondi PT, et al. Evidence that microorganisms at the animal-water interface drive sea star wasting disease. Front Microbiol. 2020: 10.3389/fmicb.2020.610009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lloyd MM, Pespeni MH. Microbiome shifts with onset and progression of sea star wasting disease revealed through time course sampling. Sci Rep. 2018;8:12. 10.1038/s41598-017-18294-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nunez-Pons L, Work TM, Angulo-Preckler C, Moles J, Avila C. Exploring the pathology of an epidermal disease affecting a circum-Antarctic sea star. Sci Rep. 2018;8:12. 10.1038/s41598-017-18294-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hewson I, Aquino CA, deRito CM. Virome variation during sea star wasting disease progression in Pisaster ochraceus (Asteroidea, Echinodermata). Viruses. 2020;12:1332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jackson EW, Wilhelm RC, Johnson MR, Lutz HL, Danforth I, Gaydos JK, et al. Diversity of sea star-associated densoviruses and transcribed endogenized viral elements of densovirus origin. J Virol. 2020: 10.1128/JVI.01594-20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Eisenlord ME, Groner ML, Yoshioka RM, Elliott J, Maynard J, Fradkin S, et al. Ochre star mortality during the 2014 wasting disease epizootic: Role of population size structure and temperature. Phil Transact Roy Soc B 2016;371(1689):20150212. 10.1098/rstb.2015.0212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Harvell CD, Montecino-Latorre D, Caldwell JM, Burt JM, Bosley K, Keller A, et al. Disease epidemic and a marine heat wave are associated with the continental-scale collapse of a pivotal predator (Pycnopodia helianthoides). Sci Adv. 2019;5(1):eaau7042. 10.1126/sciadv.aau7042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bates AE, Hilton BJ, Harley CDG. Effects of temperature, season and locality on wasting disease in the keystone predatory sea star Pisaster ochraceus. Dis Aquat Org. 2009;86(3):245–51. 10.3354/dao02125 [DOI] [PubMed] [Google Scholar]
- 14.Aalto EA, Lafferty KD, Sokolow SH, Grewelle RE, Ben-Horin T, Boch CA, et al. Models with environmental drivers offer a plausible mechanism for the rapid spread of infectious disease outbreaks in marine organisms. Sci Rep. 2020;10(1):5975. 10.1038/s41598-020-62118-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kohl WT, McClure TI, Miner BG. Decreased temperature facilitates short-term sea star wasting disease survival in the keystone intertidal sea star Pisaster ochraceus. PLoS One. 2016;11(4):e0153670. 10.1371/journal.pone.0153670 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Menge BA, Cerny-Chipman EB, Johnson A, Sullivan J, Gravem S, Chan F. Sea star wasting disease in the keystone predator Pisaster ochraceus in Oregon: Insights into differential population impacts, recovery, predation rate, and temperature effects from long-term research. PLoS One. 2016;11(5):e0153994. 10.1371/journal.pone.0153994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hewson I. Technical pitfalls that bias comparative microbial community analyses of aquatic disease. Dis Aquat Organ. 2019;137(2):109–24. 10.3354/dao03432 [DOI] [PubMed] [Google Scholar]
- 18.Jackson EW, Pepe-Ranney C, Debenport SJ, Buckley DH, Hewson I. The microbial landscape of sea stars and the anatomical and interspecies variability of their microbiome. Front Microbiol. 2018;9:12. 10.3389/fmicb.2018.00012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kelly MS, Barker MF, McKenzie JD, Powell J. The incidence and morphology of subcuticular bacteria in the echinoderm fauna of New Zealand. Biol Bull. 1995;189(2):91–105. 10.2307/1542459 [DOI] [PubMed] [Google Scholar]
- 20.Kelly MS, McKenzie JD. Survey of the occurrence and morphology of subcuticular bacteria in shelf echinoderms from the Nrotheast Atlantic Ocean. Mar Biol. 1995;123(4):741–56. 10.1007/bf00349117 [DOI] [Google Scholar]
- 21.Holland ND, Nealson KH. Fine structure of the echinoderm cuticle and the sub-cuticular bacteria of echinoderms. Acta Zoologica. 1978;59(3–4):169–85. 10.1111/j.1463-6395.1978.tb01032.x [DOI] [Google Scholar]
- 22.Egan S, Gardiner M. Microbial Dysbiosis: Rethinking disease in marine ecosystems. Front Microbiol. 2016;7. 10.3389/fmicb.2016.00991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Holmes EC. The expanding virosphere. Cell Host Microb. 2016;20(3):279–80. 10.1016/j.chom.2016.08.007 [DOI] [PubMed] [Google Scholar]
- 24.Shi M, Lin X-D, Tian J-H, Chen L-J, Chen X, Li C-X, et al. Redefining the invertebrate RNA virosphere. Nature. 2016;540:539–43. 10.1038/nature20167 [DOI] [PubMed] [Google Scholar]
- 25.Ng TFF, Manire C, Borrowman K, Langer T, Ehrhart L, Breitbart M. Discovery of a novel single-stranded DNA virus from a sea turtle fibropapilloma by using viral metagenomics. J Virol. 2009;83(6):2500–9. 10.1128/JVI.01946-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Thurber RV, Haynes M, Breitbart M, Wegley L, Rohwer F. Laboratory procedures to generate viral metagenomes. Nature Protocols. 2009;4(4):470–83. 10.1038/nprot.2009.10 [DOI] [PubMed] [Google Scholar]
- 27.Correa AMS, Welsh RM, Thurber RLV. Unique nucleocytoplasmic dsDNA and +ssRNA viruses are associated with the dinoflagellate endosymbionts of corals. ISME J. 2013;7(1):13–27 10.1038/ismej.2012.75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tricklebank KA, Grace RV, Pilditch CA. Decadal population dynamics of an intertidal bivalve (Austrovenus stutchburyi) bed: Pre- and post- a mass mortality event. NZ J Mar Freshw Res. 2020:1–23. 10.1080/00288330.2020.1772323 [DOI] [Google Scholar]
- 29.Ng FFT, Wheeler E, Greig D, Waltzek TB, Gulland F, Breitbart M. Metagenomic identification of a novel anellovirus in Pacific harbor seal (Phoca vitulina richardsii) lung samples and its detection in samples from multiple years. J Gen Virol. 2011;92(6):1318–23. 10.1099/vir.0.029678-0 [DOI] [PubMed] [Google Scholar]
- 30.Hewson I, Johnson MR, Tibbetts IR. An unconventional flavivirus and other RNA viruses in the sea cucumber (Holothuroidea; Echinodermata) virome. Viruses. 2020;12:1057. 10.3390/v12091057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Quast C, Pruesse E, Yilmaz P, Gerken J, Schweer T, Yarza P, et al. The SILVA ribosomal RNA gene database project: Improved data processing and web-based tools. Nucleic Acids Res. 2012;41(D1):D590–D6. 10.1093/nar/gks1219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Waldron FM, Stone GN, Obbard DJ. Metagenomic sequencing suggests a diversity of RNA interference-like responses to viruses across multicellular eukaryotes. PLoS Gen. 2018;14(7):e1007533. 10.1371/journal.pgen.1007533 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kang YJ, Huang W, Zhao AL, Lai DD, Shao L, Shen YQ, et al. Densoviruses in oyster Crassostrea ariakensis. Arch Virol. 2017;162(7):2153–7. Epub 2017/03/28. 10.1007/s00705-017-3343-z [DOI] [PubMed] [Google Scholar]
- 34.Richard JC, Leis E, Dunn CD, Agbalog R, Waller D, Knowles S, et al. Mass mortality in freshwater mussels (Actinonaias pectorosa) in the Clinch River, USA, linked to a novel densovirus. Sci Rep. 2020;10(1):14498. 10.1038/s41598-020-71459-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Guo H, Zhang J, Hu Y. Complete sequence and organization of Periplaneta fuliginosa densovirus genome. Acta Virol. 2000;44(6):315–22 [PubMed] [Google Scholar]
- 36.Bochow S, Condon K, Elliman J, Owens L. First complete genome of an Ambidensovirus; Cherax quadricarinatus densovirus, from freshwater crayfish Cherax quadricarinatus. Mar Genomics. 2015;24 Pt 3:305–12. Epub 2015/08/14. 10.1016/j.margen.2015.07.009 [DOI] [PubMed] [Google Scholar]
- 37.Phan TG, Messacar K, Dominguez SR, da Costa AC, Deng X, Delwart E. A new densovirus in cerebrospinal fluid from a case of anti-NMDA-receptor encephalitis. Arch Virol. 2016;161(11):3231–5. Epub 2016/08/16. 10.1007/s00705-016-3002-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Frickhofen N, Young NS. Persistent parvovirus B19 infections in humans. Microb Pathogen. 1989;7(5):319–27. 10.1016/0882-4010(89)90035-1 [DOI] [PubMed] [Google Scholar]
- 39.Gudenkauf BM, Eaglesham JB, Aragundi WM, Hewson I. Discovery of urchin-associated densoviruses (Parvoviridae) in coastal waters of the Big Island, Hawaii. J Gen Virol. 2014;95:652–8. 10.1099/vir.0.060780-0 [DOI] [PubMed] [Google Scholar]
- 40.Molthathong S, Jitrakorn S, Joyjinda Y, Boonchird C, Witchayachamnarnkul B, Pongtippatee P, et al. Persistence of Penaeus stylirostris densovirus delays mortality caused by white spot syndrome virus infection in black tiger shrimp (Penaeus monodon). BMC Vet Res. 2013;9(1):33. 10.1186/1746-6148-9-33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jackson EW, Pepe-Ranney C, Johnson MR, Distel DL, Hewson I. A highly prevalent and pervasive densovirus discovered among sea stars from the North American Atlantic coast. Appl Environ Microbiol. 2020;86(6). 10.1128/AEM.02723-19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Liu H, Fu Y, Xie J, Cheng J, Ghabrial SA, Li G, et al. Widespread endogenization of densoviruses and parvoviruses in animal and human genomes. J Virol. 2011;85(19):9863–76. 10.1128/JVI.00828-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Newton AL, Smolowitz R. Chapter 41—Invertebrates. In: Terio KA, McAloose D, Leger JS, editors. Pathology of Wildlife and Zoo Animals: Academic Press; 2018. p. 1019–52. [Google Scholar]
- 44.Mutuel D, Rayallec M, Chabi B, Multeau C, Salmon JM, Fournier P, et al. Pathogenesis of Junonia coenia densovirus in Spodoptera frugiperda: A route of infection that leads to hypoxia. Virology. 2010;403(2):137–44. 10.1016/j.virol.2010.04.003 [DOI] [PubMed] [Google Scholar]
- 45.Roekring S, Smith DR. Induction of apoptosis in densovirus infected Aedes aegypti mosquitoes. J Invert Pathol. 2010;104(3):239–41. 10.1016/j.jip.2010.04.002 [DOI] [PubMed] [Google Scholar]
- 46.Owens L, La Fauce K, Claydon K. The effect of Penaeus merguiensis densovirus on Penaeus merguiensis production in Queensland, Australia. J Fish Dis. 2011;34(7):509–15. 10.1111/j.1365-2761.2011.01263.x [DOI] [PubMed] [Google Scholar]
- 47.Nerva L, Forgia M, Ciuffo M, Chitarra W, Chiapello M, Vallino M, et al. The mycovirome of a fungal collection from the sea cucumber Holothuria polii. Virus Res. 2019;273:197737. 10.1016/j.virusres.2019.197737 [DOI] [PubMed] [Google Scholar]
- 48.Wei X, Feng C, Li XH, Mao XX, Luo HB, Zhang DM, et al. Enantiomeric polyketides from the starfish-derived symbiotic fungus Penicillium sp. GGF16-1-2. Chem Biodiv. 2019;16(6). 10.1002/cbdv.201900052 [DOI] [PubMed] [Google Scholar]
- 49.FioRito R, Leander C, Leander B. Characterization of three novel species of Labyrinthulomycota isolated from ochre sea stars (Pisaster ochraceus). Mar Biol. 2016;163(8):10. 10.1007/s00227-016-2944-5 [DOI] [Google Scholar]
- 50.Franco OP, Patino GS, Ortiz AA. Antibacterial and antifungal activity of the starfish Oreaster reticulatus (Valvatida: Oreasteridae) and the sea urchins Mellita quinquiesperforata (Clypeasteroida: Mellitidae) and Diadema antillarum (Diadematoida: Diadematidae) from the Colombian Caribbean. Revist Biol Trop. 2015;63:329–37 [Google Scholar]
- 51.Palagiano E, Zollo F, Minale L, Iorizzi M, Bryan P, McClintock J, et al. Isolation of 20 glycosides from the starfish Henricia downeyae, collected in the Gulf of Mexico. J Natur Prod. 1996;59(4):348–54. 10.1021/np9601014 [DOI] [PubMed] [Google Scholar]
- 52.Choi DH, Shin S, Park IK. Characterization of antimicrobial agents extracted from Asterina pectinifera. Int J Antimicrob Agent. 1999;11(1):65–8. 10.1016/s0924-8579(98)00079-x [DOI] [PubMed] [Google Scholar]
- 53.Chludil HD, Seldes AM, Maier MS. Antifungal steroidal glycosides from the Patagonian starfish Anasterias minuta: Structure-activity correlations. J Natur Prod. 2002;65(2):153–7. 10.1021/np010332x [DOI] [PubMed] [Google Scholar]
- 54.Mendoza L, Taylor JW, Ajello L. The class Mesomycetozoea: A group of microorganisms at the animal-fungal boundary. Ann Rev Microbiol. 2002;56:315–44. 10.1146/annurev.micro.56.012302.160950 [DOI] [PubMed] [Google Scholar]
- 55.Baker GC, Beebee TJC, Ragan MA. Prototheca richardsi, a pathogen of anuran larvae, is related to a clade of protistan parasites near the animal-fungal divergence. Microbiol UK. 1999;145:1777–84. 10.1099/13500872-145-7-1777 [DOI] [PubMed] [Google Scholar]
- 56.Fredricks DN, Jolley JA, Lepp PW, Kosek JC, Relman DA. Rhinosporidium seeberi: A human pathogen from a novel group of aquatic protistan parasites. Emerg Infect Dis. 2000;6(3):273–82. 10.3201/eid0603.000307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Arkush KD, Mendoza L, Adkison MA, Hedrick RP. Observations on the life stages of Sphaerothecum destruens n. g., n. sp., a mesomycetozoean fish pathogen formally referred to as the rosette agent. J Euk Microbiol. 2003;50(6):430–8. 10.1111/j.1550-7408.2003.tb00269.x [DOI] [PubMed] [Google Scholar]
- 58.Blazer VS, Hitt NP, Snyder CD, Snook EL, Adams CR. Dermocystidium sp infection in blue ridge sculpin captured in Maryland. J Aquat Ani Health. 2016;28(3):143–9. 10.1080/08997659.2016.1159622 [DOI] [PubMed] [Google Scholar]
- 59.Arnott SA, Dykova I, Roumillat WA, de Buron I. Pathogenic endoparasites of the spotted seatrout, Cynoscion nebulosus: Patterns of infection in estuaries of South Carolina, USA. Parasitol Res. 2017;116(6):1729–43. 10.1007/s00436-017-5449-3 [DOI] [PubMed] [Google Scholar]
- 60.Lord JC, Hartzer KL, Kambhampati S. A nuptially transmitted Ichthyosporean symbiont of Tenebrio molitor (Coleoptera: Tenebrionidae). J Euk Microbiol. 2012;59(3):246–50. 10.1111/j.1550-7408.2012.00617.x [DOI] [PubMed] [Google Scholar]
- 61.Marshall WL, Celio G, McLaughlin DJ, Berbee ML. Multiple isolations of a culturable, motile Ichthyosporean (Mesomycetozoa, Opisthokonta), Creolimax fragrantissima n. gen., n. sp., from marine invertebrate digestive tracts. Protist. 2008;159(3):415–33. 10.1016/j.protis.2008.03.003 [DOI] [PubMed] [Google Scholar]
- 62.Marshall WL, Berbee ML. Facing unknowns: Living cultures (Pirum gemmata gen. nov., sp. nov., and Abeoforma whisleri, gen. nov., sp. nov.) from invertebrate digestive tracts represent an undescribed clade within the unicellular Opisthokont lineage Ichthyosporea (Mesomycetozoea). Protist. 2011;162(1):33–57. 10.1016/j.protis.2010.06.002 [DOI] [PubMed] [Google Scholar]
- 63.Glockling SL, Marshall WL, Gleason FH. Phylogenetic interpretations and ecological potentials of the Mesomycetozoea (Ichthyosporea). Fungal Ecol. 2013;6(4):237–47. 10.1016/j.funeco.2013.03.005. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The tree was constructed by neighbor joining and based on an 689 nucleotide alignment of eukaryotic 18S rRNAs. Shown are close matches by BLAST against the non-redundant database.
(EPS)
The tree was constructed by neighbor joining and based on an 368 nucleotide alignment of eukaryotic 28S rRNAs. Shown are close matches by BLAST against the non-redundant database.
(EPS)
The tree was constructed by neighbor joining and based on a 481 nucleotide alignment of eukaryotic 28S rRNAs. Shown are close matches by BLAST against the non-redundant database.
(EPS)
The tree was constructed by neighbor joining and based on a 506 nucleotide alignment of eukaryotic 28S rRNAs. Shown are close matches by BLAST against the non-redundant database.
(EPS)
Data Availability Statement
Sequences have been deposited in the NCBI under BioProject PRJNA636826, Short Read Archive accessions SRR11923915- SRR11923917, and contig sequences archived under accessions MW080663-MW080666.