Abstract
Introduction
Prenatal ultrasounds often yield indeterminate (incomplete or minor abnormality) findings with limited clinical utility. We evaluate impact of indeterminate findings on maternal anxiety.
Methods
A single-U.S.-center prospective cohort study administered the Perinatal Anxiety Screening Scale (PASS; control mean = 13.4; > 20 denotes clinically significant anxiety) before and after prenatal ultrasounds in February-May 2017. Ultrasound reports were coded as: normal; indeterminate; or major abnormality. Primary outcome was anxiety after indeterminate vs. normal ultrasounds. Secondary outcomes included anxiety change from pre-to-post-ultrasound and relative to women’s characteristics. Linear regression adjusted for confounders.
Results
Of 286 ultrasounds, 51.0% were normal, 40.5% indeterminate (22.0% incomplete; 18.5% minor abnormality), and 8.0% major abnormalities. Indeterminate findings were unrelated to age, race, parity, infertility, or psychiatric history, but associated with gestational age (26.6%/45.0%/52.5% for first/second/third trimesters; p < 0.001), and obesity (48.8 vs. 37.0%; p = 0.031). Pretest anxiety was highest in second/third trimesters (p = 0.029), and in subjects aged age = 24 or younger(p < 0.001), with a history of anxiety (p < 0.001),) or with prior pregnancy loss (p = 0.011). Mean anxiety score decreased pre-to-posttest across all groups. Indeterminate findings were associated with higher PASS scores than normal findings: pretest 20.1 vs. 16.4 (p = 0.026) and posttest 16.9 vs. 12.2 (p = 0.009; adjusted-p = 0.01). Versus normal ultrasounds, incomplete findings were associated with higher post-ultrasound anxiety (p = 0.007; adjusted-p = 0.01) and smaller decreases from pre-to-posttest (adjusted-p = 0.03), whereas minor abnormalities had higher pretest anxiety (p = 0.029) with larger pre-to-posttest decreases (adjusted-p =0.010).
Discussion
Indeterminate ultrasounds, especially incomplete findings, are associated with significantly higher anxiety than normal findings, suggesting need for evidence-based counseling, management and strategies for decreasing number of indeterminate results.
Keywords: Prenatal ultrasound, Maternal anxiety, Incomplete findings, Minor abnormality, Soft marker
Introduction
Nearly 4 million pregnant women in the United States undergo prenatal ultrasounds each year (Silvestri et al. 2016). Since its addition to routine prenatal care, fetal imaging has become integral to the psychosocial, phenomenological, and medical conceptions of pregnancy. While two screenings are currently recommended for uncomplicated gestations, usage has increased considerably, with insurance data indicating an average of five ultrasounds per low-risk pregnancy (Practice bulletin no. 175: Ultrasound in pregnancy 2016; O’Keeffe and Abuhamad 2013).
The impact of prenatal ultrasounds on maternal anxiety has been studied since the early 1980s (Robinson et al. 1984). Normal findings on ultrasound are thought to reduce maternal anxiety by providing reassurance and promoting bonding, whereas major abnormalities have been understandably associated with increased anxiety (Kaasen et al. 2017). Unfortunately, anxiety symptoms, as assessed by validated screening instruments, are independently associated with adverse outcomes, from fetal demise in utero and preterm delivery to postpartum depression and impaired childhood development (Pesonen et al. 2016; Staneva et al. 2015). Anxiety disorders are the most common psychiatric conditions among women, and may affect 6.6–21.7% of gravidae (Somerville et al. 2014). Identifying modifiable risk factors impacting women’s anxiety during pregnancy may lead to improved symptom management and reduction of associated complications.
Maternal anxiety appears to be higher with a major abnormality that has an uncertain prognosis than with a major abnormality for which the outcomes and plan of care are well defined (Aite et al. 2011). For example, women diagnosed with fetal cystic adenomatoid malformation, with its unpredictable and wide-ranging outcomes, have higher anxiety than women diagnosed with congenital diaphragmatic hernia, a well-defined, but more severe, condition.
Meanwhile, advancing technology has drastically increased sensitivity for minor fetal abnormalities, and anatomy protocols have become more detailed over time (Practice bulletin no. 175: Ultrasound in pregnancy 2016). Likewise, increased rates of obesity have made incomplete ultrasounds more common (Pasko et al. 2016). Notably, both minor abnormalities and incomplete ultrasounds add uncertainty to an overall favorable prognosis, and thus may be considered ‘indeterminate’ (Hurt et al. 2014). Conservative estimates suggest there may be 700,000 or more indeterminate ultrasounds annually in the U.S.; their impact on maternal anxiety, however, is unknown (Silvestri et al. 2016; Viaux-Savelon et al. 2012). We hypothesize that indeterminate ultrasound findings, whose clinical value may be limited, are associated with significantly higher maternal anxiety than normal findings.
Methods
The Pregnancy Outcomes, Mother’s Mood, and Sonograms (PrO MoMS) Study is a prospective cohort study of pregnant women presenting for scheduled prenatal ultrasounds at the Johns Hopkins Hospital prenatal ultrasound suite between February and March 2017. The Johns Hopkins University Institutional Review Board approved this study (#00116293). Eligibility required current pregnancy at any gestational age, English proficiency, 18 years of age or older, and arrival on time for scheduled appointment. Women presenting for any non-emergent, outpatient maternal fetal medicine ultrasound, including routine dating/viability, first trimester or anatomy screening, and specialized/targeted ultrasounds (e.g., cervical length, interval growth) were eligible. Convenience sampling was used depending on availability of research staff with consecutive recruitment of presenting patients while staff were available.
Women meeting eligibility criteria were recruited consecutively with a standardized script during ultrasound registration. Demographics of women who declined to participate were collected to compare with participants. All subjects provided written informed consent and were assigned a random ID number prior to participating. Code sheets with random IDs and corresponding patient IDs were stored in a secure lockbox. Participants received packets with their informed consent documents, a pretest questionnaire assessing demographics, pregnancy and anxiety risk factor history, and a pre-ultrasound Perinatal Anxiety Screening Scale (PASS) form.
The Perinatal Anxiety Screening Scale (PASS) is validated specifically for identifying clinically problematic anxiety (score ≥ 21) in antenatal and postpartum populations (Somerville et al. 2014, 2015). The PASS takes approximately 6 min to complete and includes 31 questions assessing anxiety domains of (1) acute anxiety and adjustment, (2) general worry and specific fears, (3) perfectionism, control and trauma, and (4) social anxiety (see supplemental file). Each item measured presence and severity of symptoms with a Likert scale, ranging from “not at all” (zero points), “sometimes” (one point), “often” (two points), and “almost always” (three points). The PASS is intended to be a durable measure of anxiety over the course of a month. Thus, we considered a 3-point difference between pre-and-post ultrasound PASS scores to be clinically meaningful because it represents a change from one extreme to the opposite extreme within one item or a change in up to three different items over a short period of time during which scores would be expected to remain stable. Pilot data (n = 76) were collected and analyzed to determine target sample size. For 90% power to detect a 3-point difference in PASS score, using standard deviations from validating studies, a two-sided test, p-value 0.05, and assuming 70% completion of post-ultrasound PASS/posttest, a minimum total sample size of 150 participants was required.
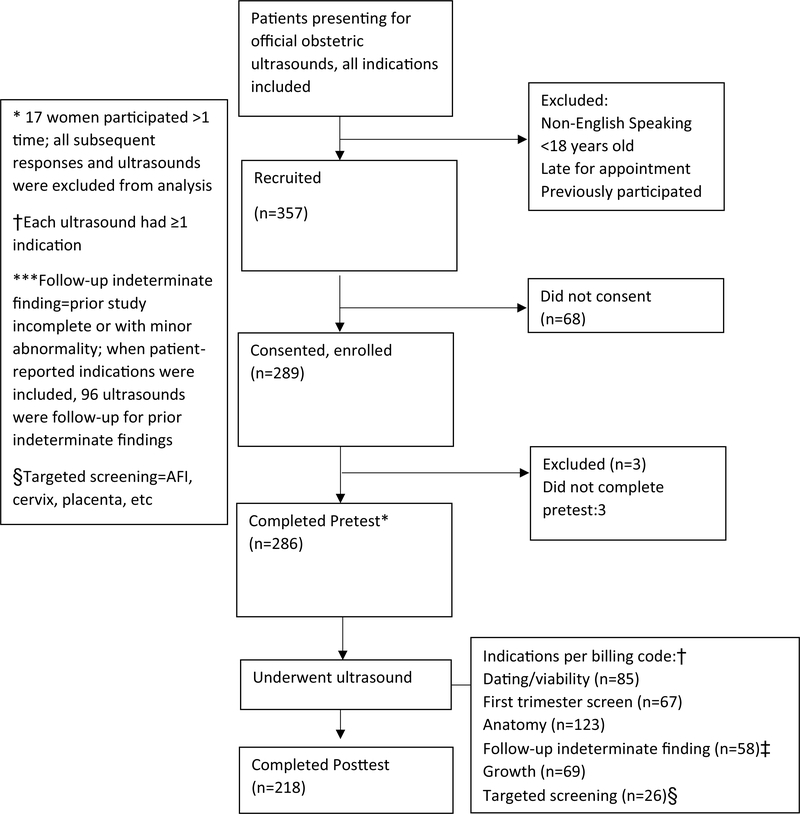
With the exception of adding the screening instrument, the ultrasound appointment proceeded without changes to the standard practices of the unit, including routine review of findings and counseling by sonographers, with additional counseling by the supervising maternal fetal medicine specialist when indicated. At the conclusion of the appointment, participants received the post-ultrasound PASS form and a qualitative posttest. Participants were instructed to complete and submit the forms in sealed lock-boxes located throughout the suite. See Fig. 1 for study flow diagram. Pretests, posttests, and PASS forms were then collected and the de-identified data were entered into a secure web application for building and managing online surveys and databases (REDCap).
Fig. 1.
Diagram of study recruitment and flow of participants through the study
The range of possible ultrasound findings was discussed in advance by investigators, and categories of findings were defined as normal, indeterminate, or major abnormality (see Box 1). Findings were deemed indeterminate if they were either incomplete or noted minor anomalies or anatomic variants of uncertain clinical significance. Using participant identifiers recorded on the secure code sheet, an experienced sonographer and study investigator then reviewed the finalized ultrasound reports for the index ultrasounds. Findings were coded by verbatim text and pilot data were reviewed to confirm inter-observer agreement of categorization. For concurrent findings, the more clinically severe category was selected (e.g., if both minor and major abnormalities were noted, the study was coded as major abnormality; if incomplete and minor abnormalities were noted, the study was coded as a minor abnormality). Reports were also surveyed for additional clinical data, including indication for the ultrasound, pregnancy dating and complications, and women’s comorbidities and pregnancy history. Data were then entered into REDCap and then questionnaire and PASS data were correlated with ultrasound findings using secure code and random IDs. For women who participated more than once, only their first ultrasound and corresponding survey responses were included in the analysis.
Box 1. Categories of ultrasound findings.
| Normal |
| Indeterminate |
| Incomplete |
| Minor anomaly |
| Anatomic variant |
| Isolated soft marker |
| Major abnormality |
| Good prognosis |
| Poor prognosis |
| Shortened cervix |
The primary outcome is post-ultrasound anxiety scores for women with indeterminate findings as compared to women with normal findings (control). Pre-ultrasound anxiety as well as the difference between pre-and-post-ultrasound anxiety scores contextualizes the posttest PASS scores and helps to better characterize the phenomenon of anxiety related to prenatal ultrasounds. Sub-analysis stratified indeterminate findings as either incomplete or minor anomaly and compared indeterminate findings to major abnormalities. Secondary outcomes included: analysis of pre-and-post-ultrasound anxiety scores as a function of age, trimester, and history of mood disorders or pregnancy loss. Qualitative data were assessed pre-and-post-ultrasound and will be reported elsewhere.
Differences between groups were assessed using ANOVA for continuous variables and chi-squared tests for count variables. A p-value < 0.05 was used to define statistical significance and all t-tests were two-tailed. Overall and pairwise linear regressions were performed, adjusting for potential confounders including age, trimester, psychiatric history, race, body habitus, parity and fertility history. Analysis was performed with Stata (Version 14).
Results
A total of 286 women participated (Fig. 1). Mean age was 30 ± 5.5 years, similar to non-participants (p = 0.810). While overall participation was > 80%, white women had a higher participation rate than women from other racial groups (p < 0.001). Table 1 shows demographics and distribution of ultrasound findings. Of 286 ultrasounds, 146 (51.0%) were normal, 116 (40.5%) were indeterminate (22.0% incomplete; 18.5% minor abnormalities), and 25 (8%) had major abnormalities. Women with normal findings were most likely to complete posttests (82.1%), vs. women with indeterminate findings (72.4%) or major abnormalities (58.3%; p < 0.001). Distribution of ultrasound findings (normal, indeterminate, and major abnormalities) was unrelated to age, race, parity, history of mood disorders or infertility. However, the proportion of ultrasounds with indeterminate findings increased significantly with gestational age (26.6% in first trimester, vs. 45.0% in second trimester and 52.5% in third trimester; p < 0.001). Indeterminate findings occurred more commonly among obese vs. non-obese participants (48.8 vs. 37.0%, p = 0.031); this difference was, driven by incomplete ultrasounds (31.4 vs. 18.0%), with minor abnormalities similar between groups (17.4 vs. 19.5%).
Table 1.
Demographics and distribution of ultrasound findings
| Maternal characteristic | Total (n = 286) | Ultrasound finding categories |
||||
|---|---|---|---|---|---|---|
| Normala (n = 146 [51.0%]) | Indeterminateb (n = 116 [40.5%]) | Pc | Major abnormalityd (n = 24 [8.0%]) | Pc | ||
| Participation | ||||||
| Pretest only | 68 (23.8) | 26 (17.8) | 32 (27.6) | 0.058 | 10 (41.7) | 0.010 |
| Pretest and posttest | 218 (76.2) | 120 (82.2) | 84 (72.4) | 14 (58.3) | ||
| Trimester | ||||||
| 1st | 94 (32.9) | 65 (44.5) | 25 (21.6) | <0.001 | 4 (16.7) | < 0.001 |
| 2nd | 131 (45.8) | 62 (42.5) | 59 (50.9) | 10 (41.7) | ||
| 3rd | 61 (21.3) | 19 (13.0) | 32 (27.6) | 10 (41.7) | ||
| Age | ||||||
| < = 24 | 54 (18.9) | 30 (20.6) | 19 (16.4) | 0.691 | 5 (20.8) | 0.250 |
| 25–33 | 152 (53.2) | 78 (53.4) | 65 (56.0) | 9 (37.5) | ||
| 34+ | 80 (28.0) | 38 (26.0) | 32 (27.6) | 10 (41.7) | ||
| Race | ||||||
| Black | 113 (39.5) | 58 (39.7) | 44 (37.9) | 0.450 | 11 (45.8) | 0.640 |
| White | 141 (49.3) | 74 (50.7) | 55 (47.4) | 12 (50.0) | ||
| Other | 32 (11.2) | 14 (9.6) | 17 (14.7) | 1 (4.2) | ||
| Psychiatric history | ||||||
| None | 144 (52.4) | 78 (54.9) | 53 (48.6) | 0.373 | 13 (54.2) | 0.710 |
| Anxiety | 50 (18.2) | 21 (14.8) | 24 (22.0) | 5 (20.8) | ||
| Depression | 24 (8.7) | 15 (10.6) | 8 (7.3) | 1 (4.2) | ||
| Anxiety + depression | 57 (20.7) | 28 (19.7) | 24 (22.0) | 5 (20.8) | ||
| Gravidity | ||||||
| Multigravida | 159 (59.6) | 80 (57.6) | 61 (58.7) | 0.864 | 18 (75.0) | 0.110 |
| Primigravida | 108 (40.5) | 59 (42.5) | 43 (41.4) | 6 (25.0) | ||
| Body mass index | ||||||
| < 30 | 200 (69.9) | 111 (76.0) | 74 (63.8) | 0.031 | 15 (62.5) | 0.160 |
| ≥ 30 | 86 (30.1) | 35 (24.0) | 42 (36.2) | 9 (37.5) | ||
| Infertility | ||||||
| No | 211 (79.3) | 110 (79.7) | 84 (80.0) | 0.956 | 17 (73.9) | 0.530 |
| Yes | 55 (20.7) | 28 (20.3) | 21 (20.0) | 6 (26.1) | ||
Data are N(%) unless otherwise specified; % in columns may not add to exactly 100% due to rounding
Reference group
Indeterminate = incomplete or minor abnormalities (including anatomic variants, isolated soft markers, e.g., isolated choroid plexus cyst), which are of uncertain clinical import
P values obtained from chi-squared tests comparing distribution of indeterminate findings and major abnormalities to normal findings with respect to various maternal characteristics
Major abnormality = ultrasound finding clinically significant for the fetus/pregnancy (including findings with a good prognosis, e.g. transposition of the great arteries, poor prognosis, e.g. anencephaly, or shortened cervix (< 25 mm)
According to documented billing codes, 62/65 women undergoing follow-up for prior indeterminate findings (32/34 with prior incomplete studies and 30/31 with prior minor abnormalities) correctly identified “follow-up for prior incomplete or minor abnormalities” as the indication for their current ultrasound on pretests. Several women also indicated that they were currently undergoing follow-up ultrasounds despite absence of “follow-up” as a billed indication for their study. When patient-reported “follow-up” indications were included, 96/261 (36.8%) of all women with either normal or indeterminate findings on index ultrasound were undergoing follow-up for prior indeterminate findings. Compared to women with normal results, women with indeterminate findings had more prior official maternal fetal medicine ultrasounds (mean 2.3 vs. 1.7, not counting any office, emergency department or triage ultrasounds; p = 0.004), and were more likely to be undergoing follow-up for a prior indeterminate result (49.1 vs. 26.7%; p < 0.001). Within the indeterminate group, women with minor abnormalities had slightly more prior ultrasounds on average, although prevalence of prior indeterminate findings was similar for women with minor abnormalities and incomplete findings (48.1 and 55.6%, respectively).
In this population, 70/286 (24.5%) of women had clinically significant anxiety (PASS > 20) on pretests compared to 33/218 (15.1%) on posttests. Pretest and posttest PASS scores according to finding category are shown in Table 2. Mean anxiety scores were higher on pretests than on posttests across all finding categories; this difference reached statistical significance for the overall population (p = 0.001) and for women with normal findings (p = 0.002). Compared to normal findings, indeterminate findings were associated with significantly higher anxiety both before (p = 0.026) and after the ultrasound (p = 0.009), with posttest anxiety remaining significantly higher than for the normal group after adjusting for potential confounders: trimester, age, psychiatric history, race, parity, body habitus, and fertility history (p = 0.01). Sensitivity analyses excluding women who did not complete posttests did not substantially affect magnitude or significance of pretest or post-pretest group comparisons.
Table 2.
Perinatal anxiety screening scale (PASS) scores before and after ultrasounds with normal, indeterminate and major abnormalities
| PASS score | Ultrasound findings (n) |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| All (286) | Normal (146) Refa | Indeterminateb (116) | P-valuec (p-adjusted) | Minor Abnormality (53) | P-valuec (p-adjusted) | Incomplete (63) | P-valuec (p-adjusted) | Major abnormality (24) | P-valuec (p-adjusted) | |
| Pretest | 18.2 ± 13.6 | 16.4 ± 11.6 | 20.1 ± 15.4 | 0.026 (0.11) | 20.9 ± 16.0 | 0.029 (0.08) | 19.4 ± 14.9 | .114 (.36) | 20.3 ± 14.9 | .150 (0.35) |
| Posttest | 14.3 ± 12.7 | 12.2 ± 10.3 | 16.9 ± 14.9 | 0.009 (0.01) | 15.5 ± 13.7 | 0.116 (0.17) | 17.8 ± 15.8 | 0.007 (0.01) | 16.5 ± 15.2 | .161 (0.24) |
| Posttest – pretest | −3.6 ± 5.8 | −3.7 ± 5.4 | −3.7 ± 6.5 | 0.928 (0.97) | −5.6 ± 8.4 | 0.119 (0.09) | −2.2 ± 4.2 | 0.081 (0.03) | −1.9 ± 5.3 | .215 (0.15) |
| Pretest vs. posttest p-valued | 0.001 | 0.002 | 0.143 | 0.105 | 0.588 | 0.456 | ||||
PASS score > 20 denotes clinically significant anxiety
Data are mean ± SD unless otherwise stated
PASS scores from normal finding group (pretest, posttest and posttest-pretest) are the reference values
“Indeterminate” category is subdivided into “minor abnormality” and “incomplete”
P-values calculated with t-tests (unpaired, two-tailed) comparing scores with analogous normal finding scores as reference. P-values in parentheses are adjusted by pairwise linear regressions correcting for trimester, maternal age, psychiatric history, race, parity, body habitus and fertility history
P-values calculated with t-tests (paired, two-tailed) comparing pretest and posttest scores within each finding category
Major abnormalities and indeterminate findings were associated with similar pretest and posttest anxiety scores. On sub-analysis of indeterminate findings, posttest anxiety was higher (p = 0.007; p-adjusted = 0.01), and pre-to-posttest decrease in anxiety was smaller for incomplete compared to normal ultrasound findings (p-adjusted = 0.03). Minor abnormalities were associated with higher pretest anxiety (p = 0.029) than normal findings and had significantly larger pre-to-posttest decrease in anxiety compared to incomplete findings (adjusted-p = 0.01).
On PASS sub-scale domains (1- acute anxiety and adjustment, 2- general worry and specific fears, 3- perfectionism, control and trauma, and 4- social anxiety), Sect. 1 was most affected by ultrasound results. Mean posttest sub-scale scores for acute anxiety and adjustment demonstrated stepwise increases, from 5.5 after normal findings, 7.6 after indeterminate findings, and 8.8 after major abnormalities (p = 0.006 for trend across all groups). Indeterminate findings were associated with higher pretest anxiety than normal ultrasounds in Sects. 1 (p = 0.028) and 3 (p = 0.049). Indeterminate findings were associated with higher posttest scores than normal ultrasounds in Sects. 1 (p = 0.006), 2 (p = 0.041) and 3 (p = 0.025). Within the indeterminate group, minor abnormalities were associated with higher pretest anxiety in Sect. 3 (p = 0.042), while incomplete findings were associated with higher posttest anxiety in Sects. 1 (p = 0.009), 2 (p = 0.007) and 3 (p = 0.037). When comparing pre-to-posttest differences, incomplete findings were associated with negligible change in Sect. 2 (p = 0.020), and minor abnormalities were associated with larger decreases in Sect. 4 (p = 0.025).
Table 3 shows PASS scores before and after ultrasounds for relevant characteristics. Women with normal or indeterminate findings who were undergoing follow-up for prior indeterminate findings had higher pretest anxiety (p = 0.028) and greater differences between pre-and-posttests than women with routine indications for the study (p < 0.001). Pretest scores for women with prior indeterminate findings were similar for women who did and who did not complete posttests. Mean pretest anxiety increased with gestational age from first through third trimesters (p = 0.029). Posttest anxiety was also lower in the first trimester (p = 0.041). Women ≤ 24 years had significantly higher pretest and posttest anxiety, and larger differences between pre-and-posttests, than women 25–33 and ≥ 34. Nulliparas and multiparas had similar pretest anxiety, however nulliparas had larger decreases in pre-to-posttest anxiety (p < 0.001). Compared to women without psychiatric history, women with anxiety or anxiety plus depression had higher mean PASS scores on pretests and posttests (all p < 0.001). Finally, prior pregnancy loss was associated with higher anxiety before and after ultrasounds, and smaller pre-to-posttest decrease in anxiety compared to women without a prior loss.
Table 3.
Perinatal anxiety screening scale (PASS) scores before and after ultrasounds by maternal characteristics
| Maternal characteristics | Pretest PASS score | Posttest PASS score | Postest-pretest PASS score |
|---|---|---|---|
| Trimesters | |||
| First | 15.2 ± 9.7a | 11.8 ± 8.4a | −3.4 ± 4.1 |
| Second Ref | 19.4 ± 15.3 | 15.8 ± 14.9 | −3.9 ± 7.2 |
| Third | 20.3 ± 14.4 | 15.1 ± 13.1 | −3.34 ± 4.9 |
| P-value across all groupsb | 0.029 | 0.113 | 0.826 |
| Age | |||
| ≤ 24 | 24.5 ± 14.8ǂ | 18.7 ± 13.6† | −5.9 ± 6.3† |
| 25–33 Ref | 16.5 ± 13.7 | 13.3 ± 13.4 | −3.1 ± 6.2 |
| ≥ 34 | 17.2 ± 11.5 | 13.5 ± 10.6 | −3.2 ± 4.4 |
| P-value across all groupsb | 0.001 | 0.074 | 0.031 |
| Psychiatric history | |||
| None Ref | 12.8 ± 8.9 | 9.3 ± 7.8 | −3.6 ± 5.3 |
| Anxiety | 23.7 ± 12.8c | 18.1 ± 10.7c | −4.0 ± 5.9 |
| Depression | 15.3 ± 11.4 | 11.2 ± 9.1 | −5.2 ± 10.6 |
| Anxiety and depression | 28.0 ± 17.1c | 25.4 ± 17.7c | −2.3 ± 4.2 |
| P-value across all groupsb | < 0.001 | < 0.001 | 0.322 |
| Gravidity | |||
| Primigravid Ref | 18.0 ± 11.9 | 13.1 ± 9.6 | −4.9 ± 1.6 |
| Multigravida | 18.4 ± 14.3 | 15.3 ± 14.4 | −3.0 ± 1.8 |
| P-value across all groupsd | 0.832 | 0.221 | < 0.001 |
| Prior pregnancy loss | |||
| No Ref | 17.5 ± 13.0 | 13.2 ± 11.8 | −4.3 ± 1.2 |
| Yes | 21.0 ± 15.6 | 18.7 ± 15.2 | −2.4 ± 3.1 |
| P-value across all groupsd | 0.082 | 0.011 | 0.029 |
| Prior indeterminate ultrasounde | |||
| No Ref | 16.4 ± 12.0 | 12.8 ± 10.8 | −3.6 ± 1.3 |
| Yes | 20.9 ± 15.4 | 16.7 ± 15.2 | −4.2 ± 2.2 |
| P-value across all groupsd | 0.006 | 0.028f | 0.012 |
PASS score > 20 denotes clinically significant anxiety
Data are mean ± SD unless otherwise stated
Individual P-values for pairwise comparison to reference category < .05
P-values generated from ANOVA
Individual P-values for pairwise comparison to reference category < .001
P-values generated from unpaired t-test
Includes women for whom the index ultrasound was performed to follow up a previous indeterminate ultrasound (incomplete or minor abnormality), including billing code and patient reported indications. Analysis adjusted for trimester demonstrated similar findings
Posttest means were similar and comparison remained significant when participants with major abnormalities on index ultrasound were excluded
Discussion
This study found significantly higher anxiety in pregnant women after indeterminate ultrasounds when compared to women with normal results (adjusted-p = 0.01). This difference was not only statistically significant but also clinically meaningful, with over a four-point difference on a scale for which three points represents a clinically meaningful increment. Mean anxiety scores were higher before ultrasounds and lower afterward across all finding categories, however this trend does not demonstrate that ultrasound decreases anxiety per se (Da Silva et al. 2012; Kowalcek et al. 2003; Simó et al. 2018). Posttest scores after normal findings were significantly lower than pretest scores (p = 0.002) and comparable to the control group of pregnant women in validating studies (12.2 vs. 13.4). Indeed, ultrasounds may start affecting anxiety in anticipation of the study, with return to baseline following normal findings. (Harpel 2008)14. Conversely, women undergoing follow-up for indeterminate findings had mean pretest scores above the threshold for psychiatric impairment (score > 20) and significantly higher pretest and posttest anxiety than women without prior indeterminate results (p = 0.006 and p = 0.028; see Table 3). Clinically significant anxiety was also twice as common after indeterminate findings vs. normal results on the current ultrasound. Critically, adverse obstetric outcomes increase with degree of anxiety symptoms as measured by scales such as the PASS, suggesting that increasing symptoms even for women with anxiety levels within normal limits may be harmful.
Longitudinal studies of women with major fetal abnormalities show that post-ultrasound increases in anxiety may be sustained through pregnancy, and even postpartum (Anne Kaasen et al. 2017; Titapant and Chuenwattana 2015). Whether higher posttest anxiety related to indeterminate findings is sustained or sufficient to affect clinical outcomes remains unknown, as this study lacks long-term follow-up. However, higher pretest and posttest anxiety among women undergoing follow-up for prior indeterminate findings suggests that these anxiety changes may persist. Higher anxiety before indeterminate findings on the index ultrasound may also reflect this group being nearly twice as likely to have prior indeterminate findings as women with normal results (49.1% vs. 26.7%; p < 0.001). Likewise, mean pretest anxiety was higher than posttest anxiety for major abnormalities: while posttest anxiety may be underestimated by greater loss-to-follow-up (Table 1), this trend may also reflect an exacerbation of pretest anticipatory anxiety and a high frequency of prior abnormal or indeterminate findings (19/25, 76%). Higher loss-to-follow-up among those with indeterminate vs. normal and major abnormalities vs. indeterminate also highlights the possibility of higher anxiety among those who did not complete posttests, suggesting that actual differences in pre-to-posttest anxiety between groups may have been even greater than captured in our data.
This prospective study utilized a validated survey instrument specifically for anxiety in pregnancy and included a large sample size relative to similar studies. To date, research on maternal anxiety related to prenatal ultrasounds has focused on women with either normal findings or major abnormalities. To our knowledge, the impact of incomplete ultrasound findings on maternal anxiety has not been studied. Meanwhile, a few small, qualitative studies have evaluated the effect of specific minor abnormalities on anxiety; participants in those studies, however, are selected retrospectively, often remote from the index ultrasound, compromising their ability to shed light on acute anxiety changes (Åhman et al. 2010; Carolan and Hodnett 2009; Cristofalo et al. 2006; Murphy and Phillippi 2015; Oscarsson et al. 2015; Viaux-Savelon et al. 2012). To strengthen the conclusions of this study, future investigations should assess anxiety at more points in time, for example at prior and subsequent office visits, particularly as magnitude of baseline anxiety may affect ultrasounds’ impact.
Formalizing the category of ‘indeterminate’ findings, in which uncertainty is imposed on an otherwise reassuring ultrasound by incomplete visualization or anatomic variations, is a novel contribution of this study. Though minor abnormalities and incomplete findings may affect anxiety differently, both may have adverse effects. This classification captures an array of findings which may be of limited utility to clinicians yet disproportionately anxiogenic for patients, potentially generating unmet needs for counseling and support. To illustrate, women with indeterminate findings had pre-and-post-ultrasound anxiety comparable to that of women with major abnormalities despite vastly different prognoses, though our sample size was underpowered for relatively rare major abnormalities. This trend has been observed among women presenting with abdominal pain and/or vaginal bleeding in early first trimester: non-viable pregnancies and uncertain diagnoses (e.g., unknown location or uncertain viability) had similarly high anxiety immediately post-ultrasound (Richardson et al. 2017). On follow-up 48–72 h later, anxiety remained equally high with uncertain diagnoses but decreased significantly with non-viable pregnancies. This finding is consistent with significantly increased anxiety after incomplete ultrasounds in our study (adjusted-p = 0.01), as they may represent greater uncertainty than specific minor abnormalities, illustrating a key disconnect between clinical prognosis and maternal anxiety response.
In this study of over 280 maternal fetal medicine ultrasounds 41% were indeterminate: 22% incomplete and 19% minor abnormalities. Historically, just 5% of pregnancies were thought to be affected by minor fetal abnormalities, possibly due to less advanced ultrasound technology (Practice bulletin no. 175: Ultrasound in pregnancy 2016; Viaux-Savelon et al. 2012). A recent retrospective review of 16,300 anatomy surveys in a high-volume academic center found 13.2% were incomplete (Silvestri et al. 2016). Though convenience sampling based on research staff availability, exclusion for late appointment arrival, and disclosure of study subject matter during recruitment may have biased our sample by inadvertently excluding representative segments of our population, other demographics and obesity were similar, and did not explain the discrepancy between rates of incomplete findings, even when applying identical selection criteria to the two populations. Institution-level factors, such as amount of time allotted to each ultrasound, may influence incidence of indeterminate findings. Although our adjusted analysis corrected for effects of trimester, age, psychiatric history, race, gravidity, obesity and infertility on maternal anxiety, racial differences in participation rates and unaccounted for socioeconomic factors may represent residual biases as the sample may deemphasize experiences of some women in our patient population.
We also did not control for sonographer or maternal fetal medicine specialist, whose bedside manner and counseling may substantively influence maternal anxiety responses (Watson et al. 2002). At present, guidance for providers regarding disclosure and follow-up of indeterminate findings is limited, and practice varies widely (Hayat Roshanai et al. 2015; Nabhan and Aflaifel 2015). For example, nearly 90% of incomplete findings were followed up in one study, vs. approximately 50% in a similar center (Silvestri et al. 2016; Waller et al. 2013). In some cases, fear of litigation may prompt disclosure of indeterminate findings even when the likelihood of clinical significance is very low (Waller et al. 2013). In this regard, indeterminate ultrasound findings may be akin to “incidentalomas,” low-risk incidental radiologic findings which have been shown to drive “unnecessary testing, invasive procedures, and overtreatment, with associated financial, psychological, and clinical consequences” (Kang et al. 2016; O’Brien et al. 2017; Petersen and Jahn 2008; Westerfield et al. 2014). Notably, Silvestri et al. found that follow-up imaging was more likely to detect abnormalities in systems previously cleared as normal (0.9%) than in systems which were previously incomplete (0.5%). Patient-driven demand for prenatal ultrasound and financial factors further complicate the issue.
We believe this study helps to characterize maternal anxiety as an underappreciated risk of indeterminate prenatal ultrasounds, one that should be factored into counseling both in the clinic and the ultrasound suite and may be considered alongside prospective costs and benefits of follow-up imaging. Women with a history of anxiety or prior pregnancy loss, younger women, and women who are later in gestation appear to have greater risk of clinically problematic anxiety associated with ultrasounds, may be most vulnerable to harm from indeterminate findings, and thus may be most likely to benefit from targeted counseling or other interventions. Evidence-based strategies to minimize ultrasound’s adverse impact on maternal anxiety are needed, particularly in the setting of low-risk indeterminate findings. Next steps may involve developing specific standards for counseling and management, and efforts to decrease the number of indeterminate ultrasounds: improving the ability to complete studies, collecting data to decrease the ambiguity regarding minor abnormalities, and advancing practice guidelines aimed at reducing follow-up imaging with marginal clinical utility.
Significance Statement.
Prenatal ultrasounds are increasingly performed despite limited evidence that benefits of screening outweigh risks. Indeed, major abnormalities on ultrasound are linked to increased maternal anxiety, and anxiety increases risk for adverse pregnancy outcomes. Meanwhile, indeterminate ultrasounds, with incomplete findings or minor abnormalities, are increasingly common given improved technological sensitivity and obesity’s association with incomplete findings, yet their impact on anxiety remains unknown. We examine the impact of indeterminate maternal fetal medicine ultrasounds on pregnant women’s anxiety, finding that indeterminate, especially incomplete, findings are associated with significantly higher anxiety despite their overall good prognosis, suggesting need for evidence-based guidance.
Acknowledgements
This original study was conducted at the above institution and was supported by a grant from the Howard A. Kelly Alumni Society, the Johns Hopkins Institute for Clinical and Translational Research (ICTR), and Biostatistics, Epidemiology and Data Management (BEAD) Core. We have no other financial disclosures or conflicts of interest. Permission to use the Perinatal Anxiety Screening Scale was provided by S. Somerville and the Western Australian Department of Health. Pilot data was presented March 9–11, 2017 at the National Perinatal Association conference in Atlanta, Georgia. Study protocol and raw data are available by contacting the corresponding author at the included email.
Footnotes
Compliance with Ethical Standards
Conflict of interest The authors declare that they have no conflict of interest.
Ethical Approval This study was approved by the Johns Hopkins University Institutional Review Board (#00116293) and was performed in accordance with the ethical standards laid down in the operative version of the Declaration of Helsinki. Informed consent was obtained from all individual participants included in the study.
References
- Åhman A, Runestam K, & Sarkadi A (2010). Did I really want to know this? pregnant women’s reaction to detection of a soft marker during ultrasound screening. Patient Education and Counseling, 81(1), 87–93. 10.1016/j.pec.2009.12.011. [DOI] [PubMed] [Google Scholar]
- Aite L, Zaccara A, Trucchi A, Nahom A, Capolupo I, Mobili L, & Bagolan P (2011). Is counselling for CCAM that difficult? Learning from parental experience. Journal of Prenatal Medicine, 5(3), 65–68. [PMC free article] [PubMed] [Google Scholar]
- Carolan M, & Hodnett E (2009). Discovery of soft markers on fetal ultrasound: Maternal implications. Midwifery, 25(6), 654–664. 10.1016/j.midw.2007.11.002. [DOI] [PubMed] [Google Scholar]
- Cristofalo EA, Dipietro JA, Costigan KA, Nelson P, & Crino J (2006). Women’s response to fetal choroid plexus cysts detected by prenatal ultrasound. Journal of Perinatology, 26(4), 215–223. [DOI] [PubMed] [Google Scholar]
- Da Silva ECP, Silva SVL, Damião R, Fonseca EB, Garcia S, & Lippi UG (2012). Stress and anxiety in pregnant women exposed to ultrasound. The Journal of Maternal-Fetal & Neonatal Medicine, 25(3), 295–298. 10.3109/14767058.2011.574299 [DOI] [PubMed] [Google Scholar]
- Harpel TS (2008). Fear of the unknown: Ultrasound and anxiety about fetal health. Clinical Trial. 10.1177/1363459308090050. [DOI] [PubMed] [Google Scholar]
- Hayat Roshanai A, Ingvoldstad C, & Lindgren P (2015). Fetal ultrasound examination and assessment of genetic soft markers in sweden: Are ethical principles respected? Acta Obstetricia Et Gynecologica Scandinavica, 94(2), 141–147. 10.1111/aogs.12554. [DOI] [PubMed] [Google Scholar]
- Hurt L, Wright M, Brook F, Thomas S, Dunstan F, Fone D, … Paranjothy S. (2014). The welsh study of mothers and babies: Protocol for a population-based cohort study to investigate the clinical significance of defined ultrasound findings of uncertain significance. BMC Pregnancy and Childbirth, 14(1), 164. 10.1186/1471-2393-14-164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaasen A, Helbig A, Malt UF, Næs T, Skari H, & Haugen G (2017). Maternal psychological responses during pregnancy after ultrasonographic detection of structural fetal anomalies: A prospective longitudinal observational study. PLoS One, 12(3), e0174412. 10.1371/journal.pone.0174412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang SK, Spector-Bagdady K, Caplan AL, & Braithwaite RS (2016). Exome and genome sequencing and Parallels in Radiology: Searching for patient-centered management of Incidental and Secondary findings. Journal of the American College of Radiology: JACR, 13(12 Pt A), 1467–1472. 10.1016/j.jacr.2016.06.050 [DOI] [PubMed] [Google Scholar]
- Kowalcek I, Huber G, Lammers C, Brunk J, Bieniakiewicz I, & Gembruch U (2003). Anxiety scores before and after prenatal testing for congenital anomalies. Archives of Gynecology and Obstetrics, 267(3), 126–129. 10.1007/s00404-002-0295-6. [DOI] [PubMed] [Google Scholar]
- Murphy H, & Phillippi JC (2015). Isolated intracardiac echogenic focus on routine ultrasound: Implications for practice. Journal of Midwifery and Women’s Health, 60(1), 83–88. 10.1111/jmwh.12282. [DOI] [PubMed] [Google Scholar]
- Nabhan AF, & Aflaifel N (2015). High feedback versus low feedback of prenatal ultrasound for reducing maternal anxiety and improving maternal health behaviour in pregnancy. The Cochrane Database of Systematic Reviews, (8), CD007208. 10.1002/14651858.CD007208.pub3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Brien K, Shainker SA, Modest AM, Spiel MH, Resetkova N, Shah N, & Hacker MR (2017). Cost analysis of following up incomplete low-risk fetal anatomy ultrasounds. Birth (Berkeley, Calif.), 44(1), 35–40. 10.1111/birt.12262 [DOI] [PubMed] [Google Scholar]
- O’Keeffe DF, & Abuhamad A (2013). Obstetric ultrasound utilization in the united states: Data from various health plans. Seminars in Perinatology, 37(5), 292–294. 10.1053/j.semperi.2013.06.003. [DOI] [PubMed] [Google Scholar]
- Oscarsson M, Gottvall T, & Swahnberg K (2015). When fetal hydronephrosis is suspected antenatally—a qualitative study. BMC Pregnancy and Childbirth, 15, 349. 10.1186/s12884-015-0791-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pasko DN, Wood SL, Jenkins SM, Owen J, & Harper LM (2016). Completion and sensitivity of the second-trimester fetal anatomic survey in obese gravidas. Journal of Ultrasound in Medicine: Official Journal of the American Institute of Ultrasound in Medicine. 10.7863/ultra.15.11057 [DOI] [PubMed] [Google Scholar]
- Pesonen AK, Lahti M, Kuusinen T, Tuovinen S, Villa P, Hamalainen E, … Raikkonen K. (2016). Maternal prenatal positive affect, depressive and anxiety symptoms and birth outcomes: The PREDO study. PloS One, 11(2), e0150058. 10.1371/journal.pone.0150058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen J, & Jahn A (2008). Suspicious findings in antenatal care and their implications from the mothers’ perspective: A prospective study in germany. Birth, 35(1), 41–49. 10.1111/j.1523-536X.2007.00210.x. [DOI] [PubMed] [Google Scholar]
- Practice bulletin no. 175: Ultrasound in pregnancy. (2016). Obstetrics and Gynecology, 128(6), e256. 10.1097/AOG.0000000000001815 [DOI] [PubMed] [Google Scholar]
- Richardson A, Raine-Fenning N, Deb S, Campbell B, & Vedhara K (2017). Anxiety associated with diagnostic uncertainty in early pregnancy. Ultrasound in Obstetrics and Gynecology, 50(2), 247–254. 10.1002/uog.17214. [DOI] [PubMed] [Google Scholar]
- Robinson JO, Hibbard BM, & Laurence KM (1984). Anxiety during a crisis: Emotional effects of screening for neural tube defects. Journal of Psychosomatic Research, 28(2), 163–169. [DOI] [PubMed] [Google Scholar]
- Silvestri MT, Pettker CM, Raney JH, Xu X, & Ross JS (2016). Frequency and importance of incomplete screening fetal anatomic sonography in pregnancy. Journal of Ultrasound in Medicine, 35(12), 2665–2673. [DOI] [PubMed] [Google Scholar]
- Simó S, Zúñiga L, Izquierdo MT, & Rodrigo MF (2018). Effects of ultrasound on anxiety and psychosocial adaptation to pregnancy. Archives of Women’s Mental Health. 10.1007/s00737-018-0918-y. [DOI] [PubMed] [Google Scholar]
- Somerville S, Byrne SL, Dedman K, Hagan R, Coo S, Oxnam E, … Page AC. (2015). Detecting the severity of perinatal anxiety with the perinatal anxiety screening scale (PASS). Journal of Affective Disorders, 186, 18–25. 10.1016/j.jad.2015.07.012 [DOI] [PubMed] [Google Scholar]
- Somerville S, Dedman K, Hagan R, Oxnam E, Wettinger M, Byrne S, … Page AC. (2014). The perinatal anxiety screening scale: Development and preliminary validation. Archives of Women’s Mental Health, 17(5), 443–454. 10.1007/s00737-014-0425-8] [DOI] [PubMed] [Google Scholar]
- Staneva A, Bogossian F, Pritchard M, & Wittkowski A (2015). The effects of maternal depression, anxiety, and perceived stress during pregnancy on preterm birth: A systematic review. Women and Birth: Journal of the Australian College of Midwives, 28(3), 179–193. 10.1016/j.wombi.2015.02.003. [DOI] [PubMed] [Google Scholar]
- Titapant V, & Chuenwattana P (2015). Psychological effects of fetal diagnoses of non-lethal congenital anomalies on the experience of pregnant women during the remainder of their pregnancy. The Journal of Obstetrics and Gynaecology Research, 41(1), 77–83. 10.1111/jog.12504. [DOI] [PubMed] [Google Scholar]
- Viaux-Savelon S, Dommergues M, Rosenblum O, Bodeau N, Aidane E, Philippon O, … Cohen D. (2012). Prenatal ultrasound screening: False positive soft markers may alter maternal representations and mother-infant interaction. PloS One, 7(1), e30935. 10.1371/journal.pone.0030935 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waller SA, O’Connell K, Carter A, Gravett MG, Dighe M, Richardson ML, & Dubinsky TJ (2013). Incidence of fetal anomalies after incomplete anatomic surveys between 16 and 22 weeks. Ultrasound Quarterly, 29(4), 307–312. 10.1097/RUQ.0b013e31829a6ad3. [DOI] [PubMed] [Google Scholar]
- Watson MS, Hall S, Langford K, & Marteau TM (2002). Psychological impact of the detection of soft markers on routine ultrasound scanning: A pilot study investigating the modifying role of information. Prenatal Diagnosis, 22(7), 569–575. 10.1002/pd.373. [DOI] [PubMed] [Google Scholar]
- Westerfield L, Darilek S, & van den Veyver IB (2014). Counseling challenges with variants of uncertain significance and incidental findings in prenatal genetic screening and diagnosis. Journal of Clinical Medicine, 3(3), 1018–1032. 10.3390/jcm3031018. [DOI] [PMC free article] [PubMed] [Google Scholar]