Abstract
Objective:
In patients with stable coronary artery disease (CAD), the risk of major adverse cardiovascular events (MACE) remains elevated despite treatment. The role of microvascular dysfunction on MACE beyond traditional risk indicators and inflammation is not well established. We examined whether peripheral microvascular dysfunction is associated with MACE in patients with CAD.
Approach and Results:
Microvascular function was measured with the Reactive Hyperemia Index (RHI) using digital peripheral arterial tonometry in 546 patients with CAD, who were followed 7 years for incident MACE. The primary endpoint included cardiovascular death or myocardial infarction (MI); the secondary endpoint included cardiovascular death, MI or heart failure hospitalization. Hazard models for competing risk were used to estimate the association between RHI and MACE adjusting for age, sex, race, traditional risk factors, medications, and CAD severity. We also examined the association of baseline interleukin-6, C-reactive protein, monocyte chemoattractant protein-1 and matrix metallopeptidase-9 with RHI.
Mean age was 62 ± 9 years. Mean RHI was 2.1 ± 0.63. After adjustment, for each 1-SD decrease in RHI, there was a 40% increase in the primary endpoint (HR 1.4, 95% CI: 1.1–1.9, p=0.01) and a similar increase in the secondary endpoint (HR 1.3, 95% CI: 1.1–1.7, p=0.006). Inflammatory biomarker levels were associated with greater RHI impairment (p<0.05) but did not affect the relationship between RHI and MACE.
Conclusions:
Peripheral microvascular dysfunction is associated with increased risk of MACE in patients with stable CAD, implicating the role of microvascular disease in the pathogenesis of adverse outcomes in CAD patients.
Keywords: Microvascular function, reactive hyperemia index, cardiovascular outcomes, coronary artery disease
Graphical abstract
Introduction
In patients with stable coronary artery disease (CAD), the risk of recurrent cardiovascular events remains elevated despite optimal medical therapy and revascularization.1–3 Current treatment approaches target traditional cardiovascular risk factors such as dyslipidemia, diabetes and hypertension, combined with coronary revascularization. However, considerable residual risk of cardiovascular morbidity and mortality remains in this population.4–8
Abnormalities in the function of the vascular endothelium occurs at the early stages of atherosclerosis and has been linked with multiple traditional cardiovascular risk factors.9, 10 Persistent impairment in vascular function after optimization of medical therapy is a predictor of adverse cardiac outcomes in individuals with and without CAD.11–13 Most studies, however, examined macrovascular endothelial function as measured by flow-mediated vasodilation,14 rather than microvascular function.
At a microvascular level, the use of peripheral arterial tonometry (PAT) to measure arterial pulsatile changes in the fingertip during reactive hyperemia, has emerged as a non-invasive method to evaluate peripheral microvascular function.15–17 The reactive hyperemia index (RHI), obtained with this device, provides an estimate of peripheral microvascular vasodilator capacity and has been linked to a wide range of traditional cardiovascular risk factors including obesity, hyperlipidemia, and hypertension.17 It has also been shown to predict cardiovascular events in individuals without known CAD, those referred for evaluation to rule out CAD,18, 19 or those with heart failure.20–22 Less is known about whether RHI predicts adverse outcomes in stable patients with CAD. A low RHI was related to adverse cardiac events in two selected samples of Japanese patients with CAD,23, 24 but whether these findings can be generalized to a broader patient population with stable CAD remains unclear.
Augmentation of the pulse amplitude after reactive hyperemia in the microcirculation reflects both changes in microvascular flow and in diameter. This phenomenon is only partly dependent on nitric oxide,25 and is affected by other mediators such as inflammatory processes, which may contribute to the progression of atherosclerosis and recurrent CAD events. Elevated serum inflammatory biomarkers such as interleukin-6 (IL-6), monocyte chemoattractant protein-1 (MCP-1), matrix metallopeptidase-9 (MMP-9), and high sensitivity C-reactive protein (CRP) have all been associated with increased risk of adverse cardiovascular outcomes in individuals with CAD26–29 as well as with microvascular disease and endothelial function.30, 31 Understanding the link between impaired microvascular function and inflammation may help to elucidate the potential mechanisms that can lead to adverse cardiac outcomes in patients with CAD with impaired microvascular function.
In this study, we assessed whether impairment in peripheral microvascular vasodilator function, as determined by the RHI, is associated with risk of subsequent adverse cardiovascular outcomes in patients with stable CAD, and whether this relationship is mediated by inflammation.
Methods
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Study population
The study population has been described previously in detail.32 We recruited 695 patients with clinically-confirmed CAD between June 2011 and August 2014 from hospitals and clinics affiliated with Emory University in Atlanta, Georgia into the Mental Stress Ischemic Prognosis Study. Patients were eligible if they were 30 to 79 years old and had a documented diagnosis of CAD, defined by any of the following: an abnormal coronary angiogram demonstrating evidence of atherosclerosis with at least luminal irregularities; previous percutaneous or surgical coronary revascularization; a previous myocardial infarction (MI), or a positive nuclear stress test. Patients were excluded if they had an acute coronary syndrome or decompensated heart failure in the previous 2 months, end stage renal disease, or severe, unstable psychiatric conditions that would affect study assessments, such as schizophrenia. Clinical information, including prior MI, cardiovascular risk factors, coronary angiography results, and current medications were obtained using standardized questionnaires and medical chart reviews. CAD severity was determined using the Gensini score based on angiographic data.33The research protocol was approved by the Emory University Institutional Review Board and all participants provided informed consent.
RHI Measurement
PAT was performed using the EndoPAT 2000 device (Itamar Medical Inc, Israel) to estimate peripheral microvascular function as previously described.16, 34–36 RHI reproducibility has been demonstrated before.37 All vasoactive medications were held for at least 24 hours prior to testing. Specially designed digital probes, comprised of inflatable latex air cuffs connected to an inflating device controlled by a computer algorithm, were placed on the middle finger of each subject’s hand to detect pulsatile volume changes in arterial blood flow induced by the pressure alterations in the cuff. The pulsatile volume changes were then transmitted to and recorded by the EndoPAT device; a decrease in digital arterial blood flow results in a decrease in the pulsatile amplitude. The standard protocol consists of a 5-minute baseline pre-occlusion measurement, followed by a blood pressure cuff inflation to 60 mmHg above the systolic blood pressure or at least 200 mmHg for 5 min to occlude arterial blood flow, resulting in a PAT tracing of zero. After 5 min, the cuff is deflated, and the PAT tracing is recorded for another 6 minutes. RHI is calculated using a computer algorithm which compares the ratio of the PAT signal post cuff release to the pre-occlusion signal of the tested arm and indexed to the contralateral control arm. Lower RHI values are indicative of impaired peripheral microvascular vasodilatory function.
Outcomes
Subjects were prospectively followed for major adverse cardiovascular events including cardiovascular death, MI, and heart failure hospitalization. Cardiovascular death was defined as death attributable to an ischemic cardiovascular cause (fatal MI), cardiac arrhythmia or cardiac arrest, or heart failure. Outcome event data were collected through clinic follow up visits at year 1 and 2, and phone calls at year 3 and 5 as well as medical chart reviews and querying of the Social Security Death Index. All events were independently adjudicated by experienced cardiologists blinded to other study data. The primary outcome of the study was a combined end point of cardiovascular death and MI. A secondary composite outcome also included hospitalization for heart failure.
Inflammatory measures
Inflammatory biomarkers were measured from venous blood samples collected at rest prior to vascular function testing and included IL-6, MCP-1, MMP-9, and CRP. These biomarkers have been previously shown to be associated with cardiovascular risk and with vascular dysfunction.38, 39 Venous blood was collected in ice-cooled citrate tubes and immediately centrifuged at 4°C; obtained plasma was frozen at −70°C until further processing. We employed the MesoScale system (Meso Scale Diagnostics Rockville, Maryland) using the SECTOR Imager 2400 to measure IL-6, MCP-1, MMP-9, and CRP according to the protocols supplied by the manufacturer. The Mesoscale multiplex assay system uses electrochemiluminescence for high sensitivity and broad dynamic range. All biomarkers were in the range of detection. The inter-assay coefficient of variations for midpoint standards were 5.78% for IL-6, 4.99% for MCP-1, 9.38% for MMP-9, and 3.06% for CRP. The intra-assay coefficients of variation were 3.29% for IL-6, 3.45% for MCP-1, 5.95% for MMP-9, and 2.33% for CRP.
Statistical analyses
We present normally distributed data as mean ± standard deviation (SD) and non-normally distributed data as median and interquartile range (IQR). We used linear regression to examine the unadjusted associations between RHI (expressed as standardized units) and various covariates including demographic factors, lifestyle and clinical risk factors, and CAD severity. We also used linear regression to determine the unadjusted and adjusted association between RHI and inflammatory biomarkers, which were log-transformed and standardized via Z-score. Models adjusted for sociodemographic factors, including age, sex, race, and income, and lifestyle and clinical risk factors including smoking, body mass index (BMI), diabetes, hypertension, hyperlipidemia, previous MI, and history of heart failure, as well as angiographic CAD severity using Gensini score.
To describe the association between RHI and adverse cardiovascular events, RHI was examined as a continuous standardized variable (per 1-SD change) in Fine & Gray’s models for competing risks defined as non-cardiovascular deaths, to derive subdistribution hazard ratios (sHR). To illustrate the results, the incidence of adverse cardiovascular events was examined based on tertiles of RHI. All analyses were conducted before and after adjusting for possible confounding factors considered a priori and included in sequential models after the unadjusted model (Model 1). First, we added demographic variables including age, sex, and race, and income (Model 2), followed by lifestyle and clinical risk factors known to affect vascular function (smoking, BMI, diabetes, hypertension, hyperlipidemia, previous MI, and heart failure), CAD severity (Gensini score) with a dummy variable to account for those without angiography data, and medication use (aspirin, clopidogrel, angiotensin converting enzyme inhibitors or angiotensin receptor blockers, beta-blockers and statins) (Model 3). To address possible model overfitting, in sensitivity analysis (Model 4), we used a propensity score to account for all the covariates added in Model 3. In a final model, we adjusted for inflammatory biomarkers (Model 5).40, 41 All statistical analyses were conducted using SAS version 9.4 (SAS Institute, Cary, NC).
Results
Patient Characteristics
Of the 695 patients with CAD recruited into the study, 546 (79%) completed the RHI protocol and therefore were included in the present analysis. Those who were not included had similar characteristics to the included group, including similar distribution of demographic factors and risk factors. The mean age was 62 ± 9 years; 25% were women, and 30% were Black (Table 1). Overall, 37% of the cohort had a history of MI; 77% had previous revascularization, and 23% had a history of heart failure. RHI was normally distributed, with a mean (SD) of 2.1 (0.63). Relationship of RHI with baseline patient characteristics and inflammatory biomarkers
Table 1.
Baseline Demographics and Clinical Characteristics of the Study Population (N = 546)
| Demographics | |
| Age, years, mean (SD) | 62.4 (9.3) |
| African American, n (%) | 164 (30.0) |
| Female, n (%) | 138 (25.3) |
| Income 20,000/year, n (%) | 86 (15.9) |
| Cardiovascular Risk Factors | |
| Body Mass Index, kg/m2, mean (SD) | 29.9 (5.5) |
| History of Ever Smoking, n (%) | 82 (15.1) |
| Diabetes, n (%) | 170 (31.1) |
| Hypertension, n (%) | 421 (77.1) |
| Dyslipidemia, n (%) | 453 (83.0) |
| Medications | |
| Clopidogrel, n (%) | 192 (35.2) |
| Aspirin, n (%) | 462 (84.8) |
| Beta Blocker, n (%) | 412 (75.6) |
| ACE Inhibitors/ARBs, n (%) | 253 (46.6) |
| Statins, n (%) | 472 (86.9) |
| Clinical Characteristics | |
| Previous Myocardial Infarction, n (%) | 203 (37.2) |
| Heart Failure History, n (%) | 123 (22.7) |
| Revascularization, n (%) | 421 (77.1) |
| Ejection Fraction, mean (SD) | 67.7 (8.4) |
| Gensini Score, median (IQR) | 25.0 (9.5, 60.0) |
| Reactive Hyperemia Index, mean (SD) | 2.1 (0.63) |
Abbreviations: ACE=angiotensin converting enzyme; ARB=angiotensin receptor blocker; IQR= interquartile range; SD= standard deviation.
Statistical tests: Categorical variables: Chi-square; Continuous variables: Student’s T-test or Wilcoxon-Mann Whitley U test when appropriate
RHI was negatively associated with BMI and history of diabetes and positively associated with prior revascularization and statin use (Table 2). There was no significant association between RHI and age, sex, race, or CAD severity indicators (ejection fraction and Gensini score). RHI was negatively associated with IL-6, CRP, and MMP-9 but not MCP-1 levels in unadjusted models with RHI as predictor and inflammatory biomarkers as the outcome variable (Table 3). All associations, except MMP-9, became attenuated with adjustments for traditional risk factors and comorbidities.
Table 2.
Factors Associated with Reactive Hyperemia Index (Unadjusted Analysis)
| Reactive Hyperemia Index | ||
|---|---|---|
| Β* (95% CI) | p-value | |
| Age, years | −0.001 (−0.007, 0.005) | 0.72 |
| African American | −0.05 (−0.17, 0.06) | 0.38 |
| Female | 0.005 (−0.12, 0.13) | 0.94 |
| Income below Poverty (<$20K/year) | −0.14 (−0.28,0.006) | 0.060 |
| Body Mass Index | −0.01 (−0.02, −0.001) | 0.029 |
| Ever Smoking | 0.002 (−0.15, 0.15) | 0.98 |
| Diabetes | −0.11 (−0.22, 0.003) | 0.055 |
| Hypertension | 0.03 (−0.09, 0.16) | 0.62 |
| Dyslipidemia | −0.04 (−0.18, 0.10) | 0.55 |
| Prior Myocardial Infarction | 0.07 (−0.04, 0.18) | 0.23 |
| Heart Failure History | −0.03 (−0.16, 0.09) | 0.61 |
| Prior Revascularization | 0.13 (0.01, 0.26) | 0.038 |
| Aspirin | 0.14 (−0.01, 0.28) | 0.068 |
| Clopidogrel | 0.11 (−0.004, 0.22) | 0.059 |
| Beta-Blocker | −0.04 (−0.16, 0.09) | 0.55 |
| ACEI/ARB | −0.003 (−0.11, 0.10) | 0.95 |
| Statins | 0.16 (0.002, 0.32) | 0.048 |
| Gensini Score | 0.0004 (−0.001, 0.001) | 0.41 |
Abbreviations: ACEI = Angiotensin-Converting Enzyme Inhibitor; ARB=Angiotensin Receptor Blocker; CI = Confidence Interval.
Beta-coefficient in bivariate analysis comparing each factor above to RHI expressed in standard deviation units
Table 3. Associations between Reactive Hyperemia Index (Predictor) and Circulating Inflammatory Markers (Outcomes).
Values for both inflammatory biomarkers and RHI are modeled in standard deviation units.
| B (95% CI) | p-value | |
|---|---|---|
| IL-6, pg/mL | ||
| Model 1 | −0.07 (−0.12, −0.01) | 0.01 |
| Model 2 | −0.07 (−0.12, −0.01) | 0.02 |
| Model 3 | −0.05 (−0.11, 0.002) | 0.06 |
| CRP, pg/mL | ||
| Model 1 | −0.07 (−0.12, −0.01) | 0.02 |
| Model 2 | −0.06 (−0.12, −0.01) | 0.02 |
| Model 3 | −0.05 (−0.10, 0.01) | 0.10 |
| MCP-1, pg/mL | ||
| Model 1 | −0.03 (−0.09, 0.02) | 0.28 |
| Model 2 | −0.02 (−0.08, 0.03) | 0.40 |
| Model 3 | −0.02 (−0.08, 0.04) | 0.53 |
| MMP-9, ng/mL | ||
| Model 1 | −0.06 (−0.12, −0.01) | 0.02 |
| Model 2 | −0.07 (−0.13, −0.02) | 0.01 |
| Model 3 | −0.07 (−0.13, −0.02) | 0.01 |
| Model 1: Unadjusted | ||
| Model 2: Adjusted for age, sex, race, and income | ||
| Model 3: Model 2 variables + clinical factors (hypertension, diabetes, dyslipidemia, smoking status, BMI, history of MI, history of heart failure, Gensini score, and medication use, including beta blockers, ACEI/ARBs and statins) | ||
Abbreviations: CRP= C-Reactive Protein; IL-6= Interleukin-6; MCP-1=Monocyte Chemoattractant Protein-1; MMP-9=Matrix Metallopeptidase
The B-coefficient expresses the difference (in standard deviation units) in inflammatory biomarkers per each standard deviation increase in RHI.
For each standard deviation decrease in RHI, there is a 0.07 standard deviation increase in IL-6.
Relationship of RHI with Adverse Cardiovascular Outcomes
Patients were followed up for a median (IQR) of 6.2 (5.6–6.7) years. A total of 87 patients had adverse cardiovascular events, including 30 cardiovascular deaths, 40 MIs, and 36 hospitalizations for heart failure. The mean RHI for patients who developed any adverse cardiac events was 1.9 (0.49), which was significantly lower than the RHI of those without adverse cardiac events, 2.1 (0.63), p=0.02.
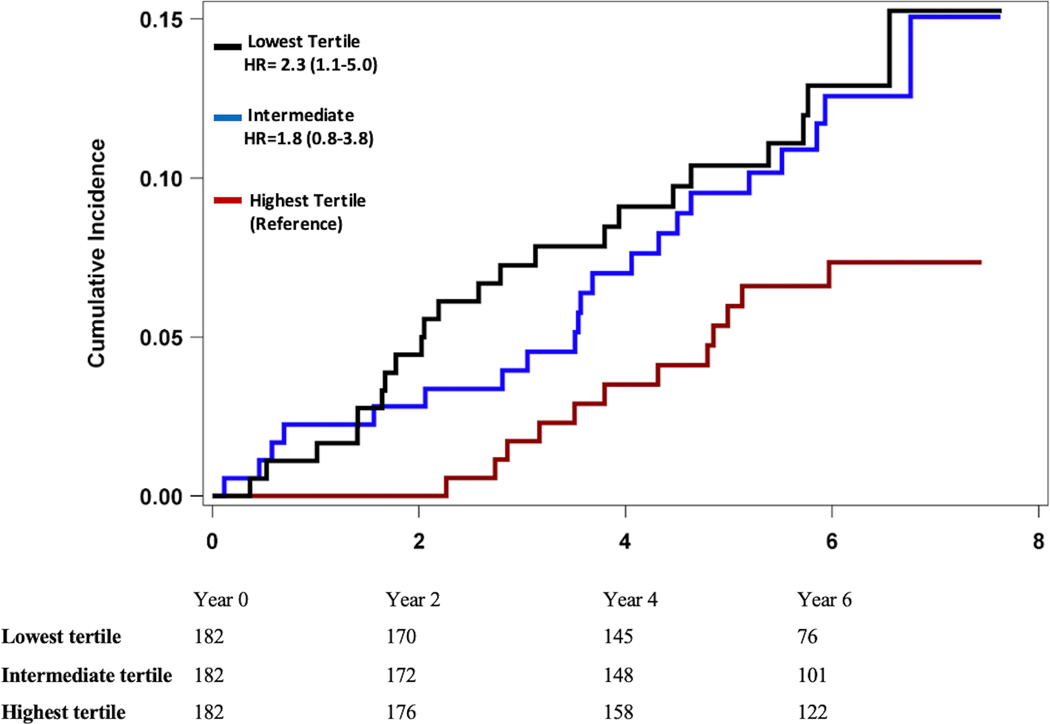
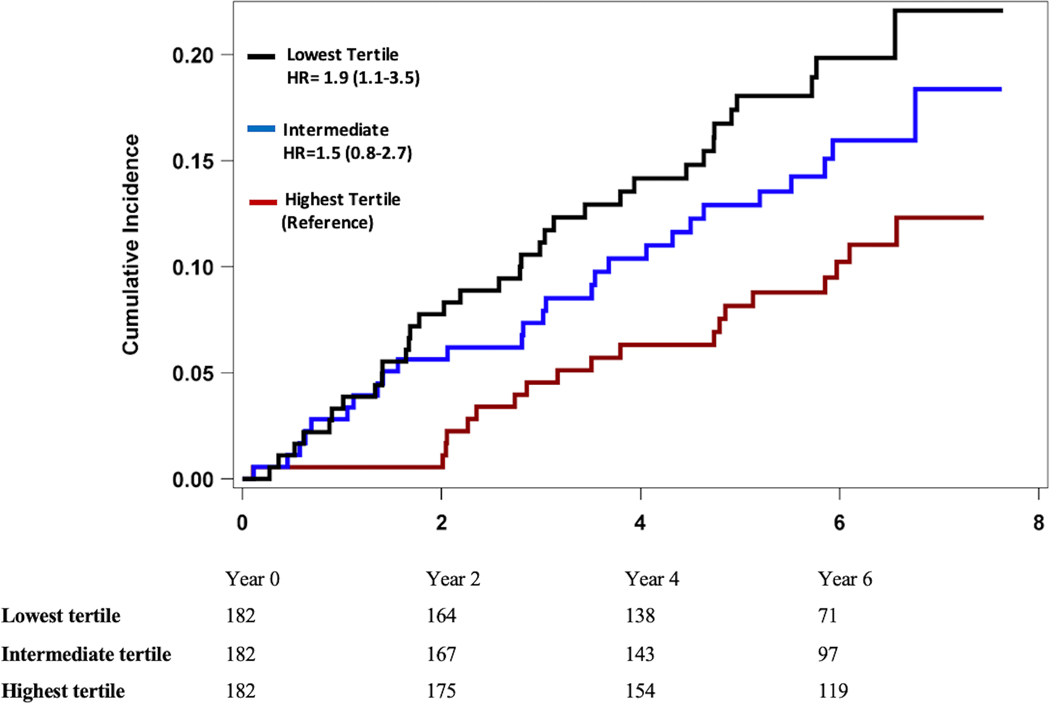
In Fine & Gray’s proportional sub-distribution models, a lower baseline RHI was associated with higher risk of adverse cardiovascular events (Table 4). For each standard deviation decrease in RHI, there was a 40% (95% CI 1.1–1.9) unadjusted increase in the hazard for the primary composite endpoint of cardiovascular death or MI (Model 1). When analyzed by tertiles of RHI values, those in the lowest tertile had >2 fold higher risk of incident cardiovascular death and MI (sHR 2.3, 95% CI 1.1–5.0) as well as incident cardiovascular death, MI, and heart failure hospitalizations (sHR 1.9, 95% CI 1.1–3.5) compared to those in the highest RHI tertile, Figure 1. In a receiver operating characteristic curve analysis, an RHI value of 1.75 was identified as the optimal cutoff to differentiate between individuals with high vs low risk of adverse coronary outcomes. These associations remained significant after adjustment for demographics factors, lifestyle and clinical risk factors, CAD severity, as well as medication use (sHR 1.4, 95% CI 1.1–1.9, Model 3). A similar association was found for the secondary endpoint of cardiovascular death, MI, or hospitalization for heart failure, with a 30% increase in the hazard of adverse cardiovascular events for every SD decrease in RHI after adjustment for covariates (sHR 1.3, 95% CI 1.1–1.7, Model 3). Additional adjustment for aspirin or P2Y12 inhibitors did not change these results (sHR 1.4, 95% CI 1.1–1.9, primary outcome and sHR 1.4, 95% CI 1.1–1.7 for secondary outcome). These were not included due to concern for overfitting the model. Similar results were found in sensitivity analyses using a propensity score adjusted model (Model 4), therefore ruling out concerns about model overfitting. Further adjustment for inflammatory biomarkers did not materially alter the relationship between RHI and the study endpoints (Model 5).
Table 4. Association between Digital Reactive Hyperemia Index (RHI) and Risk of Major Adverse Cardiovascular Events in Unadjusted and Adjusted Fine & Gray’s Proportional Sub-Distribution Hazards Models.
Subdistribution hazard ratios are expressed per standard deviation decrease in RHI.
| Primary Composite Endpoint (CV Death/MI) | Secondary Composite Endpoint (CV Death/MI/HF Hospitalization) | |||
|---|---|---|---|---|
| HR (95% CI) | p-value | HR (95% CI) | p-value | |
| Model 1 | 1.4 (1.1, 1.9) | 0.007 | 1.4 (1.1, 1.8) | 0.001 |
| Model 2 | 1.4 (1.1, 1.9) | 0.007 | 1.4 (1.1, 1.8) | 0.002 |
| Model 3 | 1.4 (1.1, 1.9) | 0.01 | 1.3 (1.1, 1.7) | 0.006 |
| Model 4 | 1.4 (1.1, 1.9) | 0.02 | 1.4 (1.1, 1.9) | 0.02 |
| Model 5 | 1.5 (1.1, 1.9) | 0.02 | 1.3 (1.1, 1.7) | 0.02 |
| Model 1: Unadjusted | ||||
| Model 2: Adjusted for age, sex, race, and income | ||||
| Model 3: Model 2 variables + clinical risk factors (hypertension, diabetes, dyslipidemia, smoking status, BMI, history of MI, history of heart failure, Gensini score, and medication use including beta blockers, ACEI/ARB and statins) | ||||
| Model 4: Model 2 variables + Propensity score including hypertension, diabetes, dyslipidemia, smoking status, BMI, history of MI, history of heart failure, Gensini score, and medication use (beta blockers, ACEI/ARBs and statins) | ||||
| Model 5: Model 3 variables + MMP-9 | ||||
Abbreviations: ACEI = angiotensin converting enzyme inhibitor; ARB = angiotensin receptor blocker; BMI = body mass index; C.I. = confidence intervals, CV = cardiovascular; HR = hazard ratio; HF = heart failure; MI = myocardial infarction.
Figure 1. Kaplan Meier Analysis of Major Adverse Cardiovascular Outcomes and Reactive Hyperemia Index by Tertiles (Unadjusted).
Panel A. Cumulative Incidence of Cardiovascular Death and Myocardial Infarction
Panel B. Cumulative Incidence of Cardiovascular Death, Myocardial Infarction, and Heart Failure Hospitalization
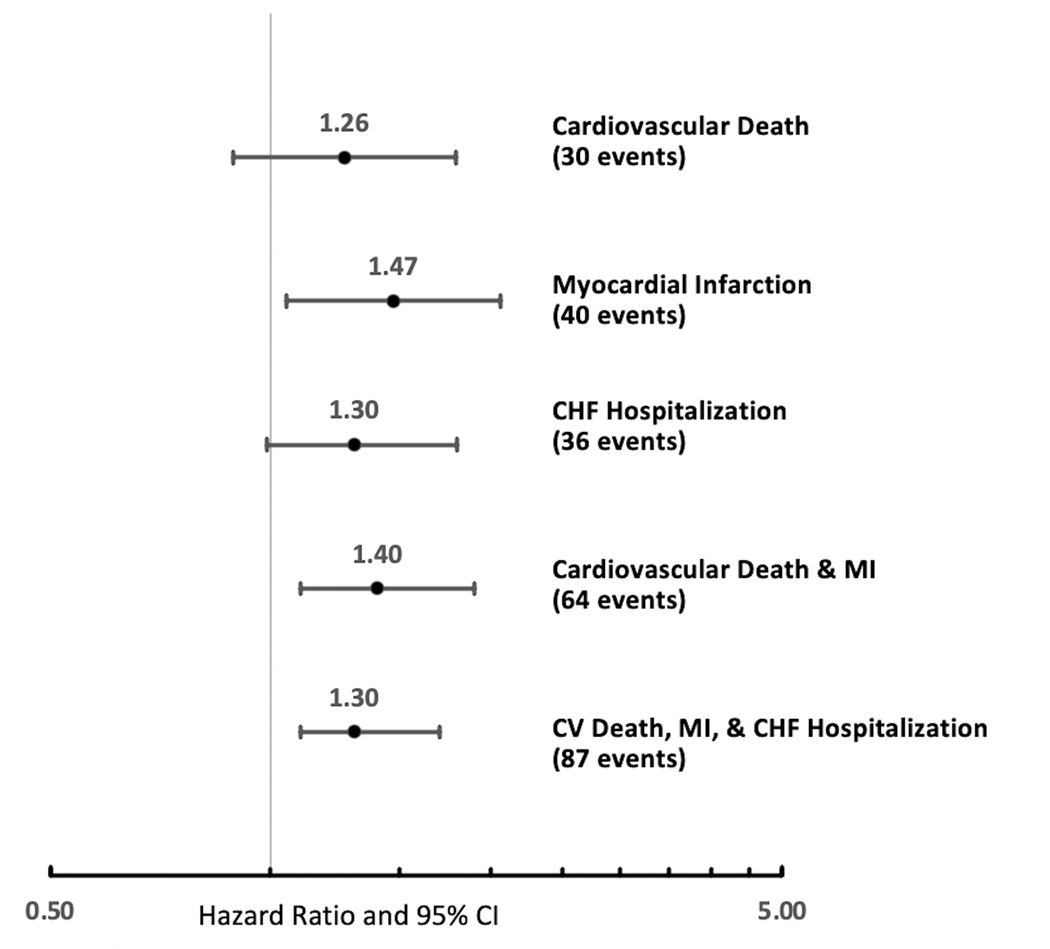
The relationship of RHI with each of the endpoint components is shown in Figure 2. Each standard deviation decrement in baseline RHI was associated with a 26% increase in risk of cardiovascular death, 47% increase in recurrent MI, and 30% increase in risk heart failure hospitalization.
Figure 2. Forest Plot for the Adjusted Association of Reactive Hyperemia Index with Individual Components of the Composite Cardiac Endpoints. Values Shown are Subdistribution Hazard Ratios and 95% Confidence Intervals per Standard Deviation Decrease in Reactive Hyperemia Index.
Estimates are adjusted for age, sex, race, income, hypertension, diabetes, dyslipidemia, smoking status, BMI, history of MI, heart failure, Gensini score, and medication use (beta blockers, angiotensin converting enzyme inhibitors/angiotensin receptor blockers or statins)
In the present study, we found that impaired peripheral microvascular function estimated as low RHI in patients with stable CAD is associated with increased risk of recurrent adverse cardiac events, including cardiovascular death, MI, and heart failure hospitalizations. The association between lower RHI and adverse events was independent of traditional risk factors and CAD severity. Although impaired microvascular function was associated with higher levels of inflammatory biomarkers, the relationship between RHI and cardiovascular events was independent of systemic inflammation. These findings suggest the potential role of microvascular dysfunction as an independent risk factor in recurrent adverse outcomes in stable CAD beyond the traditional cardiovascular risk indicators and inflammation.
Our results are in line with prior reports that found peripheral microvascular dysfunction, measured as a low RHI, to be an independent predictor of cardiovascular events in individuals without a prior diagnosis of CAD referred for evaluation for cardiac symptoms.18, 19, 42, 43 For example, Rubinshtein et al.18 found that lower RHI was prognostic for adverse cardiovascular outcomes in patients with chest pain without obstructive CAD. Whether RHI is related to adverse outcomes among patients with stable CAD has received less attention, apart from two small studies in Japan.23, 24 In a large broadly selected sample of stable CAD patients treated with secondary prevention therapy, we show that lower RHI is an independent predictor of subsequent adverse clinical outcomes.
Peripheral microvascular dysfunction may contribute to disease progression and worse outcome in CAD patients through several mechanisms such as oxidative stress which reduces nitric oxide activity in the endothelium, responsible for vasodilation.44–48 Endothelial dysfunction is an established component of atherogenesis and contributes to the development of clinical cardiovascular diseases.49 Endothelial dysfunction in the peripheral microvascular circulation, the resistance vessels, can be a marker for cardiovascular risk burden and atherosclerosis progression.18, 19, 34 However, peripheral microvascular function could also be a surrogate for the function of the coronary microcirculation. There is evidence that peripheral microvascular endothelial function is related to coronary microvascular endothelial function, as there is an association between peripheral changes in vascular resistance with acetylcholine and similar changes in the coronary circulation, as well as with coronary flow reserve.50
Our findings of a robust inverse association between RHI and the level of several inflammatory biomarkers suggest that systemic inflammation could represent a mechanistic link between peripheral microvascular impairment and outcomes. However, there was no substantial change to the relationship between RHI and outcomes after adjustment for inflammatory biomarker levels, further demonstrating the independent prognostic role of microvascular dysfunction in this high-risk population.
Peripheral microvascular function assessment using RHI is a simple, non-invasive method that could be evaluated for clinical implementation. Our findings suggest the potential clinical significance of incorporating RHI measurements in addition to traditional prognostic indicators in the risk assessment of stable patients with CAD. The effectiveness of this approach in a clinical setting, however, needs further evaluation.
There are our important strengths to this study. Our large sample size and long follow-up time enabled us to capture a substantial incidence of hard cardiac events such as cardiovascular death and recurrent MI that were independently adjudicated. The main limitation of this study is that it was based on a single center experience and therefore independent replication is needed. Furthermore, because this is an observational study, causation in the relation of RHI and cardiac events cannot be proven.
In conclusion, impaired peripheral microvascular function as assessed by digital RHI is an independent risk factor for adverse cardiovascular events in patients with CAD. Studies are urgently needed to establish whether the evaluation of microvascular function using RHI is a useful clinical tool for the identification of patients at risk for adverse events.
Supplementary Material
HIGHLIGHTS.
In patients with clinically confirmed stable coronary artery disease, impaired peripheral microvascular function is a powerful predictor of adverse cardiovascular events.
Our results underscore the importance of impaired peripheral microvascular function in the outcome of this at-risk population and suggest the need to intervene beyond traditional risk factors to mitigate further risk.
Further studies to determine the effectiveness of incorporating RHI measurements in the clinical setting are needed.
ACKNOWLEDGEMENTS
We would like to acknowledge Nancy Murrah and Lucy Shallenberger for their contributions to the study including recruitment, data collection, data management, and study operation.
SOURCES OF FUNDING
This work was supported by the NIH (P01 HL101398, P20HL113451-01, P01HL086773-06A1, R56HL126558-01, R01 HL109413, R01HL109413-02S1, UL1TR000454, KL2TR000455, K23HL127251, K24HL077506, K24 MH076955, K12HD085850, L30HL148912, K01HL149982, UL1TR002378 and T32 HL130025A).
Abbreviations
- BMI
body mass index
- CAD
coronary artery disease
- CRP
C-reactive protein
- IL-6
interleukin-6
- IQR
interquartile range
- MACE
major adverse cardiovascular events
- MCP-1
monocyte chemoattractant protein-1
- MI
myocardial infarction
- MMP-9
matrix metallopeptidase-9
- PAT
peripheral arterial tonometry
- RHI
reactive hyperemia index
- SD
standard deviation
- s-HR
subdistribution hazard ratios
Footnotes
DISCLOSURES
None.
REFERENCES
- 1.Furman MI, Dauerman HL, Goldberg RJ, Yarzebski J, Lessard D and Gore JM. Twenty-two year (1975 to 1997) trends in the incidence, in-hospital and long-term case fatality rates from initial Q-wave and non-Q-wave myocardial infarction: a multi-hospital, community-wide perspective. J Am Coll Cardiol. 2001;37:1571–80. [DOI] [PubMed] [Google Scholar]
- 2.Boden WE, O’Rourke RA, Teo KK, Maron DJ, Hartigan PM, Sedlis SP, Dada M, Labedi M, Spertus JA, Kostuk WJ, Berman DS, Shaw LJ, Chaitman BR, Mancini GB, Weintraub WS and Investigators CT. Impact of optimal medical therapy with or without percutaneous coronary intervention on long-term cardiovascular end points in patients with stable coronary artery disease (from the COURAGE Trial). Am J Cardiol. 2009;104:1–4. [DOI] [PubMed] [Google Scholar]
- 3.Malenka DJ, Kaplan AV, Lucas FL, Sharp SM and Skinner JS. Outcomes following coronary stenting in the era of bare-metal vs the era of drug-eluting stents. JAMA. 2008;299:2868–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kostis JB, Breazna A, Deedwania PC, LaRosa JC, Treating to New Targets Steering C and Investigators. The benefits of intensive lipid lowering in patients with stable coronary heart disease with normal or high systolic blood pressure: an analysis of the Treating to New Targets (TNT) study. J Clin Hypertens (Greenwich). 2008;10:367–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Smith SC, Benjamin EJ, Bonow RO, Braun LT, Creager MA, Franklin BA, Gibbons RJ, Grundy SM, Hiratzka LF and Jones DW. AHA/ACCF secondary prevention and risk reduction therapy for patients with coronary and other atherosclerotic vascular disease: 2011 update: a guideline from the American Heart Association and American College of Cardiology Foundation endorsed by the World Heart Federation and the Preventive Cardiovascular Nurses Association. Journal of the American college of cardiology. 2011;58:2432–2446. [DOI] [PubMed] [Google Scholar]
- 6.D’Agostino RB, Russell MW, Huse DM, Ellison RC, Silbershatz H, Wilson PW and Hartz SC. Primary and subsequent coronary risk appraisal: new results from the Framingham study. Am Heart J. 2000;139:272–81. [DOI] [PubMed] [Google Scholar]
- 7.Fruchart JC, Sacks F, Hermans MP, Assmann G, Brown WV, Ceska R, Chapman MJ, Dodson PM, Fioretto P, Ginsberg HN, Kadowaki T, Lablanche JM, Marx N, Plutzky J, Reiner Z, Rosenson RS, Staels B, Stock JK, Sy R, Wanner C, Zambon A and Zimmet P. The Residual Risk Reduction Initiative: a call to action to reduce residual vascular risk in patients with dyslipidemia. Am J Cardiol. 2008;102:1K–34K. [DOI] [PubMed] [Google Scholar]
- 8.Canto JG, Kiefe CI, Rogers WJ, Peterson ED, Frederick PD, French WJ, Gibson CM, Pollack CV Jr., Ornato JP, Zalenski RJ, Penney J, Tiefenbrunn AJ, Greenland P and Investigators N. Number of coronary heart disease risk factors and mortality in patients with first myocardial infarction. JAMA. 2011;306:2120–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brunner H, Cockcroft JR, Deanfield J, Donald A, Ferrannini E, Halcox J, Kiowski W, Luscher TF, Mancia G, Natali A, Oliver JJ, Pessina AC, Rizzoni D, Rossi GP, Salvetti A, Spieker LE, Taddei S, Webb DJ, Working Group on E and Endothelial Factors of the European Society of H. Endothelial function and dysfunction. Part II: Association with cardiovascular risk factors and diseases. A statement by the Working Group on Endothelins and Endothelial Factors of the European Society of Hypertension. J Hypertens. 2005;23:233–46. [DOI] [PubMed] [Google Scholar]
- 10.Celermajer DS, Sorensen KE, Gooch VM, Spiegelhalter DJ, Miller OI, Sullivan ID, Lloyd JK and Deanfield JE. Non-invasive detection of endothelial dysfunction in children and adults at risk of atherosclerosis. Lancet. 1992;340:1111–5. [DOI] [PubMed] [Google Scholar]
- 11.Kitta Y, Obata JE, Nakamura T, Hirano M, Kodama Y, Fujioka D, Saito Y, Kawabata K, Sano K, Kobayashi T, Yano T, Nakamura K and Kugiyama K. Persistent impairment of endothelial vasomotor function has a negative impact on outcome in patients with coronary artery disease. J Am Coll Cardiol. 2009;53:323–30. [DOI] [PubMed] [Google Scholar]
- 12.Lerman A and Zeiher AM. Endothelial function: cardiac events. Circulation. 2005;111:363–8. [DOI] [PubMed] [Google Scholar]
- 13.Matsuzawa Y, Kwon TG, Lennon RJ, Lerman LO and Lerman A. Prognostic value of flow‐mediated vasodilation in brachial artery and fingertip artery for cardiovascular events: a systematic review and meta‐analysis. Journal of the American Heart Association. 2015;4:e002270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Quyyumi AA. Endothelial function in health and disease: new insights into the genesis of cardiovascular disease. Am J Med. 1998;105:32S–39S. [DOI] [PubMed] [Google Scholar]
- 15.Kuvin JT, Patel AR, Sliney KA, Pandian NG, Sheffy J, Schnall RP, Karas RH and Udelson JE. Assessment of peripheral vascular endothelial function with finger arterial pulse wave amplitude. Am Heart J. 2003;146:168–74. [DOI] [PubMed] [Google Scholar]
- 16.Patvardhan EA, Heffernan KS, Ruan JM, Soffler MI, Karas RH and Kuvin JT. Assessment of vascular endothelial function with peripheral arterial tonometry: information at your fingertips? Cardiology in review. 2010;18:20–28. [DOI] [PubMed] [Google Scholar]
- 17.Hamburg NM, Keyes MJ, Larson MG, Vasan RS, Schnabel R, Pryde MM, Mitchell GF, Sheffy J, Vita JA and Benjamin EJ. Cross-sectional relations of digital vascular function to cardiovascular risk factors in the Framingham Heart Study. Circulation. 2008;117:2467–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rubinshtein R, Kuvin JT, Soffler M, Lennon RJ, Lavi S, Nelson RE, Pumper GM, Lerman LO and Lerman A. Assessment of endothelial function by non-invasive peripheral arterial tonometry predicts late cardiovascular adverse events. Eur Heart J. 2010;31:1142–8. [DOI] [PubMed] [Google Scholar]
- 19.Matsuzawa Y, Sugiyama S, Sumida H, Sugamura K, Nozaki T, Ohba K, Matsubara J, Kurokawa H, Fujisue K, Konishi M, Akiyama E, Suzuki H, Nagayoshi Y, Yamamuro M, Sakamoto K, Iwashita S, Jinnouchi H, Taguri M, Morita S, Matsui K, Kimura K, Umemura S and Ogawa H. Peripheral endothelial function and cardiovascular events in high-risk patients. J Am Heart Assoc. 2013;2:e000426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Matsue Y, Suzuki M, Nagahori W, Ohno M, Matsumura A, Hashimoto Y, Yoshida K and Yoshida M. Endothelial dysfunction measured by peripheral arterial tonometry predicts prognosis in patients with heart failure with preserved ejection fraction. Int J Cardiol. 2013;168:36–40. [DOI] [PubMed] [Google Scholar]
- 21.Akiyama E, Sugiyama S, Matsuzawa Y, Konishi M, Suzuki H, Nozaki T, Ohba K, Matsubara J, Maeda H, Horibata Y, Sakamoto K, Sugamura K, Yamamuro M, Sumida H, Kaikita K, Iwashita S, Matsui K, Kimura K, Umemura S and Ogawa H. Incremental prognostic significance of peripheral endothelial dysfunction in patients with heart failure with normal left ventricular ejection fraction. J Am Coll Cardiol. 2012;60:1778–86. [DOI] [PubMed] [Google Scholar]
- 22.Paine NJ, Hinderliter AL, Blumenthal JA, Adams KF Jr., Sueta CA, Chang PP, O’Connor CM and Sherwood A. Reactive hyperemia is associated with adverse clinical outcomes in heart failure. Am Heart J. 2016;178:108–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ikonomidis I, Kadoglou NN, Tritakis V, Paraskevaidis I, Dimas K, Trivilou P, Papadakis I, Tzortzis S, Triantafyllidi H, Parissis J, Anastasiou-Nana M and Lekakis J. Association of Lp-PLA2 with digital reactive hyperemia, coronary flow reserve, carotid atherosclerosis and arterial stiffness in coronary artery disease. Atherosclerosis. 2014;234:34–41. [DOI] [PubMed] [Google Scholar]
- 24.Matsue Y, Yoshida K, Nagahori W, Ohno M, Suzuki M, Matsumura A, Hashimoto Y and Yoshida M. Peripheral microvascular dysfunction predicts residual risk in coronary artery disease patients on statin therapy. Atherosclerosis. 2014;232:186–90. [DOI] [PubMed] [Google Scholar]
- 25.Nohria A, Gerhard-Herman M, Creager MA, Hurley S, Mitra D and Ganz P. Role of nitric oxide in the regulation of digital pulse volume amplitude in humans. J Appl Physiol (1985). 2006;101:545–8. [DOI] [PubMed] [Google Scholar]
- 26.Blankenberg S, Rupprecht HJ, Poirier O, Bickel C, Smieja M, Hafner G, Meyer J, Cambien F, Tiret L and AtheroGene I. Plasma concentrations and genetic variation of matrix metalloproteinase 9 and prognosis of patients with cardiovascular disease. Circulation. 2003;107:1579–85. [DOI] [PubMed] [Google Scholar]
- 27.Daniels LB. Pretenders and Contenders: Inflammation, C-Reactive Protein, and Interleukin-6. J Am Heart Assoc. 2017;6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Held C, White HD, Stewart RAH, Budaj A, Cannon CP, Hochman JS, Koenig W, Siegbahn A, Steg PG, Soffer J, Weaver WD, Ostlund O, Wallentin L and Investigators S. Inflammatory Biomarkers Interleukin-6 and C-Reactive Protein and Outcomes in Stable Coronary Heart Disease: Experiences From the STABILITY (Stabilization of Atherosclerotic Plaque by Initiation of Darapladib Therapy) Trial. J Am Heart Assoc. 2017;6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ridker PM. Role of inflammatory biomarkers in prediction of coronary heart disease. Lancet. 2001;358:946–8. [DOI] [PubMed] [Google Scholar]
- 30.Stenvinkel P. Endothelial dysfunction and inflammation—is there a link? Nephrology Dialysis Transplantation. 2001;16:1968–1971. [DOI] [PubMed] [Google Scholar]
- 31.Sandoo A, Kitas GD, Carroll D and Veldhuijzen van Zanten JJ. The role of inflammation and cardiovascular disease risk on microvascular and macrovascular endothelial function in patients with rheumatoid arthritis: a cross-sectional and longitudinal study. Arthritis Res Ther. 2012;14:R117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hammadah M, Al Mheid I, Wilmot K, Ramadan R, Shah AJ, Sun Y, Pearce B, Garcia EV, Kutner M, Bremner JD, Esteves F, Raggi P, Sheps DS, Vaccarino V and Quyyumi AA. The Mental Stress Ischemia Prognosis Study: Objectives, Study Design, and Prevalence of Inducible Ischemia. Psychosom Med. 2017;79:311–317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gensini GG. A more meaningful scoring system for determining the severity of coronary heart disease. Am J Cardiol. 1983;51:606. [DOI] [PubMed] [Google Scholar]
- 34.Bonetti PO, Pumper GM, Higano ST, Holmes DR Jr., Kuvin JT and Lerman A. Noninvasive identification of patients with early coronary atherosclerosis by assessment of digital reactive hyperemia. J Am Coll Cardiol. 2004;44:2137–41. [DOI] [PubMed] [Google Scholar]
- 35.Kim JH, Almuwaqqat Z, Hammadah M, Liu C, Ko YA, Lima B, Sullivan S, Alkhoder A, Abdulbaki R, Ward L, Bremner JD, Sheps DS, Raggi P, Sun YV, Shah AJ, Vaccarino V and Quyyumi AA. Peripheral Vasoconstriction During Mental Stress and Adverse Cardiovascular Outcomes in Patients With Coronary Artery Disease. Circ Res. 2019;125:874–883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Morris AA, Patel RS, Binongo JN, Poole J, Al Mheid I, Ahmed Y, Stoyanova N, Vaccarino V, Din-Dzietham R, Gibbons GH and Quyyumi A. Racial differences in arterial stiffness and microcirculatory function between Black and White Americans. J Am Heart Assoc. 2013;2:e002154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.McCrea CE, Skulas-Ray AC, Chow M and West SG. Test-retest reliability of pulse amplitude tonometry measures of vascular endothelial function: implications for clinical trial design. Vasc Med. 2012;17:29–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Suhrs HE, Schroder J, Bove KB, Mygind ND, Frestad D, Michelsen MM, Lange T, Gustafsson I, Kastrup J and Prescott E. Inflammation, non-endothelial dependent coronary microvascular function and diastolic function-Are they linked? PLoS One. 2020;15:e0236035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zanoli L, Briet M, Empana JP, Cunha PG, Maki-Petaja KM, Protogerou AD, Tedgui A, Touyz RM, Schiffrin EL, Spronck B, Bouchard P, Vlachopoulos C, Bruno RM, Boutouyrie P, Association for Research into Arterial Structure PStESoHWGoVS, Function and the European Network for Noninvasive Investigation of Large A. Vascular consequences of inflammation: a position statement from the ESH Working Group on Vascular Structure and Function and the ARTERY Society. J Hypertens. 2020;38:1682–1698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hirano K and Imbens GW. The propensity score with continuous treatments. Applied Bayesian modeling and causal inference from incomplete-data perspectives. 2004;226164:73–84. [Google Scholar]
- 41.Rubin DB and Thomas N. Combining propensity score matching with additional adjustments for prognostic covariates. Journal of the American Statistical Association. 2000;95:573–585. [Google Scholar]
- 42.Xu Y, Arora RC, Hiebert BM, Lerner B, Szwajcer A, McDonald K, Rigatto C, Komenda P, Sood MM and Tangri N. Non-invasive endothelial function testing and the risk of adverse outcomes: a systematic review and meta-analysis. Eur Heart J Cardiovasc Imaging. 2014;15:736–46. [DOI] [PubMed] [Google Scholar]
- 43.Shechter M, Matetzky S, Prasad M, Goitein O, Goldkorn R, Naroditsky M, Koren-Morag N and Lerman A. Endothelial function predicts 1-year adverse clinical outcome in patients hospitalized in the emergency department chest pain unit. Int J Cardiol. 2017;240:14–19. [DOI] [PubMed] [Google Scholar]
- 44.Heitzer T, Baldus S, von Kodolitsch Y, Rudolph V and Meinertz T. Systemic endothelial dysfunction as an early predictor of adverse outcome in heart failure. Arterioscler Thromb Vasc Biol. 2005;25:1174–9. [DOI] [PubMed] [Google Scholar]
- 45.Heitzer T, Schlinzig T, Krohn K, Meinertz T and Munzel T. Endothelial dysfunction, oxidative stress, and risk of cardiovascular events in patients with coronary artery disease. Circulation. 2001;104:2673–8. [DOI] [PubMed] [Google Scholar]
- 46.Landmesser U, Hornig B and Drexler H. Endothelial function: a critical determinant in atherosclerosis? Circulation. 2004;109:II27–33. [DOI] [PubMed] [Google Scholar]
- 47.Neunteufl T, Heher S, Katzenschlager R, Wolfl G, Kostner K, Maurer G and Weidinger F. Late prognostic value of flow-mediated dilation in the brachial artery of patients with chest pain. Am J Cardiol. 2000;86:207–10. [DOI] [PubMed] [Google Scholar]
- 48.Perticone F, Ceravolo R, Pujia A, Ventura G, Iacopino S, Scozzafava A, Ferraro A, Chello M, Mastroroberto P, Verdecchia P and Schillaci G. Prognostic significance of endothelial dysfunction in hypertensive patients. Circulation. 2001;104:191–6. [DOI] [PubMed] [Google Scholar]
- 49.Widlansky ME, Gokce N, Keaney JF Jr., and Vita JA. The clinical implications of endothelial dysfunction. J Am Coll Cardiol. 2003;42:1149–60. [DOI] [PubMed] [Google Scholar]
- 50.Al-Badri A, Kim JH, Liu C, Mehta PK and Quyyumi AA. Peripheral Microvascular Function Reflects Coronary Vascular Function. Arterioscler Thromb Vasc Biol. 2019;39:1492–1500. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.