Abstract
Objectives:
Most patients with pancreatic cancer have high symptom burdens and poor outcomes. Palliative care (PC) can improve the quality of care through expert symptom management, although the optimal timing of palliative care referral is still poorly understood. We aimed to assess the association of early PC on healthcare utilization and charges of care for pancreatic cancer patients.
Methods:
We selected patients with pancreatic cancer diagnosed between 2000 and 2009 who received at least one PC encounter using the SEER-Medicare. Patients who had unknown follow-up were excluded. We defined ‘early PC’ if the patients received PC within 30 days of diagnosis.
Results:
A total of 3166 patients had a PC encounter; 28% had an early PC. Patients receiving early PC were more likely to be female and have older age compared with patients receiving late PC (p<.001). Patients receiving early PC had fewer ED visits (2.6 vs. 3.0 visits, p=.004) and lower total charges of ED care ($3158 vs. $3981, p<.001) compared to patients receiving late PC. Patients receiving early PC also had lower ICU admissions (0.82 vs. 0.98 visits, p=.006) and total charges of ICU care ($14,466 vs. $18,687, p=.01). On multivariable analysis, patients receiving early PC were significantly associated with fewer ED visits (p=.007) and lower charges of ED care (p=.018) for all patients.
Conclusions:
Early PC referrals were associated with lower ED visits and ED related charges. Our findings support oncology society guideline recommendations for early palliative care in patients with advanced malignancies such as pancreatic cancer.
Keywords: Palliative care, Medicare, pancreatic neoplasms, propensity score, health care utilization
INTRODUCTION
A majority of patients with pancreatic cancer have locally advanced or metastatic disease at the time of initial diagnosis and suffer from high symptom burden, especially near the end of life 1–4. Palliative care (PC) plays an important role in improving the quality of life for seriously ill cancer patients 5–8. It has been shown to reduce pain and other symptom burdens, improve advance care planning, and lower healthcare costs by avoiding preventable admissions and non-beneficial treatment, particularly at the end of life 9,10. Given these benefits, PC is increasingly recognized as an integral component of high-quality cancer care, and oncology societies recommend that all patients with advanced cancer receive a PC consultation early in their disease course 11.
In a prior study by our group, we found that PC encounters for Medicare beneficiaries with pancreatic cancer have increased in recent years 12. However, most encounters occurred near the end of life and were not associated with reduced healthcare utilization or healthcare charges 12. Previous studies looking at other cancers have suggested that earlier referrals are more likely to be associated with lower health care utilization and lower healthcare costs at the end of life, suggesting that the timing of PC referral may be crucial 13,14. It is poorly understood how the timing of PC consultation influences clinical, health, and economic outcomes in a US population of pancreatic cancer patients. With this study, we assess the association of early PC consultations on healthcare utilization and charges of care for patients with pancreatic cancer in the US.
MATERIALS AND METHODS
Study Population
We have previously reported details on our study cohort 12. Briefly, we used the National Cancer Institute Surveillance, Epidemiology, and End Results (SEER)-Medicare linked database for this analysis. SEER-Medicare database is a linkage of patient records from the SEER registries with Medicare claims 15. SEER is a national US cancer program, and Medicare claim files include sociodemographic, tumor specific, clinical, and treatment information as well as diagnostic and procedure details for linked patients aged 65 or more. Medicare files contain medical claims from outpatient and inpatient care. We used claims from the following files: 1) Medical Provider Analysis and Review (MEDPAR) file which is a 100% utilization file with a unique record for each inpatient hospitalization covered under Medicare part A, 2) National Claims History (NCH)/Carrier Claims and 3) outpatient files.
We included Medicare beneficiaries with a new diagnosis of pancreatic cancer between 2000 and 2009. To include beneficiaries who received at least one year of Medicare benefits before analysis, we limited enrollment to age 66 years and above 12. As the objective of our study was to assess health care utilization close to the end of life, we limited the analysis to patients with known date of death 12. We used the International Classification of Diseases, ninth revision codes (ICD-9) to identify the procedures received during the hospital stay. Palliative care encounter was identified using ICD-9 palliative care encounter code V66.7 12,16. Only the first PC instance was included for patients with more than one PC encounter. The ‘early PC’ group included patients whose first PC encounter occurred within 30 days of diagnosis, and ‘late PC’ included patients whose first PC encounter occurred after that time point 17. Year of diagnosis, age, race, sex, marital status, and stage were assessed from the SEER-Medicare file.
Statistical Methods
We assessed the following measures of health care utilization: number of ED visits, ED associated charges, number of ICU admissions, mean length of stay in the ICU, and ICU associated charges. ED and ICU associated charges were calculated by taking a sum of all ED/ICU charges (respectively) for all patients in the group (early vs. late PC) and then dividing it by the number of patients in that group 12.
Chi-square tests and Student’s t-tests analyses were conducted to investigate differences in patient demographics and health care utilization between early and late PC groups. We then compared early and late PC groups for differences in health care utilization in the last 30 days of life for all patients. The overall survival of pancreatic cancer cases is poor, and in our cohort, a large proportion of PC encounters were offered towards the end of life. The health care utilization of patients with very poor survival is not expected to be modified by a PC encounter. Therefore, we performed an additional analysis to compare health care utilization between the two groups after limiting to patients with survival of more than 30 days.
Stepwise linear regression and stepwise quantile regression analyses were conducted to identify significant independent factors associated with outcomes, including the number of ED visits, ICU days, and ICU admissions, charges of care in ED, and ICU. Statistical analyses were performed with SAS version 9.4 (SAS Institute, Inc., Cary, NC). A p-value of < 0.05 as considered statistically significant.
Propensity Score (PS) Adjustment
The propensity score was derived from logistic regression using state, age, sex, race, site, grade, and stage of the disease to control for potential selection bias caused by the nonrandom assignment of early or late PC. We specifically used the inverse probability of treatment weight, in which the weights were calculated as the inverse of the PS 18–21. A subgroup analysis was performed for health care utilization in the last 30 days of life for all patients and patients with the survival of more than 30 days.
RESULTS
Patient cohort
Our cohort included 3166 pancreatic cancer patients that were associated with at least one PC encounter (Figure). In this cohort, 57% were female, 79% white, and 39% were 80 years of age or older. Early PC took place in 28% of patients, and 72% received late PC. Patients receiving early PC were more likely to be female (64% vs. 54%) and older (85+) (37% vs. 13%), but less likely to be married (36% vs. 54%) compared to patients receiving late PC (p<.001). After propensity adjustment, age and marital status remained statistically significant. Initial palliative care encounters occurred within 30 days from death 72% of the time. Baseline characteristics are summarized in table 1.
Figure.
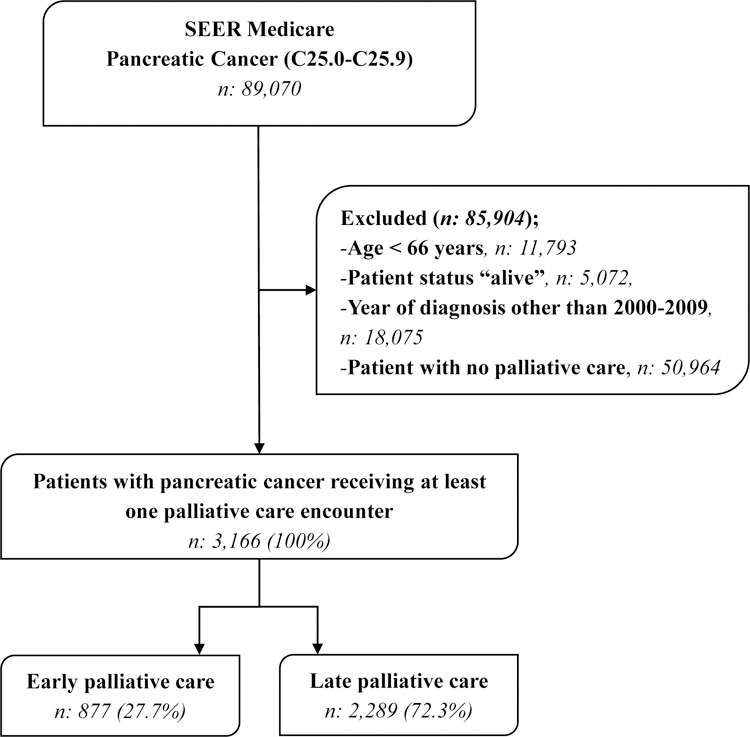
Flow diagram for the patient selection process
Table 1.
Patient demographics
| Characteristics | Early Palliative Care n(%) |
Late Palliative Care n (%) |
Total (%) | p-value | Propensity Score Adjustment p-value |
|---|---|---|---|---|---|
| Total patients | 877 (27.7) | 2,289 (72.3) | 3,166 (100.0) | ||
| Age (years) | <0.001 | 0.03 | |||
| 65–69 | 67 (7.6) | 427 (18.7) | 494 (15.6) | ||
| 70–74 | 144 (16.4) | 580 (25.3) | 724 (22.9) | ||
| 75–79 | 142 (16.2) | 564 (24.6) | 706 (22.3) | ||
| 80–84 | 196 (22.4) | 408 (17.8) | 604 (19.1) | ||
| 85+ | 328 (37.4) | 310 (13.5) | 638 (20.1) | ||
| Sex | <0.001 | NS | |||
| Male | 313 (35.7) | 1,046 (45.7) | 1,359 (42.9) | ||
| Female | 564 (64.3) | 1,243 (54.3) | 1,807 (57.1) | ||
| Race | NS | NS | |||
| White | 711 (81.1) | 1,788 (78.1) | 2,499 (78.9) | ||
| Black | 84 (9.6) | 237 (10.4) | 321 (10.1) | ||
| Asian | >37 (>4.2) | >119 (>5.1) | 164 (5.2) | ||
| Hispanic | 17 (1.9) | 63 (2.8) | 80 (2.5) | ||
| Native | <11 (<1.2) | <11 (<0.4) | 14 (0.4) | ||
| Other | 17 (1.9) | 71 (3.1) | 88 (2.8) | ||
| Marital status | <0.001 | <0.001 | |||
| Single | 70 (8.0) | 163 (7.1) | 233 (7.4) | ||
| Married | 323 (36.8) | 1,240 (54.2) | 1,563 (49.4) | ||
| Separated | <11 (<1.2) | 14 (0.6) | 22 (0.7) | ||
| Divorced | >73 (>8.3) | 147 (6.4) | 223 (7.0) | ||
| Widowed | 374 (42.6) | 654 (28.6) | 1,028 (32.5) | ||
| Unknown | 26 (3.0) | 71 (3.1) | 97 (3.0) | ||
| Stage at diagnosis | <0.001 | NS | |||
| Stage I | 45 (8.0) | 109 (7.2) | 154 (7.5) | ||
| Stage II | 65 (11.7) | 436 (28.9) | 501 (24.2) | ||
| Stage III | 31 (5.6) | 158 (10.5) | 189 (9.1) | ||
| Stage IV | 415 (74.4) | 804 (53.3) | 1,219 (59.0) | ||
| Time from diagnosis to palliative care consult | N/A | N/A | |||
| <30 days | 877 (100) | - | 877 (27.7) | ||
| 31 – 60 days | - | 491 (21.5) | 491 (15.5) | ||
| 61 – 90 days | - | 275 (12.0) | 275 (8.7) | ||
| 91 – 180 days | - | 473 (20.7) | 473 (14.9) | ||
| 181 – 365 days | - | 558 (24.4) | 558 (17.6) | ||
| 366+ days | - | 492 (21.5) | 492 (15.5) | ||
| Time from palliative care consult to death | <0.001 | <0.001 | |||
| <30 days | 598 (68.2) | 1,694 (74.0) | 2,292 (72.4) | ||
| 31 – 60 days | 105 (12.0) | 288 (12.5) | 393 (12.4) | ||
| 61 – 90 days | 39 (4.4) | 95 (4.2) | 134 (4.2) | ||
| 91 – 180 days | 66 (7.5) | 124 (5.4) | 190 (6.0) | ||
| 181 – 365 days | 29 (3.3) | 58 (2.5) | 87 (2.8) | ||
| 366+ days | 40 (4.6) | 30 (1.3) | 70 (2.2) | ||
NS: Not significant, N/A: Not available
Outcome measures
Patients receiving early PC had fewer ED visits (2.6 vs. 3.0 visits, p=.004) and lower total charges of ED care ($3158 vs. $3981, p<.001) compared to patients receiving late PC. On evaluating ICU care, patients receiving early PC had lower number of ICU admissions (0.82 vs. 0.98 visits, p=.006) and total charges of ICU care ($14,466 vs. $18,687, p=.01). There was no difference in the number of days in ICU between the two groups. After propensity score adjustment, the difference between the two groups in ED visits and the charges of ED care remained statistically significant, but ICU admissions and ICU charges did not (table 2A).
Table 2.
Health care utilization
| Early Palliative Care n (%) |
Late Palliative Care n (%) |
Total (%) | p-value | Propensity Score Adjustment p-value |
|
|---|---|---|---|---|---|
| A. Health care utilization for all patients | |||||
| Total patients | 861 (98.2) | 2,210 (96.5) | 3,071 (97.0) | ||
| Number of ED Visits | 2.59 +/− 2.77 | 2.99 +/− 2.86 | .004 | .021 | |
| Charge of care in ED ($) | 3,158 +/− 4,089 | 3,981 +/− 5,224 | <.001 | .003 | |
| Number of ICU admissions | 0.82 +/− 1.38 | 0.98 +/− 1.51 | .006 | NS | |
| Number of days in ICU | 3.92 +/− 8.74 | 4.31 +/− 9.32 | NS | NS | |
| Charge of care in ICU ($) | 14,466 +/− 40,295 | 18,687 +/− 50,833 | .01 | NS | |
| B. Health care utilization in the last 30 days of life for all patients | |||||
| Total patients | 639 (72.8) | 1,608 (70.2) | 2,247 (71.0) | ||
| Number of ED Visits | 0.92 +/− 0.62 | 0.95 +/− 0.62 | NS | NS | |
| Charge of care in ED ($) | 1,401 +/− 1,817 | 1,451 +/− 2,188 | NS | NS | |
| Number of ICU admissions | 0.29 +/− 0.50 | 0.25 +/− 0.48 | NS | NS | |
| Number of days in ICU | 1.19 +/− 2.95 | 1.04 +/− 2.87 | NS | NS | |
| Charge of care in ICU ($) | 5,149 +/− 15,456 | 5,598 +/− 20,454 | NS | NS | |
| C. Health care utilization for patients with survival >30 days | |||||
| Total patients | 503 (57.4) | 2,210 (96.5) | 2,713 (85.7) | ||
| Number of ED Visits | 2.65 +/− 2.81 | 2.99 +/− 2.86 | .01 | NS | |
| Charge of care in ED ($) | 3,110 +/− 3,948 | 3,981 +/− 5,224 | <.001 | .04 | |
| Number of ICU admissions | 0.76 +/− 1.39 | 0.98 +/− 1.51 | .002 | NS | |
| Number of days in ICU | 3.77 +/− 8.40 | 4.31 +/− 9.32 | NS | NS | |
| Charge of care in ICU ($) | 13,807 +/− 41,333 | 18,687 +/− 50,833 | .02 | NS | |
ED: Emergency department, ICU: Intensive care unit, NS: Not significant
We next analyzed health care utilization towards the end of life. There were no differences in the measures of ED and ICU utilization in the last 30 days of life between early PC and late PC before and after PS adjustment (table 2B).
After limiting the analysis to patients with survival greater than 30 days the number of ED visits (2.65 vs. 2.99 days, p=.01), charges of ED care ($3110 vs. $3981, p<.001), number ICU admissions (0.76 vs. 0.98 admissions, p=.002) and ICU charges of care ($13, 807 vs. $18,687, p=.02) were lower in the early PC group. After PS adjustment, the charges of ED care were lower in patients with early PC (table 2C).
Multivariable Linear and Quantile Regression Analysis
The multivariable stepwise linear analysis showed that patients receiving early PC had fewer ED visits compared to patients receiving late PC (p=.007). Early palliative care was not associated with differences in the number of ICU days and ICU admissions (Table 3A). The multivariable stepwise quantile analysis was performed for the charges of care. This analysis showed that the charges of care in ED for patients receiving early PC were $1,190 lower than patients receiving late PC (p=.018). There were no differences between the two groups for the charges of care in the ICU (Table 4A).
Table 3.
Results of stepwise linear regression model
| A. For all patients | |||
|---|---|---|---|
| Variables | Parameter Estimate | SE | p-value |
| Number of ED Visits | |||
| Early PC | −0.29 | 0.10 | .007 |
| Late PC | Ref | ||
| Number of days in ICU | |||
| Early PC | 0.26 | 0.42 | NS |
| Late PC | Ref | ||
| Number of ICU admissions | |||
| Early PC | −0.06 | 0.06 | NS |
| Late PC | Ref | ||
| B. For patients with survival > 30 days | |||
| Number of ED Visits | |||
| Early PC | −0.28 | 0.13 | .03 |
| Late PC | Ref | ||
| Number of days in ICU | |||
| Early PC | 0.42 | 0.55 | NS |
| Late PC | Ref | ||
| Number of ICU admissions | |||
| Early PC | −0.05 | 0.08 | NS |
| Late PC | Ref | ||
ED: Emergency department, ICU: Intensive care unit, NS: Not significant, Ref: Reference
Table 4.
Results of stepwise quantile regression model
| A. For all patients | |||
|---|---|---|---|
| Variables | Parameter Estimate | SE | p-value |
| Charge of care in ED ($) | |||
| Early PC | −1,190 | 503 | .018 |
| Late PC | Ref | ||
| Charge of care in ICU ($) | |||
| Early PC | −962 | 3,226 | NS |
| Late PC | Ref | ||
| B. For patients with survival > 30 days | |||
| Charge of care in ED ($) | |||
| Early PC | −647 | 239 | .006 |
| Late PC | Ref | ||
| Charge of care in ICU ($) | |||
| Early PC | −1,133 | 5,000 | NS |
| Late PC | Ref | ||
ED: Emergency department, ICU: Intensive care unit, NS: Not significant, Ref: Reference
For patients with survival >30 days, the multivariable stepwise linear analysis similarly showed that patients receiving early PC had fewer ED visits (p=.03). Early palliative care was not associated with differences in the number of ICU days and ICU admissions (Table 3B). In multivariable stepwise quantile analysis, the charges of ED care was $647 lower for those with early palliative care (p=.006) (Table 4B).
DISCUSSION
This study found that an early PC encounter, as opposed to late, in the pancreatic cancer disease course, is associated with fewer ED visits and lower ED related charges. This difference persisted after propensity score adjustment and multivariable analysis. These findings help understand the effect of the timing of PC encounters on health care utilization using Medicare claims data and provides essential insights into its role for pancreatic cancer.
Our group previously studied the trends of PC use in a US Medicare population of pancreatic cancer patients 12. We found that the proportion of patients receiving PC services increased significantly in recent years from 1.8% to 7.8%, and patients receiving PC were more likely to be female, Asian, and older age compared to patients without PC. However, most referrals were offered very close to the end of life. The number of ED visits in the last 30 days of life and the overall charges of ED care were significantly higher for patients receiving PC 12. This could reflect a referral bias where PC consults were offered to patients with high symptom burden and as referrals were placed very close to the end of life, PC referrals were unable to modify this outcome. We built on these findings to study the effect of early PC on health care utilization and have shown that early PC is associated with lower ED utilization.
Our study builds on earlier work evaluating the impact of PC interventions and health care utilization 13,22,23. Jang et al. performed a retrospective population-based cohort study using administrative data in patients with pancreatic cancer 22. In this study, PC encounters were associated with less aggressive care towards the end of life, including chemotherapy, ICU admissions, multiple ED visits, and hospital admissions. Our study contributes to the body of literature on the impact of early PC by evaluating a larger cohort of patients with pancreatic cancer. Additionally, we used propensity score adjustment to evaluate health care utilization and charges of care throughout the disease course as well as towards the end of life to minimize the effect of confounding variables.
This study supports oncology societies’ clinical guidelines, which recommend that patients with advanced cancer receive early PC referrals 24–26. There are several screening tools to identify patients likely to benefit from palliative care, including the Surprise Question (SQ) and the NECPAL CCOMS-ICO (NECPAL), but the SQ and the NECPAL tools are still underutilized. The surprise question, “Would you be surprised if the patient died in the next year?” has been shown as a simple, effective, and prognostic tool to improve the end of life care for cancer patients 27. Clinicians can use these tools to identify patients who benefit from palliative care and to design appropriate advance care planning 28,29. However, the impact of these tools in reducing end of life health care utilization needs to be studied. Previous studies have demonstrated an impact on the health and clinical outcomes of early PC for patients with advanced cancer. Temel et al. showed in a randomized controlled trial for lung cancer that an early PC intervention resulted in improved quality of life, reduced depressive symptoms, less aggressive end-of-life care, including reduced chemotherapy use and longer hospice care, all while improving overall survival 7. This single-center, non-blinded study focused solely on advanced lung cancer patients, limiting the generalizability to the general population or pancreatic cancer patients. Triplett et al. later expanded on this work with a matched retrospective cohort study of over 6,000 Medicare beneficiaries with advanced prostate, breast, lung, or colorectal cancer 14. They showed that palliative care was associated with reduced rates of hospitalization, invasive procedures, and chemotherapy administration. The earlier PC consultation was initiated, the larger the absolute reduction in healthcare utilization. This population-based study showed that the benefits of PC could be generalized to other cancer types, but pancreatic cancer remained understudied. Our study builds on these findings by evaluating the efficacy of early PC for pancreatic cancer and contributes to our understanding of the role of early PC in the real world.
Few studies have directly evaluated the impact of PC in pancreatic cancer. Jang et al. conducted the first population-based cohort study of over 5,000 patients in Ontario, Canada. Investigators found that pancreatic cancer patients with PC encounters had less aggressive care near the end of life, including less frequent chemotherapy administration, ICU admissions, ED visits, and hospitalizations 22. This study focused on the intensity of the PC intervention as defined by the number of PC visits and the rate of PC visits per month. However, it made no distinction as to whether the intervention was early or late in the disease process. Maltoni et al. later conducted the first randomized controlled trial of 207 patients with pancreatic cancer across 21 Italian medical centers and found that early systematic PC, compared to on-demand PC, resulted in improved quality of life, higher hospice service utilization and reduced chemotherapy use in the last 30 days of life 30,31. For all patients and those with the last 30 days of life, they found no significant difference in the frequency of hospitalization or the number of ED visits. Our work helps further defines the role of PC in pancreatic cancer and demonstrates the effect of timing of that care in a US-based population.
This study has limitations that are expected from a medical claims database analysis. We only included patients older than or equal to 66 years of age, but as the average age at diagnosis for pancreatic cancer is in the 70s, the results can be generalized to the greater population. We used Medicare claims to identify our cohort of PC patients. PC encounters without an associated claim could not be captured; however, the ICD for palliative care used in this study has been validated in prior studies with high specificity 32,33. We could not evaluate the nature or quality of the PC intervention from a claims database. However, studies of medical claims have an important role in health care research and have an established tool to study real-world medical practice. We performed propensity score adjustment to account for measurable variables associated with PC referrals, but it is not possible to adjust for factors not measured by a claims database. We did not analyze hospice claims and therefore cannot assess the effect of early palliative care on hospice utilization. Given the retrospective study design, we cannot make claims as to the causal relationship between PC and end-of-life healthcare utilization. Randomized controlled trials are needed to expand on this work.
CONCLUSIONS
Early palliative care referrals are associated with lower ED visits and charges. This study provides a perspective on the real-world benefit of early palliative care in US patients with pancreatic cancer and supports the ASCO guidelines that the majority of patients with advanced malignancies should receive early palliative care.
Research Support:
NCI Cancer Center Support Grant to UT Southwestern Medical Center (5P30CA142543-07) to MSB. The research reported in this publication was supported by the National Center for Advancing Translational Sciences of the National Institutes of Health under Award Number UL1TR001105. MSB is a Designated Dedman Family Scholar in Clinical Care.
Abbreviations:
- PC
Palliative care
- ED
Emergency department
- ICU
Intensive care unit
- SEER
Surveillance, Epidemiology, and End Results
- MEDPAR
Medical Provider Analysis and Review
Footnotes
Prior presentation: This manuscript has been presented in abstract form at the 2018 American Society of Clinical Oncology meeting in Chicago, IL.
Conflict of Interest: None
REFERENCES
- 1.Bilimoria KY, Bentrem DJ, Ko CY, et al. Validation of the 6th edition AJCC Pancreatic Cancer Staging System: report from the National Cancer Database. Cancer. 2007;110(4):738–744. [DOI] [PubMed] [Google Scholar]
- 2.Lee V, Cheng H, Li G, et al. Quality of life in patients with pancreatic cancer. JOP : Journal of the pancreas. 2012;13(2):182–184. [PubMed] [Google Scholar]
- 3.Torgerson S, Wiebe LA. Supportive care of the patient with advanced pancreatic cancer. Oncology (Williston Park, NY). 2013;27(3):183–190. [PubMed] [Google Scholar]
- 4.Davis MP. Integrating palliative medicine into an oncology practice. Am J Hosp Palliat Care. 2005;22(6):447–456. [DOI] [PubMed] [Google Scholar]
- 5.Bakitas M, Lyons KD, Hegel MT, et al. Effects of a palliative care intervention on clinical outcomes in patients with advanced cancer: the Project ENABLE II randomized controlled trial. JAMA. 2009;302(7):741–749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zimmermann C, Swami N, Krzyzanowska M, et al. Early palliative care for patients with advanced cancer: a cluster-randomised controlled trial. Lancet. 2014;383(9930):1721–1730. [DOI] [PubMed] [Google Scholar]
- 7.Temel JS, Greer JA, Muzikansky A, et al. Early palliative care for patients with metastatic non-small-cell lung cancer. The New England journal of medicine. 2010;363(8):733–742. [DOI] [PubMed] [Google Scholar]
- 8.Temel JS, Greer JA, El-Jawahri A, et al. Effects of Early Integrated Palliative Care in Patients With Lung and GI Cancer: A Randomized Clinical Trial. J Clin Oncol. 2017;35(8):834–841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Greer JA, Pirl WF, Jackson VA, et al. Effect of early palliative care on chemotherapy use and end-of-life care in patients with metastatic non-small-cell lung cancer. J Clin Oncol. 2012;30(4):394–400. [DOI] [PubMed] [Google Scholar]
- 10.Greer JA, Tramontano AC, McMahon PM, et al. Cost Analysis of a Randomized Trial of Early Palliative Care in Patients with Metastatic Nonsmall-Cell Lung Cancer. Journal of palliative medicine. 2016;19(8):842–848. [DOI] [PubMed] [Google Scholar]
- 11.Ferrell BR, Temel JS, Temin S, et al. Integration of Palliative Care Into Standard Oncology Care: ASCO Clinical Practice Guideline Update Summary. Journal of oncology practice. 2017;13(2):119–121. [DOI] [PubMed] [Google Scholar]
- 12.Bhulani N, Gupta A, Gao A, et al. Palliative care and end-of-life health care utilization in elderly patients with pancreatic cancer. Journal of Gastrointestinal Oncology. 2018;9(3):495–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Scibetta C, Kerr K, McGuire J, et al. The Costs of Waiting: Implications of the Timing of Palliative Care Consultation among a Cohort of Decedents at a Comprehensive Cancer Center. Journal of palliative medicine. 2016;19(1):69–75. [DOI] [PubMed] [Google Scholar]
- 14.Triplett DP, LeBrett WG, Bryant AK, et al. Effect of Palliative Care on Aggressiveness of End-of-Life Care Among Patients With Advanced Cancer. Journal of oncology practice. 2017;13(9):e760–e769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Institute NC. SEER-Medicare: How the SEER & Medicare Data are Linked. https://healthcaredelivery.cancer.gov/seermedicare/overview/linked.html. Published 2017. Accessed 09/29/2017, 2017.
- 16.Capello CF, Meier DE, Cassel CK. Payment code for hospital-based palliative care: help or hindrance? J Palliat Med. 1998;1(2):155–163. [DOI] [PubMed] [Google Scholar]
- 17.Back AL, Park ER, Greer JA, et al. Clinician roles in early integrated palliative care for patients with advanced cancer: a qualitative study. Journal of palliative medicine. 2014;17(11):1244–1248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kurth T, Walker AM, Glynn RJ, et al. Results of multivariable logistic regression, propensity matching, propensity adjustment, and propensity-based weighting under conditions of nonuniform effect. American journal of epidemiology. 2006;163(3):262–270. [DOI] [PubMed] [Google Scholar]
- 19.Spreeuwenberg MD, Bartak A, Croon MA, et al. The multiple propensity score as control for bias in the comparison of more than two treatment arms: an introduction from a case study in mental health. Medical care. 2010:166–174. [DOI] [PubMed]
- 20.Rubin DB, Thomas N. Matching using estimated propensity scores: relating theory to practice. Biometrics. 1996:249–264. [PubMed]
- 21.D’Agostino RB Jr. Propensity score methods for bias reduction in the comparison of a treatment to a non-randomized control group. Statistics in medicine. 1998;17(19):2265–2281. [DOI] [PubMed] [Google Scholar]
- 22.Jang RW, Krzyzanowska MK, Zimmermann C, et al. Palliative care and the aggressiveness of end-of-life care in patients with advanced pancreatic cancer. J Natl Cancer Inst. 2015;107(3). [DOI] [PubMed] [Google Scholar]
- 23.Habibi A, Wu SP, Gorovets D, et al. Early Palliative Care for Patients With Brain Metastases Decreases Inpatient Admissions and Need for Imaging Studies. Am J Hosp Palliat Care. 2018:1049909118765405. [DOI] [PubMed]
- 24.Sohal DPS, Mangu PB, Khorana AA, et al. Metastatic Pancreatic Cancer: American Society of Clinical Oncology Clinical Practice Guideline. 2016.
- 25.Balaban EP, Mangu PB, Khorana AA, et al. Locally Advanced, Unresectable Pancreatic Cancer: American Society of Clinical Oncology Clinical Practice Guideline. Journal of Clinical Oncology. 2016;34(22):2654–2668. [DOI] [PubMed] [Google Scholar]
- 26.Ferrell BR, Temel JS, Temin S, et al. Integration of Palliative Care Into Standard Oncology Care: American Society of Clinical Oncology Clinical Practice Guideline Update. J Clin Oncol. 2017;35(1):96–112. [DOI] [PubMed] [Google Scholar]
- 27.Moss AH, Lunney JR, Culp S, et al. Prognostic significance of the “surprise” question in cancer patients. Journal of palliative medicine. 2010;13(7):837–840. [DOI] [PubMed] [Google Scholar]
- 28.Gómez-Batiste X, Martínez-Muñoz M, Blay C, et al. Utility of the NECPAL CCOMS-ICO© tool and the Surprise Question as screening tools for early palliative care and to predict mortality in patients with advanced chronic conditions: A cohort study. Palliative medicine. 2017;31(8):754–763. [DOI] [PubMed] [Google Scholar]
- 29.Moroni M, Zocchi D, Bolognesi D, et al. The ‘surprise’question in advanced cancer patients: a prospective study among general practitioners. Palliative medicine. 2014;28(7):959–964. [DOI] [PubMed] [Google Scholar]
- 30.Maltoni M, Scarpi E, Dall’Agata M, et al. Systematic versus on-demand early palliative care: A randomised clinical trial assessing quality of care and treatment aggressiveness near the end of life. Eur J Cancer. 2016;69:110–118. [DOI] [PubMed] [Google Scholar]
- 31.Maltoni M, Scarpi E, Dall’Agata M, et al. Systematic versus on-demand early palliative care: results from a multicentre, randomised clinical trial. Eur J Cancer. 2016;65:61–68. [DOI] [PubMed] [Google Scholar]
- 32.Feder SL, Redeker NS, Jeon S, et al. Validation of the ICD-9 Diagnostic Code for Palliative Care in Patients Hospitalized With Heart Failure Within the Veterans Health Administration. Am J Hosp Palliat Care. 2018;35(7):959–965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hua M, Li G, Clancy C, et al. Validation of the V66.7 Code for Palliative Care Consultation in a Single Academic Medical Center. J Palliat Med. 2017;20(4):372–377. [DOI] [PMC free article] [PubMed] [Google Scholar]


