Abstract
Starting breast cancer screening at age 40 versus 50 may increase potential harms frequency with a small mortality benefit. Younger women’s screening decisions, therefore, may be complex. Shared decision-making (SDM) is recommended for women under 50 and may support women under 55 for whom guidelines vary. How women with limited health literacy (LHL) approach breast cancer screening decision-making is less understood, and most SDM tools are not designed with their input. This phenomenological study sought to characterize mammography counseling experiences among women with LHL and primary care providers (PCPs). Women ages 40–54 with LHL who had no history of breast cancer or mammogram within 9 months were approached before a primary care visit at a safety-net hospital. PCPs at this site were invited to participate. Qualitative interviews explored mammography counseling experiences. Patients also reviewed sample information materials. A constant comparison technique generated four themes salient to 25 patients and 20 PCPs: addressing family history versus comprehensive risk assessment; potential mammography harms discussions; information delivery preferences; and integrating pre-visit information tools. Findings suggest that current counseling techniques may not be responsive to patient-identified needs. Opportunities exist to improve how mammography information is shared and increase accessibility across the health literacy spectrum.
Among women in the United States, breast cancer remains one of the most commonly diagnosed cancers and the second leading cause of cancer death (Centers for Disease Control and Prevention, 2020). Breast cancer screening aims to detect breast cancer before symptoms arise, when cancer is more treatable, but involves tradeoffs, particularly for younger women. Potential screening benefits include improved survival and avoiding intensive, costly treatments, while potential screening harms include overdiagnosis, overtreatment, false positives, radiation exposure, and costs associated with additional testing (Mandelblatt et al., 2015; 2016; Siu, 2016). Though guidelines agree that women should initiate breast cancer screening by age 50, the balance of potential mammography benefits/harms for women in their forties is less clear (Gøtzsche & Jørgensen, 2013; Howlader et al., 2019; Tonelli, Connor Gorber, & Joffres, 2011). Recent estimates indicate that, for an average-risk woman, the difference between initiating mammography at age 40 versus 50 is one fewer death from breast cancer per 1,000 women, while annual screening from ages 40 to 69 yields almost double the number of false-positive results compared to biennial screening (Mandelblatt et al., 2009). However, uncertainty persists about the benefits and harms of different breast cancer screening protocols (Myers et al., 2015). Potentially small or uncertain mortality benefits, when considered alongside additional years of potential screening harms, may alter the balance of people’s preferences. As such, professional guidelines about mammography initiation and spacing vary for women in their forties, and spacing recommendations for women up to age 55 remain contested (Siu, 2016).
Subsequently, shared decision-making for breast cancer screening decisions is recommended for women under 50 and may support women under 55 for whom varied guidelines leave room for choice (Oeffinger et al., 2015; Siu, 2016). Shared decision-making (SDM) is a consultation model that promotes collaborative decision-making between patients and clinicians (Durand et al., 2014), and is most useful when multiple healthcare choices and/or varied evidence are available. Optimally, for breast cancer screening, SDM includes discussing a woman’s personal breast cancer risk, available screening options and their evidence bases, potential screening benefits and harms, and a woman’s values and preferences (Croes et al., 2020; DuBenske et al., 2018).
Among patients, understanding of breast cancer risk varies. While patients often define breast cancer risk primarily as a function of family history (Buxton et al., 2003; Davis, Stewart, & Bloom, 2004; Haber, Ahmed, & Pekovic, 2012; McCaul & O’Donnell, 1998), other dimensions such as physical symptoms (e.g. breast pain) and how personal risk compares to others’ influence perceptions of breast cancer risk (Gillespie, 2012; Gunn et al., 2019). Women often inaccurately assess their breast cancer risk, with studies demonstrating over- and underestimation for various patient groups (Costanza et al., 1992; Davis et al., 2004; Evans et al., 1993; Smith et al., 1996; Woloshin et al., 1999), and identifying challenges patients have interpreting numeric information commonly used to characterize risk (Davis et al., 2004; Gillespie, 2012). Women are typically more aware of potential mammography benefits than harms (Hoffmann & Del Mar, 2015; Shi et al., 2019; Yu et al., 2017). Among potential harms, women are more likely to be aware of false positives than overdiagnosis or overtreatment (DeFrank et al., 2012; Nagler, Franklin Fowler, & Gollust, 2017). Data also show positive associations between awareness of potential harms, confusion about screening recommendations, and ambivalence about mammography completion (DeFrank et al., 2012).
Recent research exploring SDM for women considering breast cancer screening endorsed the importance of PCPs counseling about risk factors, including patients’ personal risk, as well as potential mammography benefits/harms (Croes et al., 2020). Evidence suggests, however, that PCPs often demonstrate limited engagement in comprehensive breast cancer risk assessments and discussions of potential mammography benefits/harms with patients. One study indicates that less than half of PCPs report usually or always asking patients about risk factors beyond family history, 76% report never calculating risk, and only 9% feel confident or very confident utilizing a risk calculator (Sabatino et al., 2007). PCPs’ limited knowledge of certain risk factors, like breast density, and time-constrained clinic visits may also curb robust breast cancer risk discussions (Brown et al., 2019; DuBenske et al., 2017; Haas et al., 2016; Martinez et al., 2017). Additionally, PCPs often underestimate mammography’s potential harms (Hoffmann & Del Mar, 2017; Martinez et al., 2017; Shi et al., 2019), and both patients and clinicians report few discussions of potential harms during mammography counseling (Haas et al., 2016; Hoffman et al., 2010; Wegwarth & Gigerenzer, 2011).
Another relevant dimension of mammography counseling is health literacy. Health literacy is defined traditionally as the “degree to which individuals have the capacity to obtain, process, and understand basic health information and services needed to make appropriate health decisions” (Institute of Medicine, 2004). Paasche-Orlow and Wolf (2007) expanded this definition to include how healthcare system attributes to influence individuals’ ability to understand and act upon information. As such, health literacy may directly and indirectly influence women’s understanding of relevant health information and participation in SDM (Berkman et al., 2011; Gaglio, Glasgow, & Bull, 2012; Golbeck et al., 2005; Stacey et al., 2017).
To date, breast cancer screening SDM research has been conducted predominantly among college educated and white populations (DuBenske et al., 2018; Elkin et al., 2017; Han et al., 2018). One recent systematic review documents racial and ethnic minority groups’ general SDM preferences, challenges, and facilitators (Jolles, Richmond, & Thomas, 2019). Other research highlights the specific need for culturally appropriate cancer screening SDM tools and reports that older African American women are interested in breast cancer screening SDM and find related decision aids helpful (Hawley & Morris, 2016; Salzman et al., 2020). Less research, however, has examined whether this approach is responsive to women with limited health literacy, a salient concern given that concepts covered during mammography screening decision-making are complex. Patients with limited health literacy (LHL) may identify the purpose of cancer screenings with less accuracy (Morris et al., 2013), possess insufficient understanding of breast cancer risk and potential mammography benefits/harms (Brewer et al., 2008; Davis et al., 2002), and ask fewer questions during counseling (Katz et al., 2007). Visual aids may clarify nuanced concepts, but further research can identify relevant informational needs and delivery formats (Brewer et al., 2008; Mazor et al., 2016). PCPs may struggle with the types and amount of breast cancer screening information to share, fearing confusion, mammography deterrence, and patient–provider relationship ruptures among patients with LHL (Gunn et al., 2020). However, the extent to which perceptions of breast cancer risk and potential mammography benefits/harms influence mammography decision-making among women with LHL is less known.
This phenomenological study, therefore, sought to characterize the lived experience of mammography counseling among women ages 40–54 with LHL and PCPs. Additionally, it collected formative patient feedback on the content and format of sample mammography informational materials. Results may clarify mammography information needs and delivery preferences among patients with LHL and PCPs, and inform development of interventions that enhance mammography counseling specifically for women with LHL.
Methods
From November 2018 to May 2019, we conducted a qualitative, interview-based study in six outpatient primary care practices located at a safety-net hospital to explore patient and PCP mammography counseling experiences. Paasche-Orlow and Wolf’s conceptual model provided theoretical grounding for the study design. The model outlines how health literacy, through access to care, patient-provider communication, and self-care, influences health outcomes (Paasche-Orlow & Wolf, 2007). The framework’s patient-provider communication domain, emphasizing knowledge, beliefs, and decision-making participation, informed literature searches that prioritized perceptions and discussions of breast cancer risk and mammography’s potential benefits/harms among patients and PCPs, and shaped interview guides for both groups. The Boston University Medical Center Institutional Review Board approved all activities and materials.
Patient Interviews
We sought 25 eligible patients among women ages 40–54 with no mammogram in the prior 9 months and no history of breast cancer. The age range for eligibility was chosen based on professional guidelines, which vary in mammography initiation and spacing until age 55, rendering the review of potential benefits and harms particularly salient. We identified women with upcoming primary care visits at a Boston-based academic, safety-net practice. If present and interested, women completed the Health Literacy Skills Instrument-10, a 10-item validated measure of health literacy focused on decision-making skills (Bann et al., 2012). We invited women who scored less than 7, suggesting limited health literacy, to complete a qualitative interview. Interviews occurred immediately after primary care visits or at participants’ convenience. We purposively sampled at least 10 participants with and 10 without prior mammograms.
Patient interview guides were pilot tested with five women with LHL. Topics included knowledge of and experiences with mammography and mammography discussions with providers, familiarity with breast cancer risk and potential mammography benefits/harms, and information delivery preferences. Seeking formative evaluations of information materials to guide development of a decision aid for LHL women, patient interviews also included the presentation and guided discussion of four images representing common elements of mammography counseling and three screenshots from an online mammography decision aid. One displayed a woman getting a mammogram; two others showed breast anatomy in detailed and simplified forms, and the final was a set of mammogram images displaying breast density levels. The decision-aid pages were from Breast Screening Decisions (Weill Cornell Medicine, 2016) and covered mammogram definitions, a sample risk assessment results page, and a review of mammogram benefits and potential harms. A trained research assistant (AM) conducted 45–60 minute patient interviews in a private space. Patients received 40 USD as an incentive.
Primary Care Provider Interviews
Eligible PCPs included physicians and nurse practitioners who practiced in family medicine or outpatient general internal medicine clinics. Approximately 150 PCPs were invited via e-mail and through in-person invitation at a practice-wide meeting. PCPs who participated were entered into a raffle to receive 200 USD, which occurred after recruitment completion.
Clinician input informed development of PCP interview guides, which were pilot tested with clinical study team members before enrollment commenced. PCP interview topics included general counseling practices, familiarity with and use of risk estimates, patient preference elicitation, experiences implementing SDM for mammography, and recommendations for facilitating SDM. An investigator (CG) conducted 30-min PCP interviews via phone or in-person in a private location.
Data Analysis
Interviews were audio-recorded and professionally transcribed verbatim. Transcripts were verified against the audio files to ensure accuracy, de-identified, assigned pseudonyms to protect confidentiality, and uploaded into NVivo 12.1.0 qualitative data management software. Codebooks included deductive codes based on our guiding conceptual framework (Paasche-Orlow & Wolf, 2007), interview guides, and relevant literature, plus inductive codes sourced from interview responses. We linked patients’ responses to sample materials in NVivo to facilitate content analysis of images (QSRInternational, 2020). Two investigators (CG, AM) independently coded three patients and three PCP interviews, compared coding results, and achieved consensus in coding definitions and applications. One investigator (AM) coded remaining transcripts, and another investigator (CG) reviewed six randomly selected coded interviews to ensure quality.
Utilizing a content analysis approach, codes were compared within and across patient and PCP interview groups to generate themes. For patient interviews, CG and AM created summary maps that represented content from key themes for each participant (Miles, Huberman, & Saldana, 2020). These, along with post-interview memos, established that theoretical saturation had been achieved within 23 patient and 17 PCP interviews. Two more patient interviews and three PCP interviews were completed to confirm that no new thematic variations arose in either group. A constant comparison technique was used to refine final themes and identify representative quotes (Charmaz, 2006).
Results
Figure 1 displays the patient recruitment process. Two hundred and thirteen potentially eligible women with upcoming appointments were identified. Of the 65 women who completed screening, 10 were ineligible due to HLSI-10 > 6, and 13 self-reported mammograms in the prior 9 months or spoke limited English; 14 women were eligible but declined enrollment, were unable to be re-contacted, or did not complete interviews before sample size was reached; 25 women enrolled in the study. Of the 150 invited PCPs, 26 responded, 20 of whom completed interviews. Table 1 presents the demographics of the 25 enrolled patients and 20 PCPs. Four themes related to mammography counseling were identified as salient for women and PCPs: 1. Addressing family history versus comprehensive risk assessment; 2. Discussion of potential harms associated with mammography; 3. Preferences for information delivery; and, 4. Integration of tools to facilitate mammography discussion.
Figure 1.
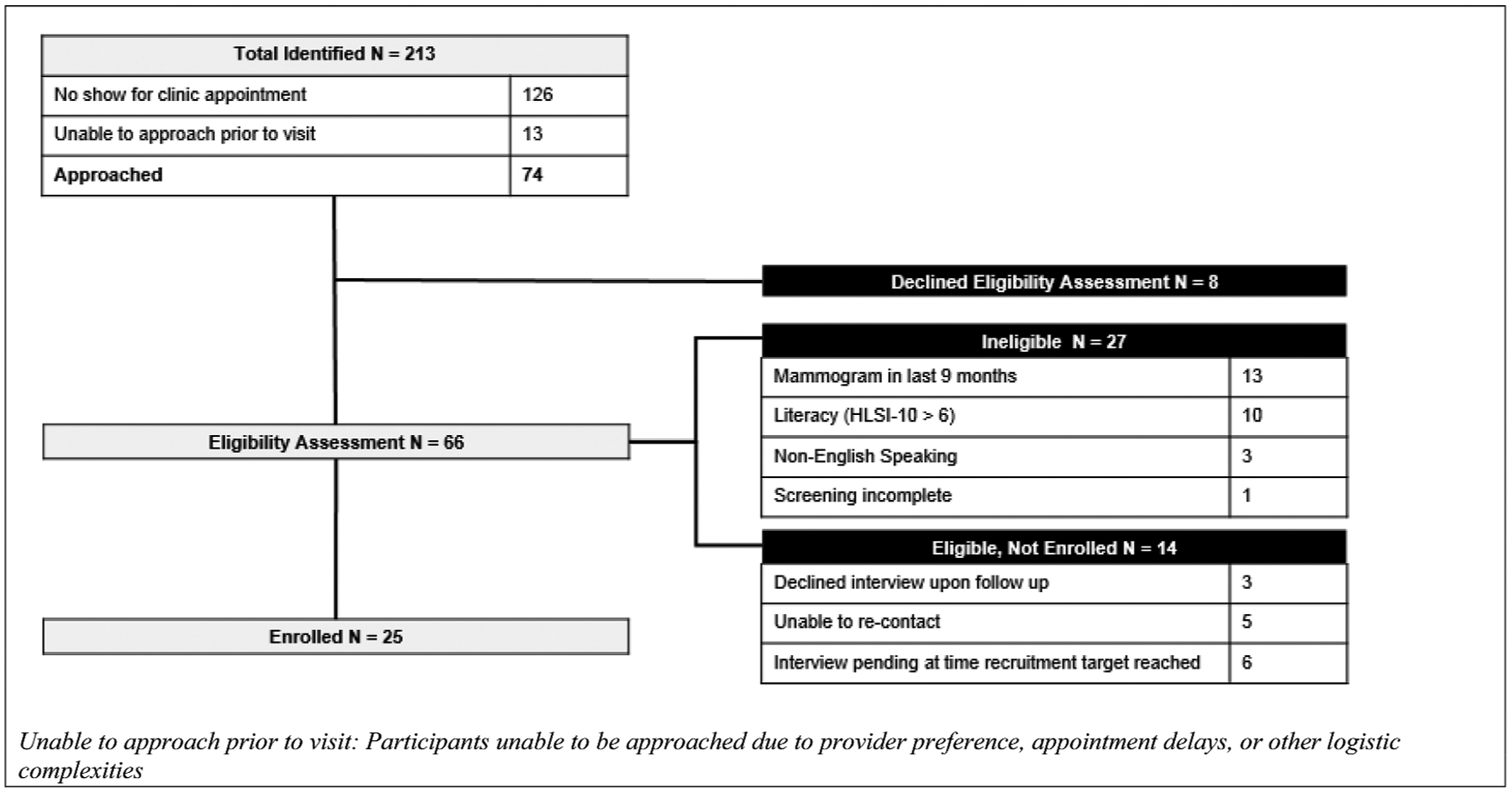
Patient recruitment.
Table 1.
Participant characteristics
| Women with Limited Health Literacy (N = 25) | |
|---|---|
| Age (average = 46) | n (%) |
| 40–45 | 10 (40) |
| 46–50 | 9 (36) |
| 51–54 | 6 (24) |
| HLSI-10 Score | n (%) |
| 0 | 1 (4) |
| 1 | 2 (8) |
| 2 | 1 (4) |
| 3 | 6 (24) |
| 4 | 3 (12) |
| 5 | 6 24) |
| 6 | 6 (24) |
| Mammography History | n (%) |
| Prior Mammogram | 12 (48) |
| No Prior Mammogram | 13 (52) |
| Race | n (%) |
| Black/African American | 18 (72) |
| White | 2 (8) |
| Not Available | 5 (20) |
| Ethnicity | n (%) |
| Hispanic/Latina | 3 (12) |
| Non-Hispanic/Latina | 22 (88) |
| Primary Care Providers (N = 20) | |
| Gender | n (%) |
| Female | 15 (75) |
| Male | 5 (25) |
| Number of Years in Practice | n (%) |
| ≤5 years | 8 (40) |
| 6–10 years | 6 (30) |
| 11–20 years | 2 (10) |
| > 20 years | 4 (20) |
| Educational Materials Provided to Patients | n(%) |
| None | 17 (85) |
| In office handouts | 2 (10) |
| Out of office referrals | 1 (5) |
Addressing Family History versus Comprehensive Risk Assessments
PCP Responses
Though some PCPs discussed a range of breast cancer risk factors, such as smoking and exercise, most PCPs prioritized family history.
“I think providers don’t do a great job of conveying risks for breast cancer. I think family history is pretty well understood, but otherwise, not as much.”
– PCP 1
“So, I think family history in my mind drives so much more than any model, whether that’s right or wrong, but in my mind family history just drives the bus.”
– PCP 2
Infrequent use of comprehensive risk assessment was attributed by some PCPs, in part, to limited familiarity with breast cancer risk estimators. Of the 20 PCPs interviewed, only two described regularly using risk calculators. Nine PCPs never used them, while eight PCPs incorporated calculators only when they highly suspected patients’ increased risk, triggered most often by family history of breast cancer. Among those who used risk calculators, some reported struggling to interpret results, and others described results as being difficult to explain to patients. This led a few PCPs to refer patients elsewhere to receive support.
“The percentage is very hard to explain to the patient. Maybe it’s because of my own lack of understanding fully, but it comes up a lot when we try to do the risk evaluations.”
– PCP 3
“In the last years, maybe I’ve used [a risk calculator] one or three times … But it took me so long to figure out how to use it … it’s like, okay, I’m not going to spend time on this. I’m just going to send them somewhere else.”
– PCP 4
Patient Responses
Aligned with PCPs reporting limited comprehensive risk assessment use, most patients, regardless of mammogram history, did not recall discussing personal risk with their PCPs. Most patients cited family history as the primary source of breast cancer risk, often characterizing personal risk as a function of family history alone. Prior mammogram experience did not necessarily translate into awareness of risk factors or personal risk, as Patient 1 and Patient 2 (prior mammograms), respectively, asked “What are the risks besides what you hear on TV?” and “What would put me at risk?” When reviewing a sample risk assessment results page from a decision-aid (Figure 2), most patients described limited awareness of and confusion about risk factors other than family history. They desired risk information that not only named additional risk factors, but also explained how they impacted their individual risk. Many patients also demonstrated challenges interpreting percentages and sought clarity about how percentages were calculated.
Figure 2.
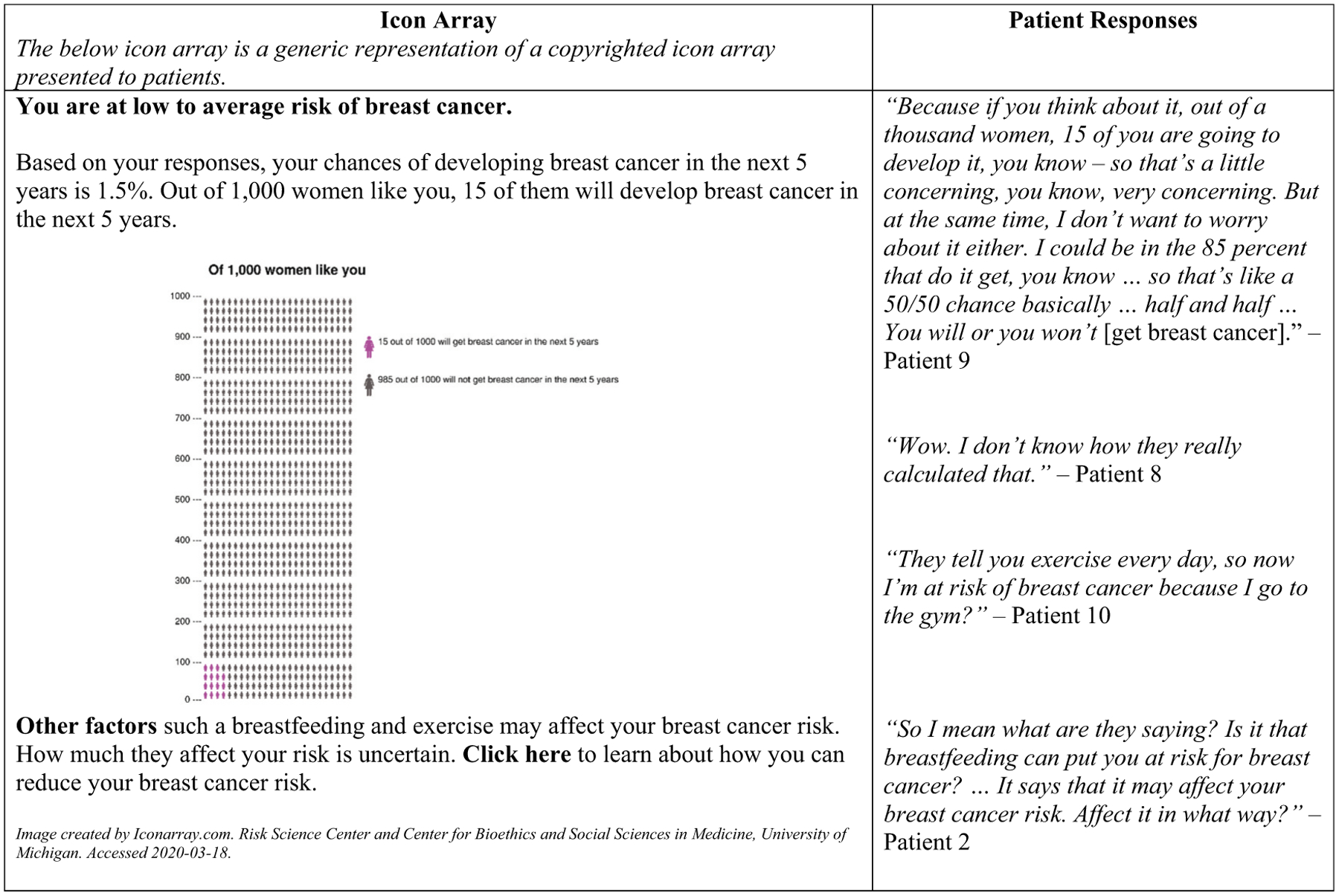
Patient responses to sample breast cancer risk assessment.
Discussion of Potential Harms Associated with Mammography
PCP Responses
Most PCPs addressed potential mammography benefits and harms during counseling. Among the 13 PCPs who discussed potential harms, the most cited potential harms were false positives, psychological stress or anxiety, logistical inconvenience of additional testing, and pain associated with mammogram machines. A minority of providers actively avoided potential harms discussions. These PCPs, seeking mammography adherence among patients, worried that describing potential harms or uncertainty about benefits would deter patients from care.
“I don’t tend to get into those [potential harms] details because I feel like it gets us off track and then people are less likely to be willing to do it.”
– PCP 2
“[Spending] a lot of time going into all the negatives of mammogram I don’t feel like is helpful. Because ultimately, we want to promote everybody to get it. That’s still the message. And the confusing message is when we say, ‘Oh well they say different and they say don’t get a mammogram and they say you have dense breasts so don’t … ‘ that’s when people get confused.”
– PCP 3
Patient Responses
Most patients described at least one mammography benefit, typically early detection when breast cancer is treatable. Though two patients (both no prior mammograms) described pain from the mammogram machine as a potential harm, most patients with and without mammogram experience were unable to report potential mammography harms. Patient 3 said, “There wouldn’t be a downside.” Patient 4 likewise added, “I don’t think there’s any risk of you letting your breasts up there. I don’t think nothing’s going to hurt you.” Upon reviewing a decision aid page about potential mammography harms, many patients like Patient 5 expressed surprise when learning about harms: “There’s harms? Wow, I’m gonna read that first.”
False positives were a challenging topic for patients and PCPs. Only one patient, who had prior mammogram experience, named false positives as a potential mammography harm, but was unsure why they occur. When reading false-positive information provided via the sample decision aid, some patients struggled to articulate what the result meant, sometimes misinterpreting “positive” as a good outcome.
“False positive, let me see if I’m right … Me, I understand false positive means that they did a test but the test came like not sure if it was positive … like if you do the test, but you’re not sure, you’re not 100 percent sure if it’s false or if it’s contagious or whatever, true or false or whatever, how do say it, positive I mean. So that’s why they do it.”
– Patient 6
Though PCPs believed that patients should have false-positive information, many struggled to address this issue, and others, adequately during brief clinic visits.
“When you’re in the room 20 minutes and you’re trying to discuss about a test … that sounds great but then how do you describe the false positive result and what kind of anxiety that can bring … how do you explain that to them or show it to them visually … I don’t have a great aid that does that.”
– PCP 5
Preferences for Information Delivery
PCP Responses
Most PCPs preferred describing risk with smaller numeric units and whole numbers, which they believed were easier for patients to digest. A few PCPs found analogies rooted in patients’ everyday experience, like lottery tickets or traffic navigation, effectively conveyed breast cancer risk information.
“[Y]ou can say, ‘So 1 in 12 women, on average, will be diagnosed with breast cancer over the course of their life. Your risk is double that … so a woman like you had a 1 in 6 chance of getting breast cancer over the course of the life-time.’ So trying to use units that are understandable.”
– PCP 6
“When I talk about medications, it’s like yeah it’s not going to make it zero, but talking about playing in the street, you can get hit by a car, but it’s much worse if you’re on the highway. Just take me from the highway to a side street. People seem to like that … ‘I understand. I can still get hit by a car but it’s less risky.’”
– PCP 5
Patient Responses
Aligned with PCPs, many patients preferred simpler data visualizations like pie charts, rather than icon arrays.
“A pie chart, graphs, I’m more so that kind of person, but [the icon array] doesn’t do nothing for me.”
– Patient 5
When reviewing sample images, patients called out visual detail, imagery, and color as informational cues. Patients compared a black-and-white, detailed, front and side medical illustration of a breast against a color, simplified, side-only drawing of a breast (Figure 3). Associating red coloring with sickness/danger and branch-like renderings of lobules and ducts with damage, many patients suggested that the colored, simplified image represented an unhealthy breast, perceptions potentially misaligned with the visual aid’s intended messaging. Some patients noted that detail made illustrations feel realistic and thus led to better associations with their own bodies.
Figure 3.
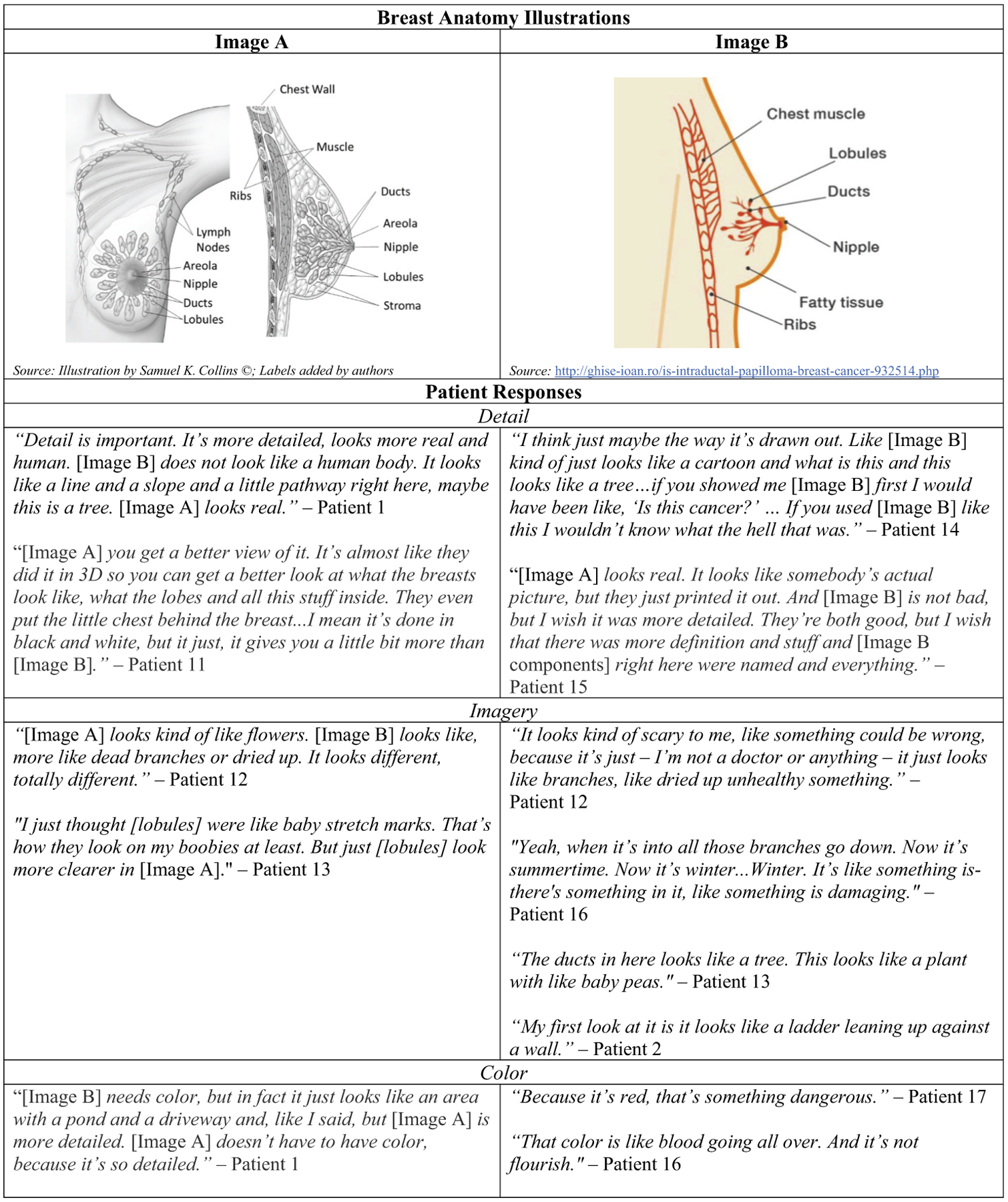
Patient responses to breast anatomy illustrations.
Integration of Tools to Facilitate Mammography Discussion
PCP Responses
Several PCPs noted that patients arrive to appointments unable to recall or unaware of their families’ cancer histories. These PCPs desired mammography tools that elicited patients’ family histories prior to counseling.
“I think very often when we do family history in the moment, we hear, ‘I had a relative with cancer, I don’t remember what type,’ or ‘I don’t remember what side of the it was on’ … There might be an opportunity for patients to come in prepared to share that history, which would be really helpful.”
– PCP 1
For some PCPs, the waiting room setting was discussed as an ideal intervention space because it allowed sufficient time for information exposure and would be immediately followed by an in-person conversation. Thus, if patients experienced concern or identified questions, PCPs could respond expediently.
“I think the ideal thing is [patients] would do this in the waiting room and kind of come with their little printout of the information … and had a way to interact with that information before the visit with the provider.”
– PCP 7
Patient Responses
Some patients similarly suggested that multi-media information tools available in clinical spaces prior to encounters could set them up for successful mammography conversations.
“[T]he computer is just amazing … so if you can even project [graphics] in the office, the doctor’s office, some people now will be using things just mid-screen, hit a button, and it’s all happening in front of us. So it’s quick data … and I think the more data that we receive, the better we have confidence.”
– Patient 7
Both groups also recommended using electronic patient-provider portals to relay information to patients and equip PCPs with relevant information to optimize discussions. In reference to the patient portal, Patient 8 stated, “It’s helpful. I mean, even if they just put in information for patients and materials to read on what you should be doing, next steps, preventive care.” PCP 8 further suggested:
“I think it would be good if there was an opportunity after the patient has seen a video, read something, to ask questions online, so that could actually be incorporated into [the electronic patient portal] for the visit and populated before-hand so you actually have some of that kind of data to work with.”
Discussion
This study explored breast cancer risk and potential mammography benefits/harms perceptions and discussions among women with LHL and PCPs, and elicited patient feedback on sample mammography information formats. We found that family history was the primary risk factor that PCPs discussed and that patients associated with breast cancer risk, consistent with other studies (Buxton et al., 2003; Collins et al., 2014; Davis et al., 2004; Haber et al., 2012; McCaul & O’Donnell, 1998; Sabatino et al., 2007). Quantitative risk assessment was infrequently used by PCPs, with patients, regardless of mammogram history, similarly lacking knowledge of their own risk. Potential mammography harms were unfamiliar to most patients, and proved challenging for PCPs to address. Both PCPs and patients preferred mammography information materials with plain, relatable language and simple numeric values. Patients highlighted the importance of illustration detail, imagery, and color in aiding understanding. PCPs and patients recommended utilizing pre-visit settings, such as waiting rooms, to elicit family history and provide mammography information.
Supports that address PCPs’ discomfort with elements of breast cancer risk assessment and potential mammography benefits/harms discussions may enhance counseling efforts. In our study, many PCPs stated that they felt limited comfort using risk calculators, as others have found (Collins et al., 2014; Martinez et al., 2017; Petrova, Garcia-Retamero, & Cokely, 2015; Sabatino et al., 2007). Some avoided potential harms discussions, also aligned with prior work (Haas et al., 2016; Hoffman et al., 2010; Wegwarth & Gigerenzer, 2011). In addition to concerns about mammography deterrence cited by PCPs in our study, uncertainty about the evidence supporting potential mammography harms may challenge individual counseling efforts (Siedlikowski, Ells, & Bartlett, 2018). Though professional guidance suggests providing balanced information, current cancer screening guidelines present potential benefits/harms in unbalanced and non-quantified ways (Caverly et al., 2016). Reformatting guidelines may be one way to model balanced presentation of ambiguous evidence for PCPs. Integrating user-friendly guidelines and promoting use of systems-level tools, such as electronic medical record reminders and performance reports, may reinforce provider uptake of SDM- and guideline- concordant activities (Schapira et al., 2016). Additional research may explore the provider motivations, provider knowledge, tools, and environmental conditions necessary to support PCP-initiated risk and potential benefits/harms discussions. These efforts may be particularly salient to safety-net settings, where PCPs often serve patient populations with higher rates of LHL and fewer personal resources to access to mammography information beyond healthcare encounters.
Our study contributes to existing literature exploring which counseling elements are best conveyed by specific information formats (e.g., numbers, narratives), particularly how women with LHL respond to visual aids describing risk assessment results, potential benefits and harms, and breast anatomy. Generally, simple graphical formats like single line or pie charts may enhance patient understanding, and packaging graphs together may improve accuracy of patient risk perception (Brown et al., 2011). Additionally, international decision-aid standards recommend presenting information in a side-by-side format to visually signal balance and enhance probabilistic understanding (Abhyankar et al., 2013). However, further efforts may parse the unique influences of health literacy, numeracy, and graphicacy on visual format comprehension. A small body of research indicates that pictographs accompanied by plain language explanations may enhance knowledge acquisition among individuals with LHL (Fagerlin, Zikmund-Fisher, & Ubel, 2011; Tait et al., 2012) and pictographs alone may enhance knowledge and acceptability across varied numeracy levels (Hawley et al., 2008; Tait et al., 2010). Information presented in numeric, versus graphical, terms, may better serve individuals with limited graphicacy (Gaissmaier et al., 2012). Though our study did not assess the numeracy and graphicacy of patients, findings suggest that our cohort of women with LHL may ascribe unanticipated meaning to visual aids based on design features such as color, detail, and imagery. Referencing our conceptual framework’s patient-provider communication domain, these preliminary findings may suggest that, absent information-sharing from providers, responses to materials’ design features may influence patients’ beliefs, behaviors, and health outcomes in unintended ways. Additional research may explore the relationship between limited health literacy, numeracy, and graphicacy; characterize the availability heuristics of women with LHL when reviewing visual formats; consider the extent to which such associations influence understanding, acceptability, and impact of materials; and pilot test materials to adequately address patient needs.
Despite evidence that patients with LHL may require tailored support, most available patient decision aids were developed without input from this population. A recent review indicates that only 3 of 97 patient decision aid trials either included individuals with limited education or health literacy or used tools explicitly designed to meet the needs of these patient groups (McCaffery et al., 2013). Additionally, only a few cancer screening and prevention interventions report effects by health literacy (Han et al., 2017; Heckel et al., 2018). Two interventions assessing decision aids in colorectal cancer screening contexts found that a patient decision aid/video combination improved screening knowledge, and a web-based decision aid increased readiness to receive screening and identify screening preferences (Miller et al., 2011; Smith et al., 2014; 2010). While a subset of studies demonstrate improvements in knowledge (Volk et al., 2008) and recall (Freed et al., 2013; Meppelink, Smit et al., 2015; Meppelink, van Weert et al., 2015) for LHL groups, most investigations observe improvements in individuals with adequate, rather than limited, health literacy. This raises concern that efforts may be widening health literacy disparities and underscores the need for interventions designed for and tested with LHL groups of varied backgrounds, including race, ethnicity, and gender identity.
As a qualitative study conducted at a single institution, findings may be context-specific and have limited generalizability. Patients’ demographics and experiences with processes of care at a safety-net institution are indicative, respectively, of the socio-demographic characteristics of the patients that this specific institution serves and this institution’s processes of care. Furthermore, providers’ shared processes of care experiences may be related to characteristics of the institution. These experiences may not be reflective of other hospitals’ patient populations and processes. Patients and PCPs were not clinical dyads, and we were unable to compare patient and provider data directly. Additionally, some topics may not be comprehensively represented in the data. For example, while interview guides asked patients about breast cancer information sources, we did not ask where or from whom specific breast cancer risk or potential harm or benefit information was acquired. Sample size also limited intersectional analysis of patients’ literacy status and other factors that may also influence SDM. Finally, this is an observational study conducted at a single point in time, which relied upon patient and provider accounts and recall bias remains a potential threat.
Conclusion
This exploratory study sought to characterize breast cancer risk and potential mammography benefits/harms perceptions and discussions among women with LHL and PCPs to inform development of decision tools for LHL populations. PCPs infrequently used risk assessment tools, and some PCPs avoided potential harms discussions. Patients, regardless of mammogram history, generally were unaware of breast cancer risk beyond family history and had limited knowledge of potential mammography harms. Visual aids may better relay specific concepts to patients with LHL, but should be piloted tested with this population to optimize understanding. Integrating informational tools into clinical settings prior to PCP counseling may enhance SDM.
Ultimately, this study identified discordance between the potential screening benefits/harms information that patients with LHL desired and PCPs’ described approaches to mammography counseling. It also identified information delivery preferences shared by both groups, but not readily feasible in clinical contexts. These findings reveal that current forms of breast cancer screening counseling may not be responsive to patient or PCP needs. The experiences captured offer worth-while foci for future interventions that explicitly address breast cancer screening SDM, for example decision-aid testing with LHL groups, and provide opportunities to enhance SDM across the health literacy spectrum.
Funding
This work was supported entirely by the National Cancer Institute (1K07CA221899).
Footnotes
Declarations Of Interest
Dr. Christine Gunn reports support from National Cancer Institute (1K07CA221899) supporting the conduct of this study. Remaining authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Abhyankar P, Volk RJ, Blumenthal-Barby J, Bravo P, Buchholz A, Ozanne E, … Stalmeier P (2013). Balancing the presentation of information and options in patient decision aids: An updated review. BMC Medical Informatics and Decision Making, 13(2). doi: 10.1186/1472-6947-13-S2-S6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bann CM, McCormack LA, Berkman ND, & Squiers LB (2012). The health literacy skills instrument: A 10-item short form. Journal of Health Communication, 17(Suppl3), 191–202. doi: 10.1080/10810730.2012.718042 [DOI] [PubMed] [Google Scholar]
- Berkman ND, Sheridan SL, Donahue KE, Halpern DJ, & Crotty K (2011). Low health literacy and health outcomes: An updated systematic review. Annals of Internal Medicine, 155(2), 97–107. doi: 10.7326/0003-4819-155-2-201107190-00005 [DOI] [PubMed] [Google Scholar]
- Brewer NT, Tzeng JP, Lillie SE, Edwards AS, Peppercorn JM, & Rimer BK (2008). Health literacy and cancer risk perception: Implications for genomic risk communication. Medical Decision Making, 29(2), 157–166. doi: 10.1177/0272989X08327111 [DOI] [PubMed] [Google Scholar]
- Brown J, Soukas C, Lin JJ, Margolies L, Santiago-Rivas M, & Jandorf L (2019). Physician knowledge, attitudes, and practices regarding breast density. Journal of Women’s Health, 28(9), 1193–1199. doi: 10.1089/jwh.2018.7429 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown SM, Culver JO, Osann KE, MacDonald DJ, Sand S, Thornton AA, … Weitzel JN (2011). Health literacy, numeracy, and interpretation of graphical breast cancer risk estimates. Patient Education and Counseling, 83(1), 92–98. doi: 10.1016/j.pec.2010.04.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buxton JA, Bottorff JL, Balneaves LG, Richardson C, McCullum M, Ratner PA, & Hack T (2003). Women’s perceptions of breast cancer risk: Are they accurate? Canadian Journal of Public Health, 94(6), 422–426. doi: 10.1007/BF03405078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caverly TJ, Hayward RA, Reamer E, Zikmund-Fisher BJ, Connochie D, Heisler M, & Fagerlin A (2016). Presentation of benefits and harms in US cancer screening and prevention guidelines: Systematic review. Journal of the National Cancer Institute, 108(6), djv436. doi: 10.1093/jnci/djv436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention. (2020). Basic information about breast cancer. Retrieved October 2, 2020 from https://www.cdc.gov/cancer/breast/basic_info/index.htm
- Charmaz K (2006). Constructing grounded theory: A practical guide through qualitative analysis. London; Thousand Oaks, California: SAGE Publications, Inc. [Google Scholar]
- Collins IM, Steel E, Mann GB, Emery JD, Bickerstaffe A, Trainer A, … Keogh LA (2014). Assessing and managing breast cancer risk: Clinicians’ current practice and future needs. Breast, 23(5), 644–650. doi: 10.1016/j.breast.2014.06.014 [DOI] [PubMed] [Google Scholar]
- Costanza ME, Zapka JG, Harris DR, Hosmer D, Barth R, Gaw VP, … Stoddard AM (1992). Impact of a physician intervention program to increase breast cancer screening. Cancer Epidemiology Biomarkers & Prevention, 1(7), 581–589. Retrieved May 6 2020 https://cebp.aacrjournals.org/content/cebp/1/7/581.full.pdffrom [PubMed] [Google Scholar]
- Croes KD, Jones NR, DuBenske LL, Schrager SB, Mahoney JE, Little TA, & Burnside ES (2020). Core elements of shared decision-making for women considering breast cancer screening: Results of a modified delphi survey. Journal of General Internal Medicine, 35(6), 1668–1677. doi: 10.1007/s11606-019-05298-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis S, Stewart S, & Bloom J (2004). Increasing the accuracy of perceived breast cancer risk: Results from a randomized trial with Cancer Information Service callers. Preventive Medicine, 39(1), 64–73. doi: 10.1016/j.ypmed.2004.02.043 [DOI] [PubMed] [Google Scholar]
- Davis TC, Williams MV, Marin E, Parker RM, & Glass J (2002). Health literacy and cancer communication. CA: A Cancer Journal for Clinicians, 52(3), 134–149. doi: 10.3322/canjclin.52.3.134 [DOI] [PubMed] [Google Scholar]
- DeFrank JT, Rimer BK, Bowling JM, Earp JA, Breslau ES, & Brewer NT (2012). Influence of false-positive mammography results on subsequent screening: Do physician recommendations buffer negative effects? Journal of Medical Screening, 19(1), 35–41. doi: 10.1258/jms.2012.011123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- DuBenske LL, Schrager S, McDowell H, Wilke LG, Trentham-Dietz A, & Burnside ES (2017). Mammography screening: Gaps in patient’s and physician’s needs for shared decision-making. The Breast Journal, 23(2), 210–214. doi: 10.1111/tbj.12779 [DOI] [PMC free article] [PubMed] [Google Scholar]
- DuBenske LL, Schrager SB, Hitchcock ME, Kane AK, Little TA, McDowell HE, & Burnside ES (2018). Key elements of mammography shared decision-making: A scoping review of the literature. Journal of General Internal Medicine, 33(10), 1805–1814. doi: 10.1007/s11606-018-4576-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durand MA, Carpenter L, Dolan H, Bravo P, Mann M, Bunn F, & Elwyn G (2014). Do interventions designed to support shared decision-making reduce health inequalities? A systematic review and meta-analysis. PLoS One, 9(4), e94670–e94670. doi: 10.1371/journal.pone.0094670 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elkin EB, Pocus VH, Mushlin AI, Cigler T, Atoria CL, & Polaneczky MM (2017). Facilitating informed decisions about breast cancer screening: Development and evaluation of a web-based decision aid for women in their 40s. BMC Medical Informatics and Decision Making, 17(29). doi: 10.1186/s12911-017-0423-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans DGR, Burnell LD, Hopwood P, & Howell A (1993). Perception of risk in women with a family history of breast cancer. British Journal of Cancer, 67(3), 612–614. doi: 10.1038/bjc.1993.112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fagerlin A, Zikmund-Fisher BJ, & Ubel PA (2011). Helping patients decide: Ten steps to better risk communication. JNCI Journal of the National Cancer Institute, 103(19), 1436–1443. doi: 10.1093/jnci/djr318 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freed E, Long D, Rodriguez T, Franks P, Kravitz RL, & Jerant A (2013). The effects of two health information texts on patient recognition memory: A randomized controlled trial. Patient Education and Counseling, 92(2), 260–265. doi: 10.1016/j.pec.2013.03.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaglio B, Glasgow RE, & Bull SS (2012). Do patient preferences for health information vary by health literacy or numeracy? A qualitative assessment. Journal of Health Communication, 17(Suppl3), 109–121. doi: 10.1080/10810730.2012.712616 [DOI] [PubMed] [Google Scholar]
- Gaissmaier W, Wegwarth O, Skopec D, Müller AS, Broschinski S, & Politi MC (2012). Numbers can be worth a thousand pictures: Individual differences in understanding graphical and numerical representations of health-related information. Health Psychology, 31(3), 286–296. doi: 10.1037/a0024850 [DOI] [PubMed] [Google Scholar]
- Gillespie C (2012). The experience of risk as ‘measured vulnerability’: Health screening and lay uses of numerical risk. Sociology of Health & Illness, 34(2), 194–207. doi: 10.1111/j.1467-9566.2011.01381.x [DOI] [PubMed] [Google Scholar]
- Golbeck AL, Ahlers-Schmidt CR, Paschal AM, & Dismuke SE (2005). A definition and operational framework for health numeracy. American Journal of Preventive Medicine, 29(4), 375–376. doi: 10.1016/j.amepre.2005.06.012 [DOI] [PubMed] [Google Scholar]
- Gøtzsche P, & Jørgensen K (2013). Screening for breast cancer with mammography. Cochrane Database Syst Rev. doi: 10.1002/14651858.CD001877.pub5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunn CM, Bokhour B, Parker VA, Parker PA, Blakeslee S, Bandos H, & Holmberg C (2019). Exploring explanatory models of risk in breast cancer risk counseling discussions: NSABP/NRG oncology decision-making project 1. Cancer Nursing, 42(1), 3–11. doi: 10.1097/ncc.0000000000000517 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunn CM, Maschke A, Paasche-Orlow MK, Kressin NR, Schonberg MA, & Battaglia TA (2020). Engaging women with limited health literacy in mammography decision-making: Perceptions of patients and primary care providers. Journal of General Internal Medicine. doi: 10.1007/s11606-020-06213-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haas JS, Sprague BL, Klabunde CN, Tosteson ANA, Chen JS, Bitton A, … Schapira MM; on behalf of the, P. C. (2016). Provider attitudes and screening practices following changes in breast and cervical cancer screening guidelines. Journal of General Internal Medicine, 31(1), 52–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haber G, Ahmed NU, & Pekovic V (2012). Family history of cancer and its association with breast cancer risk perception and repeat mammography. American Journal of Public Health, 102(12), 2322–2329. doi: 10.2105/AJPH.2012.300786 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han HR, Song Y, Kim M, Hedlin HK, Kim K, Ben Lee H, & Roter D (2017). Breast and cervical cancer screening literacy among Korean American women: A community health worker-led intervention. American Journal of Public Health, 107(1), 159–165. doi: 10.2105/AJPH.2016.303522 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han J, Jungsuwadee P, Abraham O, & Ko D (2018). Shared decision-making and women’s adherence to breast and cervical cancer screenings. International Journal of Environmental Research and Public Health, 15(7), 1509. doi: 10.3390/ijerph15071509 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawley ST, & Morris AM (2016). Cultural challenges to engage patients in shared decision making. Patient Education and Counseling, 100(1), 18–24. doi: 10.1016/j.pec.2016.07.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawley ST, Zikmund-Fisher B, Ubel P, Jancovic A, Lucas T, & Fagerlin A (2008). The impact of the format of graphical presentation on health-related knowledge and treatment choices. Patient Education and Counseling, 73(3), 448–455. doi: 10.1016/j.pec.2008.07.023 [DOI] [PubMed] [Google Scholar]
- Heckel L, Fennell KM, Reynolds J, Boltong A, Botti M, Osborne RH, … Livingston PM (2018). Efficacy of a telephone outcall program to reduce caregiver burden among caregivers of cancer patients [PROTECT]: A randomised controlled trial. BMC Cancer, 18 (1), 59. doi: 10.1186/s12885-017-3961-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman RM, Elmore JG, Fairfield KM, Gerstein BS, Levine CA, & Pignone MP (2014). Lack of shared decision making in cancer screening discussions: Results from a national survey. American Journal of Preventive Medicine, 47(3), 251–259. doi: 10.1016/j.amepre.2014.04.011 [DOI] [PubMed] [Google Scholar]
- Hoffman RM, Lewis CL, Pignone MP, Couper MP, Barry MJ, Elmore JG, … Zikmund-Fisher BJ (2010). Decision-making processes for breast, colorectal, and prostate cancer screening: The DECISIONS survey. Medical Decision Making, 30(5 Suppl), 53–64. doi: 10.1177/0272989X10378701 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffmann TC, & Del Mar C (2015). Patients’ expectations of the benefits and harms of treatments, screening, and tests: A systematic review. JAMA Internal Medicine, 175(2), 274–286. doi: 10.1001/jamainternmed.2014.6016 [DOI] [PubMed] [Google Scholar]
- Hoffmann TC, & Del Mar C (2017). Clinicians’ expectations of the benefits and harms of treatments, screening, and tests: A systematic review. JAMA Internal Medicine, 177(3), 407–419. doi: 10.1001/jamainternmed.2016.8254 [DOI] [PubMed] [Google Scholar]
- Howlader N, Noone A, Krapcho M, Miller D, Brest A, Yu M, … Cronin K (2019). SEER cancer statistics review, 1975–2016. Bethesda, MD: National Cancer Institute. SEER data submission, posted to the SEER web site, April 2019.Retrieved from https://seer.cancer.gov/csr/1975_2016/ [Google Scholar]
- Institute of Medicine. (2004). Health literacy: A prescription to end confusion. Washington, DC: The National Academies Press. doi: 10.17226/10883 [DOI] [PubMed] [Google Scholar]
- Jolles MP, Richmond J, & Thomas KC (2019). Minority patient preferences, barriers, and facilitators for shared decision-making with health care providers in the USA: A systematic review. Patient Education and Counseling, 102(7), 1251–1262. doi: 10.1016/j.pec.2019.02.003 [DOI] [PubMed] [Google Scholar]
- Katz MG, Jacobson TA, Veledar E, & Kripalani S (2007). Patient literacy and question-asking behavior during the medical encounter: A mixed-methods analysis. Journal of General Internal Medicine, 22(6), 782–786. doi: 10.1007/s11606-007-0184-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandelblatt JS, Cronin K, Bailey S, Berry D, de Koning H, Draisma G, … Feur E (2009). Effects of mammography screening under different screening schedules: Model estimates of potential benefits and harms. Annals of Internal Medicine, 151(10), 738–747. doi: 10.7326/0003-4819-151-10-200911170-00010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandelblatt JS, Cronin K, deKoning H, Miglioretti DL, Schechter C, & Stout N (2015). Collaborative modeling of U.S. breast cancer screening strategies: Technical report. Agency for Healthcare Research and Quality. Rockville, MD. No 14–05201-EF–4. Retrieved May 6, 2020 from https://www.uspreventiveservices-taskforce.org/home/getfilebytoken/nmcMhEwWT9BncBBBQQN6Uf [Google Scholar]
- Mandelblatt JS, Stout NK, Schechter CB, van den Broek JJ, Miglioretti DL, Krapcho M, … Cronin KA (2016). Collaborative modeling of the benefits and harms associated with different U.S. breast cacner screening strategies. Annals of Internal Medicine, 164(4), 215–225. doi: 10.7326/M15-1536 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez KA, Deshpande A, Ruff AL, Bolen SD, Teng K, & Rothberg MB (2017). Are providers prepared to engage younger women in shared decision-making for mammography? Journal of Women’s Health, 27(1), 24–31. doi: 10.1089/jwh.2016.6047 [DOI] [PubMed] [Google Scholar]
- Mazor KM, Rubin DL, Roblin DW, Williams AE, Han PKJ, Gaglio B, … Wagner JL (2016). Health literacy–listening skill and patient questions following cancer prevention and screening discussions. Health Expectations, 19(4), 920–934. doi: 10.1111/hex.12387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCaffery KJ, Holmes-Rovner M, Smith SK, Rovner D, Nutbeam D, Clayman ML, … Sheridan SL (2013). Addressing health literacy in patient decision aids. BMC Medical Informatics and Decision Making, 13(2), S10–S10. doi: 10.1186/1472-6947-13-s2-s10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCaul K, & O’Donnell S (1998). Naive beliefs about breast cancer risk. Women’s Health, 4(1), 93–101. [PubMed] [Google Scholar]
- Meppelink CS, Smit EG, Buurman BM, & van Weert JC (2015). Should we be afraid of simple messages? The effects of text difficulty and illustrations in people with low or high health literacy. Health Communication, 30(12), 1181–1189. doi: 10.1080/10410236.2015.1037425 [DOI] [PubMed] [Google Scholar]
- Meppelink CS, van Weert JC, Haven CJ, & Smit EG (2015). The effectiveness of health animations in audiences with different health literacy levels: An experimental study. Journal of Medical Internet Research, 17(1), e11. doi: 10.2196/jmir.3979 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miles MB, Huberman AM, & Saldana J (2020). Qualitative data analysis: A methods sourcebook (4th ed.). Thousand Oaks, California: Sage Publications. [Google Scholar]
- Miller DP, Spangler JG, Case LD, Goff DC, Singh S, & Pignone MP (2011). Effectiveness of a web-based colorectal cancer screening patient decision aid: A randomized controlled trial in a mixed-literacy population. American Journal of Preventive Medicine, 40(6), 608–615. doi: 10.1016/j.amepre.2011.02.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris NS, Field TS, Wagner JL, Cutrona SL, Roblin DW, Gaglio B, … Mazor KM (2013). The association between health literacy and cancer-related attitudes, behaviors, and knowledge. Journal of Health Communication, 18(Suppl 1), 223–241. doi: 10.1080/10810730.2013.825667 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myers E, Moorman P, Gierisch J, Havrilesky L, Grimm L, Ghate S, … Sanders G (2015). Benefits and harms of breast cancer screeing: A systematic review. JAMA, 314(15), 1615–1634. doi: 10.1001/jama.2015.13183 [DOI] [PubMed] [Google Scholar]
- Nagler RH, Franklin Fowler E, & Gollust SE (2017). Women’s awareness of and responses to messages about breast cancer overdiagnosis and overtreatment: Results from a 2016 national survey. Medical Care, 55(10), 879–885. doi: 10.1097/MLR.0000000000000798 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oeffinger KC, Fontham ET, Etzioni R, Herzig A, Michaelson JS, Shih YC, … Wender R (2015). Breast cancer screening for women at average risk: 2015 guideline update from the American Cancer Society. JAMA, 314(15), 1599–1614. doi: 10.1001/jama.2015.12783 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paasche-Orlow MK, & Wolf MS (2007). The causal pathways linking health literacy to health outcomes. American Journal of Health Behavior, 31(Suppl 1), S19–26. doi: 10.5555/ajhb.2007.31.supp.S19 [DOI] [PubMed] [Google Scholar]
- Petrova D, Garcia-Retamero R, & Cokely ET (2015). Understanding the harms and benefits of cancer screening: A model of factors that shape informed decision making. Medical Decision Making, 35(7), 847–858. doi: 10.1177/0272989X15587676 [DOI] [PubMed] [Google Scholar]
- QSRInternational. (2020). Creating a picture log. QSR International. Retrieved May 6, 2020 from http://help-nv11.qsrinternational.com/desktop/procedures/create_a_picture_log.htm [Google Scholar]
- Sabatino SA, McCarthy EP, Phillips RS, & Burns RB (2007). Breast cancer risk assessment and management in primary care: Provider attitudes, practices, and barriers. Cancer Detection and Prevention, 31(5), 375–383. doi: 10.1016/j.cdp.2007.08.003 [DOI] [PubMed] [Google Scholar]
- Salzman B, Bistline A, Cunningham A, Silverio A, & Sifri R (2020). Breast cancer screening shared decision-making in older African-American women. Journal of the National Medical Association. In Press. 10.1016/j.jnma.2020.05.007 [DOI] [PubMed] [Google Scholar]
- Schapira MM, Sprague BL, Klabunde CN, Tosteson ANA, Bitton A, Chen JS, … Haas JS (2016). Inadequate systems to support breast and cervical cancer screening in primary care practice. Journal of General Internal Medicine, 31(10), 1148–1155. doi: 10.1007/s11606-016-3726-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi W, Nagler RH, Fowler EF, & Gollust SE (2019). Predictors of women’s awareness of the benefits and harms of mammography screening and associations with confusion, ambivalence, and information seeking. Health Commun. Nov, 5, 1–12. doi: 10.1080/10410236.2019.1687129 [DOI] [PubMed] [Google Scholar]
- Siedlikowski S, Ells C, & Bartlett G (2018). Scrutinizing screening: A critical interpretive review of primary care provider perspectives on mammography decision-making with average-risk women. Public Health Reviews, 39(1), 15–20. doi: 10.1186/s40985-018-0092-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siu AL (2016). Screening for breast cancer: U.S. preventive services task force recommendation statement. Annals of Internal Medicine, 164 (4), 279–296. doi: 10.7326/m15-2886 [DOI] [PubMed] [Google Scholar]
- Smith BL, Gadd MA, Lawler C, MacDonald DJ, Grudberg SC, Chi FS, … Souba WW (1996). Perception of breast cancer risk among women in breast center and primary care settings: Correlation with age and family history of breast cancer. Surgery, 120(2), 297–303. doi: 10.1016/S0039-6060(96)80301-1 [DOI] [PubMed] [Google Scholar]
- Smith SK, Kearney P, Trevena L, Barratt A, Nutbeam D, & McCaffery KJ (2014). Informed choice in bowel cancer screening: A qualitative study to explore how adults with lower education use decision aids. Health Expectations, 17(4), 511–522. doi: 10.1111/j.1369-7625.2012.00780.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith SK, Trevena L, Simpson JM, Barratt A, Nutbeam D, & McCaffery KJ (2010). A decision aid to support informed choices about bowel cancer screening among adults with low education: Randomised controlled trial. BMJ, 341, c5370. doi: 10.1136/bmj.c5370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stacey D, Hill S, Caffery K, Boland L, Lewis K, & Horvat L (2017). Shared decision making interventions: Theoretical and empirical evidence with implications for health literacy. IOS Press, Studies in Health Technologies and Informatics, 240, 263–283. doi: 10.3233/978-1-61499-790-0-263 [DOI] [PubMed] [Google Scholar]
- Tait A, Voepel-Lewis T, Zikmund-Fisher B, & Fagerlin A (2010). The effect of format on parents’ understanding of the risks and benefits of clinical research: A comparison between text, tables, and graphics. Journal of Health Communication, 15(5), 487–501. doi: 10.1080/10810730.2010.492560 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tait AR, Voepel-Lewis T, Brennan-Martinez C, McGonegal M, & Levine R (2012). Using animated computer-generated text and graphics to depict the risks and benefits of medical treatment. The American Journal of Medicine, 125(11), 1103–1110. doi: 10.1016/j.amjmed.2012.04.040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tonelli M, Connor Gorber S, & Joffres M (2011). Recommendations on screening for breast cancer in average-risk women aged 40–74 years. CMAJ, 183(17), 1991–2001. doi: 10.1503/cmaj.110334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volk RJ, Jibaja-Weiss ML, Hawley ST, Kneuper S, Spann SJ, Miles BJ, & Hyman DJ (2008). Entertainment education for prostate cancer screening: A randomized trial among primary care patients with low health literacy. Patient Education and Counseling, 73(3), 482–489. doi: 10.1016/j.pec.2008.07.033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wegwarth O, & Gigerenzer G (2011). “There is nothing to worry about”: Gynecologists’ counseling on mammography. Patient Education and Counseling, 84(2), 251–256. doi: 10.1016/j.pec.2010.07.025 [DOI] [PubMed] [Google Scholar]
- Weill Cornell Medicine. (2016). Breast screening decisions.Retrieved February 1, 2020 from https://bsd.weill.cornell.edu/#/
- Woloshin S, Schwartz LM, Black WC, & Welch HG (1999). Women’s perceptions of breast cancer risk: How you ask matters. Medical Decision Making, 19(3), 221–229. doi: 10.1177/0272989X9901900301 [DOI] [PubMed] [Google Scholar]
- Yu J, Nagler RH, Fowler EF, Kerlikowske K, & Gollust SE (2017). Women’s awareness and perceived importance of the harms and benefits of mammography screening: Results from a 2016 national survey. JAMA Internal Medicine, 177(9), 1381–1382. doi: 10.1001/jamainternmed.2017.2247 [DOI] [PMC free article] [PubMed] [Google Scholar]


