Abstract
Background
There have been several reports of secondary anemia associated with Graves’ disease. There are no reports of secondary anemia resulting from thyrotoxicosis due to painless thyroiditis (silent thyroiditis). We report the case of a patient with pancreatic diabetes who developed anemia caused by thyrotoxicosis due to painless thyroiditis.
Case presentation
The patient was a 37-year-old man who visited the hospital complaining of fatigue, palpitations, and dyspnea. His hemoglobin was 110 g/l (reference range, 135–176), and mean corpuscular volume was 81.5 fl (81.7–101.6). His free thyroxine (FT4) was high, at 100.4 pmol/l (11.6–21.9); the free triiodothyronine (FT3) was high, at 27.49 pmol/l (3.53–6.14); TSH was low, at < 0.01 mIU/l (0.50–5.00); and TSH receptor antibody was negative. Soluble IL-2 receptor (sIL-2R) was high, at 1340 U/ml (122–496); C-reactive protein (CRP) was high, at 6900 μg/l (< 3000); and reticulocytes was high, at 108 109 /l (30–100). Serum iron (Fe) was 9.5 (9.1–35.5), ferritin was 389 μg/l (13–401), haptoglobin was 0.66 g/l (0.19–1.70. Propranolol was prescribed and followed up. Anemia completely disappeared by 12 weeks after disease onset. Thyroid hormones and sIL-2R had normalized by 16 weeks after onset. He developed mild hypothyroidism and was treated with L-thyroxine at 24 weeks.
Conclusions
This is the first case report of transient secondary anemia associated with thyrotoxicosis due to painless thyroiditis. The change in sIL-2R was also observed during the clinical course of thyrotoxicosis and anemia, suggesting the immune processes in thyroid gland and bone marrow.
Keywords: Painless thyroiditis, Anemia, siIL-2R, And thyrotoxicosis
Background
Secondary anemia associated with hyperthyroidism is a relatively rare complication [1]. Many reports have shown that although anemia progresses with hyperthyroidism in Graves’ disease, it is often transient, improving with treatment of Graves’ disease [2–4]. There are no reports of secondary anemia resulting from thyrotoxicosis due to subacute thyroiditis, painless thyroiditis (silent thyroiditis) [5], or other causes. Here, we report a case of transient anemia that developed after the onset of painless thyroiditis in a patient with pancreatic diabetes treated with insulin.
Case presentation
A 37-year-old man visited our hospital due to gradually progressive fatigue, dyspnea, and palpitations of approximately 14 days duration. His pulse was 104 beats/min, and his body temperature was 36.8 °C. No increase in sweating was observed, goiter was not palpable, and no exophthalmos or ocular movement dysfunction was observed. Laboratory examination showed anemia (Hgb 110 g/l [reference rage, 135–176], mean corpuscular volume [MCV] 81.5 fl [81.7–101.6]), and serum iron (9.5 μmol/l) and ferritin (389 μg/l) were within the normal ranges. Reticulocytes were increased at the time of onset (108 109 /l; reference rage, 30–100), C-reactive protein (CRP) was 6900 μg/l (< 3000), and his haptoglobin was within normal range (0.66 g/l; reference range, 0.19–1.70). The FT4 (free thyroxine) level was high, at 100.4 pmol/l (11.6–21.9), the FT3 (free triiodothyronine) level was high, at 27.49 pmol/l (3.53–6.14), and his TSH was < 0.01 mIU/l (0.50–5.00). TSH receptor antibody, anti-TPO antibody or anti-thyroglobulin antibody were negative. In other clinical tests, soluble interleukin-2 receptor (sIL-2R), measured for differentiation of hematologic malignancies, was high, at 1340 U/ml (122–496); his low-density lipoprotein cholesterol (LDL-C) was low, at 0.78 mmol/l (1.68–3.59); and his high-density lipoprotein cholesterol (HDL-C) was low, at 0.75 mmol/l (1.03–2.48) (Table 1).
Table 1.
Profile of laboratory data for a diabetes patient with thyrotoxicosis due to painless thyroiditis
| Variables | Reference range | Day − 56 | Day −28 | Day 0 | Day 14 | Day 28 | Day 56 | Day 84 | Day 112 | Day 140 | Day 168 |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Red blood cells, 1012/l | 4.27–5.70 | 4.80 | 4.96 | 3.78 | 3.77 | 3.84 | 4.50 | 4.77 | 4.93 | 4.88 | 4.66 |
| Hemoglobin (Hgb), g/l | 135–176 | 144 | 146 | 110 | 107 | 109 | 126 | 136 | 142 | 146 | 140 |
| Hematocrit (Hct), /l | 0.398–0.518 | 0.399 | 0.408 | 0.308 | 0.305 | 0.306 | 0.352 | 0.377 | 0.392 | 0.386 | 0.408 |
| Mean corpuscular volume (MCV), fl | 81.7–101.6 | 83.1 | 82.3 | 81.5 | 80.9 | 79.7 | 78.2 | 79.0 | 79.5 | 82.8 | 83.4 |
| White blood cells (WBC), 109 /l | 3.5–9.8 | 6.4 | 7.4 | 5.3 | 7.2 | 6.5 | 8.8 | 8.6 | 8.8 | 5.8 | 7.4 |
| Platelets, 109 /l | 130–369 | 272 | 315 | 254 | 198 | 228 | 289 | 266 | 283 | 322 | 333 |
| Reticulocytes, 109 /l | 30–100 | 108 | 68 | ||||||||
| Thyroid stimulating hormone (TSH), mIU/l | 0.50–5.00 | < 0.01 | < 0.01 | < 0.01 | < 0.01 | 10.1 | 15.2 | 18.5 | |||
| Free triiodothyronine (FT3), pmol/l | 3.53–6.14 | 27.49 | 12.61 | 9.37 | 3.93 | 4.35 | 4.22 | ||||
| Free thyroxine (FT4), pmol/l | 11.6–21.9 | 100.4 | 91.4 | 51.5 | 33.1 | 14.0 | 13.1 | 14.0 | |||
| TSH receptor antibody (TRAb), mIU/l | < 2.0 | 0.4 | |||||||||
| Anti-TPO antibody, IU/ml | < 16 | 9 | |||||||||
| Ant-thyroglobulin antibody, IU/ml | < 28 | 11 | |||||||||
| C-reactive protein (CRP), μg/l | < 3000 | 6900 | 12,100 | 5700 | 3700 | 1200 | |||||
| Iron (Fe), μmol/l | 9.1–35.5 | 9.5 | 9.5 | ||||||||
| TIBC, μmol/l | 43.2–69.0 | 34.2 | 40.8 | ||||||||
| Ferritin, μg/l | 13–401 | 389 | 376 | 396 | |||||||
| Haptoglobin, g/dl | 0.19–1.70 | 0.66 | |||||||||
| Soluble IL-2 receptor (sIL-2R), U/ml | 122–496 | 1340 | 1010 | 808 | 472 | 365 | |||||
| HDL-C, mmol/l | 1.03–2.48 | 1.27 | 1.09 | 0.75 | 0.98 | 1.27 | 1.42 | 1.14 | 1.16 | 1.14 | |
| LDL-C, mmol/l | 1.68–3.59 | 1.99 | 1.63 | 0.78 | 1.45 | 2.12 | 2.82 | 2.87 | 2.90 | 2.82 | |
| Triglycerides (TG), mmol/l | 0.34–1.68 | 0.46 | 0.59 | 0.54 | 0.84 | 0.68 | 0.75 | 0.84 | 0.59 | 0.60 | |
| HbA1c (NGSP), % | 4.6–6.2 | 6.9 | 7.1 | 7.6 | 6.5 | 6.6 | 6.9 | 7.7 | 7.6 | 7.5 | |
| AST, IU/l | 13–33 | 19 | 18 | 21 | 25 | 30 | 25 | 22 | 27 | 23 | |
| ALT, IU/l | 6–30 | 13 | 14 | 19 | 28 | 29 | 21 | 15 | 22 | 21 | |
| γGTP, IU/l | 10–47 | 13 | 14 | 21 | 42 | 57 | 37 | 26 | 20 | 19 | |
| Albumin, g/l | 40–50 | 41 | 42 | 31 | 32 | 36 | 36 | 35 | 41 | 41 | |
| eGFR, ml/min/1.73 m2 | > 60 | 103 | 97 | 111 | 115 | 104 | 90 | 88 | 79.0 | 80.0 | |
| Drug treatment |
Propranolol 30 mg |
Propranolol 30 mg |
LT4 12.5 μg |
Bold data is abnormal value. eGFR estimated glomerular filtration rate
The patient was previously diagnosed with pancreatic diabetes due to alcoholic pancreatitis at age 25 years, and thus, he was treated with a combination of insulin glargine and insulin aspart. Regular clinical examinations were performed every 28 days, and no abnormalities were found in his biochemical data or complete blood count, other than his plasma glucose and HbA1c. Propranolol (30 mg/day) was prescribed at day 14 and stopped on day 56. Thyroid ultrasonography was performed on day 28, and hypoechoic regions were observed throughout the thyroid gland (Fig. 1).
Fig. 1.
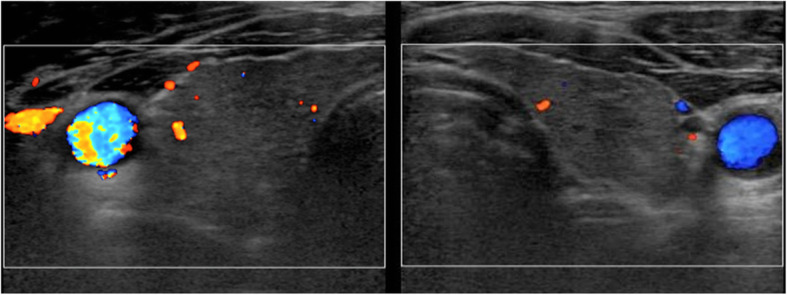
Thyroid ultrasonography findings. Hypoechoic regions are scattered throughout the thyroid gland. No increase in blood flow in the thyroid gland was observed
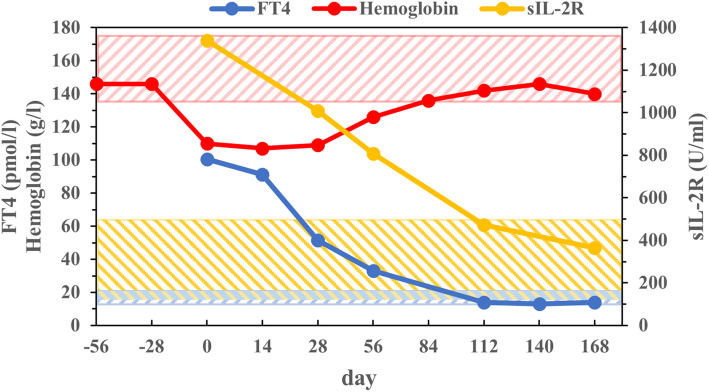
The anemia disappeared by day 84. The patient’s thyroid hormones and sIL-2R normalized by day 112, and CRP normalized by day 140. The clinical courses of Hgb, FT4, and sIL-2R were shown in Fig. 2. There was slight decrease in LDL-C and HDL-C, and increases in liver enzymes at disease onset, but these changes disappeared by day 140 (Table 1). His serum albumin was low at onset but normalized by day 140. The patient’s insulin regimen was not changed over the entire clinical course. He developed mild hypothyroidism on day 112 and was started on 12.5 μg of L-thyroxine replacement therapy on day 168 (Table 1).
Fig. 2.
Profile of FT4, hemoglobin, and sIL-2R. The patient’s hemoglobin had normalized by day 84. FT4 and sIL-2R returned to be normal by day 112. The shaded area shows the reference range for each variable
Discussion and conclusions
We reported a case of painless thyroiditis-induced thyrotoxicosis that suddenly led to anemia within 4 weeks after the patient’s last visit to our hospital. Although it was not possible to prove reduced iodine uptake using thyroid scintigram, negative TSH receptor antibody, the mild increase of CRP, lack of tenderness in thyroid gland, and the diffuse destructive findings on thyroid ultrasonography indicated painless thyroiditis [5]. In addition, FT3/FT4 ratio (0.301) was low, suggesting painless thyroiditis, because the median (IQR) of FT3/FT4 ratio in painless thyroiditis was reported as 0.310 (0.203–0.608) [6]. This is the first report of secondary anemia associated with painless thyroiditis. Although the levels remained within the normal range, slight decrease in WBC and platelets presented at disease onset [1]. Anemia, and the decrease in WBC and platelets completely disappeared by 12 weeks after onset, with spontaneous remission of thyrotoxicosis.
In addition to the clinical course of thyrotoxicosis and associated anemia, the present case provided interesting laboratory data. First, sIL-2R was high at onset but normalized by 16 weeks after onset of anemia. Elevated levels of sIL-2R have been reported in hyperthyroidism of Graves’ disease and it was suggested that thyroid hormones directly enhance sIL-2R production in lymphocytes [7]. The levels in sIL-2R also increased in patients with the thyrotoxicosis due to painless thyroiditis [8]. Second, LDL-C and HDL-C levels decreased due to thyrotoxicosis. Excessive thyroid hormone levels lower serum LDL-C and HDL-C levels via several mechanisms [9–11]. We previously reported that increased sIL-2R cause significant decreases levels in HDL-C and LDL-C in patients with hematologic malignancies [12]. Increased cytokines were recently reported to be associated with hypolipidemia in COVID-19 patients [13]. Presumably, increases in levels of both thyroid hormones and sIL-2R are associated with the decreases in HDL-C and LDL-C.
Painless thyroiditis (silent thyroiditis) is a self-limiting inflammatory disorder of the thyroid gland characterized by an early thyrotoxicosis phase caused by the release of thyroid hormones and a late hypothyroidism phase, with complete resolution in most cases [5, 14]. The pathophysiologic mechanism of painless thyroiditis is unknown, but the possibility of immune disorder involvement has been suggested [5, 7, 8, 15]. Painless thyroiditis generally manifests as a lymphocyte infiltration of the thyroid follicles, causing thyroid follicular cell damage [5]. In our case, we observed a typical course of painless thyroiditis. The secondary anemia caused by thyrotoxicosis has improved, but we would like to carefully follow up on the continuation of thyroid hormone replacement therapy.
The mechanism by which anemia develops in thyrotoxicosis is not clear. Shortened erythrocyte survival or ineffective erythropoiesis have been suggested as potential causes of anemia in thyrotoxicosis [2–4]. Moreover, slight decrease in WBC and platelets was observed in present case, which could have been due to a variety of mechanisms. The involvement of autoantibodies in leukocytes and platelets resulting in increased destruction of hematopoietic cells by immunological mechanisms has been reported [16]. The increase in sIL-2R, which had been increasing at the onset of painless thyroiditis, may suggest the result of immune process abnormality rather than the increase in thyroid hormones [8, 17]. The involvement of immune processes in the onset of painless thyroiditis could help explain the pathophysiology of anemia [18]. In present case, however, both anti-TG and anti-TPO antibodies were negative. Anti-thyroglobulin and anti-TPO antibodies prevalence in painless thyroiditis have been reported to be 70 and 30%, respectively [19]. The patient had an history of acute pancreatitis. It is interesting to assume the immune mechanisms as the pathogenesis of acute and chronic pancreatitis [20], and it is natural to assume that the immune mechanism was involved in the development of silent thyroiditis and anemia in present case.
In conclusion, we reported the case of a diabetes patient with secondary anemia resulting from thyrotoxicosis. Thyrotoxicosis was caused by painless thyroiditis, but there have been no reports of secondary anemia induced by painless thyroiditis. The change in sIL-2R was also observed during the clinical course of thyrotoxicosis and anemia.
Acknowledgements
We would like to thank FORTE (www.forte-science.co.jp) for English language editing.
Abbreviations
- TSH
Thyroid stimulating hormone
- FT4
Free thyroxine
- FT3
Free triiodothyronine
- sIL-2R
Soluble interleukin-2 receptor
- HDL-C
High-density lipoprotein cholesterol
- LDL-C
Low-density lipoprotein cholesterol
Authors’ contributions
TW has full access to all of the data from the study and takes responsibility for the integrity of the data. IK and TT were involved in study design, interpreting data, statistical analysis, creating tables and figures, and drafting the manuscript. NY and GO were involved in interpreting data and supervised the work. All authors have contributed significantly to this work. All the authors have read the manuscript and have approved this submission.
Funding
The authors received no specific funding for this work.
Availability of data and materials
The collection of data that supports the findings in this study is available from Okinawa Medical Hospital. Data are available from the authors upon reasonable request and with permission of Okinawa Medical Hospital.
Declarations
Ethics approval and consent to participate
No applicable.
Consent for publication
Regarding publication of the case report, we explained that we could not reveal personal information and obtained verbal consent from the patient.
Competing interests
The authors declare no conflict of interest.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Ford HC, Carter JM. The haematology of hyperthyroidism: abnormalities of erythrocytes, leucocytes, thrombocytes and haemostasis. Postgrad Med J. 1998;64:735–742. doi: 10.1136/pgmj.64.756.735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Szczepanek-Parulska E, Hernik A, Ruchała M. Anemia in thyroid diseases. Pol Arch Intern Med. 2017;127(5):352–360. doi: 10.20452/pamw.3985. [DOI] [PubMed] [Google Scholar]
- 3.Gianoukakis AG, Leigh MJ, Richards P, Christenson PD, Hakimian A, Fu P, et al. Characterization of the anaemia associated with Graves’ disease. Clin Endocrinol (Oxf) 2009;70:781–787. doi: 10.1111/j.1365-2265.2008.03382.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Das KC, Mukherjee M, Sarkar TK, Dash RJ, Rastogi GK. Erythropoiesis and erythropoietin in hypo- and hyperthyroidism. J Clin Endocrinol Metab. 1975;40(2):211–220. doi: 10.1210/jcem-40-2-211. [DOI] [PubMed] [Google Scholar]
- 5.Mizukami Y, Michigishi T, Hashimoto T, Tonami N, Hisada K, Matsubara F, Takazakura E. Silent thyroiditis: a histologic and immunohistochemical study. Hum Pathol. 1988;19(4):423–431. doi: 10.1016/S0046-8177(88)80492-1. [DOI] [PubMed] [Google Scholar]
- 6.Yoshimura JN, Momotani N, Fukada S, Ito K, Miyauchi A, Amino N. Ratio of serum free triiodothyronine to free thyroxine in Graves’ hyperthyroidism and thyrotoxicosis caused by painless thyroiditis. Endocr J. 2005;52(5):537–542. doi: 10.1507/endocrj.52.537. [DOI] [PubMed] [Google Scholar]
- 7.Koukkou E, Panayiotidis P, Alevizou-Terzaki V, Thalassinos N. High levels of serum soluble interleukin-2 receptors in hyperthyroid patients: correlation with serum thyroid hormones and independence from the etiology of the hyperthyroidism. J Clin Endocrinol Metab. 1991;73(4):771–776. doi: 10.1210/jcem-73-4-771. [DOI] [PubMed] [Google Scholar]
- 8.Hamamoto K, Inaba M, Yamada S, Yoda M, Yoda K, Goto H, Kurajoh M, Koyama H. Increased soluble IL-2 receptor levels in serum from a patient with painless thyroiditis. Thyroid Res. 2013;6(1):12. doi: 10.1186/1756-6614-6-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lopez D, Socarrás JFA, Bedi M, Ness GC. Activation of the hepatic LDL receptor promoter by thyroid hormone. Biochim Biophys Acta. 1771;2007:1216–1225. doi: 10.1016/j.bbalip.2007.05.001. [DOI] [PubMed] [Google Scholar]
- 10.Bonde Y, Breuer O, Lütjohann D, Sjöberg S, Angelin B, Rudling M. Thyroid hormone reduces PCSK9 and stimulates bile acid synthesis in humans. J Lipid Res. 2014;55(11):2408–2415. doi: 10.1194/jlr.M051664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tan KC, Shiu SW, Kung AW. Plasma cholesteryl ester transfer protein activity in hyper- and hypothyroidism. J Clin Endocrinol Metab. 1998;83(1):140–143. doi: 10.1210/jcem.83.1.4491. [DOI] [PubMed] [Google Scholar]
- 12.Komiya I, Tomoyose T, Ouchi G, Yara T, Higa S. Low level of serum HDL-cholesterol with increased sIL-2R predicts a poor clinical outcome for patients with malignant lymphoma and adult T-cell leukemia-lymphoma. Cytokine. 2018;105:57–62. doi: 10.1016/j.cyto.2018.02.005. [DOI] [PubMed] [Google Scholar]
- 13.Wei X, Zeng W, Su J, Wan H, Yu X, Cao X, Tan W, Wang H. Hypolipidemia is associated with the severity of COVID-19. J Clin Lipidol. 2020;14(3):297–304. doi: 10.1016/j.jacl.2020.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Strakosch CR. Thyroiditis. Aust NZ J Med. 1986;16(1):91–100. doi: 10.1111/j.1445-5994.1986.tb01138.x. [DOI] [PubMed] [Google Scholar]
- 15.Takasu N, Komiya I, Nagasawa Y, Asawa T, Yamada T. Exacerbation of autoimmune thyroid dysfunction after unilateral adrenalectomy in patients with Cushing's syndrome due to an adrenocortical adenoma. N Engl J Med. 1990;322(24):1708–1712. doi: 10.1056/NEJM199006143222404. [DOI] [PubMed] [Google Scholar]
- 16.Garla VV, Salim SA, Yanes-Cardozo LL. Pancytopenia: a rare complication of Graves’ disease. BMJ Case Rep. 2018;2018:bcr2017223887. doi: 10.1136/bcr-2017-223887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dik WA, Heron M. Clinical significance of soluble interleukin-2 receptor measurement in immune-mediated diseases. Neth J Med. 2020;78(5):220–231. [PubMed] [Google Scholar]
- 18.Nobili V, Liaskos C, Luigi G, Guidi R, Francalanci P, Marcellini M. Autoimmune thyroiditis associated with autoimmune hepatitis. Thyroid. 2005;15(10):1193–1195. doi: 10.1089/thy.2005.15.1193. [DOI] [PubMed] [Google Scholar]
- 19.Nishihara E, Amino N, Kudo T, Ito M, Fukata S, Nishikawa M, Nakamura H, Miyauchi A. Comparison of thyroglobulin and thyroid peroxidase antibodies measured by five different kits in autoimmune thyroid diseases. Endocr J. 2017;64(10):955–961. doi: 10.1507/endocrj.EJ17-0164. [DOI] [PubMed] [Google Scholar]
- 20.Lee B, Zhao Q, Habtezion A. Immunology of pancreatitis and environmental factors. Curr Opin Gastroenterol. 2017;33(5):383–389. doi: 10.1097/MOG.0000000000000387. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The collection of data that supports the findings in this study is available from Okinawa Medical Hospital. Data are available from the authors upon reasonable request and with permission of Okinawa Medical Hospital.