Abstract
Paramyxoviruses, including the mumps virus, measles virus, Nipah virus and Sendai virus (SeV), have non-segmented single-stranded negative-sense RNA genomes which are encapsidated by nucleoproteins into helical nucleocapsids. Here, we reported a double-headed SeV nucleocapsid assembled in a tail-to-tail manner, and resolved its helical stems and clam-shaped joint at the respective resolutions of 2.9 and 3.9 Å, via cryo-electron microscopy. Our structures offer important insights into the mechanism of the helical polymerization, in particular via an unnoticed exchange of a N-terminal hole formed by three loops of nucleoproteins, and unveil the clam-shaped joint in a hyper-closed state for nucleocapsid dimerization. Direct visualization of the loop from the disordered C-terminal tail provides structural evidence that C-terminal tail is correlated to the curvature of nucleocapsid and links nucleocapsid condensation and genome replication and transcription with different assembly forms.
Subject terms: Cryoelectron microscopy, Virus structures
The authors present the reconstruction of the Sendai Virus N-RNA complex at atomic resolution, including the helical part and a clam-shaped joint, resolved to 2.9 and 3.9 Å respectively, via cryo-EM. They also demonstrate the presence of an additional, ring like structural motif stabilising Nprotein interactions.
Introduction
The family of Paramyxoviridae consists of many human viruses such as measles, parainfluenza, and mumps viruses, and animal-derived pathogens including Newcastle disease and Sendai viruses. Paramyxoviruses have single-stranded negative-sense RNA genomes, and their non-segmented viral genomes are encapsulated by many copies of a nucleoprotein (N), as well as other viral proteins, forming long and helical nucleocapsids (NC) that act as scaffolds for virus assembly and as templates for genome transcription and replication1,2. During viral RNA synthesis, the viral phosphoprotein recognizes the C-terminal tail (N-tail) of a nucleoprotein to guide RNA polymerase on the nucleocapsid to synthesize daughter RNA3,4. The nascent RNA strand is immediately enwrapped by nucleoproteins for protection against possible digestions by nucleases in the host cell, before being packed into virions; this packing is also known to be mediated by nucleoproteins5.
Despite of poor sequence conservation, paramyxovirus nucleoproteins exhibit well-conserved architectures2. Specifically, paramyxovirus nucleoproteins typically consist of two lobes—an N-terminal domain (NTD) and a C-terminal domain (CTD)—with a cleft between the lobes. The interdomain cleft comprises conserved positively charged residues which function to promote clamping to nucleotides of the RNA genome, with no apparent specificity5. Both the NTD and CTD have subdomains known as arms (N-arm and C-arm, respectively), which enable paramyxovirus nucleoproteins to undertake a “domain swapping” process that enables their assembly into either ring-like structures in parainfluenza virus 5 (PIV5) or helical filaments in measles virus (MeV)6–8.
Expanding beyond the known helical nucleocapsids, we recently described a clam-shaped assembly of the nucleoprotein from Newcastle disease virus (NDV), wherein two single-turn spirals are packed in a tail-to-tail way9. Each single-turn spiral in NDV clam-shaped assembly is similar to MeV helical nucleocapsid and enwraps one RNA molecule between NTD and CTD in a “3-bases-in, 3-bases-out” conformation. Surprisingly, there is an obvious seam between two single-turn spirals, which disconnects two RNA molecules. The clam-shaped assemblies of NDV nucleoproteins are mediated by loops (residues 114–120) of vertically adjacent nucleoproteins in the clam-shaped core. Intriguingly, this clam-shaped architecture suggested the possibility of acting as a seed to nucleate the further formation of a double-headed type of nucleocapsid9.
SeV is responsible for a respiratory tract infection among murine rodents via both airborne and direct contact routes, potentially transmissible to humans as many animal-derived pathogens such as SARS, MERS, and COVID-1910–12. Similar to PIV513, SeV virion is highly pleomorphic and has been shown to range in diameter from ~110 to 540 nm, indicating that some virions may contain multiple copies of their genomes14. Furthermore, SeV has been proven infectious to many human cancer cell lines and has been shown to have oncolytic properties in animal models15,16. Given the understanding that biomedical technologies based on SeV have great potential for treating various cancers, obtaining detailed information for the SeV nucleoprotein structure and assembly process will both deepen a basic understanding of the underlying mechanism of SeV pathogenesis and guide efforts to develop novel anti-cancer therapies.
Results
Structure of double-headed SeV nucleocapsid
Following previous reports, SeV nucleoproteins were purified from Escherichia coli after tandem affinity and gel-filtration chromatography. Similar to NDV and MeV9,17, SeV nucleoprotein exhibits structural variability from filaments to ring-like structures. The early-eluting fraction at 8.7 mL mainly consists of filaments; all the filaments have a diameter at ~19 nm, and 60% of the nucleocapsids observed are of the double-headed type, wherein two herringbone-like filaments are joined as a clam-shaped structure in a tail-to-tail manner (Supplementary Fig. 1a–d). The reconstruction strategy is to divide double-headed SeV nucleocapsids into helical stems and clam-shaped joints based on the symmetries, reconstruct their respective high-resolution structures, and then splice them together.
The relatively straight helical stems of the double-headed SeV nucleocapsids were selected for our helical reconstruction. After three-dimensional (3D) classification, two conformers were resolved, exhibiting slight differences in the extent of their helical twist and rise, with the respective resolutions of 4.1 and 4.6 Å (Supplementary Figs. 2,3a and Table 1). Following the previous treatments on mumps virus (MuV) phosphoproteins and nucleoproteins4,18, the purified SeV nucleoproteins were placed at 4 °C for 5 weeks. This approach limited the cleavage of residual impurities only onto the N-tail (404-524) of nucleoproteins and yielded much straighter filaments (hereafter denoted as Ncleaved, with the respective nucleocapsid denoted as NCcleaved and the uncleaved nucleoprotein and nucleocapsid denoted as NWT and NCWT) (Supplementary Fig. 1e). These NCcleaved samples yielded a much higher resolution nucleocapsid at 2.9 Å (Supplementary Figs. 3b, 4a–c and Supplementary Movie 1).
Table 1.
Cryo-EM data collection and data processing statistics.
| NCWT helix-1 (EMDB-30066) (PDB-6M7D) | NCWT helix-2 (EMDB-30065) (PDB-6M7D) | NCcleaved helix (EMDB-30129) (PDB-6M7D) | NCWT Clam-shaped (EMDB-30064) (PDB-6M7D) | |
|---|---|---|---|---|
| Data collection and processing | ||||
| Microscope | Titan Krios G2 | Titan Krios G2 | Titan Krios G3i | Titan Krios G2 |
| Voltage (kV) | 300 | 300 | 300 | 300 |
| Camera |
Gatan K2 summit |
Gatan K2 summit |
Gatan K3 BioQuantum |
Gatan K2 summit |
| Magnification | 18,000 | 18,000 | 81,000 | 18,000 |
| Electron exposure (e−/Å2) | 40 | 40 | 40 | 40 |
| Defocus range (μm) | 1.5–3 | 1.5–3 | 1.5–3 | 1.5–3 |
| Pixel size (Å) | 0.65 | 0.65 | 0.53 | 0.65 |
| Symmetry imposed | Helical | Helical | Helical | C2 |
| Initial particle images (no.) | 94,916 | 94,916 | 1,686,748 | 436,797 |
| Final particle images (no.) | 4285 | 14,598 | 134,042 | 104,212 |
| Map resolution (Å) | 4.1 | 4.6 | 2.9 | 3.9 |
| FSC threshold | 0.143 | 0.143 | 0.143 | 0.143 |
| Map resolution range (Å) | 4–5.5 | 4–6.5 | 2.5–3 | 3.5–6 |
| Refinement | ||||
| Initial model used (PDB code) | 4UFT | 4UFT | 4UFT | 4UFT |
| Model resolution (Å) | 4.3 | 4.3 | 4.3 | 4.3 |
| FSC threshold | 0.143 | 0.143 | 0.143 | 0.143 |
| Map sharpening B factor (Å2) | −135.99 | −226.73 | −104.14 | −178.62 |
| Model composition | ||||
| Non-hydrogen atoms | 3280 | 3280 | 3280 | 3280 |
| Protein residues | 418 | 418 | 418 | 418 |
| Ligands | 0 | 0 | 0 | 0 |
| B factors (Å2) | ||||
| Protein | 8.5 | 8.5 | 8.5 | 8.5 |
| Ligand | / | / | / | / |
| R.m.s. deviations | ||||
| Bond lengths (Å) | 0.01 | 0.01 | 0.01 | 0.01 |
| Bond angles (°) | 1.025 | 1.025 | 1.025 | 1.025 |
| Validation | ||||
| MolProbity score | 1.78 | 1.78 | 1.78 | 1.78 |
| Clashscore | 15.48 | 15.48 | 15.48 | 15.48 |
| Poor rotamers (%) | 2 | 2 | 2 | 2 |
| Ramachandran plot | ||||
| Favored (%) | 98 | 98 | 98 | 98 |
| Allowed (%) | 2 | 2 | 2 | 2 |
| Disallowed (%) | 0 | 0 | 0 | 0 |
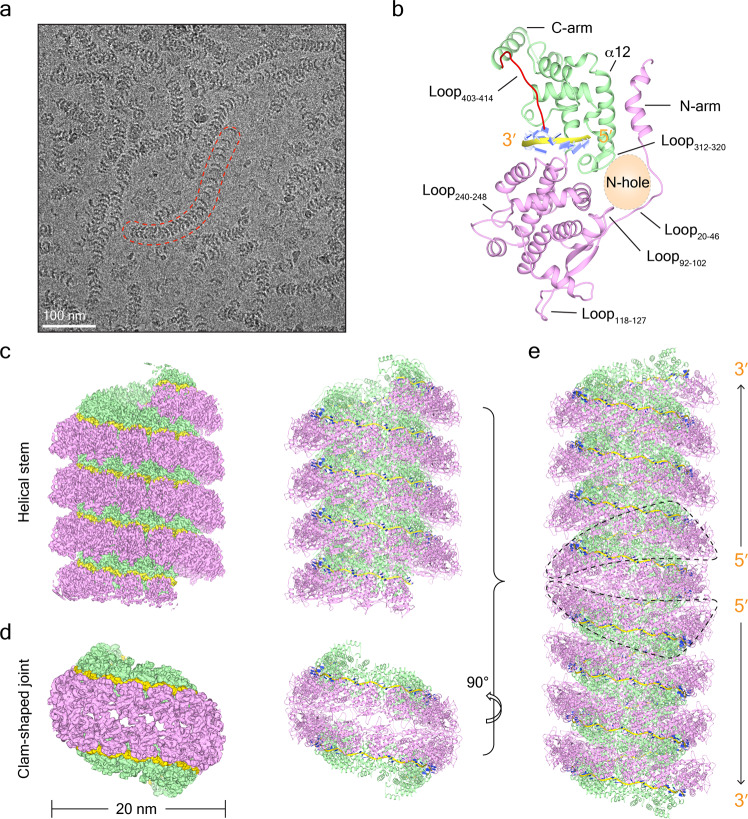
An atomic model of the SeV nucleoprotein was successfully built covering residues 3 to 414 based on the model of MeV nucleoprotein with 27% sequence identity (Fig. 1b, c and Supplementary Fig. 4d), our SeV nucleoprotein structure was coupled with RNA, without apparent sequence specificity. Poly-Uracil was docked into the NCcleaved EM map to mimic cellular RNA, with both the sugar-phosphate backbones and nitrogenous bases were clearly resolved. (Fig. 1b, c and Supplementary Fig. 4e). Structural comparison between SeV nucleoprotein and other paramyxovirus nucleoproteins such as NDV, MuV, PIV5, MeV, and Nipah virus (NiV)6–9,19, indicates high structural conservation with the RMSD values <1.3 Å (Supplementary Fig. 5).
Fig. 1. Structure of double-headed SeV nucleocapsids.
a A typical cryo-EM micrograph of double-headed SeV nucleocapsids. One curved SeV nucleocapsid with different assembly forms is highlighted in red. b The atomic model of one SeV nucleoprotein with the accompanied RNA. NTD and CTD are colored in pink and green, respectively. Loop403–414 in N-tail is colored in red. N-hole formed by Loop20–46, Loop92–102, and Loop312–320 is depicted in light orange. RNA is depicted with the backbones in gold and the bases in blue. The same color code is used for the rest of the figures. c 3D reconstruction of the helical stem of double-headed SeV nucleocapsids and the respective atomic model. d 3D reconstruction of the clam-shaped joint of double-headed SeV nucleocapsids and the respective atomic model. e Pseudo-atomic model of the double-headed SeV nucleocapsid. The clam-shaped structure is depicted in a dashed line and the RNA direction of two embedded RNA strands is labeled.
The two helical stems were joined as a clam-shaped structure. Similar to NDV9, there are many dispersed ring-like particles in the late-eluting fraction during the purification of SeV nucleoprotein (Supplementary Fig. 1a–c). Direct 2D classification indicates a strong preferred orientation of dispersed ring-like particles. To increase side views of clam-shaped structures, joints between two helical stems in each filament were manually picked up and combined with dispersed ring-like particles. 3D reconstruction on the merged particle sets yields a clam-shaped structure at 5.6 Å resolution, no matter whether two-fold symmetry is enforced or not (Supplementary Figs. 6 and 7a–c). The top-on view of the clam-shaped structure highlights its crescent shape, in which the protomers further away from the gap are resolved much better than those closer to the gap due to the structural wobbling (Supplementary Fig. 7d). Based on the local symmetry, we employed a strategy to improve resolution by masking and averaging the well-resolved 5 pairs of consecutive protomers; the final cryo-EM map reaches 3.9 Å of the resolution, into which the atomic model of the SeV nucleoprotein got from the above helical stem was fitted well via rigid-body docking (Fig. 1d and Supplementary Figs. 7e, f, 8a, b, Supplementary Movie 2). Clear RNA densities are also evident in the clam-shaped EM map, and poly-Uracil is readily docked into the density.
Interestingly, we noted that the helical parameters of the single-turn helices in the clam-shaped structure fall between those for the helical stem structures, indicating the compatibility of the helical stems and the clam-shaped structure (Supplementary Fig. 8c, d). Thus, we combined both the helical stem and clam-shaped structures together and built an atomic model of double-headed SeV nucleocapsid in which two helices are packed in a tail-to-tail manner (Fig. 1e).
Assembly mechanism of SeV nucleocapsid
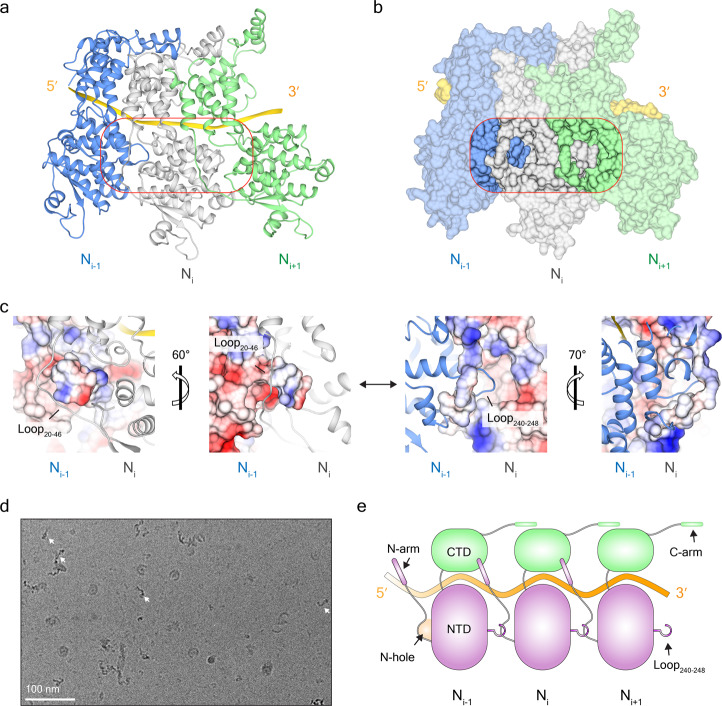
In double-headed SeV nucleocapsids, the helical stems are stranded by successive protomers together with RNA (Fig. 1c and Supplementary Figs. 2a–c, 4a–c). Similar to other nucleoproteins6,20–25, SeV nucleocapsids also employ a domain swapping process in which the N-arm and the C-arm interact with neighboring protomers (Fig. 2a, b and Supplementary Fig. 9a, b). The first interface comes from the N-arm from Ni, which lies between two α12 helices from Ni and Ni–1 to assemble into a bundle with three anti-parallel helices. The other side of the α12 helix from Ni is unoccupied and can interact with the N-arm and α12 helix from Ni+1 (Supplementary Fig. 9a, b). The second interface comprises the C-arm from Ni interacts with α16 helix of Ni+1 to capture the subsequent nucleoprotein (Supplementary Fig. 9a, c).
Fig. 2. The assembly mechanism of the helical stem of double-headed SeV nucleocapsids.
a The atomic model of three neighboring protomers in the helical stem. Protomers are colored in blue, gray, and green, respectively, and RNA is colored in gold. An unnoticed swapped interface between neighboring protomers is boxed in red. b Molecular surfaces of three neighboring protomers with the same view as in a. The same box is applied for the new interface. The transparency values of the boxed area and the other part are set to 0% and 40%, respectively. c Electrostatic interaction between N-hole from Ni–1 and Loop240–248 from Ni. On the left two images, Ni–1 is represented in the contour surfaces of electrostatic potential, and Ni is displayed in the ribbon. On the right two images, Ni–1 is displayed in the ribbon, and Ni is represented in the contour surfaces of electrostatic potential. d Threading thin filaments formed by Loop240–248 replacement mutant. Typical threading filaments were marked with arrows. e A model illustrates domain swapping mechanism in neighboring protomers.
Beyond the N-arm/C-arm domain swapping interface, our structural data support the occurrence of a previously unnoticed additional interface between neighboring protomers. Specifically, we observed that the SeV nucleoprotein has an extended loop (Loop20–46) connecting the N-arm and the core of NTD (Fig. 1b). Loop20–46, along with a loop from CTD (Loop312–320) and a loop from the NTD (Loop92–102) assembles into a closed-hole adjacent to N-arm (denoted as N-hole) (Fig. 1b and Supplementary Fig. 4d); Loop240–248 popping out from NTD of Ni–1 can become inserted into the N-hole from Ni (Fig. 2a, b). Detailed structural analysis shows that Loop240–248 is about 8 Å in diameter, and fits well with the N-hole in size. The surface of the N-hole is overall positively charged, due to the existence of several cationic residues (K21, R32, K100, and K317); Loop240–248 and its adjacent area have several negatively charged residues including E235 and E251, which keeps the affinity between them via electrostatic interaction (Fig. 2c). Loop240–248 replacement of all negatively charged residues to Alanine abolishes the electrostatic interaction with N-hole, and yields some threading thin filaments (Fig. 2d), hinting at a specific role in the assembly of helical nucleocapsids. Very interestingly, such N-hole-like structures also exist in NDV, PIV5, and MeV6–9. Detailed structural analyses indicate the occurrence of electrostatic interaction between N-holes and the extended loops in these paramyxovirus nucleoproteins (Supplementary Fig. 10). Thus, these conserved interfaces between N-holes and the extended loops resemble a gate latch and bolt, and apparently function to tightly anchor the positions of neighboring nucleoprotein protomers. Therefore, N-hole adopts the same domain swapping process as N-arm and C-arm, and contributes to the assembly of helical nucleocapsids in the family of Paramyxoviridae (Fig. 2e).
It is notable that all of these interfaces involved by N-arm, C-arm, and N-hole occur in the interior of SeV nucleocapsids. Similar to most nucleocapsids6,7,20–26, RNA cleft between NTD and CTD is facing the outsides of SeV nucleocapsids (Supplementary Fig. 9b, d). Recombinant SeV nucleoproteins can enwrap RNA from host cells, which is supported by our data of the absorbance of OD260/OD280 at ~1.2 during nucleoprotein purification (Supplementary Fig. 1a) and clear EM densities of RNA in both high-resolution structures of NCWT and NCcleaved (Fig. 1c and Supplementary Fig. 4c). In SeV nucleocapsids, negatively charged RNA interacts with nucleoprotein residues K180, R195, and R354, and six nucleotides are precisely associated with each protomer, with a typical “3-base-in” and “3-base-out” conformation (Supplementary Fig. 9d and Supplementary Movie 3).
N-tail correlates with SeV nucleocapsid curvature
Positioned immediately following the C-arm, the N-tail of Hendra virus nucleoprotein is known to function in the regulation of gene replication and transcription via binding to the C-terminal X domain of phosphoprotein27. To date, no structural information is available about the N-tails of the paramyxovirus nucleoproteins, likely owing to the intrinsic flexibility of these tails28. In our high-resolution structures of SeV NCcleaved, the first 12 residues (403–414) of the nucleoprotein N-tail are resolved (Figs. 1b and 3a). These 12 residues assemble into a loop (Loop403–414) that is orientated in the direction of the C-arm, extends about 36 Å, and points toward the outsides of nucleocapsids (Fig. 3b). Considering that the inner contact sites between neighboring rungs in helical stems of SeV nucleocapsids are filled with C-arm and Loop403–414, there is virtually no free space available to accommodate the residual N-tail (415–524) in a way that would allow it to turn back and penetrate the contact regions to the inside of nucleocapsids (Fig. 3a, b).
Fig. 3. N-tail correlates with nucleocapsid curvature.
a The first 12 residues (403–414) of N-tail are resolved in SeV NCcleaved. Loop403–414 is colored in red and the residue names are labeled along the loop. b Different views of 6 protomers from two neighboring rungs are shown. The inner contact site between neighboring rungs is marked in a dashed blue box. The unidentified residues (415–525) are denoted as dotted curved lines. c Curved nucleocapsid formed by NCWT. Two typical curved filaments are cut and zoomed in. d Straight nucleocapsid assembled by NCcleaved. Two typical straight filaments are cut and zoomed in. e The straightening of nucleocapsid is tightly correlated to the removal of N-tail from SeV nucleocapsid.
The cavity between two neighboring rungs of SeV nucleocapsids has a volume of ~11,000 Å3, which is too small to accommodate the residual N-tail of ~18,000 Å3. Some regions of the N-tail such as the MoRE motif, are expected to exist at the outsides of nucleocapsids and bind phosphoprotein to regulate gene replication and transcription3,29. Purified SeV NCWT are frequently curved (Fig. 3c), no matter long or short, just as reported in MeV nucleocapsid30. The persistence length for SeV NCWT has a value of ~288 nm. Interestingly, the removal of most N-tail from SeV NCWT either after 5 weeks storage at 4 °C or via trypsin digestion yields straighter filaments as revealed by cryo-EM (Fig. 3d and Supplementary Fig. 11a, b), with a bigger persistence length value at ~877 nm. Semi-quantification analysis on trypsin digested SeV nucleocapsids at different time points further shows that the straightening of nucleocapsid is tightly correlated with the removal of N-tail (Fig. 3e and Supplementary Fig. 11c).
Hyper-closed SeV clam-shaped structure
Double-headed SeV nucleocapsid assembles in a tail-to-tail manner, similar to our previous observation for the clam-shaped NDV nucleocapsid structure9. However, compared to the relatively loose interface in NDV nucleocapsids, the clam-shaped joint in SeV nucleocapsids adopts a tightly crisscrossed pattern; this tighter and engaged pattern clearly impacts the capacity for lateral sliding between two opposite single-turn spirals (Fig. 4a). The distance between two opposite protomers in the SeV clam-shaped structure is about 30 Å, only half of the distance compared to the NDV clam-shaped structure9. Accordingly, the empty space between two opposite protomers is reduced from ~1045 Å2 in NDV to only ~330 Å2 in SeV (Fig. 4a and Supplementary Movie 4). Given the much smaller space between two single-turn spirals, SeV clam-shaped structure nucleocapsid is depicted as a “hyper-closed” form.
Fig. 4. Hyper-closed clam-shaped joint in double-headed SeV nucleocapsid.
a Hyper-closed SeV clam-shaped assembly compared with NDV clam-shaped structure. The interface between opposite protomers in SeV clam-shaped structure is depicted in the dashed curve line. b Interface analysis of SeV clam-shaped structure. Two contact sites are marked in the solid box (left) and dotted box (right), respectively. Positively charged residues in the upper helix are labeled and the contour surface of electrostatic potential is shown in the opposite rung. c Loop118–127 is not involved in the assembly of the helical stem. d Sequence alignment of Loop118–127 of SeV nucleoprotein in members of paramyxoviruses. The critical residues from 118 to 121 are shaded in green.
The contact site between two opposite protomers of SeV clam-shaped structure comprises residues from 118 to 127 (Loop118–127) (Fig. 4b). The top of Loop118–124 from Ni′ is negatively charged, which binds to positively charged areas composed of R32, K100, and R129 from the opposite protomer Ni via electrostatic interaction. There is another interface involved by several residues including positively charged K108, K111, and K121 from Ni′, and negatively charged Loop118–127 from the opposite protomer Ni–1 (Fig. 4b and Supplementary Movie 5). Due to the two-fold symmetry owned by the clam-shaped structure, such interface and the interaction force will be doubled to enhance the clam assembly.
Loop118–127 seems only involved in the maintenance of clam-shaped joints between two helical stems. In the helical stems, Loop118–127 does not have significant interaction with neighboring protomers from upper/lower rungs (Fig. 4c). Residues between 118 and 127 are replaced with all Alanine and the loop mutant was purified as usual. Loop mutation abolishes the formation of double-headed filaments but keeps single-headed filaments (Supplementary Fig. 12). Mutation mapping on individual residue between 118 and 127 shows that F118 has a marked influence on clam-shaped structure assembly, which might be caused by a conformational change of Loop118–127 and the derived charge distribution change (Supplementary Fig. 12). Detailed sequence alignment shows a hydrophobic residue “F/P/M” followed by a positive-charged residue “R/K” in Loop118–127, highly conserved in several viruses including SeV, NDV, PIV5, and NiV, which indicates that clam-shaped structures might be popular in members of paramyxoviruses (Fig. 4d and Supplementary Fig. 13).
Discussion
In the present study, we detected a double-headed SeV nucleocapsid purified from E. coli, and resolved both its helical stems and its clam joint at near-atomic resolutions. Similar to the clam-shaped structure from NDV9, SeV utilizes its clam-shaped structure as the nucleator for the formation of double-headed nucleocapsids, wherein paramyxovirus nucleoproteins enwrap viral RNA to form a highly stable assembly. Not limited to our discoveries from NDV and SeV, the formation of clam-shaped assemblies of nucleoproteins was recently reported in NiV after overexpression in bacteria, which is also mediated by inter-subunit interactions involving several nucleoprotein loop regions19. Considering that not all viruses in the family of Paramyxoviridae have been found to contain such structures, the formation of clam-shaped structures might be due to preparative techniques. To check this, SeV nucleoprotein was over-expressed in HEK293F cells. Following a two-step ultracentrifugation process of cell lysate, fractions containing nucleoproteins were directly subjected to cryo-EM analysis. Notably, double-headed nucleocapsids (with the exact same diameter at 19 nm) were also present among nucleoproteins isolated from HEK293F cells (Supplementary Fig. 14a).
Furthermore, we attempted to verify the occurrence of clam-shaped structures in SeV virion. After Triton lysis of SeV virions, SeV nucleocapsids spread out on cryo-EM grids and clam-shaped structures surrounded by two herringbone structures packing in a tail-to-tail mode can be captured (Supplementary Fig. 14b). Electron tomography on intact SeV virions and tomography averaging of nucleocapsids are supposed as a better way to verify the occurrence of clam-shaped structures in situ.
Although small quantities of double-headed nucleocapsid structures have been visualized in multiple paramyxoviruses, their biological relevance is unclear at this point. Such structures are speculated to confer benefits for genome stability, polyploid genome organization or genome condensation14,31–35. Thus, it will be interesting to explore both the occurrence of such double-headed structures throughout the family of Paramyxoviridae and to examine the specific cellular conditions in which this nucleocapsid type is preferentially formed.
There is extensive in vitro and in vivo evidence showing that curved nucleocapsids occur commonly in paramyxoviruses, with examples of curvature for both single-headed and double-headed nucleocapsids6,7,17,29,32,36,37. In one SeV nucleocapsid, clam-shaped joint and straight/condensed filaments coexist with loosed or even uncoiled filaments (Fig. 5a). Our results establish that partial removal of the N-tail yields much straighter filaments, and demonstrate that N-tail is involved in curvature regulation of double-headed nucleocapsids. Comparing to straight fragments, curved ones might have imbalanced states of N-tail between the inside side and the opposite outside side38. This difference might get the phosphoprotein binding site exposed, and thereafter regulate gene transcription and replication. Actually, highly curved or even bent nucleocapsids will be vital for genome packing into virions. All these results point to a bold hypothesis that different assembly forms in double-headed SeV nucleocapsid represent distinct functions, which might be applied to other paramyxoviruses (Fig. 5b).
Fig. 5. A model illustrating the hypothesized distinct functions of different assembly forms.
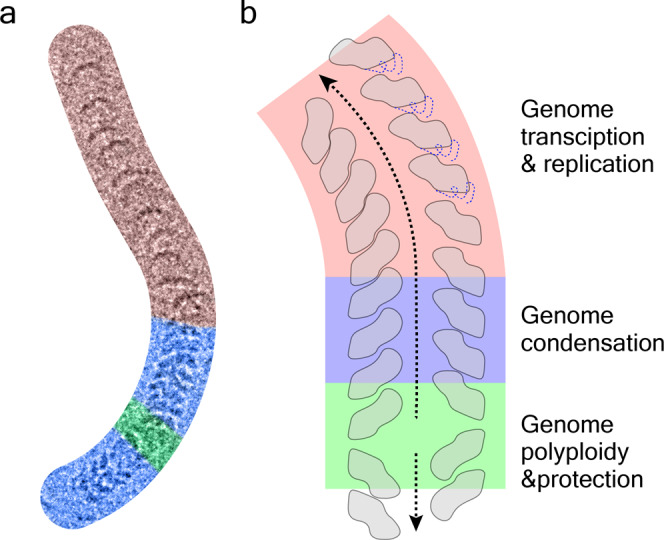
a Clam-shaped structure (light green), helical stems (light blue), and loosed coiled filament (light red) in one SeV nucleocapsid are highlighted. b A model illustrating the proposed distinct functions of different assembly forms. The same color strategy is used as in a.
Methods
Sequence alignment
Nucleoprotein sequences including Sendai virus (NP_056871.1; access numbers were obtained from the NCBI protein database), Newcastle disease virus (YP_009513194.1), mumps virus (NP_054707), Nipah virus (NP_112021.1), Parainfluenza virus 5 (YP_138511.1), and measles virus (NP_056918.1) were downloaded from PubMed in FASTA format. Geneious was used to align the sequences39, and the alignment was displayed via ESPript40. The secondary structure prediction on N-tail of SeV nucleoprotein was fulfilled in PSIPRED41.
Plasmids and gene expression
NWT was cloned into pCAGGS plasmid with 6× His-tag on C-termini for protein expression in mammalian cells. NWT and its derivatives with two 6× His-tags on both N- and C-termini were synthesized into pET28b plasmids for gene expression in Escherichia coli. All plasmids were verified via gene sequencing before gene expression.
NWT in pCAGGS plasmid were transfected into HEK293F cells with Lipofectamine 2000, when the cell density reached 4 × 106 cells/mL. The transfected cells were cultured at 37 °C, 6% CO2, 180 rpm for another 3 days and then harvested via centrifugation at 1000 × g for 15 min.
NWT and its derived mutants expressed in E. coli BL21 (DE3) cells were used for high-yield protein expression. In details, cells containing the respective plasmids were cultured in LB media at 37 °C until OD600 reached 0.8. Target proteins were induced with 1 mM IPTG (isopropyl-β-d-1-thiogalactopyranoside) at 16 °C, 220 rpm for 20 h. The cells were harvested by centrifugation at 4680 × g for 20 min to obtain the cell pellets.
Protein isolation and purification
NWT in HEK293F cells were lysed in lysis buffer (20 mM Tris-HCl (pH7.4), 150 mM NaCl, 1 mM CaCl2, 5 mM β-Mercaptoethanol and protease inhibitor cocktail (Roche, USA)). The lysate was clarified by centrifugation at 3200 × g for 20 min. The supernatant was loaded onto a double sucrose cushion (90% (w/v) sucrose is beneath 20% (w/v) sucrose) and ultracentrifuged in a Beckman SW32 Ti rotor at 130,000×g for 3 h. 1 mL fractions were manually collected via bottom puncture, 10 μL of which were subjected to western-blot analysis. Fractions containing nucleoproteins were collected into dialysis tubing with the molecular weight cutoff at 1 MDa, and dialyzed overnight in the sucrose-free lysis buffer to remove sucrose. The dialyzed sample was concentrated and subjected to another round of ultracentrifugation. Specifically, 1.5 mL of the condensed protein was loaded onto a 25–60% continuous sucrose gradient and was spun in a SW41 Ti rotor at 160,000 × g for 4 h. Samples were automatically fractionated by Fraction FC-203B collector (Gilson, USA), and the fractions containing nucleoproteins were collected based on western-blotting against 6×His-tag before overnight dialysis to remove sucrose. The dialyzed sample was concentrated and immediately applied to cryo-EM sample preparation.
NWT and its derived mutants expressed in E. coli were purified via tandem affinity and gel-filtration chromatography. Specifically, pelleted cells were resuspended in simplified lysis buffer (20 mM Tris-HCl (pH 7.4), 150 mM NaCl), and disrupted via ultrasonic homogenizers (JNBIO, China). After centrifugation at 47,850×g for 30 min, the supernatant was loaded onto a 5 mL HisTrapTM HP column (GE Healthcare LifeSciences, USA), preequilibrated with simplified lysis buffer. The column was washed with 50 mL simplified lysis buffer with a step gradient of imidazole at 20, 50, and 100 mM. Finally, proteins were eluted using a simplified lysis buffer containing 500 mM imidazole. Proteins were concentrated and loaded onto a 24 mL Superose 6 increase 10/300 GL chromatography column (GE Healthcare Lifesciences, USA) preequilibrated with simplified lysis buffer. 0.2 mL fractions were collected, 10 μL of which were subjected to SDS-PAGE analysis.
All protein samples were freshly made in the following assays except that purified NWT was stored at 4 °C for 5 weeks for the cleavage assay.
Negative stain EM
4 μL of protein samples (~0.1 mg/mL) were applied to glow-discharged EM grids covered with a thin layer of continuous carbon film and stained with 2% (w/v) uranyl acetate. Negatively stained grids were imaged on a Talos L120C TEM (Thermo Fisher Scientific, USA) operating at 120 kV. Images were recorded at a magnification of ×73,000 and a defocus set to about −2 μm, using a CetaTM 16M camera (Thermo Fisher Scientific, USA).
Cryo-EM data collection
3.5 μL of samples (~1 mg/mL) were applied to glow-discharged holey grids R2/1 (Quantifoil, Ted Pella) with a thin layer of the continuous carbon film. The grids were blotted using a Vitrobot Mark IV (ThermoFisher Scientific, USA) with a 1 s blotting time, force level of 2, and the humidity of 100% at 16 °C, immediately plunged into liquid ethane and stored under liquid nitrogen temperature for future cryo-EM imaging. Cryo-EM grids were examined in the low-dose mode on a Talos L120C TEM for screening or instant imaging. Snapshots were taken at a magnification of ×73,000 and a defocus set to about −2 μm, using a CetaTM 16 M camera.
Data collections on good grids were performed on two Titan Krios microscopes: Titan Krios G2 TEM (ThermoFisher Scientific, USA), equipped with a K2 Summit direct electron detector (Gatan, USA), which was used in the super-resolution mode with a pixel size of 0.65 Å; Titan Krios G3i TEM (ThermoFisher Scientific, USA), equipped with a K3 BioQuantum direct electron detector (Gatan, USA), which was used in the super-resolution mode with a pixel size of 0.53 Å. Special care was taken to perform a coma-free alignment on the microscopes and detailed data collection conditions were listed in Table 1. All the images were collected under the SerialEM automated data collection software package42, and data sets from two Titan Krios scopes were subjected to data analysis, separately.
Cryo-EM data processing and 3D reconstruction
Before image processing, raw frames were aligned and summed with dose weighting under MotionCor2.143 and the CTF parameters were determined by CTFFIND-444. Image processing was mainly performed in RELION 3.145, and different reconstruction strategies including helical reconstruction and single-particle analysis were applied to helical stems and clam-shaped assemblies of double-headed SeV nucleocapsid, respectively. The detailed workflows for helical reconstruction and single-particle analysis after two-dimensional classification are shown in Supplementary Fig. 3 and Supplementary Fig. 6, respectively.
-
Single-particle analysis
Ring-like particles were picked automatically in RELION and joints between two helices in each filament were manually picked in addition to increasing side views of clam-shaped structures. Obvious junks were excluded by reference-free 2D classification. NDV clam-shaped structure (EMDB, EMD-9793)9 and half of the clam (one single-turn spiral) were low-pass filtered to 60 Å and chosen as the references for 3D multiple reference alignments. Only classes with the whole clam-shaped structures were selected for further 3D auto-refinement. A 3D map was obtained after 3D refinement with the enforced two-fold symmetry, filtered, and sharpened with RELION post-processing session. An overall resolution was estimated at 5.6 Å based on gold standard Fourier Shell Correlation (FSC) 0.143 criteria.
Local resolution of the 3D structure was measured with RELION, and protomers further away from the gap of the clam-shaped structure are much better resolved than those closer to the gap due to the structural wobbling. To keep enough signal for accurate alignment, 5 pairs of consecutive protomers from clam-shaped structures, which are furthest from the gap, were chosen for local refinement. To make full use of the local circular symmetry, the particle center was switched to each pair of protomer via turning the center one protomer further using our own script. Thus, the total particle number was increased five times with the distributed Euler angles. The expanded particle set was subjected to 3D refinement with a local angular search for more accurate alignment and another round of 3D classification without alignment was applied to reduce the heterogeneity. Particles from classes with the best resolution were subjected to the 3D refinement focusing on the five pairs of protomers with a local angular search of ±6°. The final reconstruction was determined with the resolution of 3.9 Å by gold standard FSC 0.143 after polishing.
-
Helical reconstruction
Start and end points of helical stems of double-headed SeV nucleocapsids are manually specified and particles were extracted every 7 asymmetric units (about 90% overlap) along the helices. Junks and curved fragments were removed based on 2D classification. The initial model was synthesized from one single-turn spiral of SeV clam-shaped structure, which is low-pass filtered to 30 Å for 3D classification. Helical symmetry was applied during 3D classification and classes were merged into one or more groups, depending on their helical rise, twist and resolution. For NCWT, particles belonging to different classes were subjected to refinement respectively. For NCcleaved, since the variation of rise and twist was within a relatively narrow range and the particle distribution among classes was unstable through iterations, only the classes with more promising resolution were combined and subjected to refinement and Bayesian polishing. The final reconstructions were filtered and sharpened in RELION post-processing session. The resolutions were determined by gold standard FSC 0.143. Detailed helical twist and rise for each dataset are listed in Supplementary Fig. 3.
Model building and structural analysis
The homology model of SeV nucleoprotein and RNA was generated by Modeller46 using the crystal structure of measles virus nucleoprotein (RCSB, PDB-4UFT) as the template6. Pseudo-atomic model of SeV nucleoprotein was flexibly docked into the EM density of NCcleaved at the 2.9 Å resolution using Rosetta47 and Flex-EM software48. Residues (403–414) of N-tail were manually traced and placed against densities of NCcleaved and trypsin cleaved nucleocapsid with better resolved 403–408 with Coot49. The atomic model including both nucleoprotein and RNA was further optimized for better local density fitting using real-space refinement in Phenix50. The final SeV NCcleaved atomic model was duplicated to build the atomic model of SeV NCcleaved helical stem. This atomic model was also docked as a rigid body to helical stems and clam-shaped structures of double-headed SeV nucleocapsids using the University of California, San Francisco Chimera package51.
The extra EM densities enwrapped between NTD and CTD in all reconstructions were assigned as RNA and docked using poly-Uracil in Coot due to the unspecific binding of nucleoprotein to RNA49.
The structural analysis including surface electrostatic distribution and structural superimposition was fulfilled in UCSF Chimera51.
Trypsin enzymatic assay
Both NWT and NF118A were treated with trypsin to test the susceptibility. A 40 μL mixture of NWT or NF118A (1 mg/mL) with trypsin (0.003 mg/mL) was incubated at 4 °C and 5 μL fraction was taken out for SDS-PAGE analysis at 0, 3, 6, 10, 15, 120, and 240 min, respectively. Quantitation of bands at ~65, 50, and 35 kDa were analyzed densitometrically by CLIQS (TotalLab, UK).
For trypsin digestion assay on NWT under the same condition, another 3 μL fractions were taken out for cryo-EM analysis at 0, 6, 15, and 120 min, and 25 images were captured at the magnification of ×57,000 for each sample. Numbers of straight nucleocapsids were counted, and the percentage of straight nucleocapsids among all filaments was calculated at different digestion time points.
Nucleocapsids isolated from SeV virion
Wild-type SeV virion was propagated in embryonated 9-day-old chicken eggs, as described52. SeV infected allantoic fluid was harvested and stored under −80 °C until use.
The thawed allantoic fluid was centrifugated for 15 min at 4000×g under 4 °C to remove the crude debris. The supernatant was subjected to another round of centrifugation for 4 h at 48,000×g under 4 °C, and the resultant pellet was resuspended with PBS buffer (50 mM NaH2PO4, 50 mM NaCl, pH 7.2) and then loaded onto the 25–65% (w/v) continuous sucrose gradient column. After the 18 h ultracentrifugation at 150,000 g under 4 °C, the visible layer was collected and dialyzed overnight in PBS buffer to remove sucrose. The virion suspension was concentrated and lysed with 2% (v/v) Triton X-100 for 6 h under 4 °C, 3.5 μL of which was immediately applied to cryo-EM sample preparation. 500 good micrographs were collected on Titan Krios G3i TEM (ThermoFisher Scientific, USA), equipped with a K3 BioQuantum direct electron detector (Gatan, USA),
Persistence length analysis
Persistence length analysis on SeV NCWT and NCcleaved was performed in ImageJ as previously reported53. Briefly, 200 filaments per each were traced using an ImageJ plugin, JFilament54, and persistence length analysis on SeV NCWT and NCcleaved were calculated in ImageJ.
Reporting summary
Further information on research design is available in the Nature Research Reporting Summary linked to this article.
Supplementary information
Descriptions of Additional Supplementary Files
Acknowledgements
We thank Prof. Yifan Cheng from UCSF and Prof. Yun Bai from ShanghaiTech University for thorough discussions. We are grateful to Kang Li, Dianli Zhao, and Ceng Gao from the CryoEM facility for Marine Biology at QNLM for our cryo-EM data collection. We also thank the Electron Microscopy Facility of ShanghaiTech University for sample preparation and data collection. This work was supported by the National Key R&D Program of China, 2017YFA0504800 (Q.S.), National Key R&D Program of China, 2018YFC1406700 (Q.S.), National Natural Science Foundation of China, 31870743 (Q.S.) and Young Scientists Fund of the National Natural Science Foundation of China, 31800617 (R.W.).
Author contributions
N.Z. expressed and purified proteins, and prepared and screened EM grids. N.Z., H.S., and M.L. collected cryo-EM data sets. H.S. and N.Z. did data processing and carried out model building and refinement with the assistance of Y.L. T.L., L.Y., X.C, R.L., X.S., L.Q., R.W., and W.S. helped with input and discussion during the course of the work. N.Z., H.S., and Q.S. analyzed all the data. Q.S. supervised the work. Q.S. wrote the manuscript with input from N.Z., H.S., and T.L.
Data availability
The cryo-EM density maps of double-headed SeV nucleocapsids were deposited in Electron Microscopy Data Bank (EMDB) with the accession numbers 30064 (clam-shaped structure), 30065 (helical stem-1), 30066 (helical stem-2), and 30129 (NCcleaved), respectively. And the atom coordinates of a single N subunit were deposited in the Protein Data Bank (PDB) with the PDB ID code 6M7D. All other data are available in the main text or the supplementary materials or available from the corresponding author upon reasonable request.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Na Zhang, Hong Shan, Mingdong Liu, Tianhao Li.
Supplementary information
The online version contains supplementary material available at 10.1038/s42003-021-02027-y.
References
- 1.Ruigrok RW, Crepin T, Kolakofsky D. Nucleoproteins and nucleocapsids of negative-strand RNA viruses. Curr. Opin. Microbiol. 2011;14:504–510. doi: 10.1016/j.mib.2011.07.011. [DOI] [PubMed] [Google Scholar]
- 2.Jamin M, Yabukarski F. Nonsegmented negative-sense RNA viruses-structural data bring new insights into nucleocapsid assembly. Adv. Virus Res. 2017;97:143–185. doi: 10.1016/bs.aivir.2016.09.001. [DOI] [PubMed] [Google Scholar]
- 3.Guryanov SG, Liljeroos L, Kasaragod P, Kajander T, Butcher SJ. Crystal structure of the measles virus nucleoprotein core in complex with an N-terminal region of phosphoprotein. J. Virol. 2015;90:2849–2857. doi: 10.1128/JVI.02865-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Severin C, et al. Releasing the genomic RNA sequestered in the Mumps virus nucleocapsid. J. Virol. 2016;90:10113–10119. doi: 10.1128/JVI.01422-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Whelan SP, Barr JN, Wertz GW. Transcription and replication of nonsegmented negative-strand RNA viruses. Curr. Top. Microbiol. Immunol. 2004;283:61–119. doi: 10.1007/978-3-662-06099-5_3. [DOI] [PubMed] [Google Scholar]
- 6.Gutsche I, et al. Structural virology. Near-atomic cryo-EM structure of the helical measles virus nucleocapsid. Science. 2015;348:704–707. doi: 10.1126/science.aaa5137. [DOI] [PubMed] [Google Scholar]
- 7.Desfosses, A. et al. Assembly and cryo-EM structures of RNA-specific measles virus nucleocapsids provide mechanistic insight into paramyxoviral replication. Proc. Natl Acad. Sci. USA10.1073/pnas.1816417116 (2019). [DOI] [PMC free article] [PubMed]
- 8.Alayyoubi M, Leser GP, Kors CA, Lamb RA. Structure of the paramyxovirus parainfluenza virus 5 nucleoprotein-RNA complex. Proc. Natl Acad. Sci. USA. 2015;112:E1792–E1799. doi: 10.1073/pnas.1503941112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Song, X. et al. Self-capping of nucleoprotein filaments protects Newcastle disease virus genome. Elife8, 10.7554/eLife.45057 (2019). [DOI] [PMC free article] [PubMed]
- 10.Cui J, Li F, Shi ZL. Origin and evolution of pathogenic coronaviruses. Nat. Rev. Microbiol. 2019;17:181–192. doi: 10.1038/s41579-018-0118-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Khan G. A novel coronavirus capable of lethal human infections: an emerging picture. Virol. J. 2013;10:66. doi: 10.1186/1743-422X-10-66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang LF, Eaton BT. Bats, civets and the emergence of SARS. Curr. Top. Microbiol. Immunol. 2007;315:325–344. doi: 10.1007/978-3-540-70962-6_13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Terrier O, et al. Parainfluenza virus type 5 (PIV-5) morphology revealed by cryo-electron microscopy. Virus Res. 2009;142:200–203. doi: 10.1016/j.virusres.2008.12.017. [DOI] [PubMed] [Google Scholar]
- 14.Loney C, Mottet-Osman G, Roux L, Bhella D. Paramyxovirus ultrastructure and genome packaging: cryo-electron tomography of sendai virus. J. Virol. 2009;83:8191–8197. doi: 10.1128/JVI.00693-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Saga K, Kaneda Y. Oncolytic Sendai virus-based virotherapy for cancer: recent advances. Onco. Virother. 2015;4:141–147. doi: 10.2147/OV.S66419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Matveeva OV, Kochneva GV, Netesov SV, Onikienko SB, Chumakov PM. Mechanisms of oncolysis by Paramyxovirus sendai. Acta Nat. 2015;7:6–16. doi: 10.32607/20758251-2015-7-2-6-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Desfosses A, Goret G, Estrozi LF, Ruigrok RWH, Gutsche I. Nucleoprotein-RNA orientation in the measles virus nucleocapsid by three-dimensional electron microscopy. J. Virol. 2011;85:1391–1395. doi: 10.1128/JVI.01459-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cox R, et al. Structural and functional characterization of the mumps virus phosphoprotein. J. Virol. 2013;87:7558–7568. doi: 10.1128/JVI.00653-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ker, D.-S., Jenkins, H. T., Greive, S. J. & Antson, A. A. CryoEM structure of the Nipah virus nucleocapsid assembly. Preprint at bioRxiv10.1101/2020.01.20.912261 (2020). [DOI] [PMC free article] [PubMed]
- 20.Su Z, et al. Electron cryo-microscopy structure of Ebola virus nucleoprotein reveals a mechanism for nucleocapsid-like assembly. Cell. 2018;172:966–978 e912. doi: 10.1016/j.cell.2018.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sugita, Y., Matsunami, H., Kawaoka, Y., Noda, T. & Wolf, M. Cryo-EM structure of the Ebola virus nucleoprotein-RNA complex at 3.6 A resolution. Nature10.1038/s41586-018-0630-0 (2018). [DOI] [PubMed]
- 22.Wan W, et al. Structure and assembly of the Ebola virus nucleocapsid. Nature. 2017;551:394–397. doi: 10.1038/nature24490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Albertini AA, et al. Crystal structure of the rabies virus nucleoprotein-RNA complex. Science. 2006;313:360–363. doi: 10.1126/science.1125280. [DOI] [PubMed] [Google Scholar]
- 24.Ge P, et al. Cryo-EM model of the bullet-shaped vesicular stomatitis virus. Science. 2010;327:689–693. doi: 10.1126/science.1181766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Green TJ, Zhang X, Wertz GW, Luo M. Structure of the vesicular stomatitis virus nucleoprotein-RNA complex. Science. 2006;313:357–360. doi: 10.1126/science.1126953. [DOI] [PubMed] [Google Scholar]
- 26.Kirchdoerfer RN, Saphire EO, Ward AB. Cryo-EM structure of the Ebola virus nucleoprotein-RNA complex. Acta Crystallogr. F Struct. Biol. Commun. 2019;75:340–347. doi: 10.1107/S2053230X19004424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Communie G, et al. Atomic resolution description of the interaction between the nucleoprotein and phosphoprotein of Hendra virus. PLoS Pathog. 2013;9:e1003631. doi: 10.1371/journal.ppat.1003631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jensen MR, et al. Intrinsic disorder in measles virus nucleocapsids. Proc. Natl Acad. Sci. USA. 2011;108:9839–9844. doi: 10.1073/pnas.1103270108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cox R, et al. Structural studies on the authentic mumps virus nucleocapsid showing uncoiling by the phosphoprotein. Proc. Natl Acad. Sci. USA. 2014;111:15208–15213. doi: 10.1073/pnas.1413268111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bhella D, Ralph A, Yeo RP. Conformational flexibility in recombinant measles virus nucleocapsids visualised by cryo-negative stain electron microscopy and real-space helical reconstruction. J. Mol. Biol. 2004;340:319–331. doi: 10.1016/j.jmb.2004.05.015. [DOI] [PubMed] [Google Scholar]
- 31.Goff PH, Gao Q, Palese P. A majority of infectious Newcastle disease virus particles contain a single genome, while a minority contain multiple genomes. J. Virol. 2012;86:10852–10856. doi: 10.1128/JVI.01298-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kiss G, et al. Structural analysis of respiratory syncytial virus reveals the position of M2-1 between the matrix protein and the ribonucleoprotein complex. J. Virol. 2014;88:7602–7617. doi: 10.1128/JVI.00256-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Beniac DR, et al. The organisation of Ebola virus reveals a capacity for extensive, modular polyploidy. PLoS ONE. 2012;7:e29608. doi: 10.1371/journal.pone.0029608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Liljeroos L, Huiskonen JT, Ora A, Susi P, Butcher SJ. Electron cryotomography of measles virus reveals how matrix protein coats the ribonucleocapsid within intact virions. Proc. Natl Acad. Sci. USA. 2011;108:18085–18090. doi: 10.1073/pnas.1105770108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rager M, Vongpunsawad S, Duprex WP, Cattaneo R. Polyploid measles virus with hexameric genome length. EMBO J. 2002;21:2364–2372. doi: 10.1093/emboj/21.10.2364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ke Z, et al. Promotion of virus assembly and organization by the measles virus matrix protein. Nat. Commun. 2018;9:1736. doi: 10.1038/s41467-018-04058-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Battisti AJ, et al. Structure and assembly of a paramyxovirus matrix protein. Proc. Natl Acad. Sci. USA. 2012;109:13996–14000. doi: 10.1073/pnas.1210275109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Esneau C, et al. Biochemical characterization of the respiratory syncytial virus N(0)-P complex in solution. J. Biol. Chem. 2019;294:3647–3660. doi: 10.1074/jbc.RA118.006453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kearse M, et al. Geneious basic: an integrated and extendable desktop software platform for the organization and analysis of sequence data. Bioinformatics. 2012;28:1647–1649. doi: 10.1093/bioinformatics/bts199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Robert X, Gouet P. Deciphering key features in protein structures with the new ENDscript server. Nucleic Acids Res. 2014;42:W320–W324. doi: 10.1093/nar/gku316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jones DT. Protein secondary structure prediction based on position-specific scoring matrices. J. Mol. Biol. 1999;292:195–202. doi: 10.1006/jmbi.1999.3091. [DOI] [PubMed] [Google Scholar]
- 42.de la Cruz MJ, Martynowycz MW, Hattne J, Gonen T. MicroED data collection with SerialEM. Ultramicroscopy. 2019;201:77–80. doi: 10.1016/j.ultramic.2019.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zheng SQ, et al. MotionCor2: anisotropic correction of beam-induced motion for improved cryo-electron microscopy. Nat. Methods. 2017;14:331–332. doi: 10.1038/nmeth.4193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rohou A, Grigorieff N. CTFFIND4: fast and accurate defocus estimation from electron micrographs. J. Struct. Biol. 2015;192:216–221. doi: 10.1016/j.jsb.2015.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zivanov, J. et al. New tools for automated high-resolution cryo-EM structure determination in RELION-3. Elife7, 10.7554/eLife.42166 (2018). [DOI] [PMC free article] [PubMed]
- 46.Eswar N, Eramian D, Webb B, Shen MY, Sali A. Protein structure modeling with MODELLER. Methods Mol. Biol. 2008;426:145–159. doi: 10.1007/978-1-60327-058-8_8. [DOI] [PubMed] [Google Scholar]
- 47.Lindert S, Meiler J, McCammon JA. Iterative molecular dynamics-rosetta protein structure refinement protocol to improve model quality. J. Chem. Theory Comput. 2013;9:3843–3847. doi: 10.1021/ct400260c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Joseph AP, et al. Refinement of atomic models in high resolution EM reconstructions using Flex-EM and local assessment. Methods. 2016;100:42–49. doi: 10.1016/j.ymeth.2016.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Emsley P, Lohkamp B, Scott WG, Cowtan K. Features and development of Coot. Acta Crystallogr. D Biol. Crystallogr. 2010;66:486–501. doi: 10.1107/S0907444910007493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Adams PD, et al. PHENIX: a comprehensive Python-based system for macromolecular structure solution. Acta Crystallogr. D Biol. Crystallogr. 2010;66:213–221. doi: 10.1107/S0907444909052925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Pettersen EF, et al. UCSF Chimera–a visualization system for exploratory research and analysis. J. Comput. Chem. 2004;25:1605–1612. doi: 10.1002/jcc.20084. [DOI] [PubMed] [Google Scholar]
- 52.Roux L, Holland JJ. Role of defective interfering particles of Sendai virus in persistent infections. Virology. 1979;93:91–103. doi: 10.1016/0042-6822(79)90278-2. [DOI] [PubMed] [Google Scholar]
- 53.Wisanpitayakorn, P., Mickolajczyk, K. J., Hancock, W. O., Vidali, L. & Tüzel, E. Measurement of the persistence length of cytoskeletal filaments using curvature distributions. Preprint at bioRxiv10.1101/252551 (2018). [DOI] [PMC free article] [PubMed]
- 54.Smith MB, et al. Segmentation and tracking of cytoskeletal filaments using open active contours. Cytoskeleton. 2010;67:693–705. doi: 10.1002/cm.20481. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Descriptions of Additional Supplementary Files
Data Availability Statement
The cryo-EM density maps of double-headed SeV nucleocapsids were deposited in Electron Microscopy Data Bank (EMDB) with the accession numbers 30064 (clam-shaped structure), 30065 (helical stem-1), 30066 (helical stem-2), and 30129 (NCcleaved), respectively. And the atom coordinates of a single N subunit were deposited in the Protein Data Bank (PDB) with the PDB ID code 6M7D. All other data are available in the main text or the supplementary materials or available from the corresponding author upon reasonable request.