Abstract
The most common adverse drug reaction from statins are statin-associated muscle symptoms (SAMS), such as muscle pain, weakness, and/or elevations in serum creatine kinase levels. All statins are substrates of the organic anion transporter 1B1 (OATP1B1; gene:SLCO1B1), albeit to different degrees. A genetic polymorphism in SLCO1B1, c.521T>C (rs4149056), markedly decreases OATP1B1 function. The literature is currently unclear as to whether SLCO1B1 c.521T>C is significantly associated with discontinuation of atorvastatin specifically due to SAMS. Our hypothesis was that individuals carrying the SLCO1B1 decreased function 521C allele are more likely to discontinue atorvastatin due to SAMS. This was a retrospective analysis of survey data from 379 Caucasians genotyped for rs4149056 and treated with atorvastatin for at least 12 months. Crude and multivariable logistic regression, adjusted for established risk factors for SAMS, determined the association of SLCO1B1 c.521T>C with discontinuation of atorvastatin due to SAMS (SLCO1B1 521T-homozygotes vs 521C-carriers). The sample was 51% male, with a mean age of 57 years (SD=11). Sixty-one percent of participants reported discontinuing atorvastatin due to SAMS, and 32% overall carried the 521C allele. SLCO1B1 521C-carrier status was not a significant predictor of atorvastatin discontinuation in any model: crude OR=1.07, 95% CI: 0.68-1.66 (p=0.78) and adjusted OR=1.07, 95% CI:0.68-1.69, (p=0.76). The results were similar in a sub-group of participants treated with higher doses of atorvastatin (>20 mg). In summary, SLCO1B1 c.521T>C was not significantly associated with discontinuation of atorvastatin therapy due to SAMS.
Keywords: statins, atorvastatin, muscle, adverse drug reaction, genetics, OATP1B1, SLCO1B1, transporter
Introduction
The most common adverse drug reaction from statins are statin-associated muscle symptoms (SAMS), such as muscle pain, cramps, weakness, and/or elevations in serum creatine kinase (CK).[1, 2] All statins are substrates of the organic anion transporter family member 1B1 (OATP1B1; gene: SLCO1B1), albeit to different degrees.[3] A genetic polymorphism in SLCO1B1, c.521T>C (rs4149056), markedly decreases the function of OATP1B1.[4] While evidence from previous studies supports a significant association between rs4149056 and SAMS from simvastatin,[5] the literature is not clear for atorvastatin, which is the most commonly prescribed statin. To date, most of the previous studies on rs4149056 and atorvastatin SAMS are small (n<350) and have conflicting results [5]; resulting meta-analyses of these individually small atorvastatin studies are also conflicting.[6–9]
Another limitation of the previous studies that are specific to atorvastatin is the specific outcome investigated. SAMS are subjective and therefore difficult to define and capture. Many studies included ADRs that are not specific to muscle. The largest study focusing on atorvastatin and rs4149056 (n=721) evaluated any changes in statin therapy, which could have included reasons other than SAMS.[10] Other studies included assessment of SAMS specifically, but they did not determine if the SAMS was the cause of discontinuation of therapy. In clinical practice, many patients will continue statin therapy despite experiencing SAMS. SAMS that cause discontinuation of statin therapy would be the most clinically relevant, as it would lead to the loss of the cardioprotective effects of statin therapy.
In order to fill these gaps in the current literature, herein we report the largest association analysis of rs4149056 with SAMS that led to discontinuation of atorvastatin therapy. Our hypothesis was that participants carrying the SLCO1B1 decreased function 521C allele are more likely to discontinue atorvastatin, specifically due to SAMS (i.e., survey-reported muscle symptoms and/or elevations in CK).
Methods
Multi-Center Statin Study
The methods of the overall study were previously published in detail.[11, 12] Briefly, participants were enrolled from six medical centers across the U.S. and Canada between 2004 and 2013. Individuals were included in this specific analysis if they met all of the following inclusion criteria: 1) had past treatment with atorvastatin; 2) if they were no longer currently taking atorvastatin, then they provided the reasons why; and 3) had genotype data for rs4149056. The primary outcome of this study was the discontinuation of atorvastatin specifically due to SAMS. SAMS were defined as any symptom specific to muscle that was stated by the participant: muscle symptoms (e.g., pain, weakness, cramps) and/or elevated CK levels. The secondary outcome was discontinuation of atorvastatin specifically due to elevated CK levels. The number of medications that could increase or decrease statin exposure and potentially influence the occurrence of SAMS in participants was previously assessed in the overall study.[11] There was no significant difference in the number of medications that could impact statin exposure (i.e. CYP3A4 inducers/inhibitors) between those experiencing SAMS and statin tolerant individuals [11], and thus drug-drug interactions were excluded from this analysis. Questionnaires (provided in the Supplemental Material) were completed at baseline and after 12 months of follow-up. Nurses and research coordinators at each site assisted patients in completing the questionnaires. Genomic DNA was extracted from whole blood or saliva samples, and rs4149056 was genotyped using a TaqMan® assay (C__30633906_10; ThermoFisher Scientific). The study was approved by the Institutional Review Boards at each participating study site, and all participants provided written informed consent prior to participation.
Statistical Analysis
Clinical characteristics were summarized and compared by the primary outcome and SLCO1B1 c.521T>C genotype. Continuous data were summarized by mean ± standard deviation and compared using the student’s t-test. Categorical data were summarized by counts and percentages and compared by the chi-square test (or the Fisher’s exact test as appropriate). Crude and multivariable adjusted logistic regression models were used to test the association of the genotypes, coded as the following: major allele homozygotes (SLCO1B1 T/T) vs minor allele carriers (SLCO1B1 C/T + SLCO1B1 C/C), with the primary outcome, which is discontinuation of atorvastatin due to SAMS or not. Adjusted models included covariates previously shown to be associated with SAMS[11]: family history of heart disease, obesity, hypertension, and smoking. A sub-group analysis of participants on higher doses of atorvastatin (>20 mg) was conducted to evaluate the possibility of a dose-dependent relationship. A chi-square test was used to ensure that genotypes were in Hardy-Weinberg equilibrium. We estimated 80% power to detect an odds ratio ≥1.9 for SLCO1B1 c.521T>C-carrier status. All statistical analyses were completed using SAS v9.4, and p<0.05 was considered statistically significant.
Results
In total, 379 participants met the inclusion criteria. SLCO1B1 genotypes were consistent with Hardy-Weinberg equilibrium (p=0.789). Descriptive characteristics overall and stratified by SLCO1B1 genotypes are presented in Table 1. Sixty-one percent of participants discontinued atorvastatin due to SAMS (n=233), and 32% carried the SLCO1B1 decreased function C allele (n=120). Only one characteristic, family history of heart disease, was significantly different between genotype groups (p=0.043). Table 2 presents the same clinical characteristics overall but stratified by the primary outcome. Only hypertension and inflammatory muscle disease were significantly more prevalent in participants that discontinued atorvastatin due to SAMS (p=0.013 and p=0.036, respectively).
Table 1.
Participant characteristics overall and stratified by SLCO1B1 T/T versus C-carrier genotypes
| Characteristic | All (n = 379) |
SLCO1B1 T/T (n = 259; 68%) |
SCLO1B1 C/T or C/C (n = 120; 32%) |
ap-value |
|---|---|---|---|---|
| Male sex | 193* (51%) | 138 (53%) | 55 (46%) | 0.166 |
| Age started atorvastatin (years) | 56.9 ± 10.8 | 56.9 ± 10.8 | 57.0 ± 10.9 | 0.940 |
| Atorvastatin dose (mg) | 22.3 ± 18.0 | 22.7 ± 18.4 | 21.6 ± 17.2 | 0.588 |
| Coronary artery disease | 77 (20%) | 58 (22%) | 19 (16%) | 0.140 |
| Myocardial infarction | 55 (15%) | 39 (15%) | 16 (13%) | 0.658 |
| Hypertension | 181 (48%) | 119 (46%) | 62 (52%) | 0.300 |
| Smoker | 115 (30%) | 78 (30%) | 37 (31%) | 0.888 |
| Family history of heart disease | 171 (45%) | 126 (49%) | 45 (38%) | 0.043 |
| Hypothyroidism | 42 (11%) | 27 (10%) | 15 (13%) | 0.549 |
| Heavy alcohol consumption | 5 (1.3%) | 4 (1.5%) | 1 (0.8%) | 1.000 |
| Obesity | 64 (17%) | 44 (17%) | 20 (17%) | 0.938 |
| Kidney disease | 9 (2.4%) | 6 (2.3%) | 3 (2.5%) | 1.000 |
| Diabetes | 59 (16%) | 42 (16%) | 17 (14%) | 0.609 |
| Family history of muscle disease | 27 (7.1%) | 14 (5.4%) | 13 (11%) | 0.056 |
| Metabolic muscle disease | 10 (2.6%) | 7 (2.7%) | 3 (2.5%) | 1.000 |
| Inflammatory muscle disease | 26 (6.9%) | 20 (7.7%) | 6 (5%) | 0.330 |
| Liver disease | 10 (2.6%) | 7 (2.7%) | 3 (2.5%) | 1.000 |
| Discontinued atorvastatin due to SAMS | 233 (61%) | 158 (61%) | 75 (63%) | 0.781 |
| Discontinued atorvastatin due to elevated CK | 55 (15%) | 38 (15%) | 17 (14%) | 0.897 |
Continuous variables are presented as mean ± standard deviation and compared between the two SLCO1B1 genotype groups by the student’s t-test. Categorical variables are presented as counts (%) and compared between the two SLCO1B1 genotype groups with the chi-square or Fisher’s exact test where necessary. Bolded values are for p < 0.05.
Sex was undisclosed for one participant.
CK = creatine kinase; SAMS = statin-associated muscle symptoms; SLCO1B1 = solute carrier organic anion transporter family member 1B1
Table 2.
Participant characteristics overall and stratified by whether or not the participant reported discontinuing atorvastatin due to statin-associated muscle symptoms (SAMS)
| Characteristic | All (n = 379) |
Discontinued atorvastatin in the past due to SAMS (n = 233; 61%) |
Did not discontinue atorvastatin in the past due to SAMS (n = 146; 39%) |
ap-value |
|---|---|---|---|---|
| Male sex | 193* (51%) | 124 (53%) | 69 (48%) | 0.287 |
| Age started atorvastatin (years) | 56.9 ± 10.8 | 56.9 ± 10.3 | 56.9 ± 11.7 | 0.938 |
| Atorvastatin dose (mg) | 22.3 ± 18.0 | 20.9 ± 16.8 | 24.3 ± 19.4 | 0.105 |
| Coronary artery disease | 77 (20%) | 48 (21%) | 29 (20%) | 0.862 |
| Myocardial infarction | 55 (15%) | 31 (13%) | 24 (16%) | 0.399 |
| Hypertension | 181 (48%) | 123 (53%) | 58 (40%) | 0.013 |
| Smoker | 115 (30%) | 76 (33%) | 39 (27%) | 0.224 |
| Family history of heart disease | 171 (45%) | 114 (49%) | 57 (39%) | 0.060 |
| Hypothyroidism | 42 (11%) | 30 (13%) | 12 (8.2%) | 0.160 |
| Heavy alcohol consumption | 5 (1.3%) | 4 (1.7%) | 1 (0.7%) | 0.653 |
| Obesity | 64 (17%) | 44 (19%) | 20 (14%) | 0.190 |
| Kidney disease | 9 (2.4%) | 4 (1.7%) | 5 (3.4%) | 0.315 |
| Diabetes | 59 (16%) | 37 (16%) | 22 (15%) | 0.832 |
| Family history of muscle disease | 27 (7.1%) | 17 (7.3%) | 10 (6.9%) | 0.869 |
| Metabolic muscle disease | 10 (2.6%) | 9 (3.9%) | 1 (0.7%) | 0.096 |
| Inflammatory muscle disease | 26 (6.9%) | 21 (9.0%) | 5 (3.4%) | 0.036 |
| Liver disease | 10 (2.6%) | 7 (3.0%) | 3 (2.1%) | 0.747 |
| SLCO1B1 C/C or C/T genotype | 120 (32%) | 75 (32%) | 45 (31%) | 0.821 |
Continuous variables are presented as mean ± standard deviation and compared between the two SLCO1B1 genotype groups by the student’s t-test. Categorical variables are presented as counts (%) and compared between the two SLCO1B1 genotype groups with the chi-square or Fisher’s exact test where necessary. Bolded values are for p < 0.05.
Sex was undisclosed for one participant.
SAMS = statin-associated muscle symptoms; SLCO1B1 = solute carrier organic anion transporter family member 1B1
Crude and multivariable adjusted logistic regression analyses for SLCO1B1 c.521T>C-carrier status were not statistically significant for the primary outcome: crude OR=1.07, 95% CI=0.68-1.66; p=0.781 and adjusted OR=1.07, 95% CI=0.68-1.69; p=0.759; nor the secondary outcome: crude OR=0.96, 95% CI=0.52-1.78, p=0.897 and adjusted OR=0.91, 95% CI=0.49-1.72; p=0.780. Seventy-six participants were treated with higher doses (>20mg) of atorvastatin, of which 43% discontinued atorvastatin due to SAMS. When the analysis was limited to the high-dose sub-group, the results were still not statistically significant: crude OR=1.15, 95% CI=0.44-3.05; p=0.773 and adjusted OR=0.97, 95% CI=0.33-2.85; p=0.957. Figure 1 illustrates the percentage of participants that did or did not discontinue atorvastatin due to SAMS, stratified by SLCO1B1 genotype.
Figure 1.
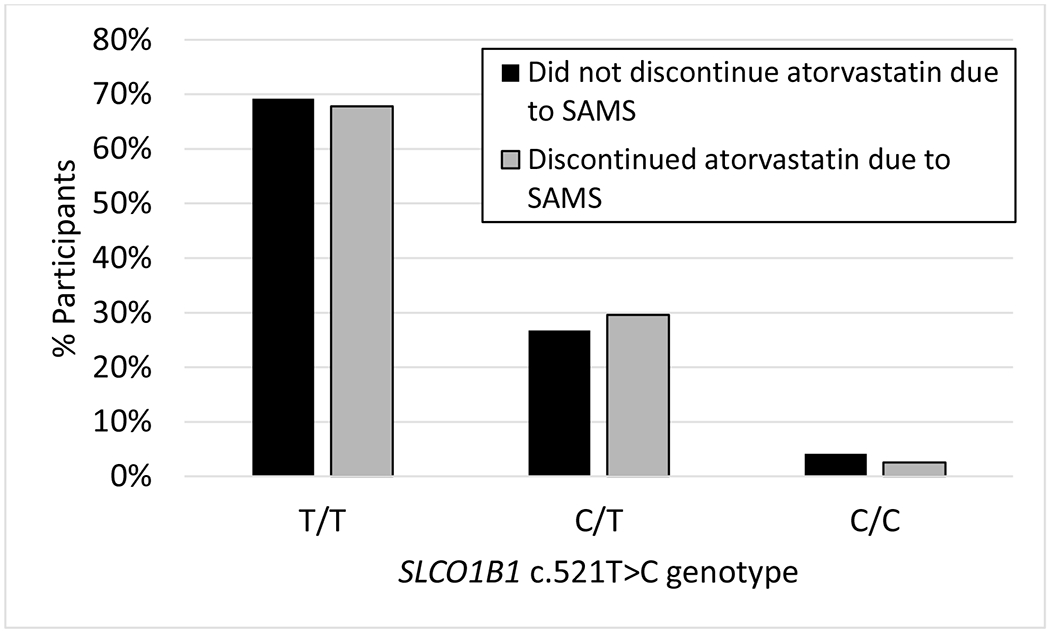
Percentage of participants that discontinued atorvastatin due to SAMS (in gray) compared to those that remained on therapy (in black), stratified by SLCO1B1 c.521T>C genotype..
Discussion
The association between SLCO1B1 c.521T>C and SAMS specifically from atorvastatin is currently unclear. Previous studies are limited by small sample sizes, not analyzing atorvastatin distinctly from other statins, and/or the use of outcomes that are either not specific to SAMS, or the SAMS evaluated were not necessarily sufficient to discontinue atorvastatin in clinical practice. This is the largest study to date that specifically investigates the association between SLCO1B1 c.521T>C and atorvastatin discontinuation due to SAMS. Despite the larger sample size, we still did not detect a significant association, which suggests that SLCO1B1 c.521T>C does not have a strong association with SAMS from atorvastatin.
Our results are consistent with three meta-analyses that did not find a significant association between SLCO1B1 c.521T>C and atorvastatin-associated ADRs.[6–8] These meta-analyses show an association of SLCO1B1 c.521T>C with SAMS from simvastatin, but not from atorvastatin. These and our findings are consistent with in vitro and pharmacokinetic studies demonstrating that OATP1B1 plays a larger role in the disposition of simvastatin than atorvastatin.[13, 14]
It is worth mentioning that our results contrast with a recent meta-analysis and the largest individual study in this area. The meta-analysis of SLCO1B1 c.521T>C and atorvastatin ADRs by Du et al found a significant association between SLCO1B1 c.521T>C and atorvastatin ADRs (total n =1,550; OR=1.57 [95% CI=1.09–2.25] p=0.01).[9] However, the difference between results could possibly be explained by the outcome assessed. Du et al evaluated the presence of any type of ADR, but did not investigate if ADRs specific to muscle were the reason to cause discontinuation of atorvastatin. De Keyser et al published the largest individual study that evaluated changes in atorvastatin therapy associated with SLCO1B1 c.521T>C (n=721).[10] They found a significant association in the sub-group of participants treated with >20 mg atorvastatin. However, restriction of our analysis to participants treated with >20 mg atorvastatin still did not yield a significant association. The different results could be due to a couple different factors. The finding by de Keyser et al may have been by chance. Their sub-group analyses made multiple comparisons (12 different tests), but they did not use methods to control the type I error rate. They had an independent validation dataset, but they did not report the higher dose sub-group analysis in the validation dataset. In addition, the de Keyser et al study defined their outcome as any change in atorvastatin therapy, such as a dose decrease or change to a different stain for any reason, whereas our outcome was specific to SAMS.
Our study has several limitations. This was an observational and retrospective study, and thus we cannot determine the true reason for discontinuation of atorvastatin. Despite being the largest study investigating this specific hypothesis to date, this study is still relatively small. We did not have CK levels; only the patient report of elevated CK levels being a reason for discontinuation. Although using patient-reported outcomes has its advantages, it also subjects our findings to recall bias. The impact of this bias was lessened however as nurses and research coordinators at each site assisted patients in filling out the questionnaires accurately. Our study includes Caucasian participants only, therefore our results may not be generalizable to other populations.
Conclusion
The literature is currently unclear as to whether or not SLCO1B1 c.521T>C is significantly associated with discontinuation of atorvastatin due to SAMS. Our study did not find either a significant association overall or one restricted to a high-dose sub-group.
Supplementary Material
Acknowledgments
Funding: This work was supported by grants from the National Institutes of Health (R01 HL085800 to GDV; R21 AR055704 to PJI; and K08 HL146990 and Loan Repayment Program L30 HL110279 to JAL); the John R. Oishei Foundation, and an Interdisciplinary Research and Creative Activities Award from the University at Buffalo Office of the Vice President for Research (to GDV).
Footnotes
Conflicts of Interest: None declared
References
- 1.Collins R, Reith C, Emberson J, Armitage J, Baigent C, Blackwell L, et al. Interpretation of the evidence for the efficacy and safety of statin therapy. Lancet. 2016;388(10059):2532–61. [DOI] [PubMed] [Google Scholar]
- 2.Cohen JD, Brinton EA, Ito MK, Jacobson TA. Understanding Statin Use in America and Gaps in Patient Education (USAGE): an internet-based survey of 10,138 current and former statin users. Journal of clinical lipidology. 2012;6(3):208–15. [DOI] [PubMed] [Google Scholar]
- 3.Niemi M, Pasanen MK, Neuvonen PJ. Organic anion transporting polypeptide 1B1: a genetically polymorphic transporter of major importance for hepatic drug uptake. Pharmacol Rev. 2011;63(1):157–81. [DOI] [PubMed] [Google Scholar]
- 4.Tirona RG, Leake BF, Merino G, Kim RB. Polymorphisms in OATP-C: identification of multiple allelic variants associated with altered transport activity among European- and African-Americans. J Biol Chem. 2001;276(38):35669–75. [DOI] [PubMed] [Google Scholar]
- 5.Talameh JA, Kitzmiller JP. Pharmacogenetics of Statin-Induced Myopathy: A Focused Review of the Clinical Translation of Pharmacokinetic Genetic Variants. J Pharmacogenomics Pharmacoproteomics. 2014;5(2). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jiang J, Tang Q, Feng J, Dai R, Wang Y, Yang Y, et al. Association between SLCO1B1 −521T>C and −388A>G polymorphisms and risk of statin-induced adverse drug reactions: A meta-analysis. SpringerPlus. 2016;5(1):1368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hou Q, Li S, Li L, Li Y, Sun X, Tian H. Association Between SLCO1B1 Gene T521C Polymorphism and Statin-Related Myopathy Risk: A Meta-Analysis of Case-Control Studies. Medicine (Baltimore). 2015;94(37):e1268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Carr DF, O’Meara H, Jorgensen AL, Campbell J, Hobbs M, McCann G, et al. SLCO1B1 Genetic Variant Associated With Statin-Induced Myopathy: A Proof-of-Concept Study Using the Clinical Practice Research Datalink. Clinical pharmacology and therapeutics. 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Du Y, Wang S, Chen Z, Sun S, Zhao Z, Li X. Association of SLCO1B1 Polymorphisms and Atorvastatin Safety and Efficacy: A Meta-analysis. Current pharmaceutical design. 2018;24(34):4044–50. [DOI] [PubMed] [Google Scholar]
- 10.de Keyser CE, Peters BJ, Becker ML, Visser LE, Uitterlinden AG, Klungel OH, et al. The SLCO1B1 c.521T>C polymorphism is associated with dose decrease or switching during statin therapy in the Rotterdam Study. Pharmacogenet Genomics. 2014;24(1):43–51. [DOI] [PubMed] [Google Scholar]
- 11.Ochs-Balcom HM, Nguyen LM, Ma C, Isackson PJ, Luzum JA, Kitzmiller JP, et al. Clinical features related to statin-associated muscle symptoms. Muscle Nerve. 2019;59(5):537–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Luzum JA, Kitzmiller JP, Isackson PJ, Ma C, Medina MW, Dauki AM, et al. GATM polymorphism associated with the risk for statin-induced myopathy does not replicate in case-control analysis of 715 dyslipidemic individuals. Cell metabolism. 2015;21(4):622–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pasanen MK, Fredrikson H, Neuvonen PJ, Niemi M. Different effects of SLCO1B1 polymorphism on the pharmacokinetics of atorvastatin and rosuvastatin. Clin Pharmacol Ther. 2007;82(6):726–33. [DOI] [PubMed] [Google Scholar]
- 14.Varma MV, Rotter CJ, Chupka J, Whalen KM, Duignan DB, Feng B, et al. pH-sensitive interaction of HMG-CoA reductase inhibitors (statins) with organic anion transporting polypeptide 2B1. Mol Pharm. 2011;8(4):1303–13. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


