Abstract
Mercury (Hg) vapor can produce kidney injury, where the proximal tubule region of the nephron is the main target of the Hg-induced oxidative stress. Hg is eliminated from the body as a glutathione conjugate. Thus, single nucleotide polymorphisms (SNPs) in glutathione-related genes might modulate the negative impact of this metal on the kidneys. Glutathione-related SNPs were tested for association with levels of Hg and renal function biomarkers between occupationally exposed (n = 160) and non-exposed subjects (n = 121). SNPs were genotyped by TaqMan assays in genomic DNA samples. Total mercury concentration was measured in blood, urine and hair samples. Regression analyses were performed to estimate the effects of SNPs on quantitative traits. Alleles GCLM rs41303970-T and GSTP1 rs4147581-C were significantly overrepresented in the exposed compared with the non-exposed group (P < 0.01). We found significant associations for GCLM rs41303970-T with higher urinary clearance rate of Hg (β = 0.062, P = 0.047), whereas GCLC rs1555903-C was associated with lower levels of estimated glomerular filtration rate in the non-exposed group (eGFR, β = − 3.22, P = 0.008) and beta-2-microglobulin in the exposed group (β-2MCG, β = − 19.32, P = 0.02). A SNP-SNP interaction analysis showed significant epistasis between GSTA1 rs3957356-C and GSS rs3761144-G with higher urinary levels of Hg in the exposed (β = 0.13, P = 0.04) but not in the non-exposed group. Our results suggest that SNPs in glutathione-related genes could modulate the pathogenesis of Hg nephrotoxicity in our study population by modulating glutathione concentrations in individuals occupationally exposed to this heavy metal.
Subject terms: Genetics, Health occupations, Nephrology
Introduction
Small-scale mining operations often use amalgamation with mercury (Hg) to recover gold; Hg is then vaporized by heating the amalgam1. This activity is the largest anthropogenic source of Hg pollution worldwide2, with Colombia being one of the main per capita Hg polluters in the world3,4. Inhalation of elemental Hg (Hg0) vapors released from burning amalgam has harmful effects on the kidneys, as it is converted to inorganic Hg (Hg1+ or Hg2+) in extrarenal tissues and then accumulates in the proximal tubule region of the nephron via mechanism of filtration–reabsorption as conjugates of glutathione with Hg5,6; causing kidney damage7,8.
Once accumulated in the kidney proximal tubule cells, inorganic Hg interrupts intracellular homeostasis by inducing oxidative stress, subcellular organelle dysfunction, and apoptosis as a consequence of reactive oxygen species (ROS) generation, which leads to kidney injury9–11. The Hg has an affinity for sulfhydryl groups (–SH) such as those found in glutathione, the most abundant non-protein thiol-containing compound in the cell, thereby mitigating its toxicity and maintaining cellular redox status12,13. Therefore, glutathione-related enzymes also play key roles in the natural detoxification process of Hg by controlling the glutathione levels14,15 and conjugates of glutathione with Hg16.
Currently, genetic variation is considered an important contributor to heavy metal body retention and metabolism17. Several epidemiological studies have suggested that genetic variants in glutathione-related genes may significantly influence Hg toxicokinetics, especially some single nucleotide polymorphisms (SNPs) in some glutathione-related enzymes which have been associated with blood and urine Hg concentrations6,18,19. However, the gene-Hg interactions have not been reported in relation to kidney injury, which needs to be addressed in order to understand the impact of genetic variation on health effects caused by this heavy metal. In this context, we carried out an epidemiological study on occupational exposure to Hg vapor on kidney function in a historically gold-mining town in Colombia. In this study, we reported that, despite higher levels of Hg in blood and urine in miners compared to a control group, the kidney function was normal and comparable between both groups20. This is especially interesting since it has been reported that SNPs in detoxification genes are associated with toxic metal tolerance and adaptation in humans21. Therefore, we hypothesized that genetic variants in glutathione-related genes could modulate the negative impact of Hg on the kidneys.
The aim of the current work was to investigate whether single nucleotide polymorphisms (SNPs) in the genes glutamate-cysteine ligase catalytic subunit (GCLC); glutamate-cysteine ligase modifier subunit (GCLM); glutathione synthetase (GSS); glutathione S-transferase alpha 1 (GSTA1); and glutathione S-transferase pi 1 (GSTP1) are associated with modulation of Hg nephrotoxicity in our gold mining population in Colombia.
Results
Study population
Sociodemographic characteristics of the study participants and measurement of biomarkers of Hg exposure and effect are presented in Table 1. All loci were in Hardy–Weinberg equilibrium in the study control group. The genotype and allele frequencies are reported in Table 2. According to the hierarchy AMOVA, no genetic structure was evidenced (FST = 0.00521; P = 0.10182 ± 0.00091) between the exposed and non-exposed groups.
Table 1.
Sociodemographic characteristics, exposure and effect biomarkers of study population.
| Variable | Exposure | Non-exposure | P value |
|---|---|---|---|
| N = 160 | N = 121 | ||
| n (%) or median (IQR) | n (%) or median (IQR) | ||
| Malea | 101 (63.12) | 59 (48.76) | 0.022 |
| Femalea | 59 (36.88) | 62 (51.24) | |
| Age (years)b | 40 (18–62) | 47 (22–59) | < 0.001 |
| Blood-Hg (μg Hg/L)b | 7.0 (3.4–11.0) | 2.5 (2.5–4.7) | < 0.001 |
| Urine-Hg (μg Hg/g creatinine)b | 3.8 (2.9–10.1) | 2.9 (2.9–3.0) | < 0.001 |
| Hair-Hg (μg Hg/g hair)b | 0.8 (0.5–1.3) | 0.4 (0.2–0.7) | < 0.001 |
| eGFR (mL/min/1.73 m2)b | 82.6 (74.5–89.6) | 75.7 (69.3–84.1) | < 0.001 |
| Urinary albumin (mg/24 h)b | 64.1 (48.6–94.6) | 87.9 (54.1–132.7) | < 0.001 |
| β-2MCG (ng/mL)b | 41.1 (23.1–62.9) | 36.6 (22.3–64.6) | 0.988 |
Hg mercury, β-2MCG β-2-microglobulin, eGFR estimated glomerular filtration rate, IQR interquartile range.
aPearson’s Chi-square test.
bMann–Whitney U test/Wilconxon rank-sum test for differences between groups.
Table 2.
Distribution of genetic variants in glutathione-related genes in Hg exposed and non-exposed groups.
| Gene | SNP | Genotype/allele | Exposed | Non-exposed | HWE P value |
P valued | P valuee | OR (95% CI) | ||
|---|---|---|---|---|---|---|---|---|---|---|
| N = 160 | N = 121 | |||||||||
| N (%) | N (%) | |||||||||
| GCLC | rs1555903 | TT | 66 (41.3) | 48 (39.7) | 0.96a | 0.96 | 0.81 | |||
| TC | 73 (45.6) | 56 (46.3) | 0.94b | 1.0 | ||||||
| CC | 21 (13.1) | 17 (14.0) | 0.94c | 0.85 | ||||||
| T | 205 (64.0) | 152 (62.8) | ||||||||
| C | 115 (36.0) | 90 (37.2) | 0.78 | 1.05 (0.75–1.49) | ||||||
| GCLM | rs41303970 | CC | 63 (39.4) | 72 (59.5) | 0.31a | 0.0015 | 0.0009 | 0.44 (0.27–0.72) | ||
| CT | 80 (50.0) | 45 (37.2) | 0.45b | 0.039 | 1.69 (1.04–2.73) | |||||
| TT | 17 (10.6) | 4 (3.3) | 0.32c | 0.026 | 3.48 (1.13–10.6) | |||||
| C | 206 (64.3) | 189 (78.0) | ||||||||
| T | 114 (35.7) | 53 (22.0) | 0.0004 | 1.97 (1.35–2.89) | ||||||
| GSS | rs3761144 | CC | 64 (40.0) | 53 (43.8) | 0.047a | 0.43 | ||||
| CG | 64 (40.0) | 51 (42.2) | 0.49b | |||||||
| GG | 32 (20.0) | 17 (14.0) | 0.036c | |||||||
| C | 192 (60.0) | 157 (64.8) | ||||||||
| G | 128 (40.0) | 85 (35.2) | 0.23 | 1.23 (0.87–1.74) | ||||||
| GSTA1 | rs3957356 | CC | 71 (44.4) | 45 (40.2) | 0.71a | 0.18 | ||||
| CT | 69 (43.1) | 59 (52.7) | 0.07b | |||||||
| TT | 20 (12.5) | 8 (7.1) | 0.45c | |||||||
| C | 211 (66.0) | 149 (66.5) | ||||||||
| T | 109 (34.0) | 75 (33.5) | 0.92 | 1.03 (0.71–1.47) | ||||||
| GSTP1 | rs4147581 | CC | 76 (47.5) | 43 (35.5) | 0.62a | 0.015 | 0.042 | 1.64 (1.01–2.66) | ||
| CG | 71 (44.4) | 55 (45.4) | 0.56b | 0.89 | ||||||
| GG | 13 (8.1) | 23 (19.1) | 0.84c | 0.012 | 0.37 (0.18–0.77) | |||||
| C | 223 (69.7) | 141 (58.3) | ||||||||
| G | 97 (30.3) | 101 (41.7) | 0.005 | 0.60 (0.43–0.86) | ||||||
Statistically significant association are shown in bold (P value < 0.05).
The statistical significance was determined using Pearson’s Chi-square test with P value simulated from 2000 permutations.
P value for Hardy–Weinberg equilibrium in exposeda and non-exposedb groups, and all samplesc.
dP value for allele and genotype associations.
eP value for each genotype versus the other two genotypes.
Association of genetic variants with Hg levels and kidney function biomarkers
Statistically significant differences in the distribution of genotypes and alleles of GCLM rs41303970 and GSTP1 rs4147581 were observed between the groups. Individuals in the exposed group had an over-representation of the rs41303970-T allele (OR = 1.97; P = 0.0004) but an under-representation of the rs4147581-G allele (OR = 0.60; P = 0.005) compared with the non-exposed group (Table 2). No other statistically significant differences were found in frequency distribution between these two groups.
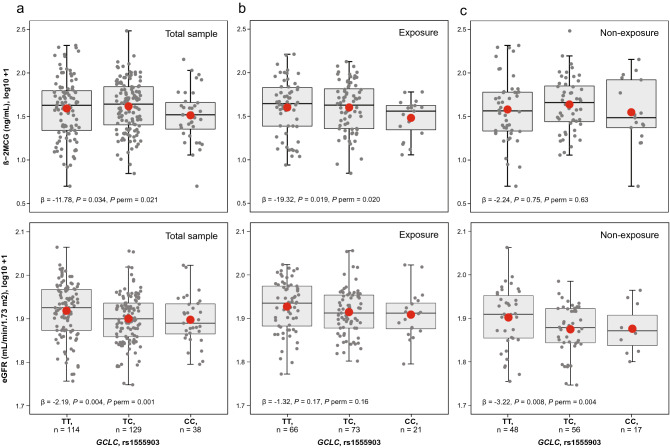
A multivariable linear regression model was used to test the effects of SNPs on Hg levels by an additive model. In the combined sample there was a statistically significantly increased urine-Hg (β = 0.062; P = 0.047 and P perm = 0.009), but not blood-Hg (P = 0.63) in relation to the GCLM rs41303970-T allele, adjusting by exposure status, sex, age, estimated glomerular filtration rate (eGFR), and hair-Hg levels (Table 3). With respect to biomarkers of renal injury, we found statistically significant associations in relation to the GCLC rs1555903-C allele by lowering the levels of eGFR and beta-2-microglobulin (β-2MCG) in the non-exposed group (β = − 3.22; P = 0.008, P perm = 0.004) and the exposed group (β = − 19.32; P = 0.02, P perm = 0.02), respectively (Table 4, Fig. 1).
Table 3.
Association of genetic variants in glutathione-related genes with Hg levels in blood and urine.
| Gene | SNP | N | Blood-Hg (µg Hg/L) | Urine-Hg (µg Hg/g creatinine) | ||||
|---|---|---|---|---|---|---|---|---|
| β | P valuea | P valueb | β | P valuea | P valueb | |||
| GCLC | rs1555903 | 281 | − 0.042 | 0.07 | 0.03 | − 0.008 | 0.76 | 0.74 |
| GCLM | rs41303970 | 281 | − 0.012 | 0.65 | 0.56 | 0.062 | 0.047 | 0.009 |
| GSS | rs3761144 | 281 | 0.014 | 0.51 | 0.55 | − 0.002 | 0.94 | 1.0 |
| GSTA1 | rs3957356 | 272 | 0.032 | 0.19 | 0.74 | − 0.012 | 0.67 | 0.32 |
| GSTP1 | rs4147581 | 281 | 0.004 | 0.86 | 0.88 | − 0.003 | 0.92 | 1.0 |
Statistically significant association are shown in bold (P value < 0.05).
Each SNP was examined using multivariable linear regression models assuming additive models of inheritance adjusted by exposure status, age, sex, eGFR, and hair-Hg levels (µg Hg/g hair).
aP value from a generalized linear model.
bEmpirical P value determined based on 5000 permutations from a generalized linear model.
Table 4.
Association of genetic variants in glutathione-related genes with kidney function biomarkers.
| Gene | SNP | Biomarker | Total sample N = 272 |
Exposure N = 155 |
Non-exposure N = 117 |
||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| β | P valuea | P valueb | β | P valuea | P valueb | β | P valuea | P valueb | |||
| GCLC | rs1555903 | eGFR | − 2.19 | 0.004 | 0.001 | − 1.32 | 0.17 | 0.16 | − 3.22 | 0.008 | 0.004 |
| Albumin | − 48.18 | 0.15 | 0.12 | − 68.59 | 0.25 | 0.10 | − 21.71 | 0.06 | 0.04 | ||
| β-2MCG | − 11.78 | 0.034 | 0.021 | − 19.32 | 0.02 | 0.02 | − 2.24 | 0.75 | 0.63 | ||
| GCLM | rs41303970 | eGFR | − 1.54 | 0.085 | 0.07 | − 0.73 | 0.49 | 0.39 | − 3.15 | 0.05 | 0.07 |
| Albumin | − 52.49 | 0.18 | 0.11 | − 71.46 | 0.28 | 0.17 | − 21.42 | 0.15 | 0.09 | ||
| β-2MCG | 2.78 | 0.67 | 0.96 | 2.94 | 0.74 | 1.0 | 2.85 | 0.75 | 1.0 | ||
| GSS | rs3761144 | eGFR | − 0.03 | 0.97 | 1.0 | − 0.26 | 0.76 | 0.94 | 0.28 | 0.82 | 0.82 |
| Albumin | 55.39 | 0.08 | 0.05 | 81.47 | 0.13 | 0.07 | 15.65 | 0.18 | 0.54 | ||
| β-2MCG | − 3.41 | 0.51 | 0.58 | − 1.77 | 0.81 | 1.0 | − 4.41 | 0.54 | 1.0 | ||
| GSTA1 | rs3957356 | eGFR | 0.95 | 0.23 | 0.12 | 0.54 | 0.58 | 0.60 | 1.78 | 0.21 | 0.19 |
| Albumin | 66.02 | 0.07 | 0.05 | 103.89 | 0.08 | 0.14 | − 9.64 | 0.49 | 0.57 | ||
| β-2MCG | 6.57 | 0.26 | 0.21 | 7.89 | 0.33 | 0.14 | 0.51 | 0.95 | 0.96 | ||
| GSTP1 | rs4147581 | eGFR | 0.32 | 0.77 | 0.66 | 0.72 | 0.48 | 0.96 | − 0.12 | 0.92 | 0.91 |
| Albumin | − 36.53 | 0.29 | 1.0 | − 64.89 | 0.31 | 0.68 | − 7.33 | 0.54 | 0.62 | ||
| β-2MCG | − 1.82 | 0.74 | 1.0 | − 11.26 | 0.19 | 0.09 | 8.53 | 0.24 | 0.61 | ||
Statistically significant association are shown in bold (P value < 0.05).
Each SNP was examined using multivariable linear regression models assuming additive model of inheritance adjusted by age, sex, blood-Hg levels (µg Hg/L), urine-Hg levels (µg Hg/g creatinine), hair-Hg levels (µg Hg/g hair), and duration of exposure (years). Having lower eGFR levels, higher albumin levels or higher β-2MCG levels are indicative of kidney injury.
aP value from a generalized linear model.
bEmpirical P value determined based on 5000 permutations from a generalized linear model.
Figure 1.
Beta-2-microglobulin (β-2MCG) and estimated glomerular filtration rate (eGFR) in relation to GCLC rs1555903 genotypes. The boxplot shows a significantly decreasing trend of both β-2MCG and eGFR in the total sample (a, P < 0.05). Analysis by exposure status also shows a similar trend in the exposure group with β-2MCG (b, β = − 19.32, P = 0.019, P perm = 0.020) and in the non-exposure group with eGFR (c, β = − 3.22, P = 0.008, P perm = 0.004). Each SNP was examined using multivariable linear regression models assuming additive model of inheritance adjusted by age, sex, blood-Hg levels (µg Hg/L), urine-Hg levels (µg Hg/g creatinine), hair-Hg levels (µg Hg/g hair), and duration of exposure (years).
Considering that sex-differences in the toxic effect of chemicals that people are exposed to in the working and general environment are to be expected22, we assessed the effect modification by sex for the association with kidney function of the GCLC rs1555903 and GCLM rs41303970 SNPs. While sex showed statistically significant difference in the study population (Table 1), there was no evidence of effect modification by sex for this genetic variant (P > 0.7 for eGFR and P > 0.3 for β-2MCG). In our multivariable linear regression model, the GCLC rs1555903 SNP remained statistically significantly associated with the biomarkers of renal injury, but the magnitude of the regression coefficient decreased by 9.8% with eGFR (β value from − 2.43 to − 2.19) and by 7.5% with β-2MCG (β value from − 20.88 to − 19.32) after adjustment for sex.
Interaction effects of genetic variants on Hg levels and kidney function biomarkers
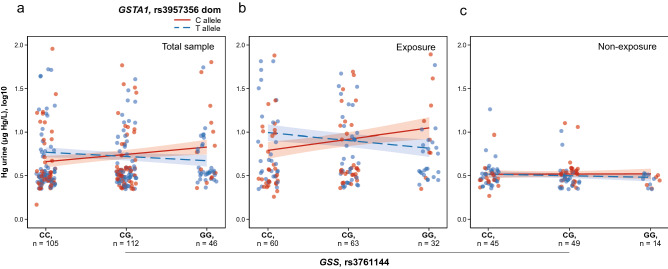
To identify epistasis between genetic loci, we used a gene interaction analysis between all pairs of individual SNPs by generalized linear regression models. We identified a significant epistatic interaction between GSS rs3761144 and GSTA1 rs3957356 on urine-Hg levels (P = 0.015, P perm = 0.017). A simple slope analysis showed that the effect of the rs3761144-G allele on the increase in urine-Hg levels only exists when individuals carry the rs3957356-C allele in the exposed group (β = 0.13, P = 0.04) but not in the non-exposed group (β = 0.01, P = 0.95) (Fig. 2). No significant interactions were found with any of the kidney function biomarkers assessed.
Figure 2.
Interaction effects of genetic variants in a multivariable model of urine-Hg. The urine-Hg concentration was defined as a function of the interaction between GSS rs3761144 under an additive model and GSTA1 rs3857356 under a dominant model. The interaction plot shows a significantly increasing trend of urine-Hg levels when individuals carry both rs3761144-G and rs3957356-C alleles (P = 0.015, P perm = 0.017) in the total sample (a). A simple slope analysis showed that the association between urine-Hg and GSS rs3761144 was stronger in individuals with rs3957356-C allele compared to individuals with rs3957356-T allele in the exposed group (β = 0.13, P = 0.04) (b) but not in the non-exposed group (c). All interaction analyses were adjusted by age, sex, eGFR, and hair-Hg levels (µg Hg/g hair). Total sample was also adjusted by exposure status.
Discussion
In the kidneys, inorganic Hg species accumulate mainly in the proximal tubule cells, causing renal injury by oxidative stress mechanisms9. While glutathione is certainly an important physiological antioxidant of Hg, the glutathione-Hg conjugates might also contribute to intracellular retention of Hg since these conjugates may not be transported easily out of the proximal tubule cells23,24. Our study suggests that SNPs in glutathione-related genes might influence toxic effects of Hg by modulating glutathione concentrations in individuals occupationally exposed to this heavy metal.
Studies have shown that GCL is the main determinant of cellular glutathione levels since it catalyzes the first and rate-limiting step in glutathione synthesis25. Glutathione de novo synthesis is thought to be induced primarily by transcriptional regulation26,27. In this regard, the SNPs GCLM rs41303970 and GCLC rs1555903, located in the 5′-flanking regions of their respective genes, might affect gene expression at a transcriptional level. On the one hand, the C-to-T substitution at GCLM rs41303970 leads to less GCLM enzyme production28. We also found that the T-allele of this SNP was associated with higher urine-Hg levels (Table 3), which is in agreement with previous reports on mining settings6,29. On the other hand, the T-to-C substitution at GCLC rs1555903 leads to higher GCLC gene expression, as shown in a small set of kidney cortex samples (N = 73, β = 0.73, P = 0.008) from the Genotype-Tissue Expression (GTEx) dataset30. A study reported that the rs1555903-C allele is associated with methyl-Hg retention in the umbilical cord31. We did not find an association between this SNP and blood- or urine-Hg levels (Table 3). However, we found that the C-allele of this SNP was associated with lower levels of β-2MCG in the exposed group but not in the non-exposed group (Table 4, Fig. 1), suggesting a better performance of the kidney tubular function.
While GCLC and GCLM are the subunits that constitute the GCL holoenzyme, they are not necessarily present in equimolar amounts within the cell27. GCLM interacts with GCLC, making the GCL holoenzyme kinetically more efficient in glutathione synthesis since GCLC has a very high Km for l-glutamate compared to when it is complexed with GCLM32,33. In this context, a low expression level of GCLM due to the rs41303970-T allele might result in a low synthesis rate of glutathione and hence the formation of Hg-glutathione conjugates6. At the kidney level, uptake of glutathione-Hg conjugates occurs mainly at the luminal plasma membrane of proximal tubular epithelial cells12,34. In Vivo findings have suggested that this process depends greatly on the actions of both γ-glutamyltransferase and cysteinylglycinase, which form conjugates of l-cysteine (e.g., dicysteinylmercury) that are internalized through amino acid transporters via a mechanism of molecular homology9,34. Therefore, a reduction in the formation of Hg-glutathione conjugates minimizes its uptake rate into the kidneys after glomerular filtration of Hg contained in the primary urine, thus favoring the excretion of Hg (Fig. 3).
Figure 3.
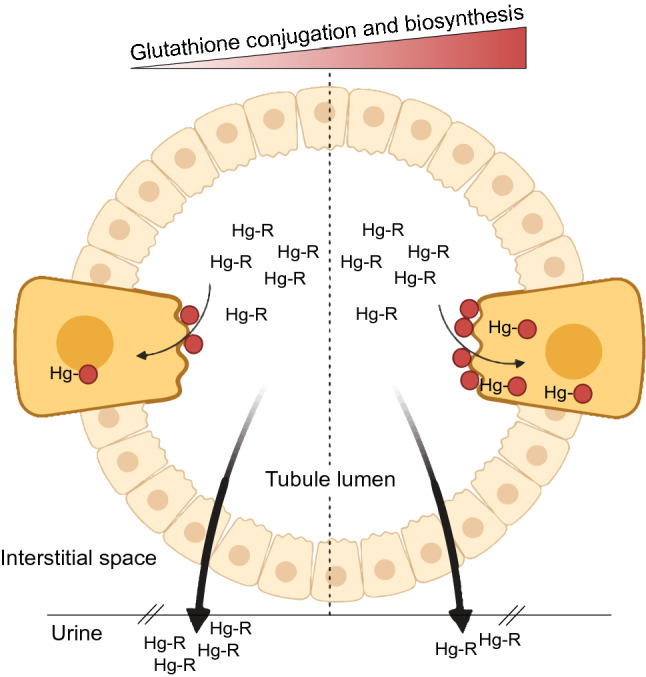
Graphical representation of contributing biosynthesis and conjugation of glutathione underlying Hg-kidney toxicity. The graph shows a cross-section of the proximal tubule of neprhon, where two cells were magnified to represent the reabsorption of glutathione-Hg conjugates. Hg contained in the primary urine after glomerular filtration is presented as Hg-R, where R is any molecule attached to it (e.g., albumin). The dotted line split the graph in two sections according to the level of biosynthesis and conjugation of glutathione (red circles): lower levels on the left and high levels on the right. At the level of the luminal plasma membrane of proximal tubular epithelial cells, glutathione-Hg conjugates are internalized by a mechanism of molecular homology such as dicysteinylmercury (cysteine-Hg-cysteine). Thus, decreasing both biosynthesis (as in GCLM rs41303970-T carriers) and/or conjugation (as in GSTP1 rs4147581-C carriers) of glutathione could increase the Hg elimination in the urine due to a lower rate in the uptake of glutathione-Hg conjugates, mitigating kidney injury by this heavy metal. Created with BioRender.com and edited with Adobe Illustrator.
In the same direction, we found that the rs4147581-C allele in the glutathione conjugation gene GSTP1 is significantly more frequent in the exposed than in the non-exposed group (Table 2). This allele has been associated with DNA methylation35, which might be down-regulating the GSTP1 gene expression36. These data could explain, at least in part, why we found lower eGFR levels in relation to the GCLC rs1555903 SNP in the non-exposed but not in the exposed group (Table 4). Thus, having the alleles GCLC rs1555903-C and GCLM rs41303970-C drives to more glutathione production, which could be efficiently conjugated to Hg by members of the GST-superfamily such GSTP1—the most widely expressed GST enzyme according to the Human Protein Atlas37, including in the kidneys. The GSTP1 rs4147581 is in linkage disequilibrium with rs1695 (D′ = 0.99, R2 = 0.44 in Admixed American populations, including Colombians), a common nonsynonymous SNP that changes the 105th amino acid from isoleucine to valine, altering the substrate binding site of GSTP138. It has been reported that the GSTP1 Val105 not only has a significantly lower affinity for glutathione, but also is more sensitive to inhibition by Hg species38. Since the GSTP1 Val105 correlates with rs4147581-C allele (Chi-square = 63.17, P < 0.0001) in Colombians from the 1000 Genomes Project39 as calculated in the LDlink web-based application40, these genetic variants could be conferring some protection to the exposed group by negatively affecting the formation of Hg-glutathione conjugates (Fig. 3).
Considering that gene–gene interactions may contribute to inter-individual variation in complex traits41,42, we tested whether epistatic interactions for SNP pairs can contribute to regulating Hg body retention and nephrotoxicity. We identified a significant SNP-SNP interaction between the GSS rs3761144-G and GSTA1 rs3957356-C alleles with a clearance of urine-Hg at a higher rate in the exposed but not in the non-exposed group (Fig. 2). Both alleles, located in the 5′-flanking regions, down-regulate the expression of their respective genes43,44. In this context, low expression of these two genes would lead not only to decreased glutathione synthesis by the GSS enzyme, but also to decreased glutathione conjugation by the GSTA1 enzyme16,45. It has been reported that the rs3761144-G allele is associated with higher levels of hair methyl-Hg due to fish consumption44, but the rs3957356-C allele did not show a similar trend with levels of methyl-Hg in erythrocytes18.
Taken together, these pieces of evidence suggest that the biosynthesis rate of glutathione might be involved in nephrotoxicity by increasing cellular retention of Hg, altering the redox balance and leading to cytotoxicity46,47, possibly via mitochondria dysfunction by altering the ratio of reduced (GSH) to oxidized (GSSG) glutathione in this organelle48,49. Therefore, decreasing both biosynthesis and conjugation of glutathione could positively impact the body’s ability to clear Hg. This is supported by the observations that: (1) inorganic Hg accumulation in the proximal tubule region of the nephron is mainly handled by filtration–reabsorption as glutathione-Hg conjugates5,6, and (2) the renal uptake of glutathione-Hg conjugates occurs mainly at the luminal membrane via a molecular mimicry mechanism using animo acid transporters9,12. The study population offers some unique opportunity to explore whether genes can provide protection to a specific disease-related environmental exposure. This is because, contrary to expectations, we did not find meaningful associations between occupational Hg vapor exposure and altered kidney function monitoring parameters20. It is important to mention that both the exposed and the non-exposed groups are not a migrant population but a stationary population. Indeed, they have been living for several generations in those towns (i.e., since Colonial times), where historically they have practiced the same economic activity50. This population feature helped us to capture some inherited genetic differences that might be modifying the toxic effect of chronic Hg exposure. In this regard, in humans, it has been suggested that an increase in the frequencies of protective variants in detoxification genes might be due to a mechanism of adaptation to toxic environmental metal tolerance21.
A few remarks can be made regarding the limitations of this study. While the study included a small sample size, it represented almost the entire non-genetically structured population from the mining and non-mining communities. We selected SNPs in glutathione-related genes based on their reported involvement in Hg metabolism. However, other SNPs in linkage disequilibrium or otherwise might also be influencing the glutathione-Hg metabolism. In addition, since multiple factors underlie the complex pathogenesis of Hg toxicity including genetic and environmental factors, their interactions could also be contributing to the associations found in our study. Hopefully, similar studies in well-characterized cohorts might ultimately enlighten the role of glutathione-related genetic variants in Hg toxicity (e.g., via meta-analysis).
Our genetic epidemiological findings suggest that in the historically artisanal and small‐scale gold mining study population, the genetic variants analyzed in the glutathione-related genes could modulate the pathogenesis of Hg nephrotoxicity by controlling the glutathione concentrations. Replication studies for genetic variants in these and in other glutathione-related genes are necessary to clarify the role of glutathione in Hg toxicity.
Methods
Study design, population, and sample collection
A cross-sectional study was performed in mining and non-mining groups with similar socio-demographic characteristics from northeastern Colombia. In an unmatched population-based case–control approach, we estimated a sample size of 258 individuals with QUANTO software, version 1.2.4, assuming a log-additive inheritance model at 5% significance and 80% power and using the following parameters based on our previous study20: an environmental exposure to Hg of 2.5%, a population risk of reduced estimated glomerular filtration rate (eGFR; < 76.4 mL/min per 1.73 m2) of 25%, an environmental effect of reduced eGFR per tenfold increase in blood Hg level of 1.3, an estimated genetic effect of 1.8, an estimated gene-environment interaction effect of 1.2, and a minor allele frequency of 0.2 using data of Colombian-ancestry individuals (CLM) from the 1000 Genomes Project39.
The study population was comprised of 281 participants: 160 in the exposed and 121 in the non-exposed group. Similar socio-demographic characteristics were found in the two communities (Table 1). Both clinical and epidemiological information was collected for each participant through a detailed personal interview. An excess of heterozygosity was found (FIS = − 0.0530, P = 0.96), indicating an absence of endogamy in this population. All participants were 18–62 years old and provided informed consent to participate in the study. Individuals in the exposed group have been residents in the gold mining districts for at least the last 5 years prior to the study and had direct contact with Hg vapors in the last year. Individuals in the non-exposed group were permanent residents of non-mining towns and had no life history of direct contact with Hg vapors. All relevant information about study design, population, and sample collection characteristics was previously described in detail51.
Quantification of mercury and kidney function biomarkers
We utilized data from our previously published studies20,51, which detail methods and analyses. Briefly, total blood-Hg and urine-Hg were measured using a S4 atomic absorption spectrometer equipped with a VP100 hydride generation system (Thermo Electron Co., Cambridge, UK), whereas total hair-Hg concentration was quantified using an RA-915+ atomic absorption spectrometer mercury analyzer with Zeeman background correction and coupled to a RP-91C pyrolysis chamber (Lumex, St. Petersburg, Russia)51. Total Hg was used as biomonitoring data since it can give precise information on the total internal exposure of an individual at a given point in time, whereas total hair-mercury was used as a confounder for the effects of methyl-Hg due to fish/seafood consumption52,53. We reported medians and interquartile ranges for concentrations of total Hg in the exposed group and the non-exposed group (Table 1)51. Testing was carried out at the Industrial Consultation Laboratory of the Universidad Industrial de Santander that has been accredited according to ISO/IEC 17025:2005, performing well in international quality control programs.
The glomerular function was evaluated by determining serum and 24-h urine creatinine, urinary albumin in the first-morning sample, and by estimating glomerular filtration rate (eGFR) with the CKD-EPI formula54. Creatinine was measured by spectrophotometry using the Selectra JR Clinical Chemistry Analyser (Vital Scientific, France). Urinary albumin was measured by a competitive immunoassay using the Siemens Immulite One analyzer (Siemens Healthcare Diagnostics, Germany). The tubular function was evaluated by determining urinary excretion of the beta-2-microglobulin (β-2MCG) by an immunometric assay using the Siemens Immulite One analyzer (Siemens Healthcare Diagnostics, Germany)20.
DNA isolation and genotyping
Total genomic DNA was extracted from 5 ml of EDTA-treated peripheral whole blood using the standard salting-out method55. DNA concentration was determined with a Nanodrop One Spectrophotometer (Thermo Fisher Scientific, USA) and adjusted to 20 ng/μL with TE buffer. The SNPs glutamate-cysteine ligase catalytic subunit, GCLC, rs1555903; glutamate-cysteine ligase modifier subunit, GCLM, rs41303970; glutathione synthetase, GSS, rs3761144; glutathione S-transferase alpha 1, GSTA1, rs3957356; and glutathione S-transferase pi 1, GSTP1, rs4147581 were genotyped by TaqMan-based allelic discrimination assay (Applied Biosystems, USA) using a CFX96 Touch Real-Time PCR detection system (Bio-Rad, USA).
Statistical analysis
To determine the presence of population genetic structure, an analysis of molecular variance (AMOVA) was performed with Arlequin, version 3.556. The differences between the genotypic and allelic frequencies of the study groups were compared by the Pearson Chi-square test, and odds ratios (OR) were established with a 95% confidence interval (95% CI). The Fisher’s exact test was used when the genotypic and allelic frequencies were less than 5%. Multivariable generalized linear regression models were used to assess the effects of SNPs on Hg levels and on kidney function biomarkers: eGFR, urinary albumin, and β-2MCG while adjusting for possible confounders such as sex and age as well as hair-Hg levels and duration of exposure to minimize the overestimation of Hg0 exposure57,58. Each SNP was coded as 0, 1 or 2 according to the count of their minor allele. P values less than 0.05 (P < 0.05) were considered statistically significant. The nominal significance level was retained when significant empirical P values were obtained through 5000 replicate permutations59,60. Interactions between SNPs were examined by logistic regression to analyze their combined effects on Hg levels and kidney function biomarkers. The significant interaction terms were decomposed by a simple slope analysis. Statistical analyses were performed with R programming language, version 3.5.1.
Ethics approval and consent to participate
The study was conducted under protocols approved by the Scientific Research Committee of the Universidad Industrial de Santander and complied with the Colombian Medical Code of Ethics, which is in accordance with the ethical standards laid down in the 1964 Declaration of Helsinki.
Acknowledgements
The authors want to thank all people who participated in the study by allowing access to the samples. Dr. Ludmila Prokunina-Olsson, Laboratory of Translational Genomics, DCEG, NCI is acknowledged for critical comments. Thanks also go to Tim Ring for his profreading of the manuscript and suggestions.
Author contributions
O.M.P., investigation, data curation, formal analysis, writing—original draft. O.FV., conceptualization, software, formal analysis, visualization, writing—original draft. G.R.C., investigation, methodology, writing—review and editing. F.R.G., methodology, formal analysis, writing—review and editing. L.R.M., investigation, methodology, writing—review and editing. L.S.R., conceptualization, methodology, supervision, project administration, funding acquisition, writing—review and editing.
Funding
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: this work was funded by the Ministry of Science, Technology and Innovation (COLCIENCIAS), Grant no. 1102-744-55577-2016 and the Universidad Industrial de Santander, Bucaramanga, Colombia. The funder of this study had no role in study design; the collection, analysis, and interpretation of data; the writing of the report or the decision to submit the report for publication.
Data availability
The data that support the findings of this study are available from the corresponding author upon reasonable request. The data are not publicly available due to the nature of the questions asked in this study, them containing information that could compromise research participant consent.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Olga Marcela Medina Pérez and Oscar Flórez-Vargas.
References
- 1.Esdaile LJ, Chalker JM. The mercury problem in artisanal and small-scale gold mining. Chemistry. 2018;24:6905–6916. doi: 10.1002/chem.201704840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Obrist D, et al. A review of global environmental mercury processes in response to human and natural perturbations: Changes of emissions, climate, and land use. Ambio. 2018;47:116–140. doi: 10.1007/s13280-017-1004-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.De Miguel E, Clavijo D, Ortega MF, Gomez A. Probabilistic meta-analysis of risk from the exposure to Hg in artisanal gold mining communities in Colombia. Chemosphere. 2014;108:183–189. doi: 10.1016/j.chemosphere.2014.01.035. [DOI] [PubMed] [Google Scholar]
- 4.Güiza L, Aristizábal JD. Mercury and gold mining in Colombia: A failed state. Univ. Sci. 2013;18:33–39. doi: 10.11144/Javeriana.SC18-2.bc. [DOI] [Google Scholar]
- 5.Berndt WO. The role of transport in chemical nephrotoxicity. Toxicol. Pathol. 1998;26:52–57. doi: 10.1177/019262339802600107. [DOI] [PubMed] [Google Scholar]
- 6.Custodio HM, Harari R, Gerhardsson L, Skerfving S, Broberg K. Genetic influences on the retention of inorganic mercury. Arch. Environ. Occup. Health. 2005;60:17–23. doi: 10.3200/AEOH.60.1.17-23. [DOI] [PubMed] [Google Scholar]
- 7.Afrifa J, Opoku YK, Gyamerah EO, Ashiagbor G, Sorkpor RD. The clinical importance of the mercury problem in artisanal small-scale gold mining. Front. Public Health. 2019;7:131. doi: 10.3389/fpubh.2019.00131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kobal AB, Flisar Z, Miklavcic V, Dizdarevic T, Sesek-Briski A. Renal function in miners intermittently exposed to elemental mercury vapour. Arh Hig Rada Toksikol. 2000;51:369–380. [PubMed] [Google Scholar]
- 9.Zalups RK. Molecular interactions with mercury in the kidney. Pharmacol. Rev. 2000;52:113–143. [PubMed] [Google Scholar]
- 10.Tchounwou PB, Ayensu WK, Ninashvili N, Sutton D. Environmental exposure to mercury and its toxicopathologic implications for public health. Environ. Toxicol. 2003;18:149–175. doi: 10.1002/tox.10116. [DOI] [PubMed] [Google Scholar]
- 11.Barnett LMA, Cummings BS. Nephrotoxicity and renal pathophysiology: A contemporary perspective. Toxicol. Sci. 2018;164:379–390. doi: 10.1093/toxsci/kfy159. [DOI] [PubMed] [Google Scholar]
- 12.Jan AT, Ali A, Haq Q. Glutathione as an antioxidant in inorganic mercury induced nephrotoxicity. J. Postgrad. Med. 2011;57:72–77. doi: 10.4103/0022-3859.74298. [DOI] [PubMed] [Google Scholar]
- 13.Rubino FM. Toxicity of glutathione-binding metals: A review of targets and mechanisms. Toxics. 2015;3:20–62. doi: 10.3390/toxics3010020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Forman HJ, Zhang H, Rinna A. Glutathione: Overview of its protective roles, measurement, and biosynthesis. Mol. Aspects Med. 2009;30:1–12. doi: 10.1016/j.mam.2008.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lushchak VI. Glutathione homeostasis and functions: Potential targets for medical interventions. J. Amino Acids. 2012;2012:736837. doi: 10.1155/2012/736837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hayes JD, Strange RC. Glutathione S-transferase polymorphisms and their biological consequences. Pharmacology. 2000;61:154–166. doi: 10.1159/000028396. [DOI] [PubMed] [Google Scholar]
- 17.Joneidi Z, et al. The impact of genetic variation on metabolism of heavy metals: Genetic predisposition? Biomed. Pharmacother. 2019;113:108642. doi: 10.1016/j.biopha.2019.108642. [DOI] [PubMed] [Google Scholar]
- 18.Custodio HM, et al. Polymorphisms in glutathione-related genes affect methylmercury retention. Arch. Environ. Health. 2004;59:588–595. doi: 10.1080/00039890409603438. [DOI] [PubMed] [Google Scholar]
- 19.Schlawicke Engstrom K, et al. Genetic variation in glutathione-related genes and body burden of methylmercury. Environ. Health Perspect. 2008;116:734–739. doi: 10.1289/ehp.10804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sanchez Rodriguez LH, et al. No effect of mercury exposure on kidney function during ongoing artisanal gold mining activities in Colombia. Toxicol. Ind. Health. 2017;33:67–78. doi: 10.1177/0748233716659031. [DOI] [PubMed] [Google Scholar]
- 21.Schlebusch CM, et al. Human adaptation to arsenic-rich environments. Mol. Biol. Evol. 2015;32:1544–1555. doi: 10.1093/molbev/msv046. [DOI] [PubMed] [Google Scholar]
- 22.Vahter M, Akesson A, Liden C, Ceccatelli S, Berglund M. Gender differences in the disposition and toxicity of metals. Environ. Res. 2007;104:85–95. doi: 10.1016/j.envres.2006.08.003. [DOI] [PubMed] [Google Scholar]
- 23.Bridges CC, Zalups RK. The aging kidney and the nephrotoxic effects of mercury. J. Toxicol. Environ. Health B Crit. Rev. 2017;20:55–80. doi: 10.1080/10937404.2016.1243501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bridges CC, Zalups RK. Mechanisms involved in the transport of mercuric ions in target tissues. Arch. Toxicol. 2017;91:63–81. doi: 10.1007/s00204-016-1803-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Franklin CC, et al. Structure, function, and post-translational regulation of the catalytic and modifier subunits of glutamate cysteine ligase. Mol. Aspects Med. 2009;30:86–98. doi: 10.1016/j.mam.2008.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dickinson DA, et al. Human glutamate cysteine ligase gene regulation through the electrophile response element. Free Radic. Biol. Med. 2004;37:1152–1159. doi: 10.1016/j.freeradbiomed.2004.06.011. [DOI] [PubMed] [Google Scholar]
- 27.Krzywanski DM, et al. Variable regulation of glutamate cysteine ligase subunit proteins affects glutathione biosynthesis in response to oxidative stress. Arch. Biochem. Biophys. 2004;423:116–125. doi: 10.1016/j.abb.2003.11.004. [DOI] [PubMed] [Google Scholar]
- 28.Nakamura S, et al. Polymorphism in the 5'-flanking region of human glutamate-cysteine ligase modifier subunit gene is associated with myocardial infarction. Circulation. 2002;105:2968–2973. doi: 10.1161/01.cir.0000019739.66514.1e. [DOI] [PubMed] [Google Scholar]
- 29.Harari R, et al. Exposure and toxic effects of elemental mercury in gold-mining activities in Ecuador. Toxicol. Lett. 2012;213:75–82. doi: 10.1016/j.toxlet.2011.09.006. [DOI] [PubMed] [Google Scholar]
- 30.Consortium, G. T The genotype-tissue expression (GTEx) project. Nat. Genet. 2013;45:580–585. doi: 10.1038/ng.2653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Julvez J, et al. Prenatal methylmercury exposure and genetic predisposition to cognitive deficit at age 8 years. Epidemiology. 2013;24:643–650. doi: 10.1097/EDE.0b013e31829d5c93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chen Y, Shertzer HG, Schneider SN, Nebert DW, Dalton TP. Glutamate cysteine ligase catalysis: Dependence on ATP and modifier subunit for regulation of tissue glutathione levels. J. Biol. Chem. 2005;280:33766–33774. doi: 10.1074/jbc.M504604200. [DOI] [PubMed] [Google Scholar]
- 33.Yang Y, et al. Initial characterization of the glutamate-cysteine ligase modifier subunit Gclm(−/−) knockout mouse. Novel model system for a severely compromised oxidative stress response. J. Biol. Chem. 2002;277:49446–49452. doi: 10.1074/jbc.M209372200. [DOI] [PubMed] [Google Scholar]
- 34.Cannon VT, Barfuss DW, Zalups RK. Molecular homology and the luminal transport of Hg2+ in the renal proximal tubule. J. Am. Soc. Nephrol. 2000;11:394–402. doi: 10.1681/ASN.V113394. [DOI] [PubMed] [Google Scholar]
- 35.Breton CV, et al. Prenatal tobacco smoke exposure affects global and gene-specific DNA methylation. Am. J. Respir. Crit. Care Med. 2009;180:462–467. doi: 10.1164/rccm.200901-0135OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Qu K, et al. Polymorphisms of glutathione S-transferase genes and survival of resected hepatocellular carcinoma patients. World J. Gastroenterol. 2015;21:4310–4322. doi: 10.3748/wjg.v21.i14.4310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Uhlen M, et al. Proteomics. Tissue-based map of the human proteome. Science. 2015;347:1260419. doi: 10.1126/science.1260419. [DOI] [PubMed] [Google Scholar]
- 38.Goodrich JM, Basu N. Variants of glutathione s-transferase pi 1 exhibit differential enzymatic activity and inhibition by heavy metals. Toxicol. In Vitro. 2012;26:630–635. doi: 10.1016/j.tiv.2012.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Siva N. 1000 Genomes project. Nat. Biotechnol. 2008;26:256. doi: 10.1038/nbt0308-256b. [DOI] [PubMed] [Google Scholar]
- 40.Machiela MJ, Chanock SJ. LDlink: A web-based application for exploring population-specific haplotype structure and linking correlated alleles of possible functional variants. Bioinformatics. 2015;31:3555–3557. doi: 10.1093/bioinformatics/btv402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cordell HJ. Detecting gene–gene interactions that underlie human diseases. Nat. Rev. Genet. 2009;10:392–404. doi: 10.1038/nrg2579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Moore JH. The ubiquitous nature of epistasis in determining susceptibility to common human diseases. Hum. Hered. 2003;56:73–82. doi: 10.1159/000073735. [DOI] [PubMed] [Google Scholar]
- 43.Coles BF, et al. Effect of polymorphism in the human glutathione S-transferase A1 promoter on hepatic GSTA1 and GSTA2 expression. Pharmacogenetics. 2001;11:663–669. doi: 10.1097/00008571-200111000-00004. [DOI] [PubMed] [Google Scholar]
- 44.Goodrich JM, et al. Glutathione enzyme and selenoprotein polymorphisms associate with mercury biomarker levels in Michigan dental professionals. Toxicol. Appl. Pharmacol. 2011;257:301–308. doi: 10.1016/j.taap.2011.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hayes JD, Flanagan JU, Jowsey IR. Glutathione transferases. Annu. Rev. Pharmacol. Toxicol. 2005;45:51–88. doi: 10.1146/annurev.pharmtox.45.120403.095857. [DOI] [PubMed] [Google Scholar]
- 46.Zhang H, et al. Glutathione-dependent reductive stress triggers mitochondrial oxidation and cytotoxicity. FASEB J. 2012;26:1442–1451. doi: 10.1096/fj.11-199869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Li M, Zhang Z, Yuan J, Zhang Y, Jin X. Altered glutamate cysteine ligase expression and activity in renal cell carcinoma. Biomed. Rep. 2014;2:831–834. doi: 10.3892/br.2014.359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Korge P, Calmettes G, Weiss JN. Increased reactive oxygen species production during reductive stress: The roles of mitochondrial glutathione and thioredoxin reductases. Biochim. Biophys. Acta. 2015;1847:514–525. doi: 10.1016/j.bbabio.2015.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hansen JM, Zhang H, Jones DP. Differential oxidation of thioredoxin-1, thioredoxin-2, and glutathione by metal ions. Free Radic. Biol. Med. 2006;40:138–145. doi: 10.1016/j.freeradbiomed.2005.09.023. [DOI] [PubMed] [Google Scholar]
- 50.Pérez Riaño P. La minería colonial en el páramo Santurbán, el caso de las Montuosas, Vetas y Páramo Rico. Bol. Hist. Antigüedades. 2014;101:517–573. [Google Scholar]
- 51.Sanchez Rodriguez LH, et al. Lack of autoantibody induction by mercury exposure in artisanal gold mining settings in Colombia: Findings and a review of the epidemiology literature. J. Immunotoxicol. 2015;12:368–375. doi: 10.3109/1547691X.2014.986591. [DOI] [PubMed] [Google Scholar]
- 52.Angerer J, Ewers U, Wilhelm M. Human biomonitoring: State of the art. Int. J. Hyg. Environ. Health. 2007;210:201–228. doi: 10.1016/j.ijheh.2007.01.024. [DOI] [PubMed] [Google Scholar]
- 53.Boerleider RZ, Roeleveld N, Scheepers PTJ. Human biological monitoring of mercury for exposure assessment. Aims Environ. Sci. 2017;4:251–276. doi: 10.3934/environsci.2017.2.251. [DOI] [Google Scholar]
- 54.Levey AS, et al. A new equation to estimate glomerular filtration rate. Ann. Intern. Med. 2009;150:604–612. doi: 10.7326/0003-4819-150-9-200905050-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Miller SA, Dykes DD, Polesky HF. A simple salting out procedure for extracting DNA from human nucleated cells. Nucleic Acids Res. 1988;16:1215. doi: 10.1093/nar/16.3.1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Excoffier L, Lischer HE. Arlequin suite ver 3.5: A new series of programs to perform population genetics analyses under Linux and Windows. Mol. Ecol. Resour. 2010;10:564–567. doi: 10.1111/j.1755-0998.2010.02847.x. [DOI] [PubMed] [Google Scholar]
- 57.Yard EE, et al. Mercury exposure among artisanal gold miners in Madre de Dios, Peru: A cross-sectional study. J. Med. Toxicol. 2012;8:441–448. doi: 10.1007/s13181-012-0252-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sherman LS, Blum JD, Franzblau A, Basu N. New insight into biomarkers of human mercury exposure using naturally occurring mercury stable isotopes. Environ. Sci. Technol. 2013;47:3403–3409. doi: 10.1021/es305250z. [DOI] [PubMed] [Google Scholar]
- 59.Knijnenburg TA, Wessels LF, Reinders MJ, Shmulevich I. Fewer permutations, more accurate P-values. Bioinformatics. 2009;25:i161–168. doi: 10.1093/bioinformatics/btp211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Clarke GM, et al. Basic statistical analysis in genetic case-control studies. Nat. Protoc. 2011;6:121–133. doi: 10.1038/nprot.2010.182. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request. The data are not publicly available due to the nature of the questions asked in this study, them containing information that could compromise research participant consent.