Abstract
Background:
Persistent inflammation and incomplete immune recovery among persons with HIV (PHIV) are associated with increased disease risk. We hypothesized that the angiotensin receptor blocker (ARB) losartan would reduce inflammation by mitigating nuclear factor (NF)κB responses and promote T-cell recovery via inhibition of transforming growth factor-beta (TGFβ)-mediated fibrosis.
Methods:
Losartan (100 mg) versus placebo over 12 months was investigated in a randomized (1 : 1) placebo-controlled trial, among PHIV age at least 50 years, receiving antiretroviral therapy (ART), with HIV RNA less than 200 copies/ml and CD4+ cell count 600 cells/μl or less. Inflammation, fibrosis and myocardial biomarkers were measured in blood using ELISA, electrochemiluminescence and immunoturbidimetric methods, and T-cell and monocyte phenotypes were assessed with flow cytometry among a subset of participants. Changes over follow-up in (log-2 transformed) biomarkers and cell phenotypes (untransformed) were compared between losartan and placebo arms using linear mixed models.
Results:
Among 108 PHIV (n = 52 to losartan; n = 56 to placebo), 97% had a month 12 visit. Median age was 57 years and baseline CD4+ cell count was 408 cells/μl. Losartan treatment was not associated with an improvement in interleukin-6 levels, or other blood measures of inflammation, immune activation, fibrosis activity or myocardial function. CD4+ and CD8+ T cells also did not differ by treatment group. Losartan reduced SBP and DBP by 6 and 5mmHg, respectively.
Conclusion:
Among older PHIV with viral suppression, losartan did not improve blood measures of inflammation nor T-cell immune recovery. Losartan treatment is unlikely to reduce inflammation associated comorbidities to a clinically meaningful degree, beyond the benefits from lowering blood pressure.
Keywords: ageing, comorbidities, fibrosis, HIV, immune recovery, inflammation
Introduction
Persons with HIV (PHIV) who are living to older ages with antiretroviral therapy (ART) are at an increased risk for comorbidities such as cardiovascular disease (CVD) and other non-AIDS defining end-organ diseases [1,2]. This increased clinical risk is due, in part, to persistent inflammation and incomplete immune recovery [3,4]. Despite suppression of plasma viremia with ART, ongoing immune activation during HIV disease results in chronic exposure to higher levels of inflammatory cytokines [e.g. interleukin-6 (IL-6)] that contributes to a wide spectrum of disease risk [4–6]. In addition, lower CD4+ cell counts during ART treatment have been associated with an increased risk for comorbid conditions such as CVD, cancer, liver disease, osteoporosis and fractures [3,7].
Although immune recovery after ART initiation is substantial among most PHIV, CD4+ cell count levels often remain lower than for uninfected persons. Approximately 15–20% of PHIV who start ART during advanced disease (e.g. CD4+ cell count <200 cells/μl) will have persistent immune depletion (e.g. CD4+ cell count <500 cells/μl) [8,9]. The pathogenesis of impaired immune recovery despite suppression of plasma viremia involves fibrosis within lymphatic tissues [10]. Collagen deposition within the parafollicular T-cell zone in lymph nodes disrupts the homeostasis of naive and central memory T cells and impairs immune recovery [11]. This pathologic collagen deposition within lymphatic tissues is mediate by transforming growth factor beta (TGFβ1) pathways as a consequence of ongoing immune activation in the context of HIV disease [12].
Losartan, an angiotensin receptor blocker (ARB), has several well established treatment effects beyond blood pressure (BP) lowering that make it a potentially useful candidate treatment to reduce inflammation and improve immune recovery among PHIV. Angiotensin receptor 1 (AT1) activates pro-inflammatory [through nuclear factor-kappa B (NF-κB) pathways] and pro-fibrotic (via TGFβ pathways) [13–15]. ARBs selectively block AT1 with resulting treatment effects that are both anti-inflammatory and antifibrotic [16–18]. For example, ARBs treatment has been shown to reduce inflammatory markers (e.g. IL-6 and C-reactive protein) [17,19]. In the context of HIV disease, ARBs also have potential to reverse tissue fibrosis through a well characterized mechanism of decreasing TGF-ß activity and thereby improve T-cell homeostasis [16,20–22]. In animal models of renal, vascular and cardiac fibrosis, losartan therapy inhibits TGF-ß activity and improves histologic fibrosis [23–25].
We studied the potential benefits of losartan as disease modifying treatment among PHIV, by conducting a randomized placebo-controlled trial of losartan dosed at 100 mg daily. The target population was PHIV at older ages (i.e. age ≥50 years old) given that absolute disease rates of the comorbid conditions that are of primary interest (e.g. CVD) increase with advancing age [26]. In addition, losartan has an excellent safety profile and may also reduce risk for CVD and other comorbidities through its effects on lowering BP [27]. The study hypothesis was that losartan would reduce levels of IL-6 and improve CD4+ T-cell counts among older PHIV taking ART.
Materials and methods
Research setting and target population
Participants were recruited at six HIV clinics within the following healthcare systems: Hennepin Healthcare (Minneapolis, Minnesota, USA); Allina Healthcare (Minneapolis, Minnesota, USA); Mayo Clinic (Rochester, Minnesota, USA); University of California San Francisco (San Francisco, California, USA); Washington DC VAMC (Washington, District of Columbia, USA); NIH Clinical Center (Bethesda, Maryland, USA). The trial protocol was approved by each site’s institutional review board for conduct of human’s research and was registered on ClinicalTrials.gov (NCT02049307). All study participants underwent a verbal and written informed consent process.
Eligibility criteria consisted of PHIV of age at least 50 years who were receiving continuous ART for at least 2 years and had maintained HIV RNA levels less than 200 copies/ml for at least 1 year. Participants also had a CD4+ cell count of 600 cells/μl or less within blood, a SBP at least 110 mmHg and no clinical indication or contraindication to taking an ARB. Participants were also excluded if they had cirrhosis, a rheumatologic disease, invasive cancer within the prior year or had been treated with immune therapy or for hepatitis C within the last six months.
Randomization and study design
We investigated the treatment effects of oral losartan (100 mg once daily) versus placebo in a randomized (1 : 1 allocation) double-blind clinical trial. Study drug (losartan and matched placebo) was provided in tablet form by Merck & Co., Inc. (Kenilworth, New Jersey, USA). After oral and written informed consent and baseline visit procedures, participants were randomized to receive active or placebo study drug. Follow-up visits occurred at months 1, 3, 6, 9 and 12. The dose of 100 mg was chosen to maximize treatment effects, and because it was not different for adverse events when compared with 50 mg in data from 20 clinical trials [27]. If participants had low BP (e.g. systolic <100 mgHg) or side effects attributed to study drug or potentially triggered by low BP, then the study drug was stopped until symptom resolution. Participants were then offered a lower dose of 50 mg once daily for the duration of the study.
The primary outcome was plasma levels of IL-6, with the main secondary outcome of CD4+ cell count in blood. Additional secondary outcomes included blood biomarkers of inflammation, immune activation, coagulation, fibrosis and myocardial function. T-cell homeostasis and recovery among memory subsets were also explored among a subset with peripheral blood mononuclear cells collection at baseline and month 6 and 12. Power for n = 100 participants was 80%, at an alpha = 0.05 and 5% missing data, to detect a 27% relative reduction in IL-6 levels; cohort data suggest this degree of IL-6 reduction would be associated with a 30% lower risk of non-AIDS conditions or death [4]. For the key secondary outcome of CD4+ cell count, power was 80% to detect a 12% difference, which corresponds to an approximate average annual increase of 30–50 cells per year in our target population. Adherence was assessed both subjectively and objectively via pill count among participants who returned study drug. Safety was evaluated through ascertainment of any adverse events of grade 3 or higher, or if it resulted in stopping study drug.
Clinical assessments
CD4+ and CD8+ cell counts, HIV RNA level, metabolic panel, complete blood count and liver enzyme levels were measured at the individual site clinical laboratory. The fibrosis-4 (FIB-4) index was calculated from inputs of age, aspartate aminotransferase (AST), platelet count, alanine aminotransferase (ALT), as a clinically available tool reflecting liver fibrosis (with a lower cutoff <1.45 indicating low risk). Frailty phenotype was also characterized at entry using a validated approach devised by Fried et al. [28]. Nonfrailty, prefrailty and frailty were defined, respectively, by the presence of 0, 1–2 and at least three of the following five frailty criteria: unintentional weight loss, physical inactivity, exhaustion/fatigue, weak grip strength and slow walk. Differential treatment effects from losartan were explored by subgroups defined by FIB-4 and frailty phenotype status at entry.
Research laboratory methods
Participants were fasting for all blood draws. Soluble (s) biomarker levels were measured from batched cryopreserved samples, blinded to treatment group. Inflammation was assessed via levels of high sensitivity IL-6 (electrochemiluminescence; Meso Scale Discovery, Rockville, Maryland, USA) and tumour necrosis factor receptor-1 (TNFr-1; ELISA; R&D Systems, Minneapolis, Minnesota, USA). Monocyte activation was estimated via measures of sCD14 (ELISA; R&D Systems), sCD163 (ELISA; R&D Systems) and neopterin (ELISA; Brahms, Oklahoma City, Oklahoma, USA). Coagulation activity assessed via D-dimer (Sta-R analyser, Liatest D-DI; Diagnostic Stago, Parsippany, New Jersey, USA). Potential tissue fibrosis was indirectly assessed via circulating levels of hyaluronic acid (ELISA; Corgenix, Broomfield, Colorado, USA), which is a main component of the extracellular matrix, beta-crosslaps (electrochemiluminescence, Roche Cobas e411; Roche Diagnostics, Indianapolis, Indiana, USA), a specific marker for the degradation of type 1 collagen and Galectin-3 (ELISA; R&D Systems), a marker of fibrogenesis and tissue repair that also has prognostic value in heart failure [29]. Finally, given the potential cardioprotective effects of losartan, myocardial function and stress was explored via levels of N-terminal pro b-type natriuretic peptide (NTproBNP; electrochemiluminescence; Roche Cobas e411) and ST2 (receptor for interleukin-33; ELISA; Critical Diagnostics Presage, San Diego, California, USA).
Immunophenotyping to identify T-cell memory populations and monocyte subsets was performed for a subset of participants (n = 33), for whom viable peripheral blood mononuclear cells (PBMCs) were cryopreserved at baseline as well as follow-up. Dead cells were identified and excluded from further analysis with LIVE/DEAD Fixable Aqua Dead Cell Stain kit (Invitrogen, Carlsbad, California, USA). PBMCs were stained using fluorescent labelled mAbs against extracellular and intracellular antigens. Samples were acquired on an LSRFortessa cytometer (BD Biosciences, San Jose, California, USA) and analysed with FlowJo 10.4.2 (BD Biosciences).
Statistical methods
Participant characteristics and laboratory measures were summarized by mean (SD) or median [IQR] for continuous variables and proportion (count) for categorical variables. Primary analyses for the treatment effect was intent-to-treat using generalized linear mixed models with log-2 transformed biomarker values as outcomes, adjusted for pretreatment biomarker level. All analyses were conducted using SAS version 9.4 (SAS Institute, Cary, North Carolina, USA) with a two-sided Type I error probability.
Results
Study participants
Figure 1 presents the study design and flow diagram for all screened participants through randomization and follow-up visits. One hundred and fifty-one participants were screened, of which 108 were randomized into the study. The most common reason for exclusion included a screening CD4+ cell count more than 600 cells/μl (n = 17), an HIV RNA level more than 200 copies/ml (n = 5) and SBP less than 110 mmHg (n = 4). All randomized participants completed at least one follow-up study visit on study drug, and 105 (97%) completed a month 12 visit.
Fig. 1.
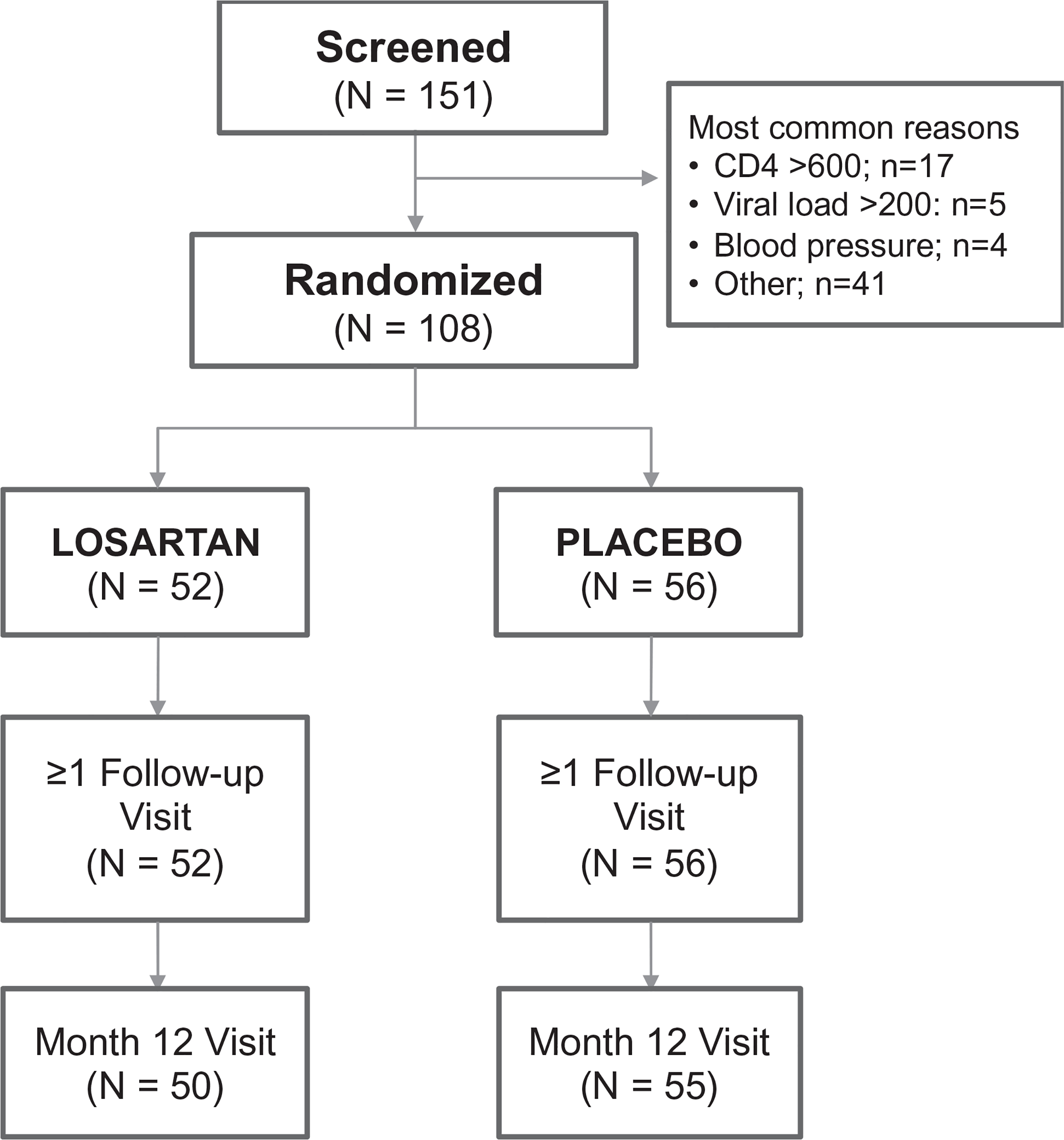
Study design and participant retention through follow-up.
Table 1 presents participant characteristics. Median age was 56 years, with 96% being male sex at birth, 57% white non-Hispanic and 34% African–American. Prevalence of CVD risk factors included 20% current smokers, 20% with hypertension and 26% prescribed lipid lowering therapy. Median (IQR) CD4+ cell count was 439 cells/μl (304–498), and 70% had prior AIDS. Median time since HIV diagnosis was 20 years (12–26), and 76% had viral suppression more than 5 years at study entry. The ART regimen consisted of an integrase strand transfer inhibitor (INSTI) in 49%, a nonnucleoside reverse transcriptase inhibitor (NNRTI) in 39% and a protease inhibitor in 35%. Finally, only four (4%) participants met criteria for frailty phenotype, with 41 (38%) classified as prefrail.
Table 1.
Baseline participant characteristics (n = 108).
| Median [IQR] or % (n) |
|||
|---|---|---|---|
| Losartan (n = 52) | Placebo (n = 56) | Overall (n = 108) | |
| Demographic characteristics | |||
| Age (years) | 56 [53, 62] | 56 [53, 61] | 56 [53, 61] |
| Male sex at birth | 96% (50) | 96% (54) | 96% (104) |
| Race/ethnicity | – | – | – |
| White | 62% (32) | 52% (29) | 57% (61) |
| Black | 29% (15) | 39% (22) | 34% (37) |
| Hispanic or Latino | 8% (4) | 4% (2) | 6% (6) |
| Clinical characteristics | |||
| Smoking, current | 15% (8) | 25% (14) | 20% (22) |
| Hypertension Diagnosis | 23% (12) | 18% (10) | 20% (22) |
| Body mass index (kg/m2) | 26.6 [24.2, 29.7] | 27.2 [23.0, 31.8] | 26.8 [23.6, 30.7] |
| Hepatitis C antibody positive | 16% (8) | 11% (6) | 13% (14) |
| SBP (mmHg) | 126 [122, 136] | 130 [121, 136] | 129 [122, 136] |
| DBP (mmHg) | 82 [73, 86] | 81 [75, 86] | 81 [74, 86] |
| Prescribed lipid lowering therapy | 23% (12) | 29% (16) | 26% (28) |
| Total cholesterol (mg/dl) | 173 [162, 195] | 184 [165, 200] | 182 [163, 198] |
| LDL cholesterol (mg/dl) | 100 [80, 123] | 103 [87, 123] | 102 [84, 123] |
| HDL cholesterol (mg/dl) | 44 [35, 60] | 46 [39, 56] | 45 [37, 57] |
| Serum creatinine (mg/dl) | 1.1 [0.9, 1.2] | 1.1 [1.0, 1.2] | 1.1 [1.0, 1.2] |
| Fibrosis-4 index ≥1.45 | 64% (33) | 48% (27) | 56% (60) |
| Frail phenotype (at least three criteria) | 2% (1) | 6% (3) | 4% (4) |
| Prefrail phenotype (one or two criteria) | 44% (23) | 32% (18) | 38% (41) |
| HIV disease characteristics | |||
| CD4+cell count, nadir (cells/μl) | 121 [46, 226] | 119 [37, 240] | 120 [37, 240] |
| CD4+cell count current (cells/μl) | 451 [300, 496] | 430 [321, 516] | 439 [304, 498] |
| CD8+cell count, current (cells/μl) | 698 [491, 879] | 683 [450, 948] | 683 [472, 906] |
| CD4:CD8 | 0.53 [0.40, 0.91] | 0.65 [0.36, 0.95] | 0.57 [0.39, 0.94] |
| Duration of HIV diagnosis (years) | 20 [7, 25] | 18 [12, 26] | 20 [12, 26] |
| Time since first ART (years) | 16 [7, 21] | 17 [10, 22] | 17 [8, 22] |
| ART includes NNRTI | 35% (18) | 43% (24) | 39% (42) |
| ART includes PI | 31% (16) | 39% (22) | 35% (38) |
| ART includes INSTI | 50% (26) | 48% (27) | 49% (53) |
| Prior AIDS | 71% (37) | 70% (39) | 70% (76) |
| Opportunistic illness | 8% (4) | 20% (11) | 14% (15) |
| CD4+cell count <200 cells/μl | 64% (33) | 50% (28) | 57% (61) |
ART, antiretroviral therapy; HDL, high-density lipoprotein; INSTI, integrase strand transfer inhibitor; LDL, low-density lipoprotein; NNRTI, nonnucleoside reverse transcriptase inhibitor; PI, protease inhibitor.
Inflammation and other blood biomarkers
Median [IQR] IL-6 levels at entry were 1.1 pg/ml [0.7–1.4]. Median levels of all blood biomarkers in losartan and placebo groups separately are reported in Supplemental Table A, http://links.lww.com/QAD/B905. Figure 2 presents the primary intention-to-treat comparisons showing that losartan did not reduce IL-6 levels, nor any of the other plasma biomarkers of inflammation, monocyte activation, fibrosis activity and myocardial function. The effect estimate for losartan versus placebo on IL-6 was 0.6%, with 95% confidence interval (95% CI) of −14.7 to 18.7, and a 99% CI of −19.0 to 25. Sensitivity analyses were conducted restricted to those that maintained HIV viral suppression throughout follow-up or those indicating 100% adherence to study medication at all visits, and results were similar with no treatment effect on any of the blood biomarkers. Finally, approximately one-third (n = 33) study participants had monocyte activation phenotypes characterized, and there was no evidence of a treatment effect on monocyte activation (Supplemental Figure A, http://links.lww.com/QAD/B905).
Fig. 2. Treatment effect of losartan versus placebo over 12 months.
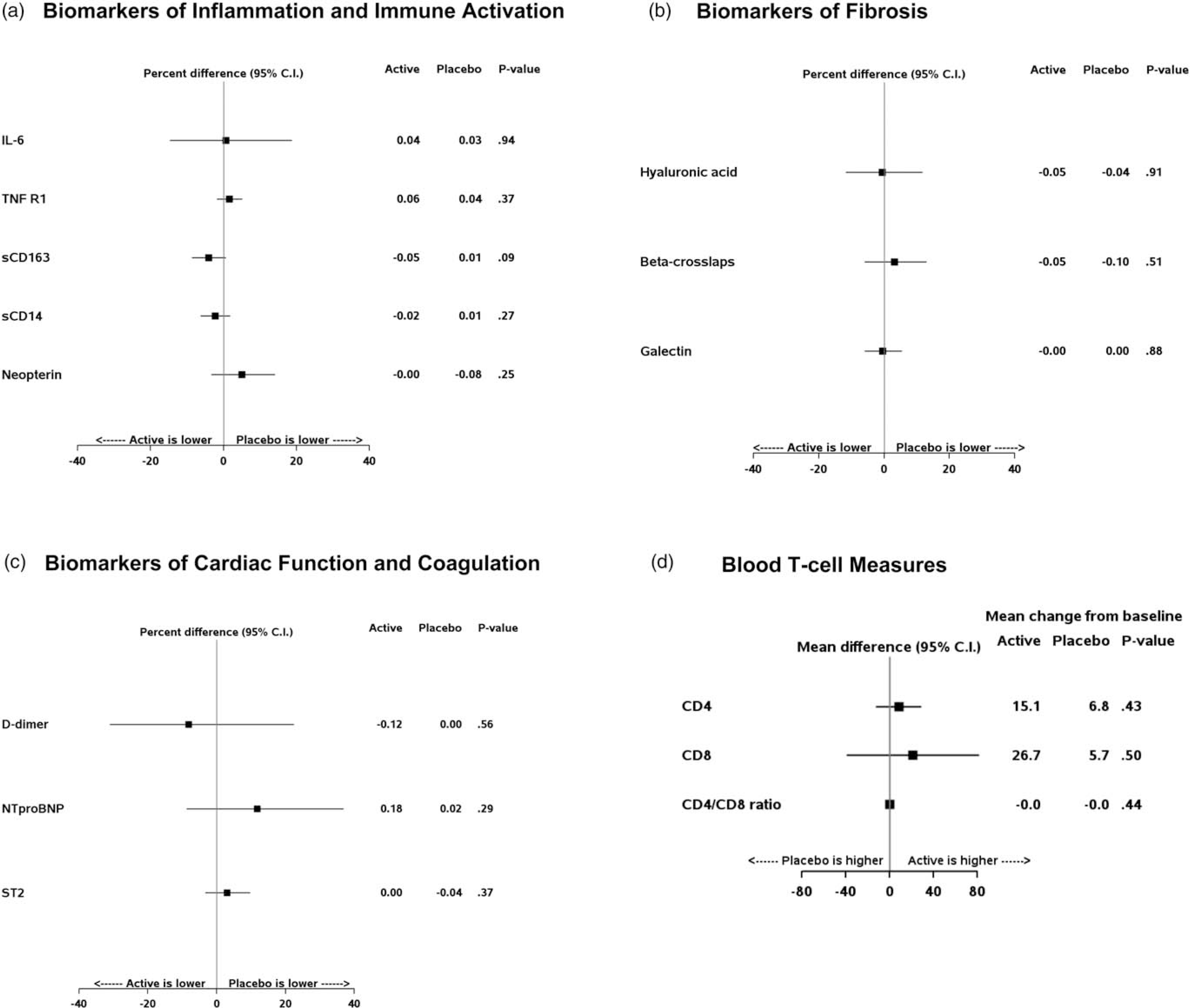
Point estimates reflect the treatment effect over follow-up of losartan versus placebo, with 95% confidence intervals. Plasma biomarkers (a–c) plot a percentage difference for the corresponding marker, whereas T-cell measures (d) plot the absolute mean difference between groups. The mean change from baseline is shown to the right, with plasma biomarkers represented on log-2 scale (a–c) and T-cell measures on the corresponding absolute scale.
Subgroup analyses are shown for IL-6 in Fig. 3, and there was no significant treatment effect within subgroups or evidence of a treatment-subgroup interaction in these factors; similarly, null results were present for subgroups defined by race/ethnicity, duration of HIV diagnosis and ART class (data not shown). Subgroup analyses for the secondary outcome biomarkers reported in Fig. 1 also did not reveal evidence for treatment interactions (data not shown).
Fig. 3. Treatment effect of losartan on IL-6 levels among key subgroups.
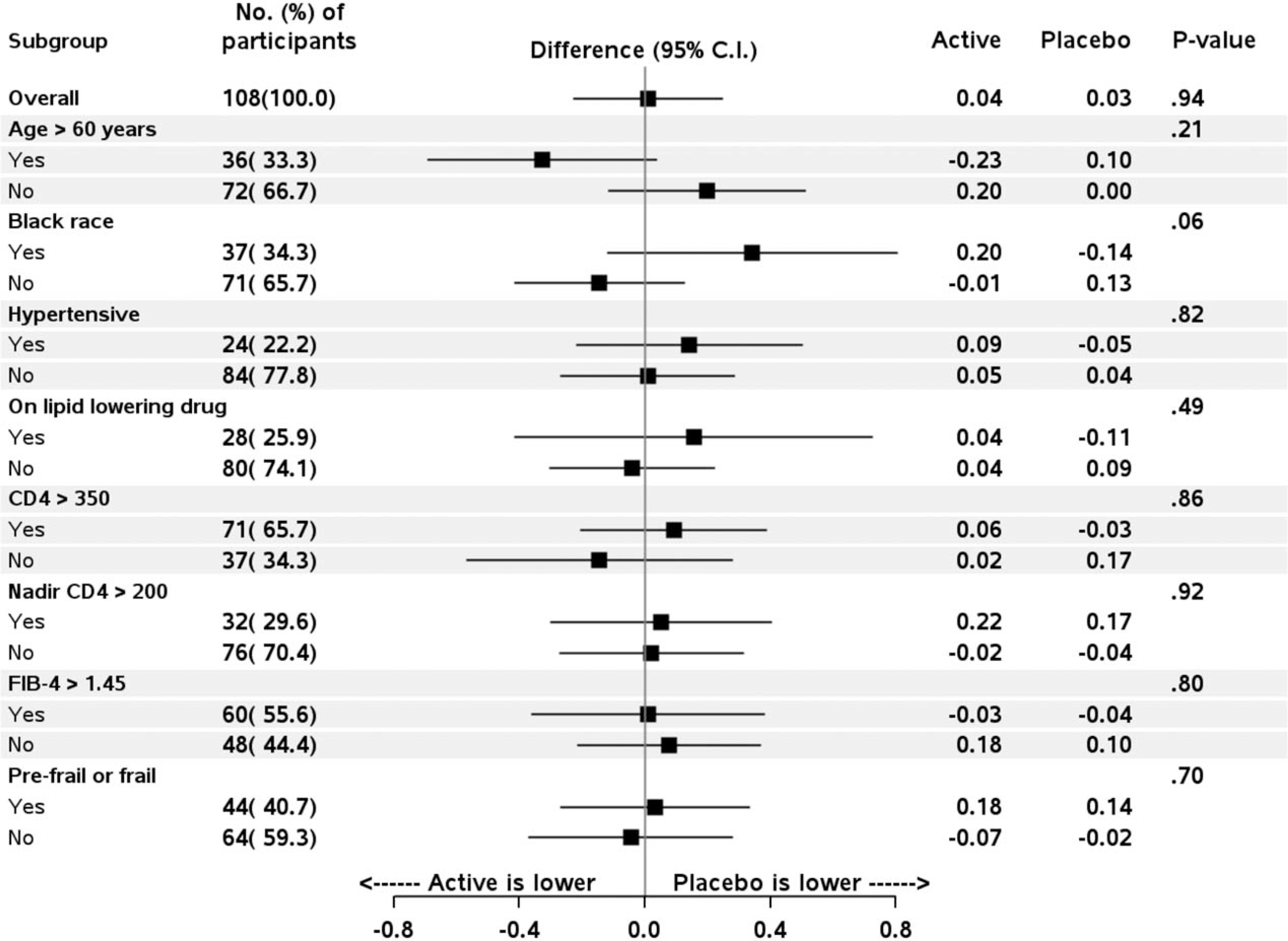
Forrest plots include point estimates reflecting the treatment effect over follow-up of losartan versus placebo, with error bars reflecting 95% confidence intervals. The treatment effect reflects the percentage difference for the study population overall (top), and then for each of the subsequent subgroups defined at study entry. The mean change from baseline over follow-up within active-losartan and placebo groups is shown to the right of the graphs for each of the corresponding measures represented on log-2 scale.
Immune recovery
Median levels of clinical T-cell measures at the baseline are reported in Table 1. Losartan treatment did not improve levels of peripheral blood CD4+ or CD8+ T cells, or the CD4:CD8 ratio. Null findings persisted for key subgroups (as studied in Fig. 3), and when restricted to those that maintained viral suppression or 100% adherence. Among the subset of n = 33 with immunophenotyping, losartan treatment did not change the percentage of CD4+ or CD8+ T-cell memory subsets (i.e. naive, central memory or effector memory; Supplemental Figure B, http://links.lww.com/QAD/B905).
Adherence and adverse events
Among 12 occurrences wherein participants stopped study drug due to possible side effects, one resumed study drug at 100 mg dose, five resumed at lower dose of 50 mg daily and six did not restart study medication. Among the 12 who stopped, two had an indication of low BP and both were in the active losartan group, and one was able to resume study medication at 50 mg daily. Among the participants expected to be taking study drug, the percentage that reported adherence on every study day was 93% during the first 3 months, but then decreased to 66% between months 9 and 12 of follow up (see supplemental Figure C, http://links.lww.com/QAD/B905). Eighty-one percent of dispensed bottles were returned facilitating objective estimate of adherence by pill count. Among this subset, the mean adherence was 97% of days during the first 3 months, decreasing to 87% of study days between months 9 and 12. There were no differences between treatment groups in study drug discontinuation, dose adjustment or subjective or objective adherence assessments. When restricting to those with high adherence (i.e. daily by subjective or >90% by objective measures), there remained no evidence of a treatment effect on IL-6 or any of the outcomes in Fig. 2.
Table 2 presents a summary adverse events and clinical assessments between losartan and placebo groups. Losartan treatment was associated with a small decline in BP and clinically insignificant change in serum creatinine and eGFR. There were more adverse events reported overall in the losartan versus placebo group, with differences not reaching statistical significance. The most frequent types of adverse events were fatigue (n = 5), dizziness (n = 4) and malaise (n = 3).
Table 2.
Clinical monitoring and adverse events.
| Clinical measures | Losartan Mean (SE) overall change | Placebo Mean (SD) overall change | P for diff. |
|---|---|---|---|
| SBP (mmHg) | −7.9 (1.0) | −1.7 (1.0) | <0.001 |
| DBP (mmHg) | −4.8 (0.8) | 0.4 (0.7) | <0.001 |
| Serum creatinine (mg/dl) | 0.05 (0.01) | 0.00 (0.01) | 0.002 |
| Serum potassium (mmol/l) | 0.04 (0.02) | −0.03 (0.02) | 0.03 |
| eGFR (ml/min per 1.73 m2) | −3.03 (0.88) | 0.02 (0.84) | 0.01 |
| Adverse events (AE) | # | # | |
| AE resulting in drug discontinuation | 3 | 4 | 0.82 |
| AE, grade 3 or 4 | 9 | 5 | 0.11 |
| SAE | 6 | 4 | 0.52 |
| Death | 0 | 0 | – |
| Any above AE | 18 | 9 | 0.06 |
AE, adverse event; BP, blood pressure; eGFR, estimated glomerular filtration rate; SAE, serious adverse event.
Discussion
In this randomized placebo-controlled trial, we tested the hypothesis that losartan given at 100 mg daily in addition to ART would reduce systemic inflammation and improve immune recovery. In our study population, losartan treatment was not associated with reductions in blood measures of IL-6 or other measures of inflammation, immune activation and fibrotic activity. There was also no evidence of a treatment effect on immune recovery either by total CD4+ cell count or within memory subsets. Losartan was well tolerated overall, but potentially associated with more adverse events in this study population that were attributable to low BPs.
Treatment with angiotensin-converting enzyme inhibition (ACE) or ARB has demonstrated anti-inflammatory effects via mechanisms both dependent and independent from mitigating angiotensin-2 effects on AT1 [19,30]. Additional mechanisms specific to ARB that may be unrelated to AT1 receptor blockade, include the unopposed stimulation of AT2 receptor activity and/or by reducing innate immune responses in circulating monocytes more broadly [e.g. as a response to bacterial lipopolysaccharide (LPS)] [19,30]. Numerous studies from the general population have demonstrated reductions in circulating cytokines and inflammatory mediators (e.g. CRP, IL-6, TNF-α) with ACE or ARB treatments, though many of these were among patients with additional risk factors or comorbidities (e.g. hypertension, metabolic syndrome, coronary artery disease, heart failure and so on). In a proof-of-concept trial of n = 34 PHIV, we previously showed a reduction in CRP and TNF-α levels from lisinopril versus placebo treatment [31].
Our current findings failed to demonstrate an anti-inflammatory effect of losartan in the setting of treated HIV disease. Recently, another well powered placebo-controlled randomized trial of losartan among older persons (age ≥70 years) in the general population also failed to demonstrate reductions in IL-6 levels [32]. Reasons for inconsistent findings when compared with prior studies may be related to differences between individual medications (i.e. lack of consistent ‘class effect’), and/or, importantly, to differences between the target populations being studied. Drivers of inflammation among persons with hypertension or cardiometabolic risk factors may be more directly related to pathways modulated by angiotensin-2, and thus, more responsive to ARB treatment. Whereas, losartan treatment effects may not sufficiently mitigate mechanisms driving persistent inflammation during ART-treated HIV disease, such as the persistence of HIV-specific immune responses, injury to mucosal effector sites with associated increase in microbial translocation and/or loss of immunologic control over other chronic copathogens (e.g. cytomegalovirus) [5,33–35]. Finally, IL-6 levels among PHIV in our study were low or modest overall, which likely diminishes the potential for detecting a meaningful effect.
The potential for losartan and other ARB to reduce fibrosis within tissues has been described in multiple end-organ diseases, such as renal interstitial fibrosis, myocardial fibrosis and aortic root dilation in Marfan’s syndrome [36,37]. The mechanism of fibrosis attenuation or reversal in these settings is largely attributed to losartan mitigating TGF-β signalling. However, two recently conducted trials among PHIV have failed to demonstrate an antifibrotic effect of ARB or ACE treatment within lymphatic tissues [38,39]. In a randomized trial of telmisartan versus placebo among PHIV with viral suppression (n = 44), there was no difference between groups in lymph node (LN) or adipose tissue collagen deposition over 1 year [38]. Similarly, lisinopril failed to demonstrate a reduction within gut associated lymphatic tissue (via rectal biopsy; n = 30), when compared with placebo [39]. In contrast, using an SIV nonhuman primate model, the potent antifibrotic drug pirfenidone was shown to mitigate LN fibrosis and improved recovery of CD4+ T-cell populations in blood [40]. Potential reasons for the lack of antifibrotic effects of ARB/ACE within lymphatic tissues in HIV studies, in part, may relate to mechanisms that are independent of TGF-β signalling and/or the degree of AT-1 blockade may be insufficient to overcome HIV-specific drivers of fibrosis. Finally, it is also worth emphasizing the inherent limitations related to the smaller samples sizes of studies evaluating fibrosis at the level of end-organ tissues. An earlier study of losartan in n = 20 patients with nonobstructive hypertrophic cardiomyopathy suggested an attenuation in myocardial fibrosis and hypertrophy by cardiac magnetic resonance, but a larger follow-up study of n = 318 patients with hypertrophic cardiomyopathy then failed to demonstrate these same effects [41,42].
This study has several limitations. The sample size remains modest for detecting smaller treatment effects, and results may also not be generalizable across sex given the very small number of women. In addition, losartan could have inflammation and immune effects on pathways not assessed, or that are only present among those with higher levels of ongoing inflammation. Specifically, treatment effects within tissues may not be detected in blood but could have important long-term implications. Evaluation of fibrosis and T-cell homeostasis with lymphatic tissues are planned among a subset of participants that underwent lymph node biopsies in this trial, and will provide additional context for these findings. Despite these limitations, our findings suggest that losartan is unlikely to have a meaningful impact on inflammatory markers among PHIV. The 95% CI of our estimated treatment difference for losartan versus placebo supports that we can rule out an effect of lowering IL-6 by at least 15% in this population. We have previously shown that an IL-6 decline less than 15% among PHIV would predict a modest reduction in risk (i.e. <17%) for serious non-AIDS events or mortality [4].
In summary, losartan treatment given in addition to ART among older persons with longstanding HIV disease did not improve blood measures of inflammation, fibrotic activity or T-cell immune recovery. These results suggest that losartan is unlikely to reduce inflammation associated end-organ complications among PHIV, beyond the established CVD risk reduction associated with lowering BP. Additional strategies to reduce inflammation and improve T-cell recovery are needed to improve the health of people living with HIV.
Supplementary Material
Acknowledgements
We would like to thank all the study participants for their commitment and support of the project.
This study was funded by the National Aging Institute (NIA/NIH: R01 AG045032). Study drug was provided by Merck Pharmaceuticals. The work of I.S., C.M. and H.M. was supported by the intramural research programme of NIAID/NIH.
Footnotes
Conflicts of interest
The authors have no conflicts of interest to disclose.
References
- 1.Mocroft A, Brettle R, Kirk O, Blaxhult A, Parkin JM, Antunes F, et al. Changes in the cause of death among HIV positive subjects across Europe: results from the EuroSIDA study. AIDS 2002; 16:1663–1671. [DOI] [PubMed] [Google Scholar]
- 2.Legarth RA, Ahlstrom MG, Kronborg G, Larsen CS, Pedersen C, Pedersen G, et al. Long-term mortality in HIV-infected individuals 50 years or older: a nationwide, population-based cohort study. J Acquir Immune Defic Syndr 2016; 71:213–218. [DOI] [PubMed] [Google Scholar]
- 3.Baker JV, Peng G, Rapkin J, Abrams Dl, Silverberg MJ, MacArthur RD, et al. CD4+ count and risk of non-AIDS diseases following initial treatment for HIV infection. AIDS 2008; 22:841–848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Grund B, Baker JV, Deeks SG, Wolfson J, Wentworth D, Cozzi-Lepri A, et al. Relevance of interleukin-6 and D-dimer for serious non-AIDS morbidity and death among HIV-positive adults on suppressive antiretroviral therapy. PLoS One 2016; 11:e0155100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Neuhaus J, Jacobs DR Jr, Baker JV, Calmy A, Duprez D, La Rosa A, et al. Markers of inflammation, coagulation, and renal function are elevated in adults with HIV infection. J Infect Dis 2010; 201:1788–1 795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tenorio AR, Zheng Y, Bosch RJ, Krishnan S, Rodriguez B, Hunt PW, et al. Soluble markers of inflammation and coagulation but not T-cell activation predict non-AIDS-defining morbid events during suppressive antiretroviral treatment. J Infect Dis 2014; 210:1248–1259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Achhra AC, Amin J, Law MG, Emery S, Gerstoft J, Gordin FM, et al. Immunodeficiency and the risk of serious clinical endpoints in a well studied cohort of treated HIV-infected patients. AIDS 2010; 24:1877–1886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Baker JV, Peng G, Rapkin J, Krason D, Reilly C, Cavert WP, et al. Poor initial CD4+ recovery with antiretroviral therapy prolongs immune depletion and increases risk for AIDS and non-AIDS diseases. J Acquir Immune Defic Syndr 2008; 48:541–546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kelley CF, Kitchen CM, Hunt PW, Rodriguez B, Hecht FM, Kitahata M, et al. Incomplete peripheral CD4+ cell count restoration in HIV-infected patients receiving long-term antiretroviral treatment. Clin Infect Dis 2009; 48:787–794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schacker TW, Nguyen PL, Beilman GJ, Wolinsky S, Larson M, Reilly C, Haase AT. Collagen deposition in HIV-1 infected lymphatic tissues and T cell homeostasis. J Clin Invest 2002; 110:1133–1139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schacker TW, Brenchley JM, Beilman GJ, Reilly C, Pambuccian SE, Taylor J, et al. Lymphatic tissue fibrosis is associated with reduced numbers of naive CD4+ T cells in human immunodeficiency virus type 1 infection. Clin Vaccine Immunol 2006; 13:556–560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zeng M, Smith AJ, Wietgrefe SW, Southern PJ, Schacker TW, Reilly CS, et al. Cumulative mechanisms of lymphoid tissue fibrosis and T cell depletion in HIV-1 and SIV infections. J Clin Invest 2011; 121:998–1008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kranzhofer R, Browatzki M, Schmidt J, Kubler W. Angiotensin II activates the proinflammatory transcription factor nuclear factor-kappaB in human monocytes. Biochem Biophys Res Commun 1999; 257:826–8281 [DOI] [PubMed] [Google Scholar]
- 14.Ruiz-Ortega M, Lorenzo O, Ruperez M, Konig S, Wittig B, Egido J. Angiotensin II activates nuclear transcription factor kappaB through AT(1) and AT(2) in vascular smooth muscle cells: molecular mechanisms. Circ Res 2000; 86:1266–1272. [DOI] [PubMed] [Google Scholar]
- 15.Ruster C, Wolf G. Angiotensin II as a morphogenic cytokine stimulating renal fibrogenesis. J Am Soc Nephrol 2011; 22:1189–1199. [DOI] [PubMed] [Google Scholar]
- 16.Yoshiji H, Kuriyama S, Fukui H. Blockade of renin-angiotensin system in antifibrotic therapy. J Gastroenterol Hepatol 2007; 22 Suppl 1:S93–S95. [DOI] [PubMed] [Google Scholar]
- 17.Tsutamoto T, Wada A, Maeda K, Mabuchi N, Hayashi M, Tsutsui T, et al. Angiotensin II type 1 receptor antagonist decreases plasma levels of tumor necrosis factor alpha, interleukin-6 and soluble adhesion molecules in patients with chronic heart failure. J Am Coll Cardiol 2000; 35:714–721. [DOI] [PubMed] [Google Scholar]
- 18.el-Agroudy AE, Hassan NA, Foda MA, Ismail AM, el-Sawy EA, Mousa O, Ghoneim MA. Effect of angiotensin II receptor blocker on plasma levels of TGF-beta 1 and interstitial fibrosis in hypertensive kidney transplant patients. Am J Nephrol 2003; 23:300–306. [DOI] [PubMed] [Google Scholar]
- 19.Dandona P, Dhindsa S, Chanim H, Chaudhuri A. Angiotensin II and inflammation: the effect of angiotensin-converting enzyme inhibition and angiotensin II receptor blockade. J Hum Hypertens 2007; 21:20–27. [DOI] [PubMed] [Google Scholar]
- 20.Ruperez M, Lorenzo O, Blanco-Colio LM, Esteban V, Egido J, Ruiz-Ortega M. Connective tissue growth factor is a mediator of angiotensin II-induced fibrosis. Circulation 2003; 108:1499–1505. [DOI] [PubMed] [Google Scholar]
- 21.Wu M, Peng Z, Zu C, et al. Losartan attenuates myocardial endothelial-to-mesenchymal transition in spontaneous hypertensive rats via inhibiting TGF-beta/Smad signaling. PLoS One 2016; 11:e01 55730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gomez-Garre D, Martin-Ventura JL, Granados R, Sancho T, Torres R, Ruano M, et al. Losartan improves resistance artery lesions and prevents CTGF and TGF-beta production in mild hypertensive patients. Kidney Int 2006; 69:1237–1244. [DOI] [PubMed] [Google Scholar]
- 23.Boffa JJ, Lu Y, Placier S, Stefanski A, Dussaule JC, Chatziantoniou C. Regression of renal vascular and glomerular fibrosis: role of angiotensin II receptor antagonism and matrix metalloproteinases. J Am Soc Nephrol 2003; 14:1132–1144. [DOI] [PubMed] [Google Scholar]
- 24.Lim DS, Lutucuta S, Bachireddy P, Youker K, Evans A, Entman M, et al. Angiotensin II blockade reverses myocardial fibrosis in a transgenic mouse model of human hypertrophic cardiomyopathy. Circulation 2001; 103:789–791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Miguel-Carrasco JL, Beaumont J, San Jose G, Moreno MU, López B, González A, et al. Mechanisms underlying the cardiac antifibrotic effects of losartan metabolites. Sci Rep 2017; 7:41865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Miller CJ, Baker JV, Bormann AM, Erlandson KM, Huppler Hullsiek K, Justice AC, et al. Adjudicated morbidity and mortality outcomes by age among individuals with HIV infection on suppressive antiretroviral therapy. PLoS One 2014; 9:e95061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Weber M. Clinical safety and tolerability of losartan. Clin Ther 1997; 19:604–61 6discussion 3. [DOI] [PubMed] [Google Scholar]
- 28.Fried LP, Tangen CM, Walston J, Newman AB, Hirsch C, Gottdiener J, et al. Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci 2001; 56:M146–M156.. [DOI] [PubMed] [Google Scholar]
- 29.Lok DJ, Van Der Meer P, de la Porte PW, Lipsic E, Van Wijngaarden J, Hillege HL, van Veldhuisen DJ. Prognostic value of galectin-3, a novel marker of fibrosis, in patients with chronic heart failure: data from the DEAL-HF study. Clin Res Cardiol 2010; 99:323–328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Larrayoz IM, Pang T, Benicky J, Pavel J, Sanchez-Lemus E, Saavedra JM. Candesartan reduces the innate immune response to lipopolysaccharide in human monocytes. J Hypertens 2009; 27:2365–2376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Baker JV, Huppler Hullsiek K, Prosser R, Duprez D, Grimm R, Tracy RP, et al. Angiotensin converting enzyme inhibitor and HMG-CoA reductase inhibitor as adjunct treatment for persons with HIV infection: a feasibility randomized trial. PLoS One 2012; 7:e46894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pahor M, Anton SD, Beavers DP, Cauley JA, Fielding RA, Kritchevsky SB, et al. Effect of losartan and fish oil on plasma IL-6 and mobility in older persons. The ENRGISE pilot randomized clinical trial. J Gerontol A Biol Sci Med Sci 2019; 74:1612–1619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Brenchley JM, Price DA, Schacker TW, Asher TE, Silvestri G, Rao S, et al. Microbial translocation is a cause of systemic immune activation in chronic HIV infection. Nat Med 2006; 12:1365–1371. [DOI] [PubMed] [Google Scholar]
- 34.Freeman ML, Mudd JC, Shive CL, Younes SA, Panigrahi S, Sieg SF, et al. CD8 T-cell expansion and inflammation linked to CMV coinfection in ART-treated HIV infection. Clin Infect Dis 2016; 62:392–396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hatano H, Jain V, Hunt PW, et al. Cell-based measures of viral persistence are associated with immune activation and programmed cell death protein 1 (PD-l)-expressing CD4+ T cells. J Infect Dis 2013; 208:50–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Brooke BS, Habashi JP, Judge DP, Patel N, Loeys B, Dietz HC 3rd. Angiotensin II blockade and aortic-root dilation in Marfan's syndrome. N Engl J Med 2008; 358:2787–2795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Shibasaki Y, Nishiue T, Masaki H, Tamura K, Matsumoto N, Mori Y, et al. Impact of the angiotensin II receptor antagonist, losartan, on myocardial fibrosis in patients with end-stage renal disease: assessment by ultrasonic integrated backscatter and biochemical markers. Hypertens Res 2005; 28:787–795. [DOI] [PubMed] [Google Scholar]
- 38.Utay NS, Kitch DW, Yeh E, Fichtenbaum CJ, Lederman MM, Estes JD, et al. Telmisartan therapy does not improve lymph node or adipose tissue fibrosis more than continued antiretroviral therapy alone. J Infect Dis 2018; 217:1770–1781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cockerham LR, Yukl SA, Harvill K, Somsouk M, Joshi SK, Sinclair E, et al. A randomized controlled trial of lisinopril to decrease lymphoid fibrosis in antiretroviral-treated, HIV-infected individuals. Pathogens Immun 2017; 2:310–334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Estes JD, Reilly C, Trubey CM, Fletcher CV, Cory TJ, Piatak M, et al. Antifibrotic therapy in simian immunodeficiency virus infection preserves CD4+ T-cell populations and improves immune reconstitution with antiretroviral therapy. J Infect Dis 2015; 211:744–754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Shimada YJ, Passeri JJ, Baggish AL, O'Callaghan C, Lowry PA, Yannekis G, et al. Effects of losartan on left ventricular hypertrophy and fibrosis in patients with nonobstructive hypertrophic cardiomyopathy. JACC Heart Fail 2013; 1:480–487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Axelsson A, Iversen K, Vejlstrup N, Ho C, Norsk J, Langhoff L, et al. Efficacy and safety of the angiotensin II receptor blocker losartan for hypertrophic cardiomyopathy: the INHERIT randomised, double-blind, placebo-controlled trial. Lancet Diabets Endocrinol 2015; 3:123–1 31. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


