Abstract
Objectives:
Literature suggests that neutrophils of patients with rheumatoid arthritis (RA) are primed to respond to N-formyl methionine group (formylated peptides). Animal models indicate that formylated peptides contribute to joint damage via neutrophil recruitment and inflammation in joints. Non-steroidal anti-inflammatory drugs are also known to inhibit formyl peptide-induced neutrophil activation. The predominant source of formylated peptides in sterile inflammatory conditions like RA is mitochondria, organelles with prokaryotic molecular signatures. However, there is no direct evidence of mitochondrial formyl peptides (mtNFPs) in the circulation of patients with RA and their potential role in neutrophil-mediated inflammation in RA, including their clinical significance.
Methods:
Levels of mtNFPs (total fMet, MT-ND6) were analyzed using ELISA in plasma and serum obtained from patients in 3 cross-sectional RA cohorts (n=275), a longitudinal inception cohort (n=192) followed for a median of 8 years, and age/gender-matched healthy controls (total n=134). Neutrophil activation assays were done in the absence or presence of formyl peptide receptor 1 (FPR1) inhibitor cyclosporine H.
Results:
Elevated levels of total fMet were observed in the circulation of patients with RA as compared to healthy controls (p<0.0001) associating with disease activity and could distinguish patients with the active disease from patients with inactive disease or patients in remission. Baseline levels of total fMet correlated with current and future joint involvement, respectively and predicted the development of rheumatoid nodules (OR=1.2, p=0.04). Further, total fMet levels improved the prognostic ability of ACPA in predicting erosive disease (OR of 7.9, p=0.001). Total fMet levels correlated with markers of inflammation and neutrophil activation. Circulating mtNFPs induced neutrophil activation in vitro through FPR1-dependent mechanisms.
Conclusions:
Circulating mtNFPs could be novel biomarkers of disease monitoring and prognosis for RA and in investigating neutrophil-mediated inflammation in RA. We propose, FPR1 as a novel therapeutic target for RA.
Keywords: Rheumatoid arthritis, mitochondria, formyl peptide receptor 1, neutrophils, clinical biomarkers
1. Introduction
Rheumatoid arthritis (RA) is a systemic chronic inflammatory disease primarily characterized by progressive joint destruction involving complex pathophysiology[1]. Now added to this complexity are mitochondrial N-formyl peptides (mtNFPs) that we hypothesize to contribute to RA pathogenesis via neutrophil activation. Chronic inflammation orchestrated by the excessive levels of inflammatory mediators released by resident and infiltrated cells contribute to tissue damage in RA [1]. Neutrophils are the most abundant immune cell type in the arthritic joint and play an essential role in the initiation and progression of RA [2, 3]. RA neutrophils, in general, have an activated phenotype characterized by enhanced oxidative burst, degranulation, and excessive release of neutrophil extracellular traps (NETs) [4–6]. NETs, mainly intended for microbial killing, are a potential source of citrullinated antigens, well-established principal targets of anti-citrullinated protein antibodies (ACPAs) in RA [7]. In a proinflammatory environment like RA, neutrophils are activated by various molecules, including immune complexes, cytokines, and damage-associated molecular patterns (DAMPs) like mitochondria [8–12]. Cell-free mitochondria, as well as mitochondrial DNA (mtDNA), have been found in the synovial fluid and circulation of RA patients suggesting mitochondrial release in the context of RA pathogenesis [13–15].
Mitochondria, owing to their microbial ancestry, possess many bacteria-like molecules, including circular mtDNA and mtNFPs. MtNFPs are known potent neutrophil chemotactic peptide which are able to induce the release of ROS from neutrophils via signaling through formyl-peptide receptor 1 (FPR1) [12, 16–20]. FPR1, a member of the G-protein coupled family of receptors (GPCRs), highly expressed by myeloid cells like neutrophils, monocytes, and macrophages exhibits strong binding affinity towards NFPs, including mtNFPs [21–23]. Signaling through FPR1 leads to numerous neutrophil effector functions, including reactive oxygen species (ROS) production, degranulation, and chemotaxis, which can contribute to tissue damage upon chronic activation of neutrophils [23]. FPRs are present both on the plasma membrane and in neutrophil granules, which can be mobilized upon priming with agents like bacterial endotoxin and TNF-α, suggesting that ongoing inflammation can amplify FPR-mediated inflammatory response of neutrophils [24–27]. Indeed, neutrophils from RA patients, but not control neutrophils, exhibit higher rates of oxidative burst upon activation by standard bacterial NFP, N-formylmethionyl-leucyl-phenylalanine (fMLP) [28]. This observation suggests that neutrophils in RA patients are primed to produce ROS in response to mtNFPs. However, the role of mtNFPs, potent neutrophil activation molecules, remains to be explored in RA pathogenesis. Specifically, it is not known whether mtNFPs are elevated in RA patients, their clinical significance, or their contribution to neutrophil-mediated inflammation in RA.
To investigate this biology, we first analyzed the circulating levels of mtNFPs in several independent RA cohorts in comparison to levels in healthy individuals. Secondly, we determined whether mtNFPs in RA patients could contribute to neutrophil activation and inflammation. This aim included an in vitro evaluation of the inflammatory potential of mtNFPs in RA plasma using FPR1 blockade experiments. Thirdly, we sought to determine the clinical relevance of mtNFPs to RA disease activity, joint damage, and their ability to predict the development of the extra-articular disease. In brief, we made the novel and significant observation that mtNFP levels were elevated in RA patients compared to healthy individuals and associated with neutrophil-mediated inflammation and disease progression, including development of rheumatoid nodules. FPR1 may thus be a novel therapeutic target in RA.
2. Materials and Methods
2.1. Patient Characteristics
Plasma samples from three independent cross-sectional RA cohorts and serum samples from one longitudinally followed inception RA cohort were analyzed in the current study. Patients with RA (Cohort 1, n=95) defined according to the American College of Rheumatology criteria 1987, and age- and gender-matched healthy individuals were recruited to participate in research studies at the University of Washington Medical Center, Seattle. The majority of the patients in cohort 1 were female (75%), white (65%), and median (range) age at the time of diagnosis was 54 (20–78) years. Clinical disease activity index (CDAI) (information available for 70% of patients) that considered tender and swollen joints, patient global assessment, and provider global assessment was 11 (0–46) reflecting moderate disease activity. Based on CDAI scores, patients are sub-grouped as follows: patients in remission (CDAI <3), low disease activity (CDAI 3–10), moderate disease activity (CDAI 11–22), and high disease activity (CDAI ≥23). The second cross-sectional cohort of patients (Cohort 2, n=87) with established RA as well as one RA inception cohort (Cohort 3, n=192) followed for a median of 8.3 years (range 4.4 – 19.8 years) following disease onset were recruited in Washington State. The study was approved by regional ethics boards (#3100 and #810), and informed written consent was obtained from all participants in accordance with the Helsinki Declaration. A fourth cross-sectional cohort (Cohort 4, n=93) selected from the Studies of the Etiology of RA (SERA) cohort in Denver, Colorado (IRB# 13–2606), was included to validate key findings from Cohort 1. Subjects were randomly selected based on availability of RA disease activity measures. Overall, subjects in Cohort 4 had low disease activity with median (range) CDAI of 8 (0–50) and DAS28-CRP of median (range) 2.11 (0.97–5.26), respectively. The median age (range) of patients at the time of diagnosis is 60 (20–78) years, with the majority of patients being female (85%) and non-Hispanic white (69%). Patient cohorts 1, 2, and 3 have been reported previously[29], with additional patient characteristics, including that of cohort 4 and SLE cohort are given in Table 1.
Table 1.
Demographic, clinical and treatment information of subjects.
| Cohort | RA 1 | RA 2 | RA 3 | RA4 | HC 1 | HC 2 | HC3 | SLE |
|---|---|---|---|---|---|---|---|---|
| Patients (#) | 95 | 87 | 192 | 93 | 36 | 48 | 50 | 44 |
| Specimen | Plasma | Plasma | Serum | Plasma | Plasma | Serum | Plasma | Plasma |
| Age in years (median, range) | 54 (20–78) | 47 (18–64) | 43 (16–64) | 60 (20–78) | 57 (26–71) | 43 (16–64) | 59 (19–59) | 33 (19–61) |
| Gender (% female) | 75 | 100 | 100 | 85 | 85 | 100 | 84 | 91 |
| Ethnicity (white, %) | 65 | 88 | 88 | 69 (non-Hispanic White) | 100 | 88 | 80 (non-Hispanic White) | 65 |
| Seropositive (%) | 80 | 68 | 71 | 100 | N/A | N/A | 0 | N/A |
| RF (%) | 72 | 52 | 59 | 69 | N/A | N/A | 4 | N/A |
| ACPA (%) | 76 | 57 | 57 | 100 | N/A | N/A | 0 | N/A |
| Outcome measure | Disease activity | Erosion | Erosion | Disease activity | N/A | N/A | N/A | N/A |
| CDAI (median, range) | 11 (0–46) | N/A | N/A | 8 (0–50) | N/A | N/A | N/A | N/A |
| Erosive disease (%) | 50 | 54 | 10 | N/A | N/A | N/A | N/A | N/A |
| Immunosuppression (%) | 78 | 38 | 1 | N/A | N/A | N/A | N/A | N/A |
| Figures | 1A,B | 4A-C | 2D | 1A | ||||
| 2A,B,E | 5F-H | 5I-J | 2C,2F | |||||
| 3A | 3B | |||||||
| 5A-E | 5K | |||||||
| 6A-C | ||||||||
| Symbol | White circle ○ | White square □ | White up-pointing triangle Δ | White down-pointing triangle ∇ | Black circle ● | Black circle ● | Black square ■ | |
2.2. ELISA-based methods
Plasma levels of mtNFPs and sPLA2 (human formyl methionine (fMet), and human mitochondrially encoded NADH dehydrogenase 6 (MT-ND6) and Human secreted phospholipase A2, My BioSource Inc., San Diego, CA) were determined by ELISA following manufacturer’s instructions. Absorbance was measured using Synergy 2, BioTek (Winooski, VT). Standards were used as reference to calculate concentrations of measured analytes in plasma. Total fMET levels were defined as high if above the 95th percentile of healthy controls. The ability of total fMet ELISA to detect mtNFPs was validated by analyzing mitochondrial lysates as positive controls, whereas non-mitochondrial fractions collected during mitochondrial isolation were included as negative controls. The mitochondrial lysates were enriched for total fMet compared to non-mitochondrial fractions, validating the fMet assay (Supplementary figure 1).
2.3. Isolation of neutrophils
To isolate neutrophils from the blood of healthy subject, heparinized blood was layered on Polymorphprep (Axis-Shield, Dundee, UK) density gradient, according to the manufacturer’s instructions, or as described previously [7, 30]. Red blood cells were lysed with RBC lysis buffer (BioLegend). Neutrophils were re-suspended in serum-free RPMI-1640 medium (Life Technologies, Waltham, MA) for in vitro assays.
2.4. ROS analysis for fMet signaling
Neutrophils, plated at 3 × 105 cells/well, were incubated with a selective inhibitor of FPR1, cyclosporine H (CsH, 5 μM) for 30 min prior to the addition of stimuli, R848 (2.5 μg/ml), N-Formyl-Met-Leu-Phe (1 μM), formylated MT-ND6 peptide (1 μM) or plasma (1:100 dilution) for an additional 60 min. DHR 123 (0.5 μM), was added during last 30 min of incubation and ROS was analyzed by flow cytometry. Published sequence of formylated MT-ND6 peptide (f-MMYALF) [31] was synthesized commercially (GenScript USA, Inc.) and was tested as endotoxin-free.
2.5. Statistics
For sample sets with a non-Gaussian distribution, non-parametric tests, Mann-Whitney U and Spearman’s correlation, were used as applicable. High modified Sharp erosion score was defined as the upper quartile within the RA inception cohort [32]. All analyses were considered statistically significant at p<0.05. Hierarchical clustering was performed using the R v4.0.2 pheatmap v1.0.12 (https://www.r-pkg.org/pkg/pheatmap).
3. Results
Levels of mtNFPs (total fMet and MT-ND6) in plasma were measured using two different ELISAs to distinguish between total fMet levels, representing the mtDNA-encoded proteome of 13 mitochondrial proteins, as well as a peptide specific to MT-ND6, a complex I protein of the mitochondrial electron transport chain, respectively. Following the validation of fMet ELISA assay for analyzing total fMet levels (detailed in methods, Supplementary Figure 1), analyses were extended to clinical samples.
3.1. Patients with RA have elevated levels of mtNFPs
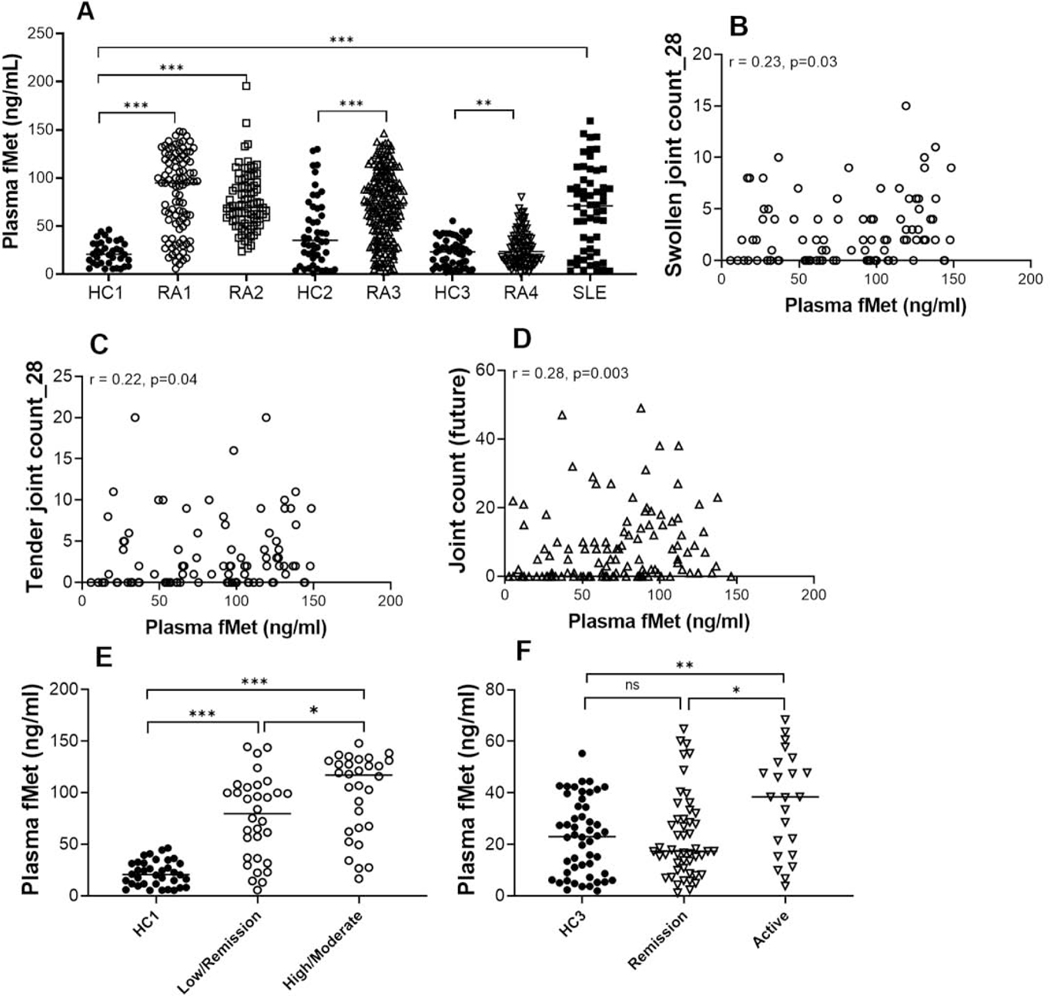
As shown in Figure 1A, total fMet levels were elevated in all three RA cohorts compared to healthy controls (p<0.0001 for all analyses). In RA cohort 4, which had overall low disease activity (median CDAI 8; media DAS28-CRP of 2.11), total fMet levels were significantly lower compared to the other three RA cohorts (median fMet 23.40 ng/mL vs. 94.69 ng/mL, 69.11 ng/mL, 71.47 ng/mL, respectively; P<0.0001 for all comparisons). However, RA4 patients with active disease (DAS28-CRP ≥ 2.6) had elevated levels of total fMet compared to healthy individuals (p=0.004). Based on the evidence of mitochondrial extrusion in SLE[33, 34], total fMet levels of SLE patients were included as a positive disease control for mtNFPs. As expected, SLE patients had elevated levels of total fMet compared to HC1 (Figure 1A, p<0.0001). Although, patients in RA1 have elevated levels of total fMet compared to SLE (94.69 vs. 70.93 ng/mL ng/mL; p<0.01), overall levels of total fMet were comparable between patients with RA and SLE, suggesting fMet levels to be elevated in conditions of inflammation. Finally, MT-ND6 levels were significantly associated with total fMet levels (r=0.27, p=0.01, Supplementary Figure 2) suggesting that individual mtNFPs are also present at measurable levels in RA plasma.
Figure 1: Levels of mtNFPs are elevated in patients with RA and associate with disease activity.
Total (fMet) and MT-ND6 specific levels of NFPs were analyzed by ELISA. A, Total fMet levels were analyzed in 4 cohorts of RA patients, a SLE cohort and 3 cohorts of healthy controls (HC). Plasma samples were analyzed in cohorts RA1, RA2, RA4, SLE, HC1 and HC3. Serum samples were analyzed in cohorts RA3 and HC2. For RA4, only fMet levels of patients with low to high DAS28-CRP (> 2.6) were considered, but not levels in patients in remission (<2.6 DAS28-CRP). Correlation analysis between levels of total fMet and swollen joints (B), tender joints (C) of RA1 and future joint count (D) in the inception cohort, RA3. Healthy controls and RA patients were analyzed based on total fMet levels. Patients were further analyzed based on CDAI in RA1 (E) and DAS28-CRP in RA4 (F). Statistical analyses were done using Mann-Whitney U test, and Spearman correlation test with * P < 0.05, ** p<0.01, *** p<0.0001, and ns, non-significant.
3.2. MtNFPs (total fMet levels) in patients with RA associate with joint damage and disease activity
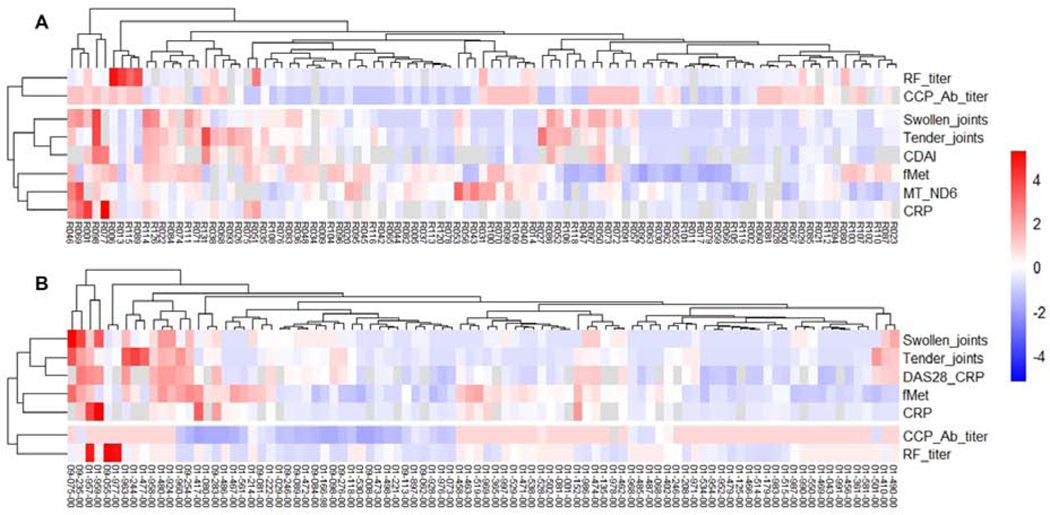
We next asked whether analysis of mtNFPs had clinical significance. Levels of total fMet correlated with markers of active disease, including swollen and tender joints (r=0.23, p=0.03, and r=0.22, p=0.04, respectively, for RA1 Figures 1C–D and r=0.25, p=0.02 for RA4, Supplementary Figure 3). For both RA cohorts 1 and 4, mtNFP levels could distinguish patients with moderate-high disease activity (CDAI ≥11; DAS28-CRP ≥3.2) from patients with low disease activity and/or in remission (CDAI ≤10; DAS28-CRP <3.2) (Figures 1E–F). In RA cohort 1, even patients in remission had significantly elevated levels of total fMet compared to HC1, suggesting ongoing subclinical inflammation. The strong association of fMet levels with disease activity in cohorts RA1 and RA4 is further demonstrated in clustering analysis, as shown in heat maps (Figures 2A–B) where fMet levels were enriched in a cluster of disease activity markers, whereas it did not associate with diagnostic markers of RA, including rheumatoid factor and anti-cyclic citrullinated peptide antibody titers. Finally, levels of mtNFPs were not only associated with current joint involvement (Figures 1B–C and Supplementary Figure 3), but also with future joint involvement, with baseline levels of mtNFPs correlating with joint involvement several years later in the disease process (r=0.28, p=0.003, Figure 1D, RA3).
Figure 2: Levels of mtNFPs (total fMet) in RA patients cluster with markers of disease activity and inflammation.
Shown in A and B are heat maps showing hierarchical clustering of various disease activity, diagnostic and inflammatory markers with levels of mtNFPs in cohorts RA1 (A) and RA4 (B). Rows and columns represent markers and patients, respectively. Hierarchical clustering was performed using the R v4.0.2 pheatmap v1.0.12
3.3. Levels of mtNFPs (total fMet levels) associate with disease progression in combination with ACPA
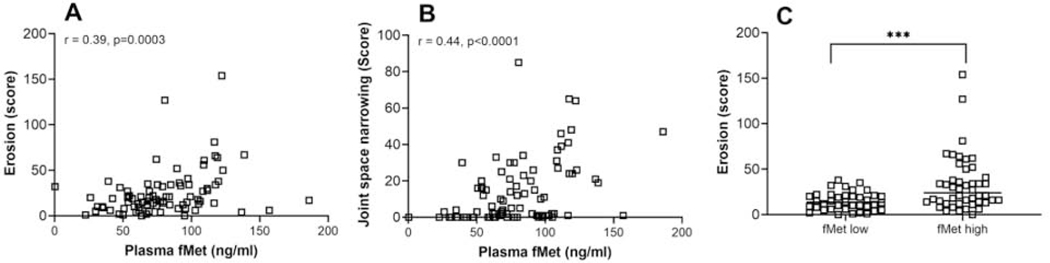
In RA cohort 2 (follow-up cohort), mtNFP levels correlated significantly with current joint erosion and joint space narrowing (r=0.39, p=0.0003, and r=0.44, p<0.0001, respectively, Figures 3A–B). We next asked whether baseline mtNFPs levels were predictive of development of erosive disease and extra-articular manifestations. We excluded patients with radiographic evidence of erosive disease at inception (10%) from this analysis. Although total fMet levels were strongly associated with erosion score at time of blood sampling as seen in the follow-up cohort, RA2 (Figure 3C), baseline fMet levels by themselves did not predict development of high modified Sharp erosion score (OR =2.3 (0.7–7.4), p=0.15). Since ACPA positivity is a strong predictor of radiographic progression of joint erosion in RA patients [35], we next explored if fMet levels improve the prognostic value of ACPA in predicting erosive disease. Patients positive for ACPA or fMet exhibited increased odds of developing erosive disease with high modified Sharp erosion score (ACPA+ or fMet + OR=7.9 (2.3–27.7), p=0.001) as compared to patients positive for ACPA alone (ACPA+ OR (95%, CI) =5.7 (2.0–15.9), p=0.001). The sensitivity and specificity for ACPA, fMet and ‘ACPA or fMet’ for development of a high modified Sharp erosion score are 32.4%, 31.6% and 31.3%, respectively, and 92.2%, 83.6% and 94.5%, respectively.
Figure 3: MtNFPs (total fMet) circulating in RA plasma associate with joint damage.
Correlation analysis between levels of total fMet and erosion score (A) joint space narrowing (B) and (C) comparison of erosion scores between patients stratified based on fMet levels as fMet low or fMet high (fMet levels above 95th percentile of HC are considered as fMet high) for follow-up cohort, RA2 white square □. Statistical analyses were done using Mann-Whitney U test, and Spearman correlation test with *** p<0.001.
Although known primarily for joint inflammation, about 50% of patients with RA will also develop extra-articular manifestations [36]. Extra-articular RA is a severe condition associating with worse disease outcomes [36]. In an inception cohort of RA (cohort 3), 23 of 165 patients (14%) developed extra-articular nodules during follow-up, a mean of 8 years later. Further univariate analyses revealed that mtNFP levels at baseline are predictive of extra-articular (nodules) development (OR=1.2, p=0.04, 95% CI 1.0–1.4). The OR is for an increase of 10 ng/ml in baseline mtNFP levels.
3.4. MtNFPs (total fMet levels) associate with neutrophil activation markers and inflammation in patients with RA
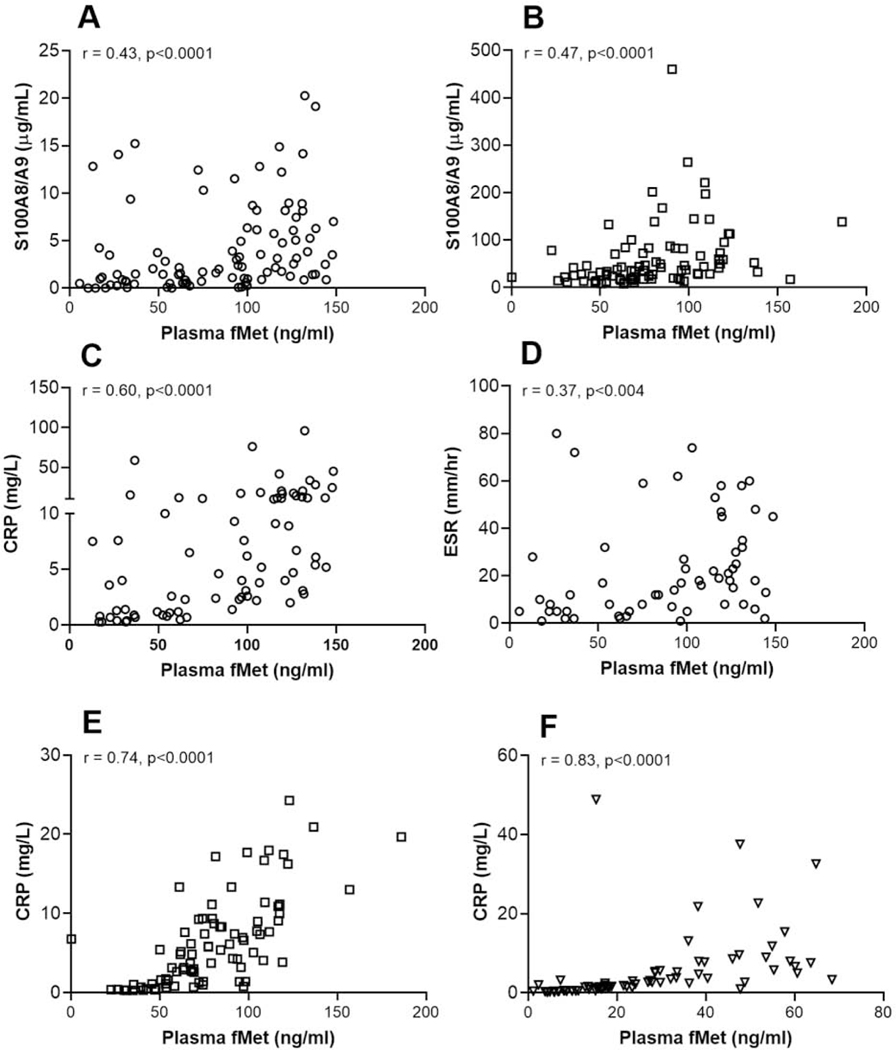
Considering the ability of mtNFPs to induce neutrophil activation, we assessed the association of mtNFP levels with neutrophil activation markers. In all three RA cohorts we found circulating mtNFP levels to be associated significantly with neutrophil activation markers including levels of S100A8/9 or calprotectin, NETs and peroxidase (fMet vs. S100A8/9 and peroxidase, for RA1: r=0.43, r=0.43, p<0.0001 for all analyses, Figure 4A and Supplementary Figure 4A; fMet vs. S100A8/9 for RA2: r=0.57, r=0.47, p<0.0001 for all analyses, Figures 4B and Supplementary Figure 4B; RA3 : r=0.25, p<0.0005, r=0.25, p<0.002, Supplementary Figures 4C–D). There were also significant correlations between mtNFP levels and clinical markers of systemic inflammation, ESR and CRP (fMet vs. CRP and ESR for RA1: r=0.60, p<0.0001, r=0.37, p<0.004, Figures 4C–D; fMet vs. CRP for RA2 and RA4: r=0.74, r=0.83, p<0.0001 for all analyses, Figures 4E–F). Further analysis revealed that total fMet levels in RA1 cluster with levels of neutrophil activation markers (peroxidase, and S100) and a clinical marker of inflammation, CRP (Supplementary Figure 4E).
Figure 4: Levels of mtNFPs (total fMet) in RA patients associate with neutrophil activation markers and clinical measures inflammation.
Total fMet and S100A8/A9 levels were analyzed by ELISA. Shown in figures A, C and D, figures B and E and figure F are data from cohorts: RA1 (white circle ○), RA2 (white square □), and RA4 (white down-pointing triangle ∇), respectively. Statistical analyses were done using Spearman correlation test.
3.5. Circulating mtNFPs (total fMet) induce ROS production from neutrophils in an FPR1-dependent manner
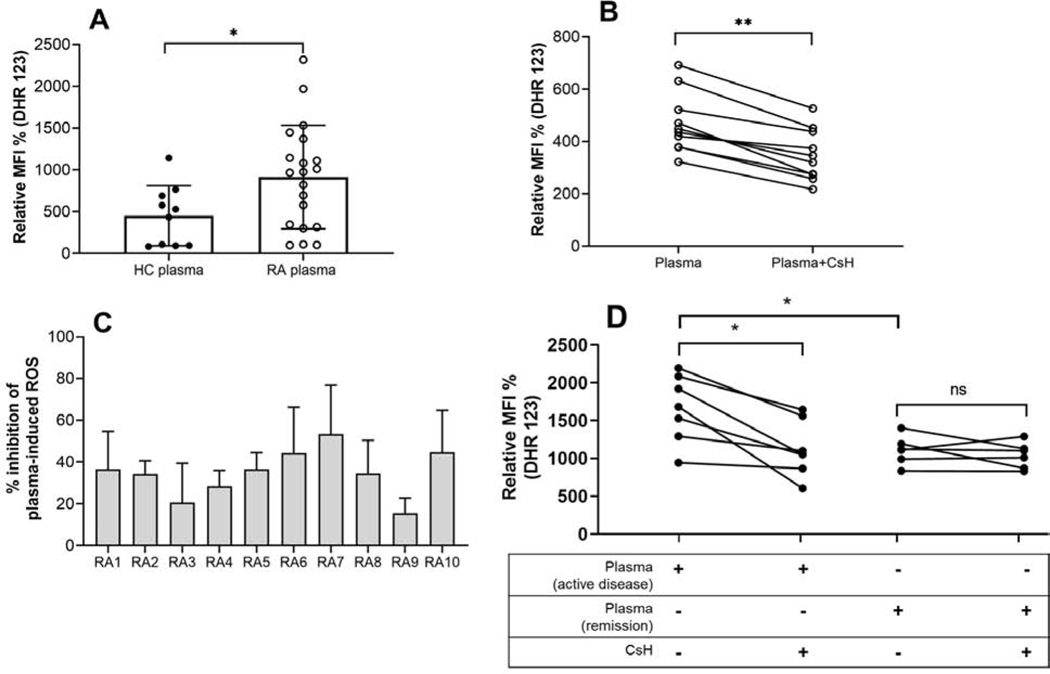
MtNFPs signal through FPRs to induce neutrophil effector functions, including ROS generation. We thus asked whether NFPs, present in RA plasma, could promote neutrophil activation, such as ROS generation in vitro. We optimized the assay with fMLP, a prototype bacterial NFP, and formylated MT-ND6 peptide that are known to induce ROS generation in neutrophils. Cyclosporine H (CsH), an FPR1 inhibitor, could completely block the ROS generation induced by fMLP and formylated MT-ND6 whereas non-FPR1 triggered ROS generation (e.g. TLR7/8 agonist) could not be inhibited, thus confirming the specificity of the inhibitor (Supplementary Figure 5). Compared to HC plasma, plasma from RA patients caused significant induction of ROS release from healthy control neutrophils (p=0.02, Figure 5A). However, this ROS induction could be due to many inflammatory mediators present in plasma, including proinflammatory cytokines. To determine whether RA plasma-induced ROS generation was mtNFP-dependent, RA plasma that were high ROS inducers (above 85th percentile of ROS induced by HC plasma) were analyzed for ROS induction by neutrophils in presence or absence of CsH. Although CsH treatment did not reduce ROS generation to baseline as for prototype NFPs, it did inhibit a significant proportion, ranging from 15% to 53% (Figure 5B), of RA plasma-induced ROS formation by neutrophils, suggesting that mtNFPs in plasma are contributing to neutrophil activation (p=0.001, Figure 5C). To further investigate the relevance of these circulating mtNFPs to disease activity, we compared neutrophil ROS-induction potential of plasma from patients with active disease and patients in remission with normal CRP (<3 mg/L) in FPR1-dependent manner i.e. by stimulating neutrophils with patient plasma in the presence or absence of CsH. Consistent with the association of mtNFPs with disease activity (Figure 1E), plasma from patients with active disease induced significantly elevated levels of ROS from neutrophils compared to plasma from patients in remission (Figure 5D). Further, neutrophil ROS-induction by plasma from patients with active disease but not by plasma from patients in remission could be significantly attenuated with FPR1-inhibition suggesting that mtNFPs primarily contribute to disease-relevant inflammation in active disease.
Figure 5: MtNFPs (total fMet) circulating in RA plasma can induce ROS release from neutrophils in FPR1-dependent manner.
(A) Neutrophil ROS induced by RA plasma compared to HC plasma. (B) Inhibition of RA plasma-induced neutrophil ROS by CsH. (C) Percent inhibition of RA plasma-induced neutrophil ROS. (D) Comparison of ROS-induction potential of plasma from patients with active disease and patients in remission from neutrophils with and without pretreatment with FPR1 inhibitor, CsH. Healthy neutrophils plated at 3 × 105 cells/well were incubated with a selective inhibitor of FPR1, CsH (5 μM) for 30 min prior to the addition of plasma (1:100 dilution) for an additional 60 min. DHR 123 (0.5 μM), was added during last 30 min of incubation and ROS was analyzed by flow cytometry. Relative MFI % was calculated as ROS induced by stimuli divided by media control x 100; % inhibition was calculated as ROS induced by plasma only – ROS induced by plasma pretreated with CsH condition divided by ROS induced by plasma only x 100. Statistical analyses were done using Mann-Whitney U test and Wilcoxon test for paired analyses with * < 0.05, and ** p<0.01. Samples belong to RA1, white circle ○.
4. Discussion
This study presents evidence of an association between mtNFP-mediated neutrophil activation in RA patients and inflammation, disease activity, and joint damage, supporting their potential role in amplifying a central disease-promoting process of RA pathogenesis via chronic neutrophil activation. Overall, we propose mtNFPs as novel clinical biomarkers for measure of disease activity and disease severity and mtNFP-mediated signaling as a potential therapeutic target of RA.
Several cell types, including neutrophils, activated platelets, mast cells, and damaged cells, are known sources of extracellular mitochondria and their derived products in sterile inflammatory pathologies like RA. Consistently Boudreau et al [15] have demonstrated that synovial fluid of RA patients, but not osteoarthritis patients, have elevated levels of platelet-derived extracellular mitochondria. MtNFPs are intramitochondrial. Potential mechanisms of mtNFPs release from mitochondria include the digestion of mitochondrial membrane by enzymes like secreted phospholipase A2 and/or complement-mediated lysis [15, 37]. Incidentally, secreted phospholipases A2 are observed at increased concentrations in the circulation of inflammatory conditions, including RA [38]. Consistent with the literature, we also found elevated levels of sPLA2 in the plasma of our RA patient cohort compared to controls (Supplementary Figure 6), demonstrating the presence of a mechanism that can potentially contribute to elevated fMet levels in RA. However, further studies are needed to determine the pathway(s) essential in promoting mitochondrial disruption in RA.
Once released into the extracellular space, mtNFPs can be sensed by high-affinity FPR1 predominantly expressed on phagocytic leukocytes, including monocytes, macrophages with a high expression in neutrophils [39]. Activation of FPR1 by mtNFPs, akin to bacterial NFPs, elicit signaling cascades that culminate in diverse neutrophil effector responses, including oxidative burst [18, 23]. While not in the RA context, mtNFPs released during sterile injury were demonstrated to cause neutrophil migration and degranulation and elicit neutrophil-mediated organ injury [18]. However, the role of mtNFPs in neutrophil activation contributing to RA pathogenesis is unknown. We have previously reported on elevated levels of neutrophil activation markers in RA patients able to predict erosive disease and joint space narrowing [29]. Our current data demonstrate that plasma from RA patients had increased ability to induce ROS from neutrophils of healthy blood donors. FPR1 blockade experiments suggested that mtNFPs circulating in RA plasma contribute significantly to this immune activation of neutrophils. As further evidence, we found that RA patients have elevated levels of mtNFPs associating with neutrophil activation markers like calprotectin (S100A8/A9), peroxidase, and NETs, all of which are reported to be increased in RA patients [29]. Considering the clinical implications, mtNFP levels were associated with CRP and ESR, inflammatory markers commonly incorporated in disease activity indexes. In the current analysis both ACPA and RF, current diagnostic markers of RA, did not associate with disease activity index. In contrast, levels of mtNFPs significantly associated with disease activity and could distinguish patients based on disease activity as demonstrated in two independent RA cohorts. Additional analysis also revealed that mtNFPs do not cluster with RF and ACPA suggesting mtNFPs to be independent indicators of disease activity. Of note, levels of mtNFPs were elevated in some patients in clinical remission (CDAI ≤2.8), suggesting mtNFP could be a sensitive marker of ongoing subclinical disease activity. This is consistent with prior work from our group, and others, demonstrating a neutrophil activation signature in RA patients in clinical remission [29, 40]. Hence, when analyzed, circulating biomarkers like mtNFPs and neutrophil-related markers in combination with conventional blood markers may be useful in clinical practice to detect low-grade inflammatory activity of RA patients in clinical remission.
One of the key findings from the current investigation relates to the capacity of mtNFPs to associate with disease progression, e.g., development of future joint damage as well as rheumatoid nodules and mtNFPs, in combination with ACPAs, showed an improved ability to predict the development of the erosive disease than ACPA alone, which is one of the strongest predictors of radiographic progression of joint erosion in RA [35]. Interestingly, NETs were shown to make similar predictions, strengthening our proposition of mtNFP-driven neutrophil activation in RA [29]. The mechanistic details of mtNFP-driven extra-articular development in RA remains to be investigated. Given the study design, causality cannot be implied. However, consistent with our findings, prior work in a mouse model of septic arthritis clearly demonstrate that bacterial formylated peptides are sufficient in mediating S. aureus-induced arthritis [41]. In that study, mice immunized with the wild-type strain of S. aureus, but not with an isogenic mutant strain lacking the ability to produce formylated peptides, developed arthritis, and severe joint destruction accompanied by the increased infiltration of neutrophils. Future studies involving arthritic animal models deficient in FPR1 are needed to characterize the mechanism of mtNFP-mediated joint injury in RA.
The study limitation includes analyses and experiments considering mtNFP-mediated activation of neutrophils alone. Although FPR1 is highly expressed on neutrophils, other immune cells implicated in RA pathogenesis, including platelets, monocytes, and macrophages, also express FPR1 suggesting the potential activation of those cells by circulating mtNFPs in vivo, warranting future biomarker and RA animal model studies analyzing these additional cell types. Further limitation includes the effect of different treatment regimens on the levels of mtNFPs considering that release mechanisms of mtNFPs are associated with inflammation. Hence, the inclusion of pre-clinical and treatment-naïve patients with RA in future studies should determine treatment effects on the levels of mtNFPs and validate the prognostic capacity of mtNFPs for disease activity. Although our longitudinal cohort allowed us to conduct predictive analyses for the joint destruction, sampling at the inception alone limited further analysis to assess the change in mtNFPs levels with disease progression and activity and substantiate the potential association of mtNFPs with joint destruction. Another potential limitation could be the alternate sources of formylated peptides other than mitochondria in the circulation of RA patients, which include bacteria, the prokaryotic ancestors of mitochondria. Given the variable evidence of increased intestinal permeability (i.e., leaky gut) in RA patients [42–44], whereby gut bacteria can gain access to the bloodstream, we cannot rule out the possibility that some of the measured NFPs in our study are potentially from bacterial sources and thus are not exclusively of mitochondrial origin. The role of the gut-joint axis in RA pathogenesis majorly encompasses the molecular mimicry between antigens of gut microbiota and host proteins that can be enabled by increased intestinal permeability. This suggests a hypothesis where bacterial NFPs in the circulation of RA patients could contribute to chronic neutrophil activation as a novel mechanism through which leaky gut and its associated risk factors such as pathological inflammation across intestinal barrier and gut dysbiosis can contribute to RA pathogenesis; this will need to be investigated in future studies.
5. Conclusion
For the first time, we have demonstrated that mtNFPs are elevated in the circulation of patients with RA and promote neutrophil activation through formyl peptide receptor 1 (FPR1). Our data demonstrate the clinical value of mtNFPs in monitoring disease activity and in predicting disease severity, including extra-articular disease in RA patients, although these observations remain to be validated in larger patient cohorts. Several non-steroidal anti-inflammatory drugs (NSAIDs) are known to inhibit fMLP-induced neutrophil activation [45–47]. Thus, it is likely that FPR1 antagonistic property of NSAIDs might contribute to, at least, some of their anti-inflammatory effects. Further, genetic deletion or pharmacological inhibition of FPR1 demonstrated significant anti-inflammatory effects in various in vitro and in vivo studies [48–50]. Hence, FPRs in the context of sterile injury and conditions of unresolved chronic inflammation like RA represent important therapeutic targets for ameliorating neutrophil-dominant inflammation.
Supplementary Material
Highlights.
Mitochondrial formyl peptides (mtNFPs) are elevated in the circulation of patients with RA.
Levels of mtNFPs are potential biomarkers of disease activity and progression in RA.
Circulating mtNFPs of patients with RA contribute to neutrophil-mediated inflammation in a formyl peptide receptor 1 -dependent manner.
Acknowledgements and Funding
This work was supported by Arthritis National Research Foundation (#632002) to CL, the National Center for Advancing Translational Sciences of the National Institutes of Health (#UL1 TR002319) to BD, the NIH grants AR066712 to MKD, and R01-AR39282 to JLN.
Footnotes
Competing interests
None declared.
Author Statement
Bhargavi Duvvuri: Conceptualization, Methodology, Formal analysis, Investigation, Writing – Original Draft, Visualization, Funding acquisition. Al Anoud Baddour: Investigation, Writing – Review & Editing. Kevin D Deane: Resources, Data Curation, Writing – Review & Editing. Marie L. Feser: Resources, Data Curation, Writing – Review & Editing. J. Lee Nelson: Resources, Data Curation, Writing – Review & Editing, Funding acquisition. M. Kristen Demoruelle: Resources, Data Curation, Writing – Review & Editing, Funding acquisition. Christian Lood: Conceptualization, Methodology, Formal analysis, Writing – Original Draft, Writing – Review & Editing, Supervision, Funding acquisition.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].McInnes IB, Schett G. The pathogenesis of rheumatoid arthritis. N Engl J Med, 2011;365:2205–19. [DOI] [PubMed] [Google Scholar]
- [2].Wipke BT, Allen PM. Essential role of neutrophils in the initiation and progression of a murine model of rheumatoid arthritis. J Immunol, 2001;167:1601–8. [DOI] [PubMed] [Google Scholar]
- [3].Tanaka D, Kagari T, Doi H, Shimozato T. Essential role of neutrophils in anti-type II collagen antibody and lipopolysaccharide-induced arthritis. Immunology, 2006;119:195–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Wright HL, Chikura B, Bucknall RC, Moots RJ, Edwards SW. Changes in expression of membrane TNF, NF-{kappa}B activation and neutrophil apoptosis during active and resolved inflammation. Ann Rheum Dis, 2011;70:537–43. [DOI] [PubMed] [Google Scholar]
- [5].Mantovani A, Cassatella MA, Costantini C, Jaillon S. Neutrophils in the activation and regulation of innate and adaptive immunity. Nat Rev Immunol, 2011;11:519–31. [DOI] [PubMed] [Google Scholar]
- [6].Amulic B, Cazalet C, Hayes GL, Metzler KD, Zychlinsky A. Neutrophil function: from mechanisms to disease. Annu Rev Immunol, 2012;30:459–89. [DOI] [PubMed] [Google Scholar]
- [7].Khandpur R, Carmona-Rivera C, Vivekanandan-Giri A, Gizinski A, Yalavarthi S, Knight JS et al. NETs are a source of citrullinated autoantigens and stimulate inflammatory responses in rheumatoid arthritis. Sci Transl Med, 2013;5:178ra40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Aleyd E, Al M, Tuk CW, van der Laken CJ, van Egmond M. IgA Complexes in Plasma and Synovial Fluid of Patients with Rheumatoid Arthritis Induce Neutrophil Extracellular Traps via FcαRI. J Immunol, 2016;197:4552–9. [DOI] [PubMed] [Google Scholar]
- [9].Robinson J, Watson F, Bucknall RC, Edwards SW. Activation of neutrophil reactive-oxidant production by synovial fluid from patients with inflammatory joint disease. Soluble and insoluble immunoglobulin aggregates activate different pathways in primed and unprimed cells. Biochem J, 1992;286 ( Pt 2):345–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Fossati G, Bucknall RC, Edwards SW. Insoluble and soluble immune complexes activate neutrophils by distinct activation mechanisms: changes in functional responses induced by priming with cytokines. Ann Rheum Dis, 2002;61:13–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Kolaczkowska E, Kubes P. Neutrophil recruitment and function in health and inflammation. Nat Rev Immunol, 2013;13:159–75. [DOI] [PubMed] [Google Scholar]
- [12].McDonald B, Pittman K, Menezes GB, Hirota SA, Slaba I, Waterhouse CC et al. Intravascular danger signals guide neutrophils to sites of sterile inflammation. Science, 2010;330:362–6. [DOI] [PubMed] [Google Scholar]
- [13].Collins LV, Hajizadeh S, Holme E, Jonsson IM, Tarkowski A. Endogenously oxidized mitochondrial DNA induces in vivo and in vitro inflammatory responses. J Leukoc Biol, 2004;75:995–1000. [DOI] [PubMed] [Google Scholar]
- [14].Hajizadeh S, DeGroot J, TeKoppele JM, Tarkowski A, Collins LV. Extracellular mitochondrial DNA and oxidatively damaged DNA in synovial fluid of patients with rheumatoid arthritis. Arthritis Res Ther, 2003;5:R234–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Boudreau LH, Duchez AC, Cloutier N, Soulet D, Martin N, Bollinger J. et al. Platelets release mitochondria serving as substrate for bactericidal group IIA-secreted phospholipase A2 to promote inflammation. Blood, 2014;124:2173–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Wenceslau CF, Szasz T, McCarthy CG, Baban B, NeSmith E, Webb RC. Mitochondrial N-formyl peptides cause airway contraction and lung neutrophil infiltration via formyl peptide receptor activation. Pulm Pharmacol Ther, 2016;37:49–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Schiffmann E, Corcoran BA, Wahl SM. N-formylmethionyl peptides as chemoattractants for leucocytes. Proc Natl Acad Sci U S A, 1975;72:1059–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Zhang Q, Raoof M, Chen Y, Sumi Y, Sursal T, Junger W. et al. Circulating mitochondrial DAMPs cause inflammatory responses to injury. Nature, 2010;464:104–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Carp H. Mitochondrial N-formylmethionyl proteins as chemoattractants for neutrophils. J Exp Med, 1982;155:264–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Rabiet MJ, Huet E, Boulay F. Human mitochondria-derived N-formylated peptides are novel agonists equally active on FPR and FPRL1, while Listeria monocytogenes-derived peptides preferentially activate FPR. Eur J Immunol, 2005;35:2486–95. [DOI] [PubMed] [Google Scholar]
- [21].Dorward DA, Lucas CD, Chapman GB, Haslett C, Dhaliwal K, Rossi AG. The role of formylated peptides and formyl peptide receptor 1 in governing neutrophil function during acute inflammation. Am J Pathol, 2015;185:1172–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Fu H, Karlsson J, Bylund J, Movitz C, Karlsson A, Dahlgren C. Ligand recognition and activation of formyl peptide receptors in neutrophils. J Leukoc Biol, 2006;79:247–56. [DOI] [PubMed] [Google Scholar]
- [23].Raoof M, Zhang Q, Itagaki K, Hauser CJ. Mitochondrial peptides are potent immune activators that activate human neutrophils via FPR-1. J Trauma, 2010;68:1328–32; discussion 32–4. [DOI] [PubMed] [Google Scholar]
- [24].Hayashi F, Means TK, Luster AD. Toll-like receptors stimulate human neutrophil function. Blood, 2003;102:2660–9. [DOI] [PubMed] [Google Scholar]
- [25].Kitchen E, Rossi AG, Condliffe AM, Haslett C, Chilvers ER. Demonstration of reversible priming of human neutrophils using platelet-activating factor. Blood, 1996;88:4330–7. [PubMed] [Google Scholar]
- [26].Sengeløv H, Boulay F, Kjeldsen L, Borregaard N. Subcellular localization and translocation of the receptor for N-formylmethionyl-leucyl-phenylalanine in human neutrophils. Biochem J, 1994;299 ( Pt 2):473–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].O’Flaherty JT, Rossi AG, Redman JF, Jacobson DP. Tumor necrosis factor-alpha regulates expression of receptors for formyl-methionyl-leucyl-phenylalanine, leukotriene B4, and platelet-activating factor. Dissociation from priming in human polymorphonuclear neutrophils. J Immunol, 1991;147:3842–7. [PubMed] [Google Scholar]
- [28].Eggleton P, Wang L, Penhallow J, Crawford N, Brown KA. Differences in oxidative response of subpopulations of neutrophils from healthy subjects and patients with rheumatoid arthritis. Ann Rheum Dis, 1995;54:916–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Bach M, Moon J, Moore R, Pan T, Nelson JL, Lood C. A Neutrophil Activation Biomarker Panel in Prognosis and Monitoring of Patients With Rheumatoid Arthritis. Arthritis Rheumatol, 2020;72:47–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Denny MF, Yalavarthi S, Zhao W, Thacker SG, Anderson M, Sandy AR et al. A distinct subset of proinflammatory neutrophils isolated from patients with systemic lupus erythematosus induces vascular damage and synthesizes type I IFNs. J Immunol, 2010;184:3284–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Kaczmarek E, Hauser CJ, Kwon WY, Rica I, Chen L, Sandler N. et al. A subset of five human mitochondrial formyl peptides mimics bacterial peptides and functionally deactivates human neutrophils. J Trauma Acute Care Surg, 2018;85:936–43. [DOI] [PubMed] [Google Scholar]
- [32].Sharp JT, Young DY, Bluhm GB, Brook A, Brower AC, Corbett M. et al. How many joints in the hands and wrists should be included in a score of radiologic abnormalities used to assess rheumatoid arthritis? Arthritis Rheum, 1985;28:1326–35. [DOI] [PubMed] [Google Scholar]
- [33].Lood C, Blanco LP, Purmalek MM, Carmona-Rivera C, De Ravin SS, Smith CK et al. Neutrophil extracellular traps enriched in oxidized mitochondrial DNA are interferogenic and contribute to lupus-like disease. Nat Med, 2016;22:146–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Caielli S, Athale S, Domic B, Murat E, Chandra M, Banchereau R. et al. Oxidized mitochondrial nucleoids released by neutrophils drive type I interferon production in human lupus. J Exp Med, 2016;213:697–713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Jilani AA, Mackworth-Young CG. The role of citrullinated protein antibodies in predicting erosive disease in rheumatoid arthritis: a systematic literature review and meta-analysis. Int J Rheumatol, 2015;2015:728610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Das S, Padhan P. An Overview of the Extraarticular Involvement in Rheumatoid Arthritis and its Management. J Pharmacol Pharmacother, 2017;8:81–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Brinkmann CR, Jensen L, Dagnæs-Hansen F, Holm IE, Endo Y, Fujita T. et al. Mitochondria and the lectin pathway of complement. J Biol Chem, 2013;288:8016–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Boilard E, Lai Y, Larabee K, Balestrieri B, Ghomashchi F, Fujioka D. et al. A novel anti-inflammatory role for secretory phospholipase A2 in immune complex-mediated arthritis. EMBO Mol Med, 2010;2:172–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Durstin M, Gao JL, Tiffany HL, McDermott D, Murphy PM. Differential expression of members of the N-formylpeptide receptor gene cluster in human phagocytes. Biochem Biophys Res Commun, 1994;201:174–9. [DOI] [PubMed] [Google Scholar]
- [40].Cedergren J, Forslund T, Sundqvist T, Skogh T. Intracellular oxidative activation in synovial fluid neutrophils from patients with rheumatoid arthritis but not from other arthritis patients. J Rheumatol, 2007;34:2162–70. [PubMed] [Google Scholar]
- [41].Gjertsson I, Jonsson IM, Peschel A, Tarkowski A, Lindholm C. Formylated peptides are important virulence factors in Staphylococcus aureus arthritis in mice. J Infect Dis, 2012;205:305–11. [DOI] [PubMed] [Google Scholar]
- [42].Maeda Y, Kurakawa T, Umemoto E, Motooka D, Ito Y, Gotoh K. et al. Dysbiosis Contributes to Arthritis Development via Activation of Autoreactive T Cells in the Intestine. Arthritis Rheumatol, 2016;68:2646–61. [DOI] [PubMed] [Google Scholar]
- [43].Mielants H, Goemaere S, De Vos M, Schelstraete K, Goethals K, Maertens M. et al. Intestinal mucosal permeability in inflammatory rheumatic diseases. I. Role of antiinflammatory drugs. J Rheumatol, 1991;18:389–93. [PubMed] [Google Scholar]
- [44].Chen J, Wright K, Davis JM, Jeraldo P, Marietta EV, Murray J. et al. An expansion of rare lineage intestinal microbes characterizes rheumatoid arthritis. Genome Med, 2016;8:43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Perianin A, Gougerot-Pocidalo MA, Giroud JP, Hakim J. Diclofenac binding to human polymorphonuclear neutrophils: effect on respiratory burst and N-formylated peptide binding. Biochem Pharmacol, 1987;36:2609–15. [DOI] [PubMed] [Google Scholar]
- [46].Colli S, Colombo S, Tremoli E, Stragliotto E, Nicosia S. Effects of tenoxicam on superoxide anion formation, beta-glucuronidase release and fMLP binding in human neutrophils: comparison with other NSAIDs. Pharmacol Res, 1991;23:367–79. [DOI] [PubMed] [Google Scholar]
- [47].Stenfeldt AL, Karlsson J, Wennerås C, Bylund J, Fu H, Dahlgren C. The non-steroidal anti-inflammatory drug piroxicam blocks ligand binding to the formyl peptide receptor but not the formyl peptide receptor like 1. Biochem Pharmacol, 2007;74:1050–6. [DOI] [PubMed] [Google Scholar]
- [48].Marques PE, Amaral SS, Pires DA, Nogueira LL, Soriani FM, Lima BH et al. Chemokines and mitochondrial products activate neutrophils to amplify organ injury during mouse acute liver failure. Hepatology, 2012;56:1971–82. [DOI] [PubMed] [Google Scholar]
- [49].Dorward DA, Lucas CD, Doherty MK, Chapman GB, Scholefield EJ, Conway Morris A. et al. Novel role for endogenous mitochondrial formylated peptide-driven formyl peptide receptor 1 signalling in acute respiratory distress syndrome. Thorax, 2017;72:928–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Cardini S, Dalli J, Fineschi S, Perretti M, Lungarella G, Lucattelli M. Genetic ablation of the fpr1 gene confers protection from smoking-induced lung emphysema in mice. Am J Respir Cell Mol Biol, 2012;47:332–9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.