Abstract
α-crystallins are small heat-shock proteins that act as holdase chaperones. In humans, αA is expressed only in the eye lens, while αB is found in many tissues. α-crystallins have a central domain flanked by flexible extensions and form dynamic, heterogenous oligomers. Structural models show that both the C- and N- terminal extensions are important for controlling oligomerization through domain-swapping. α-crystallin prevents aggregation of damaged βγ-crystallins by binding to the client protein using a variety of binding modes. α-crystallin chaperone activity can be compromised by mutation or post-translational modifications, leading to protein aggregation and cataract. Because of their high solubility and their ability to form large, functional oligomers, α-crystallins are particularly amenable to structure determination by solid-state NMR and solution NMR, as well as cryo-EM.
Keywords: α-crystallin, protein solubility, vertebrate lens protein, molecular chaperone, protein oligomer, intermolecular interactions
1. INTRODUCTION
For the eye lens to function correctly, it must be transparent and refract visible light strongly enough to form an image on the retina. The bulk of this unique tissue is made of layers of densely packed fiber cells filled with very stable proteins called crystallins. Crystallins comprise over 90% of the dry weight of the human eye lens (1). These proteins exhibit a higher refractive index than average proteins, and their refractive power is not a simple function of the amino acid sequence (2). During development, all organelles in fiber cell are degraded to minimize light scattering, leaving these cells without the machinery needed to synthesize new proteins. Thus, the crystallin proteins that are made during development remain soluble under crowded conditions for decades, and are used by the organism throughout its life. Crystallin proteins exist at very high concentrations in the lens, up to 400 mg/mL in humans (3), and even higher, over 1000 mg/mL in some aquatic species (4). If these proteins aggregate, the light-scattering aggregate formed is called a cataract, the most common cause of blindness (5). To understand how the eye lens works is to understand the physicochemical properties of crystallins, including the molecular basis of their extraordinary solubility, the relationship between sequence and structure, how protein-protein interactions are mediated under conditions of extreme macromolecular crowding, and what happens when this system fails, resulting in cataract.
The study of lens crystallins tracks with the beginning of modern chemistry. In 1830, Berzelius isolated a gelatinous substance from the eye lens that he called crystallin because of its transparency (6). This material was then further fractionated to show that it was in fact made up of more than one substance (7), but it was not until 1894 that the individual protein components were isolated and named α-crystallin and β-crystallin by Mörner (8). Since then great strides have been made in understanding the structure and function of these proteins. The crystallins themselves are categorized into three broad categories in the human lens, the α-, β-, and γ-crystallins. In addition to these ubiquitous vertebrate crystallins, many more taxon-specific crystallins exist in other organisms, but these are beyond the scope of this review. The α-crystallins are chaperone proteins that belong to the small heat-shock protein family (9), while the β- and γ- crystallins are a part of an evolutionarily distinct superfamily of proteins called the βγ-crystallin superfamily (10); they are mentioned here because they are the most common client proteins for α-crystallins in the lens. This review will discuss some history and recent advances in understanding the physical chemistry underpinnings of the unique and extraordinary properties of α-crystallins, with a particular focus on comparisons between human and fish crystallins. We discuss α-crystallin structures, their complex and dynamic oligomerization states, and most importantly, the intermolecular interactions by which they bind to damaged structural crystallins and keep them in solution. Finally, the sidebars provide an introduction to several techniques that are used to obtain this information.
2. α-crystallins are small heat-shock proteins
α-crystallins are small heat-shock proteins (sHSPs) that act as holdase chaperones, meaning they maintain the solubility of damaged client proteins, but are unable to refold them (11, 12). While αA-crystallin is primarily expressed in the lens (13), αB-crystallin is expressed in many tissues throughout the body (14, 15), and alterations to its solubility or substrate binding competence are implicated in a variety of diseases (16). Many studies of α-crystallin function have been performed using zebrafish as a model organism, as the vertebrate crystallin functions (17) and expression patterns (18) are strongly conserved. Furthermore, zebrafish embryos have the advantage of being transparent, enabling detailed studies of the developing lens (19). Mouse α-crystallin promoters were found to drive GFP expression in several organs in the zebrafish, including the lens, notochord, heart, and skeletal muscle, indicating that mammalian α-crystallin promoter activity can also be screened in this model organism (20). In zebrafish, knocking out αB-crystallin causes both lens defects and reduced cardiac stress tolerance (21), while αA was found to have the same function in the lens as it does in other vertebrates (22).
As in other sSHPs, α-crystallins have a β-sheet rich α-crystallin domain flanked by flexible N-terminal and C-terminal extensions (23). A schematic view is shown in Figure 1. The α-crystallin domain is main active part of the protein, containing the region that is responsible for substrate binding; in fact, relatively short peptides from this region, the mini-α-crystallins, display substantial chaperone activity on their own (24, 25). However, the tails also play important roles in controlling the activity, oligomeric state, and substrate recognition (26, 27), as illustrated by the different binding surfaces, corresponding to different sequence regions, that recognize amorphous aggregates and amyloid fibrils (28). Structures of truncated α-crystallins show the primary dimer interface, which is in the middle of an extended β-sheet, but also reveal how the C-terminal extensions can participate in domain-swapping, leading to oligomers of differing sizes (29). The N-terminal extension has also been implicated in binding of specific substrates (28).
Figure 1. α-crystallin binding to client protein.
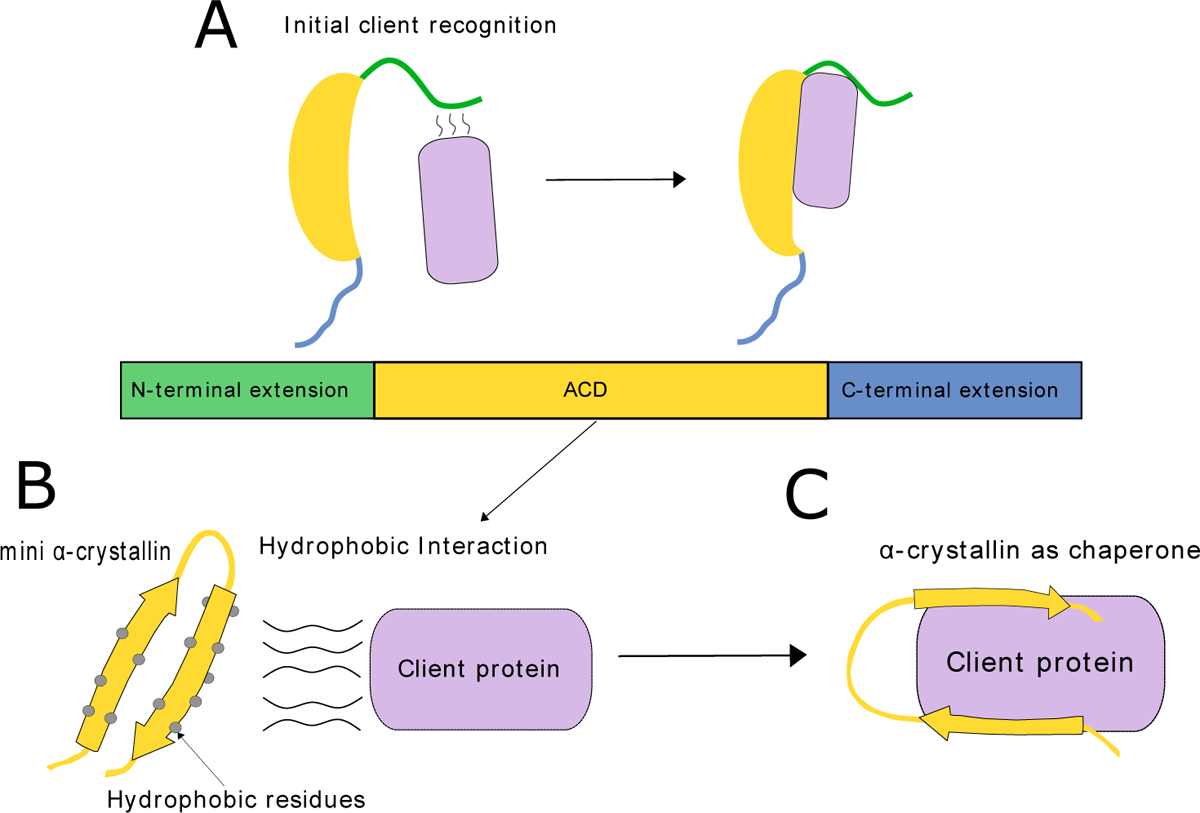
(A) The initial recognition of the client protein sometimes occurs at the N- terminal extension of the α-crystallin (116). (B) Mini αA-crystallin contains many hydrophobic residues that bind to the client protein through hydrophobic interactions (110). (C) αA-crystallin tightly binds the client protein, keeping it in solution.
3. Polydispersity is central to α-crystallin function
Understanding how α-crystallins recognize client proteins and maintain their structural integrity requires high-resolution structures not only for the monomeric proteins, but also their complexes. α-crystallins exist as polydisperse mixtures of 10–40-mers (30, 31), with the most common species in the range of 24–28 (32). Characterizing the equilibrium of oligomeric states is central to gaining full mechanistic insight into α-crystallin activity: NMR experiments have shown that polyhedra of varying sizes form, providing different environments for client proteins (33). Heterogeneous interactions are observed even in constructs of the isolated α-crystallin domain, as key residues display resonances at more than one chemical shift position, indicating multiple conformations (34). However, the importance of the C- and N-terminal extensions for controlling oligomerization has been demonstrated in several studies. The IPI motif on the C-terminal domain, embedded in the palindromic sequence “ERTIPITRE” can interact bidirectionally with the β4/β8 surface of the α-domain, and these interactions control both the oligomeric state and the ability of the protein to chaperone amyloid clients (35, 36). Peptides mimicking this sequence readily bind to the α-crystallin domain in solution, providing support for the importance of this motif for protein-protein interactions (37). Polydispersity appears to be central to α-crystallin function: not only does the formation of variable oligomers discourage crystallization or other deleterious interactions between the α-crystallin molecules themselves, but the ability of these molecules to self-organize into constantly shifting polyhedra provides a variety of surfaces that are presented to client proteins of differing sizes and shapes.
Solving the structures of α-crystallins in biologically relevant complexes is a challenging endeavor. Their dynamic and polydisperse nature makes them particularly suitable for NMR (38, 39), and in many cases hybrid structural methods are used to integrate information across different length scales. Various combinations of experimental and molecular modeling techniques have been used to model α-crystallin oligomers, sometimes with inconsistent results, possibly reflecting differences in protein constructs or sample preparation methods, or true heterogeneity.
For example, building on solid-state and solution NMR structures of smaller oligomers (40, 41), cryo-EM, solid-state NMR, and small-angle x-ray scattering (SAXS) were used to generate a model of symmetric αB-crystallin 24-mers (42) (Figure 2A). These oligomers are built using a hierarchical progression where dimers connected by the β-sheet interface assemble via their C-terminal extensions to form hexamers, which in turn associate via their N-terminal regions to create higher-order structures. Another hybrid structure of αB-crystallin 24-mers was generated using a combination of cryo-EM, solid-state NMR, and molecular modeling also came to the conclusion that the building blocks are dimers, which then associate to form hexamers (43). This structure also identifies two different dimer arrangements, consistent with the variable interactions between the palindromic sequence of one monomer and hydrophobic residues of the other observed in the crystal structures (35), but in contrast to solution-state NMR data showing only a single set of chemical shifts, suggesting that all monomers adopt the same conformation (33).
Figure 2.
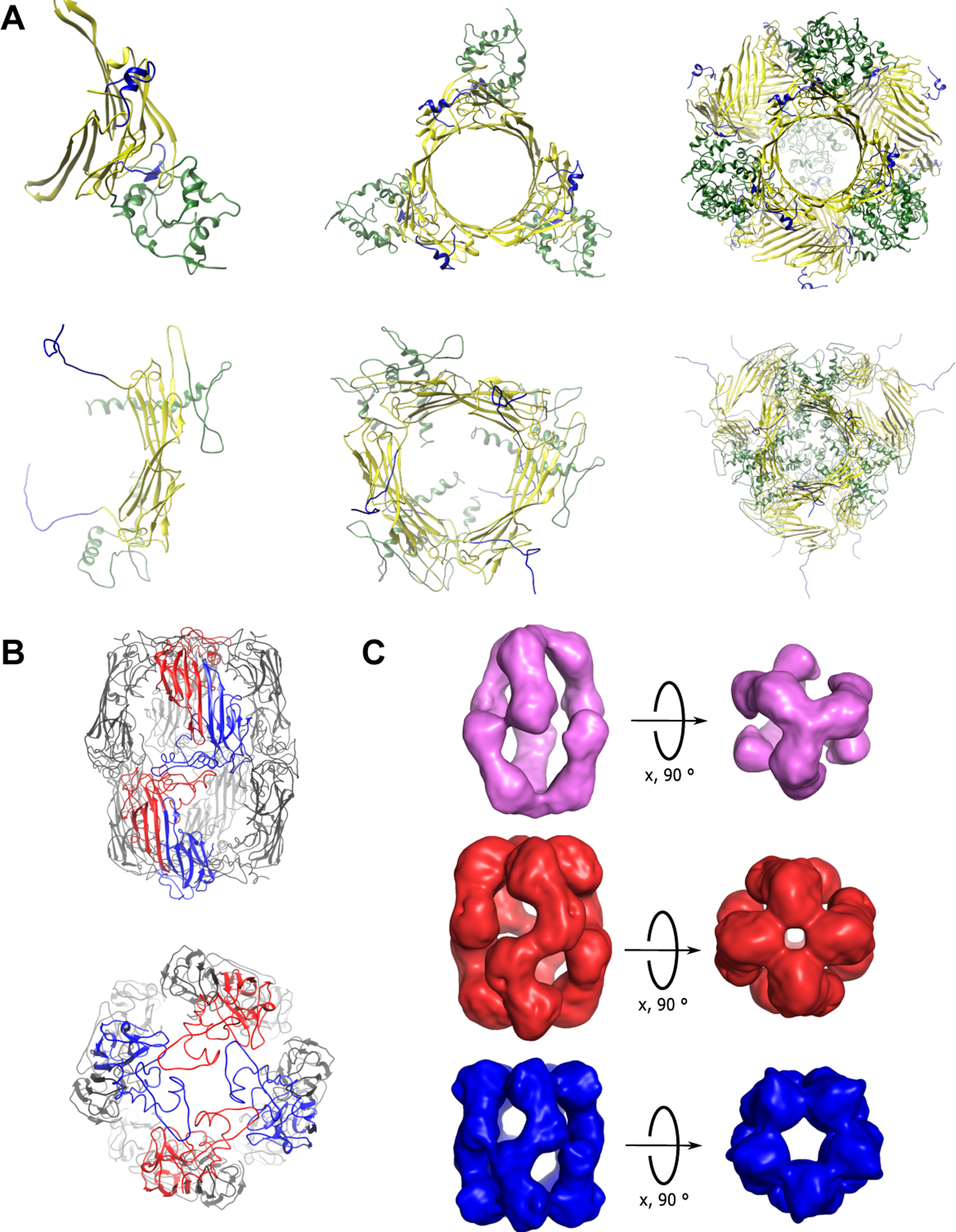
Oligomeric states of α-crystallins. (A) The Jehle (top) (31, 42) and Braun (bottom, PDB: 2YGD) (43) αB 24mer pseudoatomic models. Homodimers (left) are formed through ACD (yellow) contacts. Dimers combine to form a hexameric species (middle) through NTR (green) and ACD contacts. The C-terminal domain is highlighted in blue. Hexamers combine to form the dominant 24-mer structures (right).
(B) Pseudoatomic models of a 16-mer of wild-type reduced human αA-crystallin(49). Top: αA-crystallin monomers (blue and red) forming the β7-interface dimer. Dimers via interactions between N-terminal extensions, to form z-shaped tetramers that stack to make up the pillars of the hollow oligomer. Bottom: Monomers (red) binding through N-terminal interactions. (C) Cryo-EM density maps from the Electron Microscopy Database (EMD) (49) of the (top) 12-mer (EMD-4895), (middle) 16-mer (EMD-4894), and (bottom) 20-mer (EMD 4896) with three, four, and five-fold symmetry, respectively, from the apical axis (right). All oligomers are formed from a z-shaped tetrameric building block. Abbreviations: ACD, α-crystallin domain; cryo-EM, cryo-electron microscopy; EMD, Electron Microscopy Database; NTR, N-terminal region; PDB, Protein Data Bank.
In addition to the most common species, the 24-mers, oligomers ranging from 6-mers to 48-mers have also been observed in solution. These data and the closed, spherical shape of the higher-order oligomers suggest that the highly symmetric complexes may be for storage, whereas the binding-competent species are either smaller complexes (perhaps dimers) or incomplete spheres allowing room for client proteins. Solution NMR indicates that ms-timescale dynamics in the C-terminal extensions regulate the oligomerization state (44). Some details of the dynamic equilibrium among these complexes were revealed using deuteration-assisted small-angle neutron scattering (DA-SANS) in conjunction with electrospray ionization (ESI) native mass spectrometry (nMS): exchange of subunits between complexes was common, however oligomer collapse did not appear to occur, even for a subset of the population (45). Thus, the dynamic quaternary structure of αB-crystallin appears to be mediated by small oligomers dissociating from and re-associating with larger complexes. Other studies have suggested that monomers are present under physiological conditions (46) and have chaperone activity (32), but are compromised from a solubility standpoint (47). Beyond the size of the oligomers, the morphology of the α-crystallin oligomers can also affect chaperone function. α-crystallins can retain function after forming amyloid fibrils or amorphous aggregates, with amyloid fibrils even showing enhanced affinity for particular clients (48).
The first structural models of the lens-specific chaperone αA-crystallin in 12-, 16- and 20-mers were generated recently using a combination of cryo-EM, mass spectrometry with cross-linking, NMR spectroscopy, and molecular modeling (49)(Figure 2B). This model suggests that for αA-crystallin, the building block for oligomerization is a tetramer. As in the case of αB-crystallin, the exact extent of oligomerization is governed in part by the extent of C-terminal domain swapping (50, 51, 52), although the N-terminus is also involved in oligomerization, as its truncation shifts the equilibrium to favor smaller oligomers (53, 54). Domain-swapped oligomers are found in multimers larger than 12, whereas the dodecameric species can form without any interactions with the C-terminus (49). These results are consistent with earlier crystal structures of truncated versions of vertebrate αA-crystallins that showed conformations both with (29) and without domain-swapping (55). Recently, an alternative splicing variant of human αA-crystallin with a truncated N-terminal sequence was discovered. On its own, this isoform has only weak chaperone activity and makes only small oligomers, but it is capable of integrating into larger oligomers of the more common canonical variant, modulating oligomer size and chaperone activity (56). In contrast, an alternative splicing variant found in rodents (αAins) has a longer N-terminal domain, and has enhanced activity relative to the standard isoform (57)
4. Cataract-related α-crystallin variants can be aggregation-prone or have altered chaperone activity
Many cataract-associated variants of α-crystallins feature an altered α-domain, which results in structural changes and/or compromised chaperone activity. The αB-R69C and D109H variants are implicated in human disease, including cataract (58, 59). Both cause considerable structural changes, reduced chaperone activity, and increased aggregation propensity in vitro (60). The D109H variant is particularly disruptive, probably because the loss of the negative charge at position 109 disrupts a critical salt bridge that is required for proper formation of the anti-parallel sheet dimer interface, as does the R120G variant (61). The R12C variant of αA-crystallin is more prone to aggregation than WT, especially in the presence of calcium ions (62). Both the αA-G98R and αA-R21Q variants show decreased function, but when mutated together to make an αA-G98R/R12Q double variant, compensating for the difference in charge, much of the function and stability of the protein is rescued (63).
In other cases, the major driving force of cataract formation is how mutations in α-crystallin affect the aggregation of other proteins. For example, the R49C and R116C variants of αA-crystallin have differing affinities for the aggregation-prone client protein I4F γD-crystallin (64). The higher affinity R49C variant results in increased aggregation of γD-I4F, possibly through simple mass action, as the folded form is sequestered by the chaperone protein, thus pushing the equilibrium toward formation of the denatured form (64). In mouse models, knocking out αA and αB-crystallin leads to increased abundance and cross-linking of βB2-crystallin (65) the αB-R120G and the αA-R49C variants lead to decreased protein degradation in the lens during development (66), which may lead to an increased propensity for cataract formation, or alternatively might be an effect of cataract formation. However, not all mutations are deleterious; for example the R12C variant of αB-crystallin exhibits increased chaperone activity in addition to changes in the oligomeric state of the protein with increased population of a dimer form that is favored as a result of a disulfide bond (67). This variant also resists thermal and calcium-induced aggregation compared to WT. However, when exposed to calcium ions the chaperone activity of the variant is decreased, potentially due to structural changes, as the R12C variant shows altered chemical environments of its tryptophan residues, suggesting partial unfolding (67).
5. α-crystallins bind divalent cations, with complex effects on their function
Human αB-crystallin binds one equivalent of Cu2+, and its chaperone activity is enhanced by this interaction (68, 69, 70). αB is able to prevent aggregation and co-precipitation of Cu2+ and human γD at all Cu2+:γD ratios, suggesting that αB is acting as a chaperone rather than a chelator in this situation (71). However, there is also evidence of metal ion homeostasis through sequestration. Increasing the amount of α-crystallin bound to the client protein γD, which would occur naturally with age, results in an increase in free iron and calcium ions (72), which would increase the susceptibility of the eye lens to oxidation. Schiff bases and rutin, a naturally occurring flavonoid, were shown to inhibit copper-induced aggregation of human γD and promote the chaperone activity of αB, suggesting a possible role as a cataract therapeutic (73, 74).
In the case of Zn2+, αB-crystallin was again able to prevent metal ion-induced aggregation, but here it appears to act as a chelator rather than a chaperone, because higher concentrations of Zn2+ were still able to cause aggregation (75). Zinc ions mitigated diabetic cataract in mouse models due to their antioxidant properties and positive interactions with α-crystallin (76, 77, 78). Zn2+ interactions with α-crystallin do not restructure the protein’s secondary or tertiary arrangement, but they do increase its surface hydrophobicity, thereby enhancing the chaperone activity (76). In human lens epithelial cells, treatment with Cd2+ or Cu2+ promoted the expression of αB-crystallin, whereas Cu2+ promoted the expression of αA-crystallin (79).
6. α-crystallin post-translational modifications can cause age-related cataract
The majority of cases of cataract are caused by aging: most people are born with healthy lens proteins, which accumulate post-translational modifications (PTMs) over time, leading to cataract (80, 81). UV irradiation is associated with the formation of cataract (82). In αA-crystallin, UV damage leads to increased hydrophobic exposure, secondary structure changes, and diminished chaperone activity (83). Backbone cleavage can lead to the accumulation of insoluble peptides in the lens, providing nucleation sites for the growth of aggregates. For example, a 15-residue peptide derived from αA-crystallin aggregates in aging lenses, recruiting full-length protein and forming β-sheet rich fibril structures (84).
One of the most common PTMs in the lens is isomerization (85), which is difficult to detect because it does not change the protein mass. Serine and aspartic acid can epimerize to form their D-counterparts, and Asp can also convert to L- or D-isoAsp (86). These modifications can alter the protein structure, sometimes causing disruption that goes beyond the mutation site (87). In the eye lens, Asp isomerization increases with age, with αA-crystallin being more susceptible to isomerization than αB-crystallin (88). Isomerization in αA changes the relative populations of different oligomers (89) In αB, isomerization of Asp109 disrupts a critical salt bridge at the dimer interface, negatively impacting its solubility, while epimerization of Ser162 significantly weakens the dimerization interface between the palindromic sequence and the β4-β8 groove (90). Glycation, particularly of lysine side chains, can also occur in the crystallins, linking cataract and diabetes (91). In mice, L-lysine treatment has been shown to reduce glycation in α-crystallin after treatment with glucose (92). PTMs have even been shown to enhance α-crystallin activity. Acetylation of lysine residues have been linked to higher hydrophobic exposure and increased chaperone activity in α-crystallin (93). Recently, the link between the αA-crystallin oxidation state and oligomerization and function has been characterized. When αA-crystallin is oxidized, a destabilizing disulfide bond is formed, dispersing higher-order oligomers (49).
Another important post-translational modification that impacts the regulation of protein function is phosphorylation, which happens to α-crystallins under stress conditions. In αB-crystallin, phosophorylation occurs in a heterogenous manner, with mixed populations of proteins phosphorylated at different sites. Overall phosphorylation efficiency is low, due to the inability of kinases to act on monomers that are part of a large complex; phosphorylation appears to happen during subunit exchange (94). Phosphorylation of αB-crystallin is mostly localized to three serine residues: 19, 45, and 59 (95, 96). When these sites are phosphorylated, αB-crystallin forms smaller oligomeric complexes, and shows preferential binding to different client proteins (97). An αB-crystallin variant mimicking hyperphosphorylation of these residues showed increased chaperone function relative to wild-type, using insulin as a client (98). Low levels of phosphorylation appear to improve chaperone activity and reduce aggregation propensity, whereas hyperphosphorylation, which sometimes happens to variant proteins in vivo, leads to increased aggregation (99). Consistent with this observation, extensive phosphorylation at S45 resulted in uncontrolled aggregation in vitro (100). Epimerization and phosphorylation can also interact to alter the protein’s behavior: phosphorylation of Ser59, is precluded by epimerization of this residue, and reduced by the isomerization of the nearby residue Asp62 (90). Despite the many advances in understanding α-crystallin function since Horwitz first discovered its molecular chaperone properties, studies such as these underscore the importance of recognizing that α-crystallins may behave differently in dilute, homogenous solutions than they do in the messy, crowded environment of the cell (101).
7. Client-chaperone interactions
Recognition of misfolded, partially unfolded, or otherwise solubility-compromised proteins is central to α-crystallin function. Although the exact mechanisms of client recognition are not yet fully understood, the size, charge, size, and exposed hydrophobic surface of the client protein all appear to play a role (102). All-atom molecular dynamics (MD) simulations were used to probe the interactions of monomers and oligomers of a small peptide implicated in Alzheimer’s disease-related plaques (Aβ17−42) with an αB-crystallin ACD dimer. The dimer bound more strongly to oligomers of Aβ17−42 relative to monomers, as each peptide has only limited number of contacts with the ACD dimer, resulting in weak and transient interactions. However, oligomer formation locks the peptides into a more rigid conformation, resulting in a larger interaction surface (103). Particular residues in αA, such as D69 (104) and F71 (105) have all been proposed as being critical to chaperone activity, as have the C-terminal (106) and N-terminal tails (107). An important step in understanding what governs these protein-protein interactions was the discovery of small peptides derived from the α-crystallin domain that are active as chaperones. For example, the peptide “KFVIFLDVKHFSPEDLTVK” from αA-crystallin has chaperone activity and was thus named mini-αA-crystallin (108). In αB-crystallin a similar peptide, “DRFSVNLD-VKHFSPEELKVK” was identified, and has been called mini-αB-crystallin (see Figure 1B) (109). The discovery of these peptides has allowed for more focused probing of the molecular mechanism of the client-chaperone interaction involving the α-crystallin domain.
A common finding when investigating α-crystallin binding interactions is the importance of hydrophobic interactions. Mini αA-crystallin is notably hydrophobic, and its confirmed site of interaction with human γD-crystallin specifically involves several hydrophobic residues (110). In human γD-crystallin, both the I4F and V76D variants stabilize an aggregation-prone intermediate, leading to early onset cataract. However αB-crystallin does not recognize either of these variants, whereas it does bind the I4F/V76D double variant (111), suggesting that a minimum amount of unfolding is needed to trigger α-crystallin activity. More evidence that full-length αB-crystallin can select between native-like and strongly aggregation-prone proteins with very similar, folded structures is provided by the difference between αB-crystallin’s robust binding to the G18V variant of human γS-crystallin, but not the G18A variant, which is likewise thermally destabilized, albeit to a lesser extent (112). Similarly, hydrophobic residues in melittin mediate the interaction of full-length αA-crystallin with this peptide (113). Although hydrophobic interactions are clearly important, there is also more to the story. In αB-crystallin alone there are at least 13 peptide sequences related to chaperone activity (114), and each of these could reveal unique mechanisms that explain αB-crystallin’s prodigious ability to chaperone diverse client proteins. A schematic illustrating the general binding modes is given in Figure 3A; Figure 3B shows the location of the mini-αA peptide and several other key residues on the α-crystallin monomer.
Figure 3.
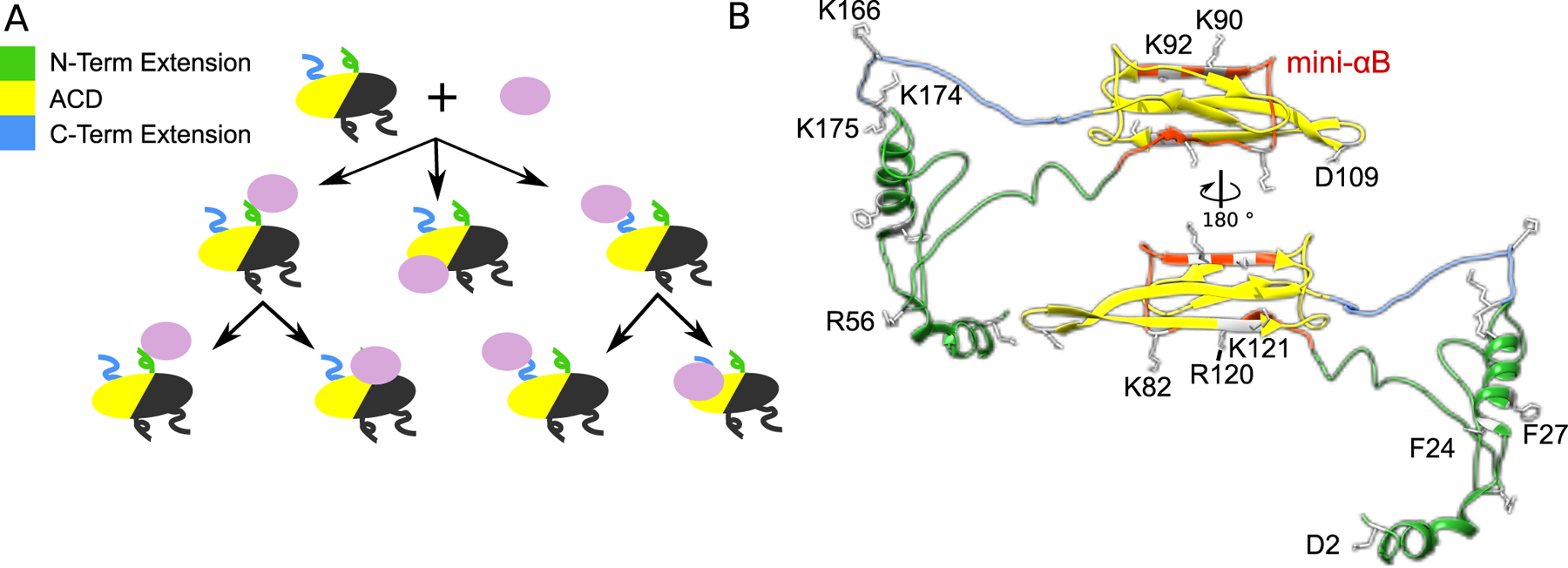
A A schematic of the interaction modes of αB-crystallin with client proteins. There are several different possible paths for client recognition and chaperone activity. Both the N- (green) and C-terminal extensions (blue) can act as site for initial recognition or even as the active holdase region. In the case of initial recognition, the client initially binds the flexible extension and then becomes bound to the α-crystallin domain. The α-crystallin domain itself can also directly interact with client proteins for holdase activity, without intermediate binding. B. Many specific residues and sequence regions are implicated in αB-crystallin holdase activity. The mini-αB peptide is highlighted in red, while the other key residues throughout the protein are labeled. These residues are D2, F24, F27 (107), R56 (157), D109 (59), R120(158), R157 (159), K82, K90, K92, K121, K166, K174, K175 (160).
Abbreviation: PDB, Protein Data Bank.
One area of current interest is how α-crystallin interacts with UV-damaged client proteins. Current work has shown that α-crystallin forms very large complexes when binding βL-crystallin, and that the size of the complex is positively correlated with the content of UV-damaged protein (115). High-resolution structural studies are needed to resolve the details of these complexes. So far studies have all mostly focused on the role and mechanism of the α-crystallin domain, but the N- and C- terminal extensions are also important, possibly for initial client recognition (116), in addition to their role in mediating oligomerization.
7.1. Species-specific adaptations
α-crystallins have species-specific adaptations that enable them to recognize particular client proteins: bovine α-crystallin effectively protects cow γ-crystallins from aggregation, while failing to interact with structural crystallins from the Antarctic toothfish, and even α-crystallins from the bigeye tuna only partially protected toothfish γ-crystallins under heat stress conditions (117). The cold adaptations required for the lifestyle of the Antarctic toothfish, which lives at a temperature of −2°C year-round provide some clues to this exquisite specificity. Like humans, the toothfish has two α-crystallin paralogs, αA and αB, although its lens protein mixture is more complicated due to the much larger number of γ-crystallin paralogs (118). Sequence comparison shows that α-crystallins from cold-adapted fish have more hydrophobic residues than their warm-water counterparts, and mutating key residues in zebrafish αA-crystallin with their counterparts in the toothfish protein altered surface hydrophobicity (measured using bis-ANS binding), oligomerization, and chaperone activity (119). Unlike humans and toothfish, zebrafish have two αB-crystallins, αBa and αBb (120). αBb is the more similar to human αB, whereas αBa has unusual oligomerization behavior and superior chaperone activity against a panel of destabilized T4 lysozyme variants (57).
7.2. Human α-crystallin chaperone activity as as a biomedical and therapeutic target
An important reason for studying the molecular mechanisms of α-crystallin chaperone action and aggregation is the long-term goal of using this information in a therapeutic context. α-crystallin itself is potentially useful as a therapeutic agent against protein deposition diseases, particularly the α-crystallin domain and component peptides. The α-crystallin domain binds to, and prevents the fibrillization of Aβ1−42 (121), a protein found in amyloid plaques in Alzheimer’s disease. The ability of α-crystallin to bind amyloid fibrils in a therapeutic context has also been examined in the context of Multiple Sclerosis (MS). MS symptoms are linked to the formation of amyloid fibrils of fibronectin, contributing to the formation of an MS lesion, so maintaining the solubility of these proteins could lead to an effective treatment for MS. In a clinical trial, doses of αB-crystallin were shown to reduce the number of MS lesions by 76% over a 9 month period (122). Delivery remains a problem with this approach: it is difficult to get α-crystallin into the cell efficiently. αB-crystallin can be fused to the glycoprotein C cell penetrating peptide, followed by stimulation of cell uptake by applying heparan sulfate (123).
Of course α-crystallin is also associated with disease states, and so it is itself a target for therapeutics. Attempts at treating α-crystallin associated cataract typically focus on resolubilizing the protein, as protein turnover in the lens is negligible so degradation in not a viable option. Aspirin nanorods have shown effectiveness in maintaining α-crystallin solubility in vitro, allowing for the possibility of an aspirin-based cataract treatment (124). Mouse models of hereditary and age-related cataract have been successfully treated with small molecules, with one compound in particular, 29, 5-cholesten-3b,25-diol, improving the solubility of α-crystallin by 63% (125). RNA aptamers can act as molecular switches to control the activity of the targeted proteins. RNA aptamers have been developed that bind specifically to αB-crystallin while not targeting the very similar αA-crystallin (126). This work marks an important first step toward selectively and specifically controlling the activity of αB-crystallin.
8. Conclusion and outlook
We have presented an overview of the current state of knowledge of the molecular mechanisms of α-crystallin oligomerization and chaperone activity. Much of this information has come from combinations of complementary methodologies, notably solid-state and solution NMR with cryo-EM. Future progress toward understanding native α-crystallin oligomers and their interactions with client proteins will require advanced biomolecular simulations. MD simulations provide a detailed picture of how proteins move, leading to insights into protein folding, enzyme activity, and solvent interactions (127, 128). MD is also an integral part of the process of refining macromolecular structures based on X-ray crystallographic (129) and NMR (130) data. MD simulations are often performed using a detailed structural model containing all the atoms in the protein as well as atomically detailed solvent molecules, although coarse-grained approaches and implicit solvent treatments are also available. Monte Carlo simulations can be used to study the equilibrium behavior of a wide range of physical systems. In this method, the system starts in an arbitrary configuration, and a perturbation is applied. Depending on whether the new configuration is lower or higher in energy, the move is accepted or rejected (131), and a wide range of thermodynamic properties can then be explored. In the context of systems containing many proteins, rigid-body models are often used to reduce the computational cost (132). Multiconformation Monte Carlo simulations can reintroduce some conformational complexity at low additional cost by using a library of predetermined protein conformations (133). For systems that are characterized by complex interactions among a large number of monomers, another alternative to computationally expensive all-atom simulations is the network Hamiltonian dynamics approach, a coarse-grained method in which a network Hamiltonian is written strictly in terms of connectivity among the protein monomers, eliding the molecular-level details. The properties being simulated are therefore characteristic of the entire system rather than of the structural details of any particular protein, allowing long-timescale simulation of systems comprising hundreds to thousands of monomers. This approach has been used to recapitulate all known types of amyloid fibrils observed in the Protein Data Bank (134) and to model the formation kinetics of several different fibrillization pathways (135). It may also provide a modeling framework for understanding α-crystallin oligomerization and the dynamic equilibrium among oligomers of different sizes, even before detailed all-atom simulations for the full system can be obtained. α-Crystallin forms complex, heterogeneous oligomers that are built up in a modular fashion based on domain-swapping interactions with its N- and C-terminal extensions. The full picture of α-crystallin chaperone behavior is very complicated, with different parts of the chaperone contact- ing its aggregation-prone substrate depending on specific properties of the client. A clear future direction in this area is the continued exploration of α-crystallin interactions with a greater variety of misfolded or destabilized proteins, as the full range of α-crystallin activity has probably not yet been characterized. Many different client protein modifications have been observed, ranging from amino acid substitutions to PTMs such as phosphorylation and isomerization. A pattern that emerges from all of these studies is that there are multiple pathways for aggregation depending on the nature of the modification. As these aggregates are formed by different mechanisms and may have different physicochemical properties, pharmaceutical interventions for medical conditions caused by these modifications will require either tailored treatments or a general solution that encompasses all of the α-crystallin functionalities observed in nature. In contrast, understanding α-crystallin properties and crystallin–crystallin interactions can also lead to improved designs for artificial lenses that more closely mimic the functionality of the biological lens.
8.1. Sidebars and Margin Notes
CRYO-EM
A major advantage of cryo-EM is that it enables structure determination of large complexes, even for proteins that resist crystallization. Smaller proteins are problematic as the current theoretical limit for high resolution cryo-EM structures is thought to be 38 kDa for single particles (136), any smaller and the noise overcomes the signal. The basic principle involves freezing a protein sample in vitreous ice. Then, the sample is bombarded with electrons and an image is generated of separated single particles of the protein or complex of interest. The individual particles are then classified based on the orientation of the sample by a computer. Each class is then averaged to generate a sharp image of that orientation. By combining all the different orientations, a 3-D model can be generated. For success with this technique, a few things are required: 1. Particles in the sample must be identical for the class averages to generate a sharp image. 2. Particles in the sample must take many different orientations, because a bias towards a certain orientations will lead to lower resolution for the less well-represented orientations.
SOLUTION-STATE NMR
Because the solubility of the crystallin proteins is so central to their function, solution-state NMR is a natural choice for solving structures of these proteins under native-like conditions. NMR makes use of the nuclear magnetic moment of atoms that have an odd number of protons or neutrons (137, 138, 139). When placed in an external magnetic field, the magnetic moment of the nucleus will precess about the external magnetic field at a characteristic frequency that is influenced by the unique magnetic environment around that atom. This includes the local magnetic fields produced by electrons in the chemical bonds as well as other nearby atoms, a phenomenon known as the chemical shift. Here, obtaining a high-resolution spectrum depends on the rapid, isotropic tumbling of the molecules of interest relative to the timescale of the experiment. This is the case for relatively small proteins (up to about 30 kDa), although specialized pulse sequences and labeling schemes can be used to extend the size range of solution-state NMR to much larger proteins or complexes (140, 141).
NMR ASSIGNMENTS AND STRUCTURE DETERMINATION
For small organic molecules, assigning each chemical shift to the atom that gives rise to it is relatively straightforward, but this is a daunting task for a protein, which has thousands of atoms. Nonetheless, the first stage in the structure determination process is to obtain a characteristic frequency for every 1H, 13C, and 15N in the protein. This process is greatly facilitated by using recombinant expression, inducing bacteria, yeast, or cell cultures to produce isotopically labeled proteins in sufficient quantities for NMR. Protein samples can be uniformly labeled with 13C and 15N, can be fully or partially deuterated, or can incorporate more specialized labeling schemes (142, 143, 144). In addition to increasing sensitivity, isotopic enrichment is crucial because it enables multidimensional experiments (145) in which two or more correlated Fourier dimensions reveal connectivity along the protein backbone (146). After the assignments are complete, pairs of intermolecular distances are measured, either indirectly via cross-relaxation effects (147), or directly through dipolar couplings in a partially oriented sample (148). These distance restraints, along with other parameters derived from the chemical shift and the protein sequence (149), are used to constrain a molecular dynamics simulation to obtain the final structure (150, 151).
SOLID-STATE NMR
Despite its utility for studying the structure and dynamics of small, soluble proteins, an obvious limitation of solution-state NMR is that the molecules of interest must be dispersed through the sample and rotating freely. For large or insoluble protein complexes, solid-state NMR is a better choice. Here, anisotropic interactions, such as chemical shift anisotropy and dipolar coupling are not averaged out. These interactions contain valuable orientational information, but the broadening they impose on the spectra present a challenge. A static solid-state NMR spectrum can be described by a “powder pattern,” which is very broad for each equivalent atom as the chemical shift, for example, can vary greatly in different orientations. In principle, fitting each powder pattern provides a wealth of detailed orientational information, however this becomes infeasible for even relatively small spin systems.
In solution NMR, anisotropic interactions are averaged to zero by the free rotation of molecules in solution. In solid-state NMR, it is possible to mimic this rotation by mechanically rotating the sample at the magic angle, 54.74° relative to the external magnetic field (152). The speed of rotation is chosen based on the instrumentation used as well as the frequency of the interaction to be averaged out; for example a 12 kHz dipolar coupling would require a rotation rate faster than 12 kHz (in practice, radiofrequency decoupling is also used to average this interaction away. Of course, the liquid-like spectra are obtained at the cost of losing the information found in the powder pattern. However, this information can be recovered using a variety of dipolar recoupling methods, including a vast library pulse sequences and switched angle spinning, which uses mechanical reorientation to selectively recover this information. Under magic angle spinning, multidimensional experiments can be used to obtain assignments and distance restraints, as in solution-state NMR (153, 154, 155, 156). Although the practical details and spin transfer pathways are different, the experiments and structure determination algorithms are conceptually very similar.
SUMMARY POINTS.
Summary point 1. α-crystallins bind to damaged structural proteins and maintain their solubility.
Summary point 2. The dynamic and heterogeneous oligomerization state of α-crystallins is critical to their functionality.
Summary point 3. Multiple sequence regions and structural features are required for complex formation.
Summary point 4. The high solubility and complex oligomerization behavior of these proteins make them particularly amenable to study by NMR and cryo-EM.
FUTURE ISSUES.
Future issue 1. Although we are beginning to understand how α-crystallin recognizes and solubilizes its client proteins, more molecular-level studies are required to develop a general model for its holdase chaperone activity.
Future issue 2. The activity of α-crystallin monomers, dimers, and larger oligomers needs further study to determine what is the most active species, or if different assemblies play different roles.
Future issue 3. The physicochemical impact of post-translational modifications under in vivo conditions is only beginning to be understood.
Future issue 4. The mini-α-crystallin peptides or even more minimal chaperones derived from them may provide a basis for development of future therapeutics against protein aggregation diseases.
ACKNOWLEDGMENTS
Our crystallin work is supported by NIH grants 2R01EY021514 to R.W.M and 1R01EY025328 to R.W.M. and D.J. Tobias and NSF grant DMR-2002837 to R.W.M. and D.J. Tobias. M.A.S.P. is supported by the HHMI Gilliam Fellowship. This work builds on discussions at the 2018 Crystallin Satellite Biophysical Society Meeting in San Francisco, and we are grateful to the participants for ideas and suggestions.
Footnotes
DISCLOSURE STATEMENT
The authors are not aware of any affiliations, memberships, funding, or financial holdings that might be perceived as affecting the objectivity of this review.
LITERATURE CITED
- 1.Dilley Keith J. and Harding John J.. Changes in proteins of the human lens in development and aging. Biochimica et Biophysica Acta (BBA) - Protein Structure, 386(2):391–408, April 1975. ISSN 0005–2795.. [DOI] [PubMed] [Google Scholar]
- 2.Khago Domarin, Bierma Jan C, Roskamp Kyle W, Kozlyuk Natalia, and Martin Rachel W. Protein refractive index increment is determined by conformation as well as composition. Journal of Physics: Condensed Matter, 30(43):435101, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Delaye Mireille and Tardieu Annette. Short-range order of crystallin proteins accounts for eye lens transparency. Nature, 302(5907):302415a0, March 1983. ISSN 1476–4687.. [DOI] [PubMed] [Google Scholar]
- 4.Kröger Ronald H. H., Campbell Melanie C. W., Munger Rejean, and Fernald Russell D.. Refractive index distribution and spherical aberration in the crystalline lens of the African cichlid fish Haplochromis burtoni. Vision Research, 34(14):1815–1822, 1994. [DOI] [PubMed] [Google Scholar]
- 5.Dawson Chandler R. and Schwab Ivan R.. Epidemiology of cataract—a major cause of preventable blindness. Bull World Health Organ, 59(4):493–501, 1981. ISSN 0042–9686. [PMC free article] [PubMed] [Google Scholar]
- 6.Berzelius JJ. Lärobok i kemien. Stockholm, P. A. Norstedt & Söner, pt. 2:p. 512, 1830. [Google Scholar]
- 7.Johann Franz Simon. Animal Chemistry: With Reference to the Physiology and Pathology of Man. Lea and Blanchard, Philadelphia, 1846. [Google Scholar]
- 8.C.T. M\:orner. Untersuchung der Proteïnsubstanzen in den lichtbrechenden Medien des Auges. Ztschr. f. physiol. Chem, 16:80, 1894. [Google Scholar]
- 9.de Jong Wilfried W., Caspers Gert-Jan, and Leunissen Jack A. M.. Genealogy of the α-crystallin—small heat-shock protein superfamily. International Journal of Biological Macromolecules, 22(3):151–162, May 1998. ISSN 0141–8130.. [DOI] [PubMed] [Google Scholar]
- 10.Slingsby C, Wistow GJ, and Clark AR. Evolution of crystallins for a role in the vertebrate eye lens. Protein Sci, 22:367–380, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Horwitz J. Alpha-crystallin can function as a molecular chaperone. Proceedings of the National Academy of Sciences of the United States of America, 89(21):10449–10453, 1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Haslbeck M and Vierling E. A first line of stress defense: Small heat shock proteins and their function in protein homeostasis. Journal of Molecular Biology, 427(7):1537–1548, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Runkle Stephanie, Hill Julie, Kantorow Marc, Horwitz Joseph, and Posner Mason. Sequence and spatial expression of zebrafish (Danio rerio) αA-crystallin. Mol. Vis, 8:45–50, 2002. [PMC free article] [PubMed] [Google Scholar]
- 14.Chepelinsky AB, Piatigorsky J, Pisano MM, Dubin RA, Wistow G, Limjoco TI, Klement JF, and Jaworski CJ. Lens protein gene expression: Alpha-crystallins and MIP. Lens and Eye Toxicity Research, 8(2–3):319, 1991. [PubMed] [Google Scholar]
- 15.Srinivasan AN, Nagineni CN, and Bhat SP. Alpha a-crystallin is expressed in non-ocular tissues. Journal of Biological Chemistry, 267(32):23337–23341, 1992. [PubMed] [Google Scholar]
- 16.Hayashi Junna and Carver John A.. The multifaceted nature of αB-crystallin. Cell Stress and Chaperones, pages 10.1007/s12192-020-01098-w, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dahlman Jason M, Margot Kelli L, Ding Linlin, Horwitz Joseph, and Posner Mason. Zebrafish α-crystallins: Protein structure and chaperone-like activity compared to their mammalian orthologs. Molecular Vision, 11:88–96, 2005. [PubMed] [Google Scholar]
- 18.Posner M, Hawke M, Lacava C, Prince CJ, Bellanco NR, and Corbin RW. A proteome map of the zebrafish (Danio rerio) lens reveals similarities between zebrafish and mammalian crystallin expression. Molecular Vision, 14:806–814, 2008. [PMC free article] [PubMed] [Google Scholar]
- 19.S Greiling TM, Aose M, and Clark JI. Cell fate and differentiation of the developng ocular lens. Investigative Ophthalmology & Visual Science, 51:1540–1546, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Posner Mason, Murray Kelly L., McDonald Matthew S., Eighinger Hayden, Andrew Brandon, Drossman Amy, Haley Zachary, Nussbaum Justin, David Larry L., and Lampi Kirsten J.. The zebrafish as a model system for analyzing mammalian and native α-crystallin promoter function. PeerJ, 5:e4093, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mishra Sanjay, Wu Shu-Yu, Fuller Alexandra W., Wang Zhen, Rose Kristie L., Schey Kevin L., and Mchaourab Hassane S.. Loss of αB-crystallin function in zebrafish reveals critical roles in the development of the lens and stress resistance of the heart. J. Biol. Chem, 293(2):740–753, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zou Ping, Wu Shu-Yu, Koteiche Hanane A., Mishra Sanjay, Levic Daniel S., Knapik Ela, Chen Wenbiao, and Mchaourab Hassane S.. A conserved role of αA-crystallin in the development of the zebrafish embryonic lens. Experimental Eye Research, 138:104–113, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sharma KK, Kaur H, and Kester K. Functional elements in molecular chaperone -crystallin: identification of binding sites in bcrystallin. Biochem. Biophys. Res. Commun, 239:217–222, 1997. [DOI] [PubMed] [Google Scholar]
- 24.Bhattacharyya J, Padmanabha Udupa EG, Wang J, and Sharma KK. Mini-αB-crystallin: a functional element of αB-crystallin with chaperone-like activity. Biochemistry, 45:3069–3076, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Raju Murugesan, Santhoshkumar Puttur, and Sharma K. Krishna. Alpha-crystallin-derived peptides as therapeutic chaperones. Biochimica et Biophysica Acta (BBA) - General Subjects, 1860(1, Part B):246–251, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ghosh JG, Shenoy AK Jr, and Clark JI. N- and C-terminal motifs in human αB crystallin play an important role in the recognition, selection, and solubilization of substrates. Biochemistry, 45:13847–13854, 2006. [DOI] [PubMed] [Google Scholar]
- 27.McHaourab Hassane S., Godar Jared A., and Stewart Phoebe L. Structure and mechanism of protein stability sensors: chaperone activity of small heat shock proteins. Biochemistry, 48 (18):3828–3837, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mainz Andi, Peschek Jirka, Stavropoulou Maria, Katrin C Back Benjamin Bardiaux, Asami Sam, Prade Elke, Peters Carsten, Weinkauf Sevil, Buchner Johannes, and Reif Bernd. The chaperone αB-crystallin uses different interfaces to capture an amorphous and an amyloid client. Nature Structural & Molecular Biology, 22:898–905, 2015. [DOI] [PubMed] [Google Scholar]
- 29.Laganowsky Arthur, Benesch Justin L. P., Landau Meytal, Ding Linlin, Sawaya Michael R., Cascio Duilio, Huang Qingling, Robinson Carol V., Horwitz Joseph, and Eisenberg David. Crystal structures of truncated αA- and αB-crystallins reveal structural mechanisms of polydispersity important for eye lens function. Protein Science, 19:1031–1043, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Clark John I and Muchowski Paul J. Small heat-shock proteins and their potential role in human disease. Current opinion in structural biology, 10(1):52–59, 2000. [DOI] [PubMed] [Google Scholar]
- 31.Delbecq Scott P and Klevit Rachel E. One size does not fit all: the oligomeric states of αb crystallin. FEBS letters, 587(8):1073–1080, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Andrew Aquilina J, Benesch Justin L. P., Bateman Orval A., Slingsby Christine, and Robinson Carol V.. Polydispersity of a mammalian chaperone: Mass spectrometry reveals the population of oligomers in αB-crystallin. Proc. Natl. Acad. Sci. U. S. A, 100(19):10611–10616, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Baldwin Andrew J., Lioe Hadi, Hilton Gillian R., Baker Lindsay A., Rubinstein John L., Kay Lewis E., and Benesch Justin L. P.. The polydispersity of αB-crystallin is rationalized by an interconverting polyhedral architecture. Structure, 19(12):1855–1863, 2011. [DOI] [PubMed] [Google Scholar]
- 34.Jehle Stefan, Barth van Rossum Joseph R. Stout, Noguchi Satoshi M., Falber Katja, Rehbein Kristina, Oschkinat Hartmut, Klevit Rachel E., and Rajagopal Ponni. αB-crystallin: A hybrid solid-state/solution-state NMR investigation reveals structural aspects of the heterogeneous oligomer. Journal of Molecular Biology, 385(5), 2009, Pages 1481–1497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bagneris C, Bateman OA, Naylor CE, Cronin N, Boelens WC, Keep NH, and Slingsby C. Crystal structures of α-crystallin domain dimers of αB-crystallin and Hsp20. J. Mol. Biol, 392:1242–1252, 2009. [DOI] [PubMed] [Google Scholar]
- 36.Liu Zhenying, Wang Chuchu, Li Yichen, Zhao Chunyu, Li Tongzhou, Li Dan, Zhang Shengnan, and Liu Cong. Mechanistic insights into the switch of αB-crystallin chaperone activity and self-multimerization. The Journal of Biological Chemistry, 293(38):14880–14890, September 2018. ISSN 1083–351X.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Delbecq Scott P., Jehle Stefan, and Klevit Rachel. Binding determinants of the small heat shock protein, αB-crystallin: recognition of the ‘IxI’ motif. EMBO Journal, 31:4587–4594, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Martin RW. NMR studies of eye lens crystallins. eMagRes (Encyclopedia of NMR). John Wiley & Sons, Ltd., The Atrium, Southern Gate, Chichester, West Sussex, PO19 8SQ, United Kingdom, 2014. [Google Scholar]
- 39.Burmann Björn M. and Hiller Sebastian. Chaperones and chaperone–substrate complexes: Dynamic playgrounds for NMR spectroscopists. Progress in Nuclear Magnetic Resonance Spectroscopy, 86–87:41–64, 2015. [DOI] [PubMed] [Google Scholar]
- 40.Jehle Stefan, Barth van Rossum Joseph R. Stout, Noguchi Satoshi M., Falber Katja, Rehbein Kristina, Oschkinat Hartmut, Klevit Rachel E., and Rajagopal Ponni. αB-crystallin: a hybrid solid-state/solution-state NMR investigation reveals structural aspects of the heterogeneous oligomer. Journal of Molecular Biology, 385(5):1481–1497, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jehle Stefan, Rajagopal Ponni, Bardiaux Benjamin, Markovic Stefan, Ronald Kühne Joseph R. Stout, Higman Victoria A., Klevit Rachel E., van Rossum Barth-Jan, and Oschkinat Hartmut. Solid-state nmr and saxs studies provide a structural basis for the activation of αb-crystallin oligomers. Nature Structural & Molecular Biology, 17(9):1037, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Jehle Stefan, Vollmar Breanna S., Bardiaux Benjamin, Dove Katja K., Rajagopal Ponni, Gonen Tamir, Oschkinat Hartmut, and Klevit Rachel E.. N-terminal domain of αB-crystallin provides a conformational switch for multimerization and structural heterogeneity. Proc. Natl. Acad. Sci. U. S. A, 108(16):6409–6414, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Braun Nathalie, Zacharias Martin, Peschek Jirka, Andreas Kastenmüller Juan Zou, Hanzlik Marianne, Haslbeck Martin, Rappsilber Juri, Buchner Johannes, and Weinkauf Sevil. Multiple molecular architectures of the eye lens chaperone αB-crystallin elucidated by a triple hybrid approach. Proceedings of the National Academy of Sciences of the United States of America, 108(51):20491–20496, December 2011. ISSN 0027–8424.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Baldwin Andrew J., Hilton Gillian R., Lioe Hadi, Claire Bagnéris Justin L. P. Benesch, and Kay Lewis E.. Quaternary dynamics of αB-crystallin as a direct consequence of localised tertiary fluctuations in the C-terminus. J. Mol. Biol, 413(2):310–320, 2011. [DOI] [PubMed] [Google Scholar]
- 45.Inoue Rintaro, Takata Takumi, Fujii Norihiko, Ishii Kentaro, Uchiyama Susumu, Sato Nobuhiro, Oba Yojiro, Wood Kathleen, Kato Koichi, Fujii Noriko, and Sugiyama Masaaki. New insight into the dynamical system of αB-crystallin oligomers. Scientific Reports, 6:29208, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rajagopal Ponni, Tse Eric, Borst Andrew J., Delbecq Scott P., Shi Lei, Southworth Daniel R., and Klevit Rachel E.. A conserved histidine modulates HSPB5 structure to trigger chaperone activity in response to stress-related acidosis. eLife, 4:e07304, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Reid Alderson T, Roche Julien, Gastall Heidi Y., Dias David M., Pritišanac Iva, Ying Jinfa, Bax Ad, Benesch Justin L. P., and Baldwin Andrew J.. Local unfolding of the HSP27 monomer regulates chaperone activity. Nature Communications, 10:1068, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Garvey Megan, Ecroyd Heath, Ray Nicholas J., Gerrard Juliet A., and Carver John A.. Functional amyloid protection in the eye lens: Retention of α-crystallin molecular chaperone activity after modification into amyloid fibrils. Biomolecules, 7(3):67, 2017.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Christoph JO Kaiser Carsten Peters, Philipp WN Schmid Maria Stavropoulou, Zou Juan, Dahiya Vinay, Evgeny V Mymrikov Beate Rockel, Asami Sam, Haslbeck Martin, et al. The structure and oxidation of the eye lens chaperone αa-crystallin. Nature Structural & Molecular Biology, 26(12):1141–1150, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Thampi P and Abraham EC. Influence of the C-terminal residues on oligomerization of αA-crystallin. Biochemistry, 42:11857–11863, 2003. [DOI] [PubMed] [Google Scholar]
- 51.Aziz A, Santhoshkumar P, Sharma KK, and Abraham EC. Cleavage of the C-terminal serine of human αA-crystallin produces αA1–172 with increased chaperone activity and oligomeric size. Biochemistry, 46:2510–2519, 2007. [DOI] [PubMed] [Google Scholar]
- 52.Treweek TM, Rekas A, Walker MJ, and Carver JA. A quantitative NMR spectroscopic examination of the flexibility of the C-terminal extensions of the molecular chaperones, αA-and αB-crystallin. Exp. Eye Res, 91:691–699, 2010. [DOI] [PubMed] [Google Scholar]
- 53.Bova MP, McHaourab HS, Han Y, and Fung BKK. Subunit exchange of small heat shock proteins. Analysis of oligomer formation of αA-crystallin and Hsp27 by fluorescence resonance energy transfer and site-directed truncations. J. Biol. Chem, 275:1035–1042, 2000. [DOI] [PubMed] [Google Scholar]
- 54.Kundu M, Sen PC, and Das KP. Structure, stability and chaperone function of αA-crystallin: role of N-terminal region. Biopolymers, 86:177–192, 2007. [DOI] [PubMed] [Google Scholar]
- 55.Laganowsky A and Eisenberg D. Non-3D domain swapped crystal structure of truncated zebrafish αA crystallin. Protein Science, 19(10):1978–1984, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Preis Waldemar, Bestehorn Annika, Buchner Johannes, and Haslbeck Martin. An alternative splice variant of human αA-crystallin modulates the oligomer ensemble and the chaperone activity of α-crystallins. Cell Stress and Chaperones, 22:541–552, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Koteiche HA, Claxton DP, Mishra S, Stein RA, McDonald ET, and McHaourab HS. Species-specific structural and functional divergence of α-crystallins: Zebrafish αBa- and rodent αA ins-crystallin encode activated chaperones. Biochemistry, 54:5949–5958, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Panda AK, Nandi SK, Chakraborty A, Nagaraj RH, and Biswas A. Differential role of arginine mutations on the structure and functions of α-crystallin. Biochimica et Biophysica Acta, 1860(1 Pt B):199–210, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Sacconi Sabrina, Léonard Féasson Jean Christophe Antoine, Christophe Pécheux Rafaelle Bernard, Ana Maria Cobo Alberto Casarin, Salviati Leonardo, Desnuelle Claude, and Urtizberea Andoni. A novel CRYAB mutation resulting in multisystemic disease. Neuromuscul. Disord, 22(1):66–72, 2012. [DOI] [PubMed] [Google Scholar]
- 60.Ghahramani Maryam, Yousefi Reza, Krivandin Alexey, Muranov Konstantin, Kurganov Boris, and Ali Akbar Moosavi-Movahedi. Structural and functional characterization of D109H and R69C mutant versions of human αB-crystallin: The biochemical pathomechanism underlying cataract and myopathy development. International Journal of Biological Macromolecules, 2019. ISSN 1879–0003.. [DOI] [PubMed] [Google Scholar]
- 61.Bova MP, Yaron O, Huang Q, Ding L, Haley DA, Stewart PL, and Horwitz J. Mutation R120G in αB-crystallin, which is linked to a desmin-related myopathy, results in an irregular structure and defective chaperone-like function. Proc. Natl. Acad. Sci. U. S. A, 96 (11):6137–6142, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Saba Sadaf, Ghahramani Maryam, and Yousefi Reza. A comparative study of the impact of calcium ion on structure, aggregation and chaperone function of human αa-crystallin and its cataract-causing r12c mutant. Protein and peptide letters, 24(11):1048–1058, 2017. [DOI] [PubMed] [Google Scholar]
- 63.Phadte Ashutosh S., Mahalingam Sundararajan, Santhoshkumar Puttur, and Sharma Krishna K.. Functional rescue of cataract-causing αA-G98R-crystallin by targeted compensatory suppressor mutations in human αA-crystallin. Biochemistry, 58(40):4148–4158, October 2019. ISSN 1520–4995.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Wu Shu-Yu, Zou Ping, Mishra Sanjay, and Mchaourab Hassane S.. Transgenic zebrafish models reveal distinct molecular mechanisms for cataract-linked αA-crystallin mutants. PloS One, 13(11):e0207540, 2018. ISSN 1932–6203.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Andley Usha P., Malone James P., Hamilton Paul D., Ravi Nathan, and R. Reid Townsend. Comparative proteomic analysis identifies age-dependent increases in the abundance of specific proteins after deletion of the small heat shock proteins αA- and αB-crystallin. Biochemistry, 52(17):2933–2948, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Andley Usha P and Goldman Joshua W. Autophagy and upr in α-crystallin mutant knock-in mouse models of hereditary cataracts. Biochimica et Biophysica Acta (BBA)-General Subjects, 1860(1):234–239, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Ragerdi Kashani M, Yousefi R, Akbarian M, Alavianmehr MM, and Ghasemi Y. Structure, Chaperone Activity, and Aggregation of Wild-Type and R12C Mutant αB-Crystallins in the Presence of Thermal Stress and Calcium Ion - Implications for Role of Calcium in Cataract Pathogenesis. Biochemistry. Biokhimiia, 81(2):122–134, February 2016. ISSN 1608–3040.. [DOI] [PubMed] [Google Scholar]
- 68.Md Faiz Ahmad Devendra Singh, Taiyab Aftab, Ramakrishna Tangirala, Raman Bakthisaran, and Ch Mohan Rao. Selective cu2+ binding, redox silencing, and cytoprotective effects of the small heat shock proteins αa-and αb-crystallin. Journal of molecular biology, 382(3):812–824, 2008. [DOI] [PubMed] [Google Scholar]
- 69.Maria Luisa Ganadu Michaela Aru, Giovanni Maria Mura Alessio Coi, Mlynarz Piotr, and Kozlowski Henryk. Effects of divalent metal ions on the αb-crystallin chaperone-like activity: spectroscopic evidence for a complex between copper (ii) and protein. Journal of inorganic biochemistry, 98(6):1103–1109, 2004. [DOI] [PubMed] [Google Scholar]
- 70.Mainz Andi, Bardiaux Benjamin, Kuppler Frank, Multhaup Gerd, Isabella C Felli Roberta Pierattelli, and Reif Bernd. Structural and mechanistic implications of metal binding in the small heat-shock protein αb-crystallin. Journal of Biological Chemistry, 287(2):1128–1138, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Quintanar Liliana, José A Domínguez-Calva Eugene Serebryany, Lina Rivillas-Acevedo Cameron Haase-Pettingell, Amero Carlos, and Jonathan A King. Copper and zinc ions specifically promote nonamyloid aggregation of the highly stable human γD- crystallin. ACS Chemical Biology, 11(1):263–272, 2016. [DOI] [PubMed] [Google Scholar]
- 72.Kalyan S Ghosh Ajay Pande, and Pande Jayanti. Binding of γ-crystallin substrate prevents the binding of copper and zinc ions to the molecular chaperone α-crystallin. Biochemistry, 50 (16):3279–3281, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Chauhan Priyanka and Ghosh Kalyan S. Inhibition of copper-induced aggregation of human γd-crystallin by rutin and studies on its role in molecular level for enhancing the chaperone activity of human αa-crystallin by using multi-spectroscopic techniques. Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy, 218:229–236, 2019. [DOI] [PubMed] [Google Scholar]
- 74.Chauhan Priyanka, Sai Brinda Muralidharan Anand Babu Velappan, Datta Dhrubajyoti, Pratihar Sanjay, Debnath Joy, and Ghosh Kalyan Sundar. Inhibition of copper-mediated aggregation of human γd-crystallin by schiff bases. JBIC Journal of Biological Inorganic Chemistry, 22(4):505–517, 2017. [DOI] [PubMed] [Google Scholar]
- 75.José Antonio Dominguez-Calva Cameron Haase-Pettingell, Serebryany Eugene, King Jonathan Alan, and Quintanar Liliana. A histidine switch for Zn-induced aggregation of γ-crystallins reveals a metal-bridging mechanism that is relevant to cataract disease. Biochemistry, 57(33):4959–4962, 2018. [DOI] [PubMed] [Google Scholar]
- 76.Biswas Ashis and Das Kali P. Zn2+ enhances the molecular chaperone function and stability of α-crystallin. Biochemistry, 47(2):804–816, 2008. [DOI] [PubMed] [Google Scholar]
- 77.Biswas A, Karmakar S, Chowdhury A, and Das KP. Interaction of α-crystallin with some small molecules and its effect on its structure and function. Biochimica et Biophysica Acta (BBA)-General Subjects, 1860(1):211–221, 2016. [DOI] [PubMed] [Google Scholar]
- 78.Barman Susmita and Srinivasan Krishnapura. Zinc supplementation ameliorates diabetic cataract through modulation of crystallin proteins and polyol pathway in experimental rats. Biological trace element research, 187(1):212–223, 2019. [DOI] [PubMed] [Google Scholar]
- 79.Hawse John R, Cumming Jonathan R, Oppermann Brian, Sheets Nancy L, Reddy Venkat N, and Kantorow Marc. Activation of metallothioneins and α-crystallin/shsps in human lens epithelial cells by specific metals and the metal content of aging clear human lenses. Investigative ophthalmology & visual science, 44(2):672–679, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Hanson SR, Hasan A, Smith DL, and Smith JB. The major in vivo modifications of the human waterinsoluble lens crystallins are disulfide bonds, deamidation, methionine oxidation and backbone cleavage. Experimental Eye Research, 71:195–207, 2000. [DOI] [PubMed] [Google Scholar]
- 81.Truscott Roger JW and Friedrich Michael G. The etiology of human age-related cataract. proteins don’t last forever. Biochimica et Biophysica Acta (BBA)-General Subjects, 1860(1): 192–198, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Löfgren Stefan. Solar ultraviolet radiation cataract. Experimental Eye Research, 156:112–116, March 2017. ISSN 1096–0007.. [DOI] [PubMed] [Google Scholar]
- 83.Anbaraki Afrooz, Ghahramani Maryam, Muranov Konstantin O., Kurganov Boris I., and Yousefi Reza. Structural and functional alteration of human αA-crystallin after exposure to full spectrum solar radiation and preventive role of lens antioxidants. International Journal of Biological Macromolecules, 118(Pt A):1120–1130, October 2018. ISSN 1879–0003.. [DOI] [PubMed] [Google Scholar]
- 84.Santhoshkumar P, Raju M, and Sharma KK. αA-crystallin peptide SDRDKFVIFLDVKHF accumulating in aging lens impairs the function of α-crystallin and induces lens protein aggregation. PLoS ONE, 6:e19291, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Hooi MY and Truscott RJ. Racemisation and human cataract. D-Ser, D-Asp/Asn and D-Thr are higher in the lifelong proteins of cataract lenses than in age-matched normal lenses. Age (Dordr.), 33:131–141, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Aswad Dana W., Paranandi Mallik V., and Schurter Brandon T.. Isoaspartate in peptides and proteins: Formation, significance, and analysis. Journal of Pharmaceutical and Biomedical Analysis, 21(6):1129–1136, 2000. [DOI] [PubMed] [Google Scholar]
- 87.Noguchi Shuji. Structural changes induced by the deamidation and isomerization of asparagine revealed by the crystal structure of Ustilago sphaerogena ribonuclease U2B. Biopolymers, 93(11):1003–1010, 2010. ISSN 1097–0282.. eprint: https://onlinelibrary.wiley.com/doi/pdf/10.1002/bip.21514. [DOI] [PubMed] [Google Scholar]
- 88.Lyon Yana A., Sabbah Georgette M., and Julian Ryan R.. Differences in α-Crystallin isomerization reveal the activity of protein isoaspartyl methyltransferase (PIMT) in the nucleus and cortex of human lenses. Experimental Eye Research, 171:131–141, June 2018. ISSN 1096–0007.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Takata T and Fujii N. Isomerization of Asp residues plays an important role in αA-crystallin dissociation. FEBS J., 283:850–859, 2016. [DOI] [PubMed] [Google Scholar]
- 90.Lyon Yana A., Collier Miranda P., Riggs Dylan L., Degiacomi Matteo T., Benesch Justin L. P., and Julian Ryan R.. Structural and functional consequences of age-related isomerization in α-crystallins. The Journal of Biological Chemistry, 294:7546–7555, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Stevens VJ, Rouzer CA, Monnier VM, and Cerami A. Diabetic cataract formation: Potential role of glycosylation of lens crystallins. Proceedings of the National Academy of Sciences of the United States of America, 75(6):2918–2922, June 1978. ISSN 0027–8424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Fereshteh Bahmani S Zahra Bathaie, S. Javid Aldavood, and Arezou Ghahghaei. Prevention of α-crystallin glycation and aggregation using l-lysine results in the inhibition of in vitro catalase heat-induced-aggregation and suppression of cataract formation in the diabetic rat. International Journal of Biological Macromolecules, 132:1200–1207, July 2019. ISSN 1879–0003.. [DOI] [PubMed] [Google Scholar]
- 93.Nandi Sandip K., Nahomi Rooban B., Harris Peter S., Michel Cole R., Fritz Kristofer S., and Nagaraj Ram H.. The absence of SIRT3 and SIRT5 promotes the acetylation of lens proteins and improves the chaperone activity of α-crystallin in mouse lenses. Experimental Eye Research, 182:1–9, May 2019. ISSN 1096–0007.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Muranova LK, Sudnitsyna MV, and Gusev NB. αB-crystallin phosphorylation: Advances and problems. Biochemistry (Moscow), 83(10):1196–1206, October 2018. ISSN 1608–3040.. [DOI] [PubMed] [Google Scholar]
- 95.Md Faiz Ahmad Bakthisaran Raman, Ramakrishna Tangirala, and Rao Ch Mohan. Effect of phosphorylation on alpha B-crystallin: Differences in stability, subunit exchange and chaperone activity of homo and mixed oligomers of alpha B-crystallin and its phosphorylation-mimicking mutant. Journal of Molecular Biology, 375(4):1040–1051, January 2008. ISSN 1089–8638.. [DOI] [PubMed] [Google Scholar]
- 96.Smith JB, Sun Y, Smith DL, and Green B. Identification of the posttranslational modifications of bovine lens alpha B-crystallins by mass spectrometry. Protein Science : A Publication of the Protein Society, 1(5):601–608, May 1992. ISSN 0961–8368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Ciano Michela, Allocca Simona, Ciardulli Maria Camilla, Volpe Lucrezia della, Bonatti Stefano, and D’Agostino Massimo. Differential phosphorylation-based regulation of αB-crystallin chaperone activity for multipass transmembrane proteins. Biochemical and Biophysical Research Communications, 479(2):325–330, October 2016. ISSN 0006–291X.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Baboolall Kashmeera D., Kaudeer Yusrah B., Gershenson Anne, and O’Hara Patricia B.. ph dependence of oligomerization and functional activity of α b crystallin. Biophysical Journal, 118(3):510a, February 2020. ISSN 0006–3495.. [Google Scholar]
- 99.Bakthisaran Raman, Kranthi Kiran Akula Ramakrishna Tangirala, and Rao Ch. Mohan. Phosphorylation of αB-crystallin: Role in stress, aging and patho-physiological conditions. Biochimica et Biophysica Acta (BBA) - General Subjects, 1860(1, Part B):167–182, January 2016.. [DOI] [PubMed] [Google Scholar]
- 100.Andrew Aquilina J, Benesch Justin L. P., Ding Lin Lin, Yaron Orna, Horwitz Joseph, and Robinson Carol V.. Phosphorylation of αB-crystallin alters chaperone function through loss of dimeric substructure. The Journal of Biological Chemistry, 279(27):28675–28680, 2004.. [DOI] [PubMed] [Google Scholar]
- 101.Haslbeck Martin, Peschek Jirka, Buchner Johannes, and Weinkauf Sevil. Structure and function of α-crystallins: Traversing from in vitro to in vivo. Biochimica et Biophysica Acta (BBA) - General Subjects, 1860(1 Part B):149–166, 2016. [DOI] [PubMed] [Google Scholar]
- 102.Basha E, Lee GJ, Breci LA, Hausrath AC, Buan NR, Giese KC, and Vierling E. The identity of proteins associated with a small heat shock protein during heat stress in vivo indicates that these chaperones protect a wide range of cellular functions. Journal of Bioloical Chemistry, 279(9), 2004. [DOI] [PubMed] [Google Scholar]
- 103.Das Payel, Seung gu Kang Sally Temple, and Belfort Georges. Interaction of amyloid inhibitor proteins with amyloid beta peptides: Insight from molecular dynamics simulations. PloS One, 9(11):e113041, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Smulders RH, Merck KB, Aendekerk J, Horwitz J, Takemoto L, Slingsby C, Bloemendal H, and De Jong WW. The mutation Asp69–>Ser affects the chaperone-like activity of α A-crystallin. European Journal of Biochemistry, 232(3):834–838, September 1995. ISSN 0014–2956.. [DOI] [PubMed] [Google Scholar]
- 105.Santhoshkumar P and Sharma KK. Phe71 is essential for chaperone-like function in alpha A-crystallin. The Journal of Biological Chemistry, 276(50):47094–47099, December 2001. ISSN 0021–9258.. [DOI] [PubMed] [Google Scholar]
- 106.Takemoto L, Emmons T, and Horwitz J. The C-terminal region of alpha-crystallin: Involvement in protection against heat-induced denaturation. The Biochemical Journal, 294 (Pt 2): 435–438, September 1993. ISSN 0264–6021.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Plater ML, Goode D, and Crabbe MJ. Effects of site-directed mutations on the chaperone-like activity of αB-crystallin. The Journal of Biological Chemistry, 271(45):28558–28566, November 1996. ISSN 0021–9258.. [DOI] [PubMed] [Google Scholar]
- 108.Krishna Sharma K, Senthil Kumar R, Suresh Kumar G, and Thomas Quinn P. Synthesis and Characterization of a Peptide Identified as a Functional Element in αA-crystallin. Journal of Biological Chemistry, 275(6):3767–3771, February 2000. ISSN 0021–9258, 1083–351X.. [DOI] [PubMed] [Google Scholar]
- 109.Jaya Bhattacharyya EG Padmanabha Udupa, Jing Wang, and K. Krishna Sharma. Mini-alphaB-crystallin: A functional element of alphaB-crystallin with chaperone-like activity. Biochemistry, 45(9):3069–3076, March 2006. ISSN 0006–2960.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Banerjee Priya R., Pande Ajay, Shekhtman Alexander, and Pande Jayanti. Molecular mechanism of the chaperone function of mini-α-crystallin, a 19-residue peptide of human α-crystallin. Biochemistry, 54(2):505–515, January 2015. ISSN 0006–2960.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Mishra Sanjay, Stein Richard A., and Mchaourab Hassane S.. Cataract-linked γD-crystallin mutants have weak affinity to lens chaperones α-crystallins. FEBS Letters, 586(4):330–336, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Sprague-Piercy Marc A., Wong Eric, Roskamp Kyle W., Fakhoury Joseph N., Freites J. Alfredo, Tobias Douglas J., and Martin Rachel W.. Human αB-crystallin discriminates between aggregation-prone and function-preserving variants of a client protein. Biochimica Et Biophysica Acta. General Subjects, 1864(3):129502, March 2020. ISSN 1872–8006.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Ramirez Lisa M., Shekhtman Alexander, and Pande Jayanti. Hydrophobic residues of melittin mediate its binding to αA-crystallin. Protein Science, 29(2):572–588, 2020. ISSN 1469–896X.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Clark John I.. Functional sequences in human alphaB crystallin. Biochimica et Biophysica Acta (BBA) - General Subjects, 1860(1, Part B):240–245, January 2016. ISSN 0304–4165.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Muranov KO, Poliansky NB, Chebotareva NA, Yu Kleimenov S, Bugrova AE, Indeykina MI, Kononikhin AS, Nikolaev EN, and Ostrovsky MA. The mechanism of the interaction of α-crystallin and UV-damaged βL-crystallin. International Journal of Biological Macromolecules, 140:736–748, November 2019. ISSN 1879–0003.. [DOI] [PubMed] [Google Scholar]
- 116.Carver John A., Grosas Aidan B., Ecroyd Heath, and Quinlan Roy A.. The functional roles of the unstructured N- and C-terminal regions in αB-crystallin and other mammalian small heat-shock proteins. Cell Stress & Chaperones, 22(4):627–638, July 2017. ISSN 1355–8145.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Kiss Andor J., Mirarefi Amir Y., Ramakrishnan Subramanian, Zukoski Charles F., DeVries Arthur L., and Cheng Chi-Hing C.. Cold-stable eye lens crystallins of the Antarctic nototheniid toothfish Dissostichus mawsoni Norman. Journal of Experimental Biology, 207:4633–4649, 2004. [DOI] [PubMed] [Google Scholar]
- 118.Kiss Andor J. and Cheng C.-H. Christina. Molecular diversity and genomic organisation of the α, β, and γ eye lens crystallins from the Antarctic toothfish Dissostichus mawsoni. Comparative Biochemistry and Physiology Part D: Genomics and Proteomics, 3(2):155–171, 2008. [DOI] [PubMed] [Google Scholar]
- 119.Posner Mason, Kiss Andor J., Skiba Jackie, Drossman Amy, Dolinska Monika B., Hejtmancik J. Fielding, and Sergeev Yuri V.. Functional validation of hydrophobic adaptation to physiological temperature in the small heat shock protein αA-crystallin. PloS One, 7(3):e34438, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Smith AA, Wyatt K, Vacha J, Vihtelic TS, Zigler JS Jr., Wistow GJ, and Posner M. Gene duplication and separation of functions in αB-crystallin from zebrafish (Danio rerio). FEBS Journal, 273:481–490, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Hochberg Georg K. A., Ecroyd Heath, Liu Cong, Cox Dezerae, Cascio Duilio, Sawaya Michael R., Collier Miranda P., Stroud James, Carver John A., Baldwin Andrew J., Robinson Carol V., Eisenberg David S., Benesch Justin L. P., and Laganowsky Arthur. The structured core domain of αB-crystallin can prevent amyloid fibrillation and associated toxicity. Proceedings of the National Academy of Sciences of the United States of America, 111(16): E1562–E1570, 2014.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.van Noort Johannes M, Bsibsi Malika, Nacken Peter J, Verbeek Richard, and Venneker Edna HG. Therapeutic intervention in multiple sclerosis with alpha b-crystallin: A randomized controlled phase iia trial. PloS one, 10(11):e0143366, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Droho Steven, Keener Mitchell E., and Mueller Niklaus H.. Heparan sulfate mediates cell uptake of αB-crystallin fused to the glycoprotein C cell penetration peptide. Biochimica Et Biophysica Acta. Molecular Cell Research, 1865(4):598–604, April 2018. ISSN 0167–4889.. [DOI] [PubMed] [Google Scholar]
- 124.Bisht Anjali, Sharma Manju, Sharma Shikha, Ali Md Ehesan, and Panda Jiban Jyoti. Carrierfree self-built aspirin nanorods as anti-aggregation agents towards alpha-crystallin-derived peptide aggregates: potential implications in non-invasive cataract therapy. Journal of Materials Chemistry B, 7(44):6945–6954, 2019. [DOI] [PubMed] [Google Scholar]
- 125.Makley Leah N., McMenimen Kathryn A., DeVree Brian T., Goldman Joshua W., McGlasson Brittney N., Rajagopal Ponni, Dunyak Bryan M., McQuade Thomas J., Thompson Andrea D., Sunahara Roger, Klevit Rachel E., Andley Usha P., and Gestwicki Jason E.. Pharmacological chaperone for α-crystallin partially restores transparency in cataract models. Science, 350 (6261):674–677, November 2015.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Mallik Prabhat K., Shi Hua, and Pande Jayanti. RNA aptamers targeted for human αA-crystallin do not bind αB-crystallin, and spare the α-crystallin domain. Biochem. Biophys. Res. Commun, 491(2):423–428, September 2017. ISSN 1090–2104.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Karplus Martin and J. Andrew McCammon. Molecular dynamics simulations of biomolecules. Nature Structural & Molecular Biology, 9:646–652, 2002. [DOI] [PubMed] [Google Scholar]
- 128.Karplus M and Kuriyan J. Molecular dynamics and protein function. Proceedings of the National Academy of Sciences of the United States of America, 102(19):6679–6685, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Brunger AT and Adams PD. Molecular dynamics applied to x-ray structure refinement. Accounts of Chemical Research, 35:404–412, 2002. [DOI] [PubMed] [Google Scholar]
- 130.Chen JH, Won HS, Im WP, Dyson HJ, and Brooks CL. Generation of native-like protein structures from limited NMR data, modern force fields and advanced conformational sampling. Journal of Biomolecular NMR, 31:59–64, 2005. [DOI] [PubMed] [Google Scholar]
- 131.Metropolis Nicholas, Rosenbluth Arianna W., Rosenbluth Marshall N., and Teller Augusta H.. Equation of state calculations by fast computing machines. Journal of Chemical Physics, 21: 1087, 1953. [Google Scholar]
- 132.Kurut Anıl, Persson Björn A., Åkesson Torbjörn, Forsman Jan, and Lund Mikael. Anisotropic interactions in protein mixtures: Self assembly and phase behavior in aqueous solution. Journal of Physical Chemistry Letters, 3(6):731–734, 2012. [DOI] [PubMed] [Google Scholar]
- 133.Prytkova Vera, Heyden Matthias, Khago Domarin, Freites J. Alfredo, Butts Carter T., Martin Rachel W., and Tobias Douglas J.. Multi-conformation Monte Carlo: A method for introducing flexibility in efficient simulations of many-protein systems. The Journal of Physical Chemistry B, 120(33):8115–8126, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Grazioli Gianmarc, Yu Yue, Unhelkar Megha H., Martin Rachel W., and Butts Carter T.. Network-based classification and modeling of amyloid fibrils. The Journal of Physical Chemistry B, 123(26):5452–5462, 2019. [DOI] [PubMed] [Google Scholar]
- 135.Grazioli Gianmarc, Yu Yue, Unhelkar Megha H., Martin RW, and Butts Carter T.. Network hamiltonian models reveal pathways to amyloid fibril formation. Scientific Reports, in press, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Henderson Richard. The potential and limitations of neutrons, electrons and x-rays for atomic resolution microscopy of unstained biological molecules. Quarterly reviews of biophysics, 28 (2):171–193, 1995. [DOI] [PubMed] [Google Scholar]
- 137.Rabi II, Zacharias JR, Millman S, and Kusch P. A new method of measuring nuclear magnetic moment. Physical Review, 53(4):318–327, 1938. [DOI] [PubMed] [Google Scholar]
- 138.Bloch F, Hansen WW, and Packard M. Nuclear induction. Physical Review, 69:127, 1946. [Google Scholar]
- 139.Purcell EM, Torrey HC, and Pound RV. Resonance absorption by nuclear moments in a solid. Physical Review, 69:37–38, 1946. [Google Scholar]
- 140.Pervushin Konstantin, Riek Roland, Wider Gerhard, and Wüthrich Kurt. Attenuated T2 relaxation by mutual cancellation of dipole-dipole coupling and chemical shift anisotropy indicates an avenue to NMR structures of very large biological macromolecules in solution. Proceedings of the National Academy of Sciences of the United States of America, 94(23): 12366–12371, 1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Tugarinov Vitali, Kanelis Voula, and Kay Lewis E.. Isotope labeling strategies for the study of high-molecular-weight proteins by solution NMR spectroscopy. Nature Protocols, 1:749–754, 2006. [DOI] [PubMed] [Google Scholar]
- 142.LeMaster David M.. Isotope labeling in solution protein assignment and structural analysis. Progress in Nuclear Magnetic Resonance Spectroscopy, 26(4):371–419, 1994. [Google Scholar]
- 143.Suzuki Rika, Sakakura Masayoshi, Mori Masaki, Fujii Moe, Akashi Satoko, and Takahashi Hideo. Methyl-selective isotope labeling using α-ketoisovalerate for the yeast Pichia pastoris recombinant protein expression system. Journal of Biomolecular NMR, 71:213–223, 2018. [DOI] [PubMed] [Google Scholar]
- 144.Velyvis Algirdas, Ruschak Amy M., and Kay Lewis E.. An economical method for production of 2H,13CH3-threonine for solution NMR studies of large protein complexes: Application to the 670 kDa proteasome. PLoS ONE, 7(9):e43725, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Aue WP, Bartholdi E, and Ernst RR. Two-dimensional spectroscopy: Application to nuclear magnetic resonance. Journal of Chemical Physics, 64:2229, 1976. [Google Scholar]
- 146.Wüthrich K, Wider G, Wagner G, and Braun W. Sequential resonance assignments as a basis for determination of spatial protein structures by high resolution nuclear magnetic resonance. Journal of Molecular Biology, 155:311–319, 1982. [DOI] [PubMed] [Google Scholar]
- 147.Kumar Anil, Ernst RR, and Wüthrich K. A two-dimensional nuclear Overhauser enhancement (2D NOE) experiment for the elucidation of complete proton-proton cross-relaxation networks in biological macromolecules. Biochemical and Biophysical Research Communications, 95:1, 1980. [DOI] [PubMed] [Google Scholar]
- 148.Tjandra Nico and Bax Ad. Direct measurement of distances and angles in biomolecules by NMR in a dilute liquid crystalline medium. Science, 278(5340):1111–1114, 1997. [DOI] [PubMed] [Google Scholar]
- 149.Cornilescu Gabriel, Delaglio Frank, and Bax Ad. Backbone angle restraints from searching a database for chemical shift and sequence homology. Journal of Biomolecular NMR, 13: 289–302, 1999. [DOI] [PubMed] [Google Scholar]
- 150.Havel TF and Wüthrich K. A distance geometry program for determining the structures of small proteins and other macromolecules from nuclear magnetic resonance measurements of intramolecular 1H-1H proximities in solution. Bulletin of Mathematical Biology, 46:673–678, 1984. [Google Scholar]
- 151.Arthanari Haribabu, Takeuchi Koh, Dubey Abhinav, and Wagner Gerhard. Emerging solution NMR methods to illuminate the structural and dynamic properties of proteins. Current Opinion in Structural Biology, 58:294–304, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Andrew ER, Bradbury A, and Eades RG. Nuclear magnetic resonance spectra from a crystal rotated at high speed. Nature, 182:1659, 958. [Google Scholar]
- 153.Castellani Federica, Barth van Rossum Annette Diehl, Schubert Mario, Rehbein Kristina, and Oschkinat Hartmut. Structure of a protein determined by solid-state magic-angle-spinning NMR spectroscopy. Nature, 420(6911):99–102, November 2002. ISSN 1476–4687.. [DOI] [PubMed] [Google Scholar]
- 154.Lange Adam, Becker Stefan, Seidel Karsten, Giller Karin, Pongs Olaf, and Baldus Marc. A concept for rapid protein-structure determination by solid-state NMR spectroscopy. Angewandte Chemie, 44(14):2089–2092, 2005. [DOI] [PubMed] [Google Scholar]
- 155.Sperling Lindsay J., Berthold Deborah A., Sasser Terry L., Jeisy-Scott Victoria, and Rienstra Chad M.. Assignment strategies for large proteins by magic-angle spinning NMR: the 21-kDa disulfide bond forming enzyme DsbA. Journal of Molecular Biology, 399(2):268–282, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Patrick CA van der Wel. New applications of solid-state NMR in structural biology. Emerging Topics in Life Sciences, 2(1):57–67, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Biswas Ashis, Goshe Jeffery, Miller Antonia, Santhoshkumar Puttur, Luckey Carol, Bhat Manjunatha B., and Nagaraj Ram H.. Paradoxical effects of substitution and deletion mutation of Arg56 on the structure and chaperone function of human alphaB-crystallin. Biochemistry, 46(5):1117–1127, February 2007. ISSN 0006–2960.. [DOI] [PubMed] [Google Scholar]
- 158.Treweek Teresa M., Rekas Agata, Lindner Robyn A., Walker Mark J., Aquilina J. Andrew, Robinson Carol V., Horwitz Joseph, Perng Ming Der, Quinlan Roy A., and Carver John A.. R120G alphaB-crystallin promotes the unfolding of reduced alpha-lactalbumin and is inherently unstable. The FEBS journal, 272(3):711–724, February 2005. ISSN 1742–464X.. [DOI] [PubMed] [Google Scholar]
- 159.Gerasimovich Evgeniia S., Strelkov Sergei V., and Gusev Nikolai B.. Some properties of three αB-crystallin mutants carrying point substitutions in the C-terminal domain and associated with congenital diseases. Biochimie, 142:168–178, November 2017. ISSN 0300–9084.. [DOI] [PubMed] [Google Scholar]
- 160.Droho Steven, Keener Mitchell E., and Mueller Niklaus H.. Changes in function but not oligomeric size are associated with αB-crystallin lysine substitution. Biochemistry and Biophysics Reports, 14:1–6, July 2018. ISSN 2405–5808.. [DOI] [PMC free article] [PubMed] [Google Scholar]


