Abstract
Few investigations have analyzed the neuroanatomical substrate of empathic capacities in healthy subjects, and most of them have neglected the potential involvement of cerebellar structures. The main aim of the present study was to investigate the associations between bilateral cerebellar macro- and micro-structural measures and levels of cognitive and affective trait empathy (measured by Interpersonal Reactivity Index, IRI) in a sample of 70 healthy subjects of both sexes. We also estimated morphometric variations of cerebral Gray Matter structures, to ascertain whether the potential empathy-related peculiarities in cerebellar areas were accompanied by structural differences in other cerebral regions. At macro-structural level, the volumetric differences were analyzed by Voxel-Based Morphometry (VBM)- and Region of Interest (ROI)-based approaches, and at a micro-structural level, we analyzed Diffusion Tensor Imaging (DTI) data, focusing in particular on Mean Diffusivity and Fractional Anisotropy. Fantasy IRI-subscale was found to be positively associated with volumes in right cerebellar Crus 2 and pars triangularis of inferior frontal gyrus. The here described morphological variations of cerebellar Crus 2 and pars triangularis allow to extend the traditional cortico-centric view of cognitive empathy to the cerebellar regions and indicate that in empathizing with fictional characters the cerebellar and frontal areas are co-recruited.
Subject terms: Neuroscience, Social neuroscience, Empathy
Introduction
“Every reader, as he reads, is actually the reader of himself”
(Marcel Proust)
The non-motor role of the cerebellum has gained increasingly convincing evidence in the neuroscientific research. In fact, converging lines of experimental, clinical, and neuroimaging findings have advanced a new conceptualization on cerebellar functionality, indicating that the cerebellum is critically involved in a wide variety of cognitive, emotional and affective functions1, and also in social cognition. Indeed, neuroscientific evidence revealed a marked activation of the cerebellum during social judgments2–10 and functional connectivity studies evidenced strong neural connectivity between cerebellum and cerebrum during social interactions11 and social inferences5,12–14 as well as in the default/mentalizing network2,3.
Social cognition is a general term to describe cognition involving others, as understanding others’ emotions, intentions and behaviors and acting towards and with them in social settings4,15. Social cognition involves understanding others and understanding with others15. One of the most advanced social functions is the empathic capacity that allows sharing the affective states of others, exerting cognitive control, predicting, and understanding others’ feelings, motivations and actions, and behaving accordingly16. In humans and non-human animals, the empathy is aimed at promoting prosocial and cooperative behaviors16–19. This psychological construct is regulated by both affective and cognitive components that produce the emotional understanding20. Affective empathy refers to the ability of sharing the state of other persons through observation or imagination of their experience. Such ability usually leads to an appropriate isomorphic emotional response as a consequence of other's state. Cognitive empathy refers to the abilities of perspective taking and Theory of Mind (ToM) that allow predicting and understanding other's mental state by using cognitive processes. Combined, these processes enable to understand beliefs, desires, and emotions of others in real-life or imaginary situations, although the subject remains aware that someone else is the source of that state.
Evidence from functional Magnetic Resonance Imaging (fMRI) studies21 and functional connectivity measures22–24 have indicated consistent activation of specific brain structures (frontal, parietal and temporal neocortical structures) associated with each component of empathy. Namely, the anterior cingulate cortex (ACC) and anterior insula (AI) are mostly recruited in the affective empathy, whereas the medial cingulate cortex and adjacent dorsomedial prefrontal cortex (MCC/DMPFC) in the cognitive empathy. While many studies have assessed empathy as a state rather than a trait focusing on the neuronal activation associated with empathy-eliciting situations25–27, few investigations have been interested in the structural underpinning of the trait empathy, and additionally most often in the presence of pathological conditions, as schizophrenia28, aggressive and antisocial behavior29, neurodegenerative disorders30. To date, very few reports have analyzed the brain substrate of empathic capacity in healthy subjects by using Voxel-Based Morphometry (VBM)31–34. It is evident that the research on the neural correlates of empathy has been so far mainly focused on the cerebrum, however the increasingly recognized involvement of cerebellum in social cognition1,6,12, of which empathy is one of the most advanced component, makes it necessary aimed investigations on the role played by the cerebellum in empathic capabilities. In support of this idea, empathy for other people’s feelings of pain is associated with cerebellar activation in fMRI studies35–38, and bilateral lesions to posterior vermis and cerebellar hemispheres result in empathy and ToM deficits39,40. Although it is widely accepted that the cerebellum is implicated in many neuropsychiatric disorders featured by social malfunctioning, such as autistic spectrum disorders, attentional deficit and hyperkinetic disorder, depression, and schizophrenia1,41,42, only recently it has been recognized that the cerebellum plays a more critical role in social thinking than assumed so far. In meta-analytic studies on fMRI studies in healthy humans on cerebellum and social cognition, a robust activation of the cerebellar areas during social judgments and mentalizing has been identified4,5,8,8,14. One may wonder whether social processes activate the cerebellum in a distributed fashion at many locations or they activate a specialized cerebellar function and recruit limited areas. Recent evidence has documented that as specific areas of the cerebellum are specialized in motor processing and others in cognitive and affective functions, there are areas in the posterior cerebellum (Crus 1 and 2) selectively recruited during social cognition. Interestingly, in these cerebellar areas a distinct mentalizing network, directly connected to the cerebral mentalizing network, has been identified3.
In the present research we investigated whether and which cerebellar regions are involved in the empathic capabilities by analyzing the associations between cerebellar macro- and micro-structural measures and levels of affective and cognitive trait empathy (measured by Interpersonal Reactivity Index, IRI)17 in a sample of 70 healthy subjects of both sexes. We also estimated the eventual empathy-related morphometric modifications in extra-cerebellar structures. While at macro-structural level the volumetric differences were analyzed through both whole brain- and Region Of Interest (ROI)-based analyses, at a micro-structural level Diffusion Tensor Imaging (DTI) was analyzed through a ROI-based approach. DTI supplies reliable physiological information on the direction and degree of water displacement in the brain, providing thus information on the obstacles encountered by diffusing water molecules43,44. Among DTI indices, Mean Diffusivity (MD) and Fractional Anisotropy (FA) were used as probes for, respectively, Gray Matter (GM) and White Matter (WM) micro-structural integrity45–48. In this perspective, DTI measures represent a reliable research tool that supplies physiological information not available on conventional MRI.
Results
Sociodemographic and Psychological Variables
Mean scores and standard deviations of each psychological variable (IRI subscales, HAM-A and HAM-D) are reported in Table 1. While gender differences on HAM-A (t = 1.11; p = 0.268) and HAM-D (t = 1.20; p = 0.231) data were not significant, those on each IRI subscale indicated that females showed significantly higher scores than males in Fantasy, Personal Distress, and Empathic Concern (Tables 1 and 2).
Table 1.
Scores on psychological instruments (IRI subscales, HAM-A and HAM-D) for all subjects, males and females (mean ± standard deviation).
| IRI subscale | All participants | Males | Females |
|---|---|---|---|
| Fantasy | 14.44 ± 5.20 | 12.80 ± 5.45 | 15.74 ± 4.66 |
| Perspective taking | 18.28 ± 3.08 | 17.58 ± 3.08 | 18.84 ± 3.00 |
| Empathic concern | 18.08 ± 2.98 | 17.19 ± 3.00 | 18.79 ± 2.80 |
| Personal distress | 9.68 ± 4.64 | 8.09 ± 3.57 | 10.94 ± 5.04 |
| HAM-A | 4.82 ± 3.81 | 4.25 ± 3.94 | 5.28 ± 3.69 |
| HAM-D | 2.78 ± 2.66 | 2.35 ± 2.78 | 3.12 ± 2.55 |
Table 2.
IRI subscales and sociodemographic variables.
| IRI Subscales | Age | Years of education | Gender | HAM-A | HAM-D | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| r | p | r | p | t | p | r | p | r | p | |
| Fantasy | − 0.03 | 0.816 | 0.10 | 0.400 | − 2.38 | 0.020 | 0.17 | 0.155 | 0.03 | 0.813 |
| Perspective taking | − 0.29 | 0.015 | 0.30 | 0.010 | − 1.72 | 0.089 | − 0.18 | 0.119 | − 0.17 | 0.159 |
| Empathic concern | 0.19 | 0.105 | − 0.00 | 0.945 | − 2.28 | 0.026 | − 0.03 | 0.826 | − 0.03 | 0.803 |
| Personal distress | 0.02 | 0.864 | − 0.04 | 0.736 | − 2.76 | 0.007 | 0.27 | 0.023 | 0.34 | 0.003 |
Significant results (p < 0.05) are in Bold.
As expected, significant direct correlations were found between some IRI subscales. Namely, the scores of Perspective Taking positively correlated with Empathic Concern (r = 0.24; p = 0.047); Fantasy positively correlated with Empathic Concern (r = 0.41; p < 0.001) and Personal Distress (r = 0.41; p < 0.001). Also, significant correlations were found between scores of the IRI subscale Perspective Taking and age and education levels. Personal Distress showed a significant direct association with HAM-A and HAM-D scores (Table 2).
ROI-based VBM
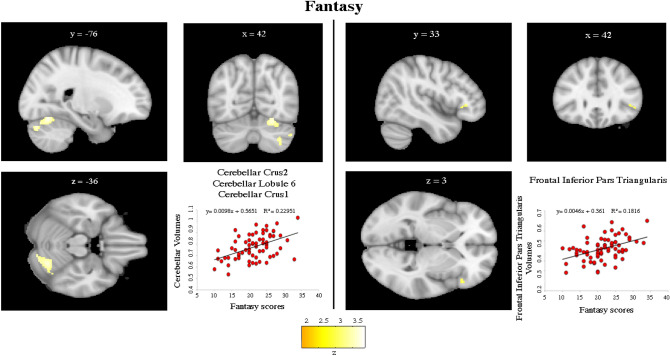
Analyses on the a priori selected areas revealed significant associations. Namely, positive associations were found between Fantasy IRI subscale and an extended (1271 voxels) cerebellar cluster in right Crus 2, Crus 1, and Lobule 6 (pFWEcorr = 0.009) (Fig. 1; Table 3). Furthermore, Fantasy IRI subscale was positively correlated with right frontal inferior pars triangularis (pFWEcorr = 0.029) (Fig. 1; Table 3).
Figure 1.
Positive association between a priori Regions of Interest (ROIs) and Fantasy IRI subscale. Coordinates are in Montreal Neurological Institute (MNI) space. Z below colorbar indicates normalized t-values. In figure left is left. Areas significantly associated with Fantasy subscale in the ROI-based analyses were used as masks to extract raw data and create scatterplot. Equation, R2 and linear fit (solid black line) are reported.
Table 3.
Regional gray matter volumes (ROI-based and voxel based morphometry analyses) and fantasy IRI subscale.
| Label for peak | Side | Extent (n voxels) | t | p | equivZ | x,y,z (mm) |
|---|---|---|---|---|---|---|
| ROI-based | ||||||
| Cerebellum Crus 2 (Crus1, Lobule 6) ↑ | R | 1271 | 3.88 | 0.009 | 3.67 | 42, − 76, − 36 |
| Inferior frontal gyrus, Pars Triangularis ↑ | R | 54 | 4.24 | 0.029 | 3.97 | 42, 33, 3 |
| Voxel based morphometry | ||||||
| Cerebellum Crus 2 ↑ | R | 1709 | 3.93 | 0.002 | 3.71 | 44, − 76, − 38 |
Results are FWE corrected.
Coordinates are in Montreal Neurological Institute (MNI) space.
L left, R right.
p = significance (FWE corrected) at the cluster level (Cerebellum Crus 2) and at the peak level (Inferior Frontal Gyrus, Pars Triangularis).
Significant results (p < 0.05) are in Bold.
The associations significant at uncorrected statistical level between IRI subscales and ROI-based VBM data are reported as Supplementary Materials S1.
Whole-brain VBM
A positive association was found between Fantasy IRI subscale and an extended (1709 voxels) cerebellar cluster in right Crus 2 (pFWEcorr = 0.002) (Table 3).
The associations significant at uncorrected statistical level between IRI subscales and whole-brain VBM data are reported as Supplementary Materials S1.
DTI Analyses
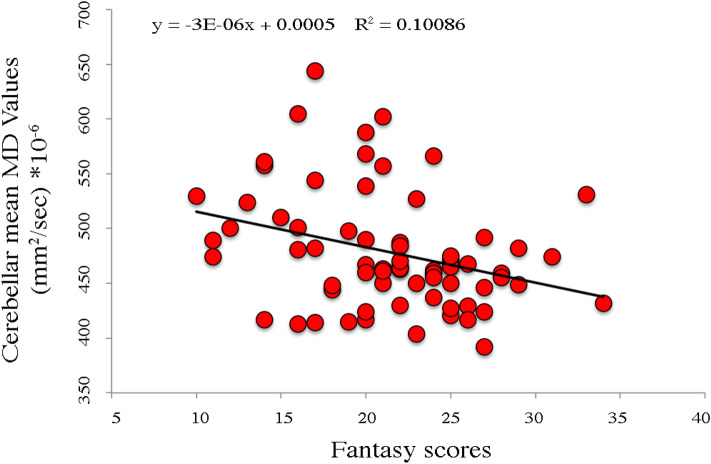
MD values of right cerebellar Crus 2 showed a significant negative association with Fantasy subscale scores (r = 0.30; p = 0.010) (Fig. 2). Such an association remained significant even when controlled for age and gender (R2 = 0.181; p = 0.028). FA values failed to reveal any significant association with cerebellar areas. No significant associations were found between Fantasy scores and both MD and FA micro-structural measures of right pars triangularis.
Figure 2.
Negative association between cerebellar Crus 2 mean Mean Diffusivity (MD) values and Fantasy IRI subscale scores. Equation, R2 and linear fit (solid black line) are reported.
Discussion
The main result of the present research is that the volumes in right cerebellar Crus 2 and pars triangularis of inferior frontal gyrus were positively associated with the Fantasy IRI-subscale scores. The increased volumes in Crus 2 were accompanied by diminished MD values. An MD decrease is generally considered to reflect increased functional capacities. In fact, lower MD values across the whole brain are associated with higher IQ scores, and lower MD values in the motivation-related subcortical areas (putamen and pallidum) are associated with greater motivational state49. Conversely, higher MD values in basal region of putamen are associated to increased anxiety-related personality traits46,50. Thus, a pattern of increased volume and diminished MD such as the one we found in Crus 2 speaks in favor of a greater functional capacity.
Since the trait empathy is expressed with variable intensity in the healthy population, we expected an effect size of cognitive and affective empathy on brain morphology to be small. Conversely, the associations of Fantasy subscale scores with cerebellar and frontal (pars triangularis) volumes were powerfully significant. Taking into account the close anatomical and functional proximity of the pars triangularis (BA45) and dorsolateral prefrontal cortex (DLPFC) (BA46), it appears notable the positive association between right DLPFC volume and Fantasy subscale scores31 and the role of right DLPFC in identification process measured with the same subscale32. Unfortunately, both studies have neglected any possible association between Fantasy subscale and cerebellar volumes.
Although healthy, all participants were also evaluated by HAM-D and HAM-A scales51,52. A positive correlation was found only between scores of Personal Distress and both HAM scales, in line with previous reports53–55. Thus, the more the self-oriented perspective traits were present, the more the anxious and depressive tendencies were evident. This finding suggests that in the presence of others’ suffering the self-orientation may induce ruminations on what it would be like to be affected oneself, resulting in the feeling of negative affect and distress56,57.
Interestingly, a recent fMRI study reports the activation of right Crus 1 and 2 when the participants viewed a short dramatic movie rich in dynamic cognitive and affective contents58. The activity in right Crus regions correlated with the presence of a structured storyline and with distinct unexpected features of the movie, such as turning points of the plot and sequences with verbal components. Convincingly, the authors have interpreted their results as an index of the cerebellar engagement in dynamic perceptual and affective processes elicited by naturalistic stimuli, such as movies, spoken or read narratives. In accordance with this evidence, the increased volumes in Crus 2 associated with high scores of Fantasy subscale may represent the structural underpinning of the processes required for empathizing with characters of immersive storylines.
Reading a book or watching a movie are non-innate and highly artificial brain activities, which may occupy a very significant part in the daily life of many people, presumably because their considerable adaptive value59. During such activities it is often opened a window into characters’ thoughts and feeling, so that people respond in though and feeling to fictive situations, as if they actually occur. Ultimately, these activities help subjects to optimize decisions and actions, learn about existing or fictive worlds, and stimulate motivation and imagination, functioning thus as a sort of “emotional gym”. In watching a movie or reading a book subjects may be so emotionally moved to get lost in the fictive happenings of the stories as if these were real, and imaginatively perceive themselves as transposed into character’s thoughts and feelings, experiencing the character’s happenings from the character’s perspective, and merging with or being that character. The cognitive empathy required for such processes is measured by the IRI subscale Fantasy.
The cerebellar involvement in cognitive empathy fits with the significant covariation of right lateral cerebellum with self-rated individual differences in empathy for pain described by Singer and colleagues38, and with the reported cerebellar involvement in social cognition4,7,60. Even the new functional parcellation of the cerebellar cortex proposed by King and colleagues61 evidenced the activation throughout right Crus 1 and 2 with ToM tasks that are essential in social interactions. Correspondingly, bilateral lesions of cerebellar posterior vermis and hemispheres result in empathy and ToM deficits40. In a pediatric brain injury sample the individual differences in cerebellar volumes predicted ToM outcomes, and the volumetric reductions in Cerebro-Cerebellar Mentalizing Network (CCMN) predicted poor ToM performances62. Some neuropsychiatric disorders exhibiting cerebellar dysfunctions display impairments in social functions. For instance, the performance of patients with various types of cerebellar damage is impaired in ToM tasks63. In children affected by autism spectrum disorders VBM analyses revealed reduced GM volumes in right Crus 1 and 2 and emphasized that the degree of cerebellar GM reductions correlated with the severity of symptoms in social interactions, communication, and repetitive behaviors41. More specifically, it has been recently reported that the size of empathic imbalance between the cognitive and emotional components is positively correlated with autism traits in neurotypical population64. Furthermore, cognitive empathy predominant on affective empathy is related to stronger connectivity in interoceptive and socio-cognitive networks including the cerebellum65. Additionally, in a fMRI study individuals with alexithymia (i.e. difficulty in recognizing and expressing one’s own emotions and in describing the emotional experience of others in hypothetical situations66) showed decreased activation in the cerebellum (and increased activation in the anterior insula) in response to empathic painful situations in comparison to healthy controls37. On the whole, these data highlight the involvement of the cerebellum in socio-cognitive processes6,14,67,68 and support the view of a “social cerebellum”, and more specifically of an “empathic cerebellum”.
In motor domain, the cerebellar networks construct internal models of motor processes to control whether actions are executed as planned; if it is not, the generation of an error signal regulates cortical processes responsible for that error, facilitating thus the enacting of the intended action. Notably, the cerebellum forms forward or inverse internal models even of mental processes without involvement of overt movements and somatosensory responses69. The internal models are neural representations that encode the context-specific dynamics of concrete or abstract representations to facilitate predictive control of the system. The cerebellar-dependent, error-based learning is used to calibrate forward models. Therefore, fundamental cerebellar functions are predicting the consequences and scope of motor and non-motor operations by reconstructing hypothetical events, and signaling prediction errors to the cortex. To successfully manage any mismatch, the co-activation of cerebellum and neocortical areas appears needed since the predictions are based on information from the cortex to the cerebellum (efferent copies), and error signals are sent from the cerebellum to the cortex. Similar to what happens in the sensorimotor system, it has been advanced that the related processes of forward modelling and error sensitivity may characterize the cerebellar function even within social realm allowing thus to anticipate the other’s behavior or one’s own reactions8,63,70,71. On the basis of the present findings we are advancing that these concepts of prediction and error processing may be advanced to understand the cerebellar contribution even to empathy with fictional characters. When the subject empathizes with fictional characters the cerebellar forward model potentially generates representations and predictions regarding the feelings of the character. Internal models develop by using past perceptual, motor, and socio-emotional experiences of the empathizer framed by the intentions, beliefs, and feelings of the character. The degree of matching between subject and character relies on such representations, but the subject can efficiently match with the state of the character to the degree that s/he has already existing representations for that state, pointing out the experience-dependence of such a process, in analogy to what previously described for the motor domain72. In their fMRI study, Calvo-Merino and colleagues have examined the brain activity of expert dancers who watched videos of ballet moves and have observed greater activity in right cerebellum and premotor areas when subjects were looking at the movements they were more familiar with, indicating that observing others’ actions engages neural pathways associated with sensorimotor internal models. Ultimately, the internal models involving cortico-cerebellar networks may also be essential for empathic responses in real-life or imaginary situations.
Notably, in addition to volumes of right Crus 2 we found that Fantasy subscale scores were powerfully associated with volumes of right pars triangularis (BA45) of the inferior frontal gyrus (BA44, BA47). As known, the functions of these frontal areas are related to the ability to live sociably and communicate with others, being key nodes of the mirror neuron system (MNS)73–75. Given its observation-execution matching properties, MNS provides the appropriate mechanism for empathy and imitation76, and allows identifying goals and intentions of others by their resemblance to stored representations for the same states (experience-dependence). MNS may facilitate thus the simulation of behavior—even social—of the other77–80, even when the other is a fictional character. Reading about or viewing a character who experiences a powerful emotion stimulates mirroring mechanisms and through the implementation of the internal models (provided by the cerebellum) might form embodied representations of that emotion grounded in perceptual, sensorimotor, and visceral control loops81. These embodiment circuitries act as a boost for subsequent socio-emotional processes, allowing the remapping of character's states into the corresponding subject’s sensorimotor and visceral brain areas, making the subject experience the same emotion of the fictional character18,82–84. The more similar the character’s state is to something the subject has already experienced, the more his/her representations will match character’s state18. Consistently with these considerations, it has been postulated that the prefrontal areas activate when two or more emotional states—such as one's own and that of the other (in our case the fictional character) are simultaneously processed and integrated to form a higher-order empathic state20. It has been described the engagement of the right inferior frontal cortex when comparing conditions in which the subject attributes a mental state to a character in a story in which s/he is featured and one in which s/he is absent85. The critical role of the right inferior frontal cortex in the inhibition of self-perspective has been also described, reporting a case of a subject with a lesion of this area who was impaired in ToM tasks that required the suppression of his own perspective but performed well if they did not86.
Empathy toward fictional characters slightly differs from empathy towards real people due to the need in the first case to overcome the difficulties in attending to internal over external stimuli and to bring character’s state into mind to succeed in feeling it. In this sense, since movies and stories offer narratives that are unfolding in time, empathizing with a fictional character requires the maintenance (or the internal rehearsal) of information in working memory, once again engaging the prefrontal networks, including the pars triangularis87. Anyway, working memory system functions only when it is driven by attention. It has been shown that top-down attention to one’s own emotions activates the prefrontal cortical areas87–90. Then, the notion that the more attentive the subjects are, the more they are drawn into the character’s emotional situation emphasizes the importance of the cerebello-frontal processing in the proper attentional maintenance (or shift) required for empathic response to occur. In fact, the cerebellum (and its connections with prefrontal areas) is necessary to execute attentional shifts, to track the state of the character, and to avoid attending to misdirection and intruders, to stay focused on the plot.
A final consideration has to be added. In watching a movie, or listening to or reading a story, it is necessary to accumulate, integrate and process a lot of information (often with high socio-emotional content) in order to gain cognitive and emotional comprehension of that story91–93. The high-order prefrontal, temporal and parietal areas activated in these situations coincide with the members of the DMN, the network that regulates the switch from an internal reference state to external target-oriented behaviors. Remarkably, even the cerebellum, and in particular Crus 2, belongs to this network as indicated by its coherent activation with the cortical areas of DMN3,5. Connectivity studies revealed that function-specific cerebellar networks are strongly connected to cortical networks serving the identical function5. Since DMN involves cortical and cerebellar activity, the volumetric increased volume in Crus 2 and frontal pars triangularis associated with the enhanced empathizing abilities with fictional characters found in the present research appears intriguing.
Conclusions
Emotionally charged narratives or movies allow re-experiencing emotions in the resonating sensory-motor systems of the subject, as if s/he were there in the same situation, experiencing the very same emotional state of the other, even if the other is a fictional character. The present findings indicate that the empathy with emotionally charged characters is enabled by the coordinated activity of cerebellar areas (Crus 2) and MNS (pars triangularis). These findings are in line with the “fiction feeling hypothesis”59 that posits that greater emotionality in a narrative results in greater feelings of empathy and immersion and in greater recruitment of networks of cognitive mentalizing empathy94.
Methods
Participants
A sample of 70 healthy subjects (31 males; mean age ± SD: 41.11 ± 12.34 years; range: 21–62) was recruited for the study. Educational level ranged from an eighth grade to a post-graduate degree (mean education years ± SD: 15.83 ± 2.86; range: 8–25). All participants were right-handed as assessed with the Edinburgh Handedness Inventory95. Inclusion criteria were: age between 18 and 65 years and suitability for MRI scanning. Exclusion criteria included: (1) cognitive impairment or dementia, based on Mini Mental State Examination (MMSE)96 scores ≤ 2497, and confirmed by clinical neuropsychological evaluation by using the Mental Deterioration Battery98 and the NINCDS-ADRDA criteria for dementia99; (2) subjective complaint of memory difficulties or of any other cognitive deficit, regardless of interference with daily activities; (3) major medical illnesses, e.g. diabetes (not stabilized), obstructive pulmonary disease, or asthma; hematologic and oncologic disorders; pernicious anemia; clinically significant gastrointestinal, renal, hepatic, endocrine, or cardio-vascular system diseases; newly treated hypothyroidism; (4) current or reported mental (assessed by SCID-I and the SCID-II)100 or neurological (assessed by clinical neurological evaluation) disorders (e.g. schizophrenia, mood disorders, anxiety disorders, stroke, Parkinson’s disease, seizure disorder, head injury with loss of consciousness, and any other significant mental or neurological disorder); (5) known or suspected history of alcoholism or drug dependence and abuse, evaluated by structured interviews (SCID I or SCID II)100,101; (6) MRI evidence of focal parenchymal abnormalities or cerebro-vascular diseases: for each subject, a trained neuroradiologist and a neuropsychologist expert in neuroimaging co-inspected all the available clinical MRI sequences (i.e. T1- and T2-weighted and FLAIR images) to ensure that the subjects were free from structural brain pathologies and vascular lesions (i.e. FLAIR or T2-weighted hyper-intensities and T1-weighted hypo-intensities).
Ethical statement
In accordance with the Declaration of Helsinki the study was approved by the Local Ethics Committee of Santa Lucia Foundation IRCCS. We confirm that the whole research was performed in accordance with relevant guidelines/regulations and that written consent was obtained from all participants after full explanation of study procedures.
Psychological Instruments
Trait empathy assessment
Empathic abilities were assessed through the Interpersonal Reactivity Index, IRI17, a widely used well-validated, multidimensional measure of trait empathy. The questionnaire is based on a self-report comprising 28 items answered on a 5-point Likert scale ranging from 0 ("Does not describe me well") to 4 ("Describes me very well"). The measure has 4 subscales, each made up of 7 different items, and for each subscale, a minimum score of 0 or maximum score of 28 is possible. The subscales that measure the affective dimension of empathy are Personal Distress and Empathic Concern, while the subscales that measure the cognitive dimension of empathy are Perspective Taking and Fantasy. In more detail, Personal Distress is “self-oriented” and is associated to aversive emotional responses in the observer (e.g. feelings of fear or discomfort at witnessing negative experiences of others) (Sample item: When I see someone who badly needs help in an emergency, I go to pieces), while Empathic Concern is “other-oriented” and is related to feelings of compassion and sympathy for observed unfortunate individuals (I often have tender, concerned feelings for people less fortunate than me). Perspective Taking examines the tendency to spontaneously adopt the psychological point of view of others in everyday life (i.e. cognitive responses) (Before criticizing somebody, I try to imagine how I would feel if I were in their place), while Fantasy examines participants' abilities to imaginatively transpose themselves into feelings and actions of fictitious characters in books, movies, and plays (When I am reading an interesting story or novel, I imagine how I would feel if the events in the story were happening to me).
Depression and anxiety assessment
Because of the known associations between empathy and depression/anxiety55,102–104, although healthy, all participants were evaluated by means of Hamilton rating scales. Namely, presence and severity of depressive symptoms were evaluated by using Hamilton depression rating scale‐17 items (HAM‐D17, indicated in the text as HAM-D). Scores < 8 indicated no depression, scores from 8 to 17 corresponded to mild depression, scores from 18 to 24 corresponded to moderate depression, and scores > 24 severe depression51. Presence and severity of anxiety symptoms were evaluated by using Hamilton anxiety rating scale (HAM-A), which consists of 14 questions. Scores < 5 indicated no anxiety, scores between 6 and 14 indicated mild anxiety, and score > 14 indicated moderate to severe anxiety52.
Image acquisition
All participants underwent the imaging protocol originally described elsewhere45,47,48. The protocol included standard clinical sequences (FLAIR, DP-T2-weighted), a volumetric whole-brain 3D high-resolution T1-weighted sequence, and a DTI scan protocol, performed with a 3-T Achieva MR imager (Siemens, Erlangen, Germany). Volumetric whole-brain T1-weighted images were obtained in the sagittal plane using a modified driven equilibrium Fourier transform (MDEFT) sequence (Echo Time/Repetition Time—TE/TR— = 2.4/7.92 ms, flip angle 15°, voxel size 1 × 1 × 1 mm3). Diffusion volumes were acquired by using echo-planar imaging (TE/TR = 89/8500 ms, bandwidth = 2126 Hz/vx; matrix size 128 × 128; 80 axial slices, voxel size 1.8 × 1.8 × 1.8 mm3) with 30 isotropically distributed orientations for the diffusion-sensitizing gradients at one b value of 1000 s mm2 and two b = 0 images. Scanning was repeated three times to increase the signal-to-noise ratio. All planar sequence acquisitions were obtained in the plane of the anterior–posterior commissure line. Since the posterior cranial fossa usually falls at the lower limit of the field of view, particular care was taken to center subjects’ head in the head coil, in order to avoid possible magnetic field dishomogeneities or artifacts at the level of the cerebellum.
Image processing
T1-weighted and DTI images were submitted to several processing steps. First, to explore the relationship between regional volumes and empathy on a voxel-by-voxel basis, T1-weighted images were processed and examined using the SPM8 software (Wellcome Department of Imaging Neuroscience Group, London, UK; http://www.fil.ion.ucl.ac.uk/spm), specifically the VBM8 toolbox (http://dbm.neuro.uni-jena.de/vbm.html) running in Matlab 2007b (MathWorks, Natick, MA, USA). The toolbox extends the unified segmentation model105 consisting of MRI field intensity inhomogeneity correction, spatial normalization, and tissue segmentation at several pre-processing steps to further improve data quality. Initially, to increase the signal-to-noise ratio, an optimized block-wise nonlocal-means filter was applied to the MRI scans using the Rician noise adaption106. Then, an adaptive maximum a posteriori segmentation approach extended by partial volume estimation was employed to separate the MRI scans into GM, WM, and cerebro-spinal fluid. The segmentation step was finished by applying a spatial constraint to the segmented tissue probability maps based on a hidden Markow Random Field model to remove isolated voxels, which unlikely were members of a certain tissue class, and to close holes in clusters of connected voxels of a certain class, resulting in a higher signal-to-noise ratio of the final tissue probability maps. Then, the iterative high- dimensional normalization approach provided by the Diffeomorphic Anatomical Registration through Exponentiated Lie Algebra (DARTEL)107 toolbox was applied to the segmented tissue maps to register them to the stereotaxic space of the Montreal Neurological Institute (MNI). The tissue deformations were used to modulate participants’ GM and WM maps to be entered in the analyses. Voxel values of the resulting normalized and modulated GM and WM segments indicated the probability (between 0 and 1) that a specific voxel belonged to the relative tissue. Finally, the modulated and normalized GM and WM segments were written with an isotropic voxel resolution of 1.5 mm3 and smoothed with a 6-mm Full-Width Half Maximum (FWHM) Gaussian kernel. The segmented, normalized, modulated and smoothed GM and WM images were used for analyses. Subsequently, DTI data were pre-processed and analyzed in Explore DTI v4.8.6108. Data were corrected for motion and eddy currents. Motion artifacts and eddy current distortions were corrected with B-matrix rotation using the approach by Leemans and Jones108. During this processing procedure, all brain scans were rigidly normalized to Montreal Neurological Institute (MNI) space during the motion-distortion correction step. A diffusion tensor model was fit at each voxel and maps of FA and MD were generated. All diffusional indexes were finally written in a resolution of 2 × 2 × 2 mm. MD and FA maps were subsequently smoothed by using a Gaussian kernel with a 6-mm FWHM. Among DTI indices, Mean Diffusivity (MD) and Fractional Anisotropy (FA) were used as probes for GM and WM micro-structural integrity, respectively45–48. MD measures the averaged diffusion of water molecules through tissues providing information on restrictions (e.g., high density of cells) that water molecules encounter. If these obstacles have coherent alignment, on average the water tends to diffuse more along a certain axis. MD reflects cellular and cyto-architectonic changes, which result in higher density of synapses, spines, and capillaries, modifications in the properties of myelin and membranes, alterations in shape of glial cells and neurons. Ultimately, decreased MD reflects increased functional adaptation, and increased MD has been linked to poor cognitive performance or psychiatric symptoms109 and to states characterized by reduced efficacy of synaptic and extra-synaptic transmission110. FA measures the anisotropy of water diffusion processes and it is positively linked to fiber density, axonal diameter and myelination in WM43. Low FA values stand for isotropic diffusion (i.e., unrestricted in all directions), while high FA values indicate diffusion fully restricted along one axis.
Statistical analysis
Sociodemographic and psychological variables
Parametric associations between IRI scores and age, years of formal education, and HAM-D and HAM-A scores, were analyzed by Pearson’s product moment correlations (Fisher’s r to z). Gender Differences in psychological variables were assessed by unpaired t test. Results of the demographic characteristics were considered significant at the p < 0.05 level.
Volumetric analyses
ROI-based VBM
We selected ROIs considering previous functional and structural neuroimaging studies on empathic abilities to constrain our anatomical hypotheses. In particular, as main aim of the present study we focused our analyses on the cerebellum. As ancillary aim we analyzed the anterior cingulate cortex38, inferior frontal gyrus26, precuneus111, insula38, somatosensory cortex112, medial prefrontal cortex26,113, supplementary motor area38, and frontal inferior pars triangularis114. These regions were selected based on the quoted functional neuroimaging studies, demonstrating their involvement in affective and cognitive empathy, and meta-analyses113 of brain regions involved in affect sharing and mentalizing31.
The MNI-oriented atlas of the human brain (Automated Anatomical Labeling Atlas, AAL)115 was used to extract GM masks of the ROIs singularly achieved by meaning all GM probability maps, obtained in the VBM8 processing steps, thresholding the relative image to a value of 0.3 (i.e. removing all voxels having a probability to belong to GM lower or equal to 29%), and manually removing all the other structures (e.g. for the cerebellum by manually removing all the non-cerebellar structures) using the AAL template, as reference. The resulting data were then fed into VBM analyses to evaluate morphological changes associated with ROIs and empathy subscales. We evaluated at the voxel-level the associations between cerebellar or neocortical (either ROIs or whole-brain VBM) structural measures and empathy scores, by using SPM8 within the framework of the General Linear Model. Multiple-regression analyses were computed by singularly using the measures of ROIs GM volumes as dependent variables, the scores of empathy IRI subscales as regressors, and age as covariate to control age impact on the brain structures. Moreover, when significantly associated to empathy IRI subscales, also gender, education years, depression or anxiety levels were used as covariates. Gender was always considered a ‘‘dummy variable’’ given its dichotomic nature.
Whole-brain VBM
As for ROI-based VBM analyses, also whole-brain VBM multiple-regression analyses were computed by singularly using the measures of GM volumes as dependent variables, the scores of IRI subscales as regressors, and age as covariate. When significantly associated to empathy IRI subscales, also gender, education years, depression or anxiety levels were used as covariates. We considered significant only the relationships whose voxels were part of a spatially contiguous cluster size of a minimum of 50 voxels, and that survived (p < 0.05) at the Family Wise Error (FWE) correction. Anyway, to avoid the risk of type II errors, in Supplementary Materials S1 we reported the areas significantly associated at uncorrected statistical level (puncorr < 0.001) to scores of empathy IRI subscales.
To obtain the precise anatomical localization of VBM results, we superimposed statistical maps onto Diedrichsen’s probabilistic atlas of the human cerebellum, which subdivides the cerebellum into ten different regions116 or onto the AAL template for extra-cerebellar ROIs and whole-brain analyses. Finally, the mean values of GM volumes significantly associated (pFWEcorr) with empathy scores in ROIs and whole-brain analyses were extracted and used to create scatterplots.
DTI analyses
The areas significantly associated (pFWEcorr) with IRI subscales at macro-structural analyses were used as masks and applied to MD and FA maps, in order to extract mean micro-structural values for each measure. Parametric associations between empathy scores and mean MD or FA values were analyzed by Pearson’s product moment correlations (Fisher’s r to z) to assess potential significant associations also with micro-structural measures. Analyses were also controlled for age, and, when significantly associated to IRI scores, also for gender, education years, depression or anxiety levels, as above described. Then, MD or FA values significantly associated with IRI scores were extracted and used to create scatterplots.
Supplementary Information
Acknowledgements
All authors declare no potential conflicts of interest, including any financial, personal, or other relationships with other people or organizations relevant to the subject of their manuscript. This work was partially supported by the Italian Ministry of Health, Ricerca Corrente. This work was supported by the Italian Ministry of Health, Ricerca Corrente (IRCCS Fondazione Santa Lucia).
Author contributions
All authors conceived and designed the study; E.P., F.P., D.V. gathered and analyzed neuroimaging data; E.P., D.L., D.C. gathered and analyzed behavioral data; all authors contributed to the interpretation of data and were involved in drafting the manuscript and revising it critically; all authors gave their approval of the manuscript version to be published.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Debora Cutuli and Gianfranco Spalletta.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-021-87861-0.
References
- 1.Schmahmann JD, Guell X, Stoodley CJ, Halko MA. The theory and neuroscience of cerebellar cognition. Annu. Rev. Neurosci. 2019;42:337–364. doi: 10.1146/annurev-neuro-070918-050258. [DOI] [PubMed] [Google Scholar]
- 2.Habas C, et al. Distinct cerebellar contributions to intrinsic connectivity networks. J. Neurosci. 2009;29:8586–8594. doi: 10.1523/JNEUROSCI.1868-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Buckner RL, Krienen FM, Castellanos A, Diaz JC, Yeo BTT. The organization of the human cerebellum estimated by intrinsic functional connectivity. J. Neurophysiol. 2011;106:2322–2345. doi: 10.1152/jn.00339.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Van Overwalle F, Baetens K, Mariën P, Vandekerckhove M. Social cognition and the cerebellum: A meta-analysis of over 350 fMRI studies. Neuroimage. 2014;86:554–572. doi: 10.1016/j.neuroimage.2013.09.033. [DOI] [PubMed] [Google Scholar]
- 5.Van Overwalle, F., D’aes, T. & Mariën, P. Social cognition and the cerebellum: A meta‐analytic connectivity analysis. Hum. Brain Mapp.36, 5137–5154 (2015). [DOI] [PMC free article] [PubMed]
- 6.Van Overwalle F, et al. The role of the cerebellum in reconstructing social action sequences: A pilot study. Soc. Cogn. Affect. Neurosci. 2019;14:549–558. doi: 10.1093/scan/nsz032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Van Overwalle F, Ma Q, Heleven E. The posterior crus II cerebellum is specialized for social mentalizing and emotional self-experiences: A meta-analysis. Soc. Cogn. Affect. Neurosci. 2020;15:905–928. doi: 10.1093/scan/nsaa124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Van Overwalle F, et al. Consensus paper: Cerebellum and social cognition. Cerebellum. 2020;19:833–868. doi: 10.1007/s12311-020-01155-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Heleven E, van Dun K, Van Overwalle F. The posterior Cerebellum is involved in constructing Social Action Sequences: An fMRI Study. Sci. Rep. 2019;9:11110. doi: 10.1038/s41598-019-46962-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pu M, et al. The posterior cerebellum supports the explicit sequence learning linked to trait attribution. Cogn. Affect. Behav. Neurosci. 2020;20:798–815. doi: 10.3758/s13415-020-00803-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jack A, Pelphrey KA. Neural correlates of animacy attribution include neocerebellum in healthy adults. Cereb. Cortex. 2015;25:4240–4247. doi: 10.1093/cercor/bhu146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Van Overwalle F, Van de Steen F, Mariën P. Dynamic causal modeling of the effective connectivity between the cerebrum and cerebellum in social mentalizing across five studies. Cogn. Affect. Behav. Neurosci. 2019;19:211–223. doi: 10.3758/s13415-018-00659-y. [DOI] [PubMed] [Google Scholar]
- 13.Van Overwalle F, Van de Steen F, van Dun K, Heleven E. Connectivity between the cerebrum and cerebellum during social and non-social sequencing using dynamic causal modelling. Neuroimage. 2020;206:116326. doi: 10.1016/j.neuroimage.2019.116326. [DOI] [PubMed] [Google Scholar]
- 14.Van Overwalle F, Mariën P. Functional connectivity between the cerebrum and cerebellum in social cognition: A multi-study analysis. Neuroimage. 2016;124:248–255. doi: 10.1016/j.neuroimage.2015.09.001. [DOI] [PubMed] [Google Scholar]
- 15.De Jaegher H, Di Paolo E, Gallagher S. Can social interaction constitute social cognition? Trends Cogn. Sci. 2010;14:441–447. doi: 10.1016/j.tics.2010.06.009. [DOI] [PubMed] [Google Scholar]
- 16.de Waal FBM, Preston SD. Mammalian empathy: Behavioural manifestations and neural basis. Nat. Rev. Neurosci. 2017;18:498–509. doi: 10.1038/nrn.2017.72. [DOI] [PubMed] [Google Scholar]
- 17.Davis MH. Measuring individual differences in empathy: Evidence for a multidimensional approach. J. Pers. Soc. Psychol. 1983;44:113–126. doi: 10.1037/0022-3514.44.1.113. [DOI] [Google Scholar]
- 18.Preston, S. D. & de Waal, F. B. M. Empathy: Its ultimate and proximate bases. Behav. Brain Sci.25, 1–20; discussion 20–71 (2002). [DOI] [PubMed]
- 19.Leblanc H, Ramirez S. Linking social cognition to learning and memory. J. Neurosci. 2020;40:8782–8798. doi: 10.1523/JNEUROSCI.1280-20.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shamay-Tsoory SG, Aharon-Peretz J, Perry D. Two systems for empathy: A double dissociation between emotional and cognitive empathy in inferior frontal gyrus versus ventromedial prefrontal lesions. Brain. 2009;132:617–627. doi: 10.1093/brain/awn279. [DOI] [PubMed] [Google Scholar]
- 21.Fan Y, Duncan NW, de Greck M, Northoff G. Is there a core neural network in empathy? An fMRI based quantitative meta-analysis. Neurosci. Biobehav. Rev. 2011;35:903–911. doi: 10.1016/j.neubiorev.2010.10.009. [DOI] [PubMed] [Google Scholar]
- 22.Bilevicius E, Kolesar T, Smith S, Trapnell P, Kornelsen J. Trait emotional empathy and resting state functional connectivity in default mode, salience, and central executive networks. Brain Sci. 2018;8:128. doi: 10.3390/brainsci8070128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Christov-Moore L, Reggente N, Douglas PK, Feusner JD, Iacoboni M. Predicting empathy from resting state brain connectivity: A multivariate approach. Front. Integr. Neurosci. 2020;14:3. doi: 10.3389/fnint.2020.00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Takeuchi H, et al. Empathizing associates with mean diffusivity. Sci. Rep. 2019;9:8856. doi: 10.1038/s41598-019-45106-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chochinov HM, et al. Eliciting personhood within clinical practice: Effects on patients, families, and health care providers. J. Pain Symptom Manage. 2015;49:974–980.e2. doi: 10.1016/j.jpainsymman.2014.11.291. [DOI] [PubMed] [Google Scholar]
- 26.Lamm C, Batson CD, Decety J. The neural substrate of human empathy: Effects of perspective-taking and cognitive appraisal. J. Cogn. Neurosci. 2007;19:42–58. doi: 10.1162/jocn.2007.19.1.42. [DOI] [PubMed] [Google Scholar]
- 27.Moore RC, Dev SI, Jeste DV, Dziobek I, Eyler LT. Distinct neural correlates of emotional and cognitive empathy in older adults. Psychiatry Res. Neuroimaging. 2015;232:42–50. doi: 10.1016/j.pscychresns.2014.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hooker CI, Bruce L, Lincoln SH, Fisher M, Vinogradov S. Theory of mind skills are related to gray matter volume in the ventromedial prefrontal cortex in schizophrenia. Biol. Psychiat. 2011;70:1169–1178. doi: 10.1016/j.biopsych.2011.07.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sterzer P, Stadler C, Poustka F, Kleinschmidt A. A structural neural deficit in adolescents with conduct disorder and its association with lack of empathy. Neuroimage. 2007;37:335–342. doi: 10.1016/j.neuroimage.2007.04.043. [DOI] [PubMed] [Google Scholar]
- 30.Butler, P. M. & Chiong, W. Neurodegenerative disorders of the human frontal lobes. Handb. Clin. Neurol. 163, 391–410 (Elsevier, 2019). [DOI] [PubMed]
- 31.Banissy MJ, Kanai R, Walsh V, Rees G. Inter-individual differences in empathy are reflected in human brain structure. Neuroimage. 2012;62:2034–2039. doi: 10.1016/j.neuroimage.2012.05.081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cheetham M, Hänggi J, Jancke L. Identifying with fictive characters: structural brain correlates of the personality trait ‘fantasy’. Soc. Cogn. Affect. Neurosci. 2014;9:1836–1844. doi: 10.1093/scan/nst179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Eres R, Decety J, Louis WR, Molenberghs P. Individual differences in local gray matter density are associated with differences in affective and cognitive empathy. Neuroimage. 2015;117:305–310. doi: 10.1016/j.neuroimage.2015.05.038. [DOI] [PubMed] [Google Scholar]
- 34.Uribe C, et al. Neuroanatomical and functional correlates of cognitive and affective empathy in young healthy adults. Front. Behav. Neurosci. 2019;13:85. doi: 10.3389/fnbeh.2019.00085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gu X, et al. Anterior insular cortex is necessary for empathetic pain perception. Brain. 2012;135:2726–2735. doi: 10.1093/brain/aws199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jackson PL, Meltzoff AN, Decety J. How do we perceive the pain of others? A window into the neural processes involved in empathy. Neuroimage. 2005;24:771–779. doi: 10.1016/j.neuroimage.2004.09.006. [DOI] [PubMed] [Google Scholar]
- 37.Moriguchi Y, et al. Empathy and judging other’s pain: An fMRI study of alexithymia. Cereb. Cortex. 2007;17:2223–2234. doi: 10.1093/cercor/bhl130. [DOI] [PubMed] [Google Scholar]
- 38.Singer T, et al. Empathy for pain involves the affective but not sensory components of pain. Science. 2004;303:1157–1162. doi: 10.1126/science.1093535. [DOI] [PubMed] [Google Scholar]
- 39.Clausi S, et al. The cerebellar predictions for social interactions: Theory of mind abilities in patients with degenerative cerebellar atrophy. Front. Cell. Neurosci. 2019;12:510. doi: 10.3389/fncel.2018.00510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Roldan Gerschcovich, E., Cerquetti, D., Tenca, E. & Leiguarda, R. The impact of bilateral cerebellar damage on theory of mind, empathy and decision making. Neurocase17, 270–275 (2011). [DOI] [PubMed]
- 41.D’Mello, A. M., Crocetti, D., Mostofsky, S. H. & Stoodley, C. J. Cerebellar gray matter and lobular volumes correlate with core autism symptoms. NeuroImage: Clin.7, 631–639 (2015). [DOI] [PMC free article] [PubMed]
- 42.Olivito G, et al. Resting-state functional connectivity changes between dentate nucleus and cortical social brain regions in autism spectrum disorders. Cerebellum. 2017;16:283–292. doi: 10.1007/s12311-016-0795-8. [DOI] [PubMed] [Google Scholar]
- 43.Basser PJ, Pierpaoli C. Microstructural and physiological features of tissues elucidated by quantitative-diffusion-tensor MRI. J. Magn. Reson. B. 1996;111:209–219. doi: 10.1006/jmrb.1996.0086. [DOI] [PubMed] [Google Scholar]
- 44.Bihan DL. The ‘wet mind’: Water and functional neuroimaging. Phys. Med. Biol. 2007;52:R57–R90. doi: 10.1088/0031-9155/52/7/R02. [DOI] [PubMed] [Google Scholar]
- 45.Laricchiuta D, et al. The embodied emotion in cerebellum: A neuroimaging study of alexithymia. Brain Struct. Funct. 2015;220:2275–2287. doi: 10.1007/s00429-014-0790-0. [DOI] [PubMed] [Google Scholar]
- 46.Laricchiuta D, et al. Linking novelty seeking and harm avoidance personality traits to basal ganglia: Volumetry and mean diffusivity. Brain Struct. Funct. 2014;219:793–803. doi: 10.1007/s00429-013-0535-5. [DOI] [PubMed] [Google Scholar]
- 47.Picerni E, et al. Cerebellar structural variations in subjects with different hypnotizability. Cerebellum. 2019;18:109–118. doi: 10.1007/s12311-018-0965-y. [DOI] [PubMed] [Google Scholar]
- 48.Picerni, E. et al. New evidence for the cerebellar involvement in personality traits. Front. Behav. Neurosci.7, (2013). [DOI] [PMC free article] [PubMed]
- 49.Takeuchi H, et al. Mean diffusivity of basal ganglia and thalamus specifically associated with motivational states among mood states. Brain Struct. Funct. 2017;222:1027–1037. doi: 10.1007/s00429-016-1262-5. [DOI] [PubMed] [Google Scholar]
- 50.Westlye LT, Bjørnebekk A, Grydeland H, Fjell AM, Walhovd KB. Linking an anxiety-related personality trait to brain white matter microstructure: Diffusion Tensor imaging and harm avoidance. Arch. Gen. Psychiatry. 2011;68:369. doi: 10.1001/archgenpsychiatry.2011.24. [DOI] [PubMed] [Google Scholar]
- 51.Hamilton M. A rating scale for depression. J. Neurol. Neurosurg. Psychiatry. 1960;23:56–62. doi: 10.1136/jnnp.23.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hamilton M. The assessment of anxiety states by rating. Br. J. Med. Psychol. 1959;32:50–55. doi: 10.1111/j.2044-8341.1959.tb00467.x. [DOI] [PubMed] [Google Scholar]
- 53.Lee SA. Does empathy mediate the relationship between neuroticism and depressive symptomatology among college students? Personal. Individ. Differ. 2009;47:429–433. doi: 10.1016/j.paid.2009.04.020. [DOI] [Google Scholar]
- 54.Peres V, Corcos M, Robin M, Pham-Scottez A. Emotional intelligence, empathy and alexithymia in anorexia nervosa during adolescence. Eat. Weight Disord. 2020;25:1–8. doi: 10.1007/s40519-018-0482-5. [DOI] [PubMed] [Google Scholar]
- 55.Schreiter, S., Pijnenborg, G. H. M. & aan het Rot, M. Empathy in adults with clinical or subclinical depressive symptoms. J. Affect. Disord.150, 1–16 (2013). [DOI] [PubMed]
- 56.Flory JD, Räikkönen K, Matthews KA, Owens JF. Self-focused attention and mood during everyday social interactions. Pers. Soc. Psychol. Bull. 2000;26:875–883. doi: 10.1177/0146167200269012. [DOI] [Google Scholar]
- 57.Banzhaf C, et al. Interacting and dissociable effects of alexithymia and depression on empathy. Psychiatry Res. 2018;270:631–638. doi: 10.1016/j.psychres.2018.10.045. [DOI] [PubMed] [Google Scholar]
- 58.Nguyen VT, et al. Distinct cerebellar contributions to cognitive-perceptual dynamics during natural viewing. Cereb. Cortex. 2017;27:5652–5662. doi: 10.1093/cercor/bhw334. [DOI] [PubMed] [Google Scholar]
- 59.Jacobs A. Towards a neurocognitive poetics model of literary reading. In: Roel W, editor. Cognitive Neuroscience of Natural Language Use. Cambridge University Press; 2015. pp. 135–195. [Google Scholar]
- 60.Baumann O, Mattingley JB. Functional topography of primary emotion processing in the human cerebellum. Neuroimage. 2012;61:805–811. doi: 10.1016/j.neuroimage.2012.03.044. [DOI] [PubMed] [Google Scholar]
- 61.King M, Hernandez-Castillo CR, Poldrack RA, Ivry RB, Diedrichsen J. Functional boundaries in the human cerebellum revealed by a multi-domain task battery. Nat. Neurosci. 2019;22:1371–1378. doi: 10.1038/s41593-019-0436-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ryan NP, et al. Uncovering the neuroanatomical correlates of cognitive, affective and conative theory of mind in paediatric traumatic brain injury: A neural systems perspective. Soc. Cogn. Affect. Neurosci. 2017;12:1414–1427. doi: 10.1093/scan/nsx066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Sokolov AA. The cerebellum in social cognition. Front. Cell. Neurosci. 2018;12:145. doi: 10.3389/fncel.2018.00145. [DOI] [Google Scholar]
- 64.Shalev I, Uzefovsky F. Empathic disequilibrium in two different measures of empathy predicts autism traits in neurotypical population. Mol. Autism. 2020;11:59. doi: 10.1186/s13229-020-00362-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Cox CL, et al. The balance between feeling and knowing: affective and cognitive empathy are reflected in the brain’s intrinsic functional dynamics. Soc. Cogn. Affect. Neurosci. 2012;7:727–737. doi: 10.1093/scan/nsr051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Bydlowski S, et al. Emotion-processing deficits in eating disorders. Int. J. Eat. Disord. 2005;37:321–329. doi: 10.1002/eat.20132. [DOI] [PubMed] [Google Scholar]
- 67.Schurz M, Radua J, Aichhorn M, Richlan F, Perner J. Fractionating theory of mind: A meta-analysis of functional brain imaging studies. Neurosci. Biobehav. Rev. 2014;42:9–34. doi: 10.1016/j.neubiorev.2014.01.009. [DOI] [PubMed] [Google Scholar]
- 68.Heleven E, Van Overwalle F. The neural basis of representing others’ inner states. Curr. Opin. Psychol. 2018;23:98–103. doi: 10.1016/j.copsyc.2018.02.003. [DOI] [PubMed] [Google Scholar]
- 69.Ito M. Control of mental activities by internal models in the cerebellum. Nat. Rev. Neurosci. 2008;9:304–313. doi: 10.1038/nrn2332. [DOI] [PubMed] [Google Scholar]
- 70.Sokolov AA, Miall RC, Ivry RB. The cerebellum: Adaptive prediction for movement and cognition. Trends Cogn. Sci. 2017;21:313–332. doi: 10.1016/j.tics.2017.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Van Overwalle F, Manto M, Leggio M, Delgado-García JM. The sequencing process generated by the cerebellum crucially contributes to social interactions. Med. Hypotheses. 2019;128:33–42. doi: 10.1016/j.mehy.2019.05.014. [DOI] [PubMed] [Google Scholar]
- 72.Calvo-Merino B, Grèzes J, Glaser DE, Passingham RE, Haggard P. Seeing or doing? Influence of visual and motor familiarity in action observation. Curr. Biol. 2006;16:1905–1910. doi: 10.1016/j.cub.2006.07.065. [DOI] [PubMed] [Google Scholar]
- 73.Rizzolatti G, Craighero L. The mirror-neuron system. Annu. Rev. Neurosci. 2004;27:169–192. doi: 10.1146/annurev.neuro.27.070203.144230. [DOI] [PubMed] [Google Scholar]
- 74.Cattaneo, L. & Rizzolatti, G. The mirror neuron system. Arch. Neurol.66, (2009). [DOI] [PubMed]
- 75.Molenberghs P, Cunnington R, Mattingley JB. Brain regions with mirror properties: A meta-analysis of 125 human fMRI studies. Neurosci. Biobehav. Rev. 2012;36:341–349. doi: 10.1016/j.neubiorev.2011.07.004. [DOI] [PubMed] [Google Scholar]
- 76.Iacoboni M. Imitation, empathy, and mirror neurons. Annu. Rev. Psychol. 2009;60:653–670. doi: 10.1146/annurev.psych.60.110707.163604. [DOI] [PubMed] [Google Scholar]
- 77.Carr L, Iacoboni M, Dubeau M-C, Mazziotta JC, Lenzi GL. Neural mechanisms of empathy in humans: A relay from neural systems for imitation to limbic areas. Proc. Natl. Acad. Sci. 2003;100:5497–5502. doi: 10.1073/pnas.0935845100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Decety J, Jackson PL. The functional architecture of human empathy. Behav. Cogn. Neurosci. Rev. 2004;3:71–100. doi: 10.1177/1534582304267187. [DOI] [PubMed] [Google Scholar]
- 79.Gallese, V., Keysers, C. & Rizzolatti, G. A unifying view of the basis of social cognition. Trends Cogn. Sci. (Regul. Ed.)8, 396–403 (2004). [DOI] [PubMed]
- 80.Kaplan JT, Iacoboni M. Getting a grip on other minds: Mirror neurons, intention understanding, and cognitive empathy. Soc. Neurosci. 2006;1:175–183. doi: 10.1080/17470910600985605. [DOI] [PubMed] [Google Scholar]
- 81.Schaefer M, Heinze H-J, Rotte M. Close to you: Embodied simulation for peripersonal space in primary somatosensory cortex. PLoS ONE. 2012;7:e42308. doi: 10.1371/journal.pone.0042308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Niedenthal PM. Embodying emotion. Science. 2007;316:1002–1005. doi: 10.1126/science.1136930. [DOI] [PubMed] [Google Scholar]
- 83.Nummenmaa L, et al. Emotions promote social interaction by synchronizing brain activity across individuals. Proc. Natl. Acad. Sci. U.S.A. 2012;109:9599–9604. doi: 10.1073/pnas.1206095109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Preston SD. A perception-action model for empathy. In: Farrow TFD, Woodruff PWR, editors. Empathy in Mental Illness. Cambridge University Press; 2007. pp. 428–447. [Google Scholar]
- 85.Vogeley K, et al. Mind Rreading: Neural mechanisms of theory of mind and self-perspective. Neuroimage. 2001;14:170–181. doi: 10.1006/nimg.2001.0789. [DOI] [PubMed] [Google Scholar]
- 86.Samson D, Apperly IA, Kathirgamanathan U, Humphreys GW. Seeing it my way: A case of a selective deficit in inhibiting self-perspective. Brain. 2005;128:1102–1111. doi: 10.1093/brain/awh464. [DOI] [PubMed] [Google Scholar]
- 87.Smith R, et al. The role of medial prefrontal cortex in the working memory maintenance of one’s own emotional responses. Sci. Rep. 2018;8:3460. doi: 10.1038/s41598-018-21896-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Ochsner KN, et al. Reflecting upon feelings: An fMRI study of neural systems supporting the attribution of emotion to self and other. J. Cogn. Neurosci. 2004;16:1746–1772. doi: 10.1162/0898929042947829. [DOI] [PubMed] [Google Scholar]
- 89.Smith R, Baxter LC, Thayer JF, Lane RD. Disentangling introspective and exteroceptive attentional control from emotional appraisal in depression using fMRI: A preliminary study. Psychiatry Res. Neuroimaging. 2016;248:39–47. doi: 10.1016/j.pscychresns.2016.01.009. [DOI] [PubMed] [Google Scholar]
- 90.Xin F, Lei X. Competition between frontoparietal control and default networks supports social working memory and empathy. Soc. Cogn. Affect. Neurosci. 2015;10:1144–1152. doi: 10.1093/scan/nsu160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Borja Jimenez KC, et al. Changes in brain activity following the voluntary control of empathy. Neuroimage. 2020;216:116529. doi: 10.1016/j.neuroimage.2020.116529. [DOI] [PubMed] [Google Scholar]
- 92.Lerner Y, Honey CJ, Silbert LJ, Hasson U. Topographic mapping of a hierarchy of temporal receptive windows using a narrated story. J. Neurosci. 2011;31:2906–2915. doi: 10.1523/JNEUROSCI.3684-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Simony E, et al. Dynamic reconfiguration of the default mode network during narrative comprehension. Nat. Commun. 2016;7:12141. doi: 10.1038/ncomms12141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Altmann U, Bohrn IC, Lubrich O, Menninghaus W, Jacobs AM. Fact vs fiction—how paratextual information shapes our reading processes. Soc. Cogn. Affect. Neurosci. 2014;9:22–29. doi: 10.1093/scan/nss098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Oldfield RC. The assessment and analysis of handedness: The Edinburgh inventory. Neuropsychologia. 1971;9:97–113. doi: 10.1016/0028-3932(71)90067-4. [DOI] [PubMed] [Google Scholar]
- 96.Folstein MF, Folstein SE, McHugh PR. Mini-mental state. J. Psychiatr. Res. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 97.Measso G, et al. Raven’s colored progressive matrices: A normative study of a random sample of healthy adults. Acta Neurol. Scand. 1993;88:70–74. doi: 10.1111/j.1600-0404.1993.tb04190.x. [DOI] [PubMed] [Google Scholar]
- 98.Carlesimo GA, et al. The mental deterioration battery: Normative data, diagnostic reliability and qualitative analyses of cognitive impairment. Eur. Neurol. 1996;36:378–384. doi: 10.1159/000117297. [DOI] [PubMed] [Google Scholar]
- 99.McKhann GM, et al. The diagnosis of dementia due to Alzheimer’s disease: Recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimer’s Dementia. 2011;7:263–269. doi: 10.1016/j.jalz.2011.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.First, M. B. & Pincus, H. A. The DSM-IV Text Revision: Rationale and potential impact on clinical practice. PS53, 288–292 (2002). [DOI] [PubMed]
- 101.Spitzer RL. The structured clinical interview for DSM-III-R (SCID): I: History, rationale, and description. Arch. Gen. Psychiatry. 1992;49:624. doi: 10.1001/archpsyc.1992.01820080032005. [DOI] [PubMed] [Google Scholar]
- 102.Cusi AM, MacQueen GM, Spreng RN, McKinnon MC. Altered empathic responding in major depressive disorder: Relation to symptom severity, illness burden, and psychosocial outcome. Psychiatry Res. 2011;188:231–236. doi: 10.1016/j.psychres.2011.04.013. [DOI] [PubMed] [Google Scholar]
- 103.de Vignemont F, Singer T. The empathic brain: How, when and why? Trends Cogn. Sci. 2006;10:435–441. doi: 10.1016/j.tics.2006.08.008. [DOI] [PubMed] [Google Scholar]
- 104.Thoma P, et al. Cognitive and affective empathy in depression linked to executive control. Psychiatry Res. 2011;189:373–378. doi: 10.1016/j.psychres.2011.07.030. [DOI] [PubMed] [Google Scholar]
- 105.Ashburner J, Friston KJ. Unified segmentation. Neuroimage. 2005;26:839–851. doi: 10.1016/j.neuroimage.2005.02.018. [DOI] [PubMed] [Google Scholar]
- 106.Wiest-Daesslé, N., Prima, S., Coupé, P., Morrissey, S. P. & Barillot, C. Rician Noise Removal by Non-Local Means Filtering for Low Signal-to-Noise Ratio MRI: Applications to DT-MRI. in Medical Image Computing and Computer-Assisted Intervention – MICCAI 2008 (eds. Metaxas, D., Axel, L., Fichtinger, G. & Székely, G.) vol. 5242 171–179 (Springer Berlin Heidelberg, 2008). [DOI] [PMC free article] [PubMed]
- 107.Ashburner J. A fast diffeomorphic image registration algorithm. Neuroimage. 2007;38:95–113. doi: 10.1016/j.neuroimage.2007.07.007. [DOI] [PubMed] [Google Scholar]
- 108.Leemans A, Jones DK. The B -matrix must be rotated when correcting for subject motion in DTI data. Magn. Reson. Med. 2009;61:1336–1349. doi: 10.1002/mrm.21890. [DOI] [PubMed] [Google Scholar]
- 109.Kantarci K, et al. Diffusion tensor imaging and cognitive function in older adults with no dementia. Neurology. 2011;77:26–34. doi: 10.1212/WNL.0b013e31822313dc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Syková E, Nicholson C. Diffusion in brain extracellular space. Physiol. Rev. 2008;88:1277–1340. doi: 10.1152/physrev.00027.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Mar RA. The neural bases of social cognition and story comprehension. Annu. Rev. Psychol. 2011;62:103–134. doi: 10.1146/annurev-psych-120709-145406. [DOI] [PubMed] [Google Scholar]
- 112.Hooker CI, Verosky SC, Germine LT, Knight RT, D’Esposito M. Mentalizing about emotion and its relationship to empathy. Soc. Cogn. Affect. Neurosci. 2008;3:204–217. doi: 10.1093/scan/nsn019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Lamm C, Decety J, Singer T. Meta-analytic evidence for common and distinct neural networks associated with directly experienced pain and empathy for pain. Neuroimage. 2011;54:2492–2502. doi: 10.1016/j.neuroimage.2010.10.014. [DOI] [PubMed] [Google Scholar]
- 114.Riem MME, et al. Oxytocin modulates amygdala, insula, and inferior frontal gyrus responses to infant crying: A randomized controlled trial. Biol. Psychiat. 2011;70:291–297. doi: 10.1016/j.biopsych.2011.02.006. [DOI] [PubMed] [Google Scholar]
- 115.Tzourio-Mazoyer N, et al. Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. Neuroimage. 2002;15:273–289. doi: 10.1006/nimg.2001.0978. [DOI] [PubMed] [Google Scholar]
- 116.Diedrichsen J, Balsters JH, Flavell J, Cussans E, Ramnani N. A probabilistic MR atlas of the human cerebellum. Neuroimage. 2009;46:39–46. doi: 10.1016/j.neuroimage.2009.01.045. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.