Abstract
The ongoing coronavirus disease 2019 (COVID-19) pandemic, caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), is a global public health problem. The SARS-CoV-2 triggers hyper-activation of inflammatory and immune responses resulting in cytokine storm and increased inflammatory responses on several organs like lungs, kidneys, intestine, and placenta. Although SARS-CoV-2 affects individuals of all age groups and physiological statuses, immune-compromised individuals such as pregnant women are considered as a highly vulnerable group. This review aims to raise the concerns of high risk of infection, morbidity and mortality of COVID-19 in pregnant women and provides critical reviews of pathophysiology and pathobiology of how SARS-CoV-2 infection potentially increases the severity and fatality during pregnancy. This article also provides a discussion of current evidence on vertical transmission of SARS-CoV-2 during pregnancy and breastfeeding. Lastly, guidelines on management, treatment, preventive, and mitigation strategies of SARS-CoV-2 infection during pregnancy and pregnancy-related conditions such as delivery and breastfeeding are discussed.
Keywords: COVID-19, SARS-CoV-2, Pregnant women, Vertical transmission, Prevention
Introduction
Coronavirus disease 2019 (COVID-19), caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), has spread rapidly across international borders and was declared as a global pandemic by the World Health Organization (WHO) on March 11, 2020 [1,2]. The pandemic has caused more than 2.9 million deaths and has infected over 135 million people as of April 11, 2021 [1]. The infected individuals initially exhibit mild respiratory symptoms, and some of them further progress to severe disease resulting in acute respiratory distress syndrome (ARDS), effecting neurological, gastrointestinal, cardiovascular systems and cause multi-organ failures [[2], [3], [4], [5]]. The accelerated risk of COVID-19 disease is documented to be provoked by the consistent activation of CD4+ and CD8+ cascades associated with the induction of an aggravated cytokine storm and inflammatory responses in different organs [6,7]. It has been suggested that ARDS and multiple organ failures are related to excessive production of cytokines such as interleukin (IL)-6, IL-8, IL-10, tumor necrosis factor (TNF), and GM-CSF [[8], [9], [10]].
SARS-CoV-2 infection could affect all groups irrespective of age and gender. However, the most severe and adverse outcomes of COVID-19 have been documented in geriatric individuals and pregnant women with chronic diseases, including hypertension, diabetes, and cardiopulmonary problems [[11], [12], [13]]. Several studies have reported more severe and a higher mortality rate due to some respiratory viral infections in pregnant women compared to non-pregnant women [[14], [15], [16], [17]]. These data suggest that pregnancy might increase the risks, vulnerability, morbidity, disease severity and the fatality of COVID-19 due to pregnancy-associated physiological changes in cardiopulmonary systems, adaptive changes in the immune system, the partial diversion of cell-mediated immunity, dysregulated immunity, and increased maternal and fetal demand for oxygen [16,18]. In addition to common deleterious effects, alteration in mothers' psychological status, including anxiety and depression, and infant’s neurobehavioral development have also been noted [[19], [20], [21]]. Some important issues of SARS-CoV-2 infection in pregnancy and pregnancy-associated conditions need to be elucidated fully by focusing in several critical aspects to minimize the impacts of this pandemic in women and the neonates [[22], [23], [24], [25], [26]]. Critical discussion is therefore required to evaluate the magnitude of COVID-19 problems in pregnant women to be able to constitute preventive measures to prevent mortality.
In this review, we aim to raise the potency of the high risk of infection, morbidity and mortality of COVID-19 in pregnant women, and critically discuss the COVID-19-associated pathophysiology and pathobiology in pregnancy including to provide the current evidence on vertical transmission of SARS-CoV-2 during pregnancy and breastfeeding. This review also summarizes the management of the cases and preventive strategies of COVID-19 during pregnancy and pregnancy-related conditions.
COVID-19 in pregnancy: raise the concerns
During severe acute respiratory syndrome (SARS) outbreaks between 2002-2004, a high incidence of maternal and neonatal complications included abortions, premature births, endotracheal intubation, admission into intensive care units, disseminated intravascular coagulopathy, and renal problems were reported [15,27]. Some pregnant women had to get tested or admitted for COVID-19, some had to get antiviral treatment for COVID-19, and in some, premature delivery was noted [28]. An increased risk of adverse birth outcomes, preterm birth, and cesarean section has been reported in COVID-19 positive pregnant women [29]. A pregnant woman with COVID-19 has been reported to be presented with multi-organ failure, hyaline membrane formation, consolidation of lungs with pleural effusion, and inflammation of the airway [30]. A population-based study in the UK reported that SARS-CoV-2 infection in pregnant women between March 1 and April 14, 2020, was 49 per 10,000 maternities [31]. This study also found that twelve out of 265 infants delivered from SARS-CoV-2 infected mothers were confirmed positive for SARS-CoV-2 RNA [31], suggesting the possibility of SARS-CoV-2 transmission during the delivery process and other following obstetric activities.
A prospective study revealed the relationship between the severity of COVID-19 and the occurrence of pre-eclampsia-like syndrome in the affected pregnant women [32]. In their study, 34 non-severe and eight cases of severe COVID-19 were enrolled, and five out of eight (62.5%) severe COVID-19 developed pre-eclampsia-like syndrome [32]. This finding crystalizes the induction of pre-eclampsia-like syndrome by COVID-19 and suggesting that the presence of COVID-19 during pregnancy might face a greater risk for maternal-related complications. A study assessing the role of comorbidities on mortality of pregnant women with COVID-19 found that patients who died were older and more likely to have co-morbidities [33]. COVID-19 associated multisystem inflammatory syndrome has been described in a pregnant woman as a rare but severe complication causing critical illness and cardiogenic shock requiring mechanical ventilation, wherein intravenous immunoglobin and high-dose corticosteroids were necessary for the recovery [34].
An analysis of COVID-19 surveillance data from January to June 2020, US Centers for Disease Control and Prevention (CDC) confirmed the likelihood of pregnant women to suffer a greater clinical burden due to COVID-19 than their non-pregnant counterparts [35]. They found that more than 2500 (31.5%) pregnant women with COVID-19 required hospitalization, much higher than non-pregnant (5.8%) [35]. Furthermore, admission to the intensive care unit (ICU) and respiratory support by mechanical ventilation were more frequently reported in the pregnancy group [35]. A systematic review and meta-analysis reported a high proportion of pregnant women with confirmed COVID-19 had preterm birth (22%) and cesarean delivery (48%) and with estimated rates of admission to the ICU among pregnant women were higher than those of non-pregnant women (7% vs 4%) [36]. These data suggest that the effects of COVID-19 on pregnancy should not be considered a trivial matter.
The deleterious effect of SARS-CoV-2 infection on the fetus might understudied. Previous studies in animal models have shown evidence of neurodevelopmental and psychological abnormalities in the fetus related to the aggravated fetal inflammatory response syndrome (FIRS) responsible for causing elevated production of cytokines (IL-1, IL-6, IL-8, and TNF-α) in the placenta [[37], [38], [39], [40]]. Studies in MERS and SARS found that these infections are associated with spontaneous abortion, teratogenic effects, and premature birth [27,41]. Elevated production of cytokines during COVID-19 might potentially induce similar conditions; however, further studies are warranted.
Considering the COVID-19 pandemic is still ongoing, data collection, curation, analysis, and reporting are subject to continuous improvement thus, findings and trends suggested so far might be modified accordingly. Recent findings and discussion presented here shall provide information regarding the potential threat of COVID-19 to pregnant women, which in turn shall raise awareness to all related parties to emphasize COVID-19 prevention measures for pregnant women and improve the protocol of maternal care during the pandemic.
Pregnancy and severity of COVID-19: pathophysiology
Pregnancy is a condition of immune modulation rather than immune suppression. Placental immune response and its tropism for specific viruses and pathogens affect the outcome of the pregnant woman’s susceptibility to and severity of certain infectious diseases [42]. Viral pneumonia is believed to be a significant cause of mortality among pregnant women [43]. Previous studies have provided evidence that infection of MERS-CoV and SARS-CoV were associated with pregnancy-associated complications [14,41]. In COVID-19, studies also have reported that pregnancy was associated with the severity of the disease [35] and increased pregnancy-related complications [32]. COVID-19 leads to an increased risk of pregnancy complications. There is intrauterine/fetal distress (14%) and premature rupture of membranes (8%). The neonatal clinical manifestations of COVID-19 commonly included shortness of breath (6%), gastrointestinal symptoms (4%), and fever (3%) [44]. Of most recent, Chen and coworkers [45], diagnosed COVID-19 in nine women during the 3rd trimester of pregnancy. In this diagnostic series, clinical symptoms were likely to be the same as observed in non-pregnant females, with cough, fever, myalgia, malaise, and sore throat in 4, 7, 3, and 2 women, respectively. Some women also showed a reduced number of lymphocytes. All the women exhibited pneumonia without requiring mechanical ventilation. Another study involving nine pregnancies and ten infants (one set of twins) reported the onset of symptoms before delivery, on the delivery day, and after delivery in 4, 2, and 3 cases, respectively [13]. Out of nine pregnancies, about 7 cesarean deliveries showed non-reassuring fetal distress, while 6 infants’ births were too early and premature. Seven COVID-19 cases were confirmed during pregnancy in New York City tertiary care center, out of which five patients showed COVID-19 symptoms such as headache, fevers, cough, chest pain, and myalgia. Two cases required additional care with intravenous fluid therapy, and two cases had no symptoms, which appeared after delivery [46].
There are some possible factors associated with susceptibility and high severity of COVID-19 during pregnancy. During the first trimester of pregnancy, a significant increase in the levels of steroidal hormones such as progesterone or other relaxants influence uterine physiology. Persistently elevated levels of steroidal hormones are characterized by the relaxation of ligaments of thoracic ribs, causing upward movement of the diaphragm [47]. These physio-anatomical changes reduce the lungs' functional residual capacity by 20–30% and contribute to anoxic conditions in the body [48]. The oxygen deficit in the body is reversed by the stimulation of the respiratory center through estrogen-dependent-progesterone receptors located in the hypothalamus [49]. More severe incidences of cardiac and pulmonary arrest in pregnant women affected with SARS-CoV-2 infection during the third trimester of pregnancy may be expected due to the rapid multiplication of virus in the respiratory system with already compromised mucociliary clearance mechanisms during pregnancy [[50], [51], [52]].
It is presumed that Th1/Th2 immuno-regulatory system partially deviates towards Th2 mediated immune response and intended to suppress the immunological response related to T cells and CD3+ T cells in the blood [[53], [54], [55], [56]]. Moreover, hormonal changes in pregnant women with COVID-19 lead to an increased immunological response to viral pathogens, in association with a diversion of the Th1/Th2 immunological responses towards a Th2 specific response, and favor more severe outcomes than those in non-pregnant individuals with COVID-19 [11]. A study found that the higher incidence of fatality among COVID-19 patients s been associated with the over-activation of the Th1 and Th2 immunological responses and the resulting overproduction of cytokines IFN-γ, IL-1β, IL-4, IL-6, and IL-10 [2]. Suppression of natural killer (NK) cells and T cells in late pregnancy is certainly linked with the slow clearance of viral etiologies [57,58].
The worrisome detrimental effects produced by SARS-CoV-2 are also directly linked with the affinity of viral S protein towards angiotensin-converting enzyme 2 (ACE2) receptors evident on the host cells (lungs, intestine, placenta, kidneys) [[59], [60], [61], [62]]. SARS-CoV-2 enters into the host cells, and the internalization of the virus is warranted after the cleavage of viral S protein by the host proteases [63,64]. In pregnant women, a 2-fold increase in the expression of ACE2 receptors is observed as in different organs, including the placenta, kidneys, and gravid uterus, compared with the non-pregnant women [65]. This might also enhance the risk of infection and the severity of the SARS-CoV-2 infection in pregnant women [66]. However, further studies are required to elucidate this.
Vertical transmission
There have been many studies regarding the possibilities of vertical transmission in COVID-19, with few revealing no vertical transmission to the possibility of vertical transmission from COVID-19 infected pregnant women to fetus [[67], [68], [69]]. Early data from nine pregnant women in China who were in the third trimester of pregnancy and without any underlying chronic disease conditions found no evidence of intrauterine transmission using samples collected from the amniotic fluid, blood from the umbilical cord, and throat swabs from the neonate [6]. A study involving 38 pregnant women also did not find any evidence of vertical transmission of the SARS-CoV-2 from mothers to their fetuses, supporting the observations made by previous studies during the MERS-CoV and SARS-CoV epidemics [70]. In one more study, out of 19 pregnant COVID-19 positive women, none of the neonates were tested positive for COVID-19 [71]. Other studies also have been conducted in China [3,6,8,11,12,72,73], Iran [74], and the US [75] and suggested no significant scientific evidence of vertical transmission of SARS-CoV-2. In another study, evidence has been provided that vertical transmission is possible, especially in the third trimester, as approximately 3.2% (22/936) of infant nasopharyngeal swab testing and SARS-CoV-2 RNA positivity ranging from 0% (0/51) in amniotic fluid and urine (0/17), 3.6% (1/28) in the cord blood, 7.7% (2/26) by placental sample analysis, 9.7% (3/31) by rectal or anal swab, and 3.7% (3/81) by serology [76]. Some investigations were unable to detect mother to fetus SARS-CoV-2 transmission, along with negative test results in breast milk, vaginal swabs, umbilical cord blood, and amniotic fluid [29,77,78]. In one study, of the 35 women patients hospitalized which were found positive for SARS-CoV-2 by RT-PCR nasopharyngeal swab testing, vaginal swabs from 2 (5.7%) women were also tested positive SARS-CoV-2, suggesting the possibility of vaginal colonization of this virus and this aspect need to be considered by clinicians during delivery, though may be uncommon [79]. Nevertheless, precise information or clinical data lacks these isolation approaches, and thus vertical maternal-fetal transmission could not be precluded [45,80].
However, a number of studies have found elevated antibodies (IgM and IgG) and cytokine levels in the blood of neonates of infected mothers, suggesting in utero transmission of SARS-CoV-2 [[81], [82], [83]]. In recent studies, elevated titers of virus-specific IgM antibodies were detected in newborn blood soon after birth, while the reverse transcription-polymerase chain reaction (RT-PCR) analysis of nasopharyngeal swab was observed negative in infants with COVID-19 positive mothers [84,85]. In utero transmission can only be confirmed by isolation of virus or virus detection by RT-PCR in the neonates immediately after delivery. The antibody and cytokines detection in neonates could be due to a passive transfer of these components from mother to the neonate or triggered by the passively transferred antigenic component of the virus. Since IgM antibodies do not transverse the placenta because of their structure, it is likely to produce IgM in the fetus following viral vertical transmission. Nevertheless, this lacks solid evidence and might be ascribed to the alterations in the placenta thus allowing IgM passage resulting in false-positive outcomes [86]. A study noted that of the 64 SARS-CoV-2 positive pregnant women, none of the fetuses tested positive which might be due to the absence of viremia and decreased co-expression and colocalization of placental ACE2 and transmembrane serine protease 2. These protective mechanisms may be responsible for the inhibition of vertical transmission [87].
The current studies suggested the possible vertical transmission of SARS-CoV-2 from pregnant women to their neonates in case reports and series as tested by RT-PCR, however, such data is still not enough, and further in-depth investigations are required [76,[88], [89], [90]]. A systematic review found that SARS-CoV-2 vertical transmission in the third trimester is approximately 3.2% by infant nasopharyngeal swab testing [76]. Altogether, current data support the hypothesis that in utero SARS-CoV-2, vertical transmission is possible, although at a low rate [88]. In a study, 14 out of 469 (3%) neonates were found positive for SARS-CoV-2 by RT-PCR on nasopharyngeal swabs at a median period of 3 h after delivery [91]. However, they quoted separation of neonates from mothers is unnecessary. This allows delayed cord clamping and skin-to-skin contact and supporting breastfeeding that is beneficial for neonates [91]. A study also reported the possibility of maternal to the fetal transmission of SARS-CoV-2 [92]. In the third trimester of pregnancy, COVID-19 infection resulted in serious renal developmental injury, which is indicated by high cystatin C and β2-microglobulin levels in all neonates.
Diagnosis of COVID-19 in pregnancy
Both conventional and advanced methods have been employed for the diagnosis of COVID-19 in pregnant women and neonates. Along with symptoms, clinical parameters, chest X-ray, histopathology, and immunohistochemistry have facilitated the diagnosis of COVID-19 [87,93]. Similar to non-pregnant groups, RT-PCR and computed tomography (CT) scan have been the routine diagnostic tools for the detection of SARS-CoV-2 during pregnancy, and these, when used together, have provided an accurate diagnosis [94]. RT-PCR is considered the gold standard for the confirmation of SARS-CoV-2, detecting the nucleocapsid (N) gene, RNA-dependent-RNA polymerase (RdRp) gene, ORF1ab gene or other genes of the virus with the help of specific probes and primers for samples collected from nasopharyngeal and oropharyngeal swabs, endotracheal aspirate, bronchoalveolar lavage, sputum, stool, and urine [8,95]. The nucleic acids of SARS-CoV-2 are not always detectable in respiratory specimens, and repeated testing may be required on two consecutive occasions at least 24 h apart to obtain a confirmatory diagnosis of COVID-19 [8,96]. In the case of neonates, testing and diagnosis of COVID-19 are still challenging [91]. RT-PCR has enabled the detection of viral RNA in nasopharyngeal swabs of neonates at a median age of 3 h after delivery (1−12 hours) [91].
Chest CT-scan is the primary diagnostic tool to diagnose COVID-19 in pregnant women using visible ground-glass opacities and may be more sensitive for detecting infection than RT-PCR [80,97,98]. One study found that RT-PCR confirmed infection in 840 samples out of a total of 975 samples (86% sensitivity) while CT-scans confirmed 580 samples out of a total of 601 samples (97% sensitivity) from individuals with COVID-19 [99]. Moreover, artificial intelligence using a three-dimensional deep learning model can also be implemented for highly sensitive and specific diagnosis of COVID-19 from the CT images [100]. Chest ultrasounds are also an accurate tool to detect lung infiltrates and avoid radiations in pregnant women with COVID-19 who require a series of exams [101,102]. In addition, ultrasound can also be performed directly at the bed-side and, therefore, could reduce the disease's risk [101,102].
IgM ELISA-based assays, along with PCR, represent non-radiation-based alternatives to CT scan and have been found to show higher sensitivity (98.6%) than RT-PCR assays alone (51.9%) [103]. Current researches have shown that clustered regularly interspaced short palindromic repeats (CRISPR)–based approaches can offer rapid, visual, specific, and ultrasensitive identification of SARS-CoV-2. These approaches have emerged as prodigious alternatives to RT-qPCR methods for accurate and efficient detection of SARS-CoV-2 [104]. A group of scientists designed a Cas12-based novel diagnostic tool to detect SARS-CoV-2 RNA sequences in an early clinical study [105]. The tool proved to be rapid, portable, and highly sensitive for visual detection of RNA. Another study developed the All-In-One Dual Cas12a assay for one-step rapid and ultrasensitive visual identification of SARS-CoV-2 [106]. This assay has demonstrated the potential to develop next-generation point-of-care molecular diagnostics. A CRISPR-based SHERLOCK (Specific High Sensitivity Enzymatic Reporter UnLOCKing) has been developed as a sensitive and more advanced technique for rapidly detecting SARS-CoV-2 without the need of many laboratory facilities [107]. In a study, a combination of techniques like CRISPR, RT-PCR, and metagenomic next-generation sequencing was reported to be effective in the clinical diagnosis of the COVID-19 [108].
Rapid antigen detection testing for universal screening of SARS-CoV-2 in pregnant women has been advocated [109]. Serological analysis might be considered as a novel tool to confirm the diagnosis of SARS-CoV-2. Many research groups have detected the antibody's responses to SARS-CoV-2 and made a comparison with other commercially available serological assays [110,111]. Considering the emerging role of the IgA isotype, a study identified the SARS‐CoV‐2 IgA antibodies in the COVID‐19 population seronegative for IgM [112]. Among the 30 patients, seven, eight, and two were positive for IgG, IgA, and IgG within the first 5–7 days of the symptom’s onset. At the 9–13 and 21–25 days time points, IgA antibodies were increased to twice as high as IgG. Therefore, the determination of IgA antibodies may overcome the serological gap of the COVID‐19. A commercial kit with chemiluminescence microparticle immunoassay was applied for determining the titer of total antibodies in serum samples. This kit can be employed for epidemiological studies as well as the diagnosis of suspected COVID-19 patients [113]. A study used a combined method including RT-PCR test and serological antibodies to detect SARS-CoV-2 infections with a superior extent of specificity and sensitivity [114]. This combined method has demonstrated usefulness for diagnosing suspected COVID-19 patients. In future, the serological testing could be applied for epidemiological researches, monitoring ongoing SARS-CoV-2 outbreaks, and evaluating the impact of vaccines.
Management and preventive strategies: general approaches and special considerations
General principles in the management of COVID-19 in pregnancy
All pregnant women have the right to a positive childbirth experience whether or not they have confirmed COVID-19. This includes proper care and management of pregnant women, antenatal care, safe delivery practices, and care for the newborn along with the mental wellbeing of the mother during the COVID-19 pandemic while adopting appropriate clinical guidelines [46,115,116]. COVID-19 suspected pregnant women need to be attended by telehealth approaches (online, phone, videoconferencing), followed by the proper wearing of protective personnel equipment when the need for attending in healthcare facility under proper protective measures [93]. Screening by checklist tool present on telephone and testing of women in hotspot districts irrespective of symptoms presenting for labor or 5 days before delivery has been recommended in some countries like India [117]. In mild cases, follow up should be scheduled 24−48 hours and weekly. After diagnostic testing, walk-in or drive-in facilities have been quoted as appropriate [93]. The initial evaluation should be through the examination box using interphone. Medical history and a physical examination need to evaluate followed by fetal heart rate auscultation, cardiotocography, or fetal ultrasound. Chest X-ray and laboratory testing are required for diagnosis and evaluating severity [93]. Pregnant women with any COVID-19 symptoms should be isolated and investigated immediately. The time and mode of delivery must be chosen depending on the gestational period, condition of the fetus, and the mother's health status [118]. Emergency cesarean delivery could be considered in the case of poor progress in labor, fetal distress, deterioration in maternal condition, septic shock, and acute organ failure [12,119]. However, the timing of delivery and emergency c-sections remains highly controversial, and many societies do not advocate for systematic c-sections in the setting of maternal clinical deterioration; therefore, clinicians should follow their local guidelines. Individuals with confirmed SARS-CoV-2 infection should be kept in a well-equipped negative pressure isolation ward with sufficient health facilities and fully trained multidisciplinary clinicians who can manage critically ill obstetric patients [119,120]. A trained multidisciplinary team comprised of neonatologists, obstetricians, specialists in intensive care medicine, anesthetists, and microbiologists should be available to evaluate and manage the risk of serious outcomes associated with COVID-19. All medical staff and health professionals must use personal protective equipment (PPE), including gloves, gown, goggles, and N95 masks, when treating patients with COVID-19.
It is of utmost importance to support pregnant women exhibiting COVID-19 symptoms, such as myalgia, pyrexia, shortness of breath, cough, and sore throat [121]. The vital parameters of pregnant women, including body temperature, respiration rate, and pulse, must be regularly monitored by maternity-care providers, alongside the provision of medical attention and advice. Inspection of vital manifestations particularly associated with heart failure and respiratory complications, renal and hepatic functions, CRP, CBC, and chest radiography, is essential. Professional psychological support must also be offered to pregnant women who have been isolated for 14 days to limit the risk of developing severe depression or anxiety. A previous qualitative study found that COVID-19 patients hope to receive online support [122]. Internet cognitive behavior therapy could be a cost-effective approach [123] and reduce the severity of psychological symptoms (e.g., insomnia) [124].
Healthcare professionals should also implement the necessary precautionary measures and standard protocols for the use and discarding of specialized clothing, gloves, masks, and medical equipment after examining pregnant women infected with SARS-CoV-2. An initial screening of healthcare workers and other individuals must be emphasized before they are allowed to enter hospitals or neonatal care units [75]. The immediate clamping of umbilical cords and separation of neonates from mothers confirmed or suspected of being infected is recommended to reduce the risk of transmitting SARS-CoV-2 [3,125]. Careful monitoring of pregnant women and fetus and appropriate precautions to avoid neonatal infection is necessary during the SARS-CoV-2 pandemic [126].
Representative guidelines for management and preventive procedures from national and international organizations are provided in Table 1 .
Table 1.
Guidelines provided by few representative organizations for the pregnant women during COVID-19 pandemic.
| Name of the organization | Guidelines for the pregnant women | References |
|---|---|---|
| World Health Organization (WHO) | • Regular checkup and monitoring • Isolation of affected women • Provision of skilled medical staff with all associated remedial provisions • Counselling to deal with the physiological and mental stress during pregnancy • Thorough counseling of pregnant women related to the breast feeding and isolation of the fetus after birth • Expert decision for the vaginal or cesarean delivery • Use of minimum essential protective gears and maintenance of proper hygiene to prevent the transmission of infection • Routine ante-natal or post-partum care of fetus and mother |
[127] |
| Centers for Disease Control and Prevention (CDC) | • Confirmed/suspected COVID-19 pregnant women should be isolated immediately and notified to obstetric unit • Immediate emergency care should be provided to the pregnant women with COVID-19 • Regular provision of counselling related to the prenatal or postpartum care • Maintenance of proper hygiene including washing of hands with soap or sanitize with 60% alcohol, using mask etc. before breast feeding the newborn baby • The women suspicious or infected with SARS-CoV-2 virus infection must be isolated from the newborn and breast pump milking should be recommended • Tdap and influenza vaccines can be used during pregnancy |
[121,128] |
| French national college of obstetricians and gynecologists (CNGOF, France) | • Assigning a triage screening area with primary facilities for the screening of symptomatic patients • All the patients and health care professions must wear PPEs • The suspicious patients must be considered positive before the final confirmation with qRT-PCR assay • The pregnant women suspicious to be affected with COVID-19 should be immediately segregated and provided with suitable obstetrical management care facilities • The general non-urgent appointment must be delayed to look after the critical cases of obstetrics • Provision of negative pressure isolation room with ultrasonography facilities for the routine checkup of the pregnant women • Use of corticosteroid therapy based upon the obstetric indications is recommended in the COVID-19 affected pregnant women even before 34 weeks • Telehealth facilities must be recommended for the postpartum visit • The proper hygiene measures must be followed by the mother before feeding or making a contact with the newborn • Breast feeding should be avoided in case the mother is infected with SARS-CoV-2 |
[[129], [130], [131]] |
| National Health Commission of the People’s Republic of China | • Thorough examination and diagnosis of mother suspicious for COVID-19 disease • Management of pyrexia and provision of treatment therapies for the affected patients • Newborn delivered by an infected mother must be isolated and observed at least for 14 days and should not be offered with breast feeding |
[132] |
| American College of Obstetricians and Gynecologists (ACOG, US) | • Regular notification of positive cases to the health department after proper testing and screening • The health care workers should wear all protective gears including face mask, gloves etc. • The person with suspicion should be instructed to avoid the contact with social groups and must be confirmed • Antenatal corticosteroids (BMZ) can be used in the patients with COVID-19 disease before 34 weeks • A skilled team of obstetrician, anesthesiologist etc. should be available every time • Due care must be offered to the newborn and the mother after delivery • A halt in the time of delivery can be imposed during late pregnancy to prevent the transmission of infection until the results of test are not available, whereas early pregnancy must be routinely handled • Telehealth facilities can be used for postpartum visit at least for 12 weeks. |
[133] |
| Royal College of Obstetricians and Gynecologists (RCOG, UK) | • Regular screening and use of protective gears • Provision of obstetric emergency facilities • Monitoring of blood parameters and isolation of suspected cases • Routine checkup of the pregnant women by using ultrasonological interventions to monitor the maternal and fetal health status • Proper cleaning of equipments after their use on suspected or positive cases • Isolation of affected patients with the provision of emergency services • Maintenance of proper hygiene measures including hand washing, use of PPEs (FFP3 masks, gloves), and use of breast pumps etc. • Telehealth facilities are recommended to ensure the postpartum visit |
[134] |
| Italian society for Ultrasound in Obstetrics and Gynecology (Italy) | • Follow self-isolation and hygiene measures in asymptomatic patients without any essentiality of PPE • PPE must be used by the symptomatic pregnant women and health care workers accompanying the patients • Use of protective gears like masks and proper hand washing before breast feeding of the newborn • Provision of isolated and designated units for the sample collection and delivery • Isolated rooms should be provided to the mother and babies with at least 60 L/s ventilation • Routine monitoring through ultrasound assessment of asymptomatic mothers • Isolation of symptomatic mothers for 14 days, regular contact through telephones and should be examined after ever 3−4 weeks for the assessment of fetal growth • Decontamination of equipments such as ultrasound machines etc. used on affected pregnant women • Critical symptomatic patients must be admitted to the intensive care units (ICUs) |
[130,131] |
| International Society of Ultrasound in Obstetrics & Gynecology (ISUOG) | • Screening of patients with symptoms in an established triage area • The tested negative samples must be rescreened with qRT-PCR after 24 h and chest CT scan can be used for the confirmation • Critical patients should be admitted to ICU or the rooms with negative pressure facilities • Examination of suspected or recovered patients with the help of ultrasonography must be done after every 2−4 weeks for the assessment of fetal growth and associated post-partum complications • The mode of delivery should be decided on the basis of health status of the patient and spectrum of infection • Water birth and delayed cord clamping should be avoided in critically ill pregnant women • The fetal and placental tissues should be handled carefully by using all the PPEs and personal hygiene guidelines • Feeding through breast pump should be encouraged |
[130,135] |
Delivery
The termination of pregnancy and mode of delivery must be determined in critical COVID-19 cases depending upon the pregnancy complications and health of the puerperal. Among asymptomatic/mild COVID-19 patients, cesarean delivery had a higher risk of clinical deterioration (increased need for oxygen supplementation after delivery), and increased risk of neonatal intensive care unit admission compared to vaginal delivery [136]. However, for severe COVID-19, cesarean delivery might be the first choice of delivery [136]. Preterm labor has been commonly observed in acute COVID-19 after 9 days of the onset of respiratory symptom [137]. The standard management protocol indicates intravenous magnesium sulfate, antepartum steroid therapy, and appropriate antibiotics to prevent the secondary bacterial infection [137]. A study found an incidence of 14.43% of COVID-19 in India [138]. Low APGAR scores (score for appearance, pulse, grimace, activity, and respiration) also have been reported in neonates of COVID-19 affected women [138].
CDC states that after delivery, the umbilical cord should be clamped, the newborn should be temporarily isolated, and breast milk should be pumped as soon as possible [121]. An aborted fetus and the placentas from pregnant women suffering from COVID-19 must be treated as infectious and be properly disposed of [135]. During delivery or during the treatment course, biomedical waste materials should be collected in double-layered medical waste bags and disinfected before disposal. Any non-disposable fabrics must be collected in double-layered medical waste bags and sent to the hospital’s disinfection center, as per the standard protocol [73].
Breastfeeding
Close and early contact and exclusive breastfeeding help babies to thrive. However, women with COVID-19 have challenges in terms of breastfeeding. A study found a negative PCR test of breastmilk samples from COVID-19 women [81]. Amniotic fluid, cord blood, and throat swabs of neonates along with breastmilk samples from six out of nine women infected with COVID-19 were found negative for the SARS-CoV-2 [6]. However, SARS-CoV-2 RNA was detected in the breast milk of a mildly symptomatic COVID-19 patient but the presence of the viable virus in breast milk is still under investigation [139]. Altogether suggest that no strong evidence of vertical transmission of SARS-CoV-2 through breastmilk. A living systematic review analyzing the evidence from 37 studies concluded that there is no evidence of SARS-CoV-2 transmission through breast milk [140].
Although not enough evidence supports SARS-CoV-2 transmission through breast milk [[140], [141], [142]], close contact during breastfeeding may contribute to the transmission of infection from mothers to their newborns [75]. Therefore, feeding of expressed mother’s milk should be considered to avoid the child’s contact with the COVID-19 infected mother [143]. Adequate precautions, including the practice of respiratory hygiene, wearing a mask, washing hands, routine cleaning, and disinfecting surfaces is recommended [141,144]. The National Health Commission of China also recommended the use of breast pumps instead of direct breastfeeding and the immediate segregation of neonates from mothers who are suspected to be infected with SARS-CoV-2 to mitigate the chances of the spread of infection [129] (Fig. 1 ). In case of favorable conditions, breastfeeding is allowed under contact and droplet precautions, including the use of a surgical mask, appropriate hand hygiene before and after contact, cleaning breast skin and contact surfaces [119,145]. In the case of infected mothers use of milk extractors under strict hygiene is recommended, and feeding of the newborn by a healthy person [93].
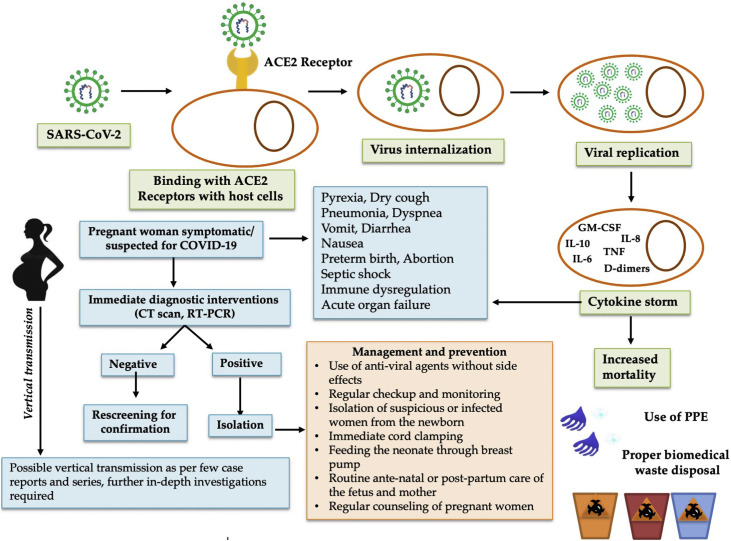
Fig. 1.
COVID-19 during pregnancy: management and prevention strategies. After SARS-CoV-2 enters into host cells and multiples in the pregnant women, it will alter responses of immune system and, in some cases, lead to cytokine storm that could increase the mortality. Pregnant women with who are suspected for COVID-19, timely diagnose strategy is need in order to provide appropriate managements to prevent maternal and neonatal complications. For those who are confirmed for COVID-19, adequate management and prevention measures need to be in placed according to international guidelines in accordance with national guideline.
Treatment
Multiple efforts are currently being made to develop effective treatment regimens against COVID-19 [146]. Several trials aimed at developing anti-viral therapies against SARS-CoV-2 currently being sponsored by several organizations excluded pregnant women from randomized trials, while the inclusion of pregnant women in treatment trials needs a global level action plan for designing better and safer therapeutic strategies [147,148]. Recently, D'Souza et al. [149] critically reviewed the progress being made in pharmacologic options especially suitable to pregnant and lactating women COVID-19 patients with evidence in support of the use of corticosteroids, magnesium sulfate, aspirin, anticoagulation after appropriate prescription, clinical suitability as well as emphasized safety issues of various therapies under investigations along with suggestion for their inclusion in clinical trials. Although no specific treatment exists against COVID-19 during pregnancy, various drugs have been used to treat the disease in pregnancy such as arbidol, remdesivir, lopinavir/ritonavir combination, dexamethasone, and combination of IFN-α-2b and arbidol as well as convalescent plasma, conservative fluid management, mechanical ventilation and other supportive therapies [150,151].
Dexamethasone has been suggested to be avoided during 1 st trimester and after 37 weeks of gestation [126]. In comparison to no corticosteroid administration, the use of antenatal corticosteroids has been found as a robust management strategy at gestational ages less than 31 weeks. Zhou et al. (2020) observed administrating antenatal corticosteroids between 24 and 30 weeks of gestation as the optimal management strategy resulting in high quality-adjusted life years compared to no corticosteroid use. A study observed that about 68% of the patients showed improvement after treatment with remdesivir, while 13% were found to die [152]. In one study on 86 pregnant and postpartum women affected with severe COVID-19, compassionate use of remdesivir resulted in higher recovery rates while rate of adverse event was recorded as low [153]. A large number of case studies and investigations have corroborated remdesivir as a safer and effective remedy for treating serious COVID-19 during pregnancy [36,154,155]. The combination of lopinavir and ritonavir (anti-proteases) was previously found to exert antiviral activity against HIV in a pregnant woman, without increasing the risk of preterm birth, fetal anomalies, or low-birth-weight of the fetus; therefore, representing a potentially safe drug regimen for the treatment of COVID-19 infection during pregnancy [8,156,157]. However, the combination of lopinavir-ritonavir has not been found to provide any significant beneficial effects against SARS-CoV-2 infection [158]. Antivirals lopinavir + ritonavir or oseltamivir can be used in high-risk women with uncontrolled diabetes, immunosuppression, or chronic diseases [117,159].
Chloroquine, a potent anti-malarial agent has been found to be effective against COVID-19 associated pneumonia [[160], [161], [162]]. An in vitro study also showed that a combination of chloroquine and remdesivir (nucleotide analog) also inhibiting SARS-CoV-2 virus replication [163]. Studies have revealed that the use of hydroxychloroquine and chloroquine presented noteworthy fetal and maternal safety and are regarded as an imperative remedy for expecting women associated with COVID-19 [113,164]. Nevertheless, an elevated dose of chloroquine also exerts negative impacts [80]. The mode of action chloroquine involves interference with the terminal glycosylation of ACE2 receptors on host cells, blockade of pH-dependent replication of the virus, and suppression of cytokine storms [161]. Although adverse side effects of chloroquine treatment in pregnant women or fetuses have been reported in the last two decades [165], at high doses, chloroquine is known to cause systolic hypertension and aggravate hemodynamic changes associated with the supine aortocaval compression of the gravid uterus [80,166]. A study has also demonstrated the clinical benefits of the combined use of azithromycin and hydroxychloroquine in COVID-19 patients [167]. However, a recent study found that the use of hydroxychloroquine, alone or with azithromycin, did not improve clinical status among hospitalized mild-to-moderate COVID-19 patients at 15 days as compared with standard care [168]. The current interim WHO Solidarity Trial results also suggested that hydroxychloroquine appeared to have little or no effect on COVID-19 mortality, initiation of ventilation, and duration of hospital stay [169]. It is worth mentioning that a recent systematic review and meta-analysis on drugs being explored for treating COVID-19 patients revealed that hydroxychloroquine, lopinavir/ritonavir, interferon-beta, and tocilizumab may not alleviate risk of death or outcome of the disease significantly as well as benefits of remdesivir remains uncertain [170]. Federation of Obstetric and Gynecological Societies of India (FOGSI) recommends use of hydroxychloroquine and azithromycin for treating infected pregnant women along with supportive treatment [117,159].
COVID-19 causes extensive damage to the lungs and has been associated with increased secondary bacterial pneumonia. As such, the immediate administration of antibiotics after culture sensitivity test is highly recommended [8]. In a case-report study of a pregnant woman with ADRS due to COVID-19, treatment with plasma transfusion and corticosteroids resulted in a clinical improvement of the patient [171]. The administration of methylprednisolone for a short duration has been found to limit the effects of ARDS in SARS-CoV-2 infected patients in China [8]; however, an assessment of the effectiveness and safety of this drug in pregnant women is needed. In premature neonates, the intramuscular administration of betamethasone at a dose of 12 mg followed by a second dose after 24 h has been found to be effective [8]. Patients with alveolar infiltrate, bacterial infection, or elevated procalcitonin may require [93].
Vaccines
Vaccine is one of the most promising preventive measures for COVID-19 [172] and COVID-19 have caused disruptions of childhood vaccination practice around the world [173]. Vaccination during pregnancy is a promising strategy to protect the mother and neonate against the disease [174,175]. Live or live attenuated vaccines may not be safe as there is a risk of developing the disease during the immunomodulated pregnancy condition; however, the inactivated or subunit and nucleic acid vaccines will be safer as there is not a risk of developing disease from the vaccine. An ideal COVID-19 vaccine for use during pregnancy should induce a balanced humoral and cell-mediated immune response without excessive activation of the maternal immune system [176]. Excessive activation of the immune system may cause an excessive inflammatory response, leading to adverse events during pregnancy. Maternal immunization is not only for protecting the mothers, but it is also critical for protecting the newborn until their immune system can develop their own defense against the viruses. Upon vaccination of pregnant women, IgG antibodies are actively transferred through the placenta to provide passive immunity to newborns [177].
Biopharmaceutical companies and research institutions are actively pursuing vaccine development strategies against SARS-CoV-2 [178]. At the time of writing, efficacy reports from phase 3 trials of three COVID-19 vaccines, BNT162b2 mRNA (BioNTech and Pfizer) [179], ChAdOx1 nCoV-19 (the University of Oxford and AstraZeneca) [180] and mRNA-1273 (Moderna and National Institutes of Health (NIH)) [181] have been published. The efficacy of the vaccines ranged from 90% for ChAdOx1 nCoV-19 [180], 94% for mRNA-1273 [181] and 95% for BNT162b2 [179]. Most recently, few of the vaccines have been developed successfully and vaccination program has been launched up in some countries with use of Sputnik V (Gamaleya Company, Russia), Covaxin (inactivated vaccine developed by Bharat Biotech Company, India), Covishield (local name in India for the Oxford-AstraZeneca vaccine developed in the UK) and other potential vaccines for emergency uses [[182], [183], [184]]. None of those studies included pregnant women as their study participants. There is a need to design dedicated vaccine trials with a larger number of men and women participants, including pregnant women and lactating women, to ensure efficacy and safety of the same in all groups [185].
Recently, some companies intend to include pregnant women in clinical trials but requested to confirm a contraception plan for weeks to months after injection of the COVID-19 vaccine. To date, very scarce information is available on efficacy and safety in pregnancy. In their clinical trial, Moderna reported 13 pregnancies, including six and seven in the vaccine and placebo group, respectively, while Pfizer enrolled 23 pregnant persons in their clinical trial, including 12 in the vaccine group. The pregnancies exposed to the COVID-19 vaccine are in progress. Since about 75% of the health care workers are women, approximately 300,000 health care personnel might be pregnant or recently postpartum at the time of vaccine implementation, according to the CDC estimates [186].
Vaccine candidates against SARS-CoV-2 involving minimal risk and potential benefit to pregnant women and fetuses should be developed and introduced into clinical trials encouraging their participation [187,188]. Pregnant women, lactating women, and women desirous of pregnancy make up a significant proportion of the population [189], and therefore exclusion of this population from trials or lack of safety and efficacy information will leave a critical gap in control of COVID-19. Pregnant women and their obstetricians need to be updated with the recent information and analyze the benefits and risks of SARS-CoV-2 vaccines until data of trials involving pregnant women is made available [186]. Though pregnant women are conceived to be at similar complications risk than non-pregnant women, there is a dire need for intense care for pregnant women with COVID-19, especially in middle and low-income countries [36]. Evidence suggests that pregnant females should take care of preventative measures, and the vaccine is among the top-priority option [187].
Conclusion and future prospects
Pregnant women are considered to be a vulnerable group during the ongoing COVID-19 pandemic due to the exacerbated detrimental effects of SARS-CoV-2 infection observed in expectant mothers and their fetuses. Multipronged factors, including physiological anatomic changes, hormonal imbalance, alterations in immune systems, and increase expression of ACE2, might associate with increased severity of COVID-19 in pregnancy. Close monitoring of body parameters, use of sensitive diagnostic protocols, and timely treatment coupled with preventive measures may help limit the transmission of COVID-19 in pregnant women and, therefore, prevent COVID-19 complications. In association with preventive measures, good management practices must be emphasized to minimize cross-infection between COVID-19-infected pregnant women, health care workers, and neonates. All possible preventive measures along with the adoption of good personal hygiene and personal protection must be ensured during delivery and breastfeeding to reduce the risk of virus transmission to newborn babies.
All the strategies including early diagnosis, timely management, and prevention measures should be employed along with robust research to generate evidence for effective strategies. Publicly available surveillance systems based on international, local, and federal guidelines should be established for pregnant women infected with SARS-CoV-2 to allow the expertise of professionals around the world to be shared to mitigate or prevent the detrimental effects of the ongoing COVID-19 pandemic.
There are many gaps of knowledge that need to be filled in SARS-CoV-2 infection during pregnancy. Neither approved therapeutic options are available to date for pregnant women with COVID-19 and not much are in the pipeline, including pregnant women in clinical trials. Therefore, there is an urgent need of a drug developed specifically for pregnant women suffering from COVID-19, and enough clinical trials, including this physiological group are highly warranted. The inclusion of pregnant and lactating women in the vaccine development process is also vital to ensure this vulnerable population is protected along with others. Learning from past efforts to develop vaccines such as influenza and the study of currently used vaccines in pregnant women will help include pregnant women in trials for vaccine development safely.
Funding
No funding sources.
Declaration of interests
None declared.
Ethical approval
Not required.
Acknowledgments
All the authors acknowledge and thank their respective institutes and universities.
Footnotes
Supplementary material related to this article can be found, in the online version, at doi:https://doi.org/10.1016/j.jiph.2021.04.005.
Appendix A. Supplementary data
The following is Supplementary data to this article:
References
- 1.WHO. WHO coronavirus disease (COVID-19) dashboard. Available from: https://covid19whoint/. [Accessed 11 April 2021].
- 2.Huang C., Wang Y., Li X., Ren L., Zhao J., Hu Y., et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395(10223):497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wang W., Tang J., Wei F. Updated understanding of the outbreak of 2019 novel coronavirus (2019-nCoV) in Wuhan, China. J Med Virol. 2020;92(4):441–447. doi: 10.1002/jmv.25689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Harapan H., Itoh N., Yufika A., Winardi W., Keam S., Te H., et al. Coronavirus disease 2019 (COVID-19): a literature review. J Infect Public Health. 2020;13(5):667–673. doi: 10.1016/j.jiph.2020.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rodriguez-Morales A.J., Cardona-Ospina J.A., Gutierrez-Ocampo E., Villamizar-Pena R., Holguin-Rivera Y., Escalera-Antezana J.P., et al. Clinical, laboratory and imaging features of COVID-19: a systematic review and meta-analysis. Travel Med Infect Dis. 2020;34 doi: 10.1016/j.tmaid.2020.101623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chen N., Zhou M., Dong X., Qu J., Gong F., Han Y., et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;395(10223):507–513. doi: 10.1016/S0140-6736(20)30211-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mehta P., McAuley D.F., Brown M., Sanchez E., Tattersall R.S., Manson J.J., et al. COVID-19: consider cytokine storm syndromes and immunosuppression. Lancet. 2020;395(10229):1033–1034. doi: 10.1016/S0140-6736(20)30628-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Liang H., Acharya G. Novel corona virus disease (COVID-19) in pregnancy: what clinical recommendations to follow? Acta Obstet Gynecol Scand. 2020;99(4):439–442. doi: 10.1111/aogs.13836. [DOI] [PubMed] [Google Scholar]
- 9.Wu C., Chen X., Cai Y., Xia J., Zhou X., Xu S., et al. Risk Factors associated with acute respiratory distress syndrome and death in patients with coronavirus disease 2019 pneumonia in Wuhan, China. JAMA Intern Med. 2020;180(7):934–943. doi: 10.1001/jamainternmed.2020.0994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Keam S., Megawati D., Patel S., Tiwari R., Dhama K., Harapan H. Immunopathology and immunotherapeutic strategies in SARS-CoV-2 infection. Rev Medical Virol. 2020;30(5):e2123. doi: 10.1002/rmv.2123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chen L., Li Q., Zheng D., Jiang H., Wei Y., Zou L., et al. Clinical characteristics of pregnant women with Covid-19 in Wuhan, China. N Engl J Med. 2020;382(25):e100. doi: 10.1056/NEJMc2009226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Favre G., Pomar L., Musso D., Baud D. 2019-nCoV epidemic: what about pregnancies? Lancet. 2020;395(10224):e40. doi: 10.1016/S0140-6736(20)30311-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhu H., Wang L., Fang C., Peng S., Zhang L., Chang G., et al. Clinical analysis of 10 neonates born to mothers with 2019-nCoV pneumonia. Transl Pediatr. 2020;9(1):51–60. doi: 10.21037/tp.2020.02.06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wong S.F., Chow K.M., de Swiet M. Severe acute respiratory syndrome and pregnancy. BJOG. 2003;110(7):641–642. doi: 10.1046/j.1471-0528.2003.03008.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lam C.M., Wong S.F., Leung T.N., Chow K.M., Yu W.C., Wong T.Y., et al. A case-controlled study comparing clinical course and outcomes of pregnant and non-pregnant women with severe acute respiratory syndrome. BJOG. 2004;111(8):771–774. doi: 10.1111/j.1471-0528.2004.00199.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kourtis A.P., Read J.S., Jamieson D.J. Pregnancy and infection. N Engl J Med. 2014;370(23):2211–2218. doi: 10.1056/NEJMra1213566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schwartz D.A. Being pregnant during the Kivu Ebola virus outbreak in DR Congo: the rVSV-ZEBOV vaccine and its accessibility by mothers and infants during humanitarian crises and in conflict areas. Vaccines. 2020;8(1):38. doi: 10.3390/vaccines8010038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yan J., Guo J., Fan C., Juan J., Yu X., Li J., et al. Coronavirus disease 2019 in pregnant women: a report based on 116 cases. Am J Obstet Gynecol. 2020;223(1):111 e111–111 e114. doi: 10.1016/j.ajog.2020.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Akgor U., Fadiloglu E., Soyak B., Unal C., Cagan M., Temiz B.E., et al. Anxiety, depression and concerns of pregnant women during the COVID-19 pandemic. Arch Gynecol Obstet. 2021 doi: 10.1007/s00404-020-05944-1. (In press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang Y., Chen L., Wu T., Shi H., Li Q., Jiang H., et al. Impact of Covid-19 in pregnancy on mother’s psychological status and infant’s neurobehavioral development: a longitudinal cohort study in China. BMC Med. 2020;18(1):347. doi: 10.1186/s12916-020-01825-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Di Mascio D., Saccone G., D’Antonio F., Berghella V. Psychopathology associated with coronavirus disease 2019 among pregnant women. Am J Obstet Gynecol MFM. 2021;3(1) doi: 10.1016/j.ajogmf.2020.100290. 100290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Etti M., Sekikubo M., Nankabirwa V., Sommerfelt H., Freyne B., Kawaza K., et al. SARS-CoV-2 infection in pregnant women and their newborns. Ann Glob Health. 2020;86(1):132. doi: 10.5334/aogh.3072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Krupa A., Schmidt M., Zborowska K., Jorg D., Czajkowska M., Skrzypulec-Plinta V. Impact of COVID-19 on pregnancy and delivery – current knowledge. Ginekol Pol. 2020;91(9):564–568. doi: 10.5603/GP.a2020.0127. [DOI] [PubMed] [Google Scholar]
- 24.Moore K.M., Suthar M.S. Comprehensive analysis of COVID-19 during pregnancy. Biochem Biophys Res Commun. 2021;538:180–186. doi: 10.1016/j.bbrc.2020.12.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ortiz E.I., Herrera E., De La Torre A. Coronavirus (COVID 19) infection in pregnancy. Colomb Med (Cali) 2020;51(2):e4271. doi: 10.25100/cm.v51i2.4271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wastnedge E.A.N., Reynolds R.M., van Boeckel S.R., Stock S.J., Denison F.C., Maybin J.A., et al. Pregnancy and COVID-19. Physiol Rev. 2021;101(1):303–318. doi: 10.1152/physrev.00024.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wong S.F., Chow K.M., Leung T.N., Ng W.F., Ng T.K., Shek C.C., et al. Pregnancy and perinatal outcomes of women with severe acute respiratory syndrome. Am J Obstet Gynecol. 2004;191(1):292–297. doi: 10.1016/j.ajog.2003.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kuehn B.M. COVID-19 poses pregnancy risks. JAMA. 2020;324(18):1819. doi: 10.1001/jama.2020.21129. [DOI] [PubMed] [Google Scholar]
- 29.Yang R., Mei H., Zheng T., Fu Q., Zhang Y., Buka S., et al. Pregnant women with COVID-19 and risk of adverse birth outcomes and maternal-fetal vertical transmission: a population-based cohort study in Wuhan, China. BMC Med. 2020;18(1):330. doi: 10.1186/s12916-020-01798-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Karami P., Naghavi M., Feyzi A., Aghamohammadi M., Novin Ms, Mobaien A., et al. Mortality of a pregnant patient diagnosed with COVID-19: a case report with clinical, radiological, and histopathological findings. Travel Med Infect Dis. 2020 doi: 10.1016/j.tmaid.2020.101665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Knight M., Bunch K., Vousden N., Morris E., Simpson N., Gale C., et al. Characteristics and outcomes of pregnant women admitted to hospital with confirmed SARS-CoV-2 infection in UK: national population based cohort study. BMJ. 2020;369:m2107. doi: 10.1136/bmj.m2107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mendoza M., Garcia-Ruiz I., Maiz N., Rodo C., Garcia-Manau P., Serrano B., et al. Pre-eclampsia-like syndrome induced by severe COVID-19: a prospective observational study. BJOG. 2020;127(11):1374–1380. doi: 10.1111/1471-0528.16339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Martinez-Portilla R.J., Sotiriadis A., Torres-Torres J., Christos C., Hawkins-Villarreal A., Villafan-Bernal J.R., et al. Risk factors for mortality in pregnant women with SARS-CoV-2 infection. medRxiv. 2020 doi: 10.1101/2020.05.31.20107276. [DOI] [Google Scholar]
- 34.Gulersen M., Staszewski C., Grayver E., Tam Tam H., Gottesman E., Isseroff D., et al. Coronavirus disease 2019 (COVID-19)-related multisystem inflammatory syndrome in a pregnant woman. Obstet Gynecol. 2021;137(3):418–422. doi: 10.1097/AOG.0000000000004256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ellington S., Strid P., Tong V.T., Woodworth K., Galang R.R., Zambrano L.D., et al. Characteristics of women of reproductive age with laboratory-confirmed sars-cov-2 infection by pregnancy status — United States, January 22–June 7, 2020. MMWR Morb Mortal Wkly Rep. 2020;69:769–775. doi: 10.15585/mmwr.mm6925a1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Khalil A., Kalafat E., Benlioglu C., O’Brien P., Morris E., Draycott T., et al. SARS-CoV-2 infection in pregnancy: a systematic review and meta-analysis of clinical features and pregnancy outcomes. EClinicalMedicine. 2020;25 doi: 10.1016/j.eclinm.2020.100446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Davies J.K., Shikes R.H., Sze C.I., Leslie K.K., McDuffie R.S., Jr, Romero R., et al. Histologic inflammation in the maternal and fetal compartments in a rabbit model of acute intra-amniotic infection. Am J Obstet Gynecol. 2000;183(5):1088–1093. doi: 10.1067/mob.2000.108888. [DOI] [PubMed] [Google Scholar]
- 38.Salaun B., Romero P., Lebecque S. Toll‐like receptors’ two‐edged sword: when immunity meets apoptosis. Eur J Immunol. 2007;37(12):3311–3318. doi: 10.1002/eji.200737744. [DOI] [PubMed] [Google Scholar]
- 39.Deverman B.E., Patterson P.H. Cytokines and CNS development. Neuron. 2009;64(1):61–78. doi: 10.1016/j.neuron.2009.09.002. [DOI] [PubMed] [Google Scholar]
- 40.Madsen-Bouterse S.A., Romero R., Tarca A.L., Kusanovic J.P., Espinoza J., Kim C.J., et al. The transcriptome of the fetal inflammatory response syndrome. Am J Reprod Immunol. 2010;63(1):73–92. doi: 10.1111/j.1600-0897.2009.00791.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Alfaraj S.H., Al-Tawfiq J.A., Memish Z.A. Middle east respiratory syndrome coronavirus (MERS-CoV) infection during pregnancy: report of two cases & review of the literature. J Microbiol Immunol Infect. 2019;52(3):501–503. doi: 10.1016/j.jmii.2018.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mor G., Cardenas I. The immune system in pregnancy: a unique complexity. Am J Reprod Immunol. 2010;63(6):425–433. doi: 10.1111/j.1600-0897.2010.00836.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Schwartz D.A., Graham A.L. Potential maternal and infant outcomes from (Wuhan) Coronavirus 2019-nCoV infecting pregnant women: lessons from SARS, MERS, and other human coronavirus infections. Viruses. 2020;12(2):194. doi: 10.3390/v12020194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Akhtar H., Patel C., Abuelgasim E., Harky A. COVID-19 (SARS-CoV-2) infection in pregnancy: a systematic review. Gynecol Obstet Invest. 2020;85(4):295–306. doi: 10.1159/000509290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chen H., Guo J., Wang C., Luo F., Yu X., Zhang W., et al. Clinical characteristics and intrauterine vertical transmission potential of COVID-19 infection in nine pregnant women: a retrospective review of medical records. Lancet. 2020;395(10226):809–815. doi: 10.1016/S0140-6736(20)30360-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Benski C., Di Filippo D., Taraschi G. Reich MR. Guidelines for pregnancy management during the COVID-19 Pandemic: a public health conundrum. Int J Environ Res Public Health. 2020;17(21):8277. doi: 10.3390/ijerph17218277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Marx G.F., Murthy P.K., Orkin L.R. Static compliance before and after vaginal delivery. Br J Anaesth. 1970;42(12):1100–1104. doi: 10.1093/bja/42.12.1100. [DOI] [PubMed] [Google Scholar]
- 48.Sharma A. The Health Sciences Publisher; New Delhi: 2016. A practical guide to third trimester of pregnancy & puerperium. [Google Scholar]
- 49.Field S.K., Bell S.G., Cenaiko D.F., Whitelaw W.A. Relationship between inspiratory effort and breathlessness in pregnancy. J Appl Physiol. 1991;71(5):1897–1902. doi: 10.1152/jappl.1991.71.5.1897. [DOI] [PubMed] [Google Scholar]
- 50.Toppozada H., Michaels L., Toppozada M., El-Ghazzawi I., Talaat M., Elwany S. The human respiratory nasal mucosa in pregnancy. An electron microscopic and histochemical study. J Laryngol Otol. 1982;96(7):613–626. doi: 10.1017/s0022215100092902. [DOI] [PubMed] [Google Scholar]
- 51.Nelson D.M., Main E., Crafford W., Ahumada G.G. Peripartum heart failure due to primary pulmonary hypertension. Obstet Gynecol. 1983;62(3 Suppl):58s–63s. [PubMed] [Google Scholar]
- 52.Bende M., Gredmark T. Nasal stuffiness during pregnancy. Laryngoscope. 1999;109(7):1108–1110. doi: 10.1097/00005537-199907000-00018. [DOI] [PubMed] [Google Scholar]
- 53.Druckmann R., Druckmann M.A. Progesterone and the immunology of pregnancy. J Steroid Biochem Mol Biol. 2005;97(5):389–396. doi: 10.1016/j.jsbmb.2005.08.010. [DOI] [PubMed] [Google Scholar]
- 54.Pierdominici M., Maselli A., Colasanti T., Giammarioli A.M., Delunardo F., Vacirca D., et al. Estrogen receptor profiles in human peripheral blood lymphocytes. Immunol Lett. 2010;132(1-2):79–85. doi: 10.1016/j.imlet.2010.06.003. [DOI] [PubMed] [Google Scholar]
- 55.Pazos M., Sperling R.S., Moran T.M., Kraus T.A. The influence of pregnancy on systemic immunity. Immunol Res. 2012;54(1-3):254–261. doi: 10.1007/s12026-012-8303-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Bharti B., Lee S.J., Lindsay S.P., Wingard D.L., Jones K.L., Lemus H., et al. Disease severity and pregnancy outcomes in women with rheumatoid arthritis: results from the organization of teratology information specialists autoimmune diseases in pregnancy project. J Rheumatol. 2015;42(8):1376–1382. doi: 10.3899/jrheum.140583. [DOI] [PubMed] [Google Scholar]
- 57.Klein S.L., Passaretti C., Anker M., Olukoya P., Pekosz A. The impact of sex, gender and pregnancy on 2009 H1N1 disease. Biol Sex Differ. 2010;1(1):5. doi: 10.1186/2042-6410-1-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Siston A.M., Rasmussen S.A., Honein M.A., Fry A.M., Seib K., Callaghan W.M., et al. Pandemic 2009 influenza A(H1N1) virus illness among pregnant women in the United States. JAMA. 2010;303(15):1517–1525. doi: 10.1001/jama.2010.479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Guo Y.R., Cao Q.D., Hong Z.S., Tan Y.Y., Chen S.D., Jin H.J., et al. The origin, transmission and clinical therapies on coronavirus disease 2019 (COVID-19) outbreak – an update on the status. Mil Med Res. 2020;7(1):11. doi: 10.1186/s40779-020-00240-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Lin L., Lu L., Cao W., Li T. Hypothesis for potential pathogenesis of SARS-CoV-2 infection-a review of immune changes in patients with viral pneumonia. Emerg Microbes Infect. 2020;9(1):727–732. doi: 10.1080/22221751.2020.1746199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Xiao F., Tang M., Zheng X., Liu Y., Li X., Shan H. Evidence for gastrointestinal infection of SARS-CoV-2. Gastroenterology. 2020;158(6) doi: 10.1053/j.gastro.2020.02.055. 1831-1833 e1833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Yan R., Zhang Y., Li Y., Xia L., Guo Y., Zhou Q. Structural basis for the recognition of SARS-CoV-2 by full-length human ACE2. Science. 2020;367(6485):1444–1448. doi: 10.1126/science.abb2762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Hoffmann M., Kleine-Weber H., Schroeder S., Krüger N., Herrler T., Erichsen S., et al. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell. 2020;181(2) doi: 10.1016/j.cell.2020.02.052. 271-280.e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Matsuyama S., Nao N., Shirato K., Kawase M., Saito S., Takayama I., et al. Enhanced isolation of SARS-CoV-2 by TMPRSS2-expressing cells. Proc Natl Acad Sci U S A. 2020;117(13):7001–7003. doi: 10.1073/pnas.2002589117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Brosnihan K.B., Neves L.A., Anton L., Joyner J., Valdes G., Merrill D.C. Enhanced expression of Ang-(1-7) during pregnancy. Braz J Med Biol Res. 2004;37(8):1255–1262. doi: 10.1590/s0100-879x2004000800017. [DOI] [PubMed] [Google Scholar]
- 66.Dhaundiyal A., Kumari P., Jawalekar S.S., Chauhan G., Kalra S., Navik U. Is highly expressed ACE 2 in pregnant women “a curse” in times of COVID-19 pandemic? Life Sci. 2021;264 doi: 10.1016/j.lfs.2020.118676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Schwartz D.A., De Luca D. The public health and clinical importance of accurate neonatal testing for Covid-19. Pediatrics. 2021;147(2) doi: 10.1542/peds.2020-036871. [DOI] [PubMed] [Google Scholar]
- 68.Luo Y., Yin K. Management of pregnant women infected with COVID-19. Lancet Infect Dis. 2020;20(5):513–514. doi: 10.1016/S1473-3099(20)30191-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Subbaraman N. Pregnancy and COVID: what the data say. Nature. 2021;591(7849):193–195. doi: 10.1038/d41586-021-00578-y. [DOI] [PubMed] [Google Scholar]
- 70.Schwartz D.A. An analysis of 38 pregnant women with COVID-19, their newborn infants, and maternal-fetal transmission of sars-cov-2: maternal coronavirus infections and pregnancy outcomes. Arch Pathol Lab Med. 2020;144(7):799–805. doi: 10.5858/arpa.2020-0901-SA. [DOI] [PubMed] [Google Scholar]
- 71.Moreno S.C., To J., Chun H., Ngai I.M. Vertical transmission of COVID-19 to the neonate. Infect Dis Obstet Gynecol. 2020;2020 doi: 10.1155/2020/8460672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Liu D., Li L., Wu X., Zheng D., Wang J., Yang L., et al. Pregnancy and perinatal outcomes of women with coronavirus disease (COVID-19) pneumonia: a preliminary analysis. AJR Am J Roentgenol. 2020;215(1):127–132. doi: 10.2214/AJR.20.23072. [DOI] [PubMed] [Google Scholar]
- 73.Liu W., Wang J., Li W., Zhou Z., Liu S., Rong Z. Clinical characteristics of 19 neonates born to mothers with COVID-19. Front Med. 2020;14(2):193–198. doi: 10.1007/s11684-020-0772-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Mardani M., Pourkaveh B. A controversial debate: vertical transmission of COVID-19 in pregnancy. Arch Clin Infect Dis. 2020;15(1) [Google Scholar]
- 75.Rasmussen S.A., Smulian J.C., Lednicky J.A., Wen T.S., Jamieson D.J. Coronavirus disease 2019 (COVID-19) and pregnancy: what obstetricians need to know. Am J Obstet Gynecol. 2020;222(5):415–426. doi: 10.1016/j.ajog.2020.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Kotlyar A.M., Grechukhina O., Chen A., Popkhadze S., Grimshaw A., Tal O., et al. Vertical transmission of coronavirus disease 2019: a systematic review and meta-analysis. Am J Obstet Gynecol. 2021;224(1) doi: 10.1016/j.ajog.2020.07.049. 35-53 e33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Fan C., Lei D., Fang C., Li C., Wang M., Liu Y., et al. Perinatal transmission of COVID-19 associated SARS-CoV-2: should we worry? Clin Infect Dis. 2021;72(5):862–864. doi: 10.1093/cid/ciaa226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Li Y., Zhao R., Zheng S., Chen X., Wang J., Sheng X., et al. Lack of vertical transmission of severe acute respiratory syndrome coronavirus 2, China. Emerg Infect Dis. 2020;26(6):1335–1336. doi: 10.3201/eid2606.200287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Schwartz A., Yogev Y., Zilberman A., Alpern S., Many A., Yousovich R., et al. Detection of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) in vaginal swabs of women with acute SARS-CoV-2 infection: a prospective study. BJOG. 2021;128(1):97–100. doi: 10.1111/1471-0528.16556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Dashraath P., Wong J.L.J., Lim M.X.K., Lim L.M., Li S., Biswas A., et al. Coronavirus disease 2019 (COVID-19) pandemic and pregnancy. Am J Obstet Gynecol. 2020;222(6):521–531. doi: 10.1016/j.ajog.2020.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Dong L., Tian J., He S., Zhu C., Wang J., Liu C., et al. Possible vertical transmission of SARS-CoV-2 from an infected mother to her newborn. JAMA. 2020;323(18):1846–1848. doi: 10.1001/jama.2020.4621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Kimberlin D.W., Stagno S. Can SARS-CoV-2 infection be acquired in utero?: more definitive evidence is needed. Jama. 2020;323(18):1788–1789. doi: 10.1001/jama.2020.4868. [DOI] [PubMed] [Google Scholar]
- 83.Zeng H., Xu C., Fan J., Tang Y., Deng Q., Zhang W., et al. Antibodies in infants born to mothers with COVID-19 pneumonia. JAMA. 2020;323(18):1848–1849. doi: 10.1001/jama.2020.4861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Zeng H., Xu C., Fan J., Tang Y., Deng Q., Zhang W., et al. Antibodies in infants born to mothers with COVID-19 pneumonia. JAMA. 2020;323(18):1848–1849. doi: 10.1001/jama.2020.4861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Dong L., Tian J., He S., Zhu C., Wang J., Liu C., et al. Possible vertical transmission of SARS-CoV-2 from an infected mother to her newborn. JAMA. 2020;323(18):1846–1848. doi: 10.1001/jama.2020.4621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Alzamora M.C., Paredes T., Caceres D., Webb C.M., Valdez L.M., La Rosa M. Severe COVID-19 during pregnancy and possible vertical transmission. Am J Perinatol. 2020;37(8):861–865. doi: 10.1055/s-0040-1710050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Edlow A.G., Li J.Z., Collier A.Y., Atyeo C., James K.E., Boatin A.A., et al. Assessment of maternal and neonatal SARS-CoV-2 viral load, transplacental antibody transfer, and placental pathology in pregnancies during the COVID-19 pandemic. JAMA Netw Open. 2020;3(12) doi: 10.1001/jamanetworkopen.2020.30455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Fenizia C., Biasin M., Cetin I., Vergani P., Mileto D., Spinillo A., et al. Analysis of SARS-CoV-2 vertical transmission during pregnancy. Nat Commun. 2020;11(1):5128. doi: 10.1038/s41467-020-18933-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Bwire G.M., Njiro B.J., Mwakawanga D.L., Sabas D., Sunguya B.F. Possible vertical transmission and antibodies against SARS-CoV-2 among infants born to mothers with COVID-19: a living systematic review. J Med Virol. 2020;93(3):1361–1369. doi: 10.1002/jmv.26622. [DOI] [PubMed] [Google Scholar]
- 90.Ashraf M.A., Keshavarz P., Hosseinpour P., Erfani A., Roshanshad A., Pourdast A., et al. Coronavirus disease 2019 (COVID-19): a systematic review of pregnancy and the possibility of vertical transmission. J Reprod Infertil. 2020;21(3):157–168. [PMC free article] [PubMed] [Google Scholar]
- 91.Sanchez-Luna M., Fernandez Colomer B., de Alba Romero C., Alarcon Allen A., Bana Souto A., Camba Longueira F., et al. Neonates born to mothers with COVID-19: data from the Spanish society of neonatology registry. Pediatrics. 2021;147(2) doi: 10.1542/peds.2020-015065. [DOI] [PubMed] [Google Scholar]
- 92.He Z., Fang Y., Zuo Q., Huang X., Lei Y., Ren X., et al. Vertical transmission and kidney damage in newborns whose mothers had coronavirus disease 2019 during pregnancy. Int J Antimicrob Agents. 2021;57(2) doi: 10.1016/j.ijantimicag.2020.106260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Lopez M., Gonce A., Meler E., Plaza A., Hernandez S., Martinez-Portilla R.J., et al. Coronavirus disease 2019 in pregnancy: a clinical management protocol and considerations for practice. Fetal Diagn Ther. 2020;47(7):519–528. doi: 10.1159/000508487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Uygun-Can B., Acar-Bolat B. Clinical properties and diagnostic methods of COVID-19 Infection in pregnancies: meta-analysis. Biomed Res Int. 2020;2020 doi: 10.1155/2020/1708267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Corman V.M., Landt O., Kaiser M., Molenkamp R., Meijer A., Chu D.K., et al. Detection of 2019 novel coronavirus (2019-nCoV) by real-time RT-PCR. Euro Surveill. 2020;25(3) doi: 10.2807/1560-7917.ES.2020.25.3.2000045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Donders F., Lonnee-Hoffmann R., Tsiakalos A., Mendling W., Martinez de Oliveira J., Judlin P., et al. ISIDOG recommendations concerning COVID-19 and pregnancy. Diagnostics (Basel) 2020;10(4) doi: 10.3390/diagnostics10040243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Ai T., Yang Z., Hou H., Zhan C., Chen C., Lv W., et al. Correlation of chest CT and RT-PCR testing in coronavirus disease 2019 (COVID-19) in China: a report of 1014 cases. Radiology. 2020;296(2):E32–E40. doi: 10.1148/radiol.2020200642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Shi H., Han X., Jiang N., Cao Y., Alwalid O., Gu J., et al. Radiological findings from 81 patients with COVID-19 pneumonia in Wuhan, China: a descriptive study. Lancet Infect Dis. 2020;20(4):425–434. doi: 10.1016/S1473-3099(20)30086-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Guan W.J., Ni Z.Y., Hu Y., Liang W.H., Ou C.Q., He J.X., et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382(18):1708–1720. doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Li L., Qin L., Xu Z., Yin Y., Wang X., Kong B., et al. Artificial intelligence distinguishes COVID-19 from community acquired pneumonia on chest CT. Radiology. 2020;296:E65–E71. doi: 10.1148/radiol.2020200905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Yassa M., Birol P., Mutlu A.M., Tekin A.B., Sandal K., Tug N. Lung ultrasound can influence the slinical treatment of pregnant women with COVID‐19. J Ultrasound Med. 2021;40:191–203. doi: 10.1002/jum.15367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Buonsenso D., Raffaelli F., Tamburrini E., Biasucci D.G., Salvi S., Smargiassi A., et al. Clinical role of lung ultrasound for the diagnosis and monitoring of COVID‐19 pneumonia in pregnant women. Ultrasound Obstet Gynecol. 2020;56(1):106–109. doi: 10.1002/uog.22055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Guo L., Ren L., Yang S., Xiao M., Chang, Yang F., et al. Profiling early humoral response to diagnose novel coronavirus disease (COVID-19) Clin Infect Dis. 2020;71(15):778–785. doi: 10.1093/cid/ciaa310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Zhang W., Liu K., Zhang P., Cheng W., Li L., Zhang F., et al. CRISPR-based approaches for efficient and accurate detection of SARS-CoV-2. Lab Med. 2021;52(2):116–121. doi: 10.1093/labmed/lmaa101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Lucia C., Federico P.-B., Alejandra G.C. An ultrasensitive, rapid, and portable coronavirus SARS-CoV-2 sequence detection method based on CRISPR-Cas12. BioRxiv. 2020 doi: 10.1101/2020.02.29.971127. [DOI] [Google Scholar]
- 106.Ding X., Yin K., Li Z., Liu C. All-in-One Dual CRISPR-Cas12a (AIOD-CRISPR) assay: a case for rapid, ultrasensitive and visual detection of novel coronavirus SARS-CoV-2 and HIV virus. bioRxiv. 2020 doi: 10.1101/2020.03.19.998724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Zhang F., Abudayyeh O.O., Gootenberg J.S. 2020. A protocol for detection of COVID-19 using CRISPR diagnostics; p. 8. A protocol for detection of COVID-19 using CRISPR diagnostics. [Google Scholar]
- 108.Ai J.W., Zhang Y., Zhang H.C., Xu T., Zhang W.H. Era of molecular diagnosis for pathogen identification of unexplained pneumonia, lessons to be learned. Emerg Microbes Infect. 2020;9(1):597–600. doi: 10.1080/22221751.2020.1738905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Rottenstreich A., Zarbiv G., Kabiri D., Porat S., Sompolinsky Y., Reubinoff B., et al. Rapid antigen detection testing for universal screening for severe acute respiratory syndrome coronavirus 2 in women admitted for delivery. Am J Obstet Gynecol. 2021 doi: 10.1016/j.ajog.2021.01.002. (In press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.GeurtsvanKessel C.H., Okba N.M.A., Igloi Z., Embregts C.W.E., Laksono B.M., Leijten L., et al. Towards the next phase: evaluation of serological assays for diagnostics and exposure assessment. MedRxiv. 2020 doi: 10.1101/2020.04.23.20077156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Lassaunière R., Frische A., Harboe Z.B., Nielsen A.C.Y., Fomsgaard A., Krogfelt K.A., et al. Evaluation of nine commercial SARS-CoV-2 immunoassays. MedRxiv. 2020 doi: 10.1101/2020.04.09.20056325. [DOI] [Google Scholar]
- 112.Infantino M., Manfredi M., Grossi V., Lari B., Fabbri S., Benucci M., et al. Closing the serological gap in the diagnostic testing for COVID-19: the value of anti-SARS-CoV-2 IgA antibodies. J Med Virol. 2021;93(3):1436–1442. doi: 10.1002/jmv.26422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Zhao J., Yuan Q., Wang H., Liu W., Liao X., Su Y., et al. Antibody responses to SARS-CoV-2 in patients with novel coronavirus disease 2019. Clin Infect Dis. 2020;71(16):2027–2034. doi: 10.1093/cid/ciaa344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Wang P. Combination of serological total antibody and RT-PCR test for detection of SARS-COV-2 infections. J Virol Methods. 2020;283 doi: 10.1016/j.jviromet.2020.113919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Goyal M., Singh P., Melana N. Review of care and management of pregnant women during COVID-19 pandemic. Taiwan J Obstet Gynecol. 2020;59(6):791–794. doi: 10.1016/j.tjog.2020.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Pavlidis P., Eddy K., Phung L., Farrington E., Connolly M., Lopes R., et al. Clinical guidelines for caring for women with COVID-19 during pregnancy, childbirth and the immediate postpartum period. Women Birth. 2020;S1871–5192(20):30372–30373. doi: 10.1016/j.wombi.2020.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Khoiwal K., Kapur D., Gaurav A., Chaturvedi J. Management of pregnant women in times of COVID-19: a review of current Literature. J Obstet Gynaecol India. 2020;70(4):262–266. doi: 10.1007/s13224-020-01342-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Qi H., Luo X., Zheng Y., Zhang H., Li J., Zou L., et al. Safe delivery for pregnancies affected by COVID-19. BJOG. 2020;127(8):927–929. doi: 10.1111/1471-0528.16231. [DOI] [PubMed] [Google Scholar]
- 119.Poon L.C., Yang H., Lee J.C.S., Copel J.A., Leung T.Y., Zhang Y., et al. ISUOG Interim Guidance on 2019 novel coronavirus infection during pregnancy and puerperium: information for healthcare professionals. Ultrasound Obstet Gynecol. 2020;55(5):700–708. doi: 10.1002/uog.22013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Ma X., Zhu J., Du L. Neonatal management during the coronavirus disease (COVID-19) outbreak: the chinese experience. Neoreviews. 2020;21(5):e293–e297. doi: 10.1542/neo.21-5-e293. [DOI] [PubMed] [Google Scholar]
- 121.CDC. Interim guidance on breastfeeding and breast milk feeds in the context of COVID-19. Available from: https://wwwcdcgov/coronavirus/2019-ncov/hcp/care-for-breastfeeding-womenhtml. [Accessed 1 June 2020].
- 122.Hao F., Tam W., Hu X., Tan W., Jiang L., Jiang X., et al. A quantitative and qualitative study on the neuropsychiatric sequelae of acutely ill COVID-19 inpatients in isolation facilities. Transl Psychiatry. 2020;10(1):355. doi: 10.1038/s41398-020-01039-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Zhang M.W., Ho R.C. Moodle: The cost effective solution for internet cognitive behavioral therapy (I-CBT) interventions. Technol Health Care. 2017;25(1):163–165. doi: 10.3233/THC-161261. [DOI] [PubMed] [Google Scholar]
- 124.Soh H.L., Ho R.C., Ho C.S., Tam W.W. Efficacy of digital cognitive behavioural therapy for insomnia: a meta-analysis of randomised controlled trials. Sleep Med. 2020;75:315–325. doi: 10.1016/j.sleep.2020.08.020. [DOI] [PubMed] [Google Scholar]
- 125.Zhou Y., Jiang S., Du L. Prospects for a MERS-CoV spike vaccine. Expert Rev Vaccines. 2018;17(8):677–686. doi: 10.1080/14760584.2018.1506702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Wang C.L., Liu Y.Y., Wu C.H., Wang C.Y., Wang C.H., Long C.Y. Impact of COVID-19 on pregnancy. Int J Med Sci. 2021;18(3):763–767. doi: 10.7150/ijms.49923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.WHO. Q&A on COVID-19, pregnancy, childbirth and breastfeeding. Available online: https://wwwwhoint/newsroom/q-a-detail/q-a-on-covid-19-pregnancy-childbirth-and-breastfeeding. [Accessed 1 April 2020].
- 128.CDC Interim considerations for infection prevention and control of coronavirus Disease 2019 (COVID-19) in inpatient obstetric healthcare settings. Acessado em. 2020;18(02) [Google Scholar]
- 129.National Health Commission of the People’s Republic of China . National Health Commission of the People’s Republic of China; 2020. Notice on strengthening maternal disease treatment and safe midwifery during the prevention and control of new coronavirus pneumonia.https://www.nhc.gov.cn/xcs/zhengcwj/202002/4f80657b346e4d6ba76e2cfc3888c630.shtml [Google Scholar]
- 130.Peyronnet V., Sibiude J., Deruelle P., Huissoud C., Lescure X., Lucet J.C., et al. SARS-CoV-2 infection during pregnancy. Information and proposal of management care. CNGOF. Gynecol Obstet Fertil Senol. 2020;48(5):436–443. doi: 10.1016/j.gofs.2020.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Narang K., Ibirogba E.R., Elrefaei A., Trad A.T.A., Theiler R., Nomura R., et al. SARS-CoV-2 in pregnancy: a comprehensive summary of current guidelines. J Clin Med. 2020;9(5) doi: 10.3390/jcm9051521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Società Italiana di Ecografia Ostetrico Ginecologica . 2020. Guia d’actuació enfront de casos d’infecció pel nou coronavirus SARS-CoV-2 en dones embarassades i nadons. Available online: https://canalsalutgencatcat/web/content/_A-Z/C/coronavirus-2019-ncov/material-divulgatiu/guia-actuacio-embarassadespdf. [Accessed 11 March 2020] [Google Scholar]
- 133.American College of Obstetricians and Gynecologists . 2020. Coronavirus COVID-19, a practice advisory. Available online: https://www.acog.org/clinical/clinical-guidance/practice-advisory/articles/2020/03/novel-coronavirus-2019. [Accessed 11 March 2020] [Google Scholar]
- 134.Obstetricians RCo, Gynecologists . Royal College of Obstetricians and Gynaecologists London; England: 2020. Coronavirus (COVID‐19) infection in pregnancy. Information for healthcare professionals. [Google Scholar]
- 135.Poon L.C., Yang H., Kapur A., Melamed N., Dao B., Divakar H., et al. Global interim guidance on coronavirus disease 2019 (COVID-19) during pregnancy and puerperium from FIGO and allied partners: information for healthcare professionals. Int J Gynaecol Obstet. 2020;149(3):273–286. doi: 10.1002/ijgo.13156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Martinez-Perez O., Vouga M., Cruz Melguizo S., Forcen Acebal L., Panchaud A., Munoz-Chapuli M., et al. Association between mode of delivery among pregnant women with COVID-19 and maternal and neonatal outcomes in Spain. JAMA. 2020:e2010125. doi: 10.1001/jama.2020.10125. (In press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Browne P.C., Linfert J.B., Perez-Jorge E. Successful treatment of preterm labor in association with acute COVID-19 infection. Am J Perinatol. 2020;37(8):866–868. doi: 10.1055/s-0040-1709993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Nayak A.H., Kapote D.S., Fonseca M., Chavan N., Mayekar R., Sarmalkar M., et al. Impact of the coronavirus infection in pregnancy: a preliminary study of 141 patients. J Obstet Gynaecol India. 2020;70(4):256–261. doi: 10.1007/s13224-020-01335-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Tam Pck, Ly Km, Kernich Ml, Spurrier N., Lawrence D., Gordon Dl, et al. Detectable severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) in human breast milk of a mildly symptomatic patient with coronavirus disease 2019 (COVID-19) Clin Infect Dis. 2020;72(1):128–130. doi: 10.1093/cid/ciaa673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Centeno-Tablante E., Medina-Rivera M., Finkelstein J.L., Rayco-Solon P., Garcia-Casal M.N., Rogers L., et al. Transmission of SARS-CoV-2 through breast milk and breastfeeding: a living systematic review. Ann N Y Acad Sci. 2021;1484(1):32–54. doi: 10.1111/nyas.14477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Lubbe W., Botha E., Niela-Vilen H., Reimers P. Breastfeeding during the COVID-19 pandemic - a literature review for clinical practice. Int Breastfeed J. 2020;15(1):82. doi: 10.1186/s13006-020-00319-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Chambers C., Krogstad P., Bertrand K., Contreras D., Tobin N.H., Bode L., et al. Evaluation for SARS-CoV-2 in breast milk from 18 infected women. JAMA. 2020;324(13):1347–1348. doi: 10.1001/jama.2020.15580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Stanojevic M. Are Covid-19-positive mothers dangerous for their term and well newborn babies? Is there an answer? J Perinat Med. 2020;48(5):441–445. doi: 10.1515/jpm-2020-0186. [DOI] [PubMed] [Google Scholar]
- 144.Peng S., Zhu H., Yang L., Cao L., Huang X., Dynes M., et al. A study of breastfeeding practices, SARS-CoV-2 and its antibodies in the breast milk of mothers confirmed with COVID-19. Lancet Reg Health-Western Pacific. 2020;4 doi: 10.1016/j.lanwpc.2020.100045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.World Health O . World Health Organization; 2020. Clinical management of severe acute respiratory infection (SARI) when COVID-19 disease is suspected: interim guidance, 13 March 2020. [Google Scholar]
- 146.Dhama K., Sharun K., Tiwari R., Dadar M., Malik Y.S., Singh K.P., et al. COVID-19, an emerging coronavirus infection: advances and prospects in designing and developing vaccines, immunotherapeutics, and therapeutics. Hum Vaccin Immunother. 2020;16(6):1232–1238. doi: 10.1080/21645515.2020.1735227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Mullard A. Flooded by the torrent: the COVID-19 drug pipeline. Lancet. 2020;395(10232):1245–1246. doi: 10.1016/S0140-6736(20)30894-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Whitehead C.L., Walker S.P. Consider pregnancy in COVID-19 therapeutic drug and vaccine trials. Lancet. 2020;395(10237):e92. doi: 10.1016/S0140-6736(20)31029-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.D’Souza R., Ashraf R., Rowe H., Zipursky J., Clarfield L., Maxwell C., et al. Pregnancy and COVID-19: pharmacologic considerations. Ultrasound Obstet Gynecol. 2021;57(2):195–203. doi: 10.1002/uog.23116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Zhao X., Jiang Y., Zhao Y., Xi H., Liu C., Qu F., et al. Analysis of the susceptibility to COVID-19 in pregnancy and recommendations on potential drug screening. Eur J Clin Microbiol Infect Dis. 2020;39(7):1209–1220. doi: 10.1007/s10096-020-03897-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Favilli A., Mattei Gentili M., Raspa F., Giardina I., Parazzini F., Vitagliano A., et al. Effectiveness and safety of available treatments for COVID-19 during pregnancy: a critical review. J Matern Fetal Neonatal Med. 2020:1–14. doi: 10.1080/14767058.2020.1774875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Grein J., Ohmagari N., Shin D., Diaz G., Asperges E., Castagna A., et al. Compassionate use of remdesivir for patients with severe Covid-19. N Engl J Med. 2020;382(24):2327–2336. doi: 10.1056/NEJMoa2007016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Burwick R.M., Yawetz S., Stephenson K.E., Collier A.-R.Y., Sen P., Blackburn B.G., et al. Compassionate use of remdesivir in pregnant women with severe coronavirus disease 2019. Clin Infect Dis. 2020:ciaa1466. doi: 10.1093/cid/ciaa1466. (In press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Mulangu S., Dodd L.E., Davey R.T., Jr., Tshiani Mbaya O., Proschan M., Mukadi D., et al. A randomized, controlled trial of Ebola virus disease therapeutics. N Engl J Med. 2019;381(24):2293–2303. doi: 10.1056/NEJMoa1910993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Naqvi M., Zakowski P., Glucksman L., Smithson S., Burwick Rm. Tocilizumab and remdesivir in a pregnant patient with coronavirus disease 2019 (COVID-19) Obstet Gynecol. 2020;136(5):1025–1029. doi: 10.1097/AOG.0000000000004050. [DOI] [PubMed] [Google Scholar]
- 156.Tookey P.A., Thorne C., van Wyk J., Norton M. Maternal and foetal outcomes among 4118 women with HIV infection treated with lopinavir/ritonavir during pregnancy: analysis of population-based surveillance data from the national study of HIV in pregnancy and childhood in the United Kingdom and Ireland. BMC Infect Dis. 2016;16:65. doi: 10.1186/s12879-016-1400-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Liu F., Xu A., Zhang Y., Xuan W., Yan T., Pan K., et al. Patients of COVID-19 may benefit from sustained lopinavir-combined regimen and the increase of eosinophil may predict the outcome of COVID-19 progression. Int J Infect Dis. 2020;95:183–191. doi: 10.1016/j.ijid.2020.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Cao B., Wang Y., Wen D., Liu W., Wang J., Fan G., et al. A trial of Lopinavir-Ritonavir in adults hospitalized with severe Covid-19. N Engl J Med. 2020;382(19):1787–1799. doi: 10.1056/NEJMoa2001282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Gandhi A., Ganatra A., Tank P. Federation of Obstetric and Gynaecological Societies of India; 2020. FOGSI GCPR on pregnancy with COVID-19 infection version 2 [internet] In. 2020. [Google Scholar]
- 160.Vincent J.P., Komaki-Yasuda K., Existe A.V., Boncy J., Kano S. No plasmodium falciparum chloroquine resistance transporter and artemisinin resistance mutations. Haiti. Emerg Infect Dis. 2018;24(11):2124–2126. doi: 10.3201/eid2411.180738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Keyaerts E., Vijgen L., Maes P., Neyts J., Van Ranst M. In vitro inhibition of severe acute respiratory syndrome coronavirus by chloroquine. Biochem Biophys Res Commun. 2004;323(1):264–268. doi: 10.1016/j.bbrc.2004.08.085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Gao J., Tian Z., Yang X. Breakthrough: chloroquine phosphate has shown apparent efficacy in treatment of COVID-19 associated pneumonia in clinical studies. Biosci Trends. 2020;14(1):72–73. doi: 10.5582/bst.2020.01047. [DOI] [PubMed] [Google Scholar]
- 163.Wang M., Cao R., Zhang L., Yang X., Liu J., Xu M., et al. Remdesivir and chloroquine effectively inhibit the recently emerged novel coronavirus (2019-nCoV) in vitro. Cell Res. 2020;30(3):269–271. doi: 10.1038/s41422-020-0282-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Whitehead C.L., Walker S.P. Consider pregnancy in COVID-19 therapeutic drug and vaccine trials. Lancet. 2020;395(10237):e92. doi: 10.1016/S0140-6736(20)31029-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Stone C.T. Safety of chloroquine in pregnancy. JAMA. 1965;192:260. doi: 10.1001/jama.1965.03080160080030. [DOI] [PubMed] [Google Scholar]
- 166.Karunajeewa H.A., Salman S., Mueller I., Baiwog F., Gomorrai S., Law I., et al. Pharmacokinetics of chloroquine and monodesethylchloroquine in pregnancy. Antimicrob Agents Chemother. 2010;54(3):1186–1192. doi: 10.1128/AAC.01269-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Gautret P., Lagier J.-C., Parola P., Meddeb L., Mailhe M., Doudier B., et al. Hydroxychloroquine and azithromycin as a treatment of COVID-19: results of an open-label non-randomized clinical trial. Int J Antimicrob Agents. 2020 doi: 10.1016/j.ijantimicag.2020.105949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Cavalcanti A.B., Zampieri F.G., Rosa R.G., Azevedo L.C.P., Veiga V.C., Avezum A., et al. Hydroxychloroquine with or without azithromycin in mild-to-Moderate Covid-19. N Engl J Med. 2020;383(21):2041–2052. doi: 10.1056/NEJMoa2019014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.WHOSolidarity Trial Consortium, Pan H., Peto R., Henao-Restrepo A.M., Preziosi M.P., Sathiyamoorthy V., et al. Repurposed antiviral drugs for Covid-19 – interim WHO solidarity trial results. N Engl J Med. 2021;384(6):497–511. doi: 10.1056/NEJMoa2023184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Siemieniuk R.A., Bartoszko J.J., Ge L., Zeraatkar D., Izcovich A., Kum E., et al. Drug treatments for covid-19: living systematic review and network meta-analysis. BMJ. 2020;370:m2980. doi: 10.1136/bmj.m2980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Soleimani Z., Soleimani A. ADRS due to COVID-19 in midterm pregnancy: successful management with plasma transfusion and corticosteroids. J Matern-Fetal Neonatal Med. 2020:1–4. doi: 10.1080/14767058.2020.1797669. [DOI] [PubMed] [Google Scholar]
- 172.Yatoo M., Hamid Z., Parray O., Wani A., Wani A., Saxena A., et al. COVID-19 - Recent advancements in identifying novel vaccine candidates and current status of upcoming SARS-CoV-2 vaccines. Hum Vaccin Immunother. 2020;16(12):2891–2904. doi: 10.1080/21645515.2020.1788310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Fahriani M., Anwar S., Yufika A., Bakhtiar B., Wardani E., Winardi W., et al. Disruption of childhood vaccination during the COVID-19 pandemic in Indonesia. Narra J. 2021;1(1):e7. doi: 10.52225/narraj.v1i1.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Saxena S., Skirrow H., Bedford H. Routine vaccination during covid-19 pandemic response. BMJ. 2020;369:m2392. doi: 10.1136/bmj.m2392. [DOI] [PubMed] [Google Scholar]
- 175.Riley L.E., Jamieson D.J. Inclusion of pregnant and lactating persons in COVID-19 vaccination efforts. Ann Intern Med. 2021:M21–173. doi: 10.7326/M21-0173. (In press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Vora K.S., Sundararajan A., Saiyed S., Dhama K., Natesan S. Impact of COVID-19 on women and children and the need for a gendered approach in vaccine development. Hum Vaccin Immunother. 2020;16(12):2932–2937. doi: 10.1080/21645515.2020.1826249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Adhikari E.H., Spong C.Y. COVID-19 vaccination in pregnant and lactating women. JAMA. 2021;325(11):1039–1040. doi: 10.1001/jama.2021.1658. [DOI] [PubMed] [Google Scholar]
- 178.Amanat F., Krammer F. SARS-CoV-2 vaccines: status report. Immunity. 2020;52(4):583–589. doi: 10.1016/j.immuni.2020.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Polack F.P., Thomas S.J., Kitchin N., Absalon J., Gurtman A., Lockhart S., et al. Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine. N Engl J Med. 2020;383(27):2603–2615. doi: 10.1056/NEJMoa2034577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Voysey M., Clemens S.A.C., Madhi S.A., Weckx L.Y., Folegatti P.M., Aley P.K., et al. Safety and efficacy of the ChAdOx1 nCoV-19 vaccine (AZD1222) against SARS-CoV-2: an interim analysis of four randomised controlled trials in Brazil, South Africa, and the UK. Lancet. 2021;397(10269):99–111. doi: 10.1016/S0140-6736(20)32661-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Baden L.R., El Sahly H.M., Essink B., Kotloff K., Frey S., Novak R., et al. Efficacy and safety of the mRNA-1273 SARS-CoV-2 vaccine. N Engl J Med. 2021;384:403–416. doi: 10.1056/NEJMoa2035389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Izda V., Jeffries M.A., Sawalha A.H. COVID-19: a review of therapeutic strategies and vaccine candidates. Clin Immunol. 2021;222 doi: 10.1016/j.clim.2020.108634. 108634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Knoll M.D., Wonodi C. Oxford-AstraZeneca COVID-19 vaccine efficacy. Lancet. 2021;397(10269):72–74. doi: 10.1016/S0140-6736(20)32623-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Draft landscape of COVID-19 candidate vaccines. Available from: https://www.who.int/publications/m/item/draft-landscape-of-covid-19-candidate-vaccines [Accessed 2 February 2021].
- 185.Klein S.L., Flanagan K.L. Sex differences in immune responses. Nat Rev Immunol. 2016;16(10):626–638. doi: 10.1038/nri.2016.90. [DOI] [PubMed] [Google Scholar]
- 186.Rasmussen S.A., Kelley C.F., Horton J.P., Jamieson D.J., Coronavirus Disease 2019 (COVID-19) vaccines and pregnancy: what obstetricians need to know. Obstet Gynecol. 2021;137(3):408–414. doi: 10.1097/AOG.0000000000004290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Heath P.T., Le Doare K., Khalil A. Inclusion of pregnant women in COVID-19 vaccine development. Lancet Infect Dis. 2020;20(9):1007–1008. doi: 10.1016/S1473-3099(20)30638-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Dashraath P., Nielsen-Saines K., Madhi S.A., Baud D. COVID-19 vaccines and neglected pregnancy. Lancet. 2020;396(10252):e22. doi: 10.1016/S0140-6736(20)31822-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Sedgh G., Singh S., Hussain R. Intended and unintended pregnancies worldwide in 2012 and recent trends. Stud Fam Plann. 2014;45(3):301–314. doi: 10.1111/j.1728-4465.2014.00393.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.