Abstract
Background:
Blood loss associated with surgical interventions can lead to several complications. Therefore, minimizing perioperative bleeding is critical to improve overall survival. Several interventions have been found to successfully reduce surgical bleeding, including the antifibrinolytic agent. After aprotinin was withdrawn from the market in 2008, TXA remained the most commonly used medication. The safety and efficacy of TXA has been well studied in other specialties. TXA has been rarely used in plastic surgery, except in craniofacial procedures. Since the last review, the number of articles examining the use of TXA has doubled; so the aim of this systematic review is to update the readers on the current knowledge and clinical recommendations regarding the efficacy of TXA in plastic surgical procedures.
Methods:
A systematic literature search was conducted in Medline, SciELO, Cochrane, and Google Scholar to evaluate all articles that discussed the use of TXA in plastic surgery in the fields of aesthetic surgery, burn care, and reconstructive microsurgery.
Results:
A total of 233 publications were identified using the search criteria defined above. After examination of titles and abstracts, and exclusion of duplicates, a total of 23 articles were selected for analysis.
Conclusions:
The literature shows a clear benefit of using TXA to decrease blood loss regardless of the administration route, with no risk of thrombosis events. Also, TXA elicits a potent anti-inflammatory response with a decrease in postoperative edema and ecchymosis, which improves recovery time. Further investigations are needed to standardize the optimal administration route and dosage of TXA.
INTRODUCTION
Blood loss associated with surgical interventions can lead to several complications such as hematoma, anemia, and, ultimately, the need for allogeneic transfusion. Although using blood products is an effective method to restore patient hemodynamic parameters and can be a life-saving measure, it is associated with several infective and noninfective complications, increasing morbidity and mortality.1,2 Therefore, minimizing perioperative bleeding is critical to reduce complications and improve overall survival.
Several interventions have been found to successfully reduce surgical bleeding.3,4 Among these interventions, a number of pharmacological agents have been used, including the antifibrinolytic agent tranexamic acid (TXA), E-aminocaproic acid, and aprotinin, which prevents fibrinolysis and increases clot stability.4
After aprotinin was withdrawn from the market in 2008 due to an increased risk in mortality and major cardiac and renal complications, TXA remained the most commonly used medication.5
TXA is a synthetic derivative of lysine that reversibly blocks the binding sites of plasminogen, thus preventing activation of plasmin and the enzymatic degradation of fibrin clot.6 Furthermore, TXA may exert an anti-inflammatory effect by blocking plasmin activation of the complement cascade and other mechanisms.7,8
The safety and efficacy of TXA has been well studied in cardiac,9 orthopedic,10 and other specialties.11 Multiple trials in elective surgical patients showed that TXA reduces the probability of receiving a blood transfusion by about one third, and the volume of blood transfused by about 1 unit, with no risk of thromboembolic or other major events.12 Also, TXA can safely reduce the risk of death in bleeding trauma patients and head-injury-related deaths after a traumatic brain injury.13
TXA has been rarely used in plastic surgery, except in craniofacial procedures.14,15 Since the last review by Rohrich et al,16 the number of articles examining the use of TXA has doubled; so the aim of this systematic review is to update the readers on the current knowledge and clinical recommendations regarding the efficacy of TXA in plastic surgical procedures.
METHOD
A systematic review was conducted to evaluate the current evidence regarding the use of TXA in Plastic Surgery. A literature search was conducted in online databases Medline, SciELO, Cochrane, and Google Scholar, for all articles on the topic published up to and including July 2020. The structured search strategy used the following terms: “TXA,” “tranexamic acid,” “plastic surgery,” “aesthetic surgery,” “rhinoplasty,” “nasal surgery,” “breast surgery,” “blepharoplasty,” “body contouring,” “liposuction,” “burn,” “microsurgery,” and “face lift.”
All randomized and nonrandomized controlled trails, retrospective cohort studies, and case reports or series involving human participants that discussed the use of TXA in plastic surgery in the fields of aesthetic surgery, burn care, and reconstructive microsurgery were included. Both English and Spanish language publications were included.
Studies not carried out in humans and studies centered on the use of TXA in other specialties were excluded, as were review articles, editorials, discussions, commentaries, and letter or viewpoints. Pediatric surgery and cranio-maxilo surgery articles were also excluded.
Studies that met inclusion and exclusion criteria were separated for full reading, critical appraisal, and data collection. The bibliographic references of the captured articles were examined to search for additional relevant citations.
Data collected for each study were as follows: author and publication year, design of the study, procedure types, dose regimen and mode of administration, main outcome data collected, and principal results recorded.
RESULTS
A total of 233 publications were identified using the search criteria defined above. After examination of titles and abstracts and exclusion of duplicates, a total of 23 articles were selected for in-depth reading and analysis. Figure 1 shows study selection through the processes of identification, screening, and eligibility, according to the Preferred Reporting Items for Systematic Reviews and Meta-analysis flow diagram (Fig. 1) (Tables 1–7).
Fig. 1.
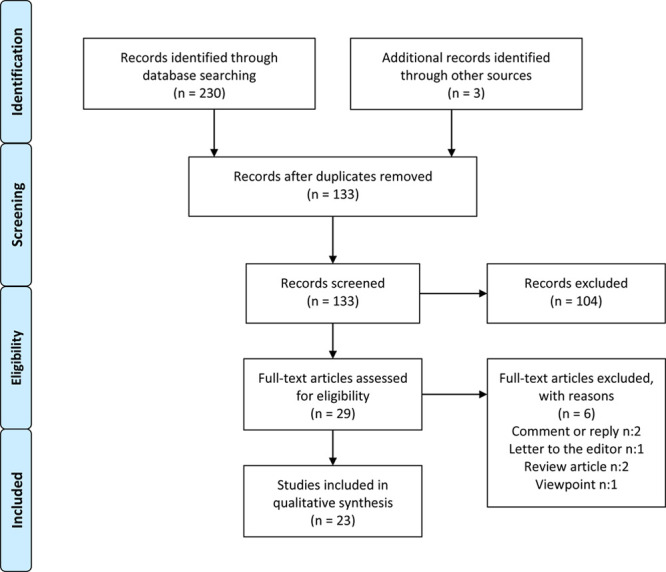
Preferred Reporting Items for Systematic Reviews and Meta-analysis flow diagram, literature search, and selection process.
Table 1.
TXA in Rhinoplasty
| Authors | Journal and Year | Study Design | Study Group (Total No.) | Surgery Type | Administration Mode and Dosage | Main Clinical Outcome | Main Results | Complications Related to TXA |
|---|---|---|---|---|---|---|---|---|
| Sakallioglu et al | Annals of Plastic Surgery 2015 | RCT | 25 (75)No significant difference among the 3 groups: control, TXA, Cs | Open septorhinoplasty | ORAL: 1 g starting 2 h before surgery, 3 g daily in divided doses (1 g, every 8 h) for 5 days | Operation timeIntraoperative bleedingPostoperative eyelid edema and periorbital ecchymosisCompare result with corticosteroids | Decrease in intraoperative bleeding compared with control and corticosteroid group (P < 0.05)Decrease in periorbital edema and ecchymosis scores compared with control group (P < 0.05)No significant difference in decreasing both periorbital edema and ecchymosis between TXA and corticosteroid | None |
| Mehdizadeh et al | Aesthetic Plastic Surgery Journal 2017 | RCT | 30 (60)No significant difference among the 4 groups: TXA, control, Cs, TXA + Cs | Primary open rhinoplasty | IV: 10 mg/kg IV 1 h before and 3 doses every 8 h after surgery | Postoperative eyelid edema and periorbital ecchymosis | Decrease in periorbital edema and ecchymosis scores compared with the control group (P < 0.01).No significant difference between TXA and Corticosteroid separately or together | None |
| Eftekharian et al | The Journal of Craniofacial Surgery 2016 | RCT | 25 (50)No significant difference between the 2 groups: control, TXA | Rhinoplasty (no specifications) | ORAL: 1 g (two 500-mg tablets) TXA orally 2 h before surgery | Intraoperative bleeding | Decrease in total blood loss (P = 0.005). Lower mean surgery duration (P = 0.017) | None |
| Beikaei et al | Biomedical & Pharmacology Journal 2015 | RCT | 48 (96)2 groups: control, TXA | Open rhinoplasty | IV: 10 mg/kg TXA during induction | Intraoperative bleeding | TXA was associated with a 15.6-mL decrease in intraoperative bleeding (P < 0.001) | None |
| Ghavimi et al | Journal of Cranio-Maxillofacial Surgery 2017 | RCT | 24 (50)No significant difference between the 2 groups: control, TXA | Closed rhinoplasty | IV: 10 mg/kg TXA before surgery | Intraoperative bleedingPostoperative eyelid edema and periorbital ecchymosis | Decrease in the mean of intraoperative bleeding (P = 0.013)Decrease in eyelid edema and periorbital ecchymosis (P = 0.03) | None |
| de Vasconcellos et al | JAMA Otolaryngology– Head & Neck Surgery 2018 | Meta-analysis | 5 RCT, including 276 patientsSignificant heterogeneity among studies (I2 = 84%) | Rhinoplasty | IV and ORAL | Intraoperative bleedingPostoperative eyelid edema and periorbital ecchymosis | TXA was associated with reduced bleeding by 42.28 mL compared with the placeboEyelid edema and ecchymosis scores in patients receiving tranexamic acid were significantly lower compared with the control group within the first postoperative weekOral tranexamic acid was associated with a greater reduction in intraoperative bleeding compared with intravenous tranexamic acid, 10 mg/kg | None |
| McGuire et al | JAMA Facial Plastic Surgery 2019 | Meta-analysis | 5 RCT, including 332 patientsLow degree of clinical heterogeneity | Rhinoplasty | IV and ORAL | Intraoperative bleedingPostoperative eyelid edema and periorbital ecchymosis | TXA treatment resulted in a mean reduction in intraoperative blood loss of −41.6 mL compared with controls (P = 0.004) | None |
Cs, Corticosteroid.
Table 7.
TXA in Microsurgery
| Authors | Journal and Year | Study Design | Study Group (Total No.) | Surgery Type | Administration Mode, Time, and Dosage | Main Clinical Outcome | Main Results | Complications Related to TXA |
|---|---|---|---|---|---|---|---|---|
| Valerio et al | Military Medicine 2015 | RC | 19 patients (173) | 100 pedicle, 73 free flaps for extremity reconstruction | IV: Pre operatory IV, dose N/A | Flap success/failure ratesComplicationsRate of VTE | No difference in flap successful rate and complication | None |
| Lardi et al | Gland Surgery 2018 | RC | 50 patients (83) | 63 free tissue transfer for immediate breast reconstruction | IV: Up to 3 g IV according to intra- and postoperative blood loss | Postoperative complications and blood loss during the first 24 h | Intraoperative and blood loss during the first 24 h was reduced significantly (P < 0.001) with the use of TXATXA did not increase the risk of thrombosis | None |
N/A, not available; RC: Retrospective Cohort; VTF, venous thromboembolic events.
RHINOPLASTY
TXA has been studied more in relation to rhinoplasty. In total, 5 Randomized controlled trials (RCT)17–21 and 2 meta-analysis22,23 were identified in the review, including 332 patients. The age of patients ranged from 16 to 42 years, with a mean age of 27 years and a mean sample size of 66, with a range between 50 and 96. TXA dosing and administration varied between studies. In 3 studies, TXA was administered intravenously (IV), and in the other 2 studies, patients received oral administration. Regarding dosage of the IV group, 2 studies administered a 10-mg/kg single dose immediately before surgery, while 1 study also injected 10 mg/kg before surgery but added 1 dose after surgery every 8 hours for 24 hours. In the oral group, 1 study administered a single 1-g dose 2 hours before surgery, and 1 study injected 1-g dose 2 hours before surgery and added 1 g 3 times daily for 5 days after surgery.
Two studies compared the use of TXA with corticosteroids for the treatment of bleeding and ecchymosis. Sakallioglu et al17 investigated the efficacy of TXA on edema and ecchymosis and compared it with the that of methylprednisolone. Seventy-five patients who underwent open septorhinoplasty were included in the study and were randomly assigned to 1 of the 3 groups: control, oral TXA (1 g 2 hours before surgery, and 3 g daily for 5 days), or a single dose of 1 mg/kg intravenous methylprednisolone. They showed that the administration of both oral TXA and IV methylprednisolone were effective in decreasing the score of both edema and ecchymosis with no significant difference between these 2 groups. However, TXA also reduced the amount of intraoperative bleeding. Mehdizadeh18 obtained similar results in a study comparing the use of EV TXA (10 mg/kg) alone, corticosteroids (8-mg dexamethasone), or a combination of both to prevent eyelid edema and ecchymosis.
Eftekharian et al19 investigated the efficacy of preoperative oral TXA in postoperative bleeding. In this double-blind, randomized, placebo-controlled clinical trial, 25 patients were assigned to received either 1 g (2 × 500 mg) TXA tablets or placebo 2 hours before surgery. The TXA group presented significantly lower total blood loss and mean surgery duration than the control group. Moreover, the surgeon was more satisfied about the surgery site quality in the TXA group.
Beikaei et al20 performed a randomized trail assessing the role of intravenous TXA in reducing intraoperative bleeding during rhinoplasty surgery. An estimated 98 subjects were randomly allocated to receiving intravenous TA at a dose of 10 mg/kg immediately after anesthesia induction, or normal saline as the placebo in open Rhinoplasty. Again, TXA was associated with a 15.6-mL decrease in intraoperative bleeding, on average. Another study21 also demonstrated that administration of 10 mg/kg TXA significantly reduced the intraoperative bleeding rate, eyelid edema, and periorbital ecchymosis in closed rhinoplasty, with no side effects.
Both meta analyses22,23 indicated that the 5 studies were of high quality and showed consistently positive results that TXA is safe and may reduce intraoperative bleeding, and postoperative eyelid edema and ecchymosis in patients undergoing rhinoplasty. Oral TXA was associated with a greater reduction in intraoperative bleeding, compared with intravenous TXA.
BLEPHAROPLASTY
For the blepharoplasty procedure, only 1 randomized clinical trial was identified.24 The authors evaluated whether preoperative subcutaneous injection of TXA reduced intra-and postoperative bleeding and ecchymoses. In total, 34 consecutive patients who underwent standard upper eyelid blepharoplasties were randomized to a preoperative local injection of lidocaine mixed with either TXA or normal saline (1:1 mixture of 2% lidocaine with 100 mg/mL of TXA or 0.9% sodium chloride) 15 minutes before incision. Although there was a trend toward smaller ecchymoses on the seventh day in the TXA group, it was not statistically significant (P = 0.072). Furthermore, there were no differences in total surgery time, cumulative cautery time, net blood weight in surgical pads, patient-reported pain level, surgeon’s assessment of hemostasis, or periocular ecchymosis on the first postoperative day.
FACE LIFT
One case series,25 2 retrospective cohort studies,26,27 1 prospective cohort,28 and 1 RCT29 were identified regarding the use of TXA in facelift procedures involving 194 patients with an average age of 61.8 years. Of these patients, 94 underwent an extended deep plane facelift, 61 had a face lift with SMAS plication. One study did not specify the type of facelift. Three different administration methods were employed: 1 study administered TXA-soaked pledgets under the skin flap, 1 study administered 1 g IV TXA before and 4 hours after surgery, and 3 studies administered TXA subcutaneously mixed with local anesthetic. The dilutions of dosages were different. Two studies used 1 mg of TXA/1 mL of local anesthetic and one study administered 9.1 mg of TXA per 1 mL of local anesthetic. In total, 3 hematomas in the TXA groups and 1 case of thromboembolism were reported.
The first description was a retrospective study performed by Butz et al,25 who placed TXA-soaked pledgets under the skin flap in 57 patients who underwent a full face and neck lift with SMAS plication. They reported 1 hematoma that required evacuation. No systemic complications related to TXA were found. They did not include the concentration of TXA used, and their results regarding reduced edema, ecchymosis, and recovery time were subjectively noted.
Schroeder et al26 designed a retrospective cohort study to determine whether local TXA reduced intraoperative bleeding and postoperative drain output in rhytidectomy. Using a 9.1 mg of TXA/1 mL of local and tumescent solution, they found that TXA decreased the drain output by 70% during the first postoperative day and the intraoperative blood loss. No differences were observed in hematoma rate. Although they reported 1 case of thromboembolic event, this was not statistically significant.
In 2019, Couto et al27 conducted a study on 27 patients who underwent a facelift and received a subcutaneous injection of TXA-lidocaine 0.5% solution before skin flap dissection. The time to gain hemostasis on each side before closure was prospectively measured in 23 of the 27 patients. Although there was no control group, they found a subjective dramatic reduction in bleeding and a total surgical time saving between 25 and 60 minutes compared with their prior experience without TXA. They also reported a subjective reduction in bleeding when compared with their previous experience with patients who receive 0.5% lidocaine with 1:200,000 epinephrine without TXA. There were no complications such as intraoperative or postoperative hematomas or seromas.
Recently, the same group28 conducted a prospective study to demonstrate that TXA with local anesthesia safely reduced the effects of rebound bleeding and postoperative drainage, and they reached similar conclusions.
The single RCT study we identified for review was designed by Cohen et al29 to determine whether intravenous TXA has an effect on intraoperative bleeding and postoperative ecchymosis and edema in patients undergoing a deep-plane facelift. Patients in the treatment group (n = 27) received 1 g of IV TXA over 15 minutes just before first skin incision. Although there was no difference in intraoperative bleeding, the TXA cohort showed a significantly reduced rate of postoperative collections. Also, the patients who received TXA were consistently rated as having less postoperative edema and ecchymosis.
BREAST SURGERY
Four studies were identified regarding the use of TXA in breast surgery30–33: 3 involving oncoplastic breast surgery, and 1 including aesthetic breast reduction. In total, 3 RCT and 1 retrospective cohort study were found. A total of 352 patients were involved. An estimated 2 studies administered IV TXA and 2 used the same as a topical solution. No thromboembolic complication was reported.
Among the oncoplastic breast articles, the first trial was published by Oerli et al30 in 1994 and was carried out in 160 women with breast cancer who underwent mastectomy or lumpectomy, including axillary clearance. The results showed that perioperative and postoperative administration of TXA 1 g 3 times daily resulted in a significant reduction in the mean postoperative drainage volume compared with patients given placebo (P < 0.001). Also, TXA statistically reduced the mean hospital stay (P < 0.02). No difference was reported in the number of postoperative hematomas and in seroma formation. To note, TXA had various side-effects when administered parenterally as a bolus injection, which were then minimized by diluting the TXA in 100-mL normal saline and infused over 20 min.
Knight et al, in 2019,31 conducted a retrospective cohort study on 304 patients who underwent mastectomy with and without implant-based reconstruction. They demonstrated a significant reduction in hematoma rate in those patients who received a single intraoperative intravenous dose of TXA (P = 0.0295).
In 2015, Ausen et al32 designed a randomized clinical trial using topical TXA in breast reduction surgery. In this study, 28 women were treated by swabbing 20 mL of 25 mg/mL TXA on 1 breast, and 20 mL of saline solution on the other breast before closing. The primary outcome was drain fluid production in the first 24 h after surgery, which was 39% lower in breasts treated with TXA compared with breasts treated with placebo (P = 0.038). Also, drain volume adjusted for resected weight was 42% lower. No adverse effects were recorded after topical TXA.
The same author33 published a similar 2-center, double-blinded, randomized clinical trial to investigate the effect of this moistening method but in 202 patients who underwent either simple mastectomy or mastectomy with sentinel node biopsy, or mastectomy with axillary lymph node clearance. The method of administration and doses was identical to those of the previous study. This time, the primary outcome was postoperative bleeding as defined by the volume of drain production in the first 24 h after surgery. Secondary outcomes were total drain production and drain time, early hematoma, postoperative complications, and seroma formation. As in the previous study, TXA significantly reduced 24-h drain production by 32.4% (P < 0.001). Moreover, they found that total drain volume was reduced by 33% (P = 0.003). Patients in the TXA group were significantly more likely to have drains removed on the first day (OR 3.00; P = 0.003) and had a significantly shorter duration of drain insertion (P = 0.017). There were no differences between groups regarding other complications. No thromboembolic events were registered.
BODY CONTOURING
Only 1 study involving body contouring procedures was found. Cansancao et al34 performed a prospective, double-blind, nonrandomized study evaluating the effects of TXA on blood loss of 20 patients who underwent liposuction. The treated group (n = 10) received a standard intravenous dose of 10 mg/kg of TXA, 30 minutes preoperatively and postoperatively. The results showed that the use of TXA decreased the total volume of blood in the total lipoaspirate by 37%, the volume of blood loss for every liter of supernatant by 56.2%, and the drop in the hematocrit by 48%. Thus, the use of TXA could allow for aspiration of 114% more fat despite comparable variations in hematocrit levels.
BURN
In total, 1 randomized controlled trial,35 1 case report,36 and 1 retrospective cohort study37 were identified in burn surgery, including a total of 96 patients. All studies involved acute burn injuries. Two studies administered TXA EV, 1 in bolus and 1 as bolus plus infusion regimen. The last study applied topical dressing gauze soaked with TXA. No thrombosis complications were reported nor hematomas.
Jennes et al35 performed a preliminary randomized controlled trial to study the effect on blood loss in 27 tangential burn excisions after a single dose of 20 mg/kg EV preoperatively. TXA group showed a reduction in blood loss. However, the significance was dependent on the calculation method used for blood loss estimation.
Tang et al36 described the use of topical TXA for control bleeding in 30 cases after burns debridement in areas where epinephrine tumescence was not appropriate or unsuccessful. They used dressing gauze soaked in 500 mg/mL of TXA diluted in 100 mL of 0.9% saline, and applied to the bleeding area after debridement for 5–10 min. No complications were reported.
In 2017, Dominguez et al37 conducted a retrospective cohort study in 52 patients with ≥20% TBSA burn injury who underwent primary wound excision and skin grafting. EV TXA was given as a loading dose of 10 mg/kg over 5 minutes, followed by continuous infusion of 1 mg/kg/hour until the end of the surgery. They found that TXA significantly reduced the incidence of transfusion and the amount of blood transfused. Also, intraoperative use of TXA was identified as an independently variable associated with intraoperative and perioperative transfusion. Interestingly, graft survival differed significantly between groups, with a higher incidence of re-grafting found in patients from the control group.
MICROSURGERY
Two retrospective cohort studies were identified for the use of TXA in microsurgery.38,39 Surgical procedures included 136 free flaps for breast and extremity reconstruction, and both studies administered EV TXA, although exact time and dose were not reported in 1 of them.
Valerio et al38 assessed the use of TXA in military trauma patients who underwent tissue transfer for extremity reconstruction. Although the number of treated patients was small, they found no significant difference in flap failure or overall flap complications in patients who received TXA, and no venous thromboembolism events were reported in patients in the study group.
In 2018, Lardi et al39 performed a retrospective single-center cohort study to evaluate the safety and benefit of using TXA in 63 free tissue transfers for immediate breast reconstruction. According to the estimated blood loss during intraoperative or postoperative period, up to 3 g of TXA was administered intravenously. There was no difference in flap failure and thrombosis rate among groups. Moreover, administration of TXA significantly reduced blood loss during the first 24 hours. Hematoma rate was nosignificantly reduced in the TXA group.
DISCUSSION
Excessive blood loss during surgery may result in hematoma, re-exploration, and postoperative anemia, which can lead ultimately to blood transfusion. Studies have shown that both anemia and blood transfusion are associated with higher morbidity and mortality in cardiac and non-cardiac procedures.40 To reduce perioperative blood loss, a number of interventions have been used, including the antifibrinolytic agents.
There are 3 main antifibrinolytic drugs: TXA, E- aminocaproic acid, and aprotinin. The first 2 are synthetic derivatives of the amino acid lysine, that inhibit fibrinolysis by attaching to the lysine-binding site of the plasminogen and by preventing the conversion to plasmin, thereby avoiding degradation of blood clots.41 They also prevent plasmin degradation of platelet receptors, thus preserving platelet function.42 The latter is a nonspecific serine protease that reversibly inhibits active serine residue in various proteases in the plasma such as trypsin, kallikrein, plasmin, elastase, and is the most potent antifibrinolytic agent.43
The 3 agents were associated with reduced perioperative blood loss and transfusion requirements, with aprotinin being slightly superior to the lysine analogs.44 However, in 2008, aprotinin was withdrawn from the market after the Blood Conservation Using Antifibrinolytic Randomized Trial, and other large nonrandomized studies showed an increased risk associated with its use, in cardio and cerebrovascular events, renal dysfunction, and possibly, mortality in cardiac surgery.45,46
TXA is about 10 times more potent than aminocaproic acid and binds much more strongly to plasminogen molecule, but no significant differences were seen in efficacy and side effects between the 2 other agents.47 Nevertheless, TXA has been gaining wide popularity and remains as the most commonly used drug.
TXA was first patented in 1957 for management of post-partum hemorrhage. Since then, its use in other surgical specialties has become commonplace particularly in cardiac, orthopedic, and trauma surgery.48
The effect of TXA in reducing bleeding has been well studied. The CRASH-2 trial was the largest study investigating the efficacy of TXA.49 This multi-centric, randomized controlled trial assessed the effects of TXA on death, vascular occlusive events, and the receipt of blood transfusion in 20211 trauma patients with, or at risk of, significant bleeding. Within 8 hours of injury, they were randomly assigned to either the TXA group or the placebo group. The results showed that early administration of TXA reduced the risk of death from hemorrhage, with no apparent increase in fatal or non-fatal vascular occlusive events. Few years later, the CRASH-3 trial provided evidence that the administration of TXA within 3 hours of injury reduced head-injury-related deaths after traumatic brain injury with no evidence of adverse effects or complications.50
A systematic review of 252 RCTs of antifibrinolytic drugs in adults scheduled for non-urgent surgery conducted by Henry et al in 2011 showed that the use of TXA was effective in reducing intra- and postoperative blood loss and significantly reduced the need for allogeneic blood transfusion by about one-third, with no evidence of any increased risk of vascular occlusive events or mortality.51
Ker,52 in 2012, in another systematic review and meta-analysis of 95 RCTs comparing TXA with no TXA or placebo in surgical patients, also found that administration of TXA reduced the probability of receiving a blood transfusion by about a third. However, there was uncertainty about the effect of TXA on thromboembolic events and mortality.
Since then, numerous studies had been investigating the prothrombotic effect of TXA. Kagoma et al53 reported no increase in the risk for thromboembolic events in their systemic review of 29 RCTs. Similarly, another systematic review showed no association between TXA and thromboembolic episodes or mortality.54 The Aspirin and Tranexamic Acid for Coronary Artery Surgery trial was one of the biggest studies involving 4631 patients. This multicenter, double-blind trial investigated patients who were scheduled to undergo coronary-artery surgery and were at an increased risk for complications. They were randomly assigned to receive TXA or placebo, and aspirin or placebo. They found that TXA was associated with a lower risk of bleeding as well as need of blood transfusion, and reoperation, without a higher risk of death or thrombotic complications within 30 days after surgery.55 In a follow-up study, TXA did not affect death or severe disability 1 year after surgery.56
However, the Aspirin and Tranexamic Acid for Coronary Artery Surgery trial found that TXA was associated with a higher risk of postoperative seizures especially among patients who underwent open-chamber surgery. At first, they used TXA at a dose of 100 mg/kg, which was later reduced to 50 mg/kg due to an increased number of studies reporting seizures associated with a high dose of TXA.57,58 Nevertheless, this dose reduction did not reduce the risk of seizure.
A retrospective analysis of 11,529 patients who underwent cardiac surgery showed that TXA was an independent predictor of postoperative convulsive seizures, particularly in patients receiving TXA as an infusion, which resulted in higher cumulative doses (>80 mg/kg) in the perioperative period.59
The reason for seizures is that TXA can cross the blood-brain barrier and reach approximately 10% of the plasma concentration in the cerebrospinal fluid.60 Depending on the concentration, TXA may cause hyperexcitability of the central nervous system. The mechanism postulated for this effect is the competitive inhibition of glycine and GABAa receptors, which abolish GABA-mediated inhibition in the central nervous system and cause an increased excitability.60 TXA-associated seizures are typically generalized tonic–clonic events that occur within the first 5–8 hours after surgery and typically persist for a few minutes with no progression into status epilepticus.61
There are several risk factors for TXA-associated seizures. This includes higher doses of TXA, preoperative cardiac arrest, high APACHE II score, preoperative neurological disease, open-chamber procedure, renal dysfunction, and previous cardiac surgery.62
As for the recommended treatment, based on preclinical studies, general anesthetics such as propofol and isofluorane may be useful to consider for the first-line treatment because they reverse the inhibitory response in glycine receptors.63 Also, the lowest effective TXA dose should be considered, and dosing should be adjusted for clinical conditions such as renal dysfunction.64 Although there are reports of seizures in non-cardiac surgery,65,66 no such cases were reported in plastic surgery.
As the method of administration, TXA can be given orally, intravenously, or topically, with none of the routes being superior to each other.67 However, the majority of studies are based on an intravenous dose. Dose regimens for TXA varied significantly between trials and thus, the optimum dose has long been debated.
In 1968, Andersson and colleagues68 reported that an 80% inhibition of tissue plasminogen activator activity required a TXA concentration of 10 μg/mL, 90% inhibition required a concentration of 25 μg/mL, and a 98%–100% inhibition required a TXA concentration of 100 μg/mL. Being 80% clinically significant, they recommended a dose of 10 mg/kg IV or 20 mg/kg oral 2–4 times a day to maintain adequate antifibrinolytic activity in serum for 7–8 hours, and in tissues for up to 17 hours.66 However, in 1995, Horrow and colleagues69 conducted a prospective, randomized, double-blind study in patients undergoing cardiac surgery to elucidate the optimum dose. They reported that 10 mg/kg followed by a maintenance dose of 1 mg/kg/hour had significantly decreased bleeding in cardiac surgery and that larger doses did not provide additional hemostatic benefits.
Although TXA was described to inhibit fibrinolysis and platelet activation for concentrations below 20 mg/mL, others found that a potential mechanism of TXA was the inhibition of thrombin formation, which required concentrations as high as 126–252 mg/mL to be therapeutic.70 For that reason, Dowd, in 2002, proposed a higher dose of 30 mg/kg of TXA administered as a bolus followed by 16 mg/kg/h to maintain the plasma concentration above 126 μg/mL.71
In 2013, Sigaut et al72 reported the first double-blind, randomized study comparing 2 regimens for TA with a loading dose followed by continuous infusion (30 mg/kg bolus followed by 16 mg/kg/hour versus 10 mg/kg bolus followed by 1 mg/kg/hour) during cardiac surgery. They found no difference in the incidence of blood products transfused during the first week between the 2 doses. Imtiaz et al73 conducted an RCT in 137 patients to compare bolus injections versus continuous infusion of 30 mg/kg TXA. They found no difference in blood loss or transfusion requirements.
In a recent meta-analysis conducted by Guo,74 49 RCT studies with 10,591 patients were analyzed to provide information on the optimal dosage and effective delivery method with the least adverse outcomes. They concluded that high-dose TXA does not further decrease transfusion rate and has a strong tendency to cause more seizure attacks, compared with the low-dose TXA. They considered low-dose TXA (bolus injection < 50 mg/kg, or 10 mg/kg + 1 mg/kg/hour) is preferable to high-dose TXA.
Due to risk of seizures and other side effects, some authors are reluctant to use intravenous TXA routinely in all surgical procedures, and there has been a raise in the use of topical administration of TXA. The main advantage is that topical administration of TXA provides a high concentration dose at the application site with a low systemic concentration, which may reduce the risk of systemic side-effects. Regarding effectiveness in reducing blood loss and safety, a meta-analysis was conducted by Ker in 2013,75 including 29 trials involving 2612 participants to assess the effects of the topical administration of tranexamic acid in the control of bleeding. The authors found that topical TXA reduced blood loss by 29% and the risk of receiving a blood transfusion by a relative 45%, with no major side effects.
In another meta-analysis, 67 RCT studies involving 6034 patients were analyzed.76 Results showed that, compared with placebo, the administration of topical TXA significantly reduced the risk of receiving a blood transfusion and the mean blood loss, with no difference in the odds of developing venous thromboembolism. When compared with the intravenous administration, there was no difference between the 2 groups in terms of transfusion requirements or blood loss, with no major differences with respect to safety.
Regarding the dose, both meta-analysis showed a wide range of TXA modes of topical administration, dose, and concentrations. Modes of topical administration of TXA include instilling a bolus into a closed wound cavity or irrigating the wound for a particular length of time and with a particular dose of TXA.
Although no systemic effects were seen with topical TXA, few studies have explored the potential local toxicity of topical TXA in chondrocytes in orthopedic arthroplasty surgery.77,78 Chondrocyte toxicity increases with both concentration and exposure time, and 20–25 mg/mL was signed as the threshold value.79
Eikebrokk et al80 conducted a study to investigate whether topical TXA might be cytotoxic or might affect wound re-epithelialization. For that, human keratinocytes and fibroblast cell cultures and an ex vivo human skin wound model were subjected to both short (limited) and long (chronic) exposure to various concentrations of TXA to mimic different modalities of topical administration. They found that in chronic exposure, cell survival decreased with increasing TXA concentration and length of exposure, whereas limited exposure to TXA did not cause significant cytotoxicity even at high concentrations. Re-epithelialization was completely absent in wounds chronically exposed to TXA concentrations of 25 mg/mL or above, and 50–100 mg/mL induced epidermolysis of normal epithelium, possibly by a nontoxic mechanism. Wound re-epithelialization was slightly delayed, but not impaired, by limited exposure to 100 mg/mL or chronic exposure to 6–25 mg/mL. With this result, they recommend that bolus administrations of topical TXA should not exceed at a concentration of 5–10 mg/mL, and propose a TXA concentration of 25–50 mg/mL when moistening a surgical wound.
In theory, topical application of TXA may be safer than intravenous administration because topically applied TXA results in a 90% reduction in plasma concentration compared with intravenous administration. Ausen et al81 published a prospective study to investigate the degree of systemic absorption of TXA in patients undergoing skin-reducing abdominoplasty after massive weight loss. They compared 3 ways of administration: moistening the wound surface before closure with 20 mL of tranexamic acid 25 mg/mL, instilling a bolus of 200 mL of 5 mg/mL TXA into the wound cavity retrogradely through drains after closure, and the standard intravenous prophylactic administration of 1 g. They found that topical application of tranexamic acid, either with moistening or by administration of a bolus into the wound cavity, resulted in mean maximum serum concentration values of 5 μg/mL, which is below the 10-μg/ mL limit considered to cause any systemic antifibrinolytic effect.
Although there are plenty of studies on TXA use mainly in cardiac and orthopedic areas, there are a few studies regarding the use of TXA in plastic surgery, except in craniofacial surgery. Studies show that up to 70% of craniomaxillofacial procedures need at least 1 unit of red blood cells, especially in craniosynostosis.82 A 2016 meta-analysis showed that tranexamic acid was effective in reducing blood loss during craniofacial surgery in both children and adults, and reduced transfusion requirements in craniosynostosis surgery, without any complications.83
The literature is scarce regarding the use of TXA in other areas of plastic surgery. The main reason is the limited amount of blood loss in the aesthetic procedures and the fear of potential thrombosis in reconstructive and microsurgery operations. However, since the last review by Rohrich et al, the number of articles involving the use of TXA has risen, showing a better understanding of safety and efficacy and other benefits of using TXA. Moreover, although the amount of blood loss is limited, TXA can help improve hemostasis and reduce bleeding due to the rebound phenomenon of epinephrine, thus reducing the amount of electrocautery required and minimizing hematoma-related complications.
Also, another benefit of TXA that is gaining more attention is the anti-inflammatory effect, which may help reduce swelling and bruising and, therefore, decrease recovery time and patient discomfort.
TXA can reduce inflammation by at least 2 mechanisms: preventing the plasminogen-fibrin interaction (which has been shown to reduce fibrin-dependent complement activation) and by reducing bleeding itself.84
Lastly, an off-label indication of TXA is in the treatment melasma and other pigmented disorders.85 Although the mechanism is still unclear, it has been postulated that TA can inhibit melanin synthesis by blocking keratinocyte-melanocyte interaction mediated by plasmin and by an anti-angiogenic effect that reduces erythema and decreases the number of vessels.86
This anti-inflammatory response may be advantageous for plastic surgeons. There are several studies in orthopedic and cardiac surgery that shows how TXA is efficient in reducing inflammation response and ameliorating postoperative pain, promoting early function recovery.87,88 In aesthetic surgery, TXA had a significant role in decreasing the intraoperative bleeding rate, eyelid edema, and periorbital ecchymosis with similar effects as those of corticosteroids. Unfortunately, edema and ecchymosis are difficult to objectively measure; so most of the studies are based on subjective reports.
This review presents some limitations. First, although the number of publications is increasing, most of the studies are based on rhinoplasty or face lift surgeries; so no generalizable conclusions can be made regarding other aesthetic procedures. Second, although TXA is effective in decreasing blood loss, there is no standardization regarding administration route or dosage with variable protocols between articles. Third, a funnel plot analysis or other methods were not performed, and the potential for publication bias was not analyzed as well as other statistical analysis. Considering the limitations of the present study, further meta-analyses are needed to reach better conclusions.
CONCLUSIONS
The present review summarizes the current literature on tranexamic acid in plastic surgery. Although there is still a paucity of information on TXA in the literature, there is a clear benefit in using TXA to decrease intra-and postoperative blood loss. Although there are no studies comparing the different routes of administration, TXA was effective when administered both intravenously and topically, as well as orally and subcutaneously. Also, although difficult to objectively measure, most of the studies noticed a decrease in edema and ecchymosis in the postoperative period and the potential anti-inflammatory response, which improves patient outcomes in aesthetic procedures. Moreover, TXA has also emerged as a promising agent for microsurgical procedures and burn care without the risk of thrombosis and flap failure.
Although the role of TXA in plastic surgery is promising, further investigations are needed to standardize its optimal administration route and dosage. Also, besides facelift and rhinoplasty, studies addressing the use of TXA in other aesthetic procedures are lacking. Breast augmentation and abdominoplasty, for example, are 2 procedures that could greatly benefit from the use of TXA, and RCTs should be encouraged. Moreover, future research should try to elucidate the anti-inflammatory response of TXA with some validated scoring instrument to objectively evaluate edema and ecchymosis.
Table 2.
TXA in Blepharoplasty
| Authors | Journal and Year | Study Design | Study Group (Total No.) | Surgery Type | Administration Mode and Dosage | Main Clinical Outcome | Main Results | Complications Related to TXA |
|---|---|---|---|---|---|---|---|---|
| Sagiv et al | Canadian Journal of Ophthalmology 2018 | RCT | 17 (34)No significant difference between the 2 groups | Skin only upper eyelid blepharoplasty | LOCAL INJECTION: 2% lidocaine with 100 mg/mL of TXA in a 1:1 mixture 15 minutes before incision | Intra- and postoperative bleeding ecchymoses on POD 1 and 7 | No difference in intraoperative or postoperative bleeding, length of surgery, or use of electrical cauteryTrend toward smaller post operative ecchymoses on POD 7 in the TXA group (P = 0.072) | None |
POD postoperative day.
Table 3.
TXA in Facelift
| Authors | Journal and Year | Study Design | Study Group (Total No.) | Surgery Type | Administration Mode and Dosage | Main Clinical Outcome | Main Results | Complications Related to TXA |
|---|---|---|---|---|---|---|---|---|
| Butz et al | PRS Global Open 2016 | Case series | 57 (57)47 women and 10 menMean age: 61.9 y | Rhytidectomy with SMAS plication | TOPIC: TXA-soaked pledgets placed under the skin flapDosage N/A | Intra- and postoperative bleedingComplications | Subjective reduction of edema, ecchymosis, and faster return to social activity1 hematoma reported | None |
| Couto et al | Aesthetic Surgery Journal 2019 | RC | 27 (27)100% womenMean age: 62.1 y | 85% extended deep plane facelift and 15% rhytidectomy with SMAS plication 100% with neck lift | LOCAL: 1.5 mL of 100 mg/mL TXA in 150 mL of 0.5 % lidocaine with 1:200,000 epinephrine for a final concentration of 1 mg of TXA/1 mL of local anesthetic. In total, 60 mL of this solution 15 min before incision. | Time to gain hemostasis | Reduction in time to gain hemostasisNo intraoperative or postoperative hematomas or seromas | None |
| Cohen et al | Aesthetic Surgery Journal 2020 | RCT | 27 (44)100% womenMean age: 59 y | Extended deep-plane facelift | EV bolus: 1 g of TXA before skin incision, and 4 hours later | Intraoperative bleedingPostoperative ecchymosis and edema | Significant decrease in postoperative collectionsStatistically significant decrease in surgeon- rated bruising | None |
| Schroeder II et al | Facial Plastic Surgery & Aesthetic Medicine 2020 | RC | 44 (76)100% womenMean age: 61.5 y | Deep plane rhytidectomy with platysmaplasty | LOCAL: 9.1 mg of TXA/1 mL of local and tumescent anesthetic | POD1 drain output, number of days to drain removal, percentage of drains removed POD1, and percentage of drains with <25 cm3 outputIntraoperative estimated blood lossComplications | POD1 drain output decreased by 70% (P < 0.001)Drains were more commonly being removed POD1 (P < 0.001)Intraoperative blood loss was reduced in TXA patients (P < 0.001) | Single thromboembolic event |
| Kochuba et al | Aesthetic Surgery Journal 2020 | PC | 39 (39)35 women and 4 menMean age: 64.9 y | Extended deep plane facelift or SMAS plication, or facelift surgery combined with ancillary facial rejuvenation procedures (37%) | LOCAL: 1–2 mg of TXA per 1 mL of 0.5% lidocaine with 1:200,000 epinephrine. In total, 60 mL of this solution 15 min before incision | Time to hemostasisDrain outputComplications | Reduction in time to gain hemostasisNo intraoperative or postoperative hematomas or seromas | None |
PC, prospective cohort; POD1, postoperative day 1; RC, retrospective cohort.
Table 4.
TXA in Breast Surgery
| Authors | Journal and Year | Study Design | Study Group (Total No.) | Surgery Type | Administration Mode and Dosage | Main Clinical Outcome | Main Results | Complications Related to TXA |
|---|---|---|---|---|---|---|---|---|
| Oerli et al | British Journal of Surgery 1994 | RCT | 79 (160) | Mastectomy or lumpectomy with or without axillary clearance | IV: 1 g of TXA 3 times during 24 h and oral dose regimen until day 5 | Drain fluid volumePostoperative complication | Reduction in the mean postoperative drainage volume (P = 0.01)Reduction in the mean hospital stay (P < 0.02) | Marked nausea, dizziness, and hypotension during bolus injection, which declined after diluting and lengthening infusion time |
| Knight et al | Breast Journal 2019 | RC | 144 (304) | Mastectomy with and without implant-based reconstruction | IV: 1 g of TXA preoperative | Hematoma rateExplantation rate | Significant reduction on hematoma rate (P = 0.0295) | None |
| Ausen et al | British Journal of Surgery 2015 | RCT | 28 (56)Average age: 45 y | Bilateral reduction Mammoplasty | TOPIC: 20 mL of 25 mg/mL tranexamic acid moistened on the wound surface before closure | Drain fluid volume in 24 hHematoma ratePostoperative pain | 42% reduction in drain production (P = 0.017) | None |
| Ausen et al | British Journal of Surgery Open 2019 | RCT | 101 (202)Patients receiving TXA were on average 3.9 years older (P = 0.033) | Simple mastectomy, mastectomy with sentinel node biopsy, or mastectomy with axillary lymph node clearance | TOPIC: 20 mL of 25 mg/mL tranexamic acid moistened on the wound surface before closure | Drain fluid volume in 24 hTotal drain production and drain timeHematoma rate | 32.4% reduction in 24 h drain production (P = 0.001)Total drain volume was reduced by 33.0% (P= 0.003) | None |
N/A, not available; RC, retrospective cohort.
Table 5.
TXA in Body Contouring
| Authors | Journal and Year | Study Design | Study Group (Total No.) | Surgery Type | Administration Mode, Time, and Dosage | Main Clinical Outcome | Main Results | Complications Related to TXA |
|---|---|---|---|---|---|---|---|---|
| Cansancao et al | Plastic & Reconstructive Surgery Journal 2018 | NRCT | 10 (20)No significant difference between the 2 groups | Liposuction | IV: 10 mg/kg of TXA 30 min preoperatively and postoperatively | Blood volume of the total lipoaspirateHematocrit level | 37% less perioperative blood loss (P < 0.05)56.2% less blood loss for every liter of supernatant (P < 0.001)48% less drop in hematocrit levels at POD 7 (P = 0.001) | None |
N/A, not available; NRCT, nonrandomized controlled trial.
Table 6.
TXA in Burn Surgery
| Authors | Journal and Year | Study Design | Study Group (Total No.) | Surgery Type | Administration Mode, Time, and Dosage | Main Clinical Outcome | Main Results | Complications Related to TXA |
|---|---|---|---|---|---|---|---|---|
| Jennes et al | J Burn Care Rehabil 2003 | Preliminary RCT | 14 patients (27) | Tangential burn excision | 20 mg/kg EV preoperative | Intraoperative and postoperative bleeding in 24 h | TXA group showed a reduction in blood loss dependent on the calculation method employed | None |
| Tang et al | Journal of Plastic, Reconstructive & Aesthetic Surgery 2012 | Case report | 30 patients | Burns debridement and grafting | Dressing gauze soaked in 500 mg/mL of TXA diluted in 100 mL of 0.9% saline applied to the bleeding area for 5–10 min | None | None | None |
| Dominguez et al | Minerva Anestesiologica 2017 | RC | 52 patients (107) | Primary burn wound excision and grafting | EV at a loading dose of 10 mg/kg over 5 min, followed by continuous infusion of 1 mg/kg/h | Incidence of allogeneic transfusion and in the number of pRCB required | ARR of 28.7% in the intraoperative and 24.2% in the postoperative need for transfusionSignificant reduction in the number of pRBC and volume (P = 0.018)Reduction in the incidence of regrafting needed (P = 0.034) | None |
ARR, absolute risk reduction; N/A, not available; pRBC, packed red blood cell transfusion, RC, retrospective cohort.
Footnotes
Published online 23 March 2021.
Disclosure: The author has no financial interest to declare in relation to the content of this article.
REFERENCES
- 1.Johnson DJ, Scott AV, Barodka VM, et al. Morbidity and mortality after high-dose transfusion. Anesthesiology. 2016; 124:387–395 [DOI] [PubMed] [Google Scholar]
- 2.Busch MP, Kleinman SH, Nemo GJ. Current and emerging infectious risks of blood transfusions. JAMA. 2003; 289:959–962 [DOI] [PubMed] [Google Scholar]
- 3.Gabay M. Absorbable hemostatic agents. Am J Health Syst Pharm. 2006; 63:1244–1253 [DOI] [PubMed] [Google Scholar]
- 4.Porte RJ, Leebeek FW. Pharmacological strategies to decrease transfusion requirements in patients undergoing surgery. Drugs. 2002; 62:2193–2211 [DOI] [PubMed] [Google Scholar]
- 5.Markin JM. Lessons learned in antifibrinolytic therapy: the BART Trial. Semin Cardiothorac Vasc Anesth. 2009; 13:127–131 [DOI] [PubMed] [Google Scholar]
- 6.McCormack PL. Tranexamic acid: a review of its use in the treatment of hyperfibrinolysis. Drugs. 2012; 72:585–617 [DOI] [PubMed] [Google Scholar]
- 7.Later AF, Sitniakowsky LS, van Hilten JA, et al. Antifibrinolytics attenuate inflammatory gene expression after cardiac surgery. J Thorac Cardiovasc Surg. 2013; 145:1611–16161616.e1 [DOI] [PubMed] [Google Scholar]
- 8.Walker PF, Foster AD, Rothberg PA, et al. Tranexamic acid decreases rodent hemorrhagic shock-induced inflammation with mixed end-organ effects. PLoS One. 2018; 13:e0208249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhang Y, Bai Y, Chen M, et al. The safety and efficiency of intravenous administration of tranexamic acid in coronary artery bypass grafting (CABG): a meta-analysis of 28 randomized controlled trials. BMC Anesthesiol. 2019; 19:104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fillingham YA, Ramkumar DB, Jevsevar DS, et al. The efficacy of tranexamic acid in total hip arthroplasty: a network meta-analysis. J Arthroplasty. 2018; 33:3083–3089.e4 [DOI] [PubMed] [Google Scholar]
- 11.Franchini M, Mengoli C, Cruciani M, et al. Safety and efficacy of tranexamic acid for prevention of obstetric haemorrhage: an updated systematic review and meta-analysis. Blood Transfus. 2018; 16:329–337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Henry DA, Carless PA, Moxey AJ, et al. Anti-fibrinolytic use for minimising perioperative allogeneic blood transfusion. Cochrane Database Syst Rev. 2007; 2011:CD001886. [DOI] [PubMed] [Google Scholar]
- 13.Brown S, Yao A, Taub PJ. Antifibrinolytic agents in plastic surgery: current practices and future directions. Plast Reconstr Surg. 2018; 141:937e–949e [DOI] [PubMed] [Google Scholar]
- 14.Goobie SM, Meier PM, Pereira LM, et al. Efficacy of tranexamic acid in pediatric craniosynostosis surgery: a double-blind, placebo-controlled trial. Anesthesiology. 2011; 114:862–871 [DOI] [PubMed] [Google Scholar]
- 15.Kurnik NM, Pflibsen LR, Bristol RE, et al. Tranexamic acid reduces blood loss in craniosynostosis surgery. J Craniofac Surg. 2017; 28:1325–1329 [DOI] [PubMed] [Google Scholar]
- 16.Rohrich RJ, Cho MJ. The role of tranexamic acid in plastic surgery: review and technical considerations. Plast Reconstr Surg. 2018; 141:507–515 [DOI] [PubMed] [Google Scholar]
- 17.Sakallioğlu Ö, Polat C, Soylu E, et al. The efficacy of tranexamic acid and corticosteroid on edema and ecchymosis in septorhinoplasty. Ann Plast Surg. 2015; 74:392–396 [DOI] [PubMed] [Google Scholar]
- 18.Mehdizadeh M, Ghassemi A, Khakzad M, et al. Comparison of the effect of dexamethasone and tranexamic acid, separately or in combination on post-rhinoplasty edema and ecchymosis. Aesthetic Plast Surg. 2018; 42:246–252 [DOI] [PubMed] [Google Scholar]
- 19.Eftekharian HR, Rajabzadeh Z. The efficacy of preoperative oral tranexamic acid on intraoperative bleeding during rhinoplasty. J Craniofac Surg. 2016; 27:97–100 [DOI] [PubMed] [Google Scholar]
- 20.Beikaei M, Ghazipour A, Derakhshande V, et al. Evaluating the effect of intravenous tranexamic acid on intraoperative bleeding during elective rhinoplasty. Biomed Pharm J. 2015; 8:753–759 [Google Scholar]
- 21.Ghavimi MA, Taheri Talesh K, Ghoreishizadeh A, et al. Efficacy of tranexamic acid on side effects of rhinoplasty: a randomized double-blind study. J Craniomaxillofac Surg. 2017; 45:897–902 [DOI] [PubMed] [Google Scholar]
- 22.de Vasconcellos SJA, do Nascimento-Júnior EM, de Aguiar Menezes MV, et al. Preoperative tranexamic acid for treatment of bleeding, edema, and ecchymosis in patients undergoing rhinoplasty: a systematic review and meta-analysis. JAMA Otolaryngol Head Neck Surg. 2018; 144:816–823 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.McGuire C, Nurmsoo S, Samargandi OA, et al. Role of tranexamic acid in reducing intraoperative blood loss and postoperative edema and ecchymosis in primary elective rhinoplasty: a systematic review and meta-analysis. JAMA Facial Plast Surg. 2019; 21:191–198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sagiv O, Rosenfeld E, Kalderon E, et al. Subcutaneous tranexamic acid in upper eyelid blepharoplasty: a prospective randomized pilot study. Can J Ophthalmol. 2018; 53:600–604 [DOI] [PubMed] [Google Scholar]
- 25.Butz DR, Geldner PD. The use of tranexamic acid in rhytidectomy patients. Plast Reconstr Surg Glob Open. 2016; 4:e716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schroeder RJ, II, Langsdon PR. Effect of local tranexamic acid on hemostasis in rhytidectomy. Facial Plast Surg Aesthet Med. 2020; 22:195–199 [DOI] [PubMed] [Google Scholar]
- 27.Couto RA, Charafeddine A, Sinclair NR, et al. Local infiltration of tranexamic acid with local anesthetic reduces intraoperative facelift bleeding: a preliminary report. Aesthet Surg J. 2019; 40:587–593 [DOI] [PubMed] [Google Scholar]
- 28.Kochuba AL, Coombs DM, Kwiecien GJ, et al. Prospective study assessing the effect of local infiltration of tranexamic acid on facelift bleeding. Aesthet Surg J. 2020; ;40:sjaa198. [DOI] [PubMed] [Google Scholar]
- 29.Cohen JC, Glasgold RA, Alloju LM, et al. Effects of intravenous tranexamic acid during rhytidectomy: a randomized, controlled, double- blind pilot study. Aesthet Surg J. 2020; 41:155–160 [DOI] [PubMed] [Google Scholar]
- 30.Oertli D, Laffer U, Haberthuer F, et al. Perioperative and postoperative tranexamic acid reduces the local wound complication rate after surgery for breast cancer. Br J Surg. 1994; 81:856–859 [DOI] [PubMed] [Google Scholar]
- 31.Knight H, Banks J, Muchmore J, et al. Examining the use of intraoperative tranexamic acid in oncoplastic breast surgery. Breast J. 2019; 25:1047–1049 [DOI] [PubMed] [Google Scholar]
- 32.Ausen K, Fossmark R, Spigset O, et al. Randomized clinical trial of topical tranexamic acid after reduction mammoplasty. Br J Surg. 2015; 102:1348–1353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ausen K, Hagen AI, Østbyhaug HS, et al. Topical moistening of mastectomy wounds with diluted tranexamic acid to reduce bleeding: randomized clinical trial. BJS Open. 2020; 4:216–224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cansancao AL, Condé-Green A, David JA, et al. Use of tranexamic acid to reduce blood loss in liposuction. Plast Reconstr Surg. 2018; 141:1132–1135 [DOI] [PubMed] [Google Scholar]
- 35.Jennes S, Degrave E, Despiegeleer X, et al. Effect of tranexamic acid on blood loss in burn surgery: a preliminary study. J Burn Care Rehabil. 2003; 24:S59 [Google Scholar]
- 36.Domínguez A, Alsina E, Landín L, et al. Transfusion requirements in burn patients undergoing primary wound excision: effect of tranexamic acid. Minerva Anestesiol. 2017; 83:353–360 [DOI] [PubMed] [Google Scholar]
- 37.Tang YM, Chapman TW, Brooks P. Use of tranexamic acid to reduce bleeding in burns surgery. J Plast Reconstr Aesthet Surg. 2012; 65:684–686 [DOI] [PubMed] [Google Scholar]
- 38.Valerio IL, Campbell P, Sabino J, et al. TXA in combat casualty care–does it adversely affect extremity reconstruction and flap thrombosis rates? Mil Med. 2015; 1803 Suppl24–28 [DOI] [PubMed] [Google Scholar]
- 39.Lardi AM, Dreier K, Junge K, et al. The use of tranexamic acid in microsurgery–is it safe? Gland Surg. 2018; 7Suppl 1S59–S63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hare GM, Baker JE, Pavenski K. Assessment and treatment of preoperative anemia: continuing professional development. Can J Anaesth. 2011; 58:569–581 [DOI] [PubMed] [Google Scholar]
- 41.Faught C, Wells P, Fergusson D, et al. Adverse effects of methods for minimizing perioperative allogeneic transfusion: a critical review of the literature. Transfus Med Rev. 1998; 12:206–225 [DOI] [PubMed] [Google Scholar]
- 42.Ng W, Jerath A, Wąsowicz M. Tranexamic acid: a clinical review. Anaesthesiol Intensive Ther. 2015; 47:339–350 [DOI] [PubMed] [Google Scholar]
- 43.Levy JH, Koster A, Quinones QJ, et al. Antifibrinolytic therapy and perioperative considerations. Anesthesiology. 2018; 128:657–670 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ortmann E, Besser MW, Klein AA. Antifibrinolytic agents in current anaesthetic practice. Br J Anaesth. 2013; 111:549–563 [DOI] [PubMed] [Google Scholar]
- 45.Fergusson DA, Hébert PC, Mazer CD, et al. ; BART Investigators. A comparison of aprotinin and lysine analogues in high-risk cardiac surgery. N Engl J Med. 2008; 358:2319–2331 [DOI] [PubMed] [Google Scholar]
- 46.Schneeweiss S, Seeger JD, Landon J, et al. Aprotinin during coronary-artery bypass grafting and risk of death. N Engl J Med. 2008; 358:771–783 [DOI] [PubMed] [Google Scholar]
- 47.Mannucci PM. Hemostatic drugs. N Engl J Med. 1998; 339:245–253 [DOI] [PubMed] [Google Scholar]
- 48.Wokes JET, Erdmann MWH, McLean NR. The role of tranexamic acid in aesthetic plastic surgery: a survey of the British association of aesthetic plastic surgeons. Aesthet Surg J. 2021; 41:244–249 [DOI] [PubMed] [Google Scholar]
- 49.CRASH-2 trial collaborators. Effects of tranexamic acid on death, vascular occlusive events, and blood transfusion in trauma patients with significant haemorrhage (CRASH-2): a randomized, placebo-controlled trial. Lancet. 2010; 376:23–32 [DOI] [PubMed] [Google Scholar]
- 50.CRASH-3 trial collaborators. Effects of tranexamic acid on death, disability, vascular occlusive events and other morbidities in patients with acute traumatic brain injury (CRASH-3): a randomised, placebo- controlled trial. Lancet. 2019; 394:1713–1723 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ker K, Edwards P, Perel P, et al. Effect of tranexamic acid on surgical bleeding: systematic review and cumulative meta-analysis. BMJ. 2012; 344:e3054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Henry DA, Carless PA, Moxey AJ, et al. Anti-fibrinolytic use for minimising perioperative allogeneic blood transfusion. Cochrane Database Syst Rev. 2011; 2011:CD001886. [DOI] [PubMed] [Google Scholar]
- 53.Kagoma YK, Crowther MA, Douketis J, et al. Use of antifibrinolytic therapy to reduce transfusion in patients undergoing orthopedic surgery: a systematic review of randomized trials. Thromb Res. 2009; 123:687–696 [DOI] [PubMed] [Google Scholar]
- 54.Gurusamy KS, Pissanou T, Pikhart H, et al. Methods to decrease blood loss and transfusion requirements for liver transplantation. Cochrane Database Syst Rev. 2011; 12:CD009052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Myles PS, Smith JA, Forbes A, et al. Tranexamic acid in patients undergoing coronary-artery surgery. N Engl J Med. 2018; 378:782. [DOI] [PubMed] [Google Scholar]
- 56.Myles PS, Smith JA, Kasza J, et al. ; ATACAS investigators and the ANZCA Clinical Trials Network. Tranexamic acid in coronary artery surgery: one-year results of the aspirin and tranexamic acid for coronary artery surgery (ATACAS) trial. J Thorac Cardiovasc Surg. 2019; 157:644–652.e9 [DOI] [PubMed] [Google Scholar]
- 57.Murkin JM, Falter F, Granton J, et al. High-dose tranexamic acid is associated with nonischemic clinical seizures in cardiac surgical patients. Anesth Analg. 2010; 110:350–353 [DOI] [PubMed] [Google Scholar]
- 58.Keyl C, Uhl R, Beyersdorf F, et al. High-dose tranexamic acid is related to increased risk of generalized seizures after aortic valve replacement. Eur J Cardiothorac Surg. 2011; 39:e114–e121 [DOI] [PubMed] [Google Scholar]
- 59.Sharma V, Katznelson R, Jerath A, et al. The association between tranexamic acid and convulsive seizures after cardiac surgery: a multivariate analysis in 11 529 patients. Anaesthesia. 2014; 69:124–130 [DOI] [PubMed] [Google Scholar]
- 60.de Faria JL, da Silva Brito J, Costa E, et al. Tranexamic acid in Neurosurgery: a controversy indication-review [published online ahead of print, 2020 June 17]. Neurosurg Rev. [DOI] [PubMed] [Google Scholar]
- 61.Manji RA, Grocott HP, Leake J, et al. Seizures following cardiac surgery: the impact of tranexamic acid and other risk factors. Can J Anaesth. 2012; 59:6–13 [DOI] [PubMed] [Google Scholar]
- 62.Lecker I, Wang DS, Whissell PD, et al. Tranexamic acid-associated seizures: causes and treatment. Ann Neurol. 2016; 79:18–26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Lecker I, Wang DS, Romaschin AD, et al. Tranexamic acid concentrations associated with human seizures inhibit glycine receptors. J Clin Invest. 2012; 122:4654–4666 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kalavrouziotis D, Voisine P, Mohammadi S, et al. High-dose tranexamic acid is an independent predictor of early seizure after cardiopulmonary bypass. Ann Thorac Surg. 2012; 93:148–154 [DOI] [PubMed] [Google Scholar]
- 65.Bhat A, Bhowmik DM, Vibha D, et al. Tranexamic acid overdosage-induced generalized seizure in renal failure. Saudi J Kidney Dis Transpl. 2014; 25:130–132 [DOI] [PubMed] [Google Scholar]
- 66.Wang CS, Yang CJ, Chen SC, et al. Generalized convulsion resulted in hyperammonemia during treatment with tranexamic acid for hemoptysis. Ir J Med Sci. 2011; 180:761–763 [DOI] [PubMed] [Google Scholar]
- 67.Palija S, Bijeljac S, Manojlovic S, et al. Effectiveness of different doses and routes of administration of tranexamic acid for total hip replacement [published online ahead of print, 2020 May 6]. Int Orthop. 2020(E-pub ahead of print) [DOI] [PubMed] [Google Scholar]
- 68.Andersson L, Nilsoon IM, Colleen S, et al. Role of urokinase and tissue activator in sustaining bleeding and the management thereof with EACA and AMCA. Ann N Y Acad Sci. 1968; 146:642–658 [DOI] [PubMed] [Google Scholar]
- 69.Horrow JC, Van Riper DF, Strong MD, et al. The dose-response relationship of tranexamic acid. Anesthesiology. 1995; 82:383–392 [DOI] [PubMed] [Google Scholar]
- 70.Grassin-Delyle S, Tremey B, Abe E, et al. Population pharmacokinetics of tranexamic acid in adults undergoing cardiac surgery with cardiopulmonary bypass. Br J Anaesth. 2013; 111:916–924 [DOI] [PubMed] [Google Scholar]
- 71.Dowd NP, Karski JM, Cheng DC, et al. Pharmacokinetics of tranexamic acid during cardiopulmonary bypass. Anesthesiology. 2002; 97:390–399 [DOI] [PubMed] [Google Scholar]
- 72.Sigaut S, Tremey B, Ouattara A, et al. Comparison of two doses of tranexamic acid in adults undergoing cardiac surgery with cardiopulmonary bypass. Anesthesiology. 2014; 120:590–600 [DOI] [PubMed] [Google Scholar]
- 73.Imtiaz A, Mujahid-ul-Islam, Ansa I, et al. Effects of bolus dose and continuous infusion of tranexamic acid on blood loss after coronary artery bypass grafting. J Ayub Med Coll Abbottabad. 2014; 26:371–375 [PubMed] [Google Scholar]
- 74.Guo J, Gao X, Ma Y, et al. Different dose regimes and administration methods of tranexamic acid in cardiac surgery: a meta-analysis of randomized trials. BMC Anesthesiol. 2019; 19:129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Ker K, Beecher D, Roberts I. Topical application of tranexamic acid for the reduction of bleeding. Cochrane Database Syst Rev. 2013; ;23:CD010562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Montroy J, Hutton B, Moodley P, et al. The efficacy and safety of topical tranexamic acid: a systematic review and meta-analysis. Transfus Med Rev. 2018; 32:165–178 [DOI] [PubMed] [Google Scholar]
- 77.Tuttle JR, Feltman PR, Ritterman SA, et al. Effects of tranexamic acid cytotoxicity on in vitro chondrocytes. Am J Orthop (Belle Mead NJ). 2015; 44:E497–E502 [PubMed] [Google Scholar]
- 78.Marmotti A, Mattia S, Mangiavini L, et al. Tranexamic acid effects on cartilage and synovial tissue: an in vitro study for a possible safe intra-articular use. J Biol Regul Homeost Agents. 2016; 304 Suppl 133–40 [PubMed] [Google Scholar]
- 79.Parker JD, Lim KS, Kieser DC, et al. Is tranexamic acid toxic to articular cartilage when administered topically? What is the safe dose? Bone Joint J. 2018; 100-B:404–412 [DOI] [PubMed] [Google Scholar]
- 80.Eikebrokk TA, Vassmyr BS, Ausen K, et al. Cytotoxicity and effect on wound re-epithelialization after topical administration of tranexamic acid. BJS Open. 2019; 3:840–851 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Ausen K, Pleym H, Liu J, et al. Serum concentrations and pharmacokinetics of tranexamic acid after two means of topical administration in massive weight loss skin-reducing surgery. Plast Reconstr Surg. 2019; 143:1169e–1178e [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Dadure C, Sauter M, Bringuier S, et al. Intraoperative tranexamic acid reduces blood transfusion in children undergoing craniosynostosis surgery: a randomized double-blind study. Anesthesiology. 2011; 114:856–861 [DOI] [PubMed] [Google Scholar]
- 83.Murphy GR, Glass GE, Jain A. The efficacy and safety of tranexamic acid in cranio-maxillofacial and plastic surgery. J Craniofac Surg. 2016; 27:374–379 [DOI] [PubMed] [Google Scholar]
- 84.Briggs GD, Balogh ZJ. Tranexamic acid and inflammation in trauma. ANZ J Surg. 2020; 90:426–428 [DOI] [PubMed] [Google Scholar]
- 85.Kim HJ, Moon SH, Cho SH, et al. Efficacy and safety of tranexamic acid in melasma: a meta-analysis and systematic review. Acta Derm Venereol. 2017; 97:776–781 [DOI] [PubMed] [Google Scholar]
- 86.Wang D, Yang Y, He C, et al. Effect of multiple doses of oral tranexamic acid on haemostasis and inflammatory reaction in total hip arthroplasty: a randomized controlled trial. Thromb Haemost. 2019; 119:92–103 [DOI] [PubMed] [Google Scholar]
- 87.Lei Y, Xie J, Huang Q, et al. Additional benefits of multiple-dose tranexamic acid to anti-fibrinolysis and anti-inflammation in total knee arthroplasty: a randomized controlled trial. Arch Orthop Trauma Surg. 2020; 140:1087–1095 [DOI] [PubMed] [Google Scholar]
- 88.Jimenez JJ, Iribarren JL, Lorente L, et al. Tranexamic acid attenuates inflammatory response in cardiopulmonary bypass surgery through blockade of fibrinolysis: a case control study followed by a randomized double-blind controlled trial. Crit Care. 2007; 11:R117. [DOI] [PMC free article] [PubMed] [Google Scholar]


