Abstract
Adverse allergic reactions due to the administration of the vaccines developed for the protection of coronavirus disease 2019 (COVID-19) have been reported since the initiation of the vaccination campaigns. Current analyses provided by the Center for Disease Control and Prevention (CDC) and Food and Drug Administration (FDA) in the United States have estimated the rates of anaphylactic reactions in 2.5 and 11.1 per million of mRNA-1273 and BNT162b2 vaccines administered, respectively. Although rather low, such rates could have importance due to the uncommon fact that a large majority of the world population will be subjected to vaccination with the aforementioned vaccines in the following months and vaccination will most likely be necessary every season as for influenza vaccines. Health regulators have advised that any subject with a previous history of allergy to drugs or any component of the vaccines should not be vaccinated, however, certain misunderstanding exists since allergy to specific excipients in drugs and vaccines are in occasions misdiagnosed due to an absence of suspicion to specific excipients as allergenic triggers or due to inaccurate labeling or nomenclature. In this review, we provide an updated revision of the most current data regarding the anaphylactic reactions described for BNT162b2 vaccine, mRNA-1273 vaccine, and AZD1222 vaccine. We extensively describe the different excipients in the vaccines with the potential to elicit systemic allergic reactions such as polyethylene glycol (PEG), polysorbates, tromethamine/trometamol, and others and the possible immunological mechanisms involved.
Keywords: Anaphylaxis, COVID-19, SARS-CoV-2, Vaccines, Vaccine allergy
Introduction
Allergic reactions to routine vaccines have been estimated to affect 1–10 per 1,000,000 administrated doses. The reactions may be caused by excipients that act as preservatives, stabilizers, or adjuvants. In that respect, excipients such as gelatine, ovalbumin from egg, chicken proteins or cow's milk proteins, that can be found in vaccines such as mumps, measles, rubella (MMR) vaccine, influenza vaccine, rabies vaccine, etc, have been described to be responsible for allergic reactions. Antigens from the infectious agent of the vaccine can also trigger allergic reactions but to a lesser extent.1 , 2
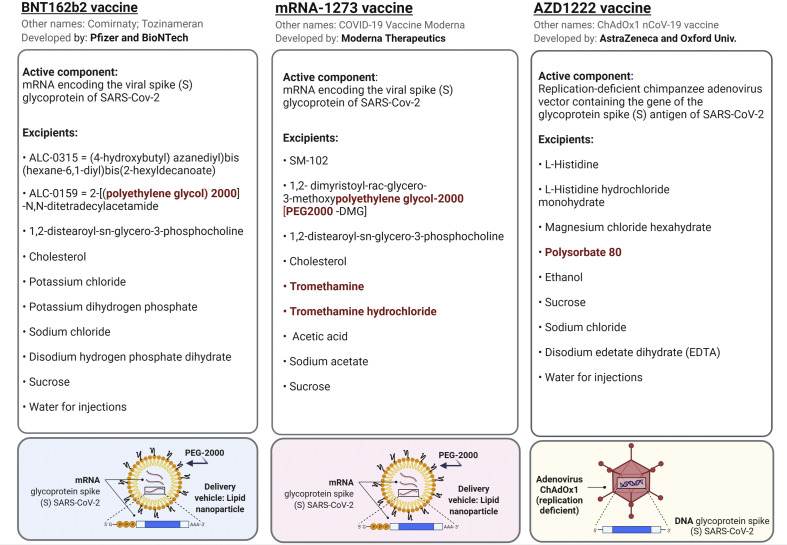
Three vaccines for the prevention of coronavirus disease 2019 (COVID-19) caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) have been approved so far by world regulatory agencies. The mRNA vaccine BNT162b2 (produced by Pfizer-BioNTech) was approved on December 2nd, 2020, by the Medicines and Healthcare Products Regulatory Agency (MHRA) in the United Kingdom (UK), followed by the United States Food and Drug Administration (FDA), European Medicines Agency (EMA) and others regulatory agencies in the world. Another mRNA vaccine, named as mRNA-1273, produced by Moderna Therapeutics has also received emergency authorizations for its use in UK, USA, or EU. The third vaccine for COVID-19 protection is a DNA vaccine, named as AZD1222 (produced by AstraZeneca-Oxford University), and is the last one to receive approval by MHRA in the UK and it is starting to be authorized by other regulatory agencies around the world. The authorizations of the vaccines were based on the results of phase–III–trials involving 44,000 participants for the BNT162b2 vaccine trial and 30,000 participants for the mRNA-1273 vaccine trial, showing that these mRNA vaccines were 95% and 94.1% effective, respectively, administrated in two dose-regimes3 , 4 (Table 1 ). In the case of the AZD1222 vaccine, the approval was given after an interim analysis of the data pooled from four ongoing phase I/II/III trials carried out in the UK, Brazil, and South Africa. The clinical trials involved over 23,000 participants who were randomized 1:1 to receive AZD1222 or placebo. The analysis showed that the vaccine efficacy was 62.1% after two standard doses containing 5 × 1010 viral particles per dose. In a subset of subjects of the UK trial who received a first low dose (half of the standard dose) followed by a standard dose, the efficacy of the vaccine reached 90.0%5 (Table 1).
Table 1.
Characteristics, components, and clinical trials of the vaccines BNT162b2, mRNA-1273, and AZD1222 for COVID-19 prevention.
| BNT162b2 | mRNA-1273 | AZD1222 | |
|---|---|---|---|
| Type of vaccine | mRNA vaccine | mRNA vaccine | DNA vaccine |
| Active component | mRNA encoding the viral spike (S) glycoprotein of SARS-Cov-2 | mRNA encoding the viral spike (S) glycoprotein of SARS-Cov-2 | DNA encoding the viral spike (S) glycoprotein of SARS-Cov-2 |
| Carrier or vector | PEGylated lipid nanoparticle | PEGylated lipid nanoparticle | Replication-deficient chimpanzee adenovirus vector ChAdOx1 |
| Excipients† |
|
|
|
| Phase-III-trials | |||
| Number of participants | 44,000 | 30,000 | 23,000 |
| Randomization (vaccine:placebo) | 1:1 | 1:1 | 1:1 |
| Regimen | Two doses | Two doses | Two doses |
| Effectiveness after second dose (%) | 95 | 94.1 | 62.1 |
Potential triggers of allergic reactions are highlighted in bold in the excipient lists of the vaccines.
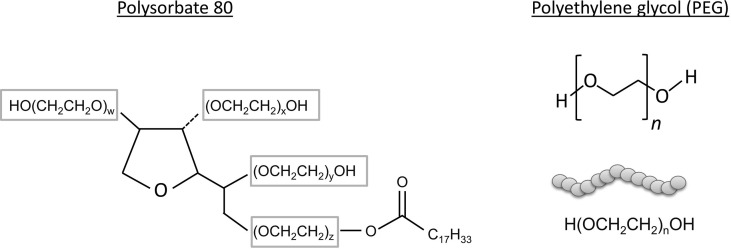
The mRNA vaccines BNT162b2 and mRNA-1273 are based on the same technology of mRNA that encodes the viral spike (S) glycoprotein of SARS-CoV-2. The mRNA molecule in these vaccines is surrounded by lipid nanoparticles (LNPs) to provide stability to the mRNA molecules. The LNPs have been additionally subjected to the chemical process of PEGylation, which consists on the chemical attachment of polyethylene glycol (PEG) to the surface of the LNPs in order to increase its efficiency and delivery to the target cells6 (Table 1). The AZD1222 vaccine is also based on the expression of the glycoprotein spike (S) antigen of SARS-CoV-2, but in this case, AZD1222 is a DNA vaccine that consists of a chimpanzee adenovirus vector (ChAdOx1) which is replication-deficient containing the gene of the glycoprotein spike (S) antigen of SARS-CoV-2. Although PEGylated LNPs or other PEG components are not found among the excipients of the AZD1222 vaccine, it does contain polyoxyethylene-80-sorbitan monooleate (Table 1), also named polysorbate 80 or Tween 80, which has structural similarities with PEG (Fig. 1 ).
Fig. 1.
Chemical structures of polysorbate 80 and PEG. The shared structures of polysorbate 80 and polyethylene glycol are included in rectangles in the chemical structure of polysorbate 80.
Soon after the initiation of the vaccination with the mRNA vaccines in December 2020, adverse allergic reactions were started to be reported and the list of excipients of the vaccines was subjected to an analysis by the scientific community in order to define the potential culprits of the reactions. Although health authorities from different countries warned from the beginning that any subject allergic to any component of the vaccines should not be vaccinated, such advice was sometimes difficult to attain, since allergy to vaccine excipients are in occasions misdiagnosed due to an absence of suspicion to specific excipients as allergenic triggers or due to inaccurate labeling or nomenclature.
Since data and information on the systemic allergic reactions to COVID-19 vaccines are being released rapidly in current times, it is our objective to review and summarize the most recent findings that exist regarding the anaphylactic reactions described for the three vaccines for COVID-19 protection and to provide an extensive description of the different excipients of the vaccines with the potential to elicit an adverse allergic reaction, as well as the potential mechanisms involved.
Anaphylactic reactions to the COVID-19 vaccines: the data
The Center for Disease Control and Prevention (CDC) and Food and Drug Administration (FDA) through the Vaccine Adverse Event Reporting System (VAERS) collected the notifications in the United States of suspected anaphylactic reactions to the BNT162b2 vaccine during December 14 and December 23, 2020. At that time 1,893,360 first doses of the vaccine were administrated. Among the 175 cases of adverse reactions reviewed using the Brighton Collaboration case definition criteria, 21 cases of anaphylaxis were found, which results in 11.1 anaphylactic cases per million vaccine doses (Table 2 ). Still, ten additional cases were either under review or had insufficient information to be cataloged as anaphylaxis. Eighteen out of the 21 confirmed reactions (86%) occurred immediately (within 30 min) of the vaccine administration. Four out of 21 patients (19%) required hospitalization and 17 out of 21 (81%) were attended in an emergency room. No deaths were reported. The majority of the patients (81%) had a previous history of allergic reactions including allergy to medicaments, insect stings, and foods. Seven out of 17 (41%) patients with a previous history of allergy had experienced anaphylactic reactions in the past including reactions to vaccines.7
Table 2.
Summary of the reports from the Center for Disease Control and Prevention (CDC) and Food and Drug Administration (FDA) regarding the anaphylactic reactions to the COVID-19 vaccines BNT162b2 and mRNA-1273.
| BNT162b2 | mRNA-1273 | |
|---|---|---|
| Time period evaluated | December 14, 2020 – December 23, 2020 |
December 21, 2020 – January 10, 2021 |
| Doses administrated (first dose) | 1,893,360 | 4,041,396 |
| Cases of adverse reactions reviewed | 175 | 108 |
| Cases of anaphylaxis (Brighton Collaboration case definition criteria) | 21 | 10 |
| Females (%) among anaphylaxis cases | 90 | 100 |
| Anaphylactic cases per million vaccine doses | 11.1 | 2.5 |
In a second recent report, CDC analyzed allergic reactions after administration of mRNA-1273 vaccine between December 21, 2020, and January 10, 2021. During that period, 4,041,396 first doses of the vaccine were administrated in the United States. Among all the reports submitted to VAERS, 108 qualified to be reviewed, and 10 of them were cataloged as anaphylactic reactions following the Brighton Collaboration case definition criteria for anaphylaxis. That sets a rate for anaphylaxis of 2.5 per million of mRNA-1273 vaccines administrated (Table 2). Yet, 47 additional cases were cataloged as allergic reactions to the vaccine although they were not anaphylactic reactions. The anaphylactic reactions occurred in a median of 7.5 min (range 1–45 min) and no deaths were reported. Six patients out of 10 required hospitalizations including four patients that were subjected to endotracheal intubation. Previous histories of allergic reactions, including drugs and contrast media, were described in most of the patients (90%), with previous anaphylaxis episodes in 5 out of 9 patients, including anaphylaxis to contrast media in two patients.8
A robust female predominance in the anaphylactic reactions was found for both mRNA-1273 and BNT162b2 vaccines (100% for mRNA-1273 vaccine and 90% for BNT162b2 vaccine). Although the vaccines were administrated to a higher percentage of women than men at the time of the analyses (61% in the case mRNA-1273 vaccine, and 64% for BNT162b2 vaccine), this fact cannot explain the high female predominance among the anaphylactic reactions.7 , 8
Initial reports started to point out which components from the vaccines could have allergenic potential and be possibly involved in the systemic allergic reactions.9 , 10 Although currently the culprit or culprits of the reactions have not been identified, the lists of excipients of the three vaccines contain some compounds with a recognized potential to elicit allergic reactions, such as PEG, polysorbates, tromethamine/trometamol, and others (Fig. 2 ). The following sections will focus on the description of those compounds, their characteristics, and their potential role in systemic allergic reactions.
Fig. 2.
Active components and full list of excipients of the vaccines. Potential triggers of allergic reactions are indicated in red color in the lists. The principles of the PEGylated-lipid nanoparticles as a delivery system for the mRNA is depicted in the lower part for BNT162b2 and mRNA-1273 vaccines. The replication-deficient adenovirus ChAdOx1 vector for the AZD1222 vaccine is also depicted. Biorender software was used to create this figure under an academic license.
Components of the vaccines for COVID-19 with allergenic potential
PEG
Definition and nomenclature
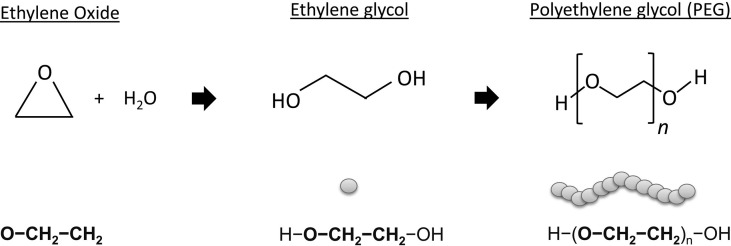
PEGs or macrogols are hydrophilic polymers derived from ethylene oxide that are extensively used as ingredients in medicaments, cosmetics, pharmaceutical, and food products (Fig. 3 ). PEGs can be covalently linked to certain drugs, a method called PEGylation, in order to enhance their molecular weight (MW), to protect the drug from degradation, and to increase their circulation and delivery to target body compartments.11 , 12 PEG polymers can have different chain lengths and, in that way, different MWs. The nomenclature of PEGs is diverse; however, the term PEG is frequently used accompanied by a figure that can denote the number of units of ethylene oxide (usually used in the cosmetic industry) or the total MW of PEG in g/mol (usually used in the pharmaceutical industry). Since the MW of a unit of ethylene oxide equals 44 g/mol, the MW of a PEG product can be calculated as n x 44, being n the number of ethylene oxide units. In that sense, PEG-2000 denotes a PEG product of an MW of 2000 g/mol, which can also be denominated as PEG-45 which denotes the number of ethylene oxide units (2000/44 = 45). The MW of PEGs usually vary from 200 to 35,000 g/mol, being PEGs of low MW (<400 g/mol) less toxic and easily absorbed in the gastrointestinal mucosa, while PEGs < 3350 g/mol can be absorbed through the cutaneous barrier. PEG has several derivatives that share similarities in their structure. That is the case of polysorbates (PEG sorbitans), poloxamers (PEG-propylene glycol copolymers), PEG ethers, PEG fatty acid esters, etc.13 The wide range of MW of PEGs and their derivatives and their extensive use as ingredients in pharmaceutical and cosmetic products opens numerous possibilities for PEG sensitization through different administration routes.
Fig. 3.
Chemical structures of ethylene oxide, ethylene glycol and PEG.
Hypersensitivity reactions to PEGs
Hypersensitivity to PEGs as part of drugs or daily life products are on occasions difficult to diagnose due to a lack of awareness among physicians of this excipient as a potential allergic sensitizer. In the scientific literature, 37 case reports of PEG allergy have been described from 1977 to 2016, being 76% of them systemic reactions,13 however a revision of FDA data has estimated that there is an average of 4 cases of anaphylaxis per year due to PEG as part of preparations such as the ones used for colonoscopy procedures or laxative preparations.14 Recently, a series of 5 cases of systemic allergic reactions to PEG was reported. The subjects had a previous diagnosis of allergy to medicaments, without an accurate study of the exact excipient to what they were allergic to. The study described the anaphylactic reactions to medicaments containing PEGs in four patients, including one whose reaction was cataloged as near-fatal. The fifth patient had a systemic reaction to PEG without airway and cardiac compromise. The reactions occurred immediately upon administration of medicaments containing PEGs of different MW. PEG allergy was confirmed by skin prick test, intradermal test, or oral challenge. Since two patients had an anaphylactic reaction due to the intradermal tests and another patient had a systemic allergic reaction due to SPT with PEG, the use of different dilutions of PEGs for such tests is highly advisable and it was included in the recommended algorithm for the diagnosis of PEG allergy.15 Other methods such as basophil activation test or dual cytometric bead assay can also be helpful as complementary tools for PEG diagnosis.16 It should be taken into account that some medications used to treat anaphylactic reactions may include among their ingredients PEG or its derives, therefore it is of vital importance to check the excipients of rescue medication before prescribing them and to have available PEG-free medicines as part of emergency kits.15 , 17
Molecular weight thresholds, PEG-2000, and COVID-19 vaccines
An individual threshold of sensitization to PEG depending on its MW and its concentration seems to exist. As mentioned before, the MW of PEGs can vary from 200 to 35,000 g/mol, in that respect, some patients react to PEG-20000 (g/mol) but tolerate PEG-6000 (g/mol), or cases of sensitization to PEG-3350 (g/mol) who are tolerant to PEG-300 (g/mol).15 , 17 , 18 A case has also been described for a subject with a previous history of anaphylaxis to PEG-3350 that experienced later an anaphylactic reaction to PEGylated liposomes.19 For that reason, it is recommended that the MW of the PEG product contained in the index medicament causing the reaction is included in the history as part of the diagnostic procedure to try to establish an individual MW threshold for PEG sensitization.
COVID-19 vaccines BNT162b2 and mRNA-1273 contain PEG-2000 as part of the PEGylation process of the LNPs that surround the mRNA molecules. The intramuscular route of administration could potentially increase the stability and bioavailability of the carrier containing PEG-2000. In the CDC reports, 52% of patients with anaphylactic reactions to the BNT162b2 vaccine and 80% of patients with anaphylaxis mRNA-1273 vaccine had a previous history of allergy to medicaments or vaccines.7 , 8 Some of these drugs can contain among their excipients PEG or its derivatives such as in the case of penicillin tablets or steroids.20 However, the specific role of PEG in the anaphylactic reactions to BNT162b2 and mRNA-1273 vaccines has not been analyzed so far.
Polysorbate 80
Polysorbate 80 (polyoxyethylene-80-sorbitan monooleate) or Tween 80 is a nonionic surfactant that is used as an emulsifier and stabilizer in the pharmaceutical and food industries.21 Polysorbates 20, 40, and 60 also exist and the figure associated with the name indicates the total numbers of ethylene oxide groups (-OCH2CH2) in the molecule (Fig. 1). Polysorbates can also be found as an excipient in certain vaccines such as Hepatitis B vaccine, influenza vaccine, or Human papillomavirus vaccine. The function is to contribute to the solubility of the vaccine.22 AZD1222 DNA vaccine contains polysorbate 80 among its excipients (Fig. 2). Other vaccines for COVID-19 protection that use adenovirus vectors or are based on the recombinant glycoprotein (S) spike antigen and that they are currently in phases I, II, or III trials, also contain polysorbates 80 and 20.10 The majority of the described cases of allergic reactions to polysorbates has been linked to medicines that contain this excipient.23 , 24 However, allergy to vaccines due to polysorbates has been reported to a lesser extent.14 , 25 Similarly, to PEG, due to the general unawareness of the allergenic potential of polysorbates, the cases of allergy to this compound that may be behind a hypersensitivity reaction to a vaccine, may be underrepresented.
Cross-reactivity between polysorbates and PEG due to shared structures such as (-OCH2CH2) or –(OCH2CH2)OH has been described (Fig. 1). In the CDC report of BNT162b2 vaccine, 2 out 21 patients with reported anaphylaxis to the BNT162b2 vaccine had past histories of anaphylaxis to vaccines such as influenza (H1N1) or rabies vaccine.7 Although PEG-2000 is an excipient that has been first utilized in BNT162b2 and mRNA-1273 vaccines, polysorbates, are frequently used in vaccines such as the ones used for influenza protection.18 Therefore, patients sensitized to polysorbates could potentially react to PEG of specific molecular weights if they are exposed to it at specific routes, such as intramuscularly. CDC reports on the anaphylactic reactions to polysorbate containing COVID-19 vaccines, such as AZD1222 DNA vaccine have not been released so far.
Tromethamine or trometamol
Tromethamine (C4H11NO3) is an excipient that can be found in the mRNA-1273 vaccine but not in the other two COVID-19 vaccines (Fig. 2). Tromethamine (or Trometamol) is an organic amine proton acceptor that is used as a biological buffer in drugs for topical, enteral, or parenteral application. It is also found in cosmetics as an emulsifier. Allergic sensitization through contact due to trometamol-containing products has been described.26 Trometamol is also a common excipient in some contrast agents such as iodinated contrast medium (IOM) or gadolinium-based contrast agents (GBCA). The risk of allergic reactions to GBCA increases when a previous history of hypersensitivity to IOM exists.27 , 28 However, an IgE cross-reactivity between both agents is unlikely since GBCAs and IOM are not structurally related. In that sense, allergic reactions to these agents are sometimes suspected to be caused by common excipients contained on them. In line with this assumption, recently an anaphylactic reaction to GBCA due to the component trometamol in its formulation has been reported. The anaphylactic reaction was described in a 23-year-old woman without previous history of allergy to drugs that was exposed to GBCA through injection. The intradermal tests (IDT) proved to be positive to the index GBCA, as well as an additional GBCA that contained trometamol, however, it was negative to GBCA without trometamol in its formulation in IDT. The patient also tested positive for trometamol in IDT.29
Interestingly, in the CDC report for adverse reactions to mRNA-1273, two out of the 10 patients that had anaphylactic reactions to the vaccine had a previous history of anaphylaxis to gadolinium, iodine, or intravenous contrast dye.8 Although the involvement of tromethamine/trometamol contained in the vaccine in such reactions could be plausible, further studies would be necessary in order to investigate the role of tromethamine/trometamol in the systemic allergic reactions caused by the mRNA-1273 vaccine.
Other excipients
It has been also proposed that other components of the vaccines different from PEG-2000, polysorbates, or tromethamine/trometamol could have a role in the allergic reactions described for the COVID-19 vaccines. In that respect, since certain phospholipids have been linked to certain allergies such as pollen allergy, the excipient 1,2-distearoyl-sn-glycero-3- phosphocholine (DSPC) which is a phospholipid in the LNPs of BNT162b2 and mRNA-1273 vaccines, has been pointed out as a possible component that can contribute to allergic reactions.30 However, such observation can be considered hypothetical at this stage since allergic reactions to DSPC have not been described to date. Furthermore, the excipient disodium edetate dihydrate (EDTA) contained in the AZD1222 vaccine has also pointed out as a possible allergenic component based on a previous case of a systemic allergic reaction to EDTA contained in local anesthetic and radiocontrast media.30 , 31
However, the role of these compounds in the allergic reactions described to COVID-19 vaccines, if they have any, could be more limited than the main excipients that have been described in the previous sections, attending to the limited scientific literature of previously described cases with these compounds.
Immune mechanisms involved
The mechanisms of the allergic reactions to PEG, polysorbates, tromethamine/trometamol, and other vaccine components might be IgE-mediated, but also non mediated by IgE. In the former, there is a recognition of the allergen by specific IgE molecules positioned on the high-affinity receptors for IgE (FcɛRI) on mast cells and basophils. Upon cross-linking, a release of mediators such as histamine, prostaglandins, leukotrienes, tryptase, proteases, serotonin, etc, is produced. Such mediators are involved in the symptomatology of allergy reactions, which may include pruritus, erythema, rashes, angioedema, coughing, wheezing, hypotension, gastrointestinal symptoms which may lead to a fatal outcome.32 In the case of allergy non-mediated by IgE, the clinical symptoms resemble IgE-mediated allergy, but in this case, the mechanisms do not involve IgE and may comprise the activation of complement in the so-called complement activation-related pseudoallergy (CARPA), or direct, non-IgE-mediated activation of mast cells, among other mechanisms. In CARPA there is an induction of the complement products C3a, C4a, C5a called anaphylatoxins that are disseminated in the bloodstream and can act as cardiovascular autonomic organ regulators.33
Statements and guidelines regarding allergy to COVID-19 vaccines
The European Academy of Allergy and Clinical Immunology (EAACI) has stated that unless there is a history of allergic reactions to any component of the COVID-19 vaccines, there is no contraindication for their administration. In that respect, a previous history of allergy to foods, aeroallergens, or insect venoms is not a contraindication for the vaccines administration.34 A previous diagnosis of allergy to medicaments or vaccines, however, should identify the specific component or excipient that triggered the reaction, in order to rule out that it is one of the excipients of the vaccines for COVID-19. Deeper information about the diagnosis and management of severe allergic reactions after COVID-19 vaccination can be found in the EAACI statement on the diagnosis, management, and prevention of severe allergic reactions to COVID-19 vaccines.34
Vaccination centers should be prepared to recognize and treat severe reactions to the administrated vaccines and to provide continuous medical supervision during the vaccination period.
Conflict of interest
The authors have no conflict of interest to declare.
Acknowledgements
This study was supported by a grant from the research program Talento Investigador of the Community of Madrid (Regional Ministry of Science, Universities, and Innovation, Madrid, Spain), and a grant from Strategic Health Action (AES 2020), Carlos III Health Institute, Spanish Ministry of Science and Innovation (to B.C) (co-funded by European Regional Development Fund) (grant number: PI20/00351). B.C. is senior researcher in the research program Talento Investigador of the Community of Madrid (number: 2019-T1/BIO-12690).
Footnotes
Peer review under responsibility of Japanese Society of Allergology.
References
- 1.Wood R.A. Allergic reactions to vaccines. Pediatr Allergy Immunol. 2013;24:521–526. doi: 10.1111/pai.12102. [DOI] [PubMed] [Google Scholar]
- 2.Nilsson L., Brockow K., Alm J., Cardona V., Caubet J.C., Gomes E., et al. Vaccination and allergy: EAACI position paper, practical aspects. Pediatr Allergy Immunol. 2017;28:628–640. doi: 10.1111/pai.12762. [DOI] [PubMed] [Google Scholar]
- 3.Polack F.P., Thomas S.J., Kitchin N., Absalon J., Gurtman A., Lockhart S., et al. Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine. N Engl J Med. 2020;383:2603–2615. doi: 10.1056/NEJMoa2034577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Baden L.R., El Sahly H.M., Essink B., Kotloff K., Frey S., Novak R., et al. Efficacy and safety of the mRNA-1273 SARS-CoV-2 vaccine. N Engl J Med. 2021;384:403–416. doi: 10.1056/NEJMoa2035389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Voysey M., Clemens S.A.C., Madhi S.A., Weckx L.Y., Folegatti P.M., Aley P.K., et al. Safety and efficacy of the ChAdOx1 nCoV-19 vaccine (AZD1222) against SARS-CoV-2: an interim analysis of four randomised controlled trials in Brazil, South Africa, and the UK. Lancet. 2021;397:99–111. doi: 10.1016/S0140-6736(20)32661-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Suk J.S., Xu Q., Kim N., Hanes J., Ensign L.M. PEGylation as a strategy for improving nanoparticle-based drug and gene delivery. Adv Drug Deliv Rev. 2016;99:28–51. doi: 10.1016/j.addr.2015.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.CDC COVID-19 Response Team. Administration FDA Allergic reactions including anaphylaxis after receipt of the first dose of Pfizer-BioNTech COVID-19 vaccine - United States, December 14-23, 2020. MMWR Morb Mortal Wkly Rep. 2021;70:46–51. doi: 10.15585/mmwr.mm7002e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.CDC COVID-19 Response Team. Administration FDA Allergic reactions including anaphylaxis after receipt of the first dose of Moderna COVID-19 vaccine - United States, December 21, 2020-January 10, 2021. MMWR Morb Mortal Wkly Rep. 2021;70:125–129. doi: 10.15585/mmwr.mm7004e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cabanillas B., Akdis C., Novak N. Allergic reactions to the first COVID-19 vaccine: a potential role of Polyethylene glycol? Allergy. 2020 doi: 10.1111/all.14711. [DOI] [PubMed] [Google Scholar]
- 10.Castells M.C., Phillips E.J. Maintaining safety with SARS-CoV-2 vaccines. N Engl J Med. 2021;384:643–649. doi: 10.1056/NEJMra2035343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Veronese F.M., Pasut G. PEGylation, successful approach to drug delivery. Drug Discov Today. 2005;10:1451–1458. doi: 10.1016/S1359-6446(05)03575-0. [DOI] [PubMed] [Google Scholar]
- 12.Turecek P.L., Bossard M.J., Schoetens F., Ivens I.A. PEGylation of biopharmaceuticals: a review of chemistry and nonclinical safety information of approved drugs. J Pharm Sci. 2016;105:460–475. doi: 10.1016/j.xphs.2015.11.015. [DOI] [PubMed] [Google Scholar]
- 13.Wenande E., Garvey L.H. Immediate-type hypersensitivity to polyethylene glycols: a review. Clin Exp Allergy. 2016;46:907–922. doi: 10.1111/cea.12760. [DOI] [PubMed] [Google Scholar]
- 14.Stone C.A., Liu Y., Relling M.V., Krantz M.S., Pratt A.L., Abreo A., et al. Immediate hypersensitivity to polyethylene glycols and polysorbates: more common than we have recognized. J Allergy Clin Immunol Pract. 2019;7:1533–1540. doi: 10.1016/j.jaip.2018.12.003. e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sellaturay P., Nasser S., Ewan P. Polyethylene glycol-induced systemic allergic reactions (anaphylaxis) J Allergy Clin Immunol Pract. 2021;9:670–675. doi: 10.1016/j.jaip.2020.09.029. [DOI] [PubMed] [Google Scholar]
- 16.Zhou Z.H., Stone C.A., Jakubovic B., Phillips E.J., Sussman G., Park J., et al. Anti-PEG IgE in anaphylaxis associated with polyethylene glycol. J Allergy Clin Immunol Pract. 2021;9:1731–1733. doi: 10.1016/j.jaip.2020.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cabanillas B., Akdis C., Novak N. COVID-19 vaccine anaphylaxis: IgE, complement or what else? A reply to: “COVID-19 vaccine anaphylaxis: PEG or not? Allergy. 2021 doi: 10.1111/all.14725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Krantz M.S., Liu Y., Phillips E.J., Stone C.A. COVID-19 vaccine anaphylaxis: PEG or not? Allergy. 2021 doi: 10.1111/all.14722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Krantz M.S., Liu Y., Phillips E.J., Stone C.A. Anaphylaxis to PEGylated liposomal echocardiogram contrast in a patient with IgE-mediated macrogol allergy. J Allergy Clin Immunol Pract. 2020;8:1416–1419. doi: 10.1016/j.jaip.2019.12.041. e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rutkowski K., Wagner A., Rutkowski R. Immediate hypersensitivity reactions to steroids and steroid containing medications. Curr Opin Allergy Clin Immunol. 2020;20:362–366. doi: 10.1097/ACI.0000000000000655. [DOI] [PubMed] [Google Scholar]
- 21.Khan B.A., Akhtar N., Khan H.M.S., Waseem K., Mahmood T., Rasul A., et al. Basics of pharmaceutical emulsions: a review. Afr J Pharm Pharmacol. 2011;5:2715–2725. [Google Scholar]
- 22.Shende N., Karale A., Bhagade S., Gulhane A., Bore P., Marathe P., et al. Evaluation of a sensitive GC-MS method to detect polysorbate 80 in vaccine preparation. J Pharm Biomed Anal. 2020;183:113126. doi: 10.1016/j.jpba.2020.113126. [DOI] [PubMed] [Google Scholar]
- 23.Perino E., Freymond N., Devouassoux G., Nicolas J.F., Berard F. Xolair-induced recurrent anaphylaxis through sensitization to the excipient polysorbate. Ann Allergy Asthma Immunol. 2018;120:664–666. doi: 10.1016/j.anai.2018.02.018. [DOI] [PubMed] [Google Scholar]
- 24.Palacios Castaño M.I., Venturini Díaz M., Lobera Labairu T., González Mahave I., Del Pozo Gil M.D., Blasco Sarramián A. Anaphylaxis due to the excipient polysorbate 80. J Investig Allergol Clin Immunol. 2016;26:394–396. doi: 10.18176/jiaci.0109. [DOI] [PubMed] [Google Scholar]
- 25.Badiu I., Geuna M., Heffler E., Rolla G. Hypersensitivity reaction to human papillomavirus vaccine due to polysorbate 80. BMJ Case Rep. 2012;2012 doi: 10.1136/bcr.02.2012.5797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Singh M., Winhoven S.M., Beck M.H. Contact sensitivity to octyldodecanol and trometamol in an anti-itch cream. Contact Dermatitis. 2007;56:289–290. doi: 10.1111/j.1600-0536.2006.00990.x. [DOI] [PubMed] [Google Scholar]
- 27.Murphy K.J., Brunberg J.A., Cohan R.H. Adverse reactions to gadolinium contrast media: a review of 36 cases. AJR Am J Roentgenol. 1996;167:847–849. doi: 10.2214/ajr.167.4.8819369. [DOI] [PubMed] [Google Scholar]
- 28.Lufkin R.B. Severe anaphylactoid reaction to Gd-DTPA. Radiology. 1990;176:879. doi: 10.1148/radiology.176.3.879-a. [DOI] [PubMed] [Google Scholar]
- 29.Lukawska J., Mandaliya D., Chan A.W.E., Foggitt A., Bidder T., Harvey J., et al. Anaphylaxis to trometamol excipient in gadolinium-based contrast agents for clinical imaging. J Allergy Clin Immunol Pract. 2019;7:1086–1087. doi: 10.1016/j.jaip.2018.08.035. [DOI] [PubMed] [Google Scholar]
- 30.Borgsteede S., Geersing T.H., Z T.-P. Other excipients than PEG might cause serious hypersensitivity reactions in COVID-19 vaccines. Allergy. 2021 doi: 10.1111/all.14774. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Russo P.A., Banovic T., Wiese M.D., Whyte A.F., Smith W.B. Systemic allergy to EDTA in local anesthetic and radiocontrast media. J Allergy Clin Immunol Pract. 2014;2:225–229. doi: 10.1016/j.jaip.2013.12.001. [DOI] [PubMed] [Google Scholar]
- 32.Schnyder B., Pichler W.J. Mechanisms of drug-induced allergy. Mayo Clin Proc. 2009;84:268–272. doi: 10.4065/84.3.268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Szebeni J. Complement activation-related pseudoallergy: a stress reaction in blood triggered by nanomedicines and biologicals. Mol Immunol. 2014;61:163–173. doi: 10.1016/j.molimm.2014.06.038. [DOI] [PubMed] [Google Scholar]
- 34.Sokolowska M., Eiwegger T., Ollert M., Torres M.J., Barber D., Del Giacco S., et al. EAACI statement on the diagnosis, management and prevention of severe allergic reactions to COVID-19 vaccines. Allergy. 2021 doi: 10.1111/all.14739. [DOI] [PMC free article] [PubMed] [Google Scholar]