Abstract
The α1,6-fucosyltransferase, FUT8, is the sole enzyme catalyzing the core-fucosylation of N-glycoproteins in mammalian systems. Previous studies using free N-glycans as acceptor substrates indicated that a terminal β1,2-GlcNAc moiety on the Man-α1,3-Man arm of N-glycan substrates is required for efficient FUT8-catalyzed core-fucosylation. In contrast, we recently demonstrated that, in a proper protein context, FUT8 could also fucosylate Man5GlcNAc2 without a GlcNAc at the non-reducing end. We describe here a further study of the substrate specificity of FUT8 using a range of N-glycans containing different aglycones. We found that FUT8 could fucosylate most of high-mannose and complex-type N-glycans, including highly branched N-glycans from chicken ovalbumin, when the aglycone moiety is modified with a 9-fluorenylmethyloxycarbonyl (Fmoc) moiety or in a suitable peptide/protein context, even if they lack the terminal GlcNAc moiety on the Man-α1,3-Man arm. FUT8 could also fucosylate paucimannose structures when they are on glycoprotein substrates. Such core-fucosylated paucimannosylation is a prominent feature of lysosomal proteins of human neutrophils and several types of cancers. We also found that sialylation of N-glycans significantly reduced their activity as substrate of FUT8. Kinetic analysis demonstrated that Fmoc aglycone modification could either improve the turnover rate or decrease the Km value depending on the nature of the substrates, thus significantly enhancing the overall efficiency of FUT8 catalyzed fucosylation. Our results indicate that an appropriate aglycone context of N-glycans could significantly broaden the acceptor substrate specificity of FUT8 beyond what has previously been thought.
Keywords: FUT8, fucosylation, substrate specificity, glycoprotein, enzyme
Introduction
Protein glycosylation is a prevalent post-translational modification that regulates the structure and functions of proteins in different ways (1). Core-fucosylation, the addition of an α1,6-linked fucose at the innermost N-acetyl-glucosamine (GlcNAc) of N-glycans, plays essential roles in many biological recognition processes, including antibody’s immune functions, cell adhesion, signal transduction, and tumor metastasis. For example, core-fucosylation regulates the activities of many cell surface receptors involved in ligand recognition and cell signaling (2–7); elevated levels of core-fucosylation are often associated with cancers such as hepatocellular carcinoma, breast carcinoma, and lung squamous cell carcinoma (8–11); and core fucosylation of Fc glycans adversely affects the affinity of antibodies for FcγRIIIa and, as a result, significantly reduces antibody-dependent cellular cytotoxicity (ADCC) (12,13). The adverse effect of core-fucosylation on ADCC has prompted glycoengineering to produce low or non-fucosylated monoclonal antibodies as more effective therapeutics for the treatment of cancer (14,15).
The α1,6-fucosyltransferase, FUT8, is the sole enzyme responsible for the core-fucosylation of N-glycans in mammalian systems (16,17). Intensive previous studies have demonstrated that FUT8 has a strict acceptor substrate specificity and requires the presence of an unmodified β−1,2-linked GlcNAc moiety on the Man-α1,3-Man arm of the N-glycans for efficient core-fucosylation (16,18–27). Thus, lack of a terminal GlcNAc moiety on the Man-α1,3-Man arm, or modification of the GlcNAc residue by galactosylation, would block core-fucosylation of the N-glycans by FUT8 (23,24). Nevertheless, some glycan characterization from isolated tissues and recombinant glycoproteins expressed from mammalian cells has demonstrated the existence of core-fucosylated high-mannose type glycans (28,29). Such discrepancy implicates a broader substrate specificity of FUT8 towards natural glycoproteins. Recently, we have revisited the substrate specificity of FUT8 and have found that FUT8 could efficiently fucosylate Man5GlcNAc2 glycan in cells and in vitro when the glycan is presented in the context of a suitable aglycone moiety (17,30). For example, the Man5GlcNAc2 on human erythropoietin could be efficiently core-fucosylated by FUT8 in HEK293 GnTI−/− cells when FUT8 is overexpressed, and a Man5GlcNAc2 glycan could be fucosylated by FUT8 in vitro when the aglycone portion is modified by a hydrophobic 9-fluorenylmethyloxycarbonyl (Fmoc) moiety, even when both lack the free GlcNAc moiety on the Man-α1,3-Man arm (17,30). We describe here an expanded study of the substrate specificity of FUT8 with a range of high-mannose type and complex type N-glycans, in the context of a modified aglycone portion. We found that FUT8 could fucosylate a wide range of high-mannose, paucimannose, and complex type N-glycans tested, including highly branched high mannose and complex type N-glycans from chicken ovalbumin, when the aglycone moiety is modified with an Fmoc tag or with a suitable peptide/protein moiety. The efficiency of the fucosylation of paucimannose structures within a glycoprotein context is relatively high. Such glycoforms are widely detected in lysosome enzymes from neutrophils and several types of cancer (31–33). In comparison, we found that terminal sialylation of N-glycans significantly reduced its substrate activity toward FUT8. Our experimental data indicate that an appropriate aglycone context could significantly enhance the substrate activity of N-glycans toward FUT8, suggesting that FUT8 has a much more broadened substrate specificity than what was previously implicated.
Results
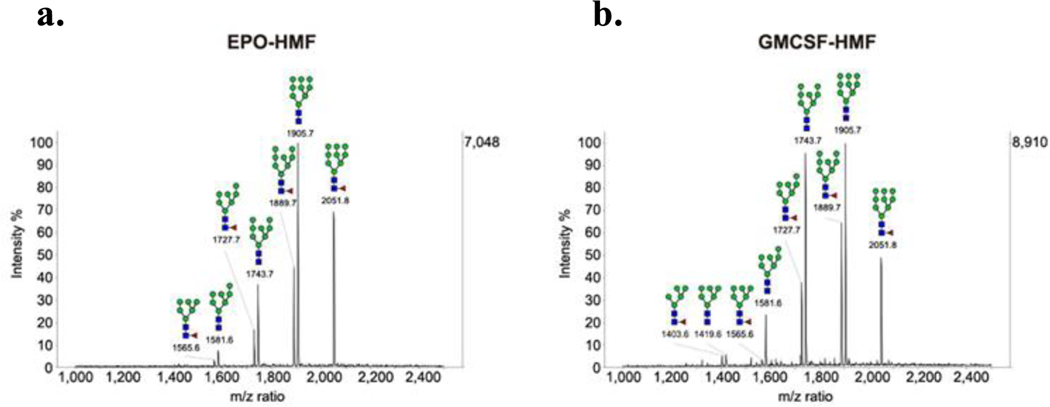
FUT8 enzymatic activity towards various high mannose-type glycans in the context of glycoproteins.
We have previously shown that FUT8 could efficiently fucosylate Man5GlcNAc2 glycan on human EPO in HEK293 GnTI−/− cells (17). To further analyze the enzymatic activity of FUT8 towards different high-mannose type glycoforms of glycoproteins, we first engineered the HEK293T cell line by overexpression of FUT8 using lentiviral transduction. Then we transfected this cell line with the erythropoietin (EPO) and the granulocyte-macrophage colony-stimulating factor (GM-CSF) expressing plasmid DNA (17,30) in the presence of 1 μg/mL of kifunensine, an α1,2-mannosidase I inhibitor. A relatively low concentration of kifunensine was used in order to obtain a range of truncated high-mannose type glycans (Man6–9GlcNAc2) on the glycoproteins (34). Three days after the transfection, the glycoproteins were purified, and the N-glycans were released by PNGase F treatment followed by MALDI-TOF MS analysis. Peaks corresponding to fucosylated high mannose glycans (1403.6, Man5GlcNAc2Fuc; 1565.6, Man6GlcNAc2Fuc; 1727.7, Man7GlcNAc2Fuc; 1889.7, Man8GlcNAc2Fuc; 2051.8, Man9GlcNAc2Fuc) were found on both EPO and GM-CSF glycoprotein products following overexpression of FUT8 in the presence of kifunensine (Figure 1). This result indicated that FUT8 could also fucosylate high-mannose type N-glycans, ranging from Man5GlcNAc2 to Man9GlcNAc2, in the context of a suitable glycoprotein aglycone (EPO or GM-CSF). Interestingly, It appeared that more core-fucosylated products were formed in the case of smaller high-mannose N-glycans (Man5GlcNAc2, Man6GlcNAc2, and Man7GlcNAc2) than those in the larger high-mannose N-glycans (Man8GlcNAc2 and Man9GlcNAc2) (Figure 1), suggesting that smaller high-mannose N-glycans are preferable substrates of FUT8 compared with the larger Man8GlcNAc2 and Man9GlcNAc2 glycoforms in the context of both human EPO and GM-CSF.
Figure 1: Cell-based study of FUT8 enzymatic activity on high-mannose type glycoform of EPO and GM-CSF.
a. MALDI-TOF MS of the N-glycans released from the EPO; b. MALDI-TOF MS of the N-glycans released from GM-CSF. The two glycoproteins were expressed in HEK 293T cells with the overexpression of FUT8 cultured in the presence of 1 μg/mL of kifunensine. Each spectrum was taken as a sum of ten random positions on the corresponding spot of the MALDI target plate.
We also tested how FUT8 would act on the high-mannose glycoform of EPO in vitro. We prepared EPO with high-mannose glycoforms (EPO-HM) by transfecting wild type HEK293T cells under 1 μg/mL kifunensine. The MALDI-TOF MS characterization of released glycans showed that the recombinant EPO carried a mixture of high-mannose type N-glycans ranging from Man5GlcNAc2 to Man9GlcNAc2 (Figure 2a). We then carried out in vitro enzymatic fucosylation experiments with EPO-HM using the conditions as previously described (23,30). After an overnight reaction, glycans were released from the glycoprotein by PNGase F treatment and analyzed by MALDI-TOF MS. Three new species appeared at m/z = 1565.7, 1727.8, and 1889.8 (Figure 2b). The mass differences between the new species and their corresponding high-mannose type glycans (Man6GlcNAc2: 1419.6, Man7GlcNAc2:1581.7, Man8GlcNAc2: 1743.8) were 146, indicating the addition of a fucose on the glycan. We did not detect the fucosylation of the Man9GlcNAc2 glycan under the condition. This result confirmed that FUT8, within the context of EPO, could core-fucosylate large high-mannose type N-glycans in vitro, but the largest Man9GlcNAc2 glycan was clearly less active toward FUT8 than the smaller ones. These obeservations confirm that the protein context plays a role in the enzymatic activity of FUT8 towards N-glycan substrates. EPO provides an effective aglycone text that facilitates core-fucosylation of high-mannose type glycans. In contrast, we have previously shown that FUT8 could not fucosylate the high-mannose N-glycans on ribonuclease B (RNase B), suggesting that the RNase B did not provide an appropriate protein context for promoting FUT8-catalyzed core-fucosylation (30).
Figure 2: In vitro core-fucosylation of high-mannose type glycoform of EPO by FUT8.
a. MALDI-TOF MS of the N-glycans released from the EPO expressed in HEK 293T cells in the presence of 1 μg/mL kifunensine. b. MALDI-TOF MS of the N-glycans released from the EPO after incubation with 0.05 mg/mL enzyme for 16 hours. Each spectrum was taken as a sum of ten random positions on the corresponding spot of the MALDI target plate.
FUT8 enzymatic activity towards various paucimannose-type glycans in the context of EPO glycosylation.
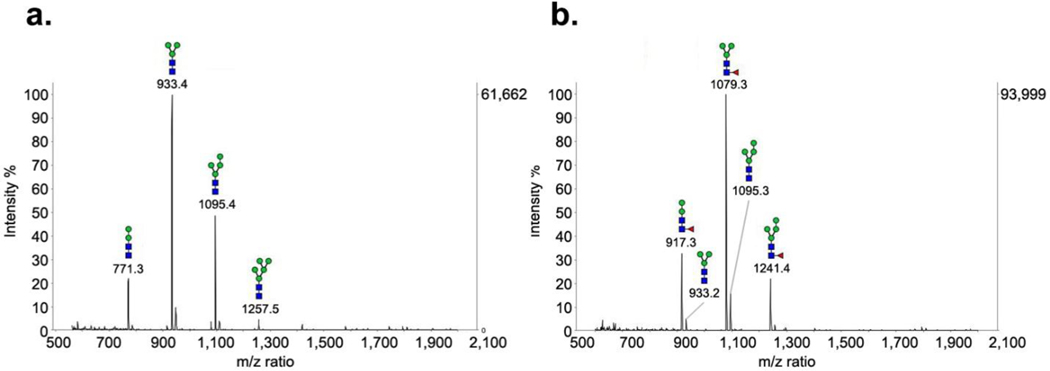
Next, we tested smaller, paucimannose glycoforms on EPO (Man2–4GlcNAc2, PM) as the substrates for FUT8. The paucimannose glycoforms were prepared by digestion of the high-mannose type glycoform of EPO with an α-mannosidase from Canavalia ensiformis to give a mixture of Man2–4GlcNAc2 glycoform (Figure 3a). Incubation of the Man2–4GlcNAc2 glycoform with FUT8 resulted in efficient core-fucosylation, as revealed by MALDI-TOF MS analysis of the PNGase F released N-glycans. The data indicate that the majority of the starting glycoform was converted by the addition of a fucose moiety (an increase of 146 Da of the molecular mass), as indicated by the appearance of three new species, m/z at 917.3 (Man2GlcNAc2Fuc), 1079.3 (Man3GlcNAc2Fuc), and 1241.4 (Man4GlcNAc2Fuc) in vitro (Figure 3b). Interestingly, our comparative studies indicated that FUT8 was much more efficient in fucosylation of smaller paucimannose (Man2–4GlcNAc2) EPO glycoforms than the larger Man7–9GlcNAc2 EPO glycoform (Figure 2b versus 3b). Steric hindrance in the binding of the larger Man7–9GlcNAc2 structures may account for the differences in the observed decreased substrate activity toward the enzyme. These data demonstrated that FUT8 could indeed core-fucosylate a range of high-mannose N-glycans in the context of glycosylated EPO substrates both in vivo and in vitro, and that the smaller paucimannose glycans were better substrates than the larger high-mannose type glycoforms. On the other hand, we also evaluated the substrate activity of FUT8 towards the innermost GlcNAc monosaccharide of EPO after deglycosylation of the high-mannose EPO with the Arthrobacter endoglycosidase (Endo A). In contrast to EPO-HM and EPO-PM, no fucose transfer was detected on EPO-GlcNAc in which only the innermost GlcNAc was attached. The results suggest that even within the EPO glycoprotein context, the core GlcNAc alone is not a substrate for FUT8.
Figure 3: In vitro core-fucosylation on paucimannose type glycoform of EPO by FUT8.
a. N-glycans released from the EPO after treatment with α-mannosidase. b. N-glycans released from the EPO after incubation with 0.05 mg/mL enzyme for 16 hours. The final spectrums were collected by adding together the intensities of ten random positions on the MALDI target plate.
FUT8 enzymatic activity towards Fmoc-labelled N-glycans derived from chicken ovalbumin.
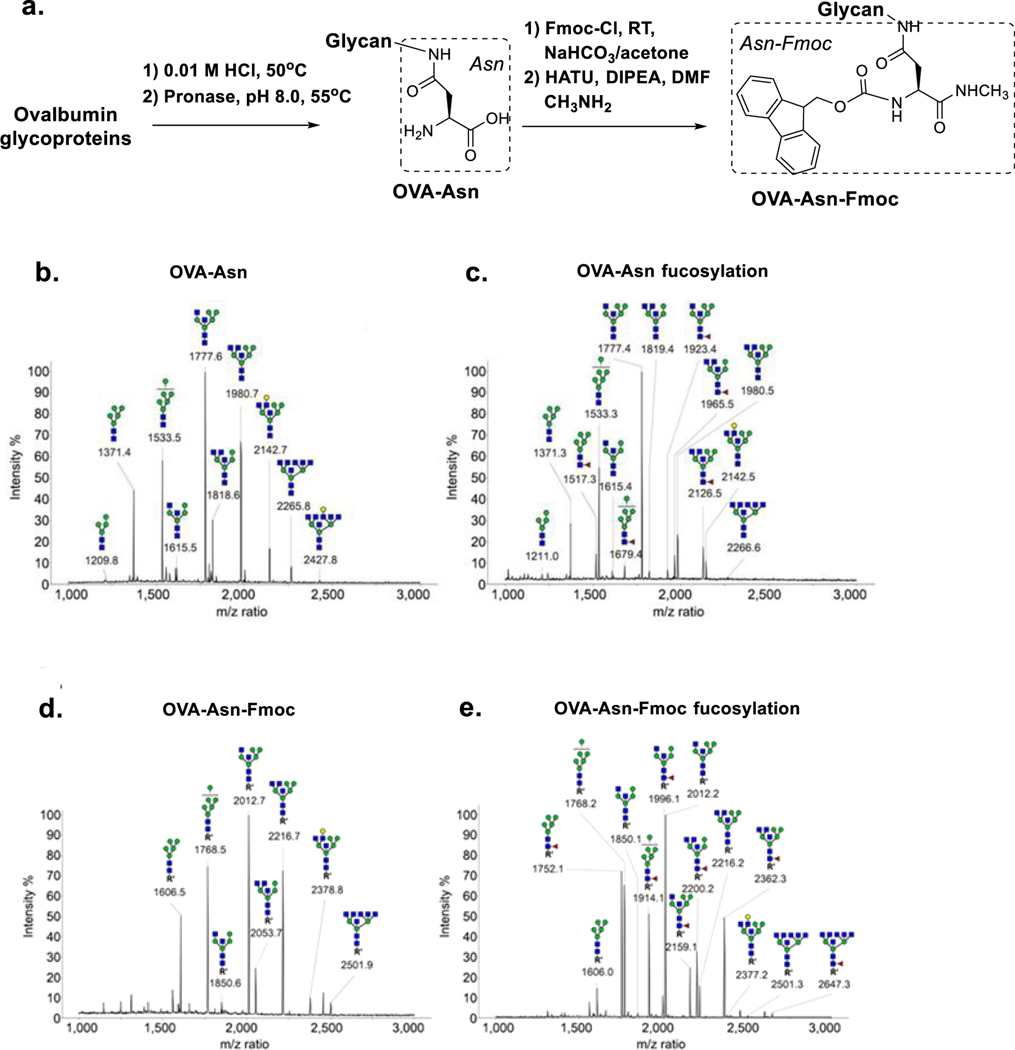
Next, we turned our attention to how FUT8 would act on other types of N-glycans. Previously, the presence of a β1,4-linked GlcNAc on the Man-α1,3-Man arm was found to reduce catalytic efficiency of FUT8 by 4.1-fold (27) and the presence of a bisecting GlcNAc moiety on the core β-mannose was found to eliminate FUT8 activity (24). Here we hypothesized that a proper aglycone modification might broaden the acceptor preference of the enzyme and partially rescue the low activity of these N-glycans as acceptors for FUT8. We prepared a library of Fmoc-labeled N-glycans derived from chicken egg white ovalbumin (OVA) that is known to carry a range of high-mannose and highly branched complex-type N-glycans, including structures containing bisecting GlcNAc residues (35–37). To prepare the Fmoc-labelled N-glycans, egg white ovalbumin (OVA) was digested with pronase to generate the Asn-linked N-glycans (OVA-Asn), which was then reacted with 9-fluorenylmethyloxycarbonyl chloride (Fmoc-Cl) (38) to give a library of Fmoc-labeled chicken ovalbumin N-glycans (OVA-Asn-Fmoc, Figure 4a).
Figure 4. Fucosylation of Fmoc-labelled N-glycans derived from chicken ovalbumin.
a. The preparation of the Fmoc-labelled Asn-linked OVA-glycan library. N-Glycans were released from OVA via exhaustive pronase digestion and then capped with Fmoc. b. MALDI-TOF MS spectrum of the free Asn-linked OVA glycans. c. MALDI-TOF MS spectrum on the free Asn-linked OVA-glycans after treatment with 0.5 mg/mL of FUT8 and GDP-Fuc overnight. d. MALDI-TOF MS spectrum of the Fmoc-labelled Asn-linked OVA glycans. e. MALDI-TOF MS spectrum of the Fmoc-labelled Asn-linked OVA-glycans after treatment with 0.5 mg/mL of FUT8 and GDP-Fuc overnight. MALDI-TOF MS data were obtained by adding the readings from ten random positions on the target plate.
The reactivity of FUT8 on free Asn-linked and the Fmoc-labeled N-glycans from ovalbulmin was evaluated by incubation of the respective compounds with FUT8 overnight, followed by MALDI-TOF MS analysis of the N-glycans in the reaction mixtures. For these free Fmoc tagged N-glycans we found that only two hybrid-type N-glycans containing both GlcNAc-β-(1–4) and GlcNAc-β-(1–2) branches on the Man-α1,3-Man arm (m/z at 1819.4 and 1980.5) showed significant amount of core-fucosylation (fucosylated peak at 1965.5 and 2126.5 respectively, Figure 4b vs. 4c), even with relatively high concentrations (0.5 mg/mL) of FUT8 enzyme. Meanwhile, Man5GlcNAc2 (1371.3), Man6GlcNAc2 (1533.3), and a bisected hybrid-type glycan (1777.4) showed only limited fucosylation under these conditions (fucosylated peak at 1517.3, 1679.4, and 1923.4 respectively, Figure 4b vs. 4c). This result is consistent with previous findings that free high-mannose type N-glycans are not substrates for FUT8, and that a bisecting β-(1–4)-linked GlcNAc moiety significantly reduces the substrate activity by FUT8 even in the presence of a β1,2-GlcNAc on the α1,3Man arm (24). However, under the same conditions, almost all of the Fmoc-tagged N-glycans from ovalbumin showed significantly enhanced fucosylation (Figure 4d vs. 4e). Specifically, the Fmoc-labelled Man5GlcNAc2-Asn, the two GlcNAc-bisected hybrid-type N-glycans (m/z at 1850.1 and 2053.1), and the penta-antennary complex-type glycan with bisecting GlcNAc residues (m/z at 2501.3) showed almost completed core-fucosylation (Figure 4d vs. 4e). These data further confirmed that the presence of a Fmoc moiety on the aglycone portion of the acceptor glycan could rescue the substrate activity for FUT8-catalyzed core-fucosylation of those otherwise “inactive” free N-glycans.
Terminal sialylation significantly decreases the FUT8-catalyzed core-fucosylation of complex type N-glycopeptides.
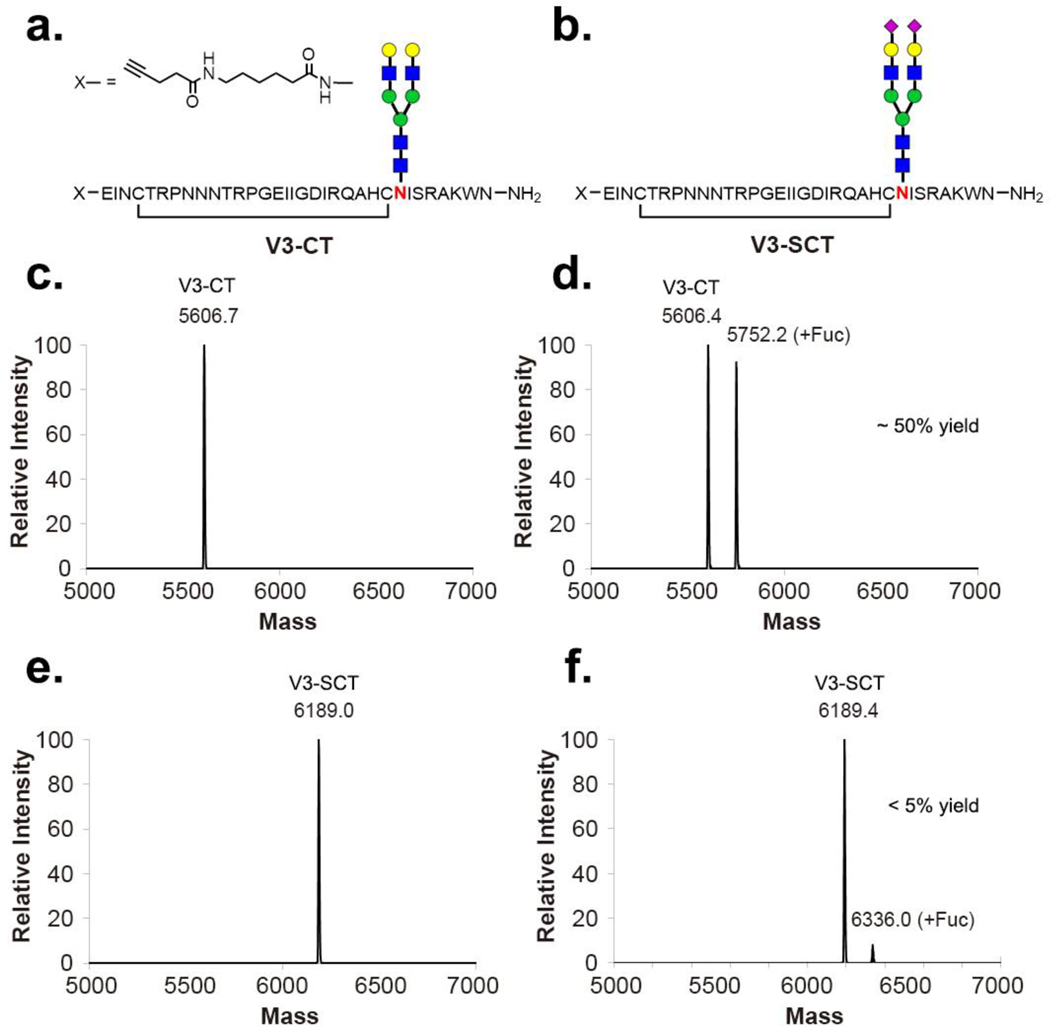
Our previous preliminary study has shown that FUT8 could fucosylate a complex-type bi-antennary N-glycan when it is present in the context of a suitable polypeptide (30). Terminal sialylation is an important modification on N-linked glycans. Thus, we sought to investigate whether terminal sialylation would affect the enzymatic activity of FUT8 on complex-type N-glycans in a peptide context. For this purpose, we synthesized a sialylated HIV-1 glycopeptide and its corresponding asialylated glycopeptide (Figure 5a–b), using a chemoenzymatic method that we have described before (39,40). The FUT8-catalyzed enzymatic reaction on the two glycopeptide substrates was performed using GDP-Fuc as the donor substrate and a relatively high concentration (0.5 mg/ml) of FUT8. It was found that FUT8 could transfer core fucose to the asialo-glycopeptide (Figure 5c vs. 5d), albeit at a slow rate whereas only marginally detectable core-fucosylation was observed for the sialylated glycopeptide even after 3-day incubation (Figure 5e vs. 5f). The fucosylated V3-CT glycopeptide was isolated via AAL lectin affinity chromatography. The isolated product appeared as a single peak in reverse phase HPLC and its identity was confirmed by ESI-MS analysis. Our result clearly indicated that terminal sialylation significantly decreased the substrate activity of N-glycans in the FUT8-catalyzed reactions.
Figure 5: ESI-MS analysis of core-fucosylation on sialylated and asialylated complex type N-glycopeptides by FUT8.
a. The V3-CT glycopeptide substrate. b. The V3-SCT glycopeptide substrate. c. The deconvoluted mass of the V3-CT peptide starting material. d. The deconvoluted mass of the enzymatic reaction of V3-CT with 0.5 mg/mL of FUT8 for 3 days. e. The deconvoluted mass of the V3-SCT peptide starting material. f. The deconvoluted mass of the enzymatic reaction of V3-SCT with 0.5 mg/mL of FUT8 for 3 days.
Kinetic analysis of the FUT8-catalyzed reaction with selectively aglycone-modified N-glycan substrates.
To determine the underlying mechanism of the aglycone-promoted enzymatic reactions, we performed kinetic analysis of FUT8-catalyzed fucosylation with selected N-glycans using the GDP-Glo assays (27). The glycan substrates included Man5GlcNAc2Asn, GlcNAc1Man5GlcNAc2Asn, GlcNAc2Man3GlcNAc2Asn (A2-Asn), and the corresponding Fmoc-tagged N-glycans. Consistent with the previous findings (23,24), the N-glycans (GlcNAc1Man5GlcNAc2Asn and A2-Asn) carrying a terminal β1,2-linked GlcNAc moiety on the Man-α1,3-Man arm are excellent substrates of FUT8, while the Man5GlcNAc2Asn acted as a poor substrate, with a much higher Km and much lower kcat (Table 1). In comparison, the Man5GlcNAc2Asn-Fmoc, in which an Fmoc moiety is attached at the Asn, resulted in an increase in catalytic efficiency of fucosylation of this substrate (~7.4-fold increase in kcat/KM) compared with Man5GlcNAc2Asn predominately through an increase in kcat. The hybrid N-glycan, GlcNAc1Man5GlcNAc2Asn-Fmoc, also had a 10.5-fold increase in kcat/KM compared with the GlcNAc1Man5GlcNAc2Asn substrate that did not contain the Fmoc substituent, largely resulting from a significantly decreased KM. Interestingly, for the highly active GlcNAc2Man3GlcNAc2Asn (A2-Asn) acceptor, the introduction of an Fmoc moiety only slightly enhanced catalytic efficiency of the FUT8-catalyzed reaction (33% increase in kcat/KM, Table 1). This moderate enhancement may be due to the relatively high enzyme affinity of the A2-Asn substrate itself. Taken together, these results suggest that the Fmoc aglycone modification could significantly enhance the overall efficiency of FUT8 catalyzed fucosylation either by improving the turnover rate or by decreasing the Km value, depending on the nature of the substrates. But for a high-affinity substrate, the effect of aglycone portion has less impact.
Table 1: Kinetic parameters of human FUT8 using GDP-Fucose as the donor substrate and different N-glycans as the acceptor substrates.
The kinetic parameters of enzyme activity was obtained via the UDP-Glo assays. The concentration range of the glycan substrates was 0–1.5 mM for the Man5GlcNAc2-Asn substrate and 0–1 mM for others.
| Acceptor Substrates | kcat | KM | kcat/KM |
|---|---|---|---|
| s−1 | μM | mM−1s−1 | |
| Man5GlcNAc2-Asn | 0.052 ± 0.005 | 2114 ± 291 | 0.025 ± 0.006 |
| Man5GlcNAc2-Asn-Fmoc | 0.214 ± 0.045 | 1151 ± 384 | 0.186 ± 0.101 |
| GlcNAc1Man5GlcNAc2Asn | 8.5 ± 0.4 | 234.9 ± 24.5 | 36.1 ± 5.3 |
| GlcNAc1Man5GlcNAc2Asn -Fmoc | 11.6 ± 0.6 | 30.6 ± 7.4 | 378.7 ± 110.4 |
| GlcNAc2Man3GlcNAc2Asn (A2-Asn) | 11.8 ± 0.4 | 52.1 ± 6.6 | 226.4 ± 36.3 |
| GlcNAc2Man3GlcNAc2Asn-Fmoc (A2-Asn-Fmoc) | 13.6 ± 0.5 | 44.9 ± 6.5 | 302.3 ± 55.4 |
Structural insights of the aglycone enhancement.
The structural basis for acceptor substrate recognition of FUT8 has been investigated in three recent X-ray crystallographic studies (25–27). Each of the studies has characterized interactions between FUT8, and the donor analog, GDP, as well as the A2-Asn acceptor. Favorable interactions have been identified between FUT8 and the GlcNAcβ1,2-Man-α1,3-Man arm of the glycan. In addition, our studies have probed FUT8 complexes with more highly branched, low affinity acceptors, A3-Asn, A3’-Asn, and GlcNAc1Man5GlcNAc2-Asn (27). The structures have revealed the roles of steric hindrance in reducing catalytic efficiency with these larger substrates. However, none of these structural studies have probed the roles of aglycone substituents in substrate interactions. Based on the FUT8:GDP:A2-Asn structure, we generated an electrostatic surface representation of FUT8 (Figure 6). It was shown that the core GlcNAc-β1,4-GlcNAc region of the structure was in tight association with the enzyme surface and the amide nitrogen of the GlcNAc-β-Asn linkage was hydrogen bonded with the carbonyl oxygen of Gly217. However, the remainder of the Asn residue extended into the solvent with no obvious adjacent hydrophobic or highly polar regions to interact with an aglycone substituent (27). Thus, these X-ray crystallographic studies provided little information on the potential contributions of the aglycone portions to the enzymatic recognition and catalytic efficiency of FUT8.
Figure 6: Electrostatic surface representation of FUT8:A2-Asn:GDP complex.
The crystal structure of FUT8 in complex with an A2-Asn acceptor and GDP donor analog (PDB 6VLD) was displayed as an electrostatic surface in Pymol (Schrodinger) using default settings. Two orientations of the enzyme-substrate complex are shown and the acceptor and GDP are illustrated in green stick form. The peptide nitrogen of the Asn residue attached to the reducing terminus of the A2-Asn acceptor is shown as a yellow sphere indicating the extension of the Asn-linked peptide into solvent.
Discussion
In this study, the acceptor substrate specificity of FUT8 was further evaluated with a library of aglycone-modified high-mannose and complex-type N-glycans. While FUT8 showed almost no activity on free N-glycans lacking an unmodified β1,2-linked GlcNAc moiety on the Man-α1,3-Man arm (16,18–26), our experimental data revealed that the substrate activity could be rescued when the N-glycans are presented as Fmoc derivatives on the attached amino acid or in a suitable peptide/protein context. Thus, FUT8 presents a much more broadened specificity than what was previously indicated, particularly for substrates with an extended glycone substituent.
FUT8 was observed to be efficient in fucosylating paucimannose glycans in a glycoprotein context. Core-fucosylated paucimannosylation has been widely detected in lysosomal enzymes from human neutrophils, key granulocytic cells of the innate immune system (33). It is reported that neutrophil elastase modulates the immune function of cells through its core-fucosylated paucimannosylation (31). Even the released core-fucosylated paucimannose glycan from elastase could directly inhibit the growth of Pseudomonas aeruginosa, a clinically relevant bacterium, in sub-micromolar concentration (41). Our observation can provide a feasible synthetic route to prepare neutrophil lysosome enzymes with core-fucosylated paucimannosylation for functional studies. In addition to their presence on human neutrophil elastase, core-fucosylated paucimannosylation has been observed and is regarded as a signature of several types of cancers (32,33). While it is conceivable to think that the core-fucosylated paucimannose glycoforms are generated by the trimming of the core-fucosylated complex type or hybrid type glycoforms in lysosomes after they leave the Golgi (33), our experimental data imply that a GnTI-independent direct core-fucosylation of paucimannose glycans could be an alternative biosynthetic pathway for the generation of these structures, particularly when a suitable protein context promotes enzymatic core fucosylation.
Our kinetic studies provide some interesting insights in the underlying mechanism of the aglycone-promoted FUT8 fucosylation of N-glycans. For poor substrates, such as Man5GlcNAc2, Fmoc moiety attached to the Asn residue increased the overall catalytic efficiency by ~7.4-fold, mainly through an increase in kcat. For the moderately active acceptor substrate, GlcNAc1Man5GlcNAc2, the presence of the Asn-linked Fmoc moiety resulted in a 10.5-fold enhancement of catalytic efficiency, largely resulting from its enhanced affinity to the enzyme (as reflected by the significantly reduced value of KM). In contrast, the high-affinity N-glycan acceptor, A2-Asn, was only modestly enhanced in catalytic efficiency as a result of the Fmoc modification, presumably because the glycan component already conferred high binding affinity. Indeed, prior crystal structures showed a tight association of the entire A2 glycan structure with the acceptor binding site while only the Asn residue beyond the glycan-bound amide nitrogen extended into solvent (25–27). Electrostatic surface analysis of FUT8 showed no obvious hydrophobic or polar regions adjacent to the Asn residue to interact with an aglycone substituent (Figure 6). Thus, the structures provide little insight into the contribution of the extended substituents (Fmoc, peptides, or larger proteins) in the enhancement of substrate recognition. Nevertheless, the results are consistent with our kinetic analysis, indicating that aglycone modification (e.g., Fmoc attachment) resulted in moderate enhancement of the reaction rate especially for low affinity substrates.
Interestingly, the structural studies (25,26) of FUT8:GDP:A2-Asn complexes noted that the GlcNAc-β1,4-GlcNAc core of the glycan acceptor complex was bound in an energetically unfavorable anti-ψ conformation for the glycosidic linkage, where the two N-acetyl groups are on the same side, in contrast to the lower free energy form of the glycosidic linkage rotated 180° (25). Therefore, one hypothesis to explain our finding is that a proper aglycone moiety can possibly stabilize the unfavorable cis conformation of the glycan or promote the conformational change for less favorable N-glycan acceptors. Further kinetic and structural analysis with suitable aglycone-modified glycan substrates is needed to clarify the mechanism.
Taken together, our experimental data suggest that an appropriate aglycone context could significantly enhance the core-fucosylation of N-glycans by FUT8, and that FUT8 has a more broadened substrate specificity than was previously appreciated. It is expected that site-directed mutagenesis or directed evolution could potentially improve enzymatic activity toward aglycone-modified acceptors and further broaden the substrate specificity of FUT8, thus providing an efficient tool for direct core-fucosylation of a range of N-glycans or glycoproteins.
Materials and Methods
Materials and reagents.
The FUT8 expression constructs were generated by inserting the FUT8 coding region (residues 41–575, Uniprot Q9BYC5) into the pGEN2-DEST vector with an 8×His-tag and a superfold GFP fusion sequence at the N-terminus. The enzyme was expressed in HEK293 cells (293-F cells, Thermo Fisher) and purified according to previously described procedures (42). The EndoM N175Q enzyme was prepared according to the previously reported procedure (43).
Cell strains and culture.
The FUT8 overexpression cell line (FUT8+) was generated from HEK 293T by lentiviral transduction (17). The stable cell line was created by infecting HEK 293T cells with lentivirus followed by selecting with 1 μg/mL of puromycin overnight. Both the HEK 293T cells and their derivatized cells were grown in suspension culture supplied with the serum-free FreeStyle™ F17 Expression Medium (ThermoFisher Scientific), with shaking at 150 rpm/min, at 37 °C with 8% CO2. Cells were subcultured every 3 days with a seeding density of 4×105 cells/mL.
In vivo core-fucosylation of high mannose type glycoform of EPO by overexpression of FUT8.
To evaluate the enzymatic activity of FUT8 on high-mannose glycans in vivo, plasmids encoding EPO and GM-CSF (17) were transfected into HEK 293T FUT8+ cells by the PEI protocol. Together with the transfection, 1 μg/mL of kifunensine was also added to the cell culture medium to obtain high-mannose type glycosylation. The cell culture medium was harvest 3 days after the transfection, and the proteins were purified by HisTrap™ HP Column (GE Healthcare Life Sciences). The N-glycan of both proteins were released by PNGase F treatment and analyzed by MALDI-TOF MS analysis, following the previously reported procedure (17).
Preparation of high-mannose glycoform of GM-CSF and EPO.
Plasmids encoding EPO and GM-CSF (17) were used to transfected into HEK 293T cells, and 1 μg/mL of kifunensine was added to the cell culture medium. Proteins were harvested 3 days after transfection and purified on a HisTrap HP column (GE Healthcare Life Sciences). Treatment of the purified EPO-HM with α1–2,3,6-mannosidase (Agilent) gave a mixture of paucimannose glycoform of EPO (EPO-PM), which was used as substrates for evaluating the substrate specificity of FUT8.
Preparation of Fmoc-labeled N-glycans from chicken ovalbumin.
1g of the chicken egg white ovalbumin (OVA, Millipore Sigma) protein was firstly denatured under 33 mL of 0.01 M HCl at 50 °C for 40 minutes. Then the total glycans of OVA were released by an exhaustive digestion of pronase at pH 8.0 for 3 days. The asparagine-linked glycans were isolated from the reaction mixture by Sephadex™ G-50 gel filtration (GE Healthcare Life Sciences) followed by a Waters 600 preparative HPLC using an Xbridge™ Prep Shield RP C18 column (Waters). The N-terminal tagging was done with 5 molar equivalents of 9-fluorenylmethyloxycarbonyl chloride (Fmoc-Cl, Millipore Sigma) in an aqueous acetone (Millipore Sigma) solution containing NaHCO3 (Millipore Sigma) at room temperature for 2 hours. Products were extracted by chloroform (Millipore Sigma) and then purified by preparative RP HPLC. Lastly, the C-terminal capping was done in dimethylformamide (DMF, Millipore Sigma) with 5 molar equivalents of 1-[Bis(dimethylamino)methylene]-1H-1,2,3-triazolo[4,5-b]pyridinium 3-oxide hexafluorophosphate (HATU, ChemPep Inc.), 5 molar equivalents of N,N-diisopropylethylamine (DIPEA, ChemPep Inc.), and 5 molar equivalents of methylamine·HCl (Millipore Sigma) at room temperature for 2 hours. Final products were purified by RP HPLC and lyophilized.
Preparation of V3 glycopeptides as FUT8 substrates.
The HIV-1 V3 GlcNAc-Peptide, (2.0 mg, 0.48 μmol), which was synthesized as described previously (39,40), was incubated together with SCT-oxazoline (3.8 mg, 1.9 mmol) and EndoM N175Q (7 μg) at 30 °C in a phosphate buffer (100 mM, pH 7.4, 40 μL). After 2 h when HPLC monitoring indicated the completion of the transglycosylation, the reaction was quenched using 0.1% aq. TFA. The product was purified by RP-HPLC to give glycopeptide V3-SCT as a white powder (2.43 mg, 82%). ESI MS: calcd. M= 6188.48; found (m/z): 1032.50 [M + 6H]6+, 1238.96 [M + 5H]5+, 1548.29 [M + 4H]4+. RP-HPLC retention time, tR = 21.25 min. The analytical HPLC was performed on a C18 column (Thermo Scientific Hypersil Gold®, 4.6 × 250 mm, 3 μm) at a flow rate of 1 mL/min using a linear gradient of 5–35% MeCN containing 0.1% TFA over 30 min. For the synthesis of the asialylated V3 glycopeptide, a solution of the glycopeptide V3-SCT (1.00 mg, 0.16 μmol) was treated with neuraminidase MvNA (44) (final concentration, 0.01 μg/μL) in 100 mM phosphate buffer (100 μL, pH 7.2) at rt for 3 h. The reaction was then quenched with 0.1% aq. TFA. The asialylated glycopeptide product was purified by RP-HPLC to give V3-CT (0.84 mg, 93%) as a white powder. ESI-MS of V3-CT: calcd., M= 5606.00; found (m/z): 935.54 [M + 6H]6+, 1122.53 [M + 5H]5+, 1402.69 [M + 4H]4+. RP-HPLC retention time, tR = 19.78 min. HPLC was performed on a C18 column (Thermo Scientific Hypersil Gold®, 4.6 × 250 mm, 3 μm) at a flow rate of 1 mL/min using a linear gradient of 5–35% MeCN containing 0.1% TFA over 30 min.
Preparation of GlcNAc2Man3GlcNAc2Asn (A2-Asn) and its Fmoc tagged derivative ( A2Asn-Fmoc).
A mixture of the sialoglycopeptide (SGP) (20 mg, 7 μmol), obtained from chicken egg yolk (45), and pronase (0.4 mg) in a Tris-HCl buffer (50 mM, 1 ml) containing CaCl2 (10 mM) and NaN3 (0.02%) NaN3 was incubated at 55 °C for 5 days. The resulting product was purified with Sephadex™ G15 (GE Healthcare Life Sciences) size-exclusion chromatography to give the Asn-linked biantennary complex type N-glycan (15 mg, 91% yield). Then the Asn-linked glycan was treated with the MvNA α2,6-sialidase (44) (0.1 mg/mL) and a β1,4-galactosidase (BgaA) (0.2 mg/mL) at room temperature for 2 h. The crude product was purified with Sephadex™ G15 (GE Healthcare Life Sciences) size-exclusion chromatography and then lyophilized to afford the desired GlcNAc2Man3GlcNAc2Asn (A2-Asn) as a white powder (8.6 mg, 94% yield). MALDI-TOF-MS: calcd for A2-Asn, M = 1430.5 Da; found (m/z), 1453.5 [M + Na]+. To prepare the A2Asn-Fmoc derivative, A2Asn (5 mg, 3.5 μmol, 20 mg/mL) was added to a solution of Fmoc-Cl (4.5 mg, 17.5 μmol, 50 mg/mL) in acetone. The reaction mixture was incubated at 37 °C for 1 h. After evaporation, the crude product was then purified with RP-HPLC and the fractions containing the product were pooled and lyophilized to afford the A2Asn-Fmoc as a white powder (5.1 mg, 88% yiled). MALDI-TOF MS: calcd for A2Asn-Fmoc, M = 1652.6 Da; found (m/z), 1675.5 [M + Na]+.
In vitro core-fucosylation with FUT8 enzyme.
All the FUT8 in vitro experiments were carried out for the indicated times in 0.1 M of MES buffer (pH 7) at 37 °C. For the reaction with glycoproteins, 1 μmol of the substrates (EPO-HM, EPO-PM, and EPO-GlcNAc) were mixed with 1 mM of GDP-Fucose (Biosynth Carbosynth) and 0.05 mg/mL of FUT8. For chemically modified N-glycans and glycopeptides, the reactions were performed with 5 molar equivalents of GDP-Fucose and 0.5 mg/mL of the FUT8 enzyme.
Liquid chromatography-mass spectrometry (LC-MS).
The fractions of samples were taken from the reaction mixture, monitored, and analyzed by LC-MS. The reaction with EPO-GlcNAc was evaluated by an ExactivePlus Orbitrap LC-MS (ThermoFisher Scientific) with an Xbridge™ Protein BEH C4, 300Å column (Waters). The method was developed with 0.1% formic acid under a 5%−90% MeCN gradient for 6 minutes. Reactions with glycopeptides were analyzed by SQ detector 2 (Waters) with an Xbridge™ BEH C18, 130Å column (Waters) under a 10-minute method with 5%−90% MeCN gradient supplied with 0.1% formic acid. Molecular weights of both the glycoprotein and the glycopeptides were deconvoluted from the m/z peaks via MagTran software.
Matrix-assisted laser desorption/ionization time-of-flight mass spectrometry (MALDI-TOF MS) analysis.
The total glycan on glycoproteins were released by PNGaseF (New England Biolabs) under the native condition and enriched by HyperSep™ Hypercarb™ SPE Cartridges (ThermoFisher Scientific) with the previously published protocol (46). The purified glycan was lyophilized and analyzed by the Bruker AutoFlex Mass Spectrometer (MALDI) with the N, N-dimethylaniline supplied 2,5-dihydroxybenzoic acid (DHB) matrix (47). Peaks were assigned by GlycoWorkbench.
Kinetic analysis of the substrates using FUT8 enzyme.
The relative enzyme activity of the substrates, A2-Asn, A2Asn-Fmoc, GlcNAc1Man5GlcNAc2-Asn, GlcNAc1Man5GlcNAc2-Asn-Fmoc, Man5GlcNAc2-Asn and Man5GlcNAc2-Asn-Fmoc, were determined by titrating with wild type Fut8 enzyme purified as reported earlier (27). Enzyme kinetics were performed using GDP-Glo™ Glycosyltransferase assay (Promega) for the substrates at a concentration range of 0–1mM (0–1.5 mM for Man5GlcNAc2-Asn) along with GDP-fucose (0.2 mM final concentration, pre-treated with Calf Intestinal alkaline-phosphatase (Promega)) as donor sugar (27). Reactions were carried out in a 10 μl reaction volume consisting of a universal buffer (200 mM each of Tris, MES, MOPS, pH 7.5) with the purified wild type enzyme at 37°C for 30 min. Reactions were stopped using 5 μl of GDP detection reagent and an equal volume of reaction mix in a polystyrene, white 384-well plate and incubating in dark for 1 h at room temperature. The luminescence values were measured using a GloMax Multi detection plate reader (Promega) and compared with a GDP standard curve to quantify the final released GDP product. The steady state parameters of KM, kcat, and kcat/KM values were determined using nonlinear curve fitting in GraphPad Prism 6 software.
Acknowledgment
This work was supported by the US National Institutes of Health (NIH grant R01 GM080374 to L.X.W. and P41GM103390, P01GM107012, and R01GM130915 to K.W.M.).
Abbreviations:
- FUT8
α1,6-fucosyltransferase
- EPO
erythropoietin
- GM-CSF
granulocyte-macrophage colony-stimulating factor
- OVA
chicken egg white ovalbumin
- Fmoc
9-flurenlymethyl chloroformate
- GlcNAc
N-acetyl-glucosamine
- LC-MS
Liquid chromatography-mass spectrometry
- MALDI-TOF
Matrix-assisted laser desorption/ionization time-of-flight
- V3
the third variable loop of HIV-1 gp120
Footnotes
Data availability statement. All data that support the findings of this study are contained within the manuscript
Conflict of interest
The authors declare that they have no conflicts of interest with the contents of this article.
References
- 1.Varki A (2017) Biological roles of glycans. Glycobiology 27, 3–49 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wang X, Inoue S, Gu J, Miyoshi E, Noda K, Li W, Mizuno-Horikawa Y, Nakano M, Asahi M, Takahashi M, Uozumi N, Ihara S, Lee SH, Ikeda Y, Yamaguchi Y, Aze Y, Tomiyama Y, Fujii J, Suzuki K, Kondo A, Shapiro SD, Lopez-Otin C, Kuwaki T, Okabe M, Honke K, and Taniguchi N (2005) Dysregulation of TGF-beta1 receptor activation leads to abnormal lung development and emphysema-like phenotype in core fucose-deficient mice. Proc Natl Acad Sci U S A 102, 15791–15796 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chandler KB, Leon DR, Kuang J, Meyer RD, Rahimi N, and Costello CE (2019) N-Glycosylation regulates ligand-dependent activation and signaling of vascular endothelial growth factor receptor 2 (VEGFR2). J Biol Chem 294, 13117–13130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Li W, Yu R, Ma B, Yang Y, Jiao X, Liu Y, Cao H, Dong W, Liu L, Ma K, Fukuda T, Liu Q, Ma T, Wang Z, Gu J, Zhang J, and Taniguchi N (2015) Core fucosylation of IgG B cell receptor is required for antigen recognition and antibody production. J. Immunol. 194, 2596–2606 [DOI] [PubMed] [Google Scholar]
- 5.Liang W, Mao S, Sun S, Li M, Li Z, Yu R, Ma T, Gu J, Zhang J, Taniguchi N, and Li W (2018) Core Fucosylation of the T Cell Receptor Is Required for T Cell Activation. Front Immunol 9, 78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Iijima J, Kobayashi S, Kitazume S, Kizuka Y, Fujinawa R, Korekane H, Shibata T, Saitoh SI, Akashi-Takamura S, Miyake K, Miyoshi E, and Taniguchi N (2017) Core fucose is critical for CD14-dependent Toll-like receptor 4 signaling. Glycobiology 27, 1006–1015 [DOI] [PubMed] [Google Scholar]
- 7.Okada M, Chikuma S, Kondo T, Hibino S, Machiyama H, Yokosuka T, Nakano M, and Yoshimura A (2017) Blockage of Core Fucosylation Reduces Cell-Surface Expression of PD-1 and Promotes Anti-tumor Immune Responses of T Cells. Cell Rep 20, 1017–1028 [DOI] [PubMed] [Google Scholar]
- 8.Keeley TS, Yang S, and Lau E (2019) The Diverse Contributions of Fucose Linkages in Cancer. Cancers (Basel) 11, 1241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen CY, Jan YH, Juan YH, Yang CJ, Huang MS, Yu CJ, Yang PC, Hsiao M, Hsu TL, and Wong CH (2013) Fucosyltransferase 8 as a functional regulator of nonsmall cell lung cancer. Proc Natl Acad Sci U S A 110, 630–635 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Miyoshi E, Moriwaki K, and Nakagawa T (2008) Biological function of fucosylation in cancer biology. J Biochem 143, 725–729 [DOI] [PubMed] [Google Scholar]
- 11.Ma T, Wang Y, Jia L, Shu J, Yu H, Du H, Yang J, Liang Y, Chen M, and Li Z (2019) Increased expression of core-fucosylated glycans in human lung squamous cell carcinoma. RSC Advances 9, 22064–22073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Niwa R, Sakurada M, Kobayashi Y, Uehara A, Matsushima K, Ueda R, Nakamura K, and Shitara K (2005) Enhanced natural killer cell binding and activation by low-fucose IgG1 antibody results in potent antibody-dependent cellular cytotoxicity induction at lower antigen density. Clin. Cancer Res. 11, 2327–2336 [DOI] [PubMed] [Google Scholar]
- 13.Shields RL, Lai J, Keck R, O’Connell LY, Hong K, Meng YG, Weikert SH, and Presta LG (2002) Lack of fucose on human IgG1 N-linked oligosaccharide improves binding to human Fcgamma RIII and antibody-dependent cellular toxicity. J. Biol. Chem. 277, 26733–26740 [DOI] [PubMed] [Google Scholar]
- 14.Jefferis R (2009) Glycosylation as a strategy to improve antibody-based therapeutics. Nat. Rev. Drug Discov. 8, 226–234 [DOI] [PubMed] [Google Scholar]
- 15.Wang LX, Tong X, Li C, Giddens JP, and Li T (2019) Glycoengineering of Antibodies for Modulating Functions. Annu Rev Biochem 88, 433–459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wilson JR, Williams D, and Schachter H (1976) The control of glycoprotein synthesis: N-acetylglucosamine linkage to a mannose residue as a signal for the attachment of L-fucose to the asparagine-linked N-acetylglucosamine residue of glycopeptide from alpha1-acid glycoprotein. Biochem Biophys Res Commun 72, 909–916 [DOI] [PubMed] [Google Scholar]
- 17.Yang Q, and Wang LX (2016) Mammalian alpha-1,6-Fucosyltransferase (FUT8) Is the Sole Enzyme Responsible for the N-Acetylglucosaminyltransferase I-independent Core Fucosylation of High-mannose N-Glycans. J Biol Chem 291, 11064–11071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Longmore GD, and Schachter H (1982) Product-identification and substrate-specificity studies of the GDP-L-fucose:2-acetamido-2-deoxy-beta-D-glucoside (FUC goes to Asn-linked GlcNAc) 6-alpha-L-fucosyltransferase in a Golgi-rich fraction from porcine liver. Carbohydr Res 100, 365–392 [DOI] [PubMed] [Google Scholar]
- 19.Shao MC, Sokolik CW, and Wold F (1994) Specificity studies of the GDP-[L]-fucose: 2-acetamido-2-deoxy-beta-[D]-glucoside (Fuc-->Asn-linked GlcNAc) 6-alpha-[L]-fucosyltransferase from rat-liver Golgi membranes. Carbohydr Res 251, 163–173 [DOI] [PubMed] [Google Scholar]
- 20.Voynow JA, Kaiser RS, Scanlin TF, and Glick MC (1991) Purification and characterization of GDP-L-fucose-N-acetyl beta-D-glucosaminide alpha 1--−−6fucosyltransferase from cultured human skin fibroblasts. Requirement of a specific biantennary oligosaccharide as substrate. J Biol Chem 266, 21572–21577 [PubMed] [Google Scholar]
- 21.Kaminska J, Glick MC, and Koscielak J (1998) Purification and characterization of GDP-L-Fuc: N-acetyl beta-D-glucosaminide alpha1-->6fucosyltransferase from human blood platelets. Glycoconj J 15, 783–788 [DOI] [PubMed] [Google Scholar]
- 22.Ihara H, Ikeda Y, and Taniguchi N (2006) Reaction mechanism and substrate specificity for nucleotide sugar of mammalian alpha1,6-fucosyltransferase--a large-scale preparation and characterization of recombinant human FUT8. Glycobiology 16, 333–342 [DOI] [PubMed] [Google Scholar]
- 23.Calderon AD, Liu Y, Li X, Wang X, Chen X, Li L, and Wang PG (2016) Substrate specificity of FUT8 and chemoenzymatic synthesis of core-fucosylated asymmetric N-glycans. Org Biomol Chem 14, 4027–4031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tseng TH, Lin TW, Chen CY, Chen CH, Lin JL, Hsu TL, and Wong CH (2017) Substrate Preference and Interplay of Fucosyltransferase 8 and N-Acetylglucosaminyltransferases. J Am Chem Soc 139, 9431–9434 [DOI] [PubMed] [Google Scholar]
- 25.Garcia-Garcia A, Ceballos-Laita L, Serna S, Artschwager R, Reichardt NC, Corzana F, and Hurtado-Guerrero R (2020) Structural basis for substrate specificity and catalysis of alpha1,6-fucosyltransferase. Nat Commun 11, 973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jarva MA, Dramicanin M, Lingford JP, Mao R, John A, Jarman KE, Grinter R, and Goddard-Borger ED (2020) Structural basis of substrate recognition and catalysis by fucosyltransferase 8. J Biol Chem 295, 6677–6688 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Boruah BM, Kadirvelraj R, Liu L, Ramiah A, Li C, Zong G, Bosman GP, Yang JY, Wang LX, Boons GJ, Wood ZA, and Moremen KW (2020) Characterizing human alpha-1,6-fucosyltransferase (FUT8) substrate specificity and structural similarities with related fucosyltransferases. J Biol Chem 295, 17027–17045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Crispin M, Harvey DJ, Chang VT, Yu C, Aricescu AR, Jones EY, Davis SJ, Dwek RA, and Rudd PM (2006) Inhibition of hybrid- and complex-type glycosylation reveals the presence of the GlcNAc transferase I-independent fucosylation pathway. Glycobiology 16, 748–756 [DOI] [PubMed] [Google Scholar]
- 29.Balog CI, Stavenhagen K, Fung WL, Koeleman CA, McDonnell LA, Verhoeven A, Mesker WE, Tollenaar RA, Deelder AM, and Wuhrer M (2012) N-glycosylation of colorectal cancer tissues: a liquid chromatography and mass spectrometry-based investigation. Mol Cell Proteomics 11, 571–585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yang Q, Zhang R, Cai H, and Wang LX (2017) Revisiting the substrate specificity of mammalian alpha1,6-fucosyltransferase reveals that it catalyzes core fucosylation of N-glycans lacking alpha1,3-arm GlcNAc. J Biol Chem 292, 14796–14803 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Loke I, Ostergaard O, Heegaard NHH, Packer NH, and Thaysen-Andersen M (2017) Paucimannose-Rich N-glycosylation of Spatiotemporally Regulated Human Neutrophil Elastase Modulates Its Immune Functions. Mol Cell Proteomics 16, 1507–1527 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chatterjee S, Lee LY, Kawahara R, Abrahams JL, Adamczyk B, Anugraham M, Ashwood C, Sumer-Bayraktar Z, Briggs MT, Chik JHL, Everest-Dass A, Forster S, Hinneburg H, Leite KRM, Loke I, Moginger U, Moh ESX, Nakano M, Recuero S, Sethi MK, Srougi M, Stavenhagen K, Venkatakrishnan V, Wongtrakul-Kish K, Diestel S, Hoffmann P, Karlsson NG, Kolarich D, Molloy MP, Muders MH, Oehler MK, Packer NH, Palmisano G, and Thaysen-Andersen M (2019) Protein Paucimannosylation Is an Enriched N-Glycosylation Signature of Human Cancers. Proteomics 19, e1900010 [DOI] [PubMed] [Google Scholar]
- 33.Tjondro HC, Loke I, Chatterjee S, and Thaysen-Andersen M (2019) Human protein paucimannosylation: cues from the eukaryotic kingdoms. Biol Rev Camb Philos Soc 94, 2068–2100 [DOI] [PubMed] [Google Scholar]
- 34.Yang Q, Li C, Wei Y, Huang W, and Wang LX (2010) Expression, glycoform characterization, and antibody-binding of HIV-1 V3 glycopeptide domain fused with human IgG1-Fc. Bioconjugate Chem. 21, 875–883 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Harvey DJ, Wing DR, Kuster B, and Wilson IB (2000) Composition of N-linked carbohydrates from ovalbumin and co-purified glycoproteins. J Am Soc Mass Spectrom 11, 564–571 [DOI] [PubMed] [Google Scholar]
- 36.Larsen MR, Hojrup P, and Roepstorff P (2005) Characterization of gel-separated glycoproteins using two-step proteolytic digestion combined with sequential microcolumns and mass spectrometry. Mol Cell Proteomics 4, 107–119 [DOI] [PubMed] [Google Scholar]
- 37.Bank S, Heller E, Memmel E, Seibel J, Holzgrabe U, and Kapkova P (2014) Matrix-assisted laser desorption/ionization tandem mass spectrometry of N-glycans derivatized with isonicotinic hydrazide and its biotinylated form. Rapid Commun Mass Spectrom 28, 1745–1756 [DOI] [PubMed] [Google Scholar]
- 38.Nakano M, Higo D, Arai E, Nakagawa T, Kakehi K, Taniguchi N, and Kondo A (2009) Capillary electrophoresis-electrospray ionization mass spectrometry for rapid and sensitive N-glycan analysis of glycoproteins as 9-fluorenylmethyl derivatives. Glycobiology 19, 135–143 [DOI] [PubMed] [Google Scholar]
- 39.Cai H, Orwenyo J, Giddens JP, Yang Q, Zhang R, LaBranche CC, Montefiori DC, and Wang LX (2017) Synthetic Three-Component HIV-1 V3 Glycopeptide Immunogens Induce Glycan-Dependent Antibody Responses. Cell Chem Biol 24, 1513–1522 e1514 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cai H, Orwenyo J, Guenaga J, Giddens J, Toonstra C, Wyatt RT, and Wang LX (2017) Synthetic multivalent V3 glycopeptides display enhanced recognition by glycan-dependent HIV-1 broadly neutralizing antibodies. Chem. Commun. (Camb) 53, 5453–5456 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Thaysen-Andersen M, Venkatakrishnan V, Loke I, Laurini C, Diestel S, Parker BL, and Packer NH (2015) Human neutrophils secrete bioactive paucimannosidic proteins from azurophilic granules into pathogen-infected sputum. J Biol Chem 290, 8789–8802 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Moremen KW, Ramiah A, Stuart M, Steel J, Meng L, Forouhar F, Moniz HA, Gahlay G, Gao Z, Chapla D, Wang S, Yang JY, Prabhakar PK, Johnson R, Rosa MD, Geisler C, Nairn AV, Seetharaman J, Wu SC, Tong L, Gilbert HJ, LaBaer J, and Jarvis DL (2018) Expression system for structural and functional studies of human glycosylation enzymes. Nat Chem Biol 14, 156–162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Umekawa M, Li C, Higashiyama T, Huang W, Ashida H, Yamamoto K, and Wang LX (2010) Efficient glycosynthase mutant derived from Mucor hiemalis endo-beta-N-acetylglucosaminidase capable of transferring oligosaccharide from both sugar oxazoline and natural N-glycan. J. Biol. Chem. 285, 511–521 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Watson JN, Dookhun V, Borgford TJ, and Bennet AJ (2003) Mutagenesis of the conserved active-site tyrosine changes a retaining sialidase into an inverting sialidase. Biochemistry 42, 12682–12690 [DOI] [PubMed] [Google Scholar]
- 45.Seko A, Koketsu M, Nishizono M, Enoki Y, Ibrahim HR, Juneja LR, Kim M, and Yamamoto T (1997) Occurence of a sialylglycopeptide and free sialylglycans in hen’s egg yolk. Biochim. Biophys. Acta 1335, 23–32 [DOI] [PubMed] [Google Scholar]
- 46.Packer NH, Lawson MA, Jardine DR, and Redmond JW (1998) A general approach to desalting oligosaccharides released from glycoproteins. Glycoconj. J. 15, 737–747 [DOI] [PubMed] [Google Scholar]
- 47.Snovida SI, Rak-Banville JM, and Perreault H (2008) On the use of DHB/aniline and DHB/N,N-dimethylaniline matrices for improved detection of carbohydrates: automated identification of oligosaccharides and quantitative analysis of sialylated glycans by MALDI-TOF mass spectrometry. J Am Soc Mass Spectrom 19, 1138–1146 [DOI] [PubMed] [Google Scholar]