Objective
In December 2020, 2 lipid nanoparticle-formulated, nucleoside-modified messenger RNA–based vaccines received emergency use authorization by the US Food and Drug Administration, after their trials demonstrated 94% to 95% efficacy in preventing coronavirus disease 2019 (COVID-19).1 Although no lactating people were included in the vaccine trials, national organizations support vaccination of this population, suggesting potential infant protection by passive transfer of maternal antibodies.1 , 2 However, there are no published data to support this theoretical benefit. We sought to characterize breast milk levels of anti–severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) antibodies in lactating people undergoing COVID-19 vaccination.
Study Design
Participants were prospectively recruited during phase IA rollout of the COVID-19 vaccine at a tertiary care center, after institutional review board approval. Inclusion criteria included lactation and planned vaccination with the Pfizer-BioNTech (Pfizer, Inc, New York, NY)/BNT162b2 vaccine (BioNTech SE, Mainz, Germany). After obtaining informed consent, participants provided frozen breast milk samples at the following time points of vaccination: before, within the first 24 hours, and the following week. Samples were assessed for SARS-CoV-2 RNA by quantitative real-time polymerase chain reaction and antispike immunoglobulin (Ig) G and IgA by an enzyme-linked immunosorbent assay.
Results
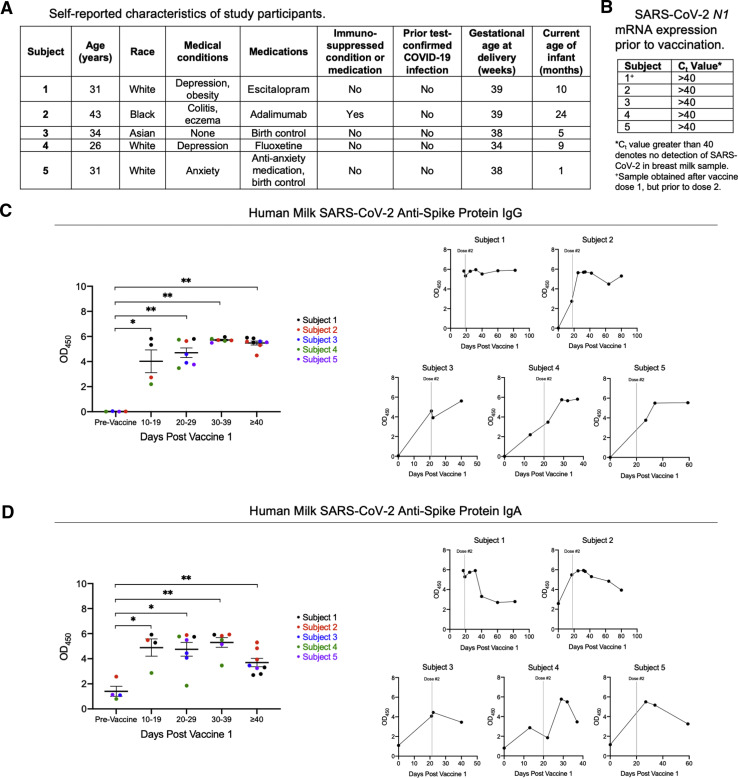
A total of 5 subjects and 29 human milk samples were included in the analysis. Subject characteristics are reported in Figure 1 , A. All prevaccine milk samples tested negative for SARS-CoV-2 RNA, as defined by the cycle threshold value of >40 for the N1 target (Figure 1, B). Antispike IgG and IgA levels were significantly elevated relative to the prevaccine baseline at all time points. Antispike protein IgG remained sustained at a significant elevation beginning at 20 days after the first dose compared with the prevaccine baseline (P=.0061), through the final milk sample (day 30–39 P=.0095, >40 days P=.0040; (Figure 1, C). Levels of antispike protein IgA were significantly elevated from baseline, starting 2 weeks after the first dose (P=.0286) through to the final sample (day 20–29 P=.0121, day 30–39 P=.0095, >40 days P=.0040); however, individual level data suggest a possible gradual decline in antispike IgA in human milk over time after the second dose (Figure 1, D).
Figure.
Breast milk levels of anti–SARS-CoV-2 antibodies after Pfizer-BioNTech/BNT162b2 vaccination
A total of 5 lactating women who received 2 doses of the Pfizer-BioNTech BNT162b2 vaccine were included in the analysis. A, Self-reported clinical data of the study subjects are shown, with Subject 2 identifying as immunocompromised; B, Prevaccine baseline milk samples were analyzed for SARS-CoV-2 RNA using the N1 target compared with RNAse P, with undetectable viral RNA defined as Ct>40. Antispike protein (C) IgG and (D) IgA antibody levels in human milk were analyzed at serial time points following the first and second vaccine doses. Delipidated human milk samples were diluted at a 1:1 ratio with sample diluent and tested in duplicate for IgG and IgA against SARS-CoV-2 full-length spike protein using ELISA Kits from Cell Signaling Technology (Catalog #20154C for IgG and Catalog #58873C for IgA). Antibody signal detections were analyzed by spectrophotometric absorbance at 450 nm. Gray vertical lines represent the timing of the administration of the second dose. Of note, the first sample from Subject 1 was obtained 17 days after the first vaccine. Data are displayed as mean±SEM and were analyzed using the Mann-Whitney U test. The single asterisk represents P<.05; the double asterisk represents P<.01.
Ct, cycle threshold; ELISA, enzyme-linked immunosorbent assay; Ig, immunoglobulin; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2; SEM, standard error of the mean.
Kelly. Severe acute respiratory syndrome coronavirus 2 antibodies in breast milk after vaccination. Am J Obstet Gynecol 2021.
Conclusion
We characterize longitudinal breast milk levels of antispike IgG/A following Pfizer-BioNTech/BNT162b2 vaccination, demonstrating sustained elevation of IgG/IgA levels. This response is similar to previous studies on maternal vaccination, which have shown high levels of breast milk IgA/G production for up to 6 months after vaccination for influenza and pertussis.3 , 4 A concurrent decrease in infant respiratory illness rates suggest that maternal vaccination confers protection against infection in breastfed infants.3 Thus, the Pfizer-BioNTech/BNT162b2 vaccination may also confer protection against COVID-19 to breastfed infants as well. Although vaccination remains one of the most crucial interventions to control infection spread, vaccine hesitancy remains a barrier to widespread uptake.5 Our study is limited by a small number of participants, but we report data that suggest a potential immune benefit to infants of lactating people up to 80 days after COVID-19 vaccination. Further studies are needed to characterize the length of antibody production in breast milk and the effect on infant infection rates after maternal COVID-19 vaccination.
Acknowledgments
The authors would like to thank Chanill Henley for her assistance in the completion of this project.
Footnotes
M.G. has received sponsored research agreement funding from Astarte Medical Partners and Takeda Pharmaceutical Company Limited. She also participated in a neonatal microbiome advisory board for Abbott Laboratories. None of these sources had any role in this study. The remaining authors report no conflict of interest.
This publication in part was supported by a grant to E.B.C. by the Foundation for Barnes-Jewish Hospital and their generous donors; and the Institute of Clinical and Translational Sciences, Washington University in St. Louis, which is, in part, supported by the Clinical and Translational Science Award under grant number UL1TR002345 from the National Institutes of Health (NIH)/National Center for Advancing Translational Sciences. J.C.K. was supported by grant number 00033770 from the PEW Charitable Trusts Community Opioid Response and Evaluation (CORE). L.S.N. was supported by grant number 5T32HD043010 from the NIH and an American Academy of Pediatrics Marshall Klaus Award. M.G. was supported by grant number R01DK118568 from the NIH, the St. Louis Children’s Hospital Foundation, the Children’s Discovery Institute of Washington University and St. Louis Children’s Hospital, and the Department of Pediatrics at Washington University School of Medicine in St. Louis.
References
- 1.Centers for Disease Control and Prevention Interim clinical considerations for use of COVID-19 vaccines currently authorized in the United States. 2021. https://www.cdc.gov/vaccines/covid-19/info-by-product/clinical-considerations.html Available at:
- 2.American College of Obstetricians and Gynecologists Vaccinating pregnant and lactating patients against COVID-19. 2020. https://www.acog.org/en/Clinical/Clinical%20Guidance/Practice%20Advisory/Articles/2020/12/Vaccinating%20Pregnant%20and%20Lactating%20Patients%20Against%20COVID%2019 Available at:
- 3.Schlaudecker E.P., Steinhoff M.C., Omer S.B., et al. IgA and neutralizing antibodies to influenza A virus in human milk: a randomized trial of antenatal influenza immunization. PLoS One. 2013;8:e70867. doi: 10.1371/journal.pone.0070867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Demers-Mathieu V., Huston R.K., Markell A.M., McCulley E.A., Martin R.L., Dallas D.C. Impact of pertussis-specific IgA, IgM, and IgG antibodies in mother’s own breast milk and donor breast milk during preterm infant digestion. Pediatr Res. 2021;89:1136–1143. doi: 10.1038/s41390-020-1031-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.McAteer J., Yildirim I., Chahroudi A. The VACCINES act: deciphering vaccine hesitancy in the time of COVID-19. Clin Infect Dis. 2020;71:703–705. doi: 10.1093/cid/ciaa433. [DOI] [PMC free article] [PubMed] [Google Scholar]