Abstract
Background
The efficacy of antibiotics in rosacea treatment suggests a role for microorganisms in its pathophysiology. Growing concern over the adverse effects of antibiotic use presents a need for targeted antimicrobial treatment in rosacea.
Objective
We performed a case–control study to investigate the skin microbiota in patients with rosacea compared to controls matched by age, sex, and race.
Methods
Nineteen participants with rosacea, erythematotelangiectatic, papulopustular, or both, were matched to 19 rosacea-free controls. DNA was extracted from skin swabs of the nose and bilateral cheeks of participants. Sequencing of the V3V4 region of the bacterial 16S ribosomal RNA gene was performed using Illumina MiSeq and analyzed using QIIME/MetaStats 2.0 software.
Results
Compared with controls, skin microbiota in erythematotelangiectatic rosacea was depleted in Roseomonas mucosa (p = 0.004). Papulopustular rosacea was enriched in Campylobacter ureolyticus (p = 0.001), Corynebacterium kroppenstedtii (p = 0.008), and the oral flora Prevotella intermedia (p = 0.001). The highest relative abundance of C. kroppenstedtii was observed in patients with both erythematotelangiectatic and papulopustular rosacea (19.2%), followed by papulopustular (5.06%) and erythematotelangiectatic (1.21%) rosacea. C. kroppenstedtii was also associated with more extensive disease, with the highest relative abundance in rosacea affecting both the cheeks and nose (2.82%), followed by rosacea sparing the nose (1.93%), and controls (0.19%).
Conclusions
The skin microbiota in individuals with rosacea displays changes from that of healthy skin, suggesting that further studies examining a potential role for the skin microbiota in the pathophysiology of rosacea may be warranted.
1. Introduction
Rosacea is a chronic, often progressive disorder characterized by a variable combination of cutaneous stigmata including facial flushing, erythema, telangiectasia, papules, pustules, and rhinophyma [1]. Four primary morphologic subtypes of rosacea are recognized: erythematotelangiectatic, papulopustular, phymatous, and ocular [2]. The pathogenesis of rosacea continues to remain disputed [2]. Proposed contributing factors include abnormalities in innate immunity, vascular hyper-reactivity, ultraviolet irradiation, gastrointestinal disorders, and genetic susceptibility [1–5].
Multiple prior studies have investigated the role of microorganisms in the etiology of rosacea. Staphylococcus epidermis has been implicated, as it has been cultured directly from pustules in PPR [6]. Studies of the Demodex folliculorum mite, present in a significantly higher density in the facial skin of patients with papulopustular rosacea (PPR) than the general population [7], indicate that the Demodex mite may act as a vector, transmitting bacteria such as Bacillus oleronius and Bartonella quintana across the skin to secondarily cause disease [8, 9].
The continued challenge to characterize the role of microorganisms in rosacea is likely related to the limitations of historic culture-dependent methods to identify and study cutaneous microbes. The highly conserved small-subunit 16S ribosomal RNA (rRNA) sequence universally present in all prokaryotes can be used as a taxonomic marker for microbiota [10]. Dysregulation of skin microbiota has been associated with a variety of skin disorders including atopic dermatitis [11], acne vulgaris [12], psoriasis [13], and seborrheic dermatitis [14].
This study investigates the differences in the skin microbiota between patients with rosacea and matched controls using microbial 16S rRNA polymerase chain reaction amplification and sequencing to determine whether associations exist between microbial dysbiosis and rosacea.
2. Methods
2.1. Study Design
An observational case–control study was approved by the Johns Hopkins Institutional Review Board. Participants were recruited from patients seen at the Johns Hopkins Outpatient Center and surrounding community throughout 2013. All participants provided written informed consent. Of the 22 patients with rosacea enrolled, 19 patients completed the study and were matched to 19 controls. The study was designed to evaluate the skin microbiota of the nose and bilateral cheeks in patients with rosacea compared to matched controls. Inclusion criteria for all subjects included: age ≥ 18 years, current diagnosis of rosacea for case patients or lack of rosacea for controls, and willingness to avoid facial washing and application of topical agents to the face for 24 h prior to skin sampling.
Exclusion criteria for all subjects included: significant medical history, recent medical treatment (within 4 weeks for topical antibiotics, corticosteroids, and other anti-inflammatory medications, and within 8 weeks for systemic antibiotics, corticosteroids, and other immunosuppressive agents), history of facial surgeries or cosmetic procedures, significant facial hair interfering with sampling, and pregnancy.
2.2. Sample Collection
Samples were obtained by separately swabbing a 2 × 2 cm area on the nose and bilateral cheeks with sterile foam-tipped swabs (Puritan Medical Products, Guilford, ME, USA) moistened with Amies medium. Each region was swabbed vigorously for 30 seconds (15 seconds per side for cheeks) while rotating the swab. Each individual swab was placed in a sample tube containing Amies medium (1 mL; Puritan Medical Products) and stored at − 80 °C within 24 h of sample collection.
2.3. DNA Extraction and Sequencing
Metagenomic DNA was extracted from skin swab samples as previously published [15, 16]. Samples were thawed and transferred aseptically to Lysing Matrix B tubes (MP Biomedicals, Solon, OH, USA). Enzymatic bacterial lysis was conducted using lysozyme, mutanolysin, proteinase K, and lysostaphin, followed by mechanical lysis through bead beating. A Zymo fecal DNA kit (Zymogen, Irvine, CA, USA) was used to further purify metagenomic DNA. DNA quality assurance was performed with spectrophotometric measurements on the NanoDrop system and gel electrophoresis. Negative extraction phosphate-buffered saline controls were also included in sample processing to confirm that contaminant DNA was not introduced into samples during the extraction process. Following extraction, the V3V4 hypervariable region of the 16S rRNA gene was polymerase chain reaction amplified and sequenced on an Illumina MiSeq 300-bp paired-reads platform (Illumina, San Diego, CA, USA) as previously described [17].
2.4. Bioinformatic Analysis
Sequencing produced between 1000 and 126,200 paired- end reads per sample (49,500 pairs on average), which were analyzed to determine metagenomic profiles of each sample, calculate intra- and inter-group diversity, and identify differentially represented taxa, using the QIIME1 and the MetaStats 2.0 software packages [18, 19]. Prior to analysis, reads were demultiplexed and filtered to remove low-quality data, vector contaminants, and chimeric reads, and the two reads in a pair were joined together based on overlap. Read assignments to operational taxonomic units were performed with QIIME1 using open clustering with the GreenGenes data-base clustered at 99% sequencing identity, and taxonomic frequency profiles were created reflecting the community’s operational taxonomic unit composition at different phylogenetic levels. Rarefaction plots of intra-sample (alpha) diversity were computed for the phylogenetic diversity whole tree metric. Comparison of alpha diversity between groups using t-tests with Monte Carlo permutations was performed with QIIME1. Between groups, beta diversity was calculated with the weighted and unweighted UniFrac similarity measures, and statistical significance was calculated using analysis of similarity on QIIME1 [20]. For both alpha and beta diversity, a maximum sample depth of 8124 was used. Last, the MetaStats 2.0 package was used to compare metagenomic profiles at each phylogenetic level, from kingdom to species, to determine taxa that were statistically enriched or depleted in one condition compared to the other. Multiple testing correction was applied. For all comparisons, p < 0.05 was considered statistically significant.
3. Results
3.1. Participant Demographics
Participants with rosacea included individuals with diagnosed erythematotelangiectatic rosacea (ETR), PPR, or both (Table 1 of the Electronic Supplementary Material). Severity of rosacea assessed using the National Rosacea Society clinical grading system ranged from mild to moderate [4]. The 11 participants with ETR, six participants with PPR, and two overlapping participants with ETR and PPR included 14 women and five men, all Caucasian, ages 23–65 years. All participants were matched to healthy rosacea-free controls by sex and age ± 5 years. Most patients with rosacea had disease affecting both the cheeks and nose, while some patients had more extensive disease affecting the lateral face, chin, and/or forehead. Symptom onset in patients with rosacea varied from 6 months to > 20 years.
3.2. Taxonomic Assignment
Our final sequence dataset contained 4,036,167 16S rRNA sequences clustered into a total of 1593 species-level operational taxonomic units. Following removal of six samples with fewer than 6089 reads and their matched samples, we identified the following distribution of unique named taxa: 29 phyla, 69 classes, 106 orders, 185 families, 380 genera, and 189 species (Figs. 1, 2). Among all subtypes of rosacea, the most abundant species was consistently Cutibacterium acnes: ETR (28.77%), PPR (16.88%), and overlapping ETR and PPR (46.46%).
Fig. 1.
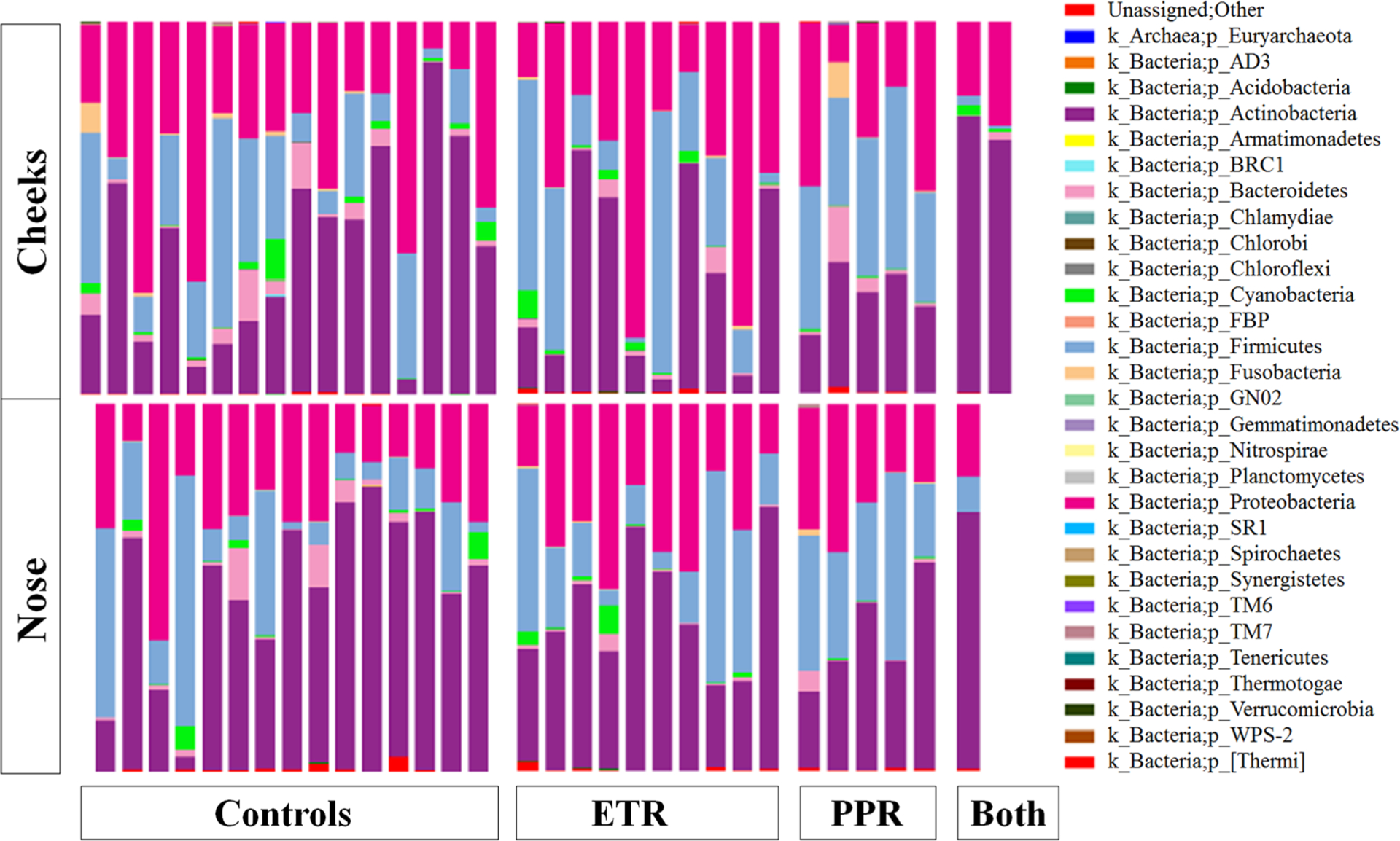
Taxonomy plot comparing microbial distributions of cheek (C) and nose (N) samples from healthy controls and patients with erythematotelangiectatic rosacea (ETR), papulopustular rosacea (PPR), or both at the phylum level
Fig. 2.
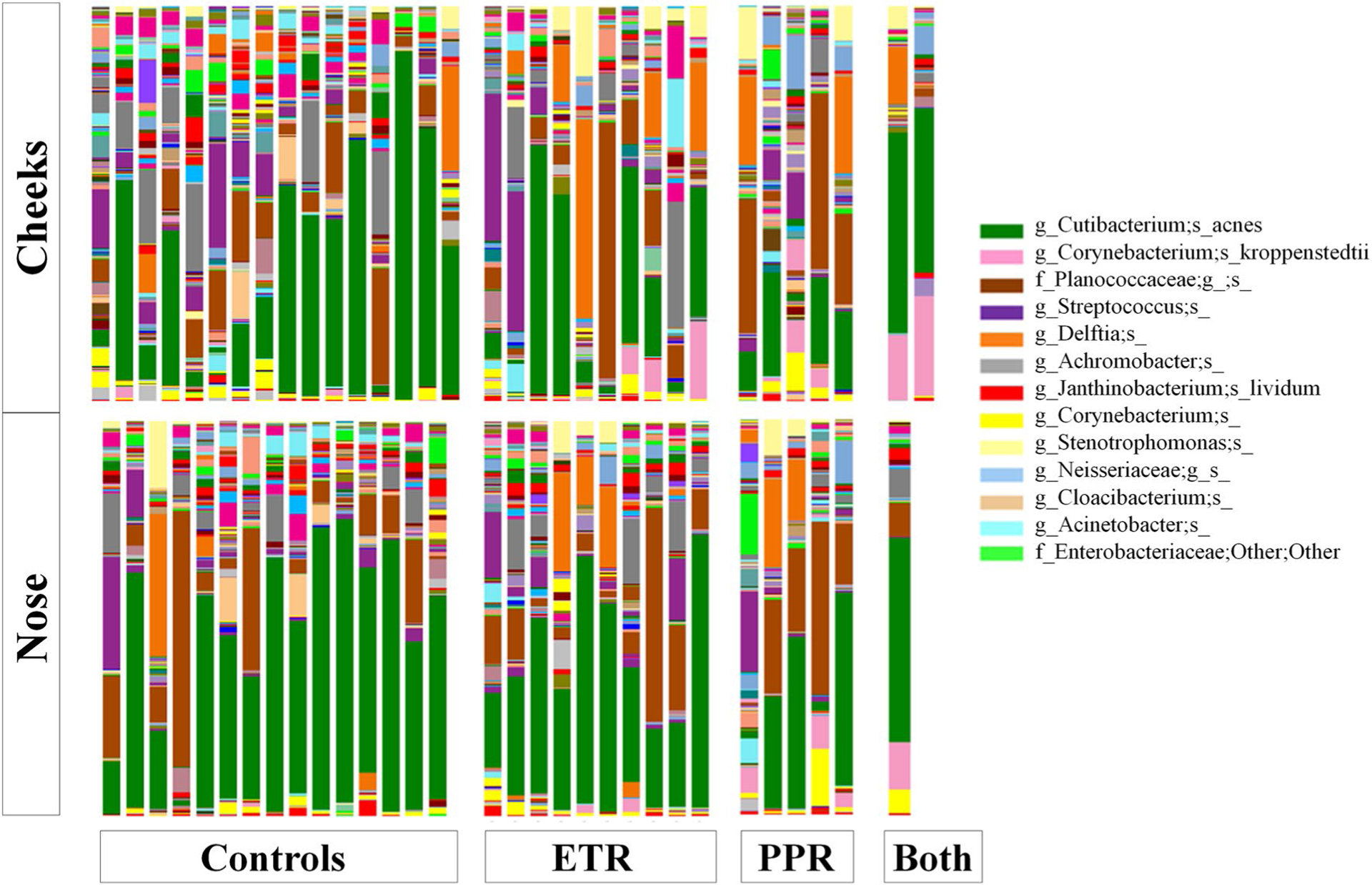
Taxonomy plot comparing microbial distributions of cheek (C) and nose (N) samples from healthy controls and patients with erythematotelangiectatic rosacea (ETR), papulopustular rosacea (PPR), or both at the species level. The legend includes only species present in the highest relative abundance
3.3. Alpha Diversity
Microbial diversity is a function of both the richness and evenness of microorganisms in a given microbial environment [21]. Using the phylogenetic diversity whole tree metric (Fig. 3), which is considered a robust measure of taxonomic richness [22], we found that the mean microbial alpha diversity at each site in patients with rosacea (cheeks = 19.837, nose = 18.764) was higher than that of controls (cheeks = 16.402, nose = 17.267), though not to a statistically significant degree (cheeks, p = 0.240, nose, p = 0.619). This remained true within rosacea subtypes, with PPR mean alpha diversity (cheeks = 22.856, nose = 18.781) being higher than that of their matched controls (cheeks = 13.723, nose = 15.184), and ETR mean alpha diversity (cheeks = 19.010, nose = 19.146) being higher than that of their controls (cheeks = 17.571, nose = 17.468), though again not to a statistically significant degree (PPR: cheeks, p = 0.191, nose, p = 0.454; ETR: cheeks, p = 0.717, nose, p = 0.699).
Fig. 3.
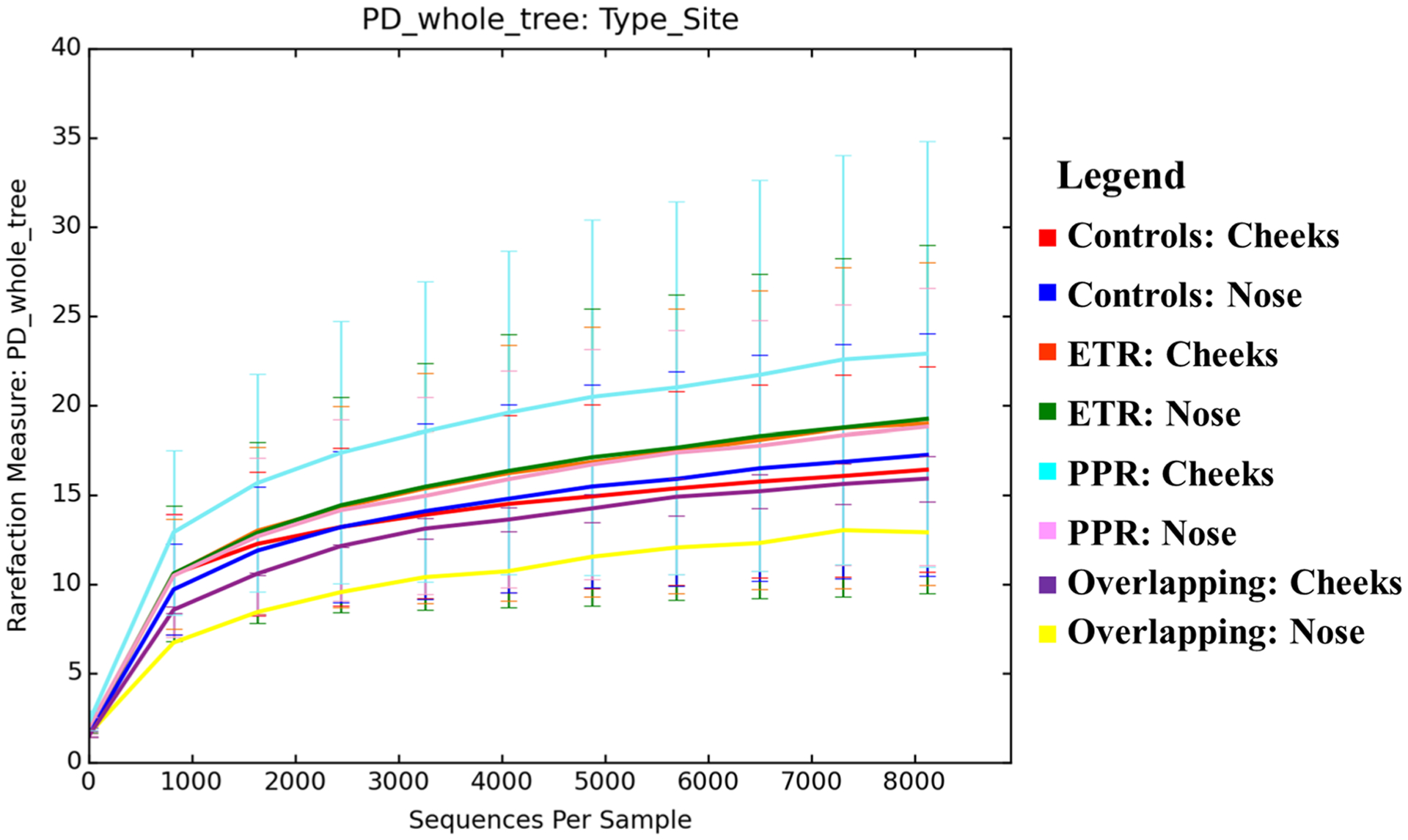
Alpha diversity using a phylogenic diversity whole tree metric across rosacea subtypes
3.4. Beta Diversity
The diversity of samples between subjects (beta diversity) measures differences in community membership over time or location [23]. We examined beta diversity of the rosacea microbiota and controls through a principal coordinates analysis of weighted UniFrac distances. Similarity between samples is represented by the distance between samples across the three principal coordinates (PC1, PC2, and PC3), with samples that cluster close to one another signifying similar bacterial composition between those samples. We did not observe clustering of samples across rosacea subtype (Fig. 4). Analysis of similarity demonstrated that there were no significant similarities in bacterial community composition between ETR and controls (R = − 0.017, p = 0.587), PPR and controls (R = 0.135, p = 0.084), or overlapping ETR/PPR with controls (R = 0, p = 0.534).
Fig. 4.
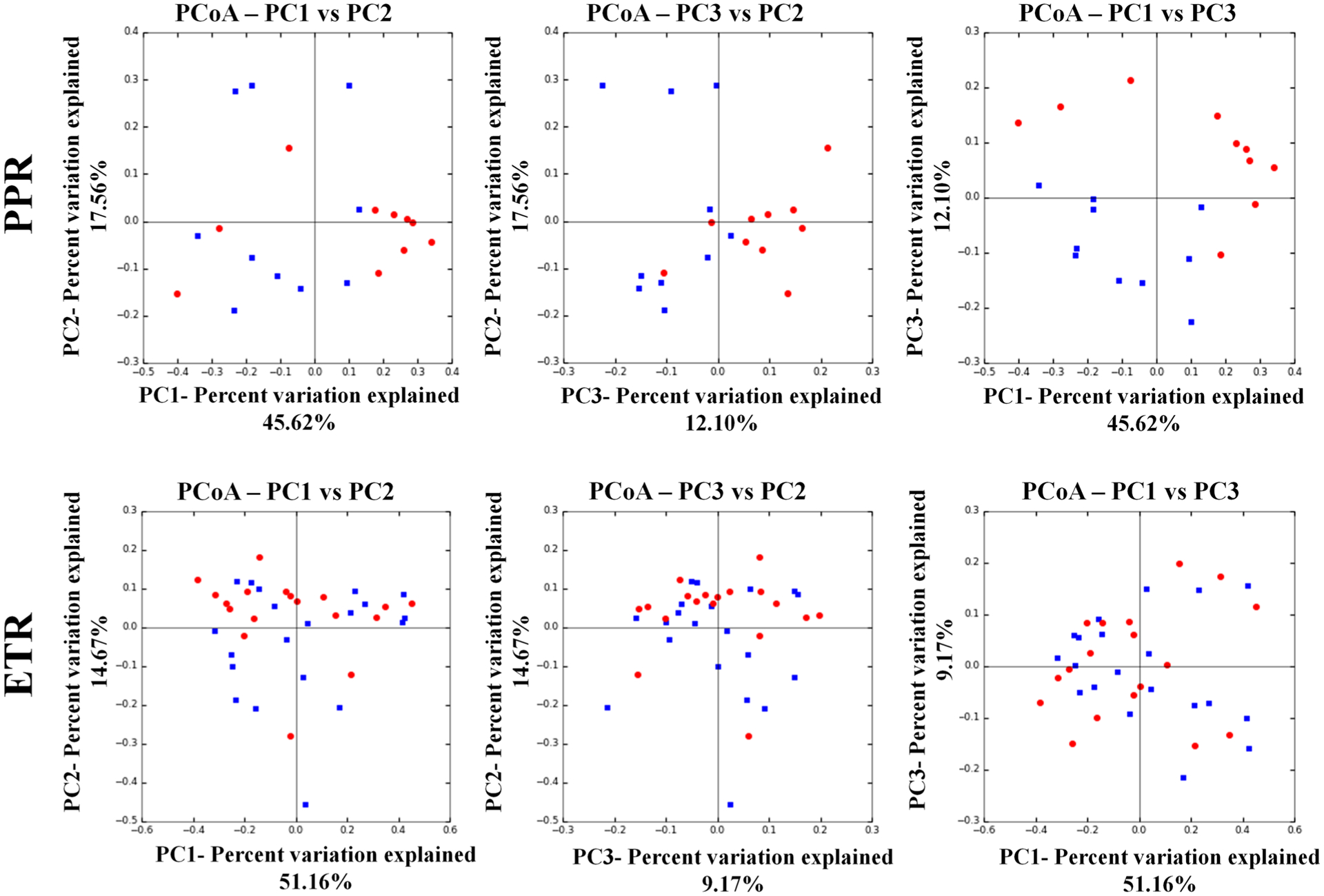
Principal coordinates analysis depicting beta diversity of patients with rosacea (blue) vs. controls (red)
3.5. Relative Abundance Distribution
Significant differences were observed in the relative abundance of certain species between each rosacea subtype compared with controls (Table 1). Species with fewer than 15 total reads within both rosacea subtype and control groups and species detected in only two samples or fewer per comparison were excluded.
Table 1.
Significantly enriched and depleted species in erythematotelangiectatic rosacea (ETR) and papulopustular rosacea (PPR) following multiple testing correction
| ETR species (p value) | PPR species (p value) |
|---|---|
| Enriched | |
| None | Actinomyces europaeus (0.001) |
| Prevotella tannerae (0.001) | |
| Prevotella intermedia (0.001) | |
| Campylobacter ureolyticus (0.001) | |
| Corynebacterium kroppenstedtii (0.008) | |
| Depleted | |
| Porphyromonas endodontalis (0.001) | Dysgonomonas gadei (0.001) |
| Azorhizobium doebereinerae (0.001) | |
| Ruminococcus gnavus (0.001) | Shewanella algae (0.001) |
| Providencia stuartii (0.001) | |
| Azorhizobium doebereinerae (0.001) | Cutibacterium granulosum (0.005) |
| Anoxybacillus kestanbolensis (0.008) | |
| Shewanella algae (0.001) | |
| Providencia stuartii (0.002) | |
| Roseomonas mucosa (0.004) |
Between men and women, the abundance of C. acnes was markedly higher in male controls (57.5%) than female controls (29.7%), while normalizing between male patients with rosacea (23.8%) and female patients with rosacea (27.8%). Across all age groups, C. acnes remained the most abundant species, but fluctuations in its relative abundance were observed (Fig. 5). The relative abundance of C. acnes in female patients was higher in controls compared with patients with rosacea in every age group, with the exception of ages 40–49 years. Because of the low sample size, male patients were separated into only two groups: young (age < 40 years) and older (age > 55 years). Among both young and older men, C. acnes was more abundant in controls than in patients with rosacea, with a greater abundance in young men (controls = 62.5%, rosacea = 39.7%) than in older men (controls = 55.4%, rosacea = 21.1%). The relative abundance of C. acnes demonstrated a weak negative correlation with National Rosacea Society severity scores (R2 = 0.0378, p = 0.124).
Fig. 5.
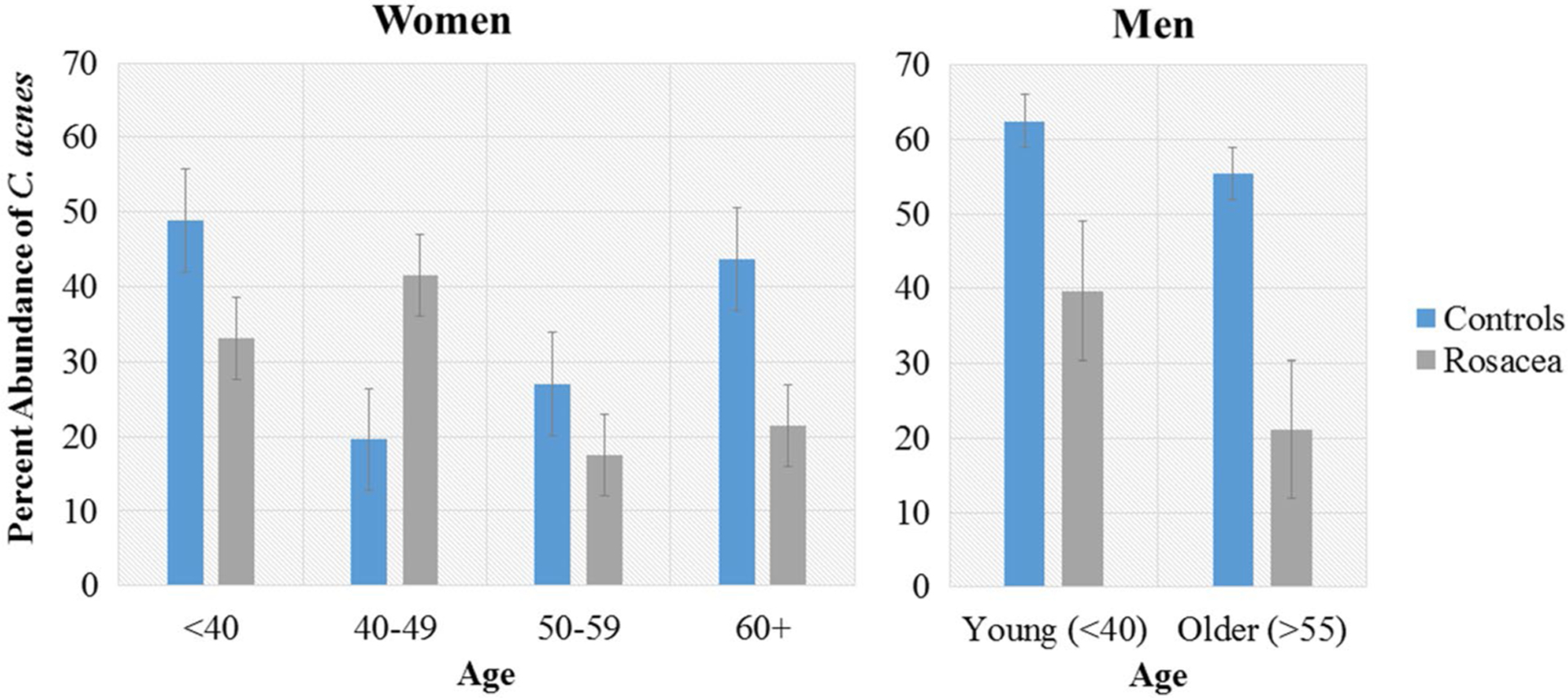
Relative abundance of Cutibacterium acnes by age groups in women and men
In patients with rosacea, the second most abundant species was Corynebacterium kroppenstedtii in all age groups, with its relative abundance peaking in the 40- to 49-year-old rosacea group (5.88%), compared with 0.007% in matched controls of the same age group. The highest relative abundance of C. kroppenstedtii was observed in patients with overlapping features of both PPR and ETR (19.2%), followed by PPR only (5.06%), ETR only (1.21%), and controls (0.285%). C. kroppenstedtii abundance in patients with rosacea was higher in cheek swabs (4.94%) than nose swabs (2.66%). Patients with limited rosacea affecting only the cheeks offered the opportunity to study the microbiota of the unaffected skin in patients with rosacea. Across all nose swabs, C. kroppenstedtii relative abundance was highest in rosacea involving the nose and cheeks (2.82%), followed by rosacea affecting the cheeks but sparing the nose (1.93%) and controls (0.189%). Linear regression analysis of C. kroppenstedtii relative abundance and National Rosacea Society severity scores demonstrated a positive correlation, though it did not achieve statistical significance (R2 = 0.0541, p = 0.0645).
4. Discussion
The overabundance of pathogenic organisms and associated loss of protective organisms is reflected by changes in the diversity of the microbiota. We found an increase, though not statistically significant, in the alpha diversity of facial microbes in patients with rosacea compared with controls. No significant similarity in beta diversity between patients with rosacea and controls was demonstrated, indicating that no one rosacea subtype displays an overall species diversity more closely aligned with controls than any other subtype. A prior study comparing the skin microbiota between twins discordant for rosacea likewise found no significant difference in the alpha or beta diversity of facial microbiota [24]. In the prior study, rosacea severity was negatively associated with alpha diversity, though not to a statistically significant degree [24], suggesting that further studies would be needed to clarify the relationship between rosacea and microbial alpha diversity.
However, we observed notable differences in the relative abundance of specific bacterial taxa between rosacea subtypes and healthy controls. While causation cannot be inferred between the presence of specific taxa and rosacea, the identified taxa may be speculated as contributory in rosacea. These results could be useful in informing researchers that are looking for new therapeutic targets for rosacea. Prior studies examining the relationship between rosacea and the relative abundance of different facial bacteria species are limited. Positive and negative correlations have been identified between rosacea severity and relative abundance of Gordonia and Geobacillus, respectively [24]. We did not observe significant enrichment or depletion of these genera in rosacea compared to controls. A study of the microbiota within the Demodex mite from rosacea and controls identified 92 bacterial species, with C. acnes predominating overall [25]. In mites from patients with rosacea and control participants, six core species represented the majority of identified clones: C. acnes, S. epidermidis, C. kroppenstedtii, Streptococcus mitis, Cutibacterium granulosum, and Snodgrassella alvi [25].
We likewise identified C. acnes as the most abundant species by far across all rosacea subtypes and controls. The density of C. acnes is known to vary with age and sex in healthy individuals, increasing between the onset of puberty to early adulthood and then remaining stable until old age [26]. C. acnes density in healthy male adults is also known to be significantly higher than in female adults [26]. We found that the relative abundance of C. acnes was higher in male controls than female controls as predicted; however, among patients with rosacea, the relative abundance of C. acnes normalized between men and women.
Cutibacterium acnes may have a protective effect in healthy skin [27]. By breaking down sebum into free fatty acids, C. acnes can prevent the colonization of skin by other microbes [28]. The lack of C. acnes in hair follicle biopsies of patients with rosacea has been suggested as evidence that C. acnes does not play a major role in the pathogenesis of rosacea [29], but perhaps it is the deficiency of C. acnes compared to healthy patients that is significant. C. acnes abundance is reduced in certain skin diseases, including atopic dermatitis and psoriasis [11, 13]. The lack of C. acnes may be owing to the effects of prior antibiotic treatments or the disruption of the skin microbiota by the colonization of more aggressive organisms. Alternatively, the loss of C. acnes as a protective barrier could promote the overgrowth of opportunists [13]. Our findings indicate that the expression of certain microbiota in rosacea is multifactorial, with different factors predominating in male rosacea vs. female rosacea in different age brackets.
The skin microbiota of patients with ETR was depleted in multiple bacterial species compared with controls. Roseomonas is a pink-pigmented, aerobic, Gram-negative cocco-bacilli [30] whose primary reservoir is the skin microbiome [31]. Topical transplantation of R. mucosa from healthy controls onto affected skin in patients with atopic dermatitis showed a significant decrease in disease severity and Staphylococcus aureus colonization, suggesting that certain strains of R. mucosa may promote healthy skin [32, 33]. Further studies would be needed to investigate whether microbiome transplantation of R. mucosa from healthy patients could decrease the severity of ETR as well.
The microbiota of PPR demonstrated a more significant derangement compared with controls than that of patients with ETR. C. kroppenstedtii, significantly enriched in PPR, is a Gram-positive lipophilic bacterium that has been isolated almost exclusively from female patients [34] presenting with either granulomatous mastitis [35–38] or breast abscesses [39–42], and one male patient with prosthetic-valve endocarditis [43]. The pathogenicity of C. kroppenstedtii has not been extensively characterized, but it may act as an opportunistic pathogen particularly in lipid-rich regions such as the breast [42] and potentially the facial skin [44]. The relative abundance of C. kroppenstedtii in rosacea was observed to be highest in affected skin, followed by unaffected skin in patients with rosacea. This interesting finding may suggest that C. kroppenstedtii levels must reach a certain threshold before PPR symptoms manifest. The skin microbiota of patients with PPR was depleted in C. granulosum, a known colonizer of normal human skin [45]. This species may play a role in maintaining the normal skin barrier by preventing the growth of potential pathogens.
Our study was limited by a low sample size in some of the rosacea subgroups including male patients and overlapping PPR/ETR. Additional studies examining the microbial communities in these subgroups would further shed light in this area. Because our study focused only on bacterial microbiota, additional studies examining viruses and fungi would be needed to fully characterize the skin microbiota of patients with rosacea. While 16S V3V4 sequencing is significantly superior to culture-based methods, it may yet underestimate the abundance of certain skin commensals, leading to an artificially low relative abundance of species such as C. acnes and S. epidermidis [46]. Last, the pathogenicity of different strains within the same bacterial species can vary, as in the case of methicillin-resistant S. aureus [47]. Different sequencing techniques in future work would be needed to confirm our findings and analyze the relative abundance distribution at the strain level.
5. Conclusions
We examined the differences in the bacterial microbiota on the skin of patients with rosacea compared to controls. While there were no significant differences in the ecologic diversity of microbiota, we did note a number of bacterial taxa that were significantly enriched or depleted across the subtypes of rosacea compared with controls with variations across age group, sex, and extent of disease.
Supplementary Material
Key Points.
Among the skin microbiota of participants with rosacea, specific bacterial taxa were significantly enriched or depleted with variations noted across age, sex, disease extent, and subtype of rosacea.
Further characterizing the relative abundance of skin microbiota in rosacea may provide clues toward understanding the pathophysiology and improving treatments for this common disease.
Funding
Research reported in this publication was supported by the National Rosacea Society and the National Institute of Arthritis and Musculoskeletal and Skin Diseases, part of the National Institutes of Health, under R01AR064297 and AR068280 to Luis A. Garza.
Footnotes
Electronic supplementary material The online version of this article (https://doi.org/10.1007/s40257-019-00471-5) contains supplementary material, which is available to authorized users.
Conflict of interest Anna L. Chien participated in Galderma’s Rosacea Medical Advisory Board Meeting in 2017. Sewon Kang is an advisory board member of Almirall and has received an honorarium. The participation was not in relation to the current article. Barbara M. Rainer, Katherine G. Thompson, Corina Antonescu, Liliana Florea, Emmanuel F. Mongodin, Jonathan Bui, Helena B. Pasieka, Alexander H. Fischer, and Luis A. Garza have no conflicts of interest that are directly relevant to the content of this article.
Ethics approval All procedures performed in studies involving human participants were in accordance with the ethical standards of the Johns Hopkins Institutional Review Board and the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Consent to participate Written informed consent was obtained from all participants included in the study.
References
- 1.Wilkin JK. Rosacea: pathophysiology and treatment. Arch Dermatol. 1994;130:359–62. [DOI] [PubMed] [Google Scholar]
- 2.Powell FC. Clinical practice: rosacea. N Engl J Med. 2005;352(8):793–800. [DOI] [PubMed] [Google Scholar]
- 3.Wilkin J, Dahl M, Detmar M, et al. Standard classification of rosacea: report of the National Rosacea Society Expert Committee on the classification and staging of rosacea. J Am Acad Dermatol. 2002;46(4):584–7. [DOI] [PubMed] [Google Scholar]
- 4.Wilkin J, Dahl M, Detmar M, et al. Standard grading system for rosacea: report of the National Rosacea Society Expert Committee on the classification and staging of rosacea. J Am Acad Dermatol. 2004;50(6):907–12. [DOI] [PubMed] [Google Scholar]
- 5.Powell FC. What’s going on in rosacea? J Eur Acad Dermatol Venereol. 2000;14:351–2. [DOI] [PubMed] [Google Scholar]
- 6.Whitfeld M, Gunasingam N, Leow LJ, et al. Staphylococcus epidermidis: a possible role in the pustules of rosacea. J Am Acad Dermatol. 2011;64(1):49–52. [DOI] [PubMed] [Google Scholar]
- 7.Bonnar E, Eustace P, Powell FC. The Demodex mite population in rosacea. J Am Acad Dermatol. 1993;28(3):443–8. [DOI] [PubMed] [Google Scholar]
- 8.Lacey N, Delaney S, Kavanagh K, Powell FC. Mite-related bacterial antigens stimulate inflammatory cells in rosacea. Br J Dermatol. 2007;157:474–81. [DOI] [PubMed] [Google Scholar]
- 9.Murillo N, Mediannikov O, Aubert J, Raoult D. Bartonella quintana detection from Demodex in erythematotelangiectatic rosacea patients. Int J Infect Dis. 2014;29:176–7. [DOI] [PubMed] [Google Scholar]
- 10.Kong HH. Skin microbiome: genomics-based insights into the diversity and role of skin microbes. Trends Mol Med. 2011;17(6):320–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kong HH, Oh J, Deming C, et al. Temporal shifts in the skin microbiome associated with disease flares and treatment in children with atopic dermatitis. Genome Res. 2012;22:850–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kong HH, Segre JA. Skin microbiome: looking back to move forward. J Investig Dermatol. 2012;132(302):933–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gao Z, Tseng C, Strober BE, et al. Substantial alterations of the cutaneous bacterial biota in psoriatic lesions. PLoS One. 2008;3(7):1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Clauvand C, Jourdain R, Bar-Hen A, et al. Dandruff is associated with disequilibrium in the proportion of the major bacterial and fungal populations colonizing the scalp. PLoS One. 2013;8(3):1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Roghman MC, Lydecker AD, Hittle L, et al. Comparison of the microbiota of older adults living in nursing homes and the community. mSphere. 2017;2(5):e00210–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bromberg JS, Hittle L, Xiong Y, et al. Gut microbiota-dependent modulation of innate immunity and lymph node remodeling affects cardiac allograft outcomes. JCI Insight. 2018;3(19):121045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fadrosh DW, Ma B, Gajer P, et al. An improved dual-indexing approach for multiplexed 16S rRNA gene sequencing on the Illumina MiSeq platform. Microbiome. 2014;2(1):6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Caporaso JG, Kuczynski J, Stombaugh J, et al. QIIME allows analysis of high-throughput community sequencing data. Nat Methods. 2010;7(5):335–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.White JR, Nagarajan N, Pop M. Statistical methods for detecting differentially abundant features in clinical metagenomic samples. PLoS Comput Biol. 2009;5(4):e1000352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Clarke KR. Non-parametric multivariate analyses of changes in community structure. Aust J Ecol. 1993;18(1):117–43. [Google Scholar]
- 21.The Human Microbiome Project Consortium. Structure, function and diversity of the healthy human microbiome. Nature. 2013;486(7402):207–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chao A, Chiu CH, Jost L. Phylogenetic diversity measures and their decomposition: a framework based on Hill numbers. In: Pellens R, Grandcolas P, editors. Biodiversity conservation and phylogenetic systematics: topics in biodiversity and conservation, vol. 14. Cham: Springer; 2016. [Google Scholar]
- 23.Hamandy M, Lozupone C, Knight R. Fast UniFrac: facilitating high-throughput phylogenetic analyses of microbial communities including analysis of pyrosequencing and PhyloChip data. ISME J. 2010;4(1):17–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zaidi AK, Spaunhurst K, Sprockett D, Thomason Y, Mann MW, Fu P, et al. Characterization of the facial microbiome in twins discordant for rosacea. Exp Dermatol. 2018;27(3):295–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Murillo N, Aubert J, Raoult D. Microbiota of Demodex mites from rosacea patients and controls. Microb Pathog. 2014;71–72:37–40. [DOI] [PubMed] [Google Scholar]
- 26.Leyden JJ, McGinley KJ, Mills OH, Kligman AM. Age-related changes in the resident bacterial flora of the human face. J Investig Dermatol. 1975;65(4):379–81. [DOI] [PubMed] [Google Scholar]
- 27.Barnard E, Shi B, Kang D, et al. The balance of metagenomics elements shapes the skin microbiome in acne and health. Sci Rep. 2016;6:39491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Marples RR, Downing DT, Kligman AM. Control of free fatty acids in human surface lipids by Corynebacterium acnes. J Investig Dermatol. 1971;56(2):127–31. [DOI] [PubMed] [Google Scholar]
- 29.Jahns AC, Lundskog B, Dahlberg I, et al. No link between rosacea and Propionibacterium acnes. APMIS. 2012;120:922–5. [DOI] [PubMed] [Google Scholar]
- 30.Rihs JD, Brenner DJ, Weaver RE, et al. Roseomonas, a new genus associated with bacteremia and other human infections. J Clin Microbiol. 1993;31(12):3275–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Romano-Bertrand S, Bourdier A, Aujoulat F, et al. Skin microbiota is the main reservoir of Roseomonas mucosa, an emerging opportunistic pathogen so far assumed to be environmental. Clin Microbiol Infect. 2016;22(737):e1–7. [DOI] [PubMed] [Google Scholar]
- 32.Myles IA, Williams KW, Reckhow JD, et al. Transplantation of human skin microbiota in models of atopic dermatitis. JCI Insight. 2016;1(10):1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Myles IA, Earland NJ, Anderson ED, et al. First-in-human topical microbiome transplantation with Roseomonas mucosa for atopic dermatitis. JCI Insight. 2018;3(9):1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tauch A, Fernandez-Natal I, Soriano F. A microbiological and clinical review on Corynebacterium kroppenstedtii. Int J Infect Dis. 2016;48:33–9. [DOI] [PubMed] [Google Scholar]
- 35.Fujii M, Mizutani Y, Sakuma T, et al. Corynebacterium kroppenstedtii in granulomatous mastitis: analysis of formalin-fixed, paraffin-embedded biopsy specimens by immunostaining using low-specificity bacterial antisera and real-time polymerase chain reaction. Pathol Int. 2018;68(7):409–18. [DOI] [PubMed] [Google Scholar]
- 36.Johnstone KJ, Robson J, Cherian SG, et al. Cystic neutrophilic granulomatous mastitis associated with Corynebacterium including Corynebacterium kroppenstedtii. Pathology. 2017;49(4):405–12. [DOI] [PubMed] [Google Scholar]
- 37.Fernandez-Natal I, Rodriguez-Lazaro D, Marrodan-Ciordia T, et al. Characterization and antimicrobial susceptibility of one antibiotic-sensitive and one multidrug-resistant Corynebacterium kroppenstedtii strain isolated from patients with granulomatous mastitis. New Microbes New Infect. 2016;14:93–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Johnson MG, Leal S, Plongla R, et al. The brief case: recurrent granulomatous mastitis due to Corynebacterium kroppenstedtii. J Clin Microbiol. 2016;54(8):1938–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Riegel P, Leigeois P, Chenard MP, et al. Case report: isolations of Corynebacterium kroppenstedtii from a breast abscess. Int J Med Microbiol. 2004;294(6):413–6. [DOI] [PubMed] [Google Scholar]
- 40.Poojary I, Kurian A, Jayalekshmi VA, et al. Corynebacterium species causing breast abscesses among patients attending a tertiary care hospital in Chennai, South India. Infect Dis (Lond). 2017;49(7):528–31. [DOI] [PubMed] [Google Scholar]
- 41.Goh A, Tan AL, Madhukhumar P, Yong WS. Recurrent Corynebacterium kroppenstedtii breast abscess in a young Asian female. Breast J. 2015;21(4):431–2. [DOI] [PubMed] [Google Scholar]
- 42.Le Fleche-Mateos A, Berthet N, Lomprez F, et al. Case report: recurrent breast abscesses due to Corynebacterium kroppenstedtii, a human pathogen uncommon in Caucasian women. Case Rep Infect Dis. 2012;2012:120968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hagemann JB, Essig A, Herrman M, et al. Early prosthetic valve endocarditis caused by Corynebacterium kroppenstedtii. Int J Med Microbiol. 2015;305:957–9. [DOI] [PubMed] [Google Scholar]
- 44.Ni Raghallaigh S, Bender K, Lacey N, et al. The fatty acid profile of the skin surface lipid layer in papulopustular rosacea. Br J Dermatol. 2012;166(2):279–87. [DOI] [PubMed] [Google Scholar]
- 45.Mak TN, Schmid M, Brzuszkiewicz E, et al. Comparative genomics reveals distinct host-interacting traits of three major human-associated Propionibacteria. BMC Genom. 2013;14:640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Meisel JS, Hannigan GD, Tyldsley AS, SanMiguel AJ, Hodkin-son BP, Zheng Q, et al. Skin microbiome surveys are strongly influenced by experimental design. J Investig Dermatol. 2016;146(5):947–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Chen CJ, Su LH, Lin TY, Huang YC. Molecular analysis of repeated methicillin-resistant Staphyloccocus aureus infections in children. PLoS One. 2010;5(12):e14431. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


