Abstract
Cancer stem cells (CSCs) are a small proportion of cancer cells with high tumorigenic activity, self-renewal ability, and multilineage differentiation potential. Standard anti-tumor therapies including conventional chemotherapy, radiation therapy, and molecularly targeted therapies are not effective against CSCs, and often lead to enrichment of CSCs that can result in tumor relapse. Therefore, it is hypothesized that targeting CSCs is key to increasing the efficacy of cancer therapies. In this review, CSC properties including CSC markers, their role in tumor growth, invasiveness, metastasis and drug resistance as well as CSC microenvironment are discussed. Further, CSC-targeted strategies including the use of targeted drug delivery systems are examined.
Keywords: Cancer stem cells (CSCs), Drug delivery system, Anti-tumor therapy, Drug-resistance, Cancer biology
Graphical Abstract
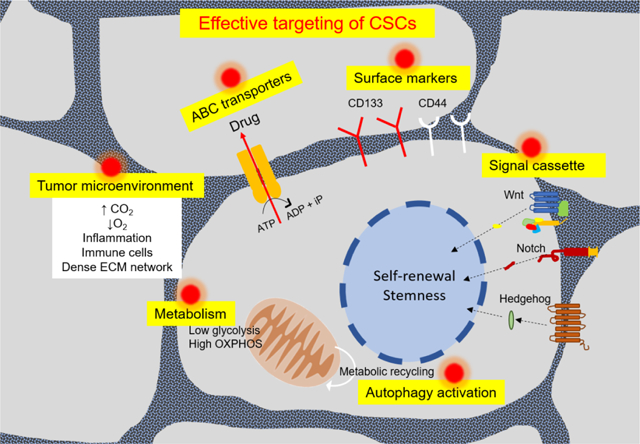
1. Introduction
Stem cells are specialized cells that possess the ability to differentiate into varied cell types. In a tumor, all tumor cells are not alike; some tumor cells possess greater differentiation capabilities than others. The notion that tumors have stem cells — cancer stem cells (CSCs) — has stirred a lot of scientific interest and greatly changed how we think about treating cancer. These self-sustaining stem cells possess characteristic self-renewal ability, enabling them to give rise to heterogenous tumor lineages. Since these cells are capable of forming an entire tumor, they are also called tumor initiating cells (TICs). The TICs differentiate into progenitor cells and further into other kinds of cells found in the tumor. Two models have been used to describe the origin of CSCs. The stochastic model (Figure 1) envisages that every tumor cell has a low but equal chance of limitless proliferation, behaving as a stem cell. As naturally acquired cell mutations are selected over time, the most aggressive tumor cell populations are selected. These populations are responsible for tumor progression and maintenance. The hierarchal model, on the other hand, proposes that only a distinct subset of tumor cells possess the potential of limitless proliferation. These cells possess the ability of self-renewal and form the top of the tumor pyramid [1]. They have the capacity for asymmetric cell division, that is, they can divide into daughter CSCs or into progenitor cells. The rapidly dividing progenitor cells form the middle of the pyramid, while the differentiated, heterogenic, non-tumorigenic cancer cells that make the bulk of the tumor are at the bottom of the pyramid (Figure 1a) [2].
Fig. 1.
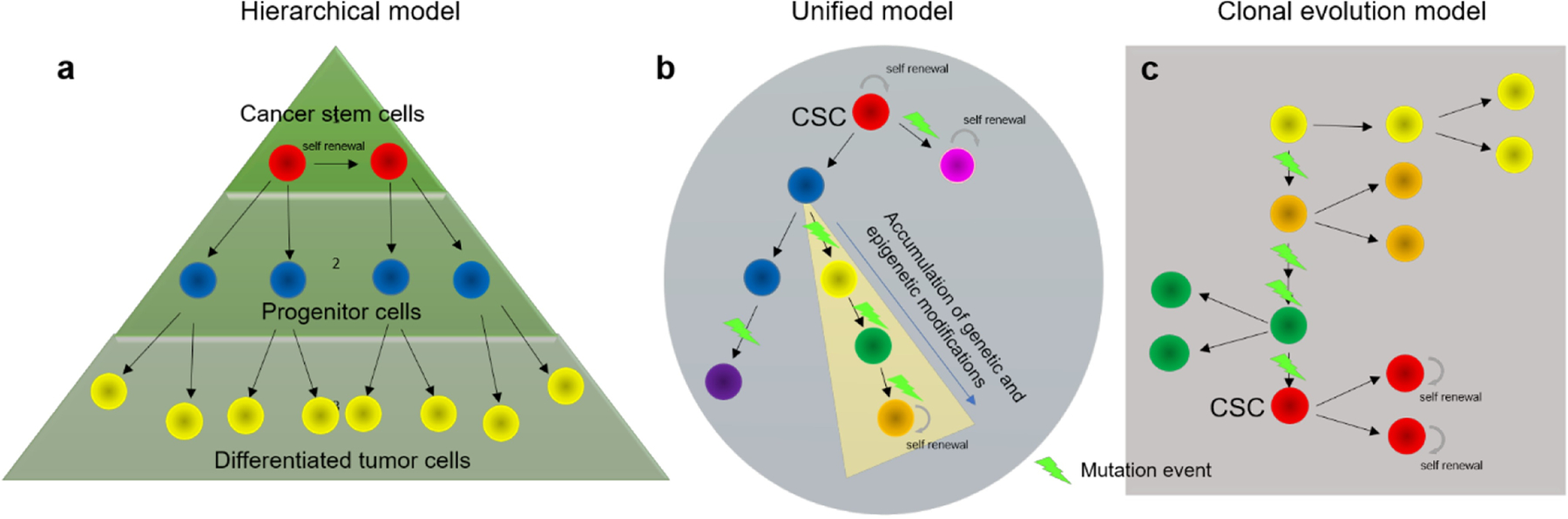
Proposed models for cancer stem cell evolution. Hierarchically organized tumors (a) have a subpopulation of self-renewing cells maintaining the clonal population at the top of the pyramid while giving rise to larger number of progenitor cells ultimately differentiating into other cells in the tumor. The tumor formed has low heterogenicity. Clonal model (c) suggests that selective pressure and continued mutations give way to a highly aggressive population of cells which are tumor initiating and can also self-renew. With different cell phenotypes present, the tumor produced has a high heterogenicity. The unified model (b) suggests stem cell plasticity where cancer stem cells can transition between non-CSCs and CSCs given the right conditions. The resulting tumor is complex, has high heterogenicity and increased resistance to most anti-cancer therapies
While the models of CSC origins were initially postulated in the late nineteenth century, it was in 1997 that Bonnet and Dick identified CSCs by demonstrating that acute myeloid leukemia (AML) arose from preexisting cells with stem-cell like features, emphasizing the hierarchical origin of CSCs. They extracted hematopoietic cells from patients with AML and serially transplanted into NOD/SCID mice to model progression of leukemia in vivo [3]. It was shown that a core population of cells expressing CD34 (similar to the cell-surface phenotype of normal SCID-repopulating cells) were responsible for generation of leukemia in xenografts. With the discovery that CD34+ cells could initiate leukemia, it was suggested that CSCs could play a role in cancer development in other cancers as well. This was later realized in solid tumors when Al-Hajj et al. identified a small portion of CD44+CD24− breast cancer cells, which when reinjected into immunodeficient mice, led to cancer incidence in 8 of 9 mice [4]. As little as 100 of the CD44+ cells resulted in a new tumor whereas a variety of other tumor cells when isolated and reinjected did not result in tumors. While the hierarchical model explains the tumor origin better, it has been additionally demonstrated that CSCs possess bi-directional differentiation ability with any cancer cell capable of becoming a CSC (or vice versa) under the right conditions [5]. This is the basis for unified CSC model widely accepted today, and extended to various human tumors with additional therapeutic implications [6]. Finally, CSCs have been defined based on the following three properties:
Differentiation – ability to form a heterogenous population, which constantly diversifies and specializes according to a hierarchical setup
Self-renewal – ability to regenerate into new identical stem cells with the same ability to proliferate, thus maintaining the stem cell population
Homeostasis control – ability to modulate self-differentiation and proliferation according to environmental stimuli and genetic obstacles
Like the hematopoietic system, other tissues need to undergo cyclic renewal for maintenance. The development of in vitro culture systems to identify various cancer cell populations has led to the observation of a hierarchical system in various tumors including those of breast, colon, pancreas, lung, prostate, brain, and skin [4, 7, 8, 9, 10]. The CSC working model is being further extended to lymphoid tumors including multiple myeloma, where studies reported a small population of CD138− cells possessing engraftment capability in NOD/SCID mice [11, 12]. The number of CSCs range from undetectableto highly abundant in tumors, irrespective of the stage of tumor development. This could be due to the limitations in identifying a set of surface markers relatively unique to CSCs. The other commonly used techniques to isolate or enrich CSC populations include detection of a side population of cells with distinct ability for Hoechst 33342 exclusion, assessment of sphere forming ability in suspension culture, and measurement of aldehyde dehydrogenase (ALDH) activity. Different techniques for detection and isolation of CSCs is discussed in detail elsewhere [13]. Regardless of the techniques utilized, the isolated or enriched CSC populations must be tested by serial transplantation/xenografting in secondary recipient/immunodeficient mice to span the full spectrum of tumor progression. The tumors that develop must represent the tumor heterogeneity of the parent tumor for the injected cells to be considered a reliable CSC population.
Current treatments for cancer largely include a combination of surgical resection, chemotherapy, targeted therapy, immunotherapy and radiotherapy. Cancer cells eventually develop resistance to most therapies. Cytotoxics also induce tumor heterogeneity further resulting in resistance. Several recent reports suggest that CSCs contribute significantly to the development of therapy resistance. Chemoresistance due to alterations in apoptotic processes, epigenetic modifications, and increased expression of anti-apoptotic proteins (all of which have been observed in CSCs) has been reported in many cancers including colorectal cancer, ovarian cancer, and glioblastoma [14]. Even after surgically removing the tumor, the presence of a very small population of CSCs may cause tumor reoccurence [15]. While treatments targeting CSCs could be an effective approach to eradicate cancer, CSCs are inherently resistant to various treatments [16]. This review examines the properties of CSCs that contribute to therapy resistance and discusses potential approaches to overcome these mechanisms.
2. CSCs and anti-cancer therapy resistance
CSCs adapt to changes in tumor microenvironment (TME) making them more resistant to anti-cancer therapies than bulk tumor cells. Mechanisms of resistance include a combination of intrinsic and extrinsic factors. CSCs are inherently resistant to radio- and chemotherapy due to their highly active anti-apoptotic machinery, protective autophagy, efficient cell cycling and DNA repair, along with highly qualified epithelial-mesenchymal transition (EMT) regulators, reactive oxygen species (ROS) scavengers, and drug transporters [17, 18]. CSC resistance is also reinforced by TME signals, which are exceptionally powerful in advanced tumor stages, thus additionally complicating tumor-targeted therapies [19]. Stressful TME (caused by hypoxia, increased EMT, etc.) promotes pluripotency and increases CSC stemness [20]. Further, a multitude of signaling pathways (including TGF-β, PI3K/AKT, NF-κB, Wnt/β-catenin) which independently or collectively induce EMT are observed to suppress the immune system, promote cell stemness and induce stem cell quiescence [21]. Various mechanisms of therapy resistance in CSCs are presented in Figure 2 [17].
Fig. 2.
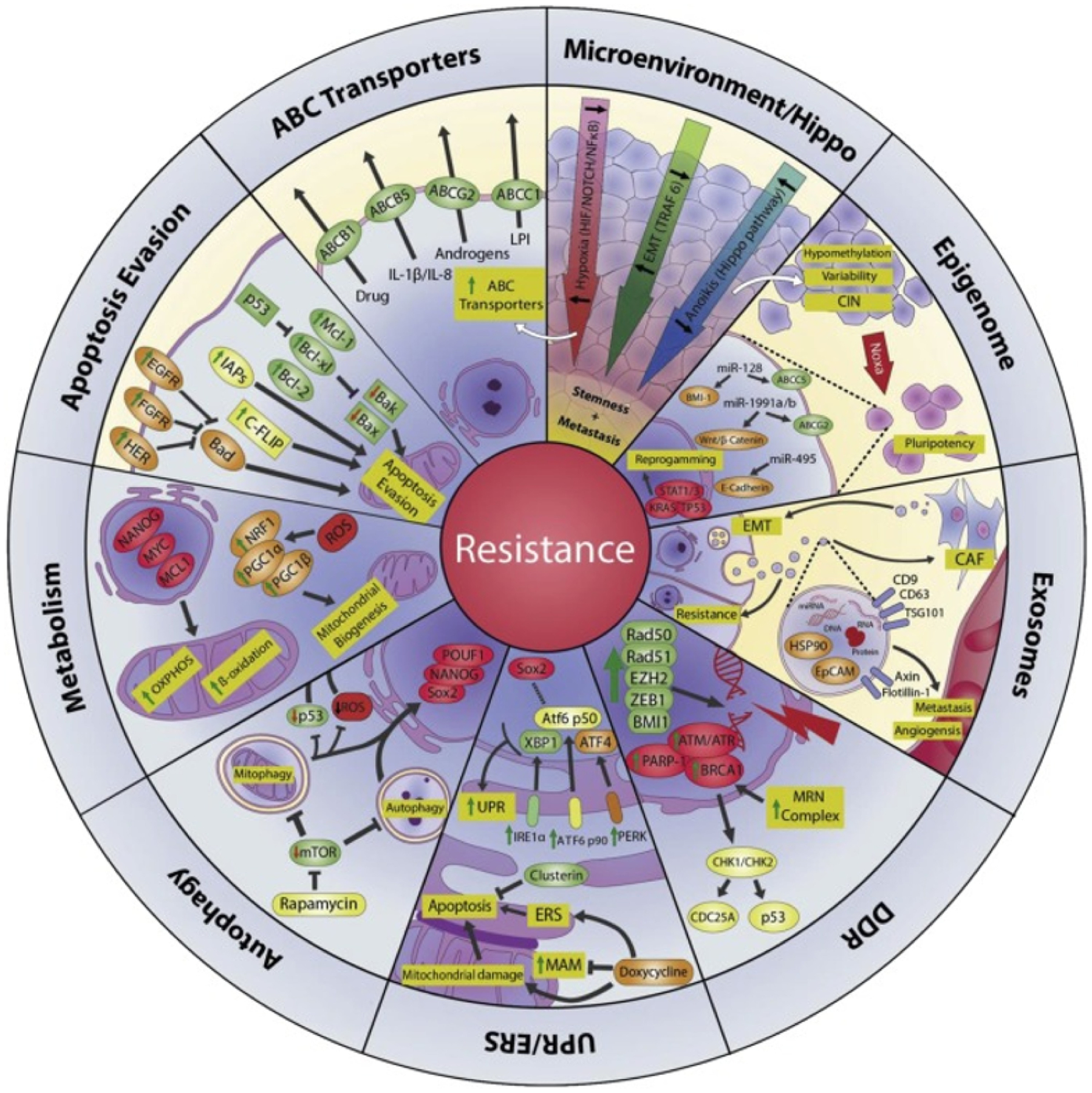
Mechanisms of therapy resistance in CSCs. ABC transporters: ABC transporters reduce the effective concentration of cytokines and administered chemotherapeutics in the cytoplasm. Microenvironment/Hippo: The tumor microenvironment (increased EMT, hypoxic conditions, inhibited Hippo pathway) contributes to increased stemness of CSCs. Epigenome: Instability of chromatin and changes in epigenetic modulators allow for phenotypic differentiation of CSCs. Exosomes: Exosomes promote a CSC phenotype in normal tumor cells by transporting MDR transport proteins to recipient drug sensitive cells. DNA damage repair (DDR): An increased DDR is observed in CSCs due to overexpression of proteins involved in repairing DNA damage (PARP-1, BRCA1, RAD 50, etc.). UPR/ERS: Endoplasmic reticulum stress (ERS) is characterized by presence of misfolded protein in the ER lumen. The unfolded protein response (UPR) signal transduction pathway which balances the ER protein folding is disturbed in the presence of certain physiological conditions (hypoxia, TME, unstable genome). Autophagy: Autophagy has been shown to increase CSC survival and aggressiveness by improving stress tolerance. Metabolism: CSCs can efficiently adapt metabolic pathways or switch pathways to maintain an energy source. This makes them thrive in adverse conditions and imparts resistance to most conventional anti-cancer treatments. Apoptosis evasion: CSCs evade apoptosis by regulating (or altering) apoptotic genes during transcription, translation or even post-translation. Figure adapted from [17]
Rapid cell proliferation is energetically taxing. Tumor cells have higher energy requirements and must adapt to survive in oxygen- and nutrient-deficient environments. One of the intrinsic adaptive measures utilized by tumor cells to survive these conditions is Unfolded Protein Response (UPR). Endoplasmic reticulum (ER) stress is generally caused when misfolded and unfolded proteins concentrate in the ER. Unresolved ER stress could lead to programmed cell death. CSCs present themselves with an adaptive UPR as the ER receptors (Inositol Requiring Enzyme 1α, Proteinkinase RNA-activated-like ER Kinase and Activating Transcription Factor 6) signal to relieve ER stress and reestablish ER homeostasis. An adaptive UPR is known to support tumor growth and increase drug efflux during chemotherapy [22].
Cancer cells, like normal cells, suffer from minor DNA damage as a result of regular wear and tear. These DNA damage events may modify transcription or replication, result in mutations, or alter chromatin arrangements. When the DNA damage is higher, the normal response is for the cell to undergo apoptosis. For CSCs, a DNA damage response (DDR) characterized by the overexpression of proteins involved in repairing DNA damage (PARP-1, BRCA1, RAD 50, etc.) addressees this problem. A highly active DDR can attenuate the effectiveness of DNA damaging anticancer drugs or radiotherapy. With a highly active DDR, CSCs may be spared apoptosis but end up with severe mutations, which further increases cancer cell heterogeneity and CSC stemness. A higher DDR and repair gene expression have been observed in CD133+ CSCs in lung carcinoma cells, breast TICs, pancreatic CSCs, glioblastoma and non-small-cell lung cancer [23].
Epigenetic modulation entails turning on and/ or off genes without altering DNA sequences. These changes in rate and frequency of genetic expression are heritable and are caused by a variety of lifestyle factors. Epigenetic modulation includes DNA methylation (capping a methyl group to part of the DNA molecule, preventing expression of certain genes), histone modification (reversible change to the N terminal of histone that affects chromatin packing) or chromatin remodeling. Overexpression of DNA repair proteins imparts effective anti-apoptotic nature to CSCs. As tumor progresses, alterations of epigenetic modulators (TP53, STAT3, STAT 1, etc.), chromatin modifications, and DNA methylation promote cell reprogramming, producing different CSC phenotypes with increased stemness [24]. Tumor progression is favored by mutated epigenetic regulators such as chromosomal rearrangement of gene KMT2A/MLL (which encodes a histone methyltransferase) in mixed lineage leukemia. Mutations in DNA methyl transferase (DNMT)1 and DNMT3A have been found in colorectal cancer and AML, respectively [25, 26]. DNMT3A mutations appear to reduce the enzyme activity, resulting in the expansion of pre-leukemic stem cells [27].
Autophagy, or cell self-digestion mechanism, is important to maintain cell homeostasis by keeping a quality check on cell organelles, especially during stressful conditions. CSCs are characterized by a high level of autophagy due to demanding conditions like hypoxia. Autophagy plays a role in various CSCs such as those in cancers of breast, pancreas, liver, ovaries, bone and brain [28]. Expression of CSC markers such as CD44 and mesenchymal markers like vimentin increases with autophagy [29]. Autophagy increases CSC survival and stemness by improving stress tolerance and supplying nutrients to meet the increased metabolic needs of CSCs.[30] Autophagy is induced with chemotherapy, radiotherapy, inhibition of mTOR, AKT or PI3K, but tumor cells evade apoptosis, as autophagy increases FOXO3A. This limits FOXO3A-dependent induction of Puma (p53 upregulated modulator of apoptosis), a Bcl-2 family pro-apoptotic protein [29].
The tumor works closely with various inflammatory molecules, signaling pathways, fibroblasts, blood vessels and extracellular matrix. The CSC TME is altered with upregulated signaling pathways and hypoxic conditions favoring tumor progression and maintenance. Understanding the complex working of CSCs is critical to designing effective therapies that target and eliminate CSCs. The implications of drug transporters, CSC surface markers and TME in anti-cancer resistance are discussed further in the subsequent sections.
2.1. Drug transporters
Decreased expression of Reduced Folate Carrier (RFC) and polymorphisms in the gene lead to reduced uptake of commonly used chemotherapeutic agents such as methotrexate, a dihydrofolate receptor (DHFR) substrate [31]. Reduced expression of transporters or mutations in the genes encoding for a transporter can result in reduced uptake of drugs [32]. ATP-dependent efflux pumps are major contributors to multi-drug resistance (MDR). ATP Binding Cassette (ABC) transporters is a superfamily of transmembrane proteins consisting of 49 MDR transporters in 7 subfamilies (designated ABCA-G). ABC transporters form a pore like structure that spans the entire thickness of the membrane, with two transmembrane domains and two nucleotide binding domains (NBD). When a substrate binds to the NBD, a molecule of ATP gets hydrolyzed to ADP while the conformational structure of the protein changes to allow movement of the substrate out of the cell. ABC transporters are involved in the movement of a variety of materials including sugar, proteins, xenobiotics and polysaccharides. Different ABC transporters (ABCB1, ABCC1 and ABCG2) are found to be overexpressed in cancers of prostate, lung, and breast, as well as in childhood neuroblastoma. [33, 34, 35, 36, 37]. It has been established that ABC transporters keep the cytoplasmic concentrations of drugs and other signaling molecules low, rendering anti-cancer treatments ineffective [37]. This also leads to MDR as cancers become resistant to other unrelated chemotherapeutics after exposure to one drug. MDR transporters are also overexpressed on CSCs compared to bulk cancer cells, making them more resistant to treatments and helping tumor propagation. Targeting CSCs using specific inhibitors of ABC transporters is an attractive strategy to target CSCs. In addition to contributing to chemoresistance, ABC transporters work in tandem with various other components of the tumor system, tumor signaling pathways and TME. ABC transporters also regulate redox cell status. Increased Nrf-2 in blood brain barrier (BBB) during oxidative stress increases protein expression and activity of ABCB1, ABCG2, and ABCC2. This decreases drug perfusion across CNS barriers [38]. Further, during oxidative stress, ABC transporters (ABCC1–4, 4, 7 and ABCG2) transport GSH and GSH conjugates, which are important for maintaining redox homeostasis. ABC transporters are also involved in lipid and cholesterol transport. ABCA1, which transports cholesterol and phospholipids to plasma membranes, is believed to be involved in cancer progression in prostate and ovarian cancers by transporting lysophosphatidylinositol and sphingosine 1-phosphate [39, 40, 41]. ABC transporters are also implicated in tumor metabolism. Mitochondrial metabolism, which is critical for tumorigenesis and ATP production, could meet the energy requirements of overexpressed ABC transporters in CSCs.
2.2. CSC surface markers
Several cytosolic and cell surface markers present on CSCs have been identified and characterized. Cell surface markers are proteins or carbohydrates on or within the cell’s plasma membrane that support intercellular communication and cell recognition. ‘Cluster of differentiation’ (CD) is the nomenclature that is used to identify cells based on a set of surface markers. CD markers can be used to differentiate cancer cells from normal cells and to identify CSCs within a cancer cell population. Surface macromolecules also present themselves as receptors or antigens for targeted drug delivery. These molecules can be recognized by magnetic micro-bead isolation techniques and detected by flow cytometry. Knowledge of unique CD markers is pivotal for diagnosis and subsequent development of therapeutic strategies targeting CSCs. Bonnet and Dick, for the first time, reported that the SCID-leukemia initiating cells (SC-ILs) from all subtypes of AML analyzed were exclusively CD34+ CD38− [3]. This seminal work led to the search for other CSC surface markers in related blood cancers as well as solid tumors. Jordan et al. demonstrated that CD123 is a unique marker for primitive leukemic stem cells [42]. As reported by Hauswirth et al., CD33, a sialic acid-dependent single transmembrane adhesion protein triggered by phosphorylation, is expressed on leukemic stem cells in AML and is unique to CSCs [43]. Thus, the combination of CD34+/CD38−/CD123+/ CD33+ is a general marker phenotype for AML CSCs. Hosen et. al. identified that CD96, which belongs to the immunoglobulin superfamily, is an important marker of AML CSCs [44]. CD96 was demonstrated to be overexpressed on a majority of CD34+/ CD38− AML cells compared to the basal expression level of CD96 on normal HSCs. A list of various CSC surface markers recognized in different cancers are listed in Table 1. Readers are directed to a detailed discussion of the structure, function and implications of CSC markers in cancer elsewhere [45].
Table 1.
Commonly expressed cancer stem cell surface markers in different cancers.
| CSC surface marker | Cancer | Reference |
|---|---|---|
| CD33 | Hematological | [46] |
| CD34 | Hematological, liver | [47] |
| CD38 | Hematological | [48] |
| CD123 | Hematological | [49] |
| CD19 | Hematological | [50] |
| CD13 | Liver | [51] |
| CD26 | Hematological, colorectal, lung | [52–54] |
| CD24 | Breast, colon, pancreas, prostrate, esophagus, nasopharynx, ovary, kidney, lungs | [55–57] |
| CD44 | Breast, prostate, pancreas, ovary, colon, liver, lung, head and neck | [55, 58, 59] |
| CD133 | Breast, colon, brain, liver, lung, pancreas, prostate, stomach, melanoma | [60] |
| Ep-CAM | Liver, pancreas | [61, 62] |
| CD90 | Brain liver esophagus | [63] |
| CD20 | Skin | [64] |
| CD271 | Head and neck, esophagus, skin | [65, 66, 44] |
| CD96 | Hematological | [44] |
As seen in Table 1, the cell surface markers for solid tumors differ from those in hematological malignancies. CD44 is one of the most commonly expressed CSC markers found in various solid malignancies including those of the bladder, colon, liver, ovaries, pancreas, and prostate. These markers are expressed individually or together with CD24, CD133, CD34, or c-Met markers [67, 68, 69, 70, 71, 72]. Al-Hajj et al., prospectively identified CD44+/CD24−/low Lin (−) phenotype to have breast cancer initiating potential. This phenotype is now correlated with high resistance to standard anti-cancer therapies [4]. CD44+ cells were also identified to be gastric colon cancer initiating cells [73, 74]. CD44 is particularly important for CSCs as it regulates cancer cell adhesion and homing, cell extravasation and migration, crosstalk between stem cells and their niche, EMT, and the suppression of cell apoptosis [45]. Further, the unique structure of CD44 (a link domain and a hyaluronic acid binding domain on its globular structure) makes it an important target for drug delivery applications.
CD133 is one of the most prominent CSC markers and was initially categorized as a marker of primitive hematopoietic and neural stem cells [75]. CD133+ subset was the first reported putative CSC population for hepatocellular carcinoma [76]. It is a transmembrane protein with 5 subunits and 865 amino acid residues. Commonly located on cell membrane protrusions, it has been implicated in membrane reorganization [77]. CD133 has now been shown to be present on tumor initiating cells in a variety of cancers including breast, brain, prostate, ovarian, lung, liver, and colon cancer [78, 79, 80, 81, 82, 83, 84]. Under stressful conditions, such as hypoxia, CD133 is significantly upregulated in human glioma cells [85]. Other surface markers, which promote CSC progression and maintenance include CD13, CD47, and epithelial cell adhesion molecules (EpCAM). EpCAM regulates Ca2+-independent homotypic cell–cell adhesion, is limited to epithelial cancers and could be either tumor promoting or tumor suppressive based on the type of cancer in question [86]. In the seminal work by Hajj-Al et al., it was shown that EpCAM+ lineage fraction had a significantly higher frequency of TICs than the EpCAM− lineage fraction. EpCAM is now a well-established marker for TICs, CSCs and cancer cells, and an attractive target for CSC targeted therapies. CD13 has been identified as a novel surface marker in liver CSCs. Using human liver cancer cell lines and clinical samples, Haraguchi and coworkers demonstrated that CD13+ cells were present majorly in the G0 phase of the cell cycle and formed cellular clusters within the tumor. Upon treatment with 5-fluorouracil, these cells survived and amplified along the fibrous capsule where liver cancers usually relapse [51]. CD47 has found to be overexpressed in many cancers including small cell lung cancer, leukemia, non-Hodgkin’s lymphoma, and breast cancer. As overexpression of CD47 helps CSCs avoid phagocytosis by macrophages, CD47 blocking treatments offer a targeted treatment approach for many cancers. [87].
Proteoglycans (PGs) are molecules formed through the covalent interaction of protein core with glycosaminoglycan (GAG) chains. The GAG chains are subdivided into (a) heparan sulfate (HS), (b) chondroitin sulfate, (c) keratin sulfate, and (d) hyaluronic acid. PGs play a key role in cancer biology as they have an affinity to bind both with ligands and receptors that modulate tumor growth and neovascularization. They can be broadly classified into pericellular, cell-surface, intracellular, and extracellular matrix PGs based on their location in the cellular environment. HS-carrying perlecan and agrin along with Collagens XVIII and XV constitute the pericellular PGs that are associated with the assembly of basement membranes. Localization of HSPGs in the basement membrane controls their barrier functions and coordinates cell-cell/ECM-cell associations. Among the cell-surface PGs, syndecans influence the growth factor receptor signaling while the glypicans can effectively modulate Hedgehog and Wnt cell signaling pathways. The intracellular PG, serglycin, has a role in inflammation and tumor progression and is overexpressed in a variety of hematological and non-hematological cancers [88, 89, 90]. It physically binds to CD44 and functions in cell signaling, lymphocyte activation, hematopoiesis and tumor metastasis. Interaction of serglycine with CXCL4 chemokine receptor contributes to angiogenesis. Perlecan, a highly glycosylated HSPG component of the extracellular matrix (ECM) plays a vital role in the presentation of growth factors to receptors. It contributes to cancer progression by establishing connections between cells and signaling molecules in the process of ECM dysregulation, angiogenesis, and invasion. Both tumor cells and the host stromal cells are known to synthesize perlecan. Interaction of perlecan with angiogenic growth factors helps in the generation of new blood vessels during cancer proliferation. Elevated expression of perlecan is observed in cancers of colon, pancreas, breast, prostate, brain and skin [91, 92]. Suppression of perlecan has been shown to downregulate invasive nature of melanoma cells and block tumor growth and angiogenesis in vivo [93]. We observed significant cell surface expression of HSPG2 in both primary tumors and metastatic lesions in triple negative breast cancer (TNBC).
2.3. CSC microenvironment and its “niche”
The CSC theory is consistent with the presence of cell communities that retain tumor growth through self-renewal and generating the bulk tumor, on the basis of the idea that tumors are organ systems regulated by aberrant development and homeostasis [94]. The TME consists of the tumor stroma that constitutes most of the tumor mass, including the ECM, mesenchymal stem cells (MSCs), endothelial cells, immune cells, as well as cytokine networks and growth factors [95, 96].’Niche’ is a term used to describe the microenvironment where CSCs reside. The role of the niche has been demonstrated to be relevant at each stage of tumorigenesis. Accumulating evidence has suggested that alterations in niche factors contribute to tumorigenesis [97]. As the niche gets established, CSCs employ tumor-associated macrophages (TAMs), cancer-associated fibroblasts (CAFs) and other stromal cells to create paracrine loops for supplying CSCs with tumor necrosis factor α (TNF-α), transforming growth factor β (TGF-β) and interleukins (ILs) for CSC maintenance. CSCs also contribute to metastatic progression by establishing perivascular niches enhanced in angiocrine factors. The adjacent stromal cells secrete ECM, which in turn frees TGF-β and other growth factors such as vascular endothelial growth factor A (VEGF-A), in order to enable further tumor growth [98]. These supportive niches also retain the characteristics of CSCs, sustain their phenotypic plasticity, safeguard them against drug-induced apoptosis, promote their metastatic ability and are critical for stemness maintenance [96, 98, 99, 100]. In order to preserve their stemness, CSCs identify and communicate with certain vascular niches. Accelerated formation of the vascular niche is assisted by vascular endothelial cells, one of the CSC niche elements [101]. Tumor neovasculature is often deficient in the basement membrane and supplied with endothelial CSC (ECs) and other related cells that may encourage tumor invasion and metastasis. Vascular niches can enhance stem-like properties of CSCs and can help CSCs escape radio-/chemo- therapy [99]. Because tumor angiogenesis is necessary for tumor development and metastasis, drugs that affect angiogenesis and vascular permeability can be effective in stopping the formation of a pre-metastatic niche, leading to the elimination of metastatic CSCs [102].
The ECM is responsible for maintaining tissue architecture and homeostasis and maintaining cell populations including immune cells, capillaries, and fibroblasts. The ECM in the CSC niche raises two problems from a therapeutic point of view. First, together with increased intratumoral pressure, ECM inhibits drug transport and shields CSCs from chemotherapy. Secondly, the active ECM environment encourages the growth of tumors through enhanced signaling and may favor cancer cell migration [103]. Therefore, modifying ECM to enhance the penetration of drugs and drug nanocarriers can improve targeting of CSCs and thus provide superior anti-tumor effects [104].
Hypoxia is a key component of the TME and potentially contributes to improved CSC survival. Activation of a subset of proteases that promote metastasis accompanies the acidic microenvironment of hypoxic cells. Also, hypoxic cells are generally located deep in the tumor and are hard to reach. Due to the inaccessibility of these areas along with anomalous angiogenesis and acidic microenvironment, therapeutically effective drug concentrations are not achieved, leading to resistance to therapy. Hypoxia inducing factors (HIFs), a family of transcription factors, are responsible for maintaining homeostasis under hypoxic conditions. Stability of HIF-α expressed in many tumors is necessary for tumor maintenance. Targeting HIFs for therapeutic elimination of CSCs and reprograming of the TME as well as targeting of the hypoxic CSC niche in conjunction with chemotherapy are attractive approaches to eradicate CSCs [105, 106, 107].
Recent studies suggest that EMT plays a significant role in disease progression and generation of distant metastasis in many solid tumors [108, 109]. EMT is a regulatory development program essential for the creation and maintenance of CSCs [110]. Acquisition of CSC characteristics is strongly linked to the progress of EMT transdifferentiation [111]. Signals from the TME tightly regulate primary tumor growth and metastasis processes. Through activation of transcription factors (TFs) such as Snail, Slug and Zeb, the malignant epithelial cell’s transcriptional program is modified and results in the loss of cell-cell attachment and the gain of mesenchymal phenotype and motility. The transition from epithelial to mesenchymal and more motile phenotype in CSCs promotes dissemination [112]. Previous studies show that EMT-related transcriptional signals have an overlapping pattern with those found in CSCs, involving TGFβ, Wnt, Hedgehog, Notch, miR-200 and miR-205 [112, 113]. The relationship between EMT and CSCs could be exploited to design novel therapeutic strategies to reduce tumor recurrence, metastasis and therapy resistance [114].
2.4. CSC-targeted treatment: the importance and need
Because of the peculiar properties of CSCs, including high tumorigenic activity, self-renewal ability, and multilineage differentiation potential [115], developing therapeutic strategies to combat CSCs has gained increasing attention. Standard cancer therapies including chemotherapy, radiation therapy, and molecularly targeted therapy aim to eliminate as many tumor cells as possible, but because they are not effective against CSCs, they fail to eliminate the tumors completely [116, 117]. CSCs are an undifferentiated cancer cell subpopulation but possess “robustness”, which has been blamed for a slow cell cycle, the ability to efflux cytotoxic agents, resistance to oxidative stress, and rapid response to DNA damage [115]. Hence, standard therapies lead to enrichment of CSCs, further promoting cancer relapse [118, 119, 120, 121]. CSC-targeted therapies are thus considered an effective and promising treatment strategy to eradicate cancer [100]. Approaches that simultaneously kill both the bulk of cancer cells and CSCs to prevent metastasis and recurrence will improve treatment outcomes. [122]. Furthermore, it is necessary to develop delivery systems for more efficient targeting and eradication of CSCs and overcoming drug resistance [123, 124, 125, 126].
3. Strategies to target CSCs
3.1. Targeting ATP-driven efflux transporters
The ABC superfamily in humans consists of genes encoding membrane proteins involved in energy-dependent transport of various substances across cell membranes. Many tumors after cancer relapse exhibit resistance to multiple drugs. ABC transporters are overexpressed in MDR tumors and lead to poor treatment outcomes [127]. Over the last three decades, expression of various transporters in tumors and their relevance in anti-cancer therapy have been examined. MDR inhibitors have been reported to target CSCs and improve chemotherapy outcomes [128, 129, 130, 131]. It is important to understand the molecular mechanisms of MDR transporters to effectively target them. The ABCB1 gene encoding P-glycoprotein (P-gp; MDR1) is the first identified and most well characterized ABC transporter that is overexpressed in MDR tumor cells [132, 133, 134]. Several of the ABCB family (also known as the MDR family of ABC transporters) members are responsible for intracellular peptide transport and confer MDR to tumor cells [135]. Most cells expressing MDR-1 are resistant to important chemotherapy agents including taxanes, doxorubicin and vinca alkaloids among others [136]. Several strategies are being pursued to overcome MDR mechanisms. These include discovery of new anticancer drugs that bypass drug efflux, MDR modulators (chemosensitizers or MDR inhibitors), monoclonal antibodies, multifunctional nanocarriers and RNA interference (RNAi) based therapies (Figure 4) [137].
Fig. 4.
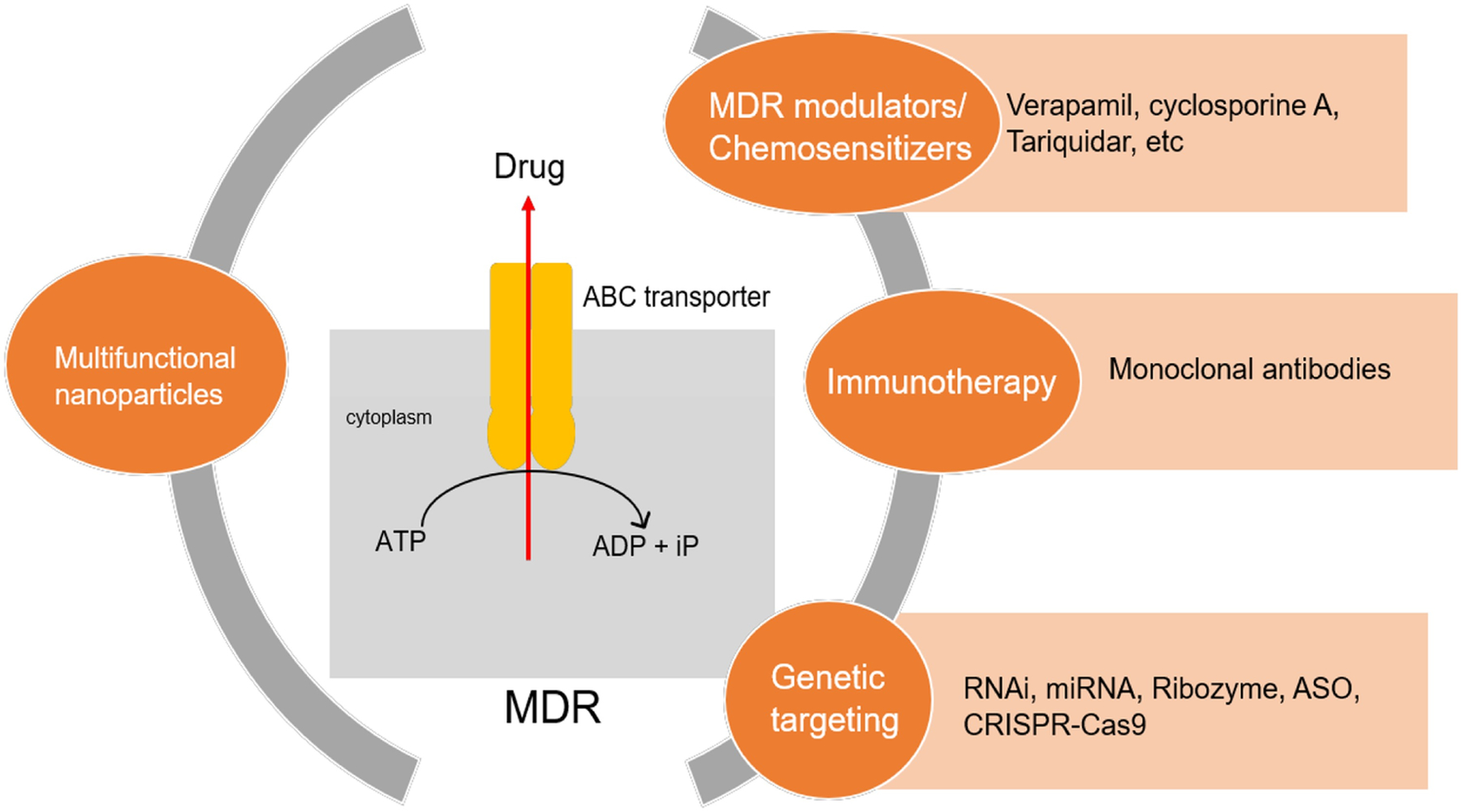
Potential approaches to combat multidrug resistance (MDR) in cancer stem cells. ABC – antigen binding cassette, RNAi – RNA interference, miRNA – microRNA, ASO – antisense oligonucleotides, CRISPR/Cas9 – Clustered randomly interspaced short palindromic repeats/Cas9
Glioblastoma stem-like cells (GSCs) are a major contributing factor to poor response in glioblastoma treatments. Torres et al. investigated the efficacy of tacrolimus as a regulator of multidrug resistance-associated protein 1 (MRP-1) protein and improved the efficacy of vincristine treatment by attenuating MRP1-mediated chemoresistance in GSCs [138]. A carboxyfluorescein diacetate (CFDA)-retention assay was used to assess MRP1 activity in GSCs and normal glioblastoma cells from U87MG (human) and C6 (rat) glioma cell line, while MTT and apoptosis were utilized to evaluate the cytotoxicity of vincristine treatment. Vincristine suffers from poor permeability across BBB because of P-gp-mediated drug efflux. Tacrolimus was seen to promote apoptosis, decrease MRP-1 activity in GSCs, and improve chemosensitization towards vincristine treatment in GSC derived tumors. Minko et al., reported that N-(2-hydroxypropyl) methacrylamide (HPMA) copolymer-adriamycin conjugate inhibited the expression of the MDR1 and β2m genes in multidrug resistant A2780/AD cells [139]. Other block co-polymers such as Pluronic P85 were studied by Alakhov et al., for their chemosensitizing effect on multi-drug resistant ovarian cancer cell lines. The cytotoxic effects of other P-gp substrate drugs (doxorubicin, epirubicin, vinblastine, and mitomycin C) increased by a factor of 20−1000 and of non-substrate drugs (methotrexate and cisplatin) increased by a factor of 2–5.5 in the presence of the co-polymer [140]. In a study reported by Venne et al., Pluronic L61 increased the sensitivity of multidrug resistant CHRC5 Chinese hamster ovary cells and MCF-7/ADR human breast carcinoma cells to the cytotoxic action of doxorubicin [141]. Taxanes are unable to kill cancer cells that overexpress P-gp. As taxanes work by stabilizing microtubule assembly resulting in cell arrest before anaphase, they must accumulate inside the tumor cells at sufficiently high concentrations to kill the cells. Several studies have shown that administering paclitaxel with P-gp inhibitors can be effective in increasing intracellular paclitaxel concentrations [142, 143]. Curcumin, a natural polyphenol has been widely studied for its chemoprotective and anti-cancer properties. One of the ways curcumin helps fight cancer is by downregulating intracellular levels of P-gp, MRP-1 and ABCG2. As a result, therapies combining various anti-cancer drugs with curcumin have been studied. One of the drawbacks with use of curcumin is its high hydrophobicity (particularly important if the anti-cancer drug in question is also hydrophobic) which limits efficient loading and release from delivery systems. Ganta et al. developed a flaxseed oil nanoemulsion to effectively load and deliver paclitaxel and curcumin in combination to wild-type SKOV3 and drug resistant SKOV3TR human ovarian adenocarcinoma cells [144]. Another study investigated curcumin delivery to sensitize breast CSCs to mitomycin C. Curcumin also improved the sensitivity of cisplatin, paclitaxel and doxorubicin in MDAMB-231 and MCF-7 cells (>2-fold reduction in half-maximal inhibitory concentration) by reducing the expression of ABCG2 [145]. ABCB5 has also been shown to be implicated in chemoresistance of CSCs. Frank et al. demonstrated that targeting ABCB5 using a specific blocking monoclonal antibody reestablishes sensitivity to doxorubicin in melanoma cells [146]. In another report epirubicin-loaded lipid microbubbles conjugated with anti-ABCG2 monoclonal antibody was shown to induce apoptosis in myeloma stem cells [147]
Nanoparticles are an efficient platform to target MDR tumors due to their passive and active targeting capabilities. Rapidly growing tumors have a leaky vasculature and poorly developed lymphatics that allows for the EPR effect. Nanoparticles may also avoid drug efflux by ABC transporters as they are internalized via either non-specific or specific endocytosis, resulting in a higher intracellular accumulation of the drug. Chen et al. studied poly(lactide-co-glycolide)/d-alpha-tocopherol polyethylene glycol 1000 succinate (PLGA/TPGS) nanoparticles loaded with doxorubicin and elacridar for targeting liver CSCs and bulk cancer cells. Elacridar, an acridone imidazole amide derivative inhibits ABCB1 and ABCG2. Synergistic cytotoxic effects with nanoparticles containing elacridar and doxorubicin (molar ratio 1:1) were demonstrated in HepG2 cells and HepG2 tumor spheroid model. Nanoparticles targeted the tumor better and showed high antitumor efficacy and low systemic toxicity in vivo [148]. Chemotherapy combined with ABC inhibition is an effective way to target both CSCs and the bulk of tumor cells. Specific inhibitors of these transporters have been established and more are being continuously developed [149]. However, an effective drug delivery system may be needed for efficient targeting of ABC transporters on CSCs. Since these transporters are also responsible for maintenance of function in normal stem cells and other mission critical barriers, their doses must be carefully titrated to limit any possible side effects.
Genetic approaches to target ABC transporters include using RNA interference (RNAi) and antisense oligonucleotides (ASO). RNAi is a powerful biological process that has been used to target MDR tumors. RNAi works by silencing specific gene sequences by destroying targeted mRNA molecules. RNAi based MDR modulation in cancer was first attempted in 2003 where synthesized siRNAs were used for MDR modulation by transient downregulation of P-gp expression. Systemic delivery of naked siRNA, however, is inefficient because of rapid degradation of the siRNA and thus siRNA is often delivered using nanocarriers. Kato et al. delivered small interfering RNA (siRNA) for O6-methylguanine-DNA methyltransferase (MGMT) encapsulated in cationic liposomes. Liposome mediated delivery bypassed the ABC transporters, thereby overcoming drug resistance conferred by these transporters [150]. Hashemitabar et al. developed an ABCG2-targeted aptamer complex that increased the efficacy of doxorubicin against ABCG2 overexpressing, mitoxantrone-resistant breast cancer cell line (MCF7/MX) [151].
MicroRNAs (miRNAs) have been found to play a role in tumorigenesis. microRNAs are regulatory noncoding short RNA sequences capable of altering gene expression. miRNAs contribute to drug resistance by modulating the expression of the genes coding for drug transporters. MicroRNA regulation has directly been correlated with chemosensitivity in HCC. Reduced miR-33a expression, which directly targets ABCA1 expression, leads to chemoresistance in HCC [152]. Overexpression of miRNA-328 was seen to increase the sensitivity of breast cancer cells to mitoxantrone by downregulating the BCRP transporter [153, 154]. In MCF-7 breast cancer cells, expression of miRNA-21 decreases the responsiveness of cancer to doxorubicin treatment. Similar effect was seen with trastuzumab in breast cancer. Role of miRNAs is further observed in a wide range of tumor types including colorectal, ovarian, prostate, lung, and hematopoietic cancers. Use of ASOs and ribozymes to overcome MDR have also been reported with limited efficacy. ASOs are small DNA sequences designed to be complementary to the genetic sequence of interest. However, ASOs were incapable of completely reversing P-gp in cell lines overexpressing high levels of P-gp [155]. The ribozyme approach also suffers from similar problems [156]. Over the last five years, genome editing using CRISPR/Cas9 has been investigated for targeting MDR mediated by ABCB1 and has shown potential, but further studies are needed to demonstrate the translational potential of this approach [157, 158, 159].
Since CSCs have various pathways to survive toxic drug concentrations, only ABC inhibition is not enough to accomplish complete tumor eradication. Further, quiescence makes CSCs less sensitive to conventional therapies, with cells being resistant to antitumor drugs even in the absence of drug transport inhibitors. Also, it is important to maintain the efficacy-safety balance by limiting any undue toxicity to normal stem cells.
3.2. Targeting surface markers
While using efflux pump inhibitors is a promising strategy to sensitize CSCs to chemotherapeutics, a more targeted approach to kill CSCs is to exploit the presence of specific surface markers on these cells. Delivery systems that utilize an antibody targeting a surface marker on CSCs have shown promising activity [160]. Existence of a molecular link between stem cell markers such as MDR-1 and the hyaluronan receptor, CD44, has been shown in breast and ovarian cancers [161]. Thus, hyaluronic acid (HA)-based drug conjugates have been investigated for targeting CSCs [162]. Once HA binds with the CD44 receptor, the receptor–polymer system is internalized, followed by degradation of HA. Further, because HA is a hydrophilic polymer, HA conjugation with anticancer drugs may improve their solubility [163]. The earliest study reporting the use of HA targeting was in 2001 when Elias et al. demonstrated that HA-targeted liposomes bound to B16F10 murine melanoma cells (which express CD44 at high levels) at greater levels than against CV-1 African green monkey kidney cells, which express low levels of CD44 [164]. Paclitaxel and curcumin loaded PLGA nanoparticles coated with a HA-lipoid were investigated for targeting breast CSCs. The nanoparticle encapsulated drugs synergistically inhibited the growth of both breast CSCs and bulk breast tumor cells in mice bearing MCF7 tumors [165]. Goodarzi et al. developed a CD44 targeted macromolecular conjugate of docetaxel linked to HA via a pH sensitive linkage. HA–docetaxel conjugates showed specific toxicity only in CD44-expressing cells in vitro, along with a decreased risk of neutropenia and dose-dependent mortality in vivo [166]. In addition to HA conjugates, anti-CD44 antibodies and aptamers have also been utilized to target CD44+ tumor cells [167–169]. Several approaches to target EpCAM have recently been studied, including the use of antibodies Catumaxomab, MT110 (Micromet GmBH) and Edrecolomab. Notably, MT110 underwent Phase I clinical trial on patients with refractory solid tumors. Dose escalation to potential therapeutic effects could not be achieved due to severity of adverse effects [170]. CD33 is expressed on leukemic blasts in 85–95% of AML patients. Mylotarg® (gemtuzumab ozogamicin, developed by Wyeth and Pfizer) is a small molecule antibody conjugate used as a targeted treatment for CD33+ AML [171]. The small molecule component of the drug is an antitumor antibiotic, N-acetyl-gamma calicheamicin while the antibody component is an anti-CD33 hP67.6 antibody. The two components are connected by a bifunctional linker which is cleaved in the acidic lysosomes of the tumor cells releasing calicheamicin. The drug forms a DNA anti-metabolite, resulting in cell cycle arrest and/or apoptosis.
Antibodies targeting specific surface proteins have been used alongside other cancer therapies for effective CSC targeting. Liu and coworkers designed multifunctional silica shell coated iron oxide nanoparticles loaded with alvespimycin (heat shock protein inhibitor and semi-synthetic derivative of the antibiotic geldanamycin) and tagged with anti-CD20 antibody [172]. Combining CSC targeting, chemotoxicity, and hyperthermia, these particles produced a synergistic response in vitro in lung CSCs (compared to individual nanoparticle components). Distribution studies in a metastatic mouse model (with lung CSCs) revealed higher accumulation of CD20 targeted nanoparticles in the lung against particles without anti-CD20 antibody. Further investigations in a mouse xenograft model showed dramatically inhibited tumor growth and no apparent regrowth (with hyperthermia + multifunctional nanoparticle treatment). In another study, CXCR4-targeted self-assembling toxin nanoparticles were investigated for their potential to selectively kill CXCR4+ human colon-CSCs compared to 5-fluorouracil and oxaliplatin treatments [173]. CXCR4 plays a critical role in the poor response to chemotherapy as CXCR4 signaling regulates the expression of CD20 on B cells. These studies revealed selective CSC targeting with CXCR4-targeted self-assembling toxin nanoparticles and pyroptosis in the absence of apoptosis, demonstrating the potential for this approach.
CD133 is another commonly targeted epitope found on the surface of CSCs in many cancers. Majority of anti-CD133 antibodies only recognize the glycosylated epitope of the CD133 protein, potentially limiting their reliability. We developed a scFv that could recognize both glycosylated and non-glycosylated forms of human CD133 [84]. The scFv immunostained Caco-2 cells (CD133+) but did not stain U87 cells (CD133−/low). Notably, the anti-CD133 scFv possessed ability to cross-react with mouse CD133 protein. This was demonstrated by co-immunostaining studies in mouse bone marrow cells, using anti-CD133 scFv-FITC and anti-mouse CD133-PE (clone 13A4) commercial antibody. Given their smaller size than the parental monoclonal antibodies, scFvs are an excellent choice for targeted drug delivery to CSCs [174]. Using the anti-CD133 scFv, our team developed a deimmunized immunotoxin (CD133KDEL). This CSC-targeted immunotoxin selectively inhibited head and neck squamous cell carcinomas (SCC), which are rich in CD133+ cells. Complete eradication of tumors in immunocompetent mice was observed with CD133KDEL [175]. Further, systemic delivery of CD133KDEL was effective in inducing tumor regression in a mouse metastatic, intrasplenic MDA-MB-231 tumor model.[176] Using the CD133 scFv as the platform, our team then developed a self-sustaining trispecific NK cell engager (TriKE) to trigger natural killer cell-mediated antibody-dependent cellular cytotoxicity (ADCC). The TriKE molecule contains an anti-CD16 scFv to induce NK cell activation and an IL-15 encoding cross linker that increases anti-cancer reactivity [177]. We also developed a novel anti-CD133 monoclonal antibody (hybridoma clone 7) with highly immunogenic amino acid residues selected from the native CD133 protein as the immunogen [77]. This antibody was able to specifically recognize CD133 protein in a range of immunological applications. We further investigated targeted paclitaxel delivery using anti-CD133 antibody conjugated PLGA nanoparticles. CD133 targeting resulted in 9-fold higher uptake of nanoparticles in Caco-2 cells, which express high levels of CD133. In vivo studies in MDA-MB-231 xenograft mouse model demonstrated significantly lower tumor regrowth with CD133 targeted nanoparticle treatment compared to that with free paclitaxel treatment [178]. Shigdar et al. identified RNA aptamers that specifically bind to CD133. They reported effective internalization of the RNA aptamers and demonstrated superior penetration into 3D tumor spheres [179]. Ma et al. prepared a CD133 aptamer modified docetaxel liposomal formulation and demonstrated improved therapeutic efficacy and reduced systemic toxicity in A549 lung cancer model [180]. Kim and coworkers demonstrated improved therapeutic efficacy with temozolomide encapsulating liposomes that were conjugated to both angiopep-2 peptide and CD133 monoclonal antibody and targeted to CD133+/ALDH1+ glioblastoma stem cells [181].
Overexpression of cell surface protein, perlecan, has been demonstrated in TNBC. Using phage display, we developed two human antibodies (clone 6 and AM6) that could bind to metastatic TNBC tumor cells. The affinity-matured antibody inhibited the growth of MDA-MB-231 tumors to a greater extent in nude mice than in NSG mice, suggesting natural killer cell-mediated antibody-dependent cell cytotoxicity. Development of therapies targeting perlecan could improve the management of metastatic TNBC. We further synthesized paclitaxel loaded PLGA nanoparticles covalently conjugated with Clone 6 and AM6 (discussed earlier) to investigate anti-tumor efficacy in TNBC [182]. Perlecan-targeted nanoparticles exhibited improved cell uptake, retention and cytotoxicity in vitro. In vivo studies in MDAMB-231-LM2 orthotopic mouse tumor model demonstrated enhanced tumor growth inhibition with AM6 nanoparticles against nanoparticles functionalized with isotype IgG.
3.3. Targeting signal cassette
Numerous signaling pathways in CSCs play specific roles in proliferation, differentiation, stemness induction and maintenance, self-renewal, and therapy resistance [183, 184, 185]. Many highly conserved signaling pathways, including the Hedgehog, Notch and Wnt/β-catenin pathways are deregulated in CSCs (Table 2) [112, 186]. Due to the importance of signaling pathways that drive oncogenesis and tumor metastasis, selective targeting of CSC signal cassette is of utmost clinical significance for drug discovery and development [184, 187]. Various ways to target CSC signaling pathways are presented in Figure 5.
Table 2.
CSCs signal pathway targeting
| Pathway | Agents | Delivery system | Reference |
|---|---|---|---|
| Hedgehog pathway | Anthothecol | PLGA nanoparticles | [202] |
| Cyclopamine | System based on HPMA | [203] | |
| Notch pathway | γ-secretase inhibitor (GSI) | Imageable mesoporous silica nanoparticles (MSNPSs); engineered glucose functionalized MSNPs | [210, 211] |
| Platinum (IV) prodrug and siRNA | Micellar nanoparticles (MNP) | [212] | |
| Wnt pathway | Salinomycin | Self-assembled nanoparticle system based on iTEP | [221, 222] |
| Peptides targeting Wnt/LRP5/6 | Small magnetic iron oxide nanoparticles (IONP) | [223] | |
| Epithelial-mesenchymal transition | miR-200c | Gelatinase-responsive nanoparticles | [225] |
| Multiple signaling pathways | Graphene oxide | [226] | |
| Ti0.8O2 nanosheets | [227] |
Fig. 5.
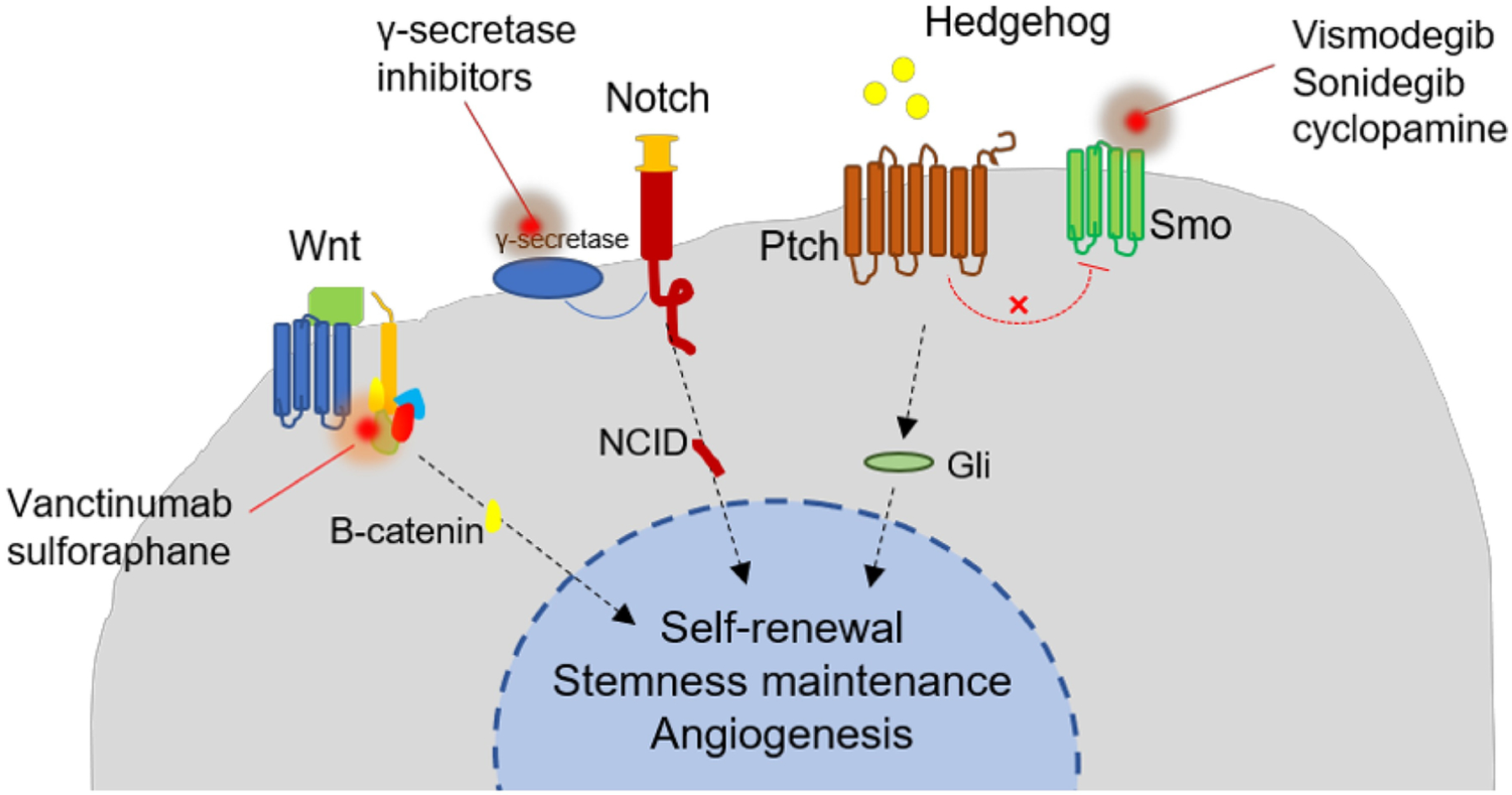
Potential ways to target signaling pathways in cancer stem cells
3.3.1. Hedgehog pathway
The Hedgehog (Hh) pathway maintains the complexity and function of stem and progenitor cells from embryonic development to the upkeep of fully grown adult organs [188, 189]. This signaling pathway has been widely reported to regulate CSCs in various cancer types [190, 191, 192, 193, 194, 195, 196, 197, 198]. It is likely that Hh signaling acts through multiple modes and is involved in interactions between CSCs, differentiated tumor cells, and the microenvironment [189]. Studies also indicate that Hh signaling is activated in human tumor samples and irradiated mouse xenografts that are resistant to radiation [185]. Hh ligands may also support the repropagation of residual tumor cells after radiation therapy [199]. Furthermore, inhibition of Hh signaling has been shown to reduce CSC stemness and chemoresistance [200].
A few small-molecule Hh inhibitors, including the FDA-approved Vismodegib and Sonidegib, are selective antagonists of Hh pathway that act by binding to Smoothened (Smo) receptor. They have been investigated in clinical trials but their usage is limited by weak Smo receptor binding (due to Smo bind site mutation) and poor systemic bioavailability [187, 201]. Therefore, various drug delivery systems have been employed to deliver new generation of antagonists to target Hh pathways, especially in the downstream of Smo. For example, anthothecol, a limonoid derived from plant Khaya anthotheca (Meliaceae), was encapsulated in PLGA nanoparticles (Antho-NPs) to improve its efficacy against pancreatic cancer cell lines. The results indicated that Antho-NPs inhibited CSC growth via the Hh signaling pathway [202]. Yan Zhou etal, developed a delivery system based on N-(2-hydroxypropyl) methacrylamide (HPMA) to enhance drug solubility and reduce the systemic toxicity of cyclopamine, a Hh inhibitor. This system showed anti-CSC effectiveness in RC-92a /hTERT cells as assessed by reduced expression of stem cell markers and viability of CSCs [203].
3.3.2. Notch pathway
Notch signaling is believed to regulate both normal stem cell and CSC function [204]. Inhibition of the Notch pathway is expected to significantly diminish self-renewal, clonogenicity, and tumorigenicity in CSCs, leading to a reduction in CSC-like subpopulations and increase in the sensitivity of CSCs to radiation-induced apoptosis [205]. In primary and metastatic tumors, Notch participates in tumor stroma and tumor endothelium interactions [206, 207,208]. Notch also interacts with multiple transcription and growth factors that participate in EMT, including snail, slug, and TGF-β [206]. Due to a significant interest in selectively targeting CSCs with novel therapeutics, Notch is one of the most intensively researched therapeutic, with several Notch inhibitors and delivery systems currently under investigation [208, 209].
Clinical use of Notch inhibitors is limited by severe side effects, thus, targeted delivery approaches have been evaluated for these molecules. Mamaeva et al. used imageable mesoporous silica nanoparticles (MSNPs) as vehicles for targeted delivery of γ-secretase inhibitors (GSIs), potent interceptors of Notch signaling [210]. Since CSC self-renewal is promoted by Notch signaling in breast cancer, they display high glycolytic activity and aggressive hormone-independent tumor growth. The same research group further utilized CSCs’ glycolytic phenotype and reliance on Notch activity, and engineered glucose functionalized MSNPs loaded with a γ-secretase inhibitor to selectively target CSCs. The data showed that these nanoparticles can be efficiently internalized by CSCs in vitro and in vivo [211].
Notch inhibition can also be used to sensitize tumors to other chemotherapeutic agents. Shen and his group developed a micellar nanoparticle (MNP) formulation to deliver platinum (IV) prodrug and siRNA targeting Notch1. They observed that siRNA-mediated suppression of Notch1 improved the sensitivity of hepatocellular carcinoma (HCC) cells to platinum drugs and reduced the proportion of HCC CSCs, resulting in improved inhibition of proliferation and induction of apoptosis in HCC cells in vitro [212].
3.3.3. Wnt pathway
The Wnt pathway controls a set of genes that are important for different biological functions. Canonical Wnt/β-catenin deregulation has been observed in various kinds of cancer, including colon, liver, lung, breast, ovarian and leukemia, making it an appealing target [213]. Wnt/β-catenin signaling has been speculated to play a key role in CSC development [198, 213, 214, 215, 216, 217]. Jang’s group found that Wnt/β-catenin regulates self-renewal and migration of CSCs to promote breast cancer development as well as metastasis/systemic dissemination. The authors suggest that Wnt/β-catenin signaling could serve as a novel target for breast CSCs (BCSCs) [218]. In order to inhibit BCSCs self-renewal, Huang et al. developed self-assembled PLGA/HA block copolymer-based nanocarriers and delivered a β-catenin inhibitor, sulforaphane (SFN) to silence the Wnt pathway in BCSCs. The results showed that SFN-loaded nanoparticles were more effective than free SFN in inhibiting BCSCs. They also found that combination therapy with docetaxel (DTX)- and SFN-loaded nanoparticles could strongly inhibit BCSC self-renewal and demonstrated targeting differentiated breast cancer cells and BCSCs simultaneously [219]. Zhi et al. found that Wnt signaling is activated in HCC, with decreased βII-Spectrin (SPTBN1, the most common non-erythrocytic member of the β-spectrin gene family). Their data indicated that loss of SPTBN1 activates Wnt signaling, promotes acquisition of stem cell-like features, and ultimately contributes to malignant tumor progression [220]. Zhao et al. also conjugated an immune tolerant elastin-like polypeptide (iTEP) to salinomycin, a Wnt/β-catenin signaling pathway inhibitor, and constructed a self-assembled nanoparticle system, which displayed significant tumor growth reduction in a 4T1 orthotopic breast cancer model [221, 222]. To efficiently treat resistant tumors, Miller-Kleinhenz et al. developed a small magnetic iron oxide nanoparticle (IONP) drug carrier combined with peptides that dually targeted Wnt/LRP5/6 and urokinase plasminogen activator receptor (uPAR). Wnt/β-catenin and cancer stem phenotype of tumor cells were inhibited by the dual receptor directed IONPs, leading to greater tumor growth inhibition [223].
3.3.4. Epithelial-mesenchymal transition
Recent studies suggest that EMT plays a significant role in disease progression and generation of distant metastasis in many solid tumors [108, 109]. EMT is a regulatory development program essential for the creation and maintenance of CSCs [110]. Acquisition of CSC characteristics is strongly linked to the progress of EMT transdifferentiation [111]. EMT is also crosslinked with multiple signaling pathways. Wnt and Hh pathways can operate together to control cell behavior across epithelialmesenchymal boundaries and are significant EMT mediators in tumor progression [185]. Inhibition of EMT as a means to target CSC has been established as a promising therapeutic strategy [224]. Liu et al. developed gelatinase-responsive nanoparticles to co-deliver CSC inhibitor miR-200c and docetaxel, to inhibit both CSCs and non-CSCs. This system showed significant inhibition of EMT characterized by greater E-cadherin expression and reduced CD44 expression. The synergistic delivery of miR-200c and docetaxel inhibited tumor growth in a synergistic manner [225].
Novel materials have recently been discovered to target CSCs via inhibition of multiple signal pathways. For instance, according to a study published by Fiorillo and coworkers, graphene oxide can selectively inhibit the proliferative expansion of CSCs through inhibition of different signal transduction pathways, including Wnt, Notch, STAT1/3 and NRF-2 [226]. Petpiroon et al. found that Ti0.8O2 nanosheets exhibit similar properties and have promising lung CSC suppression potential with minimal cytotoxicity to normal stem cells. This is due to Ti0.8O2 nanosheet treatment attenuating CSC properties through the suppression of Akt/GSK3 pathway, which in turn suppresses Akt/GSK3β/β-catenin and Notch1, as well as EMT [227].
3.4. Targeting tumor microenvironment or tumor niche
Because CSCs rely on unique niche factors, targeting the CSC niche in tumors or interfering with the CSC-niche interactions may improve treatment efficacy [98]. For example, the niche transforms into a supportive environment for leukogenic leukemia stem/progenitor cell (LSPC) survival and expansion protecting them from chemotherapy. Gul-Uludag et al. developed lipid-substituted polyethyleneimine/siRNA complexes to deliver siRNA into difficult-to-transfect myeloid leukemia cell lines. The polymeric nanoparticle-mediated silencing was able to inhibit LSPCs’ interactions with their microenvironment, resulting in the inhibition of leukemia progression [228].
The rarity of CSC population, tumor heterogeneity and dense ECM network are major challenges to intra-tumoral penetration of drugs and drug delivery systems [229, 230]. Rather than being trapped in the tumor matrix just outside tumor vessels, the drug carriers must demonstrate good tumor accumulation and tumor penetration to access CSCs [104, 230, 231, 232]. For instance, co-treatment with a TGF-β inhibitor resulted in enhanced vascular leakage and improved tumor penetration of siRNA loaded nanoparticles, followed by internalization by CSCs [104]. Deep tumor penetration can also be achieved by bioinspired strategies. Ferritin is an endogenous iron storage protein, which presents as a cage-like structure that can load a wide range of therapeutic agents [233, 234]. It was found that CSCs in brain tumor may preferably require ferritin to promote in vivo propagation and tumorigenesis [235]. By using this attribute, Tan et al. developed tumor-penetrating, biomimetic nanocages as a promising drug nanocarrier to treat metastasis and improve access to CSC fractions in the tumor [236].
Sun et al. proposed that the extra domain B of fibronectin (EDB-FN), linked to tumor angiogenesis, could be a new biomarker for BCSC detection due to a high expression of EDB-FN in breast cancer stem cell line NDY-1 cells compared to other cells [237]. They also investigated a new liposomal system (APTEDB-LS-siRNAEDB) for EDB-FN targeting and knockdown. According to the report, this system presented potent therapeutic efficacy in the BCSC-derived tumors in vivo. The siRNA-mediated EDB-FN knockdown led to lower expression of VEGF in endothelial cells [238].
It is known that CSCs are often located within hypoxic niches and HIFs play an important maintenance role. HIF inhibitors targeting CSCs can enhance the efficacy of angiogenesis inhibitors and cytotoxic chemotherapy. By using decoy oligodeoxynucleotide (ODN) and lentivirus-siRNA delivery system, Zhu et al. showed that downregulation of HIF-1α reversed chemoresistance and induced apoptosis in MDA-MB-231 cells, majority of which were CD44+CD24−/low BCSCs [239]. A metallofullerenol nanomaterial, Gd@C82(OH)22, was recently reported to have inhibitory activity against TNBC cells by blocking EMT, with resultant efficient elimination of BCSCs. Under hypoxic microenvironment, cellular uptake of Gd@C82(OH)22 increased and the nanomaterial was found to be a potent HIF-1α and TGF-β inhibitor [240].
3.5. Targeting metabolism
In recent years, metabolism has been shown to play a vital role in supporting CSC phenotype. Unlike bulk cancer cells, CSCs are thought to have a metabolic phenotype that is comparable to ordinary stem cells [241]. The metabolic singularities and the impact of diverse metabolites on CSC physiology have not been fully explored. However, some studies have showed that compared with the bulk tumor, CSCs have a distinctive metabolic phenotype. According to some studies, CSCs appear to bear a differentiated metabolic phenotype in different types of cancers and are dependent on oxidative phosphorylation (OXPHOS) and/or mitochondrial function [242, 243, 244, 245, 246]. Reports of metabolic flexibility of CSCs, including glycometabolic pattern and mitochondrial function, have also been published [247, 248]. By using the metabolic characteristics, smart and effective drug delivery systems could be designed to target therapeutic agents to CSCs. Based on the overexpression of glucose transporter 1 (GLUT1), glucose-installed sub-50-nm gold nanoparticles (Glu-NPs) were fabricated by Yi et al. to deliver siRNA to achieve sequence-specific gene silencing in BCSCs [249].
Effective and preferably selective distribution of drug to mitochondria in cancer cells is a significant consideration in designing a new CSC targeting strategies. CSCs that rely on OXPHOS demonstrate higher mitochondrial mass as well as higher mitochondrial membrane potential compared to non-CSC [243, 250]. Complex drug delivery systems or nanocarriers can be designed to establish drug accumulation in mitochondria. Studies have shown that mitochondria-targeting liposomes can enhance ROS generation, induce morphological and functional changes, enhance apoptosis or necrosis, or prevent the development of resistance [251, 252, 253, 254]. This strategy can also lead to mitochondrial drug accumulation, pro-apoptotic protein recruitment and activation, mitochondrial permeability transition pore (mPTPs) opening and mitochondrial inner membrane depolarization, eventually resulting in the activation of apoptotic machinery [255].
Previous studies have reported that following radiation exposure, reduced levels of ROS in CSCs are related to lesser DNA damage and a greater rate of cell survival [256–258]. This not only maintains CSC stemness but also confers treatment resistance [259, 260, 261, 262]. Increased ROS production and decreased oxidative defense can cause CSCs to pharmacologically lose their stemness. Reduction in ROS levels has the potential to be used as a combination therapy with standard chemotherapy [263, 264, 265, 266, 267, 268, 269, 270, 271, 272]. Notably, a combined therapy of demethoxycurcumin (DMC) and temozolomide (TMZ), which resulted in modifications in various cell signaling pathways including ROS generation and caspase-3 signaling of mitochondria-related apoptosis activation as well as inactivation of the JAK / STAT3 signaling pathway, was regarded an efficient anti-glioma stem cell treatment [273].
Recently, metal complexes containing endogenous metals and metallopeptides as well as metal-containing drug carriers have been found to have the ability to kill CSCs by targeting mitochondria, and elevating ROS levels [274]. Silver nanoparticles (AgNPs) have been reported to have a significant effect on cell viability, ROS generation and LDH leakage in A2780 ovarian cancer cells (and four different subpopulations of CSCs derived from A2780 cells). Further, ALDH+/CD133+ cells were more sensitive to the treatment than bulk cells. AgNPs have the capacity to increase ROS generation and oxidative stress. Thus, AgNPs were found to have the potential as CSCs-targeted therapeutic agents [275, 276]. Laws et al. developed a metallopeptide that can trigger mitochondrial damage in BCSCs [277]. Purushothaman et al. reported a Ru(ii) complex that can target mitochondrial and endoplasmic reticulations to effectively eliminate CSCs in MCF-7 breast cancer cells [278]. Stilgenbauer et al. developed a spermine-conjugated lipophilic Pt(IV) prodrug, which can accumulate Pt(IV) in mitochondria, reduce CSC population, and overcome drug resistance in ovarian cancer [279].
Another evolving idea in the field of CSC metabolism is that compared to the bulk cancer cells, CSCs rely highly on lipid metabolism for maintaining stemness and fulfilling energy demands [280]. The dysregulated lipid metabolism in CSCs is a result of upregulation of FA synthesis, extracellular lipid uptake, intracellular lipid droplet accumulation and β-oxidation. The increased lipogenesis in CSCs is linked to modifications in the levels of key lipogenic regulators, which could be exploited for therapeutic CSCs targeting [280, 281]. For example, CSCs are highly dependent on the operation of lipid metabolic enzymes such as 3-hydroxy-3-methylglutharyl-coenzyme A reductase (HMG-CoAR) and stearoyl-CoA desaturase 1 (SCD1). SCD1 can enhance the lipid-modified Wnt pathway and lead to both β-catenin and YAP/TAZ release. Following this, β-catenin and YAP/TAZ translocate and interact with their transcriptional partners, triggering the reprogramming of cancer cells to CSCs. Similarly, HMG-CoAR is a key point in the mevalonate pathway, a cascade culminating in the production of farnesyl pyrophosphate (FPP) and geranylgeranyl pyrophosphate (GGPP). This maintains correct membrane anchoring of Ras homologous family small guanosine triphosphatases (RHO GTPases), an essential enzyme for CSC stemness. Further, SCD1 also activates the NF-kB pathway, increases the expression of lipid desaturases and feeds a positive feedback loop. Thus, targeting of SCD1 and HMG-CoAR could be a promising therapeutic approach [282]. Finally, lipids play an important role in regulating membrane P-gp function, membrane trafficking, drug transport and endocytic function; changes in lipid biosynthesis in cancer can influence drug transport and endocytic functions [283]. Softening of the cell membrane increases drug resistance, migration and metastatic potential. Engineering the biophysical properties of the cancer cell membrane such as altering stiffness by phosphatidylcholine liposome treatment promoted caveolae recruitment and increased dynamin-dependent uptake of nanoparticles [284]. Hence, lipid-related drug delivery strategies are another approach to overcome drug resistance. In addition to these, many studies have focused on other metabolic characteristics in CSCs and revealed peculiar metabolic patterns. For example, CSCs are characterized by high cellular iron content, and enhanced iron levels correlate with sphere forming ability as well as with significant modifications in stem cell marker expression that can be reversed by iron removal [285].
3.6. Autophagy activation
Autophagy is an evolutionarily conserved mechanism that CSCs utilize to protect themselves against environmental stresses arising from hypoxia, nutrient deficiency, radiation, and chemotherapy [286, 287, 288, 289, 290]. As an adaptive mechanism, autophagy plays a crucial role in the survival, self-renewal, differentiation, and tumorgenicity of CSCs [30].
Accumulating evidence confirms the dual function of autophagy, as either a cancer inhibitor or promoter, in diverse settings and various tumor types [272, 291]. For instance, some research suggests that autophagy could induce apoptosis in breast and pancreatic CSCs through different molecular mechanisms [292, 293]. On the other hand, autophagy may enable cancer cells to survive multiple environmental stresses or promote carcinogenesis by influencing cell signaling or inducing intracellular toxicity [30]. In addition, autophagy signaling is linked to EMT, which has strong correlation with invasion and metastasis [294, 295, 296]. In some tumor types, autophagy suppression could inhibit the migration of CSCs, whereas its upregulation might restore the mesenchymal phenotype [297, 298]. Furthermore, it has been shown that the role of autophagy in regulating CSC resistance to various therapeutics is context- and cancer type-dependent [299, 300]. Some studies have shown that the combination of autophagy inhibitors and anticancer drugs could enhance the susceptibility of CSCs to chemotherapeutics [301].
Clarifying the relationship between autophagy and stem cell-like properties in various types of CSCs requires more thorough investigation. Nevertheless, application of nanoparticulate delivery systems to target autophagy in CSCs is an exciting new avenue for cancer treatment. For example, Sun et al. showed that nanocarrier-mediated autophagy inhibition markedly reduced the ‘stemness’ of human BCSCs and enhanced their susceptibility to chemotherapeutics [301]. In addition, combining AgNPs with salinomycin was found to exert a synergistic effect in inducing apoptosis and autophagy in ovarian cancer cells [302]. Furthermore, Lu et al. found that an albumin nanoparticle delivery system loaded with paclitaxel and autophagy inhibitor chloroquine, could effectively cross blood brain barrier to target glioma cells, induce apoptosis in glioma cells, and increase the sensitivity of glioma stem cells to paclitaxel [303]. Similarly, Chang et al. developed a formulation, termed 188Re-liposome, to effectively suppress the function of CSCs by targeting autophagy and mitophagy in ovarian cancer [304].
3.7. CSCs-targeted immunotherapy
Based on greater immunological characterization of CSCs and better understanding of interactions between cancer tissue and the immune system, immunotherapy, especially immunological cytotherapy, shows great promise in the treatment of cancer. In vitro generated dendritic cell (DC) vaccines have long been known for their ability to induce effective anticancer immune response. Selection of the tumor antigen and the transfection method to introduce the antigen in the DCs are critical determinants in DC-based vaccines [305]. In one previous study, DCs were pulsed with colon CSCs lysates, which acted as a potent antigen to target CSCs in colon cancer [306]. Interestingly, Szaryńska et al. found that the maturation and activation of DCs was influenced by the detailed configuration of CSC-like markers after cancer cell lysate or culture supernatant stimulation [307].
NK cells can selectively identify and lyse CSCs. Steven and coworkers found that CSCs expressing high amounts of the CSC-associated proteins like ALDH, CD133, CD24, or CD44 can be killed by activated allogeneic NK cells [308]. However, CSCs have been observed to evade NK cell mediated cytotoxicity by shedding NK cell ligands such as major histocompatibility complex (MHC) class I chain-related protein A (MICA) and MHC class I chain-related protein B (MICB). Hence, one approach to improve NK cell-mediated CSC elimination is by using CSC-epitope-specific monoclonal antibodies [309].
T cells play a central role in many immunotherapy strategies including inhibition of immunosuppressive cells (Tregs), adoptive T-cell therapy (ACT), and T cell activation through immunogenic cell death induced by oncolytic virotherapy (OVT), among others. [310]. Over the past decade, engineered CAR T-cells have showed enormous potential in tumor treatment, including targeting CSCs. CAR T-cells have been shown to eradicate CSCs selectively in several tumor models [311, 312, 313]. The blockade of immune checkpoint inhibitors, such as PD-1/PD-L1 and CTLA-4 has also shown to decrease CSC escape from immune system, thus enhancing the eradication of CSCs [314, 315, 316, 317].
4. Problems and prospects
4.1. CSCs isolation and identification
Because CSCs, normal stem cells and cancer cells share many attributes, there have been several attempts to develop methods to specifically isolate and identify CSCs [100, 318]. Serial colony-forming unit assays (replanting assay), label-retention assays and sphere-formation assays are commonly used in vitro assays to identify CSCs [319, 320, 321], while side population method, cellular markers method and establishment culture methods are utilized for CSCs isolation [322]. While each of these methods have advantages, there are also potential pitfalls that complicate interpretation of the results [321, 322]. Thus, failure to adequately recognize and isolate CSCs is still a significant obstacle to specifically target CSCs. For example, in some cases, cancers believed to have only rare tumorigenic cells may actually have common tumorigenic cells [323]. As a result, it has been suggested that multiple techniques, applied in combination or in sequence, should be used for CSC identification and isolation [321, 322, 323, 324]. In addition, efficacy of anti-CSCs drugs should also be evaluated by more relevant methods. The efficacy of CSC therapy is related more to the tumor initiating ability than the tumor size. Molecular imaging technologies could be valuable tools to observe the CSC populations [325].
4.2. In vitro CSC models
An important future direction for oncotherapeutic research is developing correlative in vitro models to improve our understanding of tumor progression. Due to the difficulties in studying CSCs through in vivo models due to high costs and ethical concerns, there is a clear need for efficient and predictive in vitro model systems and in vitro screening methodologies to study CSC targeting. One of the most widely used methods for CSC isolation is detection of CSC specific markers such as CD133, CD44, EpCAM, Nanog, Oct4, Sox2, ALDH-1, CXCL12, ABCG and bmi-1. CSCs could be sorted and separated from existing tumor lines or created cell lines by genetic manipulation or defined serum-free conditions [326, 327, 328]. CSCs proliferating in 3D culture systems display physiologically similar tumor characteristics as seen in vivo. These include similar signaling pathways, gene expression, cell–cell interactions, and drug resistance mechanisms. Using tumor spheroids (floating spheres) in vitro has been shown to improve drug efficacy and toxicity predictions. Several other approaches have been established to examine CSC populations. As EMT plays a vital role in tumor progression and drug resistance, it is essential to establish 3D culture models mimicking in vivo settings where reversibility of EMT and its role in tumorigenesis is accounted. Wang et al created a 3D bioprinted glioma cell-laden scaffold to enrich glioma stem cells and found that the stemness and EMT-related gene expression of glioma cells were increased in the 3D platform [329]. Rashidi et al. reported an in vitro model of chemoresistance and stemness in ovarian cancer using the 3D hanging drop spheroid platform [330]. Tumor spheroids can also be arranged by distinctive sources of cells, allowing in vitro studies to closely mimic the in vivo conditions and TME. Multicellular tumor spheroids (MCTS) of MCF-7 cells and the ones enriched with CSCs (eMCTS) were evaluated by Herheliuk et al [327]. The authors found that eMCTS model could improve the anti-CSC drug screening efficiency. A unique synthetic tumor microenvironment mimic system (STEMs) composed of human lung epithelial cells, pulmonary vascular endothelial cells and human marrow-derived mesenchymal stems cells (MSCs) was created for in vitro tumor biology and drug test studies [331]. A key drawback with tumor spheroids is that they do not develop the complex (yet underdeveloped) tumor vasculature. Thus, all transport (drugs, nutrients, gas exchange) occurs only from outside to inside and relies primarily on diffusion. The transport processes in a tumor tissue in vivo, on the other hand, is much more complex with diffusion, convection, and convective diffusion all playing a role. This difference in transport mechanisms is especially important while evaluating delivery systems [332, 333]. The use of microfluidic models may address some of the transport issues, but these systems lack the complexity of tumor spheroids [334, 335]. Since tumor vasculature plays a key role in management of TME, its importance in in vitro tumor models cannot be overlooked. The proximity of tumor cells to perfused blood vessels is essential to receive nutrients and oxygen. Solid tumors, hence, become angiogenic to survive and proliferate. Chemotherapy relies largely on tumor vasculature to deliver drugs to tumor cells. A quantitative understanding of tumor vasculature with in vitro models based on average vessel diameter, vascular density, and the relative vascular volume will allow for improved vascular targeting and selecting appropriate radiation therapies. A hybrid model that incorporates transport complexity of microfluidics, cellular/TME complexity of spheroids, and quantitative tumor morphology could further address the limitations of in vitro systems.
4.3. Role of drug delivery systems in CSC-targeted therapy
Improved understanding of molecular mechanisms related to CSC generation and development, CSC signaling pathways, context-dependent interactions and regulation, as well as other aspects of CSC biology will increase the possibility of designing effective therapeutic regimens [206]. Although more successful targeting and elimination of CSCs could be achieved through identification and elucidation of specific characteristics of CSCs, developing effective drug delivery platforms is still an important objective for opening up new possibilities for therapeutic intervention and improving the success of current treatments [211, 318, 335]. As discussed previously, drug delivery systems can overcome the limitations of CSC-specific agents, such as poor water solubility, short circulation time and instability and also hold great potential for overcoming the difficulties in CSC elimination (such as poor niche penetration, low drug accumulation in CSCs, radio-/chemo-therapy resistance and detrimental effects on normal somatic stem cells). Smart drug delivery platforms that respond to specific environmental cues can further enhance the performance of anticancer therapy [101, 183, 336, 337]. In addition, formulation characteristics such as particle size, shape and surface modifications could be fine-tuned to design patient specific nanomedicines [336, 338]. Incorporation of more than one agent in the same delivery system could help address the resistance mechanisms utilized by CSCs. For example, incorporation of a drug efflux inhibitor along with an anticancer drug that is a substrate for a drug efflux pump could overcome drug resistance [339]. However, it should be noted that cancer cells in general employ more than one mechanism to evade drug-induced cytotoxicity. Thus, an inhibitor with a wide-ranging mechanism of action is more likely to be successful than those that are focused on any one mechanism. In addition, physical-chemical modalities such as photodynamic and photothermal therapies that induce rapid cell kill via necrosis are less likely to develop resistance than pharmacological approaches [340]. Targeting such modalities specifically to CSCs is challenging but warrants additional investigation.
In summary, CSCs represent a formidable barrier to the success of anticancer therapies in several different cancer types. A combination of strategies that include inhibitors that can either directly kill CSCs or improve the effectiveness of co-delivered therapies as well as formulations that can target such therapies specifically to CSCs can significantly improve treatment outcomes for patients diagnosed with cancer.
Fig. 3.
Link between CSCs, tumor metastasis and epithelial-mesenchymal transition (EMT)
Financial support:
Funding was provided by the Community of Pharmaceutical Development (CPD), Office of Discovery and Translation (ODAT) at the University of Minnesota, the Randy Shaver Foundation and NIH R01EB019893.
Footnotes
Publisher's Disclaimer: This Author Accepted Manuscript is a PDF file of an unedited peer-reviewed manuscript that has been accepted for publication but has not been copyedited or corrected. The official version of record that is published in the journal is kept up to date and so may therefore differ from this version.
Animal Studies: No animal or human studies were carried out by the authors for this article
Conflict of interest: The authors, Jin Cao, Shubhmita Bhatnagar, Jiawei Wang, Xueyong Qi, Swayam Prabha, and Jayanth Panyam declare that they have no conflict of interests.
References
- 1.Sell S. Cellular origin of cancer: dedifferentiation or stem cell maturation arrest? Environmental Health Perspectives. 1993;101(suppl 5):15–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chapter 12 - Cancer Stem Cells: Where Do They Come From and Where Are They Going? In: Mummery C, van de Stolpe A, Roelen BAJ, Clevers H, editors. Stem Cells (Second Edition). Boston: Academic Press; 2014. p. 315–41. [Google Scholar]
- 3.Bonnet D, Dick JE. Human acute myeloid leukemia is organized as a hierarchy that originates from a primitive hematopoietic cell. Nat Med. 1997;3(7):730–7. [DOI] [PubMed] [Google Scholar]
- 4.Al-Hajj M, Wicha MS, Benito-Hernandez A, Morrison SJ, Clarke MF. Prospective identification of tumorigenic breast cancer cells. Proc Natl Acad Sci U S A. 2003;100(7):3983–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bomken S, Fišer K, Heidenreich O, Vormoor J. Understanding the cancer stem cell. Br J Cancer. 2010;103(4):439–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cabrera MC, Hollingsworth RE, Hurt EM. Cancer stem cell plasticity and tumor hierarchy. World J Stem Cells. 2015;7(1):27–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Li C, Heidt DG, Dalerba P, Burant CF, Zhang L, Adsay V et al. Identification of Pancreatic Cancer Stem Cells. Cancer Res. 2007;67(3):1030. [DOI] [PubMed] [Google Scholar]
- 8.Singh SK, Clarke ID, Terasaki M, Bonn VE, Hawkins C, Squire J et al. Identification of a Cancer Stem Cell in Human Brain Tumors. Cancer Res. 2003;63(18):5821. [PubMed] [Google Scholar]
- 9.Lapidot T, Sirard C, Vormoor J, Murdoch B, Hoang T, Caceres-Cortes J et al. A cell initiating human acute myeloid leukaemia after transplantation into SCID mice. Nature. 1994;367(6464):645–8. [DOI] [PubMed] [Google Scholar]
- 10.Visvader JE, Lindeman GJ. Cancer stem cells in solid tumours: accumulating evidence and unresolved questions. Nat Rev Cancer. 2008;8:755–68. [DOI] [PubMed] [Google Scholar]
- 11.Matsui W, Huff CA, Wang Q, Malehorn MT, Barber J, Tanhehco Y et al. Characterization of clonogenic multiple myeloma cells. Blood. 2004;103(6):2332–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ghosh N, Matsui W. Cancer stem cells in multiple myeloma. Cancer Lett. 2009;277(1):1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Duan J-J, Qiu W, Xu S-L, Wang B, Ye X-Z, Ping Y-F et al. Strategies for isolating and enriching cancer stem cells: well begun is half done. Stem Cells Dev. 2013;22(16):2221–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bighetti-Trevisan RL, Sousa LO, Castilho RM, Almeida LO. Cancer Stem Cells: Powerful Targets to Improve Current Anticancer Therapeutics. Stem Cells International. 2019;2019:9618065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.D’Andrea V, Guarino S, Di Matteo FM, Maugeri Saccà M, De Maria R. Cancer stem cells in surgery. G Chir. 2014;35(11–12):257–9. [PMC free article] [PubMed] [Google Scholar]
- 16.Plaks V, Kong N, Werb Z. The Cancer Stem Cell Niche: How Essential Is the Niche in Regulating Stemness of Tumor Cells? Cell Stem Cell. 2015;16(3):225–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Garcia-Mayea Y, Mir C, Masson F, Paciucci R, Lleonart ME. Insights into new mechanisms and models of cancer stem cell multidrug resistance. Semin Cancer Biol. 2020;60:166–180. [DOI] [PubMed] [Google Scholar]
- 18.Gillet J-P, Gottesman MM. Mechanisms of Multidrug Resistance in Cancer. In: Zhou J, editor. Multi-Drug Resistance in Cancer. Totowa, NJ: Humana Press; 2010. p. 47–76. [DOI] [PubMed] [Google Scholar]
- 19.Najafi M, Mortezaee K, Majidpoor J. Cancer stem cell (CSC) resistance drivers. Life Sci. 2019;234:116781. [DOI] [PubMed] [Google Scholar]
- 20.Heddleston JM, Li Z, McLendon RE, Hjelmeland AB, Rich JN. The hypoxic microenvironment maintains glioblastoma stem cells and promotes reprogramming towards a cancer stem cell phenotype. Cell Cycle. 2009;8(20):3274–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Takebe N, Harris PJ, Warren RQ, Ivy SP. Targeting cancer stem cells by inhibiting Wnt, Notch, and Hedgehog pathways. Nat Rev Clin Oncol. 2011;8(2):97. [DOI] [PubMed] [Google Scholar]
- 22.Luo B, Lee AS. The critical roles of endoplasmic reticulum chaperones and unfolded protein response in tumorigenesis and anticancer therapies. Oncogene. 2013;32(7):805–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schulz A, Meyer F, Dubrovska A, Borgmann K. Cancer Stem Cells and Radioresistance: DNA Repair and Beyond. Cancers (Basel). 2019;11(6):862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Easwaran H, Tsai H-C, Baylin Stephen B. Cancer Epigenetics: Tumor Heterogeneity, Plasticity of Stem-like States, and Drug Resistance. Mol Cell. 2014;54(5):716–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cancer Genome Atlas Research Network. Genomic and Epigenomic Landscapes of Adult De Novo Acute Myeloid Leukemia. N Engl J Med. 2013;368(22):2059–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kanai Y, Ushijima S, Nakanishi Y, Sakamoto M, Hirohashi S. Mutation of the DNA methyltransferase (DNMT) 1 gene in human colorectal cancers. Cancer Lett. 2003;192(1):75–82. [DOI] [PubMed] [Google Scholar]
- 27.Wainwright EN, Scaffidi P. Epigenetics and Cancer Stem Cells: Unleashing, Hijacking, and Restricting Cellular Plasticity. Trends Cancer. 2017;3(5):372–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nazio F, Bordi M, Cianfanelli V, Locatelli F, Cecconi F. Autophagy and cancer stem cells: molecular mechanisms and therapeutic applications. Cell Death Differ. 2019;26(4):690–702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Smith AG, Macleod KF. Autophagy, cancer stem cells and drug resistance. J Pathol. 2019;247(5):708–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nazio F, Bordi M, Cianfanelli V, Locatelli F, Cecconi F. Autophagy and cancer stem cells: molecular mechanisms and therapeutic applications. Cell Death Differ. 2019;26(4):690–702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bertino JR. Cancer research: from folate antagonism to molecular targets. Best Pract Res Clin Haematol. 2009;22(4):577–82. [DOI] [PubMed] [Google Scholar]
- 32.Noguchi K, Katayama K, Sugimoto Y. Human ABC transporter ABCG2/BCRP expression in chemoresistance: basic and clinical perspectives for molecular cancer therapeutics. Pharmgenomics Pers Med. 2014;7:53–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kim M, Turnquist H, Jackson J, Sgagias M, Yan Y, Gong M et al. The Multidrug Resistance Transporter ABCG2 (Breast Cancer Resistance Protein 1) Effluxes Hoechst 33342 and Is Overexpressed in Hematopoietic Stem Cells. Clin Cancer Res. 2002;8(1):22–8. [PubMed] [Google Scholar]
- 34.Teodori E, Dei S, Martelli C, Scapecchi S, Gualtieri F. The Functions and Structure of ABC Transporters: Implications for the Design of New Inhibitors of Pgp and MRP1 to Control Multidrug Resistance (MDR). Curr Drug Targets. 2006;7(7):893–909. [DOI] [PubMed] [Google Scholar]
- 35.Park S, Shimizu C, Shimoyama T, Takeda M, Ando M, Kohno T et al. Gene expression profiling of ATP-binding cassette (ABC) transporters as a predictor of the pathologic response to neoadjuvant chemotherapy in breast cancer patients. Breast Cancer Res Treat. 2006;99(1):9–17. [DOI] [PubMed] [Google Scholar]
- 36.Szakács G, Paterson JK, Ludwig JA, Booth-Genthe C, Gottesman MM. Targeting multidrug resistance in cancer. Nature Rev Drug Discov. 2006;5(3):219–34. [DOI] [PubMed] [Google Scholar]
- 37.Fletcher JI, Haber M, Henderson MJ, Norris MD. ABC transporters in cancer: more than just drug efflux pumps. Nature Rev Cancer. 2010;10:147–56. [DOI] [PubMed] [Google Scholar]
- 38.Wang X, Campos CR, Peart JC, Smith LK, Boni JL, Cannon RE et al. Nrf2 Upregulates ATP Binding Cassette Transporter Expression and Activity at the Blood–Brain and Blood–Spinal Cord Barriers. J Neurosci. 2014;34(25):8585–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ruban EL, Ferro R, Arifin Syamsul A, Falasca M. Lysophosphatidylinositol: a novel link between ABC transporters and G-protein-coupled receptors. Biochem Soc Trans. 2014;42(5):1372–7. [DOI] [PubMed] [Google Scholar]
- 40.Piñeiro R, Maffucci T, Falasca M. The putative cannabinoid receptor GPR55 defines a novel autocrine loop in cancer cell proliferation. Oncogene. 2011;30(2):142–52. [DOI] [PubMed] [Google Scholar]
- 41.Mitra P, Oskeritzian CA, Payne SG, Beaven MA, Milstien S, Spiegel S. Role of ABCC1 in export of sphingosine-1-phosphate from mast cells. Proc Natl Acad Sci U S A. 2006;103(44):16394–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Jordan CT, Upchurch D, Szilvassy SJ, Guzman ML, Howard DS, Pettigrew AL et al. The interleukin-3 receptor alpha chain is a unique marker for human acute myelogenous leukemia stem cells. Leukemia. 2000;14(10):1777–84. [DOI] [PubMed] [Google Scholar]
- 43.Hauswirth AW, Florian S, Printz D, Sotlar K, Krauth M-T, Fritsch G et al. Expression of the target receptor CD33 in CD34+/CD38−/CD123+ AML stem cells. Eur J Clin Invest. 2007;37(1):73–82. [DOI] [PubMed] [Google Scholar]
- 44.Hosen N, Park CY, Tatsumi N, Oji Y, Sugiyama H, Gramatzki M et al. CD96 is a leukemic stem cell-specific marker in human acute myeloid leukemia. Proc Natl Acad Sci U S A. 2007;104(26):11008–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Andrews TE, Wang D, Harki DA. Cell surface markers of cancer stem cells: diagnostic macromolecules and targets for drug delivery. Drug Deliv Transl Res. 2013;3(2):121–42. [DOI] [PubMed] [Google Scholar]
- 46.Walter RB, Appelbaum FR, Estey EH, Bernstein ID. Acute myeloid leukemia stem cells and CD33-targeted immunotherapy. Blood. 2012;119(26):6198–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sidney LE, Branch MJ, Dunphy SE, Dua HS, Hopkinson A. Concise review: evidence for CD34 as a common marker for diverse progenitors. Stem Cells. 2014;32(6):1380–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ferrero E, Malavasi F. The metamorphosis of a molecule: from soluble enzyme to the leukocyte receptor CD38. J Leukoc Biol. 1999;65(2):151–61. [DOI] [PubMed] [Google Scholar]
- 49.Su R, Chen M. CD123 Is a Useful Marker for Prediction of Clinical Outcome and Risk Stratification for Prognosis in Leukemia Patients. Am J Clin Pathol. 2015;144(suppl_2):A139. [Google Scholar]
- 50.Wang K, Wei G, Liu D. CD19: a biomarker for B cell development, lymphoma diagnosis and therapy. Exp Hematol Oncol. 2012;1(1):36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Haraguchi N, Ishii H, Mimori K, Tanaka F, Ohkuma M, Kim HM et al. CD13 is a therapeutic target in human liver cancer stem cells. J Clin Invest. 2010;120(9):3326–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Herrmann H, Sadovnik I, Cerny-Reiterer S, Rülicke T, Stefanzl G, Willmann M et al. Dipeptidylpeptidase IV (CD26) defines leukemic stem cells (LSC) in chronic myeloid leukemia. Blood. 2014;123(25):3951–62. [DOI] [PubMed] [Google Scholar]
- 53.Ghani FI, Yamazaki H, Iwata S, Okamoto T, Aoe K, Okabe K et al. Identification of cancer stem cell markers in human malignant mesothelioma cells. Biochem Biophys Res Commun 2011;404(2):735–42. [DOI] [PubMed] [Google Scholar]
- 54.Pang R, Law WL, Chu ACY, Poon JT, Lam CSC, Chow AKM et al. A Subpopulation of CD26+ Cancer Stem Cells with Metastatic Capacity in Human Colorectal Cancer. Cell Stem Cell. 2010;6(6):603–15. [DOI] [PubMed] [Google Scholar]
- 55.Jaggupilli A, Elkord E. Significance of CD44 and CD24 as Cancer Stem Cell Markers: An Enduring Ambiguity. Clin Dev Immunol. 2012;2012:1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Yang C-H, Wang H-L, Lin Y-S, Kumar KPS, Lin H-C, Chang C-J et al. Identification of CD24 as a Cancer Stem Cell Marker in Human Nasopharyngeal Carcinoma. PLoS ONE. 2014;9(6):e99412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zhu Y-Y, Yuan Z. Pancreatic cancer stem cells. Am J Cancer Res. 2015;5(3):894–906. [PMC free article] [PubMed] [Google Scholar]
- 58.Miyata T, Oyama T, Yoshimtsu T, Higa H, Kawano D, Sekimura A et al. The Clinical Significance of Cancer Stem Cell Markers ALDH1A1 and CD133 in Lung Adenocarcinoma. Anticancer Res. 2017;37(5):2541–7. [DOI] [PubMed] [Google Scholar]
- 59.Prince ME, Sivanandan R, Kaczorowski A, Wolf GT, Kaplan MJ, Dalerba P et al. Identification of a subpopulation of cells with cancer stem cell properties in head and neck squamous cell carcinoma. Proc Natl Acad Sci U S A. 2007;104(3):973–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Barzegar Behrooz A, Syahir A, Ahmad S. CD133: beyond a cancer stem cell biomarker. J Drug Target. 2019;27(3):257–69. [DOI] [PubMed] [Google Scholar]
- 61.Terris B, Cavard C, Perret C. EpCAM, a new marker for cancer stem cells in hepatocellular carcinoma. J Hepatol. 2010;52(2):280–1. [DOI] [PubMed] [Google Scholar]
- 62.Salnikov AV, Groth A, Apel A, Kallifatidis G, Beckermann BM, Khamidjanov A et al. Targeting of cancer stem cell marker EpCAM by bispecific antibody EpCAMxCD3 inhibits pancreatic carcinoma. J Cell Mol Med. 2009;13(9b):4023–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Shaikh MV, Kala M, Nivsarkar M. CD90 a potential cancer stem cell marker and a therapeutic target. Cancer Biomark. 2016;16(3):301–7. [DOI] [PubMed] [Google Scholar]
- 64.Fang D, Nguyen TK, Leishear K, Finko R, Kulp AN, Hotz S et al. A Tumorigenic Subpopulation with Stem Cell Properties in Melanomas. Cancer Res. 2005;65(20):9328–37. [DOI] [PubMed] [Google Scholar]
- 65.Redmer T, Walz I, Klinger B, Khouja S, Welte Y, Schäfer R et al. The role of the cancer stem cell marker CD271 in DNA damage response and drug resistance of melanoma cells. Oncogenesis. 2017;6(1):e291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Mochizuki M, Tamai K, Imai T, Sugawara S, Ogama N, Nakamura M et al. CD271 regulates the proliferation and motility of hypopharyngeal cancer cells. Sci Rep. 2016;6(1):1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Chan KS, Espinosa I, Chao M, Wong D, Ailles L, Diehn M et al. Identification, molecular characterization, clinical prognosis, and therapeutic targeting of human bladder tumor-initiating cells. Proc Natl Acad Sci U S A. 2009;106(33):14016–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Zalewski B, Famulski W, Sulkowska M, Sobaniec-Lotowska M, Piotrowski Z, Kisielewski W et al. CD44 expression in colorectal cancer. An immunohistochemical study including correlation with cathepsin D immunoreactivity and some tumour clinicopathological features. Folia Histochem Cytobiol. 2001;39Suppl 2:152–3. [PubMed] [Google Scholar]
- 69.Zhang S, Balch C, Chan MW, Lai H-C, Matei D, Schilder JM et al. Identification and Characterization of Ovarian Cancer-Initiating Cells from Primary Human Tumors. Cancer Res. 2008;68(11):4311–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Takada M, Yamamoto M, Saitoh Y. The significance of CD44 in human pancreatic cancer: I. High expression of CD44 in human pancreatic adenocarcinoma. Pancreas. 1994;9(6):748–52. [DOI] [PubMed] [Google Scholar]
- 71.Liu AY. Expression of CD44 in prostate cancer cells. Cancer Lett. 1994;76(1):63–9. [DOI] [PubMed] [Google Scholar]
- 72.Yan Y, Zuo X, Wei D. Concise Review: Emerging Role of CD44 in Cancer Stem Cells: A Promising Biomarker and Therapeutic Target. Stem Cells Transl Med. 2015;4(9):1033–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Takaishi S, Okumura T, Tu S, Wang SSW, Shibata W, Vigneshwaran R et al. Identification of Gastric Cancer Stem Cells Using the Cell Surface Marker CD44. Stem Cells. 2009;27(5):1006–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Todaro M, Gaggianesi M, Catalano V, Benfante A, Iovino F, Biffoni M et al. CD44v6 Is a Marker of Constitutive and Reprogrammed Cancer Stem Cells Driving Colon Cancer Metastasis. Cell Stem Cell. 2014;14(3):342–56. [DOI] [PubMed] [Google Scholar]
- 75.Glumac PM, LeBeau AM. The role of CD133 in cancer: a concise review. Clin Transl Med. 2018;7(1):18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Ma S, Chan KW, Hu L, Lee TKW, Wo JYH, Ng IOL et al. Identification and Characterization of Tumorigenic Liver Cancer Stem/Progenitor Cells. Gastroenterology. 2007;132(7):2542–56. [DOI] [PubMed] [Google Scholar]
- 77.Swaminathan SK, Olin MR, Forster CL, Cruz KSS, Panyam J, Ohlfest JR. Identification of a novel monoclonal antibody recognizing CD133. J Immunol Methods. 2010;361(1–2):110–5. [DOI] [PubMed] [Google Scholar]
- 78.Singh SK, Hawkins C, Clarke ID, Squire JA, Bayani J, Hide T et al. Identification of human brain tumour initiating cells. Nature. 2004;432(7015):396–401. [DOI] [PubMed] [Google Scholar]
- 79.Collins AT, Berry PA, Hyde C, Stower MJ, Maitland NJ. Prospective Identification of Tumorigenic Prostate Cancer Stem Cells. Cancer Res. 2005;65(23):10946. [DOI] [PubMed] [Google Scholar]
- 80.Kryczek I, Liu S, Roh M, Vatan L, Szeliga W, Wei S et al. Expression of aldehyde dehydrogenase and CD133 defines ovarian cancer stem cells. Int J Cancer. 2012;130(1):29–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Eramo A, Lotti F, Sette G, Pilozzi E, Biffoni M, Di Virgilio A et al. Identification and expansion of the tumorigenic lung cancer stem cell population. Cell Death Differ. 2007;15:504–14. [DOI] [PubMed] [Google Scholar]
- 82.Suetsugu A, Nagaki M, Aoki H, Motohashi T, Kunisada T, Moriwaki H. Characterization of CD133+ hepatocellular carcinoma cells as cancer stem/progenitor cells. Biochem Biophys Res Commun. 2006;351(4):820–4. [DOI] [PubMed] [Google Scholar]
- 83.O’Brien CA, Pollett A, Gallinger S, Dick JE. A human colon cancer cell capable of initiating tumour growth in immunodeficient mice. Nature. 2006;445:106–10. [DOI] [PubMed] [Google Scholar]
- 84.Swaminathan SK, Niu L, Waldron N, Kalscheuer S, Zellmer DM, Olin MR et al. Identification and characterization of a novel scFv recognizing human and mouse CD133. Drug Deliv Transl Res. 2013;3(2):143–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Griguer CE, Oliva CR, Gobin E, Marcorelles P, Benos DJ, Lancaster JR Jr. et al. CD133 Is a Marker of Bioenergetic Stress in Human Glioma. PLoS ONE. 2008;3(11):e3655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Baeuerle PA, Gires O. EpCAM (CD326) finding its role in cancer. Br J Cancer. 2007;96(3):417–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Huang Y, Ma Y, Gao P, Yao Z. Targeting CD47: the achievements and concerns of current studies on cancer immunotherapy. J Thorac Dis. 2017;9(2):E168–E74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Iozzo RV, Sanderson RD. Proteoglycans in cancer biology, tumour microenvironment and angiogenesis. J Cell Mol Med. 2011;15(5):1013–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Nikitovic D, Berdiaki A, Spyridaki I, Krasanakis T, Tsatsakis A, Tzanakakis GN. Proteoglycans-Biomarkers and Targets in Cancer Therapy. Front Endocrinol (Lausanne). 2018;9:69-. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Korpetinou A, Skandalis SS, Labropoulou VT, Smirlaki G, Noulas A, Karamanos NK et al. Serglycin: at the crossroad of inflammation and malignancy. Front Oncol. 2014;3:327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Elgundi Z, Papanicolaou M, Major G, Cox TR, Melrose J, Whitelock JM et al. Cancer Metastasis: The Role of the Extracellular Matrix and the Heparan Sulfate Proteoglycan Perlecan. Front Oncol. 2020;9(1482). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Kalscheuer S, Khanna V, Kim H, Li S, Sachdev D, DeCarlo A et al. Discovery of HSPG2 (Perlecan) as a Therapeutic Target in Triple Negative Breast Cancer. Sci Rep. 2019;9(1):12492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Sharma B, Handler M, Eichstetter I, Whitelock JM, Nugent MA, Iozzo RV. Antisense targeting of perlecan blocks tumor growth and angiogenesis in vivo. J Clin Invest. 1998;102(8):1599–608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Lathia JD, Heddleston JM, Venere M, Rich JN. Deadly teamwork: neural cancer stem cells and the tumor microenvironment. Cell Stem Cell. 2011;8(5):482–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Huo M, Zhao Y, Satterlee AB, Wang Y, Xu Y, Huang L. Tumor-targeted delivery of sunitinib base enhances vaccine therapy for advanced melanoma by remodeling the tumor microenvironment. J Control Release. 2016;245:81–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Ye J, Wu D, Wu P, Chen Z, Huang J. The cancer stem cell niche: cross talk between cancer stem cells and their microenvironment. Tumour Biol. 2014;35(5):3945–51. [DOI] [PubMed] [Google Scholar]
- 97.Cetinbas NM, Roper J, Yılmaz OmH. Biology and Engineering of Stem Cell Niches. The Cancer Stem Cell Niche. Academic Press; 2017. [Google Scholar]
- 98.Plaks V, Kong N, Werb Z. The cancer stem cell niche: how essential is the niche in regulating stemness of tumor cells? Cell Stem Cell. 2015;16(3):225–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Ping YF, Zhang X, Bian XW. Cancer stem cells and their vascular niche: Do they benefit from each other? Cancer Lett. 2016;380(2):561–7. [DOI] [PubMed] [Google Scholar]
- 100.Das M, Law S. Role of tumor microenvironment in cancer stem cell chemoresistance and recurrence. Int J Biochem Cell Biol. 2018;103:115–24. [DOI] [PubMed] [Google Scholar]
- 101.Dianat-Moghadam H, Heidarifard M, Jahanban-Esfahlan R, Panahi Y, Hamishehkar H, Pouremamali F et al. Cancer stem cells-emanated therapy resistance: Implications for liposomal drug delivery systems. J Control Release. 2018;288:62–83. [DOI] [PubMed] [Google Scholar]
- 102.Zhao Y, Dong QZ, Li JH, Zhang KL, Qin J, Zhao JG et al. Targeting cancer stem cells and their niche: perspectives for future therapeutic targets and strategies. Semin Cancer Biol. 2018;53:139–55. [DOI] [PubMed] [Google Scholar]
- 103.Garcia-Mazas C, Csaba N, Garcia-Fuentes M. Biomaterials to suppress cancer stem cells and disrupt their tumoral niche. Int J Pharm. 2017;523(2):490–505. [DOI] [PubMed] [Google Scholar]
- 104.Zuo ZQ, Chen KG, Yu XY, Zhao G, Shen S, Cao ZT et al. Promoting tumor penetration of nanoparticles for cancer stem cell therapy by TGF-beta signaling pathway inhibition. Biomaterials. 2016;82:48–59. [DOI] [PubMed] [Google Scholar]
- 105.Peng G, Liu Y. Hypoxia-inducible factors in cancer stem cells and inflammation. Trends Pharmacol Sci. 2015;36(6):374–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Carnero A, Lleonart M. The hypoxic microenvironment: A determinant of cancer stem cell evolution. Bioessays. 2016;38Suppl 1:S65–74. [DOI] [PubMed] [Google Scholar]
- 107.Li HJ, Yan WX, Suo XM, Peng HT, Yang XJ, Li ZH et al. Nucleus-targeted nano delivery system eradicates cancer stem cells by combined thermotherapy and hypoxia-activated chemotherapy. Biomaterials. 2019;200:1–14. [DOI] [PubMed] [Google Scholar]
- 108.Han ML, Zhao YF, Tan CH, Xiong YJ, Wang WJ, Wu F et al. Cathepsin L upregulation-induced EMT phenotype is associated with the acquisition of cisplatin or paclitaxel resistance in A549 cells. Acta Pharmacol Sin. 2016;37(12):1606–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Jin DJ, Fang YT, Li ZY, Chen ZY, Xiang JB. Epithelial-mesenchymal transition-associated microRNAs in colorectal cancer and drug-targeted therapies. Oncol Rep. 2015;33(2):515–25. [DOI] [PubMed] [Google Scholar]
- 110.Wang Z, Li Y, Ahmad A, Azmi AS, Kong D, Banerjee S et al. Targeting miRNAs involved in cancer stem cell and EMT regulation: An emerging concept in overcoming drug resistance. Drug Resist Updat. 2010;13(4–5):109–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Floor S, van Staveren WC, Larsimont D, Dumont JE, Maenhaut C. Cancer cells in epithelial-to mesenchymal transition and tumor-propagating-cancer stem cells: distinct, overlapping or same populations. Oncogene. 2011;30(46):4609–21. [DOI] [PubMed] [Google Scholar]
- 112.Agliano A, Calvo A, Box C. The challenge of targeting cancer stem cells to halt metastasis. Semin Cancer Biol. 2017;44:25–42. [DOI] [PubMed] [Google Scholar]
- 113.Nguyen LV, Vanner R, Dirks P, Eaves CJ. Cancer stem cells: an evolving concept. Nat Rev Cancer. 2012;12(2):133–43. [DOI] [PubMed] [Google Scholar]
- 114.Wang SS, Jiang J, Liang XH, Tang YL. Links between cancer stem cells and epithelial mesenchymal transition. Onco Targets Ther. 2015;8:2973–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Yoshida GJ, Saya H. Therapeutic strategies targeting cancer stem cells. Cancer Sci. 2016;107(1):5–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Yilmaz OH, Valdez R, Theisen BK, Guo W, Ferguson DO, Wu H et al. Pten dependence distinguishes haematopoietic stem cells from leukaemia-initiating cells. Nature. 2006;441(7092):475–82. [DOI] [PubMed] [Google Scholar]
- 117.Guo Q, Cao H, Qi X, Li H, Ye P, Wang Z et al. Research Progress in Reversal of Tumor Multi-drug Resistance via Natural Products. Anticancer Agents Med Chem. 2017;17(11):1466–76. [DOI] [PubMed] [Google Scholar]
- 118.Xu YY, Wang J, Li XF, Liu Y, Dai LR, Wu XC et al. Selective inhibition of breast cancer stem cells by gold nanorods mediated plasmonic hyperthermia. Biomaterials. 2014;35(16):4667–77. [DOI] [PubMed] [Google Scholar]
- 119.Ke XY, Ng VWL, Gao SJ, Tong YW, Hedrick JL, Yang YY. Co-delivery of thioridazine and doxorubicin using polymeric micelles for targeting both cancer cells and cancer stem cells. Biomaterials. 2014;35(3):1096–108. [DOI] [PubMed] [Google Scholar]
- 120.Sun TM, Wang YC, Wang F, Du JZ, Mao CQ, Sun CY et al. Cancer stem cell therapy using doxorubicin conjugated to gold nanoparticles via hydrazone bonds. Biomaterials. 2014;35(2):836–45. [DOI] [PubMed] [Google Scholar]
- 121.Du Z, Qin R, Wei C, Wang M, Shi C, Tian R et al. Pancreatic cancer cells resistant to chemoradiotherapy rich in “stem-cell-like” tumor cells. Dig Dis Sci. 2011;56(3):741–50. [DOI] [PubMed] [Google Scholar]
- 122.Krishnamurthy S, Ng VWL, Gao SJ, Tan MH, Yang YY. Phenformin-loaded polymeric micelles for targeting both cancer cells and cancer stem cells in vitro and in vivo. Biomaterials. 2014;35(33):9177–86. [DOI] [PubMed] [Google Scholar]
- 123.Chen BA, Lai BB, Cheng J, Xia GH, Gao F, Xu WL et al. Daunorubicin-loaded magnetic nanoparticles of Fe3O4 overcome multidrug resistance and induce apoptosis of K562-n/VCR cells in vivo. Int J Nanomedicine. 2009;4:201–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Chen BA, Mao PP, Cheng J, Gao F, Xia GH, Xu WL et al. Reversal of multidrug resistance by magnetic Fe3O4 nanoparticle copolymerizating daunorubicin and MDR1 shRNA expression vector in leukemia cells. Int J Nanomedicine. 2010;5:437–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Farahmand L, Darvishi B, Salehi M, Samadi Kouchaksaraei S, Majidzadeh AK. Functionalised nanomaterials for eradication of CSCs, a promising approach for overcoming tumour heterogeneity. J Drug Target. 2018;26(8):649–57. [DOI] [PubMed] [Google Scholar]
- 126.Pi Y, Zhou J, Wang J, Zhong J, Zhang L, Wang Y et al. Strategies of overcoming the physiological barriers for tumor-targeted nano-sized drug delivery systems. Curr Pharm Des. 2015;21(42):6236–45. [DOI] [PubMed] [Google Scholar]
- 127.Feng C, Rui M, Shen H, Xin Y, Zhang J, Li J et al. Tumor-specific delivery of doxorubicin through conjugation of pH-responsive peptide for overcoming drug resistance in cancer. Int J Pharm. 2017;528(1–2):322–33. [DOI] [PubMed] [Google Scholar]
- 128.Fuchs D, Daniel V, Sadeghi M, Opelz G, Naujokat C. Salinomycin overcomes ABC transporter-mediated multidrug and apoptosis resistance in human leukemia stem cell-like KG-1a cells. Biochem Biophys Res Commun. 2010;394(4):1098–104. [DOI] [PubMed] [Google Scholar]
- 129.Rabindran SK, Ross DD, Doyle LA, Yang W, Greenberger LM. Fumitremorgin C Reverses Multidrug Resistance in Cells Transfected with the Breast Cancer Resistance Protein. Cancer Res. 2000;60(1):47–50. [PubMed] [Google Scholar]
- 130.Tarasova NI, Seth R, Tarasov SG, Kosakowska-Cholody T, Hrycyna CA, Gottesman MM et al. Transmembrane Inhibitors of P-Glycoprotein, an ABC Transporter. J Med Chem. 2005;48(11):3768–75. [DOI] [PubMed] [Google Scholar]
- 131.Ling X, Wu W, Fan C, Xu C, Liao J, Rich LJ et al. An ABCG2 non-substrate anticancer agent FL118 targets drug-resistant cancer stem-like cells and overcomes treatment resistance of human pancreatic cancer. J Exp Clin Cancer Res. 2018;37(1):240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Dean M. ABC Transporters, Drug Resistance, and Cancer Stem Cells. J Mammary Gland Biol Neoplasia. 2009;14(1):3–9. [DOI] [PubMed] [Google Scholar]
- 133.Kartner N, Evernden-Porelle D, Bradley G, Ling V. Detection of P-glycoprotein in multidrug-resistant cell lines by monoclonal antibodies. Nature. 1985;316(6031):820–3. [DOI] [PubMed] [Google Scholar]
- 134.Riordan JR, Deuchars K, Kartner N, Alon N, Trent J, Ling V. Amplification of P-glycoprotein genes in multidrug-resistant mammalian cell lines. Nature. 1985;316(6031):817–9. [DOI] [PubMed] [Google Scholar]
- 135.Vasiliou V, Vasiliou K, Nebert DW. Human ATP-binding cassette (ABC) transporter family. Hum Genomics. 2009;3(3):281–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Zunino F, Cassinelli G, Polizzi D, Perego P. Molecular mechanisms of resistance to taxanes and therapeutic implications. Drug Resist Updat. 1999;2(6):351–7. [DOI] [PubMed] [Google Scholar]
- 137.Saraswathy M, Gong S. Different strategies to overcome multidrug resistance in cancer. Biotechnol Adv. 2013;31(8):1397–407. [DOI] [PubMed] [Google Scholar]
- 138.Torres Á, Arriagada V, Erices JI, Toro MdLÁ, Rocha JD, Niechi I et al. FK506 Attenuates the MRP1-Mediated Chemoresistant Phenotype in Glioblastoma Stem-Like Cells. Int J Mol Sci. 2018;19(9):2697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Minko T, Kopečková P, Pozharov V, Kopeček J. HPMA copolymer bound adriamycin overcomes MDR1 gene encoded resistance in a human ovarian carcinoma cell line. J Control Release. 1998;54(2):223–33. [DOI] [PubMed] [Google Scholar]
- 140.Alakhov VY, Moskaleva EY, Batrakova EV, Kabanov AV. Hypersensitization of Multidrug Resistant Human Ovarian Carcinoma Cells by Pluronic P85 Block Copolymer. Bioconjug Chem. 1996;7(2):209–16. [DOI] [PubMed] [Google Scholar]
- 141.Venne A, Li S, Mandeville R, Kabanov A, Alakhov V. Hypersensitizing Effect of Pluronic L61 on Cytotoxic Activity, Transport, and Subcellular Distribution of Doxorubicin in Multiple Drug-resistant Cells. Cancer Res. 1996;56(16):3626–9. [PubMed] [Google Scholar]
- 142.Jachez B, Nordmann R, Loor F. Restoration of taxol sensitivity of multidrug-resistant cells by the cyclosporine SDZ PSC 833 and the cyclopeptolide SDZ 280–446. J Natl Cancer Inst. 1993;85(6):478–83. [DOI] [PubMed] [Google Scholar]
- 143.Nanayakkara AK, Follit CA, Chen G, Williams NS, Vogel PD, Wise JG. Targeted inhibitors of P-glycoprotein increase chemotherapeutic-induced mortality of multidrug resistant tumor cells. Sci Rep. 2018;8(1):967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Ganta S, Amiji M. Coadministration of Paclitaxel and Curcumin in Nanoemulsion Formulations To Overcome Multidrug Resistance in Tumor Cells. Mol Pharm. 2009;6(3):928–39. [DOI] [PubMed] [Google Scholar]
- 145.Zhou Q, Ye M, Lu Y, Zhang H, Chen Q, Huang S et al. Curcumin Improves the Tumoricidal Effect of Mitomycin C by Suppressing ABCG2 Expression in Stem Cell-Like Breast Cancer Cells. PLoS ONE. 2015;10(8):e0136694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Frank NY, Margaryan A, Huang Y, Schatton T, Waaga-Gasser AM, Gasser M et al. ABCB5-Mediated Doxorubicin Transport and Chemoresistance in Human Malignant Melanoma. Cancer Res. 2005;65(10):4320. [DOI] [PubMed] [Google Scholar]
- 147.Shi F, Li M, Wang J, Wu D, Pan M, Guo M et al. Induction of multiple myeloma cancer stem cell apoptosis using conjugated anti-ABCG2 antibody with epirubicin-loaded microbubbles. Stem Cell Res Ther. 2018;9(1):144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Chen D, Pan X, Xie F, Lu Y, Zou H, Yin C et al. Codelivery of doxorubicin and elacridar to target both liver cancer cells and stem cells by polylactide-co-glycolide/d-alpha-tocopherol polyethylene glycol 1000 succinate nanoparticles. Int J Nanomedicine. 2018;13:6855–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Ozben T. Mechanisms and strategies to overcome multiple drug resistance in cancer. FEBS Lett. 2006;580(12):2903–9. [DOI] [PubMed] [Google Scholar]
- 150.Kato T, Natsume A, Toda H, Iwamizu H, Sugita T, Hachisu R et al. Efficient delivery of liposome-mediated MGMT-siRNA reinforces the cytotoxity of temozolomide in GBM-initiating cells. Gene Therapy. 2010;17:1363–71. [DOI] [PubMed] [Google Scholar]
- 151.Hashemitabar S, Yazdian-Robati R, Hashemi M, Ramezani M, Abnous K, Kalalinia F. ABCG2 aptamer selectively delivers doxorubicin to drug-resistant breast cancer cells. J Biosci. 2019;44(2):39. [PubMed] [Google Scholar]
- 152.Hou H, Kang Y, Li Y, Zheng Y, Ding G, Shang J. miR-33a expression sensitizes Lgr5+ HCC-CSCs to doxorubicin via ABCA1. Neoplasma. 2016;64:81–91. [DOI] [PubMed] [Google Scholar]
- 153.Pan Y-Z, Morris ME, Yu A-M. MicroRNA-328 Negatively Regulates the Expression of Breast Cancer Resistance Protein (BCRP/ABCG2) in Human Cancer Cells. Mol Pharmacol. 2009;75(6):1374–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.An X, Sarmiento C, Tan T, Zhu H. Regulation of multidrug resistance by microRNAs in anti-cancer therapy. Acta Pharm Sin B. 2017;7(1):38–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Cucco C, Calabretta B. In Vitro and in Vivo Reversal of Multidrug Resistance in a Human Leukemia-resistant Cell Line by mdr1 Antisense Oligodeoxynucleotides. Cancer Res. 1996;56(19):4332–7. [PubMed] [Google Scholar]
- 156.Puerta-Fernández E, Romero-López C, Barroso-delJesus A, Berzal-Herranz A. Ribozymes: recent advances in the development of RNA tools. FEMS Microbiol Rev. 2003;27(1):75–97. [DOI] [PubMed] [Google Scholar]
- 157.Liu T, Li Z, Zhang Q, Amorim Bernstein KD, Lozano-Calderon S, Choy E et al. Targeting ABCB1 (MDR1) in multi-drug resistant osteosarcoma cells using the CRISPR-Cas9 system to reverse drug resistance. Oncotarget. 2016;7(50):83502–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Yang Y, Qiu J-G, Li Y, Di J-M, Zhang W-J, Jiang Q-W et al. Targeting ABCB1-mediated tumor multidrug resistance by CRISPR/Cas9-based genome editing. Am J Transl Res. 2016;8(9):3986–94. [PMC free article] [PubMed] [Google Scholar]
- 159.Chen Y, Zhang Y. Application of the CRISPR/Cas9 System to Drug Resistance in Breast Cancer. Adv Sci (Weinh). 2018;5(6):1700964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Deonarain MP, Kousparou CA, Epenetos AA. Antibodies targeting cancer stem cells: a new paradigm in immunotherapy? MAbs. 2009;1(1):12–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Bourguignon LYW, Peyrollier K, Xia W, Gilad E. Hyaluronan-CD44 Interaction Activates Stem Cell Marker Nanog, Stat-3-mediated MDR1 Gene Expression, and Ankyrin-regulated Multidrug Efflux in Breast and Ovarian Tumor Cells. J Biol Chem. 2008;283(25):17635–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Goodarzi N, Ghahremani MH, Dinarvand R. Targeting CD44 by hyaluronic acid-based nano drug delivery systems may eradicate cancer stem cells in human breast cancer. Journal of Medical Hypotheses and Ideas. 2011;5:26. [Google Scholar]
- 163.Prestwich GD, Marecak DM, Marecek JF, Vercruysse KP, Ziebell MR. Controlled chemical modification of hyaluronic acid: synthesis, applications, and biodegradation of hydrazide derivatives. J Control Release. 1998;53(1):93–103. [DOI] [PubMed] [Google Scholar]
- 164.Eliaz RE, Szoka FC. Liposome-encapsulated Doxorubicin Targeted to CD44. Cancer Res. 2001;61(6):2592–601. [PubMed] [Google Scholar]
- 165.Yang Z, Sun N, Cheng R, Zhao C, Liu J, Tian Z. Hybrid nanoparticles coated with hyaluronic acid lipoid for targeted co-delivery of paclitaxel and curcumin to synergistically eliminate breast cancer stem cells. J Mater Chem B. 2017;5(33):6762–75. [DOI] [PubMed] [Google Scholar]
- 166.Goodarzi N, Ghahremani MH, Amini M, Atyabi F, Ostad SN, Shabani Ravari N et al. CD44-Targeted Docetaxel Conjugate for Cancer Cells and Cancer Stem-Like Cells: A Novel Hyaluronic Acid-Based Drug Delivery System. Chem Biol Drug Des. 2014;83(6):741–52. [DOI] [PubMed] [Google Scholar]
- 167.Gadhoum Z, Delaunay J, Maquarre E, Durand L, Lancereaux V, Qi J et al. The Effect of Anti-CD44 Monoclonal Antibodies on Differentiation and Proliferation of Human Acute Myeloid Leukemia Cells. Leuk Lymphoma. 2004;45(8):1501–10. [DOI] [PubMed] [Google Scholar]
- 168.Stroomer JWG, Roos JC, Sproll M, Quak JJ, Heider K-H, Wilhelm BJ et al. Safety and Biodistribution of 99mTechnetium-labeled Anti-CD44v6 Monoclonal Antibody BIWA 1 in Head and Neck Cancer Patients. Clin Cancer Res. 2000;6(8):3046–55. [PubMed] [Google Scholar]
- 169.Alshaer W, Hillaireau H, Vergnaud J, Ismail S, Fattal E. Functionalizing Liposomes with anti-CD44 Aptamer for Selective Targeting of Cancer Cells. Bioconjug Chem. 2015;26(7):1307–13. [DOI] [PubMed] [Google Scholar]
- 170.Kebenko M, Goebeler M-E, Wolf M, Hasenburg A, Seggewiss-Bernhardt R, Ritter B et al. A multicenter phase 1 study of solitomab (MT110, AMG 110), a bispecific EpCAM/CD3 T-cell engager (BiTE®) antibody construct, in patients with refractory solid tumors. Oncoimmunology. 2018;7(8):e1450710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Appelbaum FR, Bernstein ID. Gemtuzumab ozogamicin for acute myeloid leukemia. Blood. 2017;130(22):2373–76. [DOI] [PubMed] [Google Scholar]
- 172.Liu D, Hong Y, Li Y, Hu C, Yip T-C, Yu W-K et al. Targeted destruction of cancer stem cells using multifunctional magnetic nanoparticles that enable combined hyperthermia and chemotherapy. Theranostics. 2020;10(3):1181–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Serna N, Álamo P, Ramesh P, Vinokurova D, Sánchez-García L, Unzueta U et al. Nanostructured toxins for the selective destruction of drug-resistant human CXCR4+ colorectal cancer stem cells. J Control Release. 2020;320:96–104. [DOI] [PubMed] [Google Scholar]
- 174.Richards DA, Maruani A, Chudasama V. Antibody fragments as nanoparticle targeting ligands: a step in the right direction. Chem Sci. 2017;8(1):63–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Waldron NN, Kaufman DS, Oh S, Inde Z, Hexum MK, Ohlfest JR et al. Targeting Tumor-Initiating Cancer Cells with dCD133KDEL Shows Impressive Tumor Reductions in a Xenotransplant Model of Human Head and Neck Cancer. Mol Cancer Ther. 2011;10(10):1829–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Ohlfest JR, Zellmer DM, Panyam J, Swaminathan SK, Oh S, Waldron NN et al. Immunotoxin targeting CD133+ breast carcinoma cells. Drug Deliv Transl Res. 2013;3(2):195–204. [DOI] [PubMed] [Google Scholar]
- 177.Schmohl JU, Felices M, Oh F, Lenvik AJ, Lebeau AM, Panyam J et al. Engineering of Anti-CD133 Trispecific Molecule Capable of Inducing NK Expansion and Driving Antibody-Dependent Cell-Mediated Cytotoxicity. Cancer Res Treat. 2017;49(4):1140–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Swaminathan SK, Roger E, Toti U, Niu L, Ohlfest JR, Panyam J. CD133-targeted paclitaxel delivery inhibits local tumor recurrence in a mouse model of breast cancer. J Control Release. 2013;171(3):280–7. [DOI] [PubMed] [Google Scholar]
- 179.Shigdar S, Qiao L, Zhou S-F, Xiang D, Wang T, Li Y et al. RNA aptamers targeting cancer stem cell marker CD133. Cancer Lett. 2013;330(1):84–95. [DOI] [PubMed] [Google Scholar]
- 180.Ma J, Zhuang H, Zhuang Z, Lu Y, Xia R, Gan L et al. Development of docetaxel liposome surface modified with CD133 aptamers for lung cancer targeting. Artif Cells Nanomed Biotechnol. 2018;46(8):1864–71. [DOI] [PubMed] [Google Scholar]
- 181.Kim JS, Shin DH, Kim J-S. Dual-targeting immunoliposomes using angiopep-2 and CD133 antibody for glioblastoma stem cells. J Control Release. 2018;269:245–57. [DOI] [PubMed] [Google Scholar]
- 182.Khanna V, Kalscheuer S, Kirtane A, Zhang W, Panyam J. Perlecan-targeted nanoparticles for drug delivery to triple-negative breast cancer. Future Drug Discov. 2019;1(1):FDD8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Borah A, Raveendran S, Rochani A, Maekawa T, Kumar DS. Targeting self-renewal pathways in cancer stem cells: clinical implications for cancer therapy. Oncogenesis. 2015;4:e177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Putzer BM, Solanki M, Herchenroder O. Advances in cancer stem cell targeting: How to strike the evil at its root. Adv Drug Delivery Rev. 2017;120:89–107. [DOI] [PubMed] [Google Scholar]
- 185.Ajani JA, Song S, Hochster HS, Steinberg IB. Cancer stem cells: the promise and the potential. Semin Oncol. 2015;42Suppl 1:S3–17. [DOI] [PubMed] [Google Scholar]
- 186.Dandawate PR, Subramaniam D, Jensen RA, Anant S. Targeting cancer stem cells and signaling pathways by phytochemicals: Novel approach for breast cancer therapy. Semin Cancer Biol. 2016;40-41:192–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Hong IS, Jang GB, Lee HY, Nam JS. Targeting cancer stem cells by using the nanoparticles. Int J Nanomedicine. 2015;10:251–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Petrova R, Joyner AL. Roles for Hedgehog signaling in adult organ homeostasis and repair. Development. 2014;141(18):3445–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Merchant AA, Matsui W. Targeting Hedgehog--a cancer stem cell pathway. Clin Cancer Res. 2010;16(12):3130–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Dierks C, Beigi R, Guo GR, Zirlik K, Stegert MR, Manley P et al. Expansion of Bcr-Abl-positive leukemic stem cells is dependent on hedgehog pathway activation. Cancer Cell. 2008;14(3):238–49. [DOI] [PubMed] [Google Scholar]
- 191.Peacock CD, Wang QJ, Gesell GS, Corcoran-Schwartz IM, Jones E, Kim J et al. Hedgehog signaling maintains a tumor stem cell compartment in multiple myeloma. Proc Natl Acad Sci U S A. 2007;104(10):4048–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Ramaswamy B, Lu YZ, Teng KY, Nuovo G, Li XB, Shapiro CL et al. Hedgehog Signaling Is a Novel Therapeutic Target in Tamoxifen-Resistant Breast Cancer Aberrantly Activated by PI3K/AKT Pathway. Cancer Res. 2012;72(19):5048–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Majumder S, Lu YZ, Teng KY, Li XB, Shapiro CL, Ramaswamy B. Hedgehog pathway is a novel therapeutic target in tamoxifen resistant breast cancer that protects from autophagic cell death and is aberrantly activated by PI3K/AKT signaling. Cancer Res. 2012;72(8 Supplement):Abstract 5606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Kappler R, von Schweinitz D. A better way forward: targeting hedgehog signaling in liver cancer. Front Biosci (Scholar Edition). 2012;4:277–86. [DOI] [PubMed] [Google Scholar]
- 195.Justilien V, Fields AP. Molecular pathways: novel approaches for improved therapeutic targeting of Hedgehog signaling in cancer stem cells. Clin Cancer Res. 2015;21(3):505–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Clement V, Sanchez P, de Tribolet N, Radovanovic I, Ruiz i Altaba A. HEDGEHOG-GLI1 signaling regulates human glioma growth, cancer stem cell self-renewal, and tumorigenicity. Curr Biol. 2007;17(2):165–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Bar EE, Chaudhry A, Lin A, Fan X, Schreck K, Matsui W et al. Cyclopamine-mediated hedgehog pathway inhibition depletes stem-like cancer cells in glioblastoma. Stem Cells. 2007;25(10):2524–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Regan JL, Schumacher D, Staudte S, Steffen A, Haybaeck J, Keilholz U et al. Non-Canonical Hedgehog Signaling Is a Positive Regulator of the WNT Pathway and Is Required for the Survival of Colon Cancer Stem Cells. Cell Reports. 2017;21(10):2813–28. [DOI] [PubMed] [Google Scholar]
- 199.Sims-Mourtada J, Izzo JG, Apisarnthanarax S, Wu TT, Malhotra U, Luthra R et al. Hedgehog: an attribute to tumor regrowth after chemoradiotherapy and a target to improve radiation response. Clin Cancer Res. 2006;12(21):6565–72. [DOI] [PubMed] [Google Scholar]
- 200.Huang FT, Zhuan-Sun YX, Zhuang YY, Wei SL, Tang J, Chen WB et al. Inhibition of hedgehog signaling depresses self-renewal of pancreatic cancer stem cells and reverses chemoresistance. Int J Oncol. 2012;41(5):1707–14. [DOI] [PubMed] [Google Scholar]
- 201.Chenna V, Hu C, Pramanik D, Aftab BT, Karikari C, Campbell NR et al. A polymeric nanoparticle encapsulated small-molecule inhibitor of Hedgehog signaling (NanoHHI) bypasses secondary mutational resistance to Smoothened antagonists. Mol Cancer Ther. 2012;11(1):165–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Verma RK, Yu W, Singh SP, Shankar S, Srivastava RK. Anthothecol-encapsulated PLGA nanoparticles inhibit pancreatic cancer stem cell growth by modulating sonic hedgehog pathway. Nanomed. 2015;11(8):2061–70. [DOI] [PubMed] [Google Scholar]
- 203.Zhou Y, Yang J, Kopecek J. Selective inhibitory effect of HPMA copolymer-cyclopamine conjugate on prostate cancer stem cells. Biomaterials. 2012;33(6):1863–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Yu L, Fan Z, Fang S, Yang J, Gao T, Simoes BM et al. Cisplatin selects for stem-like cells in osteosarcoma by activating Notch signaling. Oncotarget. 2016;7(22):33055–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Lu B, Huang X, Mo J, Zhao W. Drug Delivery Using Nanoparticles for Cancer Stem-Like Cell Targeting. Front Pharmacol. 2016;7:84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Espinoza I, Pochampally R, Xing F, Watabe K, Miele L. Notch signaling: targeting cancer stem cells and epithelial-to-mesenchymal transition. Onco Targets Ther. 2013;6:1249–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Gu JW, Rizzo P, Pannuti A, Golde T, Osborne B, Miele L. Notch signals in the endothelium and cancer “stem-like” cells: opportunities for cancer therapy. Vasc Cell. 2012;4:7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Pannuti A, Foreman K, Rizzo P, Osipo C, Golde T, Osborne B et al. Targeting Notch to target cancer stem cells. Clin Cancer Res. 2010;16(12):3141–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Fan X, Khaki L, Zhu TS, Soules ME, Talsma CE, Gul N et al. NOTCH pathway blockade depletes CD133-positive glioblastoma cells and inhibits growth of tumor neurospheres and xenografts. Stem Cells. 2010;28(1):5–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Mamaeva V, Rosenholm JM, Bate-Eya LT, Bergman L, Peuhu E, Duchanoy A et al. Mesoporous Silica Nanoparticles as Drug Delivery Systems for Targeted Inhibition of Notch Signaling in Cancer. Mol Ther. 2011;19(8):1538–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Mamaeva V, Niemi R, Beck M, Ozliseli E, Desai D, Landor S et al. Inhibiting Notch Activity in Breast Cancer Stem Cells by Glucose Functionalized Nanoparticles Carrying gamma-secretase Inhibitors. Mol Ther. 2016;24(5):926–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Shen S, Sun CY, Du XJ, Li HJ, Liu Y, Xia JX et al. Co-delivery of platinum drug and siNotch1 with micelleplex for enhanced hepatocellular carcinoma therapy. Biomaterials. 2015;70:71–83. [DOI] [PubMed] [Google Scholar]
- 213.Fung TK, Leung AYH, So CWE. The Wnt/β-Catenin Pathway as a Potential Target for Drug Resistant Leukemic Stem Cells. In: Hayat MA, editor. Stem Cells and Cancer Stem Cells, Volume 10: Therapeutic Applications in Disease and Injury. Dordrecht: Springer Netherlands; 2013. p. 163–72. [Google Scholar]
- 214.Mohammadi M, Salmasi Z, Hashemi M, Mosaffa F, Abnous K, Ramezani M. Single-walled carbon nanotubes functionalized with aptamer and piperazine-polyethylenimine derivative for targeted siRNA delivery into breast cancer cells. Int J Pharm. 2015;485(1–2):50–60. [DOI] [PubMed] [Google Scholar]
- 215.Suwala AK, Hanaford A, Kahlert UD, Maciaczyk J. Clipping the Wings of Glioblastoma: Modulation of WNT as a Novel Therapeutic Strategy. J Neuropathol Exp Neurol. 2016;75(5):388–96. [DOI] [PubMed] [Google Scholar]
- 216.Aminuddin A, Ng PY. Promising Druggable Target in Head and Neck Squamous Cell Carcinoma: Wnt Signaling. Front Pharmacol. 2016;7:244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Huang W, Liu Z, Zhou G, Tian A, Sun N. Magnetic gold nanoparticle-mediated small interference RNA silencing Bag-1 gene for colon cancer therapy. Oncol Rep. 2016;35(2):978–84. [DOI] [PubMed] [Google Scholar]
- 218.Jang GB, Kim JY, Cho SD, Park KS, Jung JY, Lee HY et al. Blockade of Wnt/beta-catenin signaling suppresses breast cancer metastasis by inhibiting CSC-like phenotype. Scientific Reports. 2015;5:12465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Huang J, Tao C, Yu Y, Yu F, Zhang H, Gao J et al. Simultaneous Targeting of Differentiated Breast Cancer Cells and Breast Cancer Stem Cells by Combination of Docetaxel- and Sulforaphane-Loaded Self-Assembled Poly(D, L-lactide-co-glycolide)/Hyaluronic Acid Block Copolymer-Based Nanoparticles. J Biomed Nanotechnol. 2016;12(7):1463–77. [DOI] [PubMed] [Google Scholar]
- 220.Zhi X, Lin L, Yang S, Bhuvaneshwar K, Wang H, Gusev Y et al. betaII-Spectrin (SPTBN1) suppresses progression of hepatocellular carcinoma and Wnt signaling by regulation of Wnt inhibitor kallistatin. Hepatology. 2015;61(2):598–612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Zhao P, Dong S, Bhattacharyya J, Chen M. iTEP nanoparticle-delivered salinomycin displays an enhanced toxicity to cancer stem cells in orthotopic breast tumors. Mol Pharm. 2014;11(8):2703–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Zhao P, Xia G, Dong S, Jiang ZX, Chen M. An iTEP-salinomycin nanoparticle that specifically and effectively inhibits metastases of 4T1 orthotopic breast tumors. Biomaterials. 2016;93:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Miller-Kleinhenz J, Guo XX, Qian WP, Zhou HY, Bozeman EN, Zhu L et al. Dual-targeting Wnt and uPA receptors using peptide conjugated ultra-small nanoparticle drug carriers inhibited cancer stem-cell phenotype in chemo-resistant breast cancer. Biomaterials. 2018;152:47–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Mladinich M, Ruan D, Chan CH. Tackling Cancer Stem Cells via Inhibition of EMT Transcription Factors. Stem Cells Int. 2016;2016:5285892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Liu Q, Li RT, Qian HQ, Wei J, Xie L, Shen J et al. Targeted delivery of miR-200c/DOC to inhibit cancer stem cells and cancer cells by the gelatinases-stimuli nanoparticles. Biomaterials. 2013;34(29):7191–203. [DOI] [PubMed] [Google Scholar]
- 226.Fiorillo M, Verre AF, Iliut M, Peiris-Pages M, Ozsvari B, Gandara R et al. Graphene oxide selectively targets cancer stem cells, across multiple tumor types: Implications for non-toxic cancer treatment, via “differentiation-based nano-therapy”. Oncotarget. 2015;6(6):3553–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Petpiroon N, Bhummaphan N, Soonnarong R, Chantarawong W, Maluangnont T, Pongrakhananon V et al. Ti0.8O2 Nanosheets Inhibit Lung Cancer Stem Cells by Inducing Production of Superoxide Anion. Mol Pharmacol. 2019;95(4):418–32. [DOI] [PubMed] [Google Scholar]
- 228.Gul-Uludag H, Valencia-Serna J, Kucharski C, Marquez-Curtis LA, Jiang X, Larratt L et al. Polymeric nanoparticle-mediated silencing of CD44 receptor in CD34+ acute myeloid leukemia cells. Leuk Res. 2014;38(11):1299–308. [DOI] [PubMed] [Google Scholar]
- 229.Lu H, Clauser KR, Tam WL, Frose J, Ye X, Eaton EN et al. A breast cancer stem cell niche supported by juxtacrine signalling from monocytes and macrophages. Nat Cell Biol. 2014;16(11):1105–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Au JL, Yeung BZ, Wientjes MG, Lu Z, Wientjes MG. Delivery of cancer therapeutics to extracellular and intracellular targets: Determinants, barriers, challenges and opportunities. Adv Drug Deliv Rev. 2016;97:280–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Pattabiraman DR, Weinberg RA. Tackling the cancer stem cells - what challenges do they pose? Nat Rev Drug Discov. 2014;13(7):497–512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Dewhirst MW, Secomb TW. Transport of drugs from blood vessels to tumour tissue. Nat Rev Cancer. 2017;17(12):738–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Huang P, Rong PF, Jin A, Yan XF, Zhang MG, Lin J et al. Dye-Loaded Ferritin Nanocages for Multimodal Imaging and Photothermal Therapy. Adv Mater. 2014;26(37):6401–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Cao CQ, Wang XX, Cai Y, Sun L, Tian LX, Wu H et al. Targeted In Vivo Imaging of Microscopic Tumors with Ferritin- based Nanoprobes Across Biological Barriers. Adv Mater. 2014;26(16):2566–71. [DOI] [PubMed] [Google Scholar]
- 235.Schonberg DL, Miller TE, Wu QL, Flavahan WA, Das NK, Hale JS et al. Preferential Iron Trafficking Characterizes Glioblastoma Stem-like Cells. Cancer Cell. 2015;28(4):441–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Tan T, Wang H, Cao H, Zeng L, Wang Y, Wang Z et al. Deep Tumor-Penetrated Nanocages Improve Accessibility to Cancer Stem Cells for Photothermal-Chemotherapy of Breast Cancer Metastasis. Adv Sci (Weinh). 2018;5(12):1801012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Sun YJ, Kim HS, Park J, Li M, Tian L, Choi Y et al. MRI of Breast Tumor Initiating Cells Using the Extra Domain-B of Fibronectin Targeting Nanoparticles. Theranostics. 2014;4(8):845–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Sun Y, Kim HS, Saw PE, Jon S, Moon WK. Targeted Therapy for Breast Cancer Stem Cells by Liposomal Delivery of siRNA against Fibronectin EDB. Adv Healthc Mater. 2015;4(11):1675–80. [DOI] [PubMed] [Google Scholar]
- 239.Zhu X, Li Q, Li S, Chen B, Zou H. HIF-1α decoy oligodeoxynucleotides inhibit HIF-1α signaling and breast cancer proliferation. International journal of oncology. 2015January1;46(1):215–22. [DOI] [PubMed] [Google Scholar]
- 240.Liu Y, Chen CY, Qian PX, Lu XF, Sun BY, Zhang X et al. Gd-metallofullerenol nanomaterial as non-toxic breast cancer stem cell-specific inhibitor. Nat Commun. 2015;6:5988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Wong TL, Che N, Ma S. Reprogramming of central carbon metabolism in cancer stem cells. Biochim Biophys Acta, Mol Basis Dis. 2017;1863(7):1728–38. [DOI] [PubMed] [Google Scholar]
- 242.Peiris-Pages M, Martinez-Outschoorn UE, Pestell RG, Sotgia F, Lisanti MP. Cancer stem cell metabolism. Breast Cancer Res. 2016;18(1):55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Sancho P, Barneda D, Heeschen C. Hallmarks of cancer stem cell metabolism. Br J Cancer. 2016;114(12):1305–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Janiszewska M, Suva ML, Riggi N, Houtkooper RH, Auwerx J, Clement-Schatlo V et al. Imp2 controls oxidative phosphorylation and is crucial for preserving glioblastoma cancer stem cells. Genes Dev. 2012;26(17):1926–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Ye XQ, Li Q, Wang GH, Sun FF, Huang GJ, Bian XW et al. Mitochondrial and energy metabolism-related properties as novel indicators of lung cancer stem cells. Int J Cancer. 2011;129(4):820–31. [DOI] [PubMed] [Google Scholar]
- 246.Sancho P, Burgos-Ramos E, Tavera A, Bou Kheir T, Jagust P, Schoenhals M et al. MYC/PGC-1alpha Balance Determines the Metabolic Phenotype and Plasticity of Pancreatic Cancer Stem Cells. Cell Metab. 2015;22(4):590–605. [DOI] [PubMed] [Google Scholar]
- 247.Suda T, Takubo K, Semenza GL. Metabolic regulation of hematopoietic stem cells in the hypoxic niche. Cell Stem Cell. 2011;9(4):298–310. [DOI] [PubMed] [Google Scholar]
- 248.Zhou W, Choi M, Margineantu D, Margaretha L, Hesson J, Cavanaugh C et al. HIF1alpha induced switch from bivalent to exclusively glycolytic metabolism during ESC-to-EpiSC/hESC transition. EMBO J. 2012;31(9):2103–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Yi Y, Kim HJ, Zheng M, Mi P, Naito M, Kim BS et al. Glucose-linked sub-50-nm unimer polyion complex-assembled gold nanoparticles for targeted siRNA delivery to glucose transporter 1-overexpressing breast cancer stem-like cells. J Control Release. 2019;295:268–77. [DOI] [PubMed] [Google Scholar]
- 250.Farnie G, Sotgia F, Lisanti MP. High mitochondrial mass identifies a sub-population of stem-like cancer cells that are chemo-resistant. Oncotarget. 2015;6(31):30472–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Ma X, Zhou J, Zhang CX, Li XY, Li N, Ju RJ et al. Modulation of drug-resistant membrane and apoptosis proteins of breast cancer stem cells by targeting berberine liposomes. Biomaterials. 2013;34(18):4452–65. [DOI] [PubMed] [Google Scholar]
- 252.Wang XX, Li YB, Yao HJ, Ju RJ, Zhang Y, Li RJ et al. The use of mitochondrial targeting resveratrol liposomes modified with a dequalinium polyethylene glycol-distearoylphosphatidyl ethanolamine conjugate to induce apoptosis in resistant lung cancer cells. Biomaterials. 2011;32(24):5673–87. [DOI] [PubMed] [Google Scholar]
- 253.Yu Y, Wang ZH, Zhang L, Yao HJ, Zhang Y, Li RJ et al. Mitochondrial targeting topotecan-loaded liposomes for treating drug-resistant breast cancer and inhibiting invasive metastases of melanoma. Biomaterials. 2012;33(6):1808–20. [DOI] [PubMed] [Google Scholar]
- 254.Zhang L, Yao HJ, Yu Y, Zhang Y, Li RJ, Ju RJ et al. Mitochondrial targeting liposomes incorporating daunorubicin and quinacrine for treatment of relapsed breast cancer arising from cancer stem cells. Biomaterials. 2012;33(2):565–82. [DOI] [PubMed] [Google Scholar]
- 255.Loureiro R, Mesquita KA, Magalhaes-Novais S, Oliveira PJ, Vega-Naredo I. Mitochondrial biology in cancer stem cells. Semin Cancer Biol. 2017;47:18–28. [DOI] [PubMed] [Google Scholar]
- 256.Diehn M, Cho RW, Lobo NA, Kalisky T, Dorie MJ, Kulp AN et al. Association of reactive oxygen species levels and radioresistance in cancer stem cells. Nature. 2009;458(7239):780–U123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Lagadinou ED, Sach A, Callahan K, Rossi RM, Neering SJ, Minhajuddin M et al. BCL-2 inhibition targets oxidative phosphorylation and selectively eradicates quiescent human leukemia stem cells. Cell Stem Cell. 2013;12(3):329–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258.Ishimoto T, Nagano O, Yae T, Tamada M, Motohara T, Oshima H et al. CD44 variant regulates redox status in cancer cells by stabilizing the xCT subunit of system xc(−) and thereby promotes tumor growth. Cancer Cell. 2011;19(3):387–400. [DOI] [PubMed] [Google Scholar]
- 259.Dayem AA, Choi HY, Kim JH, Cho SG. Role of oxidative stress in stem, cancer, and cancer stem cells. Cancers (Basel). 2010;2(2):859–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260.Galadari S, Rahman A, Pallichankandy S, Thayyullathil F. Reactive oxygen species and cancer paradox: To promote or to suppress? Free Radic Biol Med. 2017;104:144–64. [DOI] [PubMed] [Google Scholar]
- 261.Haraguchi N, Ishii H, Mimori K, Tanaka F, Ohkuma M, Kim HM et al. CD13 is a therapeutic target in human liver cancer stem cells. J Clin Investig. 2010;120(9):3326–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262.Kim HM, Haraguchi N, Ishii H, Ohkuma M, Okano M, Mimori K et al. Increased CD13 expression reduces reactive oxygen species, promoting survival of liver cancer stem cells via an epithelial mesenchymal transition-like phenomenon. Ann Surg Oncol. 2012;19Suppl 3:S539–48. [DOI] [PubMed] [Google Scholar]
- 263.Guo WJ, Ye SS, Cao N, Huang J, Gao J, Chen QY. ROS-mediated autophagy was involved in cancer cell death induced by novel copper(II) complex. Exp Toxicol Pathol. 2010;62(5):577–82. [DOI] [PubMed] [Google Scholar]
- 264.Zhou D, Shao L, Spitz DR. Reactive oxygen species in normal and tumor stem cells. Adv Cancer Res. 2014;122:1–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265.Trachootham D, Alexandre J, Huang P. Targeting cancer cells by ROS-mediated mechanisms: a radical therapeutic approach? Nat Rev Drug Discov. 2009;8(7):579–91. [DOI] [PubMed] [Google Scholar]
- 266.Liou GY, Storz P. Reactive oxygen species in cancer. Free Radic Res. 2010;44(5):479–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267.Shi X, Zhang Y, Zheng J, Pan J. Reactive oxygen species in cancer stem cells. Antioxid Redox Signal. 2012;16(11):1215–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 268.Kobayashi CI, Suda T. Regulation of reactive oxygen species in stem cells and cancer stem cells. J Cell Physiol. 2012;227(2):421–30. [DOI] [PubMed] [Google Scholar]
- 269.Luo M, Wicha MS. Targeting Cancer Stem Cell Redox Metabolism to Enhance Therapy Responses. Semin Radiat Oncol. 2019;29(1):42–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 270.Wang Q, Du X, Zhou B, Li J, Lu W, Chen Q et al. Mitochondrial dysfunction is responsible for fatty acid synthase inhibition-induced apoptosis in breast cancer cells by PdpaMn. Biomed Pharmacother. 2017;96:396–403. [DOI] [PubMed] [Google Scholar]
- 271.Xu JJ, Dai XM, Liu HL, Guo WJ, Gao J, Wang CH et al. A novel 7-azaisoindigo derivative-induced cancer cell apoptosis and mitochondrial dysfunction mediated by oxidative stress. J Appl Toxicol. 2011;31(2):164–72. [DOI] [PubMed] [Google Scholar]
- 272.Lu Y, Zhang R, Liu S, Zhao Y, Gao J, Zhu L. ZT-25, a new vacuolar H(+)-ATPase inhibitor, induces apoptosis and protective autophagy through ROS generation in HepG2 cells. Eur J Pharmacol. 2015;771:130–8. [DOI] [PubMed] [Google Scholar]
- 273.Shi L, Fei X, Wang Z. Demethoxycurcumin was prior to temozolomide on inhibiting proliferation and induced apoptosis of glioblastoma stem cells. Tumour Biol. 2015;36(9):7107–19. [DOI] [PubMed] [Google Scholar]
- 274.Laws K, Suntharalingam K. The Next Generation of Anticancer Metallopharmaceuticals: Cancer Stem Cell-Active Inorganics. Chembiochem. 2018;19(21):2246–53. [DOI] [PubMed] [Google Scholar]
- 275.Choi YJ, Park JH, Han JW, Kim E, Jae-Wook O, Lee SY et al. Differential Cytotoxic Potential of Silver Nanoparticles in Human Ovarian Cancer Cells and Ovarian Cancer Stem Cells. Int J Mol Sci. 2016;17(12):2077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 276.Choi YJ, Gurunathan S, Kim JH. Graphene Oxide-Silver Nanocomposite Enhances Cytotoxic and Apoptotic Potential of Salinomycin in Human Ovarian Cancer Stem Cells (OvCSCs): A Novel Approach for Cancer Therapy. Int J Mol Sci. 2018;19(3):710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 277.Laws K, Bineva-Todd G, Eskandari A, Lu C, O’Reilly N, Suntharalingam K. A Copper(II) Phenanthroline Metallopeptide That Targets and Disrupts Mitochondrial Function in Breast Cancer Stem Cells. Angew Chem Int Ed Engl. 2018;57(1):287–91. [DOI] [PubMed] [Google Scholar]
- 278.Purushothaman B, Arumugam P, Ju H, Kulsi G, Samson AAS, Song JM. Novel ruthenium(II) triazine complex [Ru(bdpta)(tpy)]2+ co-targeting drug resistant GRP78 and subcellular organelles in cancer stem cells. Eur J Med Chem. 2018;156:747–59. [DOI] [PubMed] [Google Scholar]
- 279.Stilgenbauer M, Jayawardhana A, Datta P, Yue Z, Gray M, Nielsen F et al. A spermine-conjugated lipophilic Pt(IV) prodrug designed to eliminate cancer stem cells in ovarian cancer. Chem Commun (Camb). 2019;55(43):6106–9. [DOI] [PubMed] [Google Scholar]
- 280.Visweswaran M, Arfuso F, Warrier S, Dharmarajan A. Aberrant lipid metabolism as an emerging therapeutic strategy to target cancer stem cells. Stem Cells. 2020;38(1):6–14. [DOI] [PubMed] [Google Scholar]
- 281.El Hout M, Cosialls E, Mehrpour M, Hamai A. Crosstalk between autophagy and metabolic regulation of cancer stem cells. Mol Cancer. 2020;19(1):27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 282.Mancini R, Noto A, Pisanu ME, De Vitis C, Maugeri-Sacca M, Ciliberto G. Metabolic features of cancer stem cells: the emerging role of lipid metabolism. Oncogene. 2018;37(18):2367–78. [DOI] [PubMed] [Google Scholar]
- 283.Peetla C, Vijayaraghavalu S, Labhasetwar V. Biophysics of cell membrane lipids in cancer drug resistance: Implications for drug transport and drug delivery with nanoparticles. Adv Drug Deliv Rev. 2013;65(13–14):1686–98.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 284.Xiang S, Sarem M, Shah S, Shastri VP. Liposomal Treatment of Cancer Cells Modulates Uptake Pathway of Polymeric Nanoparticles by Altering Membrane Stiffness. Small. 2018;14(14):e1704245. [DOI] [PubMed] [Google Scholar]
- 285.Recalcati S, Gammella E, Cairo G. Dysregulation of iron metabolism in cancer stem cells. Free Radic Biol Med. 2019;133:216–20. [DOI] [PubMed] [Google Scholar]
- 286.Mizushima N. Autophagy: process and function. Genes Dev. 2007;21(22):2861–73. [DOI] [PubMed] [Google Scholar]
- 287.Choi AM, Ryter SW, Levine B. Autophagy in human health and disease. N Engl J Med. 2013;368(19):1845–6. [DOI] [PubMed] [Google Scholar]
- 288.Song YJ, Zhang SS, Guo XL, Sun K, Han ZP, Li R et al. Autophagy contributes to the survival of CD133+ liver cancer stem cells in the hypoxic and nutrient-deprived tumor microenvironment. Cancer Lett. 2013;339(1):70–81.. [DOI] [PubMed] [Google Scholar]
- 289.Lin YH, Huang YC, Chen LH, Chu PM. Autophagy in cancer stem/progenitor cells. Cancer Chemother Pharmacol. 2015;75(5):879–86. [DOI] [PubMed] [Google Scholar]
- 290.Gong C, Bauvy C, Tonelli G, Yue W, Delomenie C, Nicolas V et al. Beclin 1 and autophagy are required for the tumorigenicity of breast cancer stem-like/progenitor cells. Oncogene. 2013;32(18):2261–72, 72e 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 291.Wu T, Geng J, Guo W, Gao J, Zhu X. Asiatic acid inhibits lung cancer cell growth in vitro and in vivo by destroying mitochondria. Acta Pharm Sin B. 2017;7(1):65–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 292.Singh BN, Kumar D, Shankar S, Srivastava RK. Rottlerin induces autophagy which leads to apoptotic cell death through inhibition of PI3K/Akt/mTOR pathway in human pancreatic cancer stem cells. Biochem Pharmacol. 2012;84(9):1154–63. [DOI] [PubMed] [Google Scholar]
- 293.Kumar D, Shankar S, Srivastava RK. Rottlerin-induced autophagy leads to the apoptosis in breast cancer stem cells: molecular mechanisms. Mol Cancer. 2013;12(1):171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 294.Apel A, Herr I, Schwarz H, Rodemann HP, Mayer A. Blocked autophagy sensitizes resistant carcinoma cells to radiation therapy. Cancer Res. 2008;68(5):1485–94. [DOI] [PubMed] [Google Scholar]
- 295.Liu D, Yang Y, Liu Q, Wang J. Inhibition of autophagy by 3-MA potentiates cisplatin-induced apoptosis in esophageal squamous cell carcinoma cells. Med Oncol. 2011;28(1):105–11. [DOI] [PubMed] [Google Scholar]
- 296.Al-Hajj M, Wicha MS, Benito-Hernandez A, Morrison SJ, Clarke MF. Prospective identification of tumorigenic breast cancer cells. Proc Natl Acad Sci U S A. 2003;100(7):3983–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 297.Galavotti S, Bartesaghi S, Faccenda D, Shaked-Rabi M, Sanzone S, McEvoy A et al. The autophagy-associated factors DRAM1 and p62 regulate cell migration and invasion in glioblastoma stem cells. Oncogene. 2013;32(6):699–712. [DOI] [PubMed] [Google Scholar]
- 298.Cufi S, Vazquez-Martin A, Oliveras-Ferraros C, Martin-Castillo B, Vellon L, Menendez JA. Autophagy positively regulates the CD44(+) CD24(−/low) breast cancer stem-like phenotype. Cell Cycle. 2011;10(22):3871–85. [DOI] [PubMed] [Google Scholar]
- 299.Lomonaco SL, Finniss S, Xiang C, Decarvalho A, Umansky F, Kalkanis SN et al. The induction of autophagy by gamma-radiation contributes to the radioresistance of glioma stem cells. Int J Cancer. 2009;125(3):717–22. [DOI] [PubMed] [Google Scholar]
- 300.Zhuang WZ, Li BZ, Long LM, Chen LS, Huang Q, Liang ZQ. Induction of autophagy promotes differentiation of glioma-initiating cells and their radiosensitivity. Int J Cancer. 2011;129(11):2720–31. [DOI] [PubMed] [Google Scholar]
- 301.Sun R, Shen S, Zhang YJ, Xu CF, Cao ZT, Wen LP et al. Nanoparticle-facilitated autophagy inhibition promotes the efficacy of chemotherapeutics against breast cancer stem cells. Biomaterials. 2016;103:44–55. [DOI] [PubMed] [Google Scholar]
- 302.Zhang XF, Gurunathan S. Combination of salinomycin and silver nanoparticles enhances apoptosis and autophagy in human ovarian cancer cells: an effective anticancer therapy. Int J Nanomedicine. 2016;11:3655–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 303.Lu L, Shen XK, Tao BL, Lin CC, Li K, Luo Z et al. The nanoparticle-facilitated autophagy inhibition of cancer stem cells for improved chemotherapeutic effects on glioblastomas. J Mater Chem B. 2019;7(12):2054–62. [DOI] [PubMed] [Google Scholar]
- 304.Chang CM, Lan KL, Huang WS, Lee YJ, Lee TW, Chang CH et al. Re-188-Liposome Can Induce Mitochondrial Autophagy and Reverse Drug Resistance for Ovarian Cancer: From Bench Evidence to Preliminary Clinical Proof-of-Concept. Int J Mol Sci. 2017;18(5):903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 305.Gilboa E. DC-based cancer vaccines. J Clin Invest. 2007;117(5):1195–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 306.Fu C, Zhou N, Zhao Y, Duan J, Xu H, Wang Y. Dendritic cells loaded with CD44(+) CT-26 colon cell lysate evoke potent antitumor immune responses. Oncol Lett. 2019;18(6):5897–904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 307.Szaryńska M, Olejniczak A, Kobiela J, Laski D, Sledzinski Z, Kmiec Z. Cancer stem cells as targets for DC-based immunotherapy of colorectal cancer. Sci Rep. 2018;8:12042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 308.Grossenbacher SK, Canter RJ, Murphy WJ. Natural killer cell immunotherapy to target stem-like tumor cells. J Immunother Cancer. 2016;4:19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 309.Luna JI, Grossenbacher SK, Murphy WJ, Canter RJ. Targeting Cancer Stem Cells with Natural Killer Cell Immunotherapy. Expert Opin Biol Ther. 2017;17(3):313–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 310.Badrinath N, Yoo SY. Recent Advances in Cancer Stem Cell-Targeted Immunotherapy. Cancers (Basel). 2019;11(3). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 311.Deng Z, Wu Y, Ma W, Zhang S, Zhang YQ. Adoptive T-cell therapy of prostate cancer targeting the cancer stem cell antigen EpCAM. BMC Immunol. 2015;16:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 312.Hurton LV, Singh H, Najjar AM, Switzer KC, Mi T, Maiti S et al. Tethered IL-15 augments antitumor activity and promotes a stem-cell memory subset in tumor-specific T cells. Proc Natl Acad Sci U S A. 2016;113(48):E7788–E97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 313.Miyamoto S, Kochin V, Kanaseki T, Hongo A, Tokita S, Kikuchi Y et al. The Antigen ASB4 on Cancer Stem Cells Serves as a Target for CTL Immunotherapy of Colorectal Cancer. Cancer Immunol Res. 2018;6(3):358–69. [DOI] [PubMed] [Google Scholar]
- 314.Rajayi H, Tavasolian P, Rezalotfi A, Ebrahimi M. Cancer Stem Cells Targeting; the Lessons from the Interaction of the Immune System, the Cancer Stem Cells and the Tumor Niche. Int Rev Immunol. 2019;38(6):267–83. [DOI] [PubMed] [Google Scholar]
- 315.Wu Y, Chen M, Wu P, Chen C, Xu ZP, Gu W. Increased PD-L1 expression in breast and colon cancer stem cells. Clin Exp Pharmacol Physiol. 2017;44(5):602–4. [DOI] [PubMed] [Google Scholar]
- 316.Almozyan S, Colak D, Mansour F, Alaiya A, Al-Harazi O, Qattan A et al. PD-L1 promotes OCT4 and Nanog expression in breast cancer stem cells by sustaining PI3K/AKT pathway activation. Int J Cancer. 2017;141(7):1402–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 317.Zheng F, Dang J, Zhang H, Xu F, Ba D, Zhang B et al. Cancer Stem Cell Vaccination With PD-L1 and CTLA-4 Blockades Enhances the Eradication of Melanoma Stem Cells in a Mouse Tumor Model. J Immunother. 2018;41(8):361–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 318.Han L, Shi S, Gong T, Zhang Z, Sun X. Cancer stem cells: therapeutic implications and perspectives in cancer therapy. Acta Pharm Sin B. 2013;3(2):65–75. [Google Scholar]
- 319.Kitamura H, Okudela K, Yazawa T, Sato H, Shimoyamada H. Cancer stem cell: implications in cancer biology and therapy with special reference to lung cancer. Lung Cancer. 2009;66(3):275–81. [DOI] [PubMed] [Google Scholar]
- 320.Clarke MF, Dick JE, Dirks PB, Eaves CJ, Jamieson CH, Jones DL et al. Cancer stem cells--perspectives on current status and future directions: AACR Workshop on cancer stem cells. Cancer Res. 2006;66(19):9339–44. [DOI] [PubMed] [Google Scholar]
- 321.Bahmad HF, Cheaito K, Chalhoub RM, Hadadeh O, Monzer A, Ballout F et al. Sphere-Formation Assay: Three-Dimensional in vitro Culturing of Prostate Cancer Stem/Progenitor Sphere-Forming Cells. Front Oncol. 2018;8:347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 322.Abbaszadegan MR, Bagheri V, Razavi MS, Momtazi AA, Sahebkar A, Gholamin M. Isolation, identification, and characterization of cancer stem cells: A review. J Cell Physiol. 2017;232(8):2008–18. [DOI] [PubMed] [Google Scholar]
- 323.Magee JA, Piskounova E, Morrison SJ. Cancer stem cells: impact, heterogeneity, and uncertainty. Cancer Cell. 2012;21(3):283–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 324.Liu H, Lv L, Yang K. Chemotherapy targeting cancer stem cells. Am J Cancer Res. 2015;5(3):880–93. [PMC free article] [PubMed] [Google Scholar]
- 325.Wang AB, Qu L, Wang LH. At the crossroads of cancer stem cells and targeted therapy resistance. Cancer Lett. 2017;385:87–96. [DOI] [PubMed] [Google Scholar]
- 326.Prud’homme GJ. Cancer stem cells and novel targets for antitumor strategies. Curr Pharm Des. 2012;18(19):2838–49. [DOI] [PubMed] [Google Scholar]
- 327.Herheliuk T, Perepelytsina O, Ugnivenko A, Ostapchenko L, Sydorenko M. Investigation of multicellular tumor spheroids enriched for a cancer stem cell phenotype. Stem Cell Investig. 2019;6:21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 328.Li J, Yu Y, Wang J, Yan Z, Liu H, Wang Y et al. Establishment of a novel system for the culture and expansion of hepatic stem-like cancer cells. Cancer Lett. 2015;360(2):177–86. [DOI] [PubMed] [Google Scholar]
- 329.Wang X, Dai X, Zhang X, Ma C, Li X, Xu T et al. 3D bioprinted glioma cell-laden scaffolds enriching glioma stem cells via epithelial-mesenchymal transition. J Biomed Mater Res A. 2019;107(2):383–91. [DOI] [PubMed] [Google Scholar]
- 330.Ward Rashidi MR, Mehta P, Bregenzer M, Raghavan S, Fleck EM, Horst EN et al. Engineered 3D Model of Cancer Stem Cell Enrichment and Chemoresistance. Neoplasia. 2019;21(8):822–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 331.Lamichhane SP, Arya N, Kohler E, Xiang S, Christensen J, Shastri VP. Recapitulating epithelial tumor microenvironment in vitro using three dimensional tri-culture of human epithelial, endothelial, and mesenchymal cells. BMC Cancer. 2016;16(1):581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 332.Lazzari G, Nicolas V, Matsusaki M, Akashi M, Couvreur P, Mura S. Multicellular spheroid based on a triple co-culture: A novel 3D model to mimic pancreatic tumor complexity. Acta Biomater. 2018;78:296–307. [DOI] [PubMed] [Google Scholar]
- 333.Penfornis P, Vallabhaneni KC, Janorkar AV, Pochampally RR. Three dimensional tumor models for cancer studies. Front Biosci (Elite Ed). 2017;9:162–73. [DOI] [PubMed] [Google Scholar]
- 334.Wang HF, Ran R, Liu Y, Hui Y, Zeng B, Chen D et al. Tumor-Vasculature-on-a-Chip for Investigating Nanoparticle Extravasation and Tumor Accumulation. ACS Nano. 2018;12(11):11600–9. [DOI] [PubMed] [Google Scholar]
- 335.Shen S, Mamat M, Zhang SC, Cao J, Hood ZD, Figueroa-Cosme L et al. Synthesis of CaO2 Nanocrystals and Their Spherical Aggregates with Uniform Sizes for Use as a Biodegradable Bacteriostatic Agent. Small. 2019. [DOI] [PubMed] [Google Scholar]
- 336.Singh D, Minz AP, Sahoo SK. Nanomedicine-mediated drug targeting of cancer stem cells. Drug Discov Today. 2017;22(6):952–9. [DOI] [PubMed] [Google Scholar]
- 337.Mokhtarzadeh A, Hassanpour S, Vahid ZF, Hejazi M, Hashemi M, Ranjbari J et al. Nano-delivery system targeting to cancer stem cell cluster of differentiation biomarkers. J Control Release. 2017;266:166–86. [DOI] [PubMed] [Google Scholar]
- 338.Halappanavar S, Vogel U, Wallin H, Yauk CL. Promise and peril in nanomedicine: the challenges and needs for integrated systems biology approaches to define health risk. Wiley Interdiscip Rev Nanomed Nanobiotechnol. 2018;10(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 339.Patil Y, Sadhukha T, Ma L, Panyam J. Nanoparticle-mediated simultaneous and targeted delivery of paclitaxel and tariquidar overcomes tumor drug resistance. J Control Release. 2009;136(1):21–9. [DOI] [PubMed] [Google Scholar]
- 340.Xu L, Liu J, Xi J, Li Q, Chang B, Duan X et al. Synergized Multimodal Therapy for Safe and Effective Reversal of Cancer Multidrug Resistance Based on Low-Level Photothermal and Photodynamic Effects. Small. 2018:e1800785. [DOI] [PubMed] [Google Scholar]


