Abstract
In this study we tested the hypothesis that pharmacological modulation of glutamatergic neurotransmission could rescue behavioral deficits exhibited by mice carrying a specific mutation in the Iqsec2 gene. The IQSEC2 protein plays a key role in glutamatergic synapses and mutations in the IQSEC2 gene are a frequent cause of neurodevelopmental disorders. We have recently reported on the molecular pathophysiology of one such mutation A350V and demonstrated that this mutation downregulates AMPA type glutamatergic receptors (AMPAR) in A350V mice. Here we sought to identify behavioral deficits in A350V mice and hypothesized that we could rescue these deficits by PF-4778574, a positive AMPAR modulator. Using a battery of social behavioral tasks, we found that A350V Iqsec2 mice exhibit specific deficits in sex preference and emotional state preference behaviors as well as in vocalizations when encountering a female mouse. The social discrimination deficits, but not the impaired vocalization, were rescued with a single dose of PF-4778574. We conclude that social behavior deficits associated with the A350V Iqsec2 mutation may be rescued by enhancing AMPAR mediated synaptic transmission.
Subject terms: Autism spectrum disorders, Molecular neuroscience
Introduction
IQSEC2 is an X-linked gene that has been previously associated with ASD, ID, and drug-resistant epilepsy1,2. In this study we sought to demonstrate proof of concept for a pharmacological intervention aiming to rescue murine behavioral deficits associated with a specific missense mutation (replacement of alanine with valine at amino acid residue 350 of IQSEC2, denoted A350V IQSEC2) found in human IQSEC23. A male child carrying this mutation was diagnosed with drug-resistant epilepsy, autism spectrum disorder and severe intellectual disability and is averbal3. We have recently reported that A350V Iqsec2 CRISPR generated mice (A350V mice) exhibit several behavioral deficits, including increased locomotion in the open field test and impaired spatial learning in the Morris water maze test. However, these mice showed intact sociability and social novelty preference in the three-chamber test4.
The IQSEC2 protein contains a catalytic domain (Sec7) characteristic of all GEFS (guanine exchange factors), which promotes GDP exchange for GTP on Arf6. It also contains an IQ like domain, which has been proposed to bind calmodulin, and thereby modulate the Sec7 GEF activity of IQSEC25–7. We have previously demonstrated that the A350V IQSEC2 mutation results in the inappropriate constitutive activation of the Sec7 domain of IQSEC2, which leads to a marked downregulation of hippocampal surface AMPAR in A350V mice. We have further demonstrated that the decrease in surface AMPAR, specifically in the surface GluA2 receptor, is associated with a marked decrease in basal synaptic transmission in the murine model4. These findings thereby identify AMPAR mediated transmission as a potential target for treating the deficits associated with the A350V mutation with precise medications.
In the current study, we analyzed the social behavior of A350V male mice in detail, using a battery of different social discrimination paradigms8,9 as well as by examining several types of social vocalization. We then assessed the ability of PF-4778574, a positive allosteric modulator (PAM) of AMPAR, which serves to increase AMPAR mediated synaptic transmission10,11 to rescue impairments in these behaviors exhibited by A350V mice.
Methods and materials
Study design
The initial objective of this study was to identify deficits in social behavior exhibited by A350V Iqsec2 male mice, as compared to their WT littermates. Following the accomplishment of this objective, we defined the assessment of the short- (1–2 days) and long-term (7–11 days) effect of PF-4778574 treatment, as compared to vehicle injection, on these deficits as another objective. The initial sample size for behavioral experiments was determined to be 20 ± 5, based upon power calculations made in a previous study using C57BL/6 J mice9. For behavioral deficits in Iqsec2 mice, we repeated the results in two independent cohorts, so overall sample size was double. The effect of vehicle treatment on sex preference (SxP) and emotional state preference (ESP) in both WT and A350V mice was examined at 1–2 days and 7–8 days following injection. The effect of PF-4778574 treatment on A350V mice was examined at 1–2 days (SxP + ESP) and again at 7–8 days (SxP) or at 11 days (ESP) following treatment. The results of PF-4778574 treatment were qualitatively replicated in two independent cohorts, with slightly smaller sample size due to animal availability. No animals were excluded from experiments and no outliers were defined. A350V and WT mice were always examined together on the same day in random order. All data collection was fully automated, with no involvement of an observer.
Animals
C57BL\6J subjects were commercially obtained (Envigo, Israel) naive males (8–12 weeks old, 3–5/cage). Social stimuli were in-house grown C57BL\6J juvenile (21–30 days old males (SP, SNP) or naïve adult male and female mice (SxP, ESP). Isolated stimuli for the ESP paradigm were each individually housed for 1–2 week.
A350V Iqsec2 mice generation, maintenance, and genotyping were previously described4. All Iqsec2 animal were bred and maintained in the animal facility of the faculty of medicine of the Technion Institute of Technology until age of 8–10 weeks and then transferred to the University of Haifa mice facility for at least one week before experiments. For all studies we compared the behavior of wild type (WT) and Iqsec2 mutant littermates generated from the mating of a WT male crossed with a female heterozygous for the A350V mutation. As the Iqsec2 gene is X-linked, this mating strategy results in half of the male mice being hemizygous for the WT Iqsec2 allele and half of the male mice being hemizygous for the A350V mutant Iqsec2 allele. All animals examined in this study were 2–4 months old males (WT and hemizygous littermates), except in the case of pup vocalizations, where both male and female (WT and heterozygous littermates) pups were examined (see below).
All animals were kept on a 12-h light\12-h dark cycle, light on at 1900 hours, with ad libitum access to food and water. Behavioral experiments took place during the dark phase under dim red light. All animal protocols and experiments were approved by the institutional animal care and use committees (IL0460416; IL1691117; IL1271118).
PF-4778574 preparation and injection
PF-4778574 [N- < (3 R,4 S)-3-[4-(5-cyano-2-thienyl)phenyl]tetrahydro-2H-pyran-4-yl>propane-2-sulfonamide] was obtained as a powder from Sigma (catalog #PZ0211) and solubilized (2.5 mg/ml) in dimethyl sulfoxide (sigma cat # D2650). Aliquots were stored at −20 °C and diluted immediately prior to injection in 5% dimethyl sulfoxide, 5% Kolliphor EL (Sigma cat #C5135), and 90% dH20 as previously described11. The PF-4778574 (0.30 mg/kg) and vehicle solutions (200 ul) were administered subcutaneously and subject mice were returned to their home cage until the day of the experiment.
Behavioral assays
All social discrimination tasks were conducted using our published automated experimental system8. Social preference (SP) and social novelty preference (SNP) tests were conducted on the same day, as previously described9.
The SxP test consisted of 15 min habituation to the arena with empty chambers, followed by exposing the subject for 5 min to both adult male and female social stimuli located in individual chambers at opposite corners of the arena.
The ESP test similarly consisted of a 15 min habituation, followed by exposing the subject for 5 min to both group-housed and socially isolated (1 wk) stimuli confined to individual chambers randomly located at opposite corners of the arena. Each isolated stimulus was used for two nonconsecutive tests. All stimuli used in all four tests (SP, SNP, SxP, and ESP) were C57BL\6J mice.
Vocalizations recording
Ultrasonic vocalizations were recorded using a condenser ultrasound microphone (Polaroid/CMPA, Avisoft) placed above the experimental arena and connected to an ultra-sound recording interface (UltraSoundGate 116Hme, Avisoft), which was plugged into a computer equipped with the recording software Avi-soft Recorder USG (sampling frequency: 250 kHz; FFT-length: 1024points; 16-bit format). Vocalizations were recorded during a 10 min free interaction between 2 and 4 months old male and female, following a 10 min of habituation to the arena. For the recording of pup calls, 4–5 day-old pups were moved to a netted metal cup above which the microphone was placed and vocalizations were recorded for a period of 2 min. In these experiments, pups were recorded indiscriminately and their genotype determined later using PCR, as previously described4. Pup vocalizations were analyzed across three groups: (1) WT males and females, (2) A350 V heterozygous females, and (3) A350 V hemizygous males.
Data analysis
Video data analysis was performed by our published custom-made TrackRodent software, as previously described8. Audio data was analyzed using our TrackUSF custom-made software as described in https://www.biorxiv.org/content/10.1101/575191v1.
Statistical analysis
SPSS v21.0 (IBM) was used for statistical analysis. Following a Kolmogorov–Smirnov test for checking the normal distribution of the dependent variables and Levene’s test for homogeneity, a 2-tailed paired t-test was used to compare between parameters within a group, and a 2-tailed independent t-test was used to compare a single parameter between distinct groups. Otherwise, the non-parametric Mann-Whitney U test was used to compare between parameters in the same group. For comparison between multiple groups and parameters a mixed analysis of variance (ANOVA) model was applied to the data. This model contains one random effect (ID), one within effect, one between effect, and the interaction between them. For comparison within a group using multiple parameters, a two-way repeated measures ANOVA model was applied to data. This model contains one random effect (ID), two within effects, one between effect and the interactions between them. All ANOVA tests were followed, if main effect or interaction were significant, by post hoc Student’s t test. In case of violation of the normal distribution assumption a Mann–Whitney test was used for comparing between two groups while a Kruskal Wallis Test was used for comparing between multiple groups. Significance was set at 0.05. The parameters and results of all statistical tests are supplied in Dataset 1, while all results of the various experiments are supplied in Dataset 2.
Results
Social behavior of C57BL\6J adult male mice
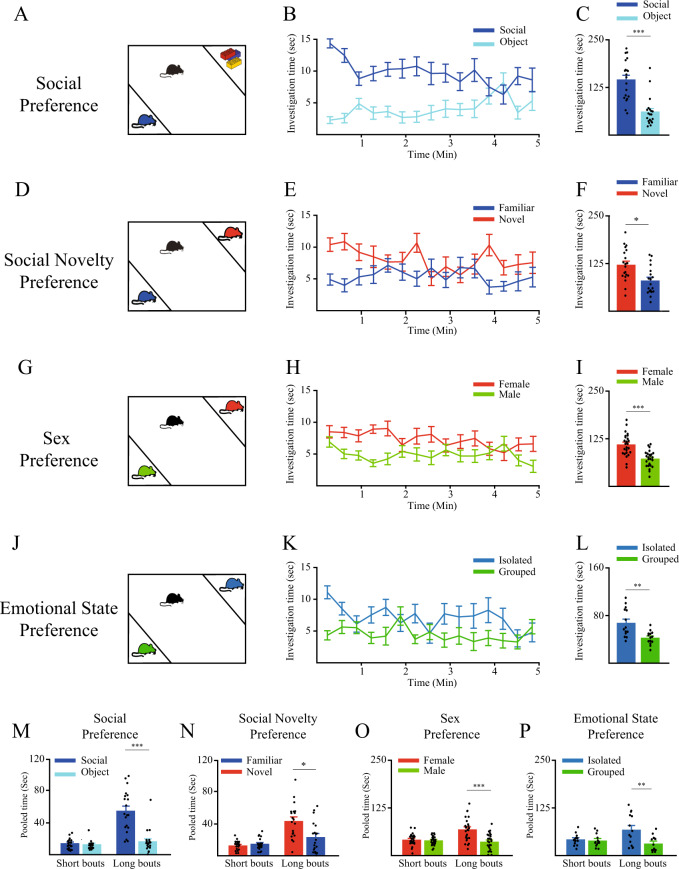
For analyzing the social behavior of mice subjects in this study, we have used our previously described experimental system which allows automated and detailed analysis of discrimination behavior8 across a battery of four social discrimination tests. First, we have used the social preference (SP) test to assess social motivation and the social novelty preference (SNP) test to assess social recognition9. We have also used the sex preference (SxP) test, in which the subject is exposed simultaneously to male and female conspecifics. Finally, we have used a novel test, termed the emotional state preference (ESP) test, in which the subject is exposed simultaneously to socially isolated and group-housed mice. Figure 1 shows the results of all these tests when conducted with C57BL/6J adult male mice. In the SP test (Fig. 1A) these mice showed a significant preference of the social stimulus over the object throughout the test (Fig. 1B, C; paired t-test - t19 = 4.475, p < 0.001). In the SNP test (Fig. 1D), the same subject mice showed a significant preference towards the novel mouse over the familiar one (Fig. 1E, F; paired t-test - t19 = −2.66, p < 0.05). In the SxP test (Fig. 1G), C57BL\6J mice spent significantly more time investigating a female over a male stimulus (Fig. 1H, I; paired t-test- t24 = 5.137, p < 0.001), indicating a preference for mice of the opposite sex. As for the ESP test (Fig. 1J), C57BL\6J subjects showed a preference to investigate the socially-isolated mouse over the group-housed mouse (Fig. 1K, L; paired t-test - t13 = 3.03, p < 0.01), suggesting a tendency to socialize more with mice which are emotionally manipulated12,13. As previously reported by us9, in all tasks the subject’s preference was expressed mainly by a strong bias in long (>6 s) investigation bouts, while we observed no preference between the stimuli when short (≤6 s) bouts were considered (Fig. 1M–P).
Fig. 1. Social discrimination tests conducted with adult C57BL\6J male mice.
A Schematic depiction of the social preference (SP) test. B Mean investigation time measured separately for each stimulus (20-s bins) across the SP test (n = 20). C Mean investigation time summed separately for each stimulus throughout the SP test. D–F As in A–C, respectively, for the social novelty preference (SNP; n = 20) test. G–I As in A–C, respectively, for the sex preference (SxP; n = 25) test. J–L As in A–C, respectively, for the emotional state preference (ESP; n = 14) test. (M–P) Mean investigation time for each of the stimuli, pooled separately for short (<7 s) and long (≥7 s) investigation bouts, for the SP (M), SxP (O), and ESP (P) tests shown above (A–L). Note that the preference for a specific stimulus is reflected only by the long bouts in all of these tests. Post hoc paired t-tests following main effect in mixed-model ANOVA test; M- short bouts: t19 = −0.576, p = 0.571, long bouts: t19 = −4.64, p < 0.001; N- short bouts: t19 = −0.93, p = 0.364, long bouts: t19 = 2.78, p < 0.05; O-short bouts: t24 = 0.973, p = 0.34, long bouts: t24 = 4.825, p < 0.001; P-short bouts: t13 = 0.708, p = 0.492, long bouts: t13 = 3.167, p < 0.01. *p < 0.05, **p < 0.01, ***p < 0.001, paired t-test following the main effect in ANOVA test. All error bars represent SEM.
A350V Iqsec2 male mice exhibit specific deficits in social behavior
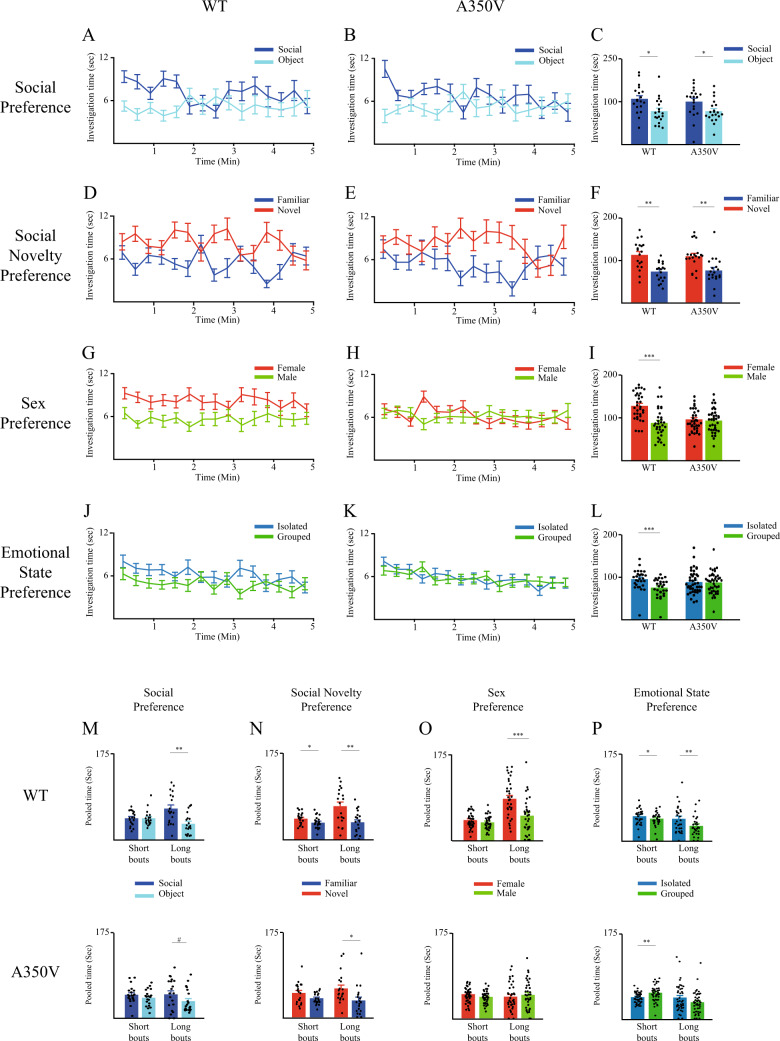
Next, we assessed the behavior of wild-type (WT) and A350V male littermates in the same battery of tests as described above for C57BL/6J mice. We found that both genotypes showed intact social preference in the SP test, with significantly greater investigation time for the social stimulus over the object (Fig. 2A–C, mixed-model ANOVA - within stimulus: F(1,37) = 10.06, p < 0.01; between genotypes: F(1,37) = 0.259, p = 0.614; stimulus × genotype: F(1,37) = 0.142, p = 0.709; post hoc paired t-test - WT: t18 = 2.178, p < 0.05; A350V:t19 = 2.357, p < 0.05). There was also no difference between the two genotypes in the amount of time spent investigating each of the stimuli (t-test - social: t36.9 = 0.557, p = 0.581; object: t37 = −0.007, p = 0.994), suggesting that the A350V Iqsec2 mutation did not affect the motivation of the animals for social interactions. In the SNP test, both genotypes showed a similar preference for investigating the novel stimulus as observed in C57BL\6J mice (Fig. 2D–F; mixed-model ANOVA - within stimulus: F(1,37) = 23.3, p < 0.001; between genotypes: F(1,37) = 0.002, p = 0.966, stimulus × genotype: F(1,37) = 0.106, p = 0.746; post hoc paired t-test - WT: t18 = −3.721, p < 0.01; A350V: t19 = −3.13 p < 0.01). Here too there was no difference between the WT and A350V littermates in the amount of time spent investigating each of the stimuli (t-test - Familiar: t37 = −0.274, p = 0.785, Novel: t37 = 0.275, p = 0.785).
Fig. 2. Deficits in social behavior of A350V Iqsec2 mice.
A, B Mean investigation time measured separately for each stimulus (20-s bins) across the SP test for WT (A, n = 19) and A350V (B, n = 20) mice. C Mean investigation time summed separately for each stimulus throughout the SP test for WT (left) and A350V (right) subjects. D–F Same in A–C, for the SNP test (WT - n = 19, A350V – n = 20). (G–I) Same in A-C, for the SxP test (WT - n = 33, A350V – n = 36). J–L Same in A-C, for the ESP test (WT - n = 28, A350V – n = 43). (M-P) Mean investigation time of WT (above) and A350V (below) mice for each of the stimuli, pooled separately for short (<7 s) and long (≥7 s) investigation bouts, for the SP (M), SNP (N), SxP (O), and ESP (P) tests shown above (A–L). Note that A350V mice showed an atypical significant preference for the group-housed stimulus when short bouts were considered. Post hoc paired t-tests following the main effect in mixed model ANOVA test; M- WT: short bouts: t18 = −0.159, p = 0.876, long bouts: t18 = 3.109, p < 0.01; A350V: short bouts: t19 = 1.277, p = 0.217, long bouts: t19 = 6.7, p < 0.001; N- WT: short bouts: t18 = −2.286, p < 0.05, long bouts: t18 = −2.897, p < 0.01; A350V: short bouts: t19 = −1.954, p = 0.066, long bouts: t19 = −2.4, p < 0.05; O- WT: short bouts: t32 = 1.157, p = 0.256, long bouts: t32 = 3.65, p < 0.001; A350V: short bouts: t35 = −0.278, p = 0.776, long bouts: t35 = −0.243, p = 0.809; P- WT: short bouts: t27 = 2.11, p < 0.05, long bouts: t27 = 3.015, p < 0.01; A350V: short bouts: t42 = −3.23, p < 0.01, long bouts: t42 = 1.76, p = 0.086. *p < 0.05, **p < 0.01, ***p < 0.001, paired t-test following the main effect in ANOVA test. All error bars represent SEM.
In contrast, we found a significant deficit displayed by A350V mice compared to WT littermates in the SxP test. While WT male mice, similarly to C57BL\6 J male mice, showed a clear preference for females, A350V male mice showed no preference for either sex (Fig. 2G–I; mixed-model ANOVA—stimulus × genotype: F(1,67) = 11.18, p < 0.001; post hoc paired t-test—WT: t33 = 3.895, p < 0.001; A350V: t35 = −.416, p = 0.68). There was no difference between the two genotypes when the total investigation time of both stimuli was considered (Mann-Whitney test—U = −0.207, p = 0.836), indicating that the deficit in sex preference behavior observed in A350V mice is not due to an overall decrease in social motivation. The difference in sex preference behavior between WT and A350V littermates was consistent across two cohorts of subjects (Fig. S1A–F). It should be noted that A350V males crossed with WT females generated on average 3.6 ± 3.1 litters (mean ± SEM; n = 9 males) as compared to WT males crossed with WT females which generated on average 4.8 ± 0.5 litters (mean ± SEM; n = 19 males). Thus, there does not seem to be any significant difference in the sexual activity of the A350V male mice as compared to the WT male mice (U = 63.5, p = 0.365, Mann–Whitney test).
A similar difference between WT and A350V littermates was observed in the ESP test. While WT mice showed a significant preference for investigating the isolated stimulus, similar to C57BL\6J mice, A350V mice showed no preference for either stimulus (Fig. 2J–L; mixed-model ANOVA - stimulus × genotype: F(1,69) = 4.329, p < 0.05; post hoc paired t-test - WT: t27 = 4.904, p < 0.001; MT: t42 = 0.171, p = 0.865). Here too, there was no difference between WT and A350V mice in the total investigation time of both stimuli (Mann-Whitney test - U = −0.353, p = 0.724). This difference in ESP between WT and A350V mice was consistent across two cohorts of subjects (Fig. S1G–L). Interestingly, when analyzing the data separately for short and long bouts (Fig. 2M–P) we found that A350V mice showed an atypical significant preference for the grouped housed stimulus (Fig. 2P-lower; 2-way ANOVA- stimulus × bout duration: F(1,42) = 12.014, p < 0.001; post hoc paired t-test- short bouts: t42 = −3.23, p < 0.01, long bouts- t24 = 1.76, p = 0.086).
We conclude that while A350V Iqsec2 mice behave the same as their WT littermates in the SP and SNP tests, they exhibit specific deficits in the SxP and ESP tests.
A350V Iqsec2 male mice exhibit a specific deficit in mating calls
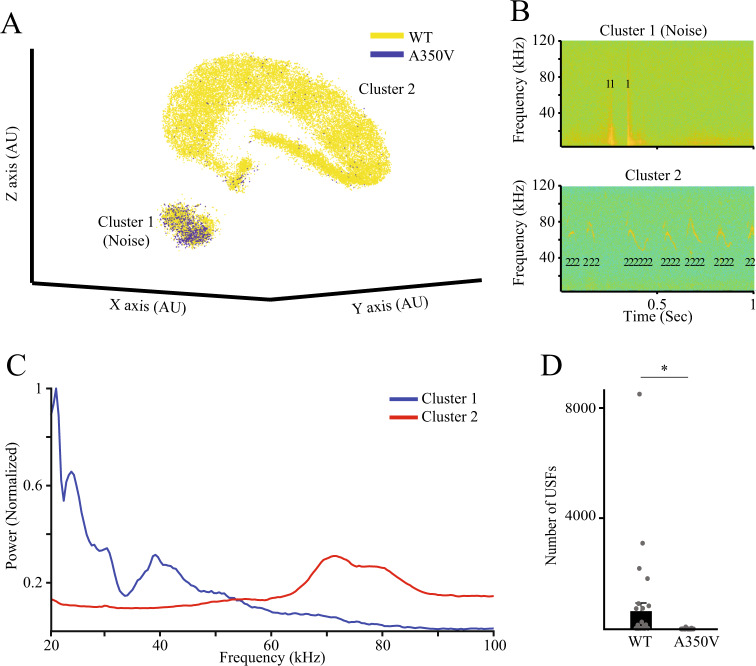
Male mice are known to emit ultrasonic vocalizations (USVs) when introduced to female mice (mating calls), but not to male mice14. We recorded mating calls of WT and A350V littermates and analyzed them using our novel analysis system (https://www.biorxiv.org/content/10.1101/575191v1) which enables separation of ultrasonic audio fragments (USFs) from noise signals and their clustering using a three-dimensional t-SNE analysis. We found that while a large number of WT animals emitted a reasonable amount of ultrasonic vocalizations, none of their A350V littermates exhibited such behavior (Fig. 3A–C) and this difference was statistically significant (Fig. 3D; Mann–Whitney test- U = 399, p < 0.05). In contrast, there was no apparent difference in the noise generated by the WT and A350V littermates (Fig. 3A), suggesting no distinction in viability and movement between the two groups. In order to demonstrate that these results were reproducible, we conducted a similar experiment with a new cohort of animals in a different laboratory by distinct research staff and obtained similar results (Fig. S2; Mann–Whitney test- U = 39, p < 0.05). In order to verify that A350V mice are capable of emitting ultrasonic vocalizations, we recorded pup separation calls from WT and MT littermates and found no significant difference between them (Fig. S3; Kruskal Wallis test- chi = 0.469, p = 0.791). Altogether, these results suggest a specific deficit exhibited by A350V Iqsec2 male mice in the emission of mating calls during interaction with a female mouse.
Fig. 3. A350V Iqsec2 male mice do not emit mating calls.
A 3D t-SNE analysis of all ultrasonic fragments emitted by WT (yellow; n = 31) and A350V (blue; n = 36) adult male mice during an encounter with a female C57BL/6J mouse. Note that while cluster 1 (noise) seem to contain similar number of fragments from both genotypes, cluster 2 (vocalizations) contains almost only fragments from WT mice. B Examples of USFs, denoted by their cluster numbers, superimposed on the spectrograms of their respective audio signals. Note that USFs of cluster 1 (upper example) represent non-vocal (noise) signals while USFs of cluster 2 (lower example) represent genuine vocalizations. C Power Spectral Density (PSD) analysis of all USFs recorded from all animals, calculated separately for each cluster. Note that in contrast to the PSD profile of cluster 1 which shows a wide range of frequencies, mainly at the lower range, the profile of cluster 2 shows a clear peak at the range of 60–90 kHz. D Comparison of the number of USFs from cluster 2 between the two genotypes. Note that A350V mice emitted significantly lower number of USFs as compared to WT animals (Mann–Whitney U test. U = 399.000, *p < 0.05). All error bars represent SEM.
Rescuing social behavior deficits with PF-4778574
We previously revealed a specific deficit in surface expression of the GluA2 AMPAR subunit in the hippocampus of A350V Iqsec2 mice, which was accompanied by a reduction in glutamatergic synaptic activity in this region4. Since the AMPAR modulator PF-4778574 was able to rescue social behavioral deficits in Cntnap2-knockout mice, which also display depressed glutamatergic transmission15, we reasoned that enhancing AMPAR activity with PF-4778574 might alleviate the behavioral impairments exhibited by A350V mice. To examine this hypothesis, we assessed the behavior of A350V mice following vehicle administration as well as 1 day and 1–2 weeks following PF-4778574 administration.
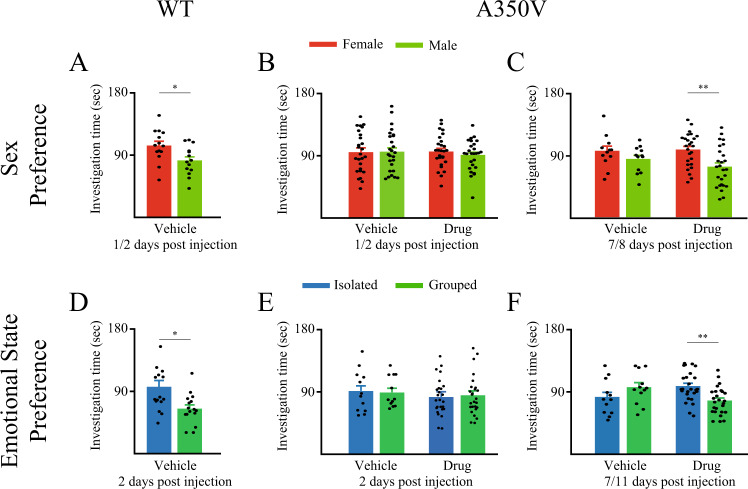
In the SxP test, WT mice showed intact sex preference 1 or 2 days following vehicle injection (Fig. 4A; t13 = 2.163, p < 0.05, paired t-test). In contrast, no such preference was observed among either vehicle or drug injected A350V mice 1 or 2 days following vehicle injection (Fig. 4B; mixed-model ANOVA - within stimulus: F(1,50) = 0.051, p = 0.821; between conditions: F(1,50) = 0.084, p = 0.773, stimulus × condition: F(1,50) = 0.353, p = 0.550). Nevertheless, 7 or 8 days after treatment drug injected A350V mice exhibited normal sex preference, in contrast to vehicle-injected animals (Fig. 4C; mixed-model ANOVA - within stimulus: F(1,36) = 8.382, p = 0.006; between conditions: F(1,36) = 0.471, p = 0.497, stimulus × condition: F(1,36) = 1.282, p = 0.265; post hoc paired t-test: vehicle: t11 = 1.974, p = 0.074; drug: t25 = 3.129, p < 0.01).
Fig. 4. PF-4778574 rescues sex social behavioral deficits in A350V Iqsec2 mice.
A Mean investigation time summed separately for the Female and Male stimuli throughout the SxP test, conducted by WT mice 2 days after vehicle injection. B Mean investigation time summed separately for the Female and Male stimuli throughout the SxP test conducted by A350V mice 1–2 days after either vehicle (left, n = 26) or PF-4778574 (right, n = 26) injection. C As in B, for the SxP test conducted by A350V mice 7–8 days after either vehicle (left, n = 12) or PF-4778574 (right, n = 26) injection. D Mean investigation time summed separately for the Isolated and Grouped stimuli in the ESP test conducted by WT mice 2 days after vehicle injection. E Mean investigation time summed separately for the Isolated and Grouped stimuli in the ESP test conducted by A350V mice 1–2 days after either vehicle (left, n = 12) or PF-4778574 (right, n = 26) injection. F As in E, for the ESP test conducted by A350V mice 7–11 days after either vehicle (left, n = 12) or PF-4778574 (right, n = 26) injection. *p < 0.05, **p < 0.01, paired t-test following the main effect in ANOVA test. All error bars represent SEM.
A similar benefit of PF-4778574 in A350V mice was obtained in the ESP test. WT mice showed clear preference towards the isolated social stimulus 1 or 2 days following vehicle injection (Fig. 4D; t15 = 2.65, p < 0.05, paired t-test). In contrast, neither vehicle nor drug injected A350V mice showed preference between the two stimuli when tested 1 or 2 days following treatment (Fig. 4E; mixed-model ANOVA - within stimulus: F(1,36) = 0.01, p = 0.922; between conditions: F(1,36) = 1.582, p = 0.217; stimulus × condition: F(1,36) = 0.04, p = 0.842). However, when tested 7 or 11 days after treatment, drug injected A350V mice exhibited a significant preference for the isolated stimulus, in contrast to vehicle injected A350V mice (Fig. 4F; mixed-model ANOVA - stimulus × condition: F(1,36) = 6.813, p = 0.013; post hoc paired t-test - vehicle: t11 = −1.28, p = 0.227, drug: t25 = 2.766, p < 0.01).
To gain further support for the dynamics of the effect of the drug, we analyzed the distance traveled by the mice during each test. While no difference was found between the vehicle and the drug 1–2 days after the injection, a significant reduction in the traveled distance of drug-injected animals, as compared to vehicle, was observed at 7–11 days for both tests (Fig. S4; for SxP: 2 way ANOVA - between treatments: F(1,87) = 2.792, p = 0.098; between times: F(1,87) = 6.524, p < 0.05, treatment × time: F(1,87) = 6.524, p < 0.05; post hoc independent t-test: 1–2 days: t51 = −0.629, p = 1.0; 7–8 days: t14.79 = 2.916, p < 0.05; for ESP: 2 way ANOVA - between treatments: F(1,72) = 15.869, p < 0.001; between times: F(1,72) = 1.504, p = 0.224, treatment × time: F(1,72) = 1.504, p = 0.224; post hoc independent t-test: 1–2 days: t36 = 1.642, p = 0.218; 7–11 days: t12.627 = 3.603, p < 0.01).
Finally, we examined the effect of PF-4778574 administration on the lack of mating calls observed in A350V mice. We found no improvement in social vocalizations 1 hour and 7 days following drug administration (Fig. S5).
Discussion
In this study, we have employed a battery of social behavioral tests to characterize the deficits in social behavior exhibited by mice with a A350V mutation in the Iqsec2 gene, a mutation that is associated with epilepsy, ID, and ASD in humans. We also demonstrated that a single administration of PF-4778574, a PAM modulator of the AMPAR, can rescue some of these deficits.
Numerous missense and nonsense mutations have been identified in the X-linked IQSEC2 gene which has been associated with ID, ASD, and drug-resistant epilepsy1. A recent review summarizing 136 subjects and 70 different mutation types revealed the prevalence of ASD to be 25% in males and 30% in females1. No human female homozygous for IQSEC2 mutation was described so far. Specific genotype-phenotype correlations have not been identified to date. Ultimately for the purposes of precision medicine treatment of IQSEC2 mutations it will be necessary to understand if the molecular pathophysiology of these mutations differs. The majority of human mutations are nonsense mutations and several recent studies in which IQSEC2 expression was manipulated in neurons6,16 or the gene knocked out in mice models17,18 may be informative in understanding the molecular pathophysiology and phenotypes of nonsense mutations as compared to the A350V IQSEC2 missense mutation4. In vitro studies in neurons in which the catalytic activity of the Sec7 domain was inhibited or constitutively activated suggested that inhibition of the Sec7 activity of IQSEC2 would lead to an impairment in Arf6 mediated AMPAR recycling and consequently increased surface AMPAR expression6,16 while mutations which appeared to increase Arf6 activation would result in downregulation of AMPAR expression as observed in A350V Iqsec2 mice4. The phenotype of mouse knockout models of Iqsec217,18 appears to have several notable differences from the A350V Iqsec2 mutation phenotype as investigated in this and prior studies. First, regarding epilepsy, both knockout models have reported seizures occurring at approximately day 90 while with the A350V mutation seizures are limited to days 14–215. Second, both knockout models have reported an increase in anxiety-related behavior which we have not observed in A350V mice4. Differences between the knockout model and the A350V model are not surprising given that the A350V mutation appears to enhance the Sec7 activity of IQSEC2. Demonstration of such differences between loss of function and gain of function in IQSEC2 implies that treatments that may be beneficial for one mutation of IQSEC2 may not be effective (or even harmful) for a different mutation in this gene. Indeed such a dichotomy has been demonstrated for another gene controlling AMPAR regulation, in which a knockout mutation in the Thorase gene resulted in an upregulation of surface AMPAR expression while a gain of function mutation in the same gene resulted in a downregulation in surface AMPAR19–21.
In a previous study using the same mouse model used here, we demonstrated normal sociability and social novelty preference in the three-chamber test4. Here, we employed a novel experimental system designed for directly monitoring social investigation behavior, to examine the behavior of these mice in a battery of four social discrimination tests. In agreement with our previous paper, we found normal behavior of A350V mice in the SP test which assesses social motivation, as well as in the SNP test which assesses social cognition. We did find, however, that A350V mice did not discriminate between the stimuli in both the SxP and ESP tests. These results suggest an impairment in the preference of A350V mice towards specific social stimuli, such as opposite sex and emotionally manipulated stimuli, which may be caused by impaired ability to recognize such stimuli or by the reduced motivation to interact with them. Future studies will be needed to reveal which one of these possibilities is the right one.
Despite its apparent association with sexual behavior, recent works suggest that the preference exhibited by male and female mice to opposite-sex as compared to same-sex stimuli is actually reflecting emotional behavior. This type of socio-emotional behavior, examined by the SxP test, was found to be dependent on brain areas associated with emotional behavior, such as the prefrontal cortex (PFC)22, nucleus accumbens23 and medial amygdala24, as well as on neuromodulators associated with emotion regulation such as serotonin and oxytocin25. Here we demonstrated, for the first time, that A350V male mice exhibit a clear deficit in the SxP test. Interestingly, several independent recent works reported atypical sexual orientation and attraction to members of the opposite sex, in association with ASD26–28.
The ESP test is a novel social behavioral test, which is similar in principle to the affective state discrimination test recently described12. As this test examines whether the subject recognizes the emotional state of another individual, it seems to be highly relevant to ASD symptoms which involve impaired theory of mind29,30. This behavior was found to be specifically impaired in Dys-1+/− mice, a clinically relevant mouse model of cognitive and psychiatric liability12. Interestingly, similar to A350V Iqsec2 mice, Dys-1+/− mice exhibit intact social preference and social novelty preference in the three-chamber test12.
The results showing that both sex preference and emotional state preference behaviors depend on activity of PFC neurons13,22 and on Oxt release in the amygdala12,24 suggest that both share common brain mechanisms. Future studies should examine if similar mechanisms are compromised in Iqsec2 A350V mice. Nevertheless, since Iqsec2 A350V mice which have a strong construct validity as they carry the same mutation linked to ASD in a human patient, show deficits specifically in the SxP and ESP tests, these tests may be more sensitive to ASD-associated impairments than the sociability and social novelty preference which are commonly used in the field. Accordingly, we observed similar results with Cntnap2-knockout mice, a well-established ASD model (unpublished data).
Our observation that A350V Iqsec2 mice had a dramatic reduction in mating calls during male-female interactions, as opposed to their WT littermates, is in agreement with a previous study characterizing Iqsec2-knockout mice18. Such a strong deficit in mating calls was previously described for several genetic mouse models of ASD31,32. Nevertheless, PF-4778574 administration did not have any effect on this impairment, despite the rescue of sex preference behavior. This suggests that the lack of mating calls in A350V Iqsec2 male mice is not due to their lack of motivation for social interactions with females, demonstrated by their impaired SxP behavior, but rather is likely due to a deficit in their social communication.
Benefits from PF-4778574 in the SxP and ESP tests were not observed immediately (1–2 days post administration) but rather 7–11 days following administration. At this point, our results differ from those previously described for Cntnap2-knockout mice, where the behavioral effects were observed already 30 min post drug administration15. Studies assessing the pharmacokinetics of PF-4778574 in mice11 have shown that the drug has a very short life in both plasma and brain (less than one hour) similar to other AMPAR PAMs that have been described33. This short half-life corresponds with the acute electrophysiological effects of AMPAR PAMs in binding to AMPAR receptors and preventing their rapid deactivation33,34. However, AMPAR PAMs also have delayed downstream effects consequent to the AMPAR activation-dependent stimulation of a specific genetic program involving new transcription and protein synthesis35. For example, PF-4778574 and other functionally similar AMPAR PAMs stimulate the production and release of neurotrophins (i.e. BDNF), promote the proliferation of neuronal progenitor cells, long-term potentiation, and dendritic remodeling for several1–10 days after treatment34–37. Notably, a recent study reported that a single administration of PF-4778574 in mice produced prolonged antidepressant actions that lasted for 7 days38, a time frame similar to what we have observed regarding behavioral changes in A350V mice following a single treatment with PF-4778574.
More studies are needed in order to reveal what is the mechanism by which PF-4778574 induces its delayed and long-lasting effect. Moreover, our study was limited to a single protocol of PF-4778574 application, with the drug’s dose and route of administration selected based on previous studies demonstrating its efficacy in rescuing AMPAR mediated memory impairments in wild type animals treated with ketamine11 and social-behavioral defects in Cntnap2-knockout mice15. Thus, future studies are needed for the development of an optimal administration protocol that will allow maximal benefits from this drug or similar modulators of AMPAR activity.
To conclude, we have utilized a battery of social discrimination tasks to analyze modified social behavior in A350V Iqsec2 mutant mice. We observed specific deficits in the social behavior of these mice, which suggest that these mice are impaired in socio-emotional behavior and social communication. Moreover, we found that a single administration of the AMPAR PAM PF-4778574 rescued the behavioral deficits, while having no effect on the deficits in social vocalization exhibited by A350V male mice. Decreased AMPAR mediated synaptic transmission appears to represent a convergent pathway for an increasing number of genetic syndromes, including Fragile X39, Rett40, Cdkl541, Rab39B42, Shank43, tuberous sclerosis44, Atad119–21, and Cntnap215 as well as in non-genetic syndromes such as neonatal hypoxia45, suggesting that it may be possible to extrapolate treatments from one of these disorders to the others. The findings of this study may therefore be broadly relevant for a large number of neurodevelopmental disorders of different etiologies and not just for this one mutation in IQSEC2.
Supplementary information
Acknowledgements
This study was supported by The Human Frontier Science Program (HFSP grant RGP0019/2015 for SW), the Israel Science Foundation (ISF grants #1350/12, 1361/17 for S.W.), by a donation by the Milgrom family for SW and by the Ministry of Science, Technology and Space of Israel (Grant #3-12068 for S.W.).
Author contributions
Study conception and design—S.W., A.P.L.; Acquisition of data – R.J., N.L., Y.A., J.H.B., A.Z.; Analysis and interpretation of data—R.J., S.N.; Drafting of manuscript—S.W., A.P.L.
Code availability
All codes used for the current study are available from the corresponding author on reasonable request. The code used for the video analysis is publicly available at the following link: [https://github.com/shainetser/TrackRodent]. The code used for vocalization analysis is publicly available at the following link: [https://github.com/shainetser/TrackUSF].
Conflict of interest
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Renad Jabarin, Nina Levy
Contributor Information
Andrew P. Levy, Email: alevy@technion.ac.il
Shlomo Wagner, Email: shlomow@research.haifa.ac.il.
Supplementary information
The online version contains supplementary material available at 10.1038/s41398-021-01347-1.
References
- 1.Shoubridge C, Harvey RJ, Dudding-Byth T. IQSEC2 mutation update and review of the female-specific phenotype spectrum including intellectual disability and epilepsy. Hum. Mutat. 2019;40:5–24. doi: 10.1002/humu.23670. [DOI] [PubMed] [Google Scholar]
- 2.Shoubridge C, et al. Mutations in the guanine nucleotide exchange factor gene IQSEC2 cause nonsyndromic intellectual disability. Nat. Genet. 2010;42:486–488. doi: 10.1038/ng.588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zipper R, et al. Developmental progression of intellectual disability, autism, and epilepsy in a child with an IQSEC2 gene mutation. Clin. Case Rep. 2017;5:1639–1643. doi: 10.1002/ccr3.1139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rogers EJ, et al. An IQSEC2 mutation associated with intellectual disability and autism results in decreased surface AMPA receptors. Front Mol. Neurosci. 2019;12:43. doi: 10.3389/fnmol.2019.00043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Levy N. S., Umanah G. K. E., Rogers E. J., Jada R., Lache O., Levy A. P. IQSEC2-associated intellectual disability and autism. Int. J. Mol. Sci. 20, 3038 (2019). [DOI] [PMC free article] [PubMed]
- 6.Myers KR, et al. Arf6-GEF BRAG1 regulates JNK-mediated synaptic removal of GluA1-containing AMPA receptors: a new mechanism for nonsyndromic X-linked mental disorder. J. Neurosci. 2012;32:11716–11726. doi: 10.1523/JNEUROSCI.1942-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shoubridge C, Walikonis RS, Gecz J, Harvey RJ. Subtle functional defects in the Arf-specific guanine nucleotide exchange factor IQSEC2 cause non-syndromic X-linked intellectual disability. Small GTPases. 2010;1:98–103. doi: 10.4161/sgtp.1.2.13285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Netser S, Haskal S, Magalnik H, Bizer A, Wagner S. A system for tracking the dynamics of social preference behavior in small rodents. J. Vis. Exp. 2019;153:e60336. doi: 10.3791/60336. [DOI] [PubMed] [Google Scholar]
- 9.Netser S, Haskal S, Magalnik H, Wagner S. A novel system for tracking social preference dynamics in mice reveals sex- and strain-specific characteristics. Mol. Autism. 2017;8:53. doi: 10.1186/s13229-017-0169-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Partin KM. AMPA receptor potentiators: from drug design to cognitive enhancement. Curr. Opin. Pharmacol. 2015;20:46–53. doi: 10.1016/j.coph.2014.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shaffer CL, et al. Positive allosteric modulation of AMPA receptors from efficacy to toxicity: the interspecies exposure-response continuum of the novel potentiator PF-4778574. J. Pharm. Exp. Ther. 2013;347:212–224. doi: 10.1124/jpet.113.204735. [DOI] [PubMed] [Google Scholar]
- 12.Ferretti V. et al. Oxytocin signaling in the central amygdala modulates emotion discrimination in mice. Curr Biol. 29,1938–1953 e1936 (2019). [DOI] [PubMed]
- 13.Scheggia D, et al. Somatostatin interneurons in the prefrontal cortex control affective state discrimination in mice. Nat. Neurosci. 2020;23:47–60. doi: 10.1038/s41593-019-0551-8. [DOI] [PubMed] [Google Scholar]
- 14.Portfors CV. Types and functions of ultrasonic vocalizations in laboratory rats and mice. J. Am. Assoc. Lab Anim. Sci. 2007;46:28–34. [PubMed] [Google Scholar]
- 15.Kim JW, et al. Pharmacological modulation of AMPA receptor rescues social impairments in animal models of autism. Neuropsychopharmacology. 2019;44:314–323. doi: 10.1038/s41386-018-0098-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Brown JC, et al. Bidirectional regulation of synaptic transmission by BRAG1/IQSEC2 and its requirement in long-term depression. Nat. Commun. 2016;7:11080. doi: 10.1038/ncomms11080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jackson M. R. et al. Heterozygous loss of function of IQSEC2/Iqsec2 leads to increased activated Arf6 and severe neurocognitive seizure phenotype in females. Life Sci. Alliance. 2, e201900386 (2019). [DOI] [PMC free article] [PubMed]
- 18.Sah M, et al. Altered excitatory transmission onto hippocampal interneurons in the IQSEC2 mouse model of X-linked neurodevelopmental disease. Neurobiol. Dis. 2020;137:104758. doi: 10.1016/j.nbd.2020.104758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ahrens-Nicklas RC, et al. Precision therapy for a new disorder of AMPA receptor recycling due to mutations in ATAD1. Neurol. Genet. 2017;3:e130. doi: 10.1212/NXG.0000000000000130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Piard J, et al. A homozygous ATAD1 mutation impairs postsynaptic AMPA receptor trafficking and causes a lethal encephalopathy. Brain. 2018;141:651–661. doi: 10.1093/brain/awx377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Umanah G. K. E. et al. Thorase variants are associated with defects in glutamatergic neurotransmission that can be rescued by Perampanel. Sci. Transl. Med. 9 eaah4985 (2017). [DOI] [PMC free article] [PubMed]
- 22.Kingsbury L. et al. Cortical representations of conspecific sex shape social behavior. Neuron. 107, 941–953 e947 (2020). [DOI] [PMC free article] [PubMed]
- 23.Beny-Shefer Y, et al. Nucleus accumbens dopamine signaling regulates sexual preference for females in male mice. Cell Rep. 2017;21:3079–3088. doi: 10.1016/j.celrep.2017.11.062. [DOI] [PubMed] [Google Scholar]
- 24.Yao S., Bergan J., Lanjuin A., Dulac C. Oxytocin signaling in the medial amygdala is required for sex discrimination of social cues. Elife6, e31373 (2017). [DOI] [PMC free article] [PubMed]
- 25.Haskal de la Zerda S, Netser S, Magalnik H, Wagner S. Impaired sex preference, but not social and social novelty preferences, following systemic blockade of oxytocin receptors in adult male mice. Psychoneuroendocrinology. 2020;116:104676. doi: 10.1016/j.psyneuen.2020.104676. [DOI] [PubMed] [Google Scholar]
- 26.Dewinter J, De Graaf H, Begeer S. Sexual orientation, gender identity, and romantic relationships in adolescents and adults with autism spectrum disorder. J. Autism Dev. Disord. 2017;47:2927–2934. doi: 10.1007/s10803-017-3199-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.May T, Pang KC, Williams K. Brief report: sexual attraction and relationships in adolescents with autism. J. Autism Dev. Disord. 2017;47:1910–1916. doi: 10.1007/s10803-017-3092-6. [DOI] [PubMed] [Google Scholar]
- 28.Qualls LR, Hartmann K, Paulson JF. Broad autism phenotypic traits and the relationship to sexual orientation and sexual behavior. J. Autism Dev. Disord. 2018;48:3974–3983. doi: 10.1007/s10803-018-3556-3. [DOI] [PubMed] [Google Scholar]
- 29.Andreou, M. & Skrimpa, V. Theory of mind deficits and neurophysiological operations in autism spectrum disorders: a review. Brain Sci. 10, 393 (2020). [DOI] [PMC free article] [PubMed]
- 30.Tiede, G. M. & Walton, K. M. Social endophenotypes in autism spectrum disorder: a scoping review. Dev. Psychopathol. 1–29. 10.1017/S0954579420000577(2020). [DOI] [PubMed]
- 31.Won H, et al. Autistic-like social behaviour in Shank2-mutant mice improved by restoring NMDA receptor function. Nature. 2012;486:261–265. doi: 10.1038/nature11208. [DOI] [PubMed] [Google Scholar]
- 32.Yang M, et al. 16p11.2 deletion syndrome mice display sensory and ultrasonic vocalization deficits during social interactions. Autism Res. 2015;8:507–521. doi: 10.1002/aur.1465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bretin S, et al. Pharmacological characterisation of S 47445, a novel positive allosteric modulator of AMPA receptors. PLoS ONE. 2017;12:e0184429. doi: 10.1371/journal.pone.0184429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Giralt A, et al. The AMPA receptor positive allosteric modulator S 47445 rescues in vivo CA3-CA1 long-term potentiation and structural synaptic changes in old mice. Neuropharmacology. 2017;123:395–409. doi: 10.1016/j.neuropharm.2017.06.009. [DOI] [PubMed] [Google Scholar]
- 35.Calabrese F, et al. Upregulation of neurotrophins by S 47445, a novel positive allosteric modulator of AMPA receptors in aged rats. Pharm. Res. 2017;121:59–69. doi: 10.1016/j.phrs.2017.04.019. [DOI] [PubMed] [Google Scholar]
- 36.Mendez-David I. et al. S 47445 Produces antidepressant-and anxiolytic-like effects through neurogenesis dependent and independent mechanisms. Front Pharmacol. 8, 462 (2017). [DOI] [PMC free article] [PubMed]
- 37.Simmons DA, et al. Up-regulating BDNF with an ampakine rescues synaptic plasticity and memory in Huntington’s disease knockin mice. Proc. Natl Acad. Sci. USA. 2009;106:4906–4911. doi: 10.1073/pnas.0811228106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Shen M, et al. Positive allosteric modulation of AMPAR by PF-4778574 produced rapid onset antidepressant actions in mice. Cereb. Cortex. 2019;29:4438–4451. doi: 10.1093/cercor/bhy324. [DOI] [PubMed] [Google Scholar]
- 39.Danesi C, Keinanen K, Castren ML. Dysregulated Ca(2+)-permeable AMPA receptor signaling in neural progenitors modeling fragile X syndrome. Front Synaptic Neurosci. 2019;11:2. doi: 10.3389/fnsyn.2019.00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Li W, Xu X, Pozzo-Miller L. Excitatory synapses are stronger in the hippocampus of Rett syndrome mice due to altered synaptic trafficking of AMPA-type glutamate receptors. Proc. Natl Acad. Sci. USA. 2016;113:E1575–E1584. doi: 10.1073/pnas.1517244113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yennawar M, White RS, Jensen FE. AMPA receptor dysregulation and therapeutic interventions in a mouse model of CDKL5 deficiency disorder. J. Neurosci. 2019;39:4814–4828. doi: 10.1523/JNEUROSCI.2041-18.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mignogna M. L. et al. The intellectual disability protein RAB39B selectively regulates GluA2 trafficking to determine synaptic AMPAR composition. Nat. Commun.6, 6504 (2015). [DOI] [PMC free article] [PubMed]
- 43.Shi R. et al. Shank proteins differentially regulate synaptic transmission. Eneuro. 4, 0163-15 (2017). [DOI] [PMC free article] [PubMed]
- 44.Talos DM, Kwiatkowski DJ, Cordero K, Black PM, Jensen FE. Cell-specific alterations of glutamate receptor expression in tuberous sclerosis complex cortical tubers. Ann. Neurol. 2008;63:454–465. doi: 10.1002/ana.21342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lippman-Bell JJ, et al. AMPA receptor antagonist NBQX attenuates later-life epileptic seizures and autistic-like social deficits following neonatal seizures. Epilepsia. 2013;54:1922–1932. doi: 10.1111/epi.12378. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All codes used for the current study are available from the corresponding author on reasonable request. The code used for the video analysis is publicly available at the following link: [https://github.com/shainetser/TrackRodent]. The code used for vocalization analysis is publicly available at the following link: [https://github.com/shainetser/TrackUSF].