Dear Editor,
The coronavirus disease 2019 (COVID-19), caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV2), accounted for more than 103 million cases worldwide as of early February 2021 [1]. Although most patients have moderate symptoms, severe acute respiratory distress syndrome develops in about 17% of cases, including 5% with critical forms, which results in hospitalization [2]. Patients are admitted to intensive care units (ICUs) [3] for unusually long periods of time [4], [5]. As a result of prolonged strict bed rest, virus infection and associated medical procedures such as endotracheal intubation and deep sedation, patients surviving this critical form of COVID-19 exhibit considerable musculoskeletal loss and dysfunction [6], [7]. In association with altered pulmonary and cardiac function along with fatigue and containment measures, this situation results in a marked reduction in physical activity, which in turn hampers clinical recovery.
Rehabilitation of patients with critical COVID-19 presents new challenges, reinforced by the novelty of the disease and the preventive measures related to patient contagiousness [8]. Functional electrical stimulation (FES) applied during cycling (FES-cycling) is used in patients with chronic lung disease to increase exercise intensity [9], and electrical stimulation is known to improve functional exercise capacity and quadriceps strength [10]. FES-cycling is widely used for patients with spinal cord injury and complete motor paralysis and allows for synchronized contractions and restoring lower-limb mobility [11]; electrical stimulation may improve muscle strength after an incomplete lesion [12].
In this 4-week pragmatic proof-of-concept controlled study, we hypothesized that FES-cycling could be safely used in combination with physiotherapy early after ICU discharge in patients with critical COVID-19 and favour erectus position restoration and spontaneous walking resumption more rapidly than rehabilitation without FES-cycling.
We included 14 patients admitted to the rehabilitation department of the Hospices Civils de Lyon during the first pandemic wave in France (i.e., from April 20, 2020 to July 16, 2020) after hospitalisation in the ICU for a critical form of COVID-19. Cycling was part of the rehabilitation program with FES for 8 patients (FES-cycling group) or without FES for 6 (cycling group). Exclusion criteria were cognitive deficit or neurological or psychiatric comorbidity. All patients signed an informed consent for the use of their clinical data for research purposes (approved by the Hospices Civils de Lyon Ethics Committee: No. 20-79).
Briefly, all patients participated in a similar rehabilitation program of 20 days for a total of 4 weeks (3 weeks as an inpatient and 1 week in outpatient facilities). The program consisted of 2 sessions of physiotherapy (30 min each) and one 30-min session of cycling per day by using the RehaMove muvi (Hasomed GmbH, Magedburg, Germany) with FES (FES-cycling group) or without FES (cycling group). During cycling, the participant sat in a standardised chair, with their feet secured to the pedals and the chair-to-cycling distance adjusted to obtain a minimum of 10° knee flexion while pedalling. The FES-cycling was performed with the RehaStim2 stimulator for FES (Hasomed GmbH, Magedburg, Germany) to which adhesive electrodes were attached to the muscle surface of the quadriceps, hamstrings, tibialis anterior and gluteus maximus or triceps surae (see movie). Using the “adaptive mode”, the stimulator progressively adjusted the current intensity to attain exercise targets (e.g., pedalling speed 60 rpm and resistance 2–3 Nm). Patients were instructed to maintain a plateau of at least 60% of the maximum stimulation intensity setting and to slow down the pedalling (i.e., > 60 rpm) to maintain a constant stimulation level. For both groups, the cycling duration was progressively extended by 5 min/session, starting from 15 min up to 30 min to ensure tolerance. Across sessions, FES-cycling training was adjusted by increasing the plateau of stimulation up to 100% of the maximum stimulation intensity setting (the maximum was then progressively increased) for the FES-cycling group or by increasing the pedalling resistance to target a rated perception of effort equal to 4–5/10 (no effort = 0, maximum effort = 10) for the cycling alone group. Because the FES-cycling tolerance was unknown, oxygen saturation and heart rate were monitored with an infrared finger sensor; dyspnea and pain were evaluated by using the Borg CR-10 scale and a visual analog scale (0–10).
Patients of both groups underwent a weekly clinical evaluation throughout the 4-week rehabilitation program. Sedentary and physical activity patterns were determined by using a triaxial accelerometer (Actigraph, wGT3X, Pensacola, FL, USA) that was worn on the right hip throughout the 24-h cycle, during both night and day, for a minimum of 2 days at the beginning of each week. Days including < 10 h of daytime registration were excluded from the analyses. After excluding non-wearing time, time spent in different sedentary/activity postures was estimated by using an automatic posture/activity recognition algorithm coupled with an activity-specific model as described [13]. For this study, the 11 initial postures/activities were merged in 4 classes: night-bedtime, sedentary daytime (lying down, reclining or sitting), standing/trampling and walking/running. The clinical parameters included strength of the deltoid, biceps brachii, wrist extensor, iliopsoas, quadriceps and tibialis anterior muscles measured with the manual muscle test of the Medical Research Council (scores 0–5/5) and forced expiratory volume in 1 sec and force vital capacity measured with a spirometer. Blinding of patients and physiotherapists was not possible because of the nature of the intervention, but the objective accelerometry posture assessment was performed without knowledge of the patient rehabilitation program.
Statistical analyses were performed with SAS 9.4 (SAS Institute, Cary, NC, USA). Baseline demographic and descriptive characteristics are summarized as mean (SD) or median (interquartile range [IQR]) for continuous variables as appropriate and as number (%) for categorical variables. Differences between groups were analysed with unpaired Student t-test, Mann–Whitney–Wilcoxon test or Chi2 test. We used linear mixed-effects models to test for the effect of FES on the 4-week net changes from baseline of the primary (sedentary daytime) and secondary (walking/running daytime) outcomes, accounting for repeated measurements among individuals, with individuals as a random effect (PROC MIXED). Fixed factors included a common intercept, indicator variables for follow-up time, and FES group by follow-up time indicator interaction variables [14]. All available patient data were used on an intention-to-treat basis; models were fit by using REML estimation and a compound symmetry covariance structure, as selected with the Bayesian information criterion. Standardized residuals from statistical models were tested for normality by using Kolmogorov–Smirnov tests. Our primary statistical inference was between-group differences in 4-week net changes in sedentary and daytime walking/running, estimated with their 95% confidence intervals (CIs) with 2-sided hypothesis tests and P < 0.05 considered statistically significant. The effect size was estimated by dividing the between-group difference in 4-week mean net changes by the estimation of their common standard deviation. Results are presented as least square means (SE) unless otherwise noted. Additional exploratory analyses were performed to test the effect of FES on the mean 4-week net changes in different clinical outcomes with similar mixed-effects models and are presented as supplementary data.
Baseline demographic and clinical characteristics of the 14 studied patients (6 cycling and 8 FES-cycling) did not differ between groups (Table 1 ). For both groups, the median (IQR) cycling training was 8.5 (7.0–13.0) sessions, corresponding to a total duration of 205.4 (154.9–334.8) min, representing 57.1 (40.8–83.0) km pedalling at a speed of 40.0 (27.5–43.5) rpm and resistance of 2.6 (2.0–2.7) Nm. The median (IQR) setting of the maximum stimulation intensity in the FES-cycling group was 46.0 (35.5–52.0) mA for the quadriceps, 41.0 (32.0–51.5) mA for hamstrings, 57.0 (41.5–60.0) mA for tibialis anterior, 46.0 (39.5–50.25) mA for gluteus maximus, and 29.0 (26.5–49.5) mA for triceps surae. During the FES-cycling, all measured SPO2 values were > 90% (96.0% [95.0–97.0]); maximal heart rate was ≤ 128 bpm (97.0 [87.5–107.3] bpm); maximal Borg CR-10 scores were ≤ 5/10 (1.0 [0.0–1.0] CR-10); and the visual analog scale score for pain was ≤ 3/10 (0.0 [0.0–0.0]).
Table 1.
Demographic and clinical baseline characteristics of the study participants according to groups: cycling alone or functional electrical stimulation with cycling (FES-cycling).
| Cycling (n = 6) |
FES-cycling (n = 8) |
|
|---|---|---|
| Sex, M/F | 5/1 | 7/1 |
| Age (years) | 64.8 (7.0) | 62.8 (9.1) |
| BMI (kg.m−2) | 27.1 (4.4) | 28.1 (4.8) |
| Cardiac chronic disease | 2 (33%) | 5 (63%) |
| Pulmonary chronic disease | 0 (0%) | 1 (13%) |
| Intubation duration (days)a | 20.5 (15.5–27.8) | 14.5 (12.0–20.5) |
| Albumin (g/L) | 31.0 (2.7) | 29.7 (4.1) |
| Prealbumin (g/L)a | 0.2 (0.2–0.3) | 0.3 (0.3–0.3) |
| HAD depression score (0 to 10) | 3.0 (2.9) | 5.1 (3.2) |
| MMT (MRC score [0 to 60]) | 50.2 (4.8) | 47.7 (9.2) |
Data are mean (SD) for non-normal variables or n (%). Analyses with unpaired Student t-test, Mann–Whitney–Wilcoxon test or Chi2 test as appropriate showed no differences between groups. FES-cycling: functional electrical stimulation applied during cycling; BMI: body mass index; HAD: Hospital Anxiety and Depression; MMT: Manual Muscle Test using the Medical Research Council score.
Data are median (IQR) for non-normal variables or n (%).
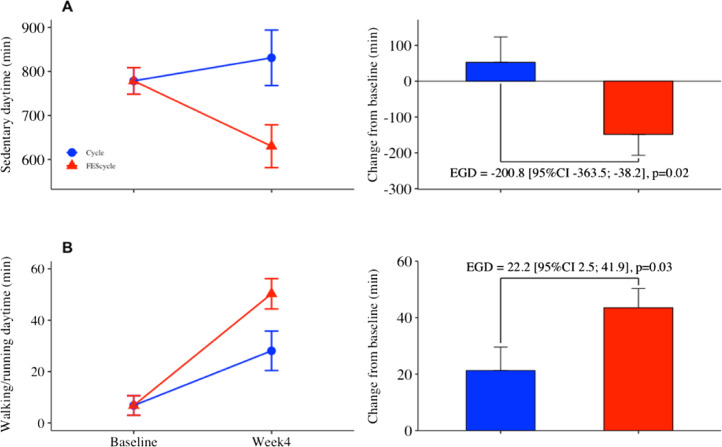
As compared with cycling alone, FES-cycling was associated with greater beneficial decline in the daytime spent sedentary (e.g. lying, reclining or sitting): 200.8 min (95% CI: −363.5; −38.2; P < 0.02) over the 4-week rehabilitation program, representing an effect size of 0.54 (moderate effect) (Fig. 1 and see supplementary Table 1). The FES-cycling group also exhibited a higher increase in time spent walking or running: 22.2 min (95% CI: 2.5; 41.9 min; P < 0.03) (effect size = 0.52). Exploratory analyses showed no effect of FES-cycling on the clinical outcomes studied.
Fig. 1.
Daytime spent sedentary (A) and walking/running (B) lsmeans (SE) at baseline and after the 4-week rehabilitation program (left panel), with corresponding estimated mean group differences (EGD) in net changes from baseline (lsmeans and 95% confidence interval) (right panel). Longitudinal mixed-effects models were used to test the effect of functional electrical stimulation (FES) on EGD, on an intention-to-treat basis (n = 6 in cycling group and n = 8 in FES-cycling group).
This pragmatic proof-of-concept study investigated whether the erectus position restoration and walking resumption in patients with a critical form of COVID-19 could be accelerated by rehabilitation combined with FES-cycling or with cycling alone in an early stage immediately after ICU discharge. All patients progressively improved across rehabilitation weeks but patients who benefitted from FES-cycling had a significantly greater daily-life physical activity recovery profile as compared with the control group. These preliminary results suggest the interest of offering rehabilitation enriched by FES-cycling to patients with critical COVID-19 as soon as they leave the ICU even though the risk of contagion requires rehabilitation under conditions of strict isolation. Nevertheless, the effect of FES-cycling on this improved physical activity recovery profile remains to be understood because it was neither explained by nor associated with an increase in muscle strength or cardiorespiratory adaptation. Instead, knowing the reported effect of electrical stimulation on muscle tone [15], one possible explanation of the FES effect could be a decrease in muscular hypotonia observed in patients with critical COVID-19 because of prolonged bed rest and viral infection.
Another important aspect of this study is the good tolerance for FES-cycling including both cardiovascular and pulmonary parameters but also the feelings related to FES administration. A related striking example was the patient who, out of breath while maintaining the standing position during the time needed to set up the electrodes on gluteus muscles, could subsequently cycle with FES for about 30 min with a small/moderate dyspnea increase. Furthermore, there was no need to adapt, change or cancel physiotherapy sessions (i.e., with no electrical stimulation), which suggests that the fatigue induced by FES-cycling was limited. Thus, FES-cycling could be combined with usual rehabilitation in patients with critical COVID-19.
FES-cycling may be a promising rehabilitation method for early mobilization during and immediately after ICU hospitalization to limit the marked reduction in physical activity and favour erectus position restoration in patients with a critical form of COVID-19 [2], [5], [7]. Further randomized controlled studies are necessary to confirm its efficacy, including for specific clinical outcomes, and to understand the underlying mechanisms.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Disclosure of interest
The authors declare that they have no competing interest.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Author contributions
All authors participated in the study conceptualization, data collection, writing the original draft and revising the manuscript. Both SM and GR supervised the study. GR provided the institutional facilities. CS performed accelerometry analyses. CS and SM performed the statistical analyses. All authors approved the final draft before submission.
Footnotes
Supplementary data associated with this article can be found, in the online version, at https://doi.org/10.1016/j.rehab.2021.101516.
Appendix A. Supplementary data
Movie. Illustrations of muscle contractions elicited by functional electrical stimulation applied during cycling by using RehaMove muvi (Hasomed GmbH, Magedburg, Germany).
References
- 1.Dong E., Du H., Gardner L. An interactive web-based dashboard to track COVID-19 in real time. Lancet Infect Dis. 2020;20:533–534. doi: 10.1016/S1473-3099(20)30120-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Carda S., Invernizzi M., Bavikatte G., Bensmaïl D., Bianchi F., Deltombe T., et al. The role of physical and rehabilitation medicine in the COVID-19 pandemic: the clinician's view. Ann Phys Rehabil Med. 2020;63:554–556. doi: 10.1016/j.rehab.2020.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chen N., Zhou M., Dong X., Qu J., Gong F., Han Y., et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;395:507–513. doi: 10.1016/S0140-6736(20)30211-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rees E.M., Nightingale E.S., Jafari Y., Waterlow N.R., Clifford S., Pearson C.A., et al. COVID-19 length of hospital stay: a systematic review and data synthesis. BMC Med. 2020;18:270. doi: 10.1186/s12916-020-01726-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Levy J., Léotard A., Lawrence C., Paquereau J., Bensmail D., Annane D., et al. A model for a ventilator-weaning and early rehabilitation unit to deal with post-ICU impairments following severe COVID-19. Ann Phys Rehabil Med. 2020;63:376–378. doi: 10.1016/j.rehab.2020.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Disser N.P., De Micheli A.J., Schonk M.M., Konnaris M.A., Piacentini A.N., Edon D.L., et al. Musculoskeletal consequences of COVID-19. J Bone Joint Surg. 2020;102:1197–1204. doi: 10.2106/JBJS.20.00847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Valenzuela P.L., Joyner M., Lucia A. Early mobilization in hospitalized patients with COVID-19. Ann Phys Rehabil Med. 2020;63:384–385. doi: 10.1016/j.rehab.2020.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.HAS, CNP de MPR, SOFMER . HAS; 2020. Rapid responses in the context of COVID-19 – Management of COVID+ patients in Physical Medicine and Rehabilitation (MPR), and on return home. [Google Scholar]
- 9.Medrinal C., Prieur G., Combret Y., Quesada A.R., Debeaumont D., Bonnevie T., et al. Functional electrical stimulation—A new therapeutic approach to enhance exercise intensity in chronic obstructive pulmonary disease patients: a randomized, controlled crossover trial. Arch Phys Med Rehabil. 2018;99:1454–1461. doi: 10.1016/j.apmr.2018.02.002. [DOI] [PubMed] [Google Scholar]
- 10.Maddocks M., Nolan C.M., Man W.D.-C., Polkey M.I., Hart N., Gao W., et al. Neuromuscular electrical stimulation to improve exercise capacity in patients with severe COPD: a randomised double-blind, placebo-controlled trial. Lancet Respir Med. 2016;4:27–36. doi: 10.1016/S2213-2600(15)00503-2. [DOI] [PubMed] [Google Scholar]
- 11.Metani A., Popović-Maneski L., Mateo S., Lemahieu L., Bergeron V. Functional electrical stimulation-cycling strategies tested during preparation for the First Cybathlon Competition – a practical report from team ENS de Lyon. Eur J Transl Myol. 2017;27:7110. doi: 10.4081/ejtm.2017.7110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.de Freitas G.R., Szpoganicz C., Ilha J. Does neuromuscular electrical stimulation therapy increase voluntary muscle strength after spinal cord injury? A systematic review. Top Spinal Cord Inj Rehabil. 2018;24:6–17. doi: 10.1310/sci16-00048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Garnotel M., Bastian T., Romero-Ugalde H.M., Maire A., Dugas J., Zahariev A., et al. Prior automatic posture and activity identification improves physical activity energy expenditure prediction from hip-worn triaxial accelerometry. J Appl Physiol. 2018;124:780–790. doi: 10.1152/japplphysiol.00556.2017. [DOI] [PubMed] [Google Scholar]
- 14.Coffman C.J., Edelman D., Woolson R.F. To condition or not condition? Analysing ‘change’ in longitudinal randomised controlled trials. BMJ Open. 2016:6. doi: 10.1136/bmjopen-2016-013096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Azman M.F., Azman A.W. The effect of electrical stimulation in improving muscle tone (clinical) IOP Conf Ser: Mater Sci Eng. 2017;260:012020. doi: 10.1088/1757-899X/260/1/012020. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Movie. Illustrations of muscle contractions elicited by functional electrical stimulation applied during cycling by using RehaMove muvi (Hasomed GmbH, Magedburg, Germany).