Abstract
Early blight is the most devastating disease in tomato which causes huge yield losses across the globe. Hence, development of specific, efficient and ecofriendly tools are required to increase the disease resistance in tomato plants. Here, we systematically investigate the defensive role and priming effect of silicon (Si) in tomato plants under control and infected conditions. Based on the results, Si-treated tomato plants showed improved resistance to Alternaria solani as there was delay in symptoms and reduced disease severity than non-Si-treated plants. To further examine the Si-mediated molecular priming in tomato plants, expression profiling of defense-related genes like PR1, PR2, WRKYII, PR3, LOXD and JERF3 was studied in control, Si-supplemented, A. solani-inoculated and Si + A. solani-inoculated plants. Interestingly, Si significantly increased the expression of jasmonic acid (JA) marker genes (PR3, LOXD and JERF3) than salicylic acid (SA) marker genes (PR1, PR2 and WRKYII). However, Si + A. solani-inoculated plants showed higher expression levels of defence genes except WRKYII than A. solani-inoculated or Si-treated plants. Furthermore, pre-supplementation of Si to A. solani-infected tomato plants showed increased activity of antioxidant enzymes viz. superoxide dismutase (SOD), catalase (CAT), ascorbate peroxidase (APX), glutathione reductase (GR) and peroxidase (POD) than control, Si-treated and A. solani-inoculated plants. Altogether, present study highlights the defensive role of Si in tomato plants in response to A. solani by increasing not only the transcript levels of defense signature genes, but also the activity of antioxidant enzymes.
Keywords: Lycopersicon esculentum, Silicon, PR genes, Early blight, Alternaria solani, Antioxidant enzymes
Introduction
Tomato (Lycopersicon esculentum Mill.) is one of the important and widely grown vegetable crops in the world (Guil-Guerrero and Rebolloso-Fuentes 2009; Khan et al. 2017; Alenazi et al. 2020) and fulfils nearly 50% of vegetable requirements of the country. It is the second most consumed vegetable after potato in the world (Foolad 2007). They are nutritionally important because of high content of vitamin A and C and also provide antioxidant lycopene pigment that protects heart diseases and cancer as well (Story et al. 2010; Singh and Goyal 2008). Unfortunately, the productivity of tomato crops has been largely affected by various biotic stresses (Pandey et al. 2017; Gerszberg and Hnatuszko-Konka 2017; Fahad et al. 2017). Among them, fungal pathogens like A. solani, Phytophthora infestans, Fusarium oxysporium, Verticilium dahlia and Septoria lycopersici are the major limiting factors of tomato production across the globe. Early blight caused by A. solani is one of the destructive diseases in tomato which causes approximately 79% yield losses (Panthee and Chen 2009; Adhikari et al. 2017). Besides huge losses, it also damages the fruit quality and market value of tomato thereby causing huge economical losses. Generally, A. solani shows unique symptoms in tomato plants which mainly depend on the organ infected like leaf blight or early blight, collar rot or stem lesions and fruit rot (Chaerani et al. 2006; Thirthamallappa and Lohithaswa 2000). Unfortunately, tomato plants lack sufficient disease resistance to A. solani. However, fungicide applications are currently used to manage early blight but are environmentally hazardous. Therefore, it is essential to find other strategies to manage early blight in tomato. Si supplementation is one of the promising and ecofriendly approach to overcome the negative effects of A. solani in tomato plants.
Si forms one of the beneficial and multifunctional elements which provides disease resistance not only to microbial pathogens but also enhances stress tolerance to multiple abiotic stresses. Plants are classified as Si accumulators or non accumulators based on their ability to absorb the element. Plants consume silicic acid as a source of Si and are known to be safe if accumulated in higher quantities (Tubaña et al. 2015; Tubana et al. 2016). Silicic acid transporters both influx (Lsi1) and efflux (Lsi2) have been identified in number of plants including rice, barley and maize, soybean and cucumber (Deshmukh et al. 2013). The pioneer work on Si transporters further provides information on Si permeability in plants (Deshmukh et al. 2015). Recently, transportation of Si through multiple transportation processes has been studied in various plant species (Mitani-ueno and Ma 2020). To defend against pathogens, plants use both preformed (structural and biochemical) as well as inducible defense responses which are regulated by a multifaceted network of signal pathways (Fraser 2000). These mechanisms involve lignin formation, callose deposition, production of reactive oxygen species (ROS), phytoalexins, phenolics, induction of pathogenesis-related (PR) proteins, peroxidase and catalase (Torres et al. 2006; Almagro et al. 2009; Das and Roychoudhury 2014; Doughari 2015). Si addition can reduce the severity of plant disease through the formation of physical barrier by fortification of the host epidermal tissue, improving biochemical mechanisms and systemic signaling pathways (Ma 2004; Fortunato et al. 2012; Van Bockhaven et al. 2013; Reynolds et al. 2016). A number of studies have revealed that silicification of the epidermal cells delayed fungal penetration, colonization and sporulation during various plant pathogen interactions such as Cucumis sativus-Colletotrichum lagenarium, Arabidopsis thaliana-Erysiphecichoracearum, Oryza sativa-Monographella albescens, Oryza sativa-Rhizoctonia solani and Triticum aestivum-P. oryzae (Araujo et al. 2016; da Silva et al. 2015; Domiciano et al. 2013; Kauss et al. 2003; Sousa et al. 2013; Fauteux et al. 2005). Generally, Si can improve disease resistance by various modes viz. preventing pathogen access through structural strengthening (Fortunato et al. 2012; Fauteux et al. 2005), activation of systemic acquired resistance (SAR), stimulates the accumulation of antimicrobial molecules like pathogenesis-related proteins and activating a cascade of defense hormonal signaling pathways (Van Bockhaven et al. 2013; Vivancos et al. 2015). It was primary reported in dicots that, Si positively declines the negative impact of powdery mildew on cucumber plants (Samuels et al. 1991; Sousa et al. 2013; Reynolds et al. 2016). It was reported earlier that Si supplementation successfully prevents penetration of Pyricularia oryzae and Bipolaris sorokiniana in wheat plants (Sousa et al. 2013; Domiciano et al. 2013). Similarly in rice plants, Si supplementation significantly prevented the penetration of two fungal pathogens namely Pyricularia grisea and Rhizoctonia solani by forming Si dense layer on leaf tissues (Rodrigues et al. 2001; Seebold et al. 2004; Hayasaka et al. 2008). (Wiese et al. 2005) showed that the addition of Si strongly enhanced barely resistance to powdery mildew infection. In addition to cell wall strengthening, Si supplementation is known to induce an array of biochemical defense responses in plants such as activating SA, JA and ET pathways, increases production of antimicrobial molecules and enzymes and enhances activity of antioxidant enzymes (Fauteux et al. 2005; Fortunato et al. 2012; Van Bockhaven et al. 2013). Classically, JA/ET pathway provides resistance to necrotrophic pathogens and herbivorous pests while as SA pathway mediated disease resistance to biotrophic pathogens (Glazebrook 2005; Bari and Jones 2009). Generally, activation of defense signaling pathways viz., SA and JA leads to the accumulation of PR proteins that minimizes pathogen load or disease onset in uninfected plant organs. There are plethora of studies which have shown that Si effectively modulates the SA and JA signaling pathways, which improves host defense machinery against the stresses. Previous reports highlighted that pre-supplementation of Si in Erysiphe cichoracearum infected Arabidopsis plants stimulates the biosynthesis of SA, JA, and ET thereby enhancing disease resistance (Fauteux et al. 2006). Similarly, Si supplementation in Ralstonia solanacearum infected tomato plants triggers the activation JA and ET signaling pathways. Interestingly, many studies have shown that Si increases disease resistance in rice plants against wide range of pathogens by activating defense pathways (Brunings et al. 2009; Van Bockhaven et al. 2015). In addition, Si is known to increase the transcript levels of pathogenesis-related genes like PR1, PR2 (glucanse), PR3 (chitinase) and other regulatory or transcription factors which provides effective resistance against broad range of pathogens. It is well documented that Si supplementation significantly increases the activity of defense-related enzymes like phenylalanine ammonia-lyase, peroxidase, polyphenoloxidase and glucanase in different crops against wide range of pathogens. In plants, Si is known to improve disease resistance by modulating defense signaling pathways (Zhang et al. 2004; Fauteux et al. 2006; Iwai et al. 2006; De Vleesschauwer et al. 2008; Brunings et al. 2009; Chen et al. 2009; Ghareeb et al. 2011; Reynolds et al. 2016). A high Si concentration in the shoots of plants infected with different pathogens was associated with a more efficient antioxidant metabolism (high APX, CAT, GR, and SOD activities), thereby enhancing the removal of ROS (Curvêlo et al. 2013; Debona et al. 2014; Pereira Domiciano et al. 2015; Fortunato et al. 2012; Li et al. 2012; Resende et al. 2012a; Polanco et al. 2014; Mohaghegh et al. 2011; Sun et al. 2010). Recently, multiomics studies on the effect of Si on different pathosystems have provided vast information to further broaden our understanding on the role of Si in disease or stress tolerance. These findings suggested that Si addition enhances resistance to plants (dicots and monocots) against pathogens. Generally, tomato plants are low Si accumulators which are mainly due to the absence of Lsi2-type transporter. However, Lsi1 is present in the root cells of tomato plants which are functional in silicon accumulation (Sun et al. 2020). The primed state of Si could be mediated via ethylene, jasmonic acid and/or reactive oxygen species signaling pathways. As there are many studies which have shown that Si increases the expression of antifungal genes in Si-non accumulator plants (Ghareeb et al. 2011; Vivancos et al. 2015; Almutairi, 2016). In this
Materials and methods
Plants and growth conditions
In the present study, the seeds of L. esculentum Mill variety Shalimar-2 were obtained from vegetable seed production section (SKAUST-K) Srinagar, Jammu and Kashmir, India. The seeds were surface sterilized with HgCl2 and grown in pots containing a mixture of sterile soil and organic manure (2:1) in a growth chamber with a temperature of 22 °C and illuminated with compact fluorescent lamps for 16 h/8 h light and dark cycles. The 30-day-old tomato seedlings were then transferred to the green house of Centre of Research for Development, University of Kashmir, India. Prior to A. solani infection, tomato plants were supplemented with Si in the form of potassium silicate (1.7 mM) as described by Ouellette et al. (2017) and control plants were treated with potassium chloride solution.
Alternaria solani infection in Lycopersicon esculentum plants and disease scoring
To study the A. solani-tomato pathosytem, A. solani strain was isolated from infected leaves of L. esculentum and cultured on potato dextrose agar (PDA) media Rodrigues et al. (2010). Si-supplemented tomato plants (45-d-old) plants were inoculated with A. solani by the method described by Ali et al. (2017a). For mock, control plants were treated with sterile distilled water and placed separately to prevent cross contamination. Both the control and infected plants were incubated at 22 °C under 100% relative humidity. Evaluation of disease resistance in Si- and non-Si-treated tomato plants in response to A. solani infection was carried out by studying following disease parameters viz. lesion appearance, lesion diameter (cm), number of lesions per leaf and percentage of disease leaf area (%DLA) (Ghosh et al. 2009; Ali et al. 2017b).
RNA isolation and reverse transcription quantitative PCR
To investigate the expression of defense marker genes such as PR1, PR2, WRKYII, PR3, LOXD and JERF3 in response to Si amendment in plants inoculated and non-inoculated with A. solani, real-time quantitative PCR was performed using gene-specific primers. Leaf samples from control, Si-treated, A. solani-inoculated and Si + A. solani-inoculated plants were collected (three technical replicates) and immediately frozen in liquid nitrogen and stored in -800C. Total RNA was extracted from control, Si-treated, A. solani-inoculated and Si + A. solani-inoculated leaf samples (100 mg) using Ambion RNA isolation kit as described by manufacturer’s protocol (Life Technologies). In this study, PR1, PR2, WRKYII, PR3, LOXD, JERF3 and alpha tubulin gene specific primers were designed by Oligoanalyzer software (Table1). For complementary DNA (cDNA) preparation, 2 µg of highly purified total RNA was used in 20 µl reaction volume containing oligo(dT) 18 primers, 10 mM deoxynucleotide (dNTPS), reverse transcriptase and water following the manufacturer’s instructions (Invitrogen, Canada). For expression analysis, qPCR was done in a mixture containing 5 µl of SYBR green real-time PCR master mix (Takara, Japan), 2 µl of cDNA and 0.5 µl (10 pmol) of each primer. The qPCR reactions were performed at 95 °C for 5 min, followed by 40 cycles at 94 °C for 30 s, at 60 °C for 30 s, and at 72 °C for 30 s. The relative expression levels of PR1, PR2, WRKYII, PR3, LOXD and JERF3 were quantified by 2¯ΔΔCt method Livak and Schmittgen (2001). All reactions were performed with three technical replicates.
Table 1.
List of primer pairs used for qRT-PCR
| Gene | Accession No | Sequence |
|---|---|---|
| PR1 | M69247 |
Sense 5′-ACTTGGCATCCCGAGCACAA-3′ Antisense 5′-CTCGGACACCCACAATTGCA-3′ |
| PR2 | M80604 |
Sense 5′-TTTCGATGCCCTTGTGGATTC-3′ Antisense 5′-GGCCAACCACTTTCCGATAC-3′ |
| PR3 | U30465 |
Sense 5′-GCGTTGTGGTTCTGGATGACA-3′ Antisense 5′-CAGCGGCAGAATCAGCAACA-3′ |
| WRKYII | AY157064 |
Sense 5′-GCTTGGAAGACGCTTCAATGC-3′ Antisense 5′-GTGATGGCAACCTGGGATGA-3′ |
| JERF3 | AY383630 |
Sense 5′-GCCATTTGCCTTCTCTGCTTC-3′ Antisense 5′-GCAGCAGCATCCTTGTCTGA-3′ |
| LOXD | AF384374 |
Sense 5′-AGATTTCTCCCGAATATGCTGAA-3′ Antisense 5′-ATACTACTGATITCATCAACGGCAT-3′ |
| α-Tubulin | NM_1003603 |
Sense 5′-GCTGGGAGCTGTACTGTCTTG-3′ Antisense 5′-CAACGGAGGTAGAGACCTGTG-3′ |
Estimation of activities of antioxidant enzymes
Leaf samples (500 mg) were harvested from control, Si-treated, A. solani-inoculated and Si + A. solani-inoculated plants after 15 days and were immediately frozen, ground in liquid nitrogen and extracted in 50 mM potassium phosphate buffer (K2HPO4) at pH 7. After centrifugation of the homogenate at 11,000 rpm for 15 min at 4 °C, the supernatant was taken and used for determination of antioxidant enzyme activities. SOD activity was carried out according to the method of Beauchamp and Fridovich (1971) by determining the ability of the enzyme to inhibit the formation of NBT from formazan. The absorbance of reduced NBT was measured at 560 nm. CAT activity was determined according to the method of Aebi (1984) and absorbance of the reaction mixture containing enzyme extract and phosphate buffer was measured at 240 nm before and after the addition of hydrogen peroxide. APX activity was determined by employing the method of Nakano and Asada (1981) to find its ability to catalyse the reduction of hydrogen peroxide into water in presence of phosphate buffer and measuring the activity as decrease in absorbance at 290 nm. GR activity was assayed by following the method of Foyer and Halliwell (1976). It was determined by measuring the decrease in absorbance at 340 nm by examining the glutathione dependent oxidation of NADPH. POD activity was assayed by following the method of Chance and Maehly (1955). It was recorded by measuring the change in absorbance of the reaction mixture at 470 nm.
Statistical analysis
The results were statistically analyzed by student’s t-test and Tukey multiple comparison test. For each experiment, three replicates were used and repeated three times. Student’s t-test was carried out to determine significant differences in gene expression in control, Si-treated, A. solani-inoculated and Si + A. solani-inoculated samples. Statistical evaluation of significant differences was determined as (p < 0.05) or extremely significant (p < 0.01).
Results
Disease development in Lycopersicon esculentum
In the present experiment first we studied the early blight disease development in L. esculentum by inoculating A. solani spores on the leaves under defined conditions. The appearance of necrotic lesions appeared on tomato leaves after 48 h of post inoculation while no symptoms were seen on non-infected leaves. These results showed the susceptibility of L. esculentum to A. solani infection as well as compatible interaction between A. solani and L. esculentum pathosystem.
Silicon improves disease resistance in tomato plants against Alternaria solani infection
In the present study, we systematically examined the role Si in improving disease resistance against early blight disease in L. esculentum plants. To further assess the resistance level of Si-treated L. esculentum, 45-day-old plants were infected with A. solani and disease scoring was done for 1–3 weeks. The disease resistance was assessed by measuring the average lesion diameter, lesion appearance and disease severity in the A. solani-infected leaves for both Si-treated and control plants from 3 to 15 dpi. After 3 days of post inoculation, small necrotic lesions began in non-Si-treated L. esculentum plants and size of the necrotic lesions increased significantly after disease progression (Fig. 1). In contrast, Si-treated plants also showed lesions but the lesion size or diameter was comparatively lower than non-treated plants days after inoculation. After 15 dpi, lesion diameter increases more significantly in non Si-treated infected plants (Fig. 2). Furthermore, our results revealed that disease severity was very high in non-Si-treated L. esculentum plants and covers approximately 75% of the total leaf area than Si-treated plants at 15th dpi (Fig. 3). The disease resistance was assessed by measuring the average lesion diameter in the A. solani infected leaves for both Si-treated and control plants and lesion diameter was 75% reduced in the former compared to non-Si-treated plants. The increased number of lesions spread in distal portions of leaves in non-treated plants than Si-treated plants after A. solani infection. Based on disease index (0–9 scale), non-silicon-treated plants showed early blight infection with disease scale of 8 (70–80%). While, Si-treated plants show disease scale of 3 (20–30%), respectively. Therefore, our results showed that Si-treated plants exhibit improved disease resistance to Alternaria early blight as there was delay in lesion appearance, size and spread of infection in comparison to non-Si-treated-inoculated plants. On the other hand, we also found the difference in the plant height and dry weight of the infected tomato plants after Si application than non-Si-treated A. solani-infected plants. As Si-treated A. solani-inoculated plants showed increase in their height and more dry weight than non-Si-treated A. solani-inoculated plants (Table 2).
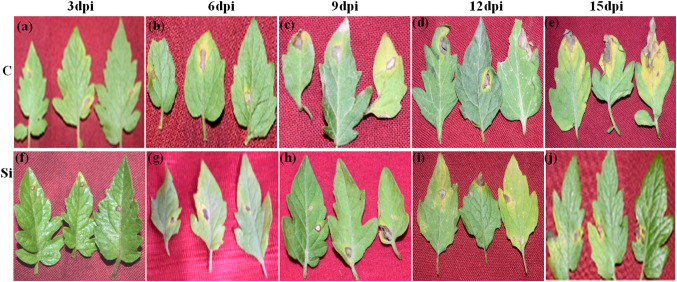
Fig. 1.
Disease resistance screening of Si-treated tomato plants after A. solani infection. Forty-five-day-old Si-treated and non-Si-treated tomato plants were infected with A. solani. Si-treated plants showed reduction and delay in disease severity than non-Si-treated-inoculated plants. C: control (a: 3 dpi, b: 6 dpi, c: 9 dpi, d: 12 dpi, e: 15 dpi) and Si: Si treated (f: 3 dpi, g: 6 dpi, h: 9 dpi, i: 12 dpi, j: 15 dpi)
Fig. 2.
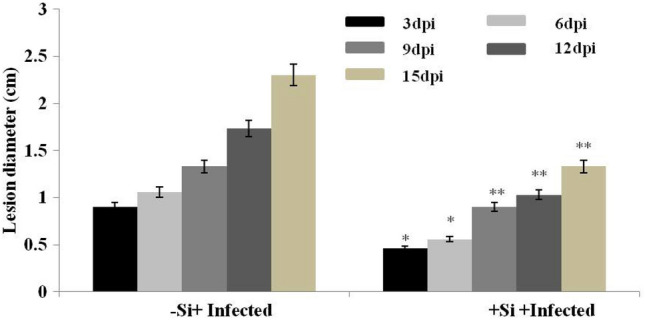
Disease scoring like lesion diameter of Si-treated and non-Si-treated plants after A. solani infection at different dpi. Three biological replicates were used. Values are mean ± SD of each condition. Statistically significant difference is shown by different asterisks on bars (*p < 0.05; **p < 0.01) between Si-treated and control-inoculated plants
Fig. 3.
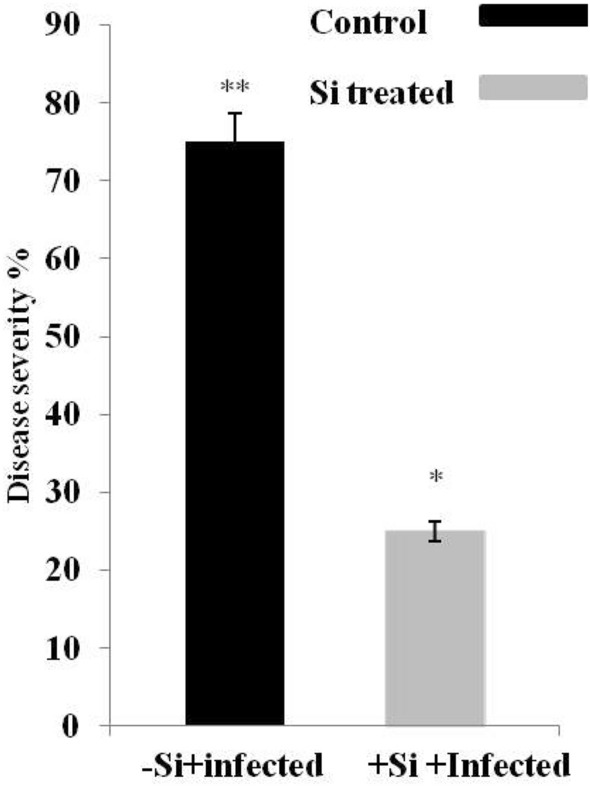
Early blight disease severity was monitored in-Si + infected and + Si + infected plants based on total leaf area infected. Values are (mean ± SD) of three replicates. Bars with different asterisks depicting statistically significant difference (*p < 0.05; **p < 0.01)
Table 2.
Phenotypic examination of Si-treated-infected and without Si-treated-infected tomato plants showed significant variations in terms of plant height and dry weight
| S. No | Plants | Plant height (cm) | Dry weight (g) |
|---|---|---|---|
| 1 | + Si + Infected | 135 ± 3a | 2.9 ± 0.9c |
| 2 | -Si + Infected | 129 ± 1b | 2.5 ± 0.1d |
Different letters indicate significant differences between the treatments at p < 0.05
Silicon modulates the expression of defense genes in tomato.
Plant defense hormones like SA and MeJA play an essential role in the regulation of plant immune responses to microbial pathogens. In this study, we systematically investigated the effect of Si, A. solani and Si + A. solani on SA and MeJA signalling marker genes (PR1, PR2, WRKYII, PR3, LOXD and JERF3) which are known to play important role in disease resistance in both model and crop plants. Based on the results, Si treatment increases the expression of PR1 (1.9 fold), PR2 (3.5 fold) WRKYII (1.8 fold) compared to control plants. However, pre supplementation of Si in A. solani-infected tomato plants further increases the expression of PR1 (2.7 fold), PR2 (4.5 fold) defense genes except WRKYII (1.2 fold) than Si or A. solani-inoculated plants (Fig. 4). On the other hand, transcript levels of JA marker genes PR3 (10.3 fold), LOXD (4.3 fold) and JERF3 (11.8 fold) increased dramatically during combined Si + A. solani treatments when compared to control, Si-treated or A. solani-inoculated plants (Fig. 4). These results provide the evidence that application of Si in tomato plants was associated with the upregulation of PR genes and defense related transcription factors which could enhance disease resistance in L. esculentum to A. solani.
Fig. 4.
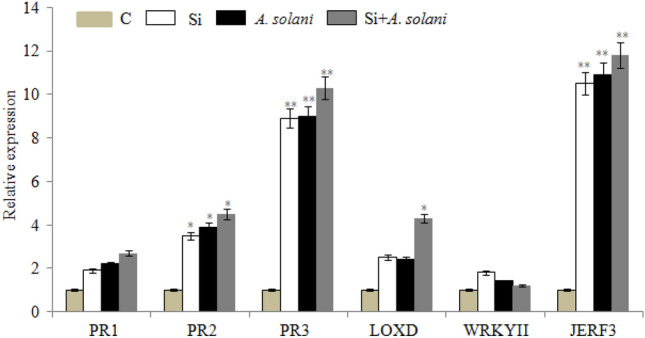
Relative expression levels of PR1, PR2, PR3, LOXD, WRKYII and JERF3 genes in L. esculentum in response to Si treatment and inoculation with A. solani determined by qRT-PCR. Expression levels were compared with an internal control alpha tubulin. Data represent mean ± S.D of three technical replicates. Asterisks indicating statistically significant differences between Si-treated, A. solani-inoculated and Si + A. solani-inoculated plants (*p < 0.05; **p < 0.01)
Improvement in the activities of antioxidant enzymes by silicon supplementation
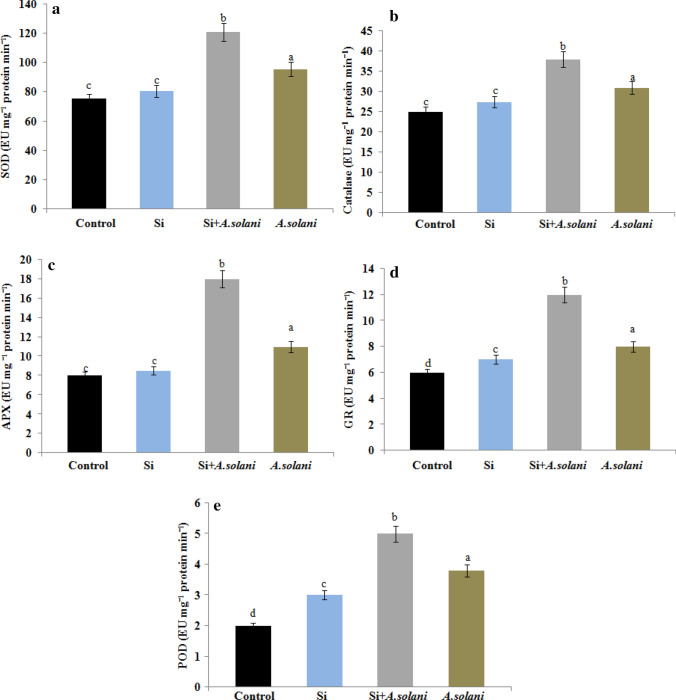
To decrease the harmful effects of reactive oxygen species (ROS) during biotic stress, plants produce an effective antioxidative defence systems or ROS scavengers such as SOD, CAT, APX, GR and POD respectively. In this study, we examined the effect of Si on the activity of antioxidant enzymes in relation to control and A. solani-infected tomato plants. The enzyme activity is expressed in enzyme units per milligram (mg) of protein. Based on our findings, Si-supplemented A. solani-infected plants dramatically increased SOD activity (121 ± 3) compared to control (75 ± 1), Si-treated (80.5 ± 2) and A. solani-inoculated plants (95.5 ± 3) (Fig. 5a). On the other hand, upon Si addition higher levels of CAT activity (38 ± 1) were observed in Si + A. solani-inoculated plants than Si-treated (27.5 ± 0.6), A. solani-inoculated (31 ± 0.9) and control plants (25 ± 0.5) (Fig. 5b). These results suggested that Si supplementation significantly increases the activity of SOD and CAT enzymes in L. esculentum plants. Interestingly, APX activity in Si + A. solani-inoculated plants was significantly higher (18 ± 1) than control (8 ± 0.4), Si-treated (8.5 ± 0.5) and A. solani-inoculated plants (11 ± 0.8) (Fig. 5c). Alternatively, there was significant difference in GR activity between control, Si-treated, Si + A. solani-inoculated and A. solani-inoculated plants and based on the results, GR activity was found to be higher in Si + A. solani-inoculated plants (12 ± 0.9) than control (6 ± 0.3), Si-treated (7 ± 0.4) and A. solani-inoculated plants (8 ± 0.6) (Fig. 5d). Generally, peroxidases are known to be the early defense enzymes produced during biotic stress response Jouili et al. (2011). Here, addition of Si significantly increases POD activity in Si + A. solani-inoculated plants (5 ± 0.4) than control (2 ± 0.1), Si-treated (3 ± 0.2) and A. solani-inoculated plants (3.8 ± 0.2) (Fig. 5e). These results strongly suggest that upon the addition of Si to L. esculentum plants, free radical production declines by the increase in the activity of antioxidant enzymes thereby declines disease progression.
Fig. 5.
Activity of antioxidant enzymes in control, Si-treated, Si + A. solani- and A. solani-inoculated L. esculentum plants a SOD, b CAT, c APX, d GR, e POD. Data presented are the mean ± SD of three replicates. Different letters on bars indicate significant differences between means at P ≤ 0.05
Discussion
Lycopersicon esculentum Mill. is one of the most economically important vegetable crop, which is very often affected by various biotic stresses. Among them, early blight caused by A. solani is one of the major diseases in L. esculentum crops with no proven source of disease resistance in any of the germplasm. In this regard, novel strategies of effective disease protection should be developed. It is well documented that Si plays a multifaceted role in plants by modulating various biochemical, physiological and molecular adaptation processes for their survival under stress conditions. Addition of Si to plants has been shown to decrease the severity of numerous diseases like root rots, leaf spots, damping off, powdery mildews, galls, root rots, rusts, and wilts (Fortunato et al. 2015; Rodrigues et al. 2015). Interestingly, in susceptible crop plants, Si supplementation enhanced disease resistance to the level of cultivars with race-specific resistance (Resende et al. 2012b; Seebold et al. 2004). Hence, present study was carried out to further investigate the positive effect of Si on improving disease resistance against early blight disease in L. esculentum. Based on our results, Si supplementation improves disease resistance in tomato plants against A. solani, by delayed incubation period, lesion number, size and reduced mean lesion diameter. Our results go in concurrent lines with the findings that Si induces resistance and enhances plant tolerance against the bacterial wilt, Fusarium crown and root rot in tomato plants (Dannon and Wydra 2004; Diogo and Wydra 2007; Huang et al. 2011). In addition, many studies have shown that Si confers resistance to both necrotrophic and biotrophic pathogens (Samuels et al. 1994; Heine et al. 2007; Ma and Yamaji 2006, 2008). Interestingly, we also found that lower rate of disease spreading to distal/non-infected parts of tomato plants after Si supplementation, thus provides the evidence of activation of SAR pathway. There is growing body of evidences that systemic resistance in plants developed by Si application through roots forms more effective against pathogen attack (Liang et al. 2005). On the other hand, we also found the significant difference in the height and dry weight of the infected tomato plants after Si supplementation. As Si + A. solani-inoculated plants showed increase in their height and more dry weight than non-Si-treated A. solani-inoculated plants. Previous reports have also highlighted the role of Si on plant growth and development (Epstein 1999, 2001; Sun et al. 2010). These findings suggested that Si amendment not only improves disease resistance to A. solani but also improves growth parameters in infected tomato plants. Our findings go in concurrent lines with earlier findings that Si supplementation improves growth parameters in tomato plants under stress conditions (Zhang et al. 2018; Li et al. 2015).
Si is known to trigger inducible defense mechanisms to overcome pathogen challenges mainly by modulating defense gene expression, series of physiological and biochemical signal transduction processes (Fauteux et al. 2005; Vivancos et al. 2015). In plants, phytohormones SA and JA are key players of inducible defense pathways that regulate the expression of an array of antimicrobial genes that minimizes pathogen load or disease onset in plant organs upon receiving pathogen infections (Balbi and Devoto 2008). To explore whether there is a significant effect on the expression of defense signaling marker genes upon Si application, A. solani infection and Si + A. solani infection in L. esculentum plants. We investigated the expression of SA marker genes (PR1, PR2, WRKYII) and JA marker genes (PR3, JERF3, LOXD), respectively. In response to Si and Si + A. solani, increased transcript levels of PR1 were observed in tomato plants. PR1 is universally known as a molecular indicator of induced plant immune system such as hypersensitivity response (HR) and systemic acquired resistance (SAR) (Jung and Hwang 2000; Jung et al. 2009). In addition, PR1 proteins isolated from tobacco and tomato possess strong in vitro antifungal activity (Niderman et al. 1995). Hence, increased expression of PR1 in Si-treated A. solani-inoculated tomato plants is responsible for enhanced disease resistance to necrotrophic fungi. On the other hand, pre supplementation of Si significantly increases the expression of PR2 (β-1,3-glucanase) gene in A. solani infected plants which is not only antifungal but also releases oligosaccharides from fungal cell walls which further elicit the host defense response and systemic acquired resistance (SAR) (Ferreria et al. 2007). β-1,3-glucanase catalyzes the hydrolysis of β-1,3-glucans found in the cell walls of many genera of fungi and so exhibit antifungal activity (Ferreria et al. 2007). Previous reports have also shown that the expression of β-1,3-glucanase was increased in different crops after Si supplementation and pathogen infection (Shwethakumari and Prakash 2018; Cruz et al. 2015). In plants, NPR1 forms a regulatory protein that is critical for the regulation of PR gene expression and the expression levels of NPR1 are in turn regulated by a transcription factor WRKY (Li et al. 2004). In the present experiment, the expression of WRKYII was significantly induced after Si supplementation but decreases during combined treatment of Si and A. solani in tomato plants. Interestingly, our results are in concurrent lines with earlier findings that upregulation of WRKY after Si treatment to L. esculentum (Menke et al. 2005; Ghareeb et al. 2011). Furthermore, our findings go in line with previous reports that Si supplementation in Arabidopsis increases the transcript levels of SA induced genes (PR1, PR2, PR5) and its biosynthetic genes (EDS1 and PAD4) (Vivancos et al. 2015).
Si can also enhance JA-dependent defense response in both model and crop plants by elevating the expression of JA biosynthetic and marker genes, which provides resistance not only to necrotrophic fungal pathogens but also to insects. Si supplementation up regulates the ubiquitin-protein ligase activity, which plays a key role on fine-tuning of JA-dependent immune response by degrading the JA-negative regulator (JAZ1) (Thines et al. 2007; Dreher and Callis 2007). In addition, previous studies have highlighted that JA promotes overall leaf silicification and the maturation of phytolith bearing silica cells by increasing Si accumulation (Fauteux et al. 2006; Ye et al. 2013). In the present study, we studied the impact of Si, Si + A. solani and A. solani on JA dependent genes viz. PR3, JERF3 and LOXD in tomato plants. JERF3 is a transcription factor which is activated in response to JA and ET signaling pathway in plants. In our study, JERF3 was significantly induced after Si treatment in A. solani-inoculated tomato plants than Si-treated or A. solani-inoculated plants thus indicating the activation and involvement of JA pathway which could trigger the immune response by activating an array of defense genes. (Ghareeb et al. 2011) showed that Si supplementation increases the transcript levels of JERF3 during R. solanacearum infection thus supporting that Si induced resistance were mediated via ET and JA signaling pathways. Chtinases or (PR3) are pathogenesis-related proteins are well known fungal cell wall hydrolyzing enzymes, restricting fungal growth and development are also upregulated by Si supplementation (Brisson et al. 1994). In our study, Si supplementation strongly increases the expression of chitinase gene in Si-supplemented A. solani-inoculated plants which contribute in Si-mediated disease resistance against early blight in tomato plants. Overexpression or elicitor-mediated studies have shown that chitinases provide effective resistance to fungal pathogens (Ignacimuthu and Ceasar 2020). Tom LOXD, a lipoxygenase gene that is involved in JA biosynthesis is localized in the autonomous organelle (choloroplast) of the cell. Overexpression of Tom LOXD leads to an enhanced resistance against necrotrophic fungal pathogens and insect herbivory attack. Our results revealed that after Si addition, expression of LOXD was highly upregulated in Si + A. solani inoculated plants, than non treated and A. solani inoculated plants, suggesting that Si plays important role in regulating defense signaling marker genes in plants, study in this line was carried by (Song et al. 2016) to determine the effect of Si on LOX genes. These results clearly indicates that Si supplementation in tomato plants leads increased expression of SA and JA marker genes and other defense-related transcription factors which could enhance disease resistance in L. esculentum not only to A. solani, but also to an array of pathogens.
Plants produce reactive oxygen species as by products under normal cellular metabolic processes (Tripathy and Oelmüller 2012). However, under stress conditions they produce higher levels of ROS such as superoxide anion, H2O2, and hydroxyl radical which severely inhibit the growth, development and productivity of plants mainly by oxidative stress (Thannickal and Fanburg 2000; Sies et al. 2017). Interestingly, plants possess a well-developed protective mechanism of scavenging ROS, which includes both enzymatic and non-enzymatic antioxidants to maintain their ROS-homeostasis and protect cellular toxicity and fine tune ROS-mediated signal transduction (Gong et al. 2005; Gechev et al. 2006; Shi et al. 2014). Si is known to modulate the activity of antioxidant enzymes during stress conditions which make it as an important factor for alleviating the harmful effects of ROS in crop plants during stress conditions. In the present study, we investigate the effect of Si on antioxidant enzymes in tomato plants after A. solani infection. Based on our findings, Si supplementation considerably increases the activities of antioxidant enzymes viz. SOD (EC 1.15.1.1), CAT (EC 1.11.1.6), APX (EC 1.11.1.11), GR (EC 1.6.4.2) and POD (EC 1.11.1.7) in A. solani-infected tomato plants. In contrast, non-Si-treated A. solani-infected tomato plants shows decreased levels of antioxidant enzymes which may increase the cell damage due to oxidative stress. Our results provide the evidence that Si plays a key role in alleviating the harmful effects of oxidative stress generated by A. solani in L. esculentum. Similar findings of increased antioxidant enzymes were also observed during powdery mildew (Guoqiang wei 2004), sheath blight (Zhang et al. 2006), and rust diseases in different crops. Previous reports have shown that Si treatment increases SOD, CAT, APX, GR and POD activities thereby, preventing the formation of hydroxyl radicals (Al-Huqail et al. 2019; Al-aghabary et al. 2005; Shi et al. 2014 and Shi et al. 2016). In another study, increased activity of SOD, GPX, APX, in Cucumber (Zhu et al. 2004), SOD, CAT, and POD in Turfgrass (He et al. 2010) and SOD, GR, and CAT in peas (Tripathi et al. 2015) were observed upon Si application. Earlier findings are in same line with our results that Si increases the antioxidant enzyme levels in plants, further suggests the potential role of Si in alleviating harmful effects during stresses (Liang et al. 2003; Shekari et al. 2017). However, contradictory results were seen in wheat plants where, Si application did not increase the levels of antioxidant enzymes but showed lower cellular damage (Debona et al. 2014; Moldes et al. 2016). Si supplementation reduces the levels of oxidative stress markers such as malondialdehyde (MDA) and hydrogen peroxides (H2O2) when added to the plants during stressed conditions. Meta analytic reports have further provided the evidence that Si supplementation in stressed plants change the activity of antioxidant enzymes which are mainly depends on the type of stress (Cooke and Leishman 2016). Similarly, in this study Si supplementation significantly decreases the harmful effects of various reactive oxygen species (ROS) during A. solani infection in tomato plants by increasing the activity of an array of antioxidant enzymes.
Conclusion
In conclusion, early blight disease is one of the major problems in tomato cultivation which causes huge yield losses. The limited availability of disease resistant varieties within the tomato germplasm is a major hindrance for the development of resistant varieties. However, fungicides are used to control fungal diseases in tomato plants but possess serious threat to the environment. In this study, we systematically studied the role of Si in improving disease resistance against Alternaria early blight in tomato plants. The supplementation of Si improves the disease resistance against A. solani by increasing the expression of defense related genes, transcriptional factors and activity of antioxidant enzymes. These findings also suggest that Si plays a key role in defense priming in tomato plants during A. solani infection. Future studies are required at genomic, metabolomic, proteomic and ionomic levels to further investigate the potential role of Si during plant pathogen interactions and stresses.
Acknowledgement
The authors would like to acknowledge the support by Centre of research for development (CORD), University of Kashmir, Srinagar. I am also thankful to the Council of Scientific & Industrial Research for financial support in the form of Junior and Senior Research Fellowships.
Declarations
Conflict of interest
The authors declare that there is no conflict of interest.
References
- Adhikari P, Oh Y, Panthee DR. Current status of early blight resistance in tomato: an update. Int J Mol Sci. 2017;18:2019. doi: 10.3390/ijms18102019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aebi H. Catalase in vitro. Methods Enzymol. 1984;105:121–126. doi: 10.1016/S0076-6879(84)05016-3. [DOI] [PubMed] [Google Scholar]
- Al-aghabary K, Zhu Z, Shi Q. Influence of silicon supply on chlorophyll content, chlorophyll fluorescence, and antioxidative enzyme activities in tomato plants under salt stress. J Plant Nutr. 2005;27:2101–2115. doi: 10.1081/pln-200034641. [DOI] [Google Scholar]
- Al-Huqail AA, Alqarawi AA, Hashem A, Ahmad Malik J. Silicon supplementation modulates antioxidant system and osmolyte accumulation to balance salt stress in Acacia gerrardii Benth. Saudi J Biol Sci. 2019;26:1856–1864. doi: 10.1016/j.sjbs.2017.11.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alenazi MM, Shafiq M, Alsadon AA, Alhelal IM, Alhamdan AM, Solieman THI, Ibrahim AA, Shady MR, Al-Selwey WA. Improved functional and nutritional properties of tomato fruit during cold storage. Saudi J Biol Sci. 2020;27:1467–1474. doi: 10.1016/j.sjbs.2020.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ali S, Chandrashekar N, Rawat S, Nayanakantha NMC, Mir ZA, Manoharan A, Sultana M, Grover A. Isolation and molecular characterization of pathogenesis related PR2 gene and its promoter from Brassica juncea. Biol Plant. 2017;61:763–773. doi: 10.1007/s10535-017-0726-7. [DOI] [Google Scholar]
- Ali S, Mir ZA, Tyagi A, Mehari H, Meena R, Bhat JA, Yadav P, Papalou P, Rawat S, Grover A. Overexpression of NPR1 in Brassica juncea confers broad spectrum resistance to fungal pathogens. Front Plant Microbe Interact. 2017 doi: 10.3389/fpls.2017.01693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Almagro L, Gómez Ros LV, Belchi-Navarro S, Bru R, Ros Barceló A, Pedreño MA. Class III peroxidases in plant defence reactions. J Exp Bot. 2009;60:370–399. doi: 10.1093/jxb/ern277. [DOI] [PubMed] [Google Scholar]
- Almutairi ZM. Effect of nano-silicon application on the expression of salt tolerance genes in germinating tomato (Solanum lycopersicum L.) seedlings under salt stress. Plant Omics. 2016;9:106–114. [Google Scholar]
- Araujo L, Paschoalino RS, Rodrigues FÁ. Microscopic aspects of silicon-mediated rice resistance to leaf scald. Phytopathology. 2016;106:132–141. doi: 10.1094/PHYTO-04-15-0109-R. [DOI] [PubMed] [Google Scholar]
- Balbi V, Devoto A. Jasmonate signalling network in Arabidopsis thaliana: crucial regulatory nodes and new physiological scenarios. New Phytol. 2008;177:301–318. doi: 10.1111/j.1469-8137.2007.02292.x. [DOI] [PubMed] [Google Scholar]
- Bari R, Jones JDG. Role of plant hormones in plant defence responses. Plant Mol Biol. 2009;69:473–488. doi: 10.1007/s11103-008-9435-0. [DOI] [PubMed] [Google Scholar]
- Beauchamp C, Fridovich I. Superoxide dismutase: improved assays and an assay applicable to acrylamide gels. Anal Biochem. 1971;4:276–287. doi: 10.1016/0003-2697(71)90370-8. [DOI] [PubMed] [Google Scholar]
- Brisson LF, Tenhaken R, Lamb C. Function of oxidative cross-linking of cell wall structural proteins in plant disease resistance. Plant Cell. 1994;6:1703–1712. doi: 10.1105/tpc.6.12.1703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunings AM, Datnoff LE, Ma JF, Mitani N, Nagamura Y, Rathinasabapathi B, Kirst M. Differential gene expression of rice in response to silicon and rice blast fungus Magnaporthe oryzae. Ann Appl Biol. 2009;155:161–170. doi: 10.1111/j.1744-7348.2009.00347.x. [DOI] [Google Scholar]
- Chaerani R, Voorrips E. Tomato early blight (Alternaria solani): the pathogen, genetics, and breeding for resistance. J Gen Plant Pathol. 2006;72:335–347. doi: 10.1007/s10327-006-0299-3. [DOI] [Google Scholar]
- Chance B, Maehly AC. Assay of catalases and peroxidases. {black small square} Methods Enzymol. 1955;2:764–775. doi: 10.1016/S0076-6879(55)02300-8. [DOI] [Google Scholar]
- Chen YY, Lin YM, Chao TC, Wang JF, Liu AC, Ho FI, Cheng CP. Virus-induced gene silencing reveals the involvement of ethylene-, salicylic acid- and mitogen-activated protein kinase-related defense pathways in the resistance of tomato to bacterial wilt. Physiol Plant. 2009;136:324–335. doi: 10.1111/j.1399-3054.2009.01226.x. [DOI] [PubMed] [Google Scholar]
- Cooke J, Leishman MR. Consistent alleviation of abiotic stress with silicon addition: a meta-analysis. Funct Ecol. 2016;30:1340–1357. doi: 10.1111/1365-2435.12713. [DOI] [Google Scholar]
- Cruz MFA, Debona D, Rios JA, Barros EG, Rodrigues FA. Potentiation of defense-related gene expression by silicon increases wheat resistance to leaf blast. Trop Plant Pathol. 2015;40:394–400. doi: 10.1007/s40858-015-0051-7. [DOI] [Google Scholar]
- Curvêlo CRS, Rodrigues FÁ, Pereira LF, Silva LC, DaMatta FM, Berger PG. Trocas gasosas e estresse oxidativo em plantas de algodoeiro supridas com silício e infectadas por Ramularia areola. Bragantia. 2013;72:346–359. doi: 10.1590/brag.2013.053. [DOI] [Google Scholar]
- da Silva WL, Cruz MFA, Fortunato AA, Rodrigues FÁ. Histochemical aspects of wheat resistance to leaf blast mediated by silicon. Sci Agric. 2015;72:322–327. doi: 10.1590/0103-9016-2014-0221. [DOI] [Google Scholar]
- Dannon EA, Wydra K. Interaction between silicon amendment, bacterial wilt development and phenotype of Ralstonia solanacearum in tomato genotypes. Physiol Mol Plant Pathol. 2004;64:233–243. doi: 10.1016/j.pmpp.2004.09.006. [DOI] [Google Scholar]
- Das K, Roychoudhury A. Reactive oxygen species (ROS) and response of antioxidants as ROS-scavengers during environmental stress in plants. Front Environ Sci. 2014;2:53. doi: 10.3389/fenvs.2014.00053. [DOI] [Google Scholar]
- De Vleesschauwer D, Djavaheri M, Bakker PAHM, Höfte M. Pseudomonas fluorescens WCS374r-induced systemic resistance in rice against Magnaporthe oryzae is based on pseudobactin-mediated priming for a salicylic acid-repressible multifaceted defense response. Plant Physiol. 2008;148:1996–2012. doi: 10.1104/pp.108.127878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Debona D, Rodrigues FA, Rios JA, Nascimento KJT, Silva LC. The effect of silicon on antioxidant metabolism of wheat leaves infected by Pyricularia oryzae. Plant Pathol. 2014;63:581–589. doi: 10.1111/ppa.12119. [DOI] [Google Scholar]
- Deshmukh RK, Vivancos J, Guérin V, Sonah H, Labbé C, Belzile F, Bélanger RR. Identification and functional characterization of silicon transporters in soybean using comparative genomics of major intrinsic proteins in Arabidopsis and rice. Plant Molec Biol. 2013;83(4–5):303–315. doi: 10.1007/s11103-013-0087-3. [DOI] [PubMed] [Google Scholar]
- Deshmukh RK, Vivancos J, Ramakrishnan G, Guérin V, Carpentier G, Sonah H, Labbé C, Isenring P, Belzile FJ, Bélanger RR. A precise spacing between the NPA domains of aquaporins is essential for silicon permeability in plants. Plant J. 2015;83:489–500. doi: 10.1111/tpj.12904. [DOI] [PubMed] [Google Scholar]
- Diogo RVC, Wydra K. Silicon-induced basal resistance in tomato against Ralstonia solanacearum is related to modification of pectic cell wall polysaccharide structure. Physiol Mol Plant Pathol. 2007;70:120–129. doi: 10.1016/j.pmpp.2007.07.008. [DOI] [Google Scholar]
- Domiciano GP, Rodrigues FA, Guerra AMN, Vale FXR. Infection process of Bipolaris sorokiniana on wheat leaves is affected by silicon. Trop Plant Pathol. 2013;38:258–263. doi: 10.1590/S1982-56762013005000006. [DOI] [Google Scholar]
- Doughari JH. An overview of plant. Immunity. 2015;6:322. doi: 10.4172/2157-7471.1000322. [DOI] [Google Scholar]
- Dreher K, Callis J. Ubiquitin, hormones and biotic stress in plants. Ann Bot. 2007;99:787–822. doi: 10.1093/aob/mcl255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epstein E. Silicon. Annu Rev Plant Physiol Plant Mol Biol. 1999;50:641–664. doi: 10.1146/annurev.arplant.50.1.641. [DOI] [PubMed] [Google Scholar]
- Epstein E. Silicon in plants: facts vs concepts. Amsterdam: Elsevier Science; 2001. p. 424. [Google Scholar]
- Fahad S, Bajwa AA, Nazir U, Anjum SA, Farooq A, Zohaib A, Sadia S, Nasim W, Adkins S, Saud S, Ihsan MZ, Alharby H, Wu C, Wang D, Huang J. Crop production under drought and heat stress: plant responses and management options. Front Plant Sci. 2017;8:1147. doi: 10.3389/fpls.2017.01147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fauteux F, Chain F, Belzile F, Menzies JG, Bélanger RR. The protective role of silicon in the Arabidopsis-powdery mildew pathosystem. Proc Natl Acad Sci USA. 2006;103:17554–17559. doi: 10.1073/pnas.0606330103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fauteux F, Rémus-Borel W, Menzies JG, Bélanger RR. Silicon and plant disease resistance against pathogenic fungi. FEMS Microbiol Lett. 2005;249:1–6. doi: 10.1016/j.femsle.2005.06.034. [DOI] [PubMed] [Google Scholar]
- Ferreira RB, Monteiro S, Freitas R, Santos CN, Chen Z, Batista LM, Duarte J, Borges A, Teixeira AR. The role of plant defence proteins in fungal pathogenesis. Mol Plant Pathol. 2007;8:677–700. doi: 10.1111/j.1364-3703.2007.00419.x. [DOI] [PubMed] [Google Scholar]
- Foolad MR. Genome mapping and molecular breeding of tomato. Int J Plant Genom. 2007 doi: 10.1155/2007/64358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fortunato AA, Rodrigues FÁ, Baroni JCP, Soares GCB, Rodriguez MAD, Pereira OL. Silicon suppresses fusarium wilt development in banana plants. J Phytopathol. 2012;160:674–679. doi: 10.1111/jph.12005. [DOI] [Google Scholar]
- Fortunato AA, Rodrigues FA, Datnoff LE. Silicon and plant diseases. Berlin: Springer; 2015. Silicon control of soil-borne and seed-borne diseases; pp. 53–66. [Google Scholar]
- Foyer CH, Halliwell B. The presence of glutathione and glutathione reductase in chloroplasts: a proposed role in ascorbic acid metabolism. Planta. 1976;133:21–25. doi: 10.1007/BF00386001. [DOI] [PubMed] [Google Scholar]
- Fraser RSS. Mechanisms of resistance to plant diseases. Dordrecht: Springer; 2000. Case studies; pp. 1–19. [Google Scholar]
- Gechev TS, Van Breusegem F, Stone JM, Denev I, Laloi C. Reactive oxygen species as signals that modulate plant stress responses and programmed cell death. BioEssays. 2006;28:1091–1101. doi: 10.1002/bies.20493. [DOI] [PubMed] [Google Scholar]
- Gerszberg A, Hnatuszko-Konka K. Tomato tolerance to abiotic stress: a review of most often engineered target sequences. Plant Growth Regul. 2017;83:175–198. doi: 10.1007/s10725-017-0251-x. [DOI] [Google Scholar]
- Ghareeb H, Bozsó Z, Ott PG, Repenning C, Stahl F, Wydra K. Transcriptome of silicon-induced resistance against Ralstonia solanacearum in the silicon non-accumulator tomato implicates priming effect. Physiol Mol Plant Pathol. 2011;75:83–89. doi: 10.1016/j.pmpp.2010.11.004. [DOI] [Google Scholar]
- Ghosh PP, Mandal D, Laha S, Dasgupta MK. Dynamics and severity model in managing fungal diseases. J Plant Protect Sci. 2009;1:55–59. [Google Scholar]
- Glazebrook J. Contrasting mechanisms of defense against biotrophic and necrotrophic pathogens. Annu Rev Phytopathol. 2005;43:205–227. doi: 10.1146/annurev.phyto.43.040204.135923. [DOI] [PubMed] [Google Scholar]
- Gong H, Zhu X, Chen K, Wang S, Zhang C. Silicon alleviates oxidative damage of wheat plants in pots under drought. Plant Sci. 2005;169:313–321. doi: 10.1016/j.plantsci.2005.02.023. [DOI] [Google Scholar]
- Guil-Guerrero JL, Rebolloso-Fuentes MM. Nutrient composition and antioxidant activity of eight tomato (Lycopersicon esculentum) varieties. J Food Compos Anal. 2009;22:123–129. doi: 10.1016/j.jfca.2008.10.012. [DOI] [Google Scholar]
- Guoqiang W. Effects of silicon supply and Sphaerotheca fuliginea inoculation on resistance of cucumber seedlings against powdery mildew|request PDF. J Appl Ecol. 2004;15:2147–2151. [PubMed] [Google Scholar]
- Hayasaka T, Fujii H, Ishiguro K. The role of silicon in preventing appressorial penetration by the rice blast fungus. Phytopath. 2008;98:1038–1044. doi: 10.1094/PHYTO-98-9-1038. [DOI] [PubMed] [Google Scholar]
- He Y, Xiao H, Wang H, Chen Y, Yu M. Effect of silicon on chilling-induced changes of solutes, antioxidants, and membrane stability in seashore Paspalum turfgrass. Acta Physiol Plant. 2010;32:487–494. doi: 10.1007/s11738-009-0425-x. [DOI] [Google Scholar]
- Heine G, Tikum G, Horst WJ. The effect of silicon on the infection by and spread of Pythium aphanidermatum in single roots of tomato and bitter gourd. J Exp Bot. 2007;58:569–577. doi: 10.1093/jxb/erl232. [DOI] [PubMed] [Google Scholar]
- Huang CH, Roberts PD, Datnoff LE. Silicon suppresses fusarium crown and root rot of tomato. J Phytopathol. 2011;159:546–554. doi: 10.1111/j.1439-0434.2011.01803.x. [DOI] [Google Scholar]
- Ignacimuthu S, Ceasar A. Development of transgenic finger millet (Eleusine coracana (L.) Gaertn.) resistant to leaf blast disease. J Biosci. 2020;37:135–147. doi: 10.1007/s12038-011-9178-y. [DOI] [PubMed] [Google Scholar]
- Iwai T, Miyasaka A, Seo S, Ohashi Y. Contribution of ethylene biosynthesis for resistance to blast fungus infection in young rice plants. Plant Physiol. 2006;142:1202–1215. doi: 10.1104/pp.106.085258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jouili H, Bouazizi H, Ferjani E. Plant peroxidases: biomarkers of metallic stress. Acta Physiol Plant. 2011;33:2075–2083. doi: 10.1007/s11738-011-0780-2. [DOI] [Google Scholar]
- Jung HW, Hwang BK. Isolation, partial sequencing, and expression of pathogenesis-related cDNA genes from pepper leaves infected by Xanthomonas campestris pv. vesicatoria. Mol Plant-Microbe Interact. 2000;13:136–142. doi: 10.1094/MPMI.2000.13.1.136. [DOI] [PubMed] [Google Scholar]
- Jung HW, Tschaplinski TJ, Wang L, Glazebrook J, Greenberg JT. Priming in systemic plant immunity. Science. 2009;80(324):89–91. doi: 10.1126/science.1170025. [DOI] [PubMed] [Google Scholar]
- Kauss H, Seehaus K, Franke R, Gilbert S, Dietrich RA, Kröger N. Silica deposition by a strongly cationic proline-rich protein from systemically resistant cucumber plants. Plant J. 2003;33:87–95. doi: 10.1046/j.1365-313X.2003.01606.x. [DOI] [PubMed] [Google Scholar]
- Khan MA, Butt SJ, Khan KA, Nadeen F, Yousaf B, Javed HU. Morphological and physico-biochemical characterization of various tomato cultivars in a simplified soilless media. Ann Agric Sci. 2017;62:139–143. doi: 10.1016/j.aoas.2017.10.001. [DOI] [Google Scholar]
- Li H, Zhu Y, Hu Y, Han W, Gong H. Beneficial effects of silicon in alleviating salinity stress of tomato seedlings grown under sand culture. Acta Physiol Plant. 2015 doi: 10.1007/s11738-015-1818-7. [DOI] [Google Scholar]
- Li J, Brader G, Palva ET. The WRKY70 transcription factor: a node of convergence for jasmonate-mediated and salicylate-mediated signals in plant defense. Plant Cell. 2004;16:319–331. doi: 10.1105/tpc.016980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li W, Bi Y, Ge Y, Li Y, Wang J, Wang Y. Effects of postharvest sodium silicate treatment on pink rot disease and oxidative stress-antioxidative system in muskmelon fruit. Eur Food Res Technol. 2012;234:137–145. doi: 10.1007/s00217-011-1611-9. [DOI] [Google Scholar]
- Liang Y, Chen Q, Liu Q, Zhang W, Ding R. Exogenous silicon (Si) increases antioxidant enzyme activity and reduces lipid peroxidation in roots of salt-stressed barley (Hordeum vulgare L.) J Plant Physiol. 2003;160:1157–1164. doi: 10.1078/0176-1617-01065. [DOI] [PubMed] [Google Scholar]
- Liang YC, Sun WC, Si J, Römheld V. Effect of foliar- and root-applied silicon on the enhancement of induced resistance to powdery mildew in Cucumis sativus. Plant Pathol. 2005;54:678–685. doi: 10.1111/j.1365-3059.2005.01246.x. [DOI] [Google Scholar]
- Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2 C T method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- Ma JF. Role of silicon in enhancing the resistance of plants to biotic and abiotic stresses. Soil Sci Plant Nutr. 2004;50:11–18. doi: 10.1080/00380768.2004.10408447. [DOI] [Google Scholar]
- Ma JF, Yamaji N. Silicon uptake and accumulation in higher plants. Trends Plant Sci. 2006;11:392–397. doi: 10.1016/j.tplants.2006.06.007. [DOI] [PubMed] [Google Scholar]
- Ma JF, Yamaji N. Functions and transport of silicon in plants. Cell Mol Life Sci. 2008;65:3049–3057. doi: 10.1007/s00018-008-7580-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menke FLH, Kang HG, Chen Z, Jeong MP, Kumar D, Klessig DF. Tobacco transcription factor WRKY1 is phosphorylated by the MAP kinase SIPK and mediates HR-like cell death in tobacco. Mol Plant-Microbe Interact. 2005;18:1027–1034. doi: 10.1094/MPMI-18-1027. [DOI] [PubMed] [Google Scholar]
- Mitani-ueno N, Ma JF. Soil science and plant nutrition linking transport system of silicon with its accumulation in different plant species. Soil Sci Plant Nutr. 2020 doi: 10.1080/00380768.2020.1845972. [DOI] [Google Scholar]
- Mohaghegh P, Khoshgoftarmanesh AH, Shirvani M, Sharifnabi B, Nili N. Effect of silicon nutrition on oxidative stress induced by Phytophthora melonis infection in cucumber. Plant Dis. 2011;95:455–460. doi: 10.1094/PDIS-05-10-0379. [DOI] [PubMed] [Google Scholar]
- Moldes CA, de Lima Filho OF, Merini LJ, Tsai SM, Camiña JM. Occurrence of powdery mildew disease in wheat fertilized with increasing silicon doses: a chemometric analysis of antioxidant response. Acta Physiol Plant. 2016;38:1–9. doi: 10.1007/s11738-016-2217-4. [DOI] [Google Scholar]
- Nakano Y, Asada K. Hydrogen peroxide is scavenged by ascorbate-specific peroxidase in spinach chloroplasts. Plant Cell Physiol. 1981;22:867–880. doi: 10.1093/oxfordjournals.pcp.a076232. [DOI] [Google Scholar]
- Niderman T, Genetet I, Bruyere T, Gees R, Stintzi A, Legrand M, Fritig B, Mosinger E. Pathogenesis-related PR-1 proteins are antifungal. Isolation and characterization of three 14-kilodalton proteins of tomato and of a basic PR-1 of tobacco with inhibitory activity against Phytophthora infestans. Plant Physiol. 1995;108:17–27. doi: 10.1104/pp.108.1.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ouellette S, Goyette MH, Labbé C, Laur J, Gaudreau L, Gosselin A, Dorais M, Deshmukh RK, Bélanger RR. Silicon transporters and effects of silicon amendments in strawberry under high tunnel and field conditions. Front Plant Sci. 2017;8:949. doi: 10.3389/fpls.2017.00949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pandey P, Irulappan V, Bagavathiannan MV, Senthil-Kumar M. Impact of combined abiotic and biotic stresses on plant growth and avenues for crop improvement by exploiting physio-morphological traits. Front Plant Sci. 2017;8:537. doi: 10.3389/fpls.2017.00537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panthee D, Chen F. Genomics of fungal disease resistance in tomato. Curr Genom. 2009;11:30–39. doi: 10.2174/138920210790217927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pereira Domiciano G, Severino Cacique I, Freitas CC, Filippi MCC, da Matta FM, do Vale FXR, Rodrigues FA. Biochemistry and cell biology alterations in gas exchange and oxidative metabolism in rice leaves infected by Pyricularia oryzae are attenuated by silicon. Biochem Cell Biol. 2015;105:738–747. doi: 10.1094/PHYTO-10-14-0280-R. [DOI] [PubMed] [Google Scholar]
- Polanco LR, Rodrigues FA, Nascimento KJT, Cruz MFA, Curvelo CRS, DaMatta FM, Vale FXR. Photosynthetic gas exchange and antioxidative system in common bean plants infected by Colletotrichum lindemuthianum and supplied with silicon. Trop Plant Pathol. 2014;39:35–42. doi: 10.1590/S1982-56762014000100005. [DOI] [Google Scholar]
- Resende RS, Rodrigues FÁ, Cavatte PC, Martins SCV, Moreira WR, Chaves ARM, DaMatta FM. Leaf gas exchange and oxidative stress in sorghum plants supplied with silicon and infected by Colletotrichum sublineolum. Phytopathol. 2012;102:892–898. doi: 10.1094/PHYTO-01-12-0014-R. [DOI] [PubMed] [Google Scholar]
- Resende RS, Rodrigues FA, Costa RV, Silva DD. Silicon and fungicide effects on anthracnose in moderately resistant and susceptible sorghum lines. J Phytopathol. 2012;161:11–17. doi: 10.1111/jph.12020. [DOI] [Google Scholar]
- Reynolds OL, Padula MP, Zeng R, Gurr GM. Silicon: potential to promote direct and indirect effects on plant defense against arthropod pests in agriculture. Front Plant Sci. 2016;7:744. doi: 10.3389/fpls.2016.00744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodrigues FA, Datnoff LE. Silicon and plant diseases, silicon and plant diseases. Berlin: Springer; 2015. pp. 1–5. [Google Scholar]
- Rodrigues FÁ, Datnoff LE, Korndörfer GH, Seebold KW, Rush MC. Effect of silicon and host resistance on sheath blight development in rice. Plant Dis. 2001;85:827–832. doi: 10.1094/pdis.2001.85.8.827. [DOI] [PubMed] [Google Scholar]
- Rodrigues TTMS, Maffia LA, Dhingra OD, Mizubuti ESG. Produção in vitro de con? Dios dev Alternaria solani. Trop Plant Pathol. 2010;35:203–212. doi: 10.1590/S1982-56762010000400001. [DOI] [Google Scholar]
- Samuels AL, Adm G, Menzies JG, Ehret DL. Silicon in cell walls and papillae of Cucumis sativus during infection by Sphaerotheca fuliginea. Physiol Mol Plant Pathol. 1994;44:237–242. doi: 10.1016/S0885-5765(05)80027. [DOI] [Google Scholar]
- Samuels AL, Glass ADM, Ehret DM, Menzies JG. Distribution of silicon in cucumber leaves during infection by powdery mildew fungus. Can J Bot. 1991;69:140–146. doi: 10.1139/b91-020. [DOI] [Google Scholar]
- Seebold KW, Datnoff LE, Correa-Victoria FJ, Kucharek TA, Snyder GH. Effects of silicon and fungicides on the control of leaf and neck blast in upland rice. Plant Dis. 2004;88:253–258. doi: 10.1094/PDIS.2004.88.3.253. [DOI] [PubMed] [Google Scholar]
- Shekari F, Abbasi A, Mustafavi SH. Effect of silicon and selenium on enzymatic changes and productivity of dill in saline condition. J Saudi Soc Agric Sci. 2017;16:367–374. doi: 10.1016/j.jssas.2015.11.006. [DOI] [Google Scholar]
- Shi Y, Zhang Y, Han W, Feng R, Hu Y, Guo J, Gong H. Silicon enhances water stress tolerance by improving root hydraulic conductance in Solanum lycopersicum L. Front Plant Sci. 2016;7:196. doi: 10.3389/fpls.2016.00196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi Y, Zhang Y, Yao H, Wu J, Sun H, Gong H. Silicon improves seed germination and alleviates oxidative stress of bud seedlings in tomato under water deficit stress. Plant Physiol Biochem. 2014;78:27–36. doi: 10.1016/j.plaphy.2014.02.009. [DOI] [PubMed] [Google Scholar]
- Shwethakumari U, Prakash NB. Effect of foliar application of silicic acid on soybean yield and seed quality under field conditions. J Indian Soc Soil Sci. 2018;66:406–414. doi: 10.5958/0974-0228.2018.00051.8. [DOI] [Google Scholar]
- Sies H, Berndt C, Jones DP. Oxidative stress. Annu Rev Biochem. 2017;86:715–748. doi: 10.1146/annurev-biochem-061516-045037. [DOI] [PubMed] [Google Scholar]
- Singh P, Goyal GK. Dietary lycopene: its properties and anticarcinogenic effects. Compr Rev Food Sci Food Saf. 2008;7:255–270. doi: 10.1111/j.1541-4337.2008.00044.x. [DOI] [PubMed] [Google Scholar]
- Song A, Xue G, Cui P, Fan F, Liu H, Yin C, Sun W, Liang Y. The role of silicon in enhancing resistance to bacterial blight of hydroponic- and soil-cultured rice. Sci Rep. 2016;6:1–13. doi: 10.1038/srep24640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sousa RS, Rodrigues FÁ, Schurt DA, Souza NFA, Cruz MFA. Cytological aspects of the infection process of Pyricularia oryzae on leaves of wheat plants supplied with silicon. Trop Plant Pathol. 2013;38:472–477. doi: 10.1590/S1982-56762013000600002. [DOI] [Google Scholar]
- Story EN, Kopec RE, Schwartz SJ, Keith Harris G. An update on the health effects of tomato lycopene. Annu Rev Food Sci Technol. 2010;1:189–210. doi: 10.1146/annurev.food.102308.124120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun H, Duan Y, Mitani-Ueno N, Che J, Jia J, Liu J, Guo J, Ma JF, Gong H. Tomato roots have a functional silicon influx transporter but not a functional silicon efflux transporter. Plant Cell Environ. 2020;43:732–744. doi: 10.1111/pce.13679. [DOI] [PubMed] [Google Scholar]
- Sun W, Zhang J, Fan Q, Xue G, Li Z, Liang Y. Silicon-enhanced resistance to rice blast is attributed to silicon-mediated defence resistance and its role as physical barrier. Eur J Plant Pathol. 2010;128:39–49. doi: 10.1007/s10658-010-9625-x. [DOI] [Google Scholar]
- Thannickal VJ, Fanburg BL. Reactive oxygen species in cell signaling. Am J Physiol Lung Cell Mol Physiol. 2000 doi: 10.1152/ajplung.2000.279.6.l1005. [DOI] [PubMed] [Google Scholar]
- Thines B, Katsir L, Melotto M, Niu Y, Mandaokar A, Liu G, Nomura K, He SY, Howe GA, Browse J. JAZ repressor proteins are targets of the SCFCOI1 complex during jasmonate signalling. Nature. 2007;448:661–665. doi: 10.1038/nature05960. [DOI] [PubMed] [Google Scholar]
- Thirthamallappa LHC. Genetics of resistance to early blight (Alternaria solani Sorauer) in tomato (Lycopersicon esculentum L.) Euphytica. 2000;113:187–193. doi: 10.1023/A:1003929303632. [DOI] [Google Scholar]
- Torres MA, Jones JDG, Dangl JL. Reactive oxygen species signaling in response to pathogens. Plant Physiol. 2006;148:373–378. doi: 10.1104/pp.106.079467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tripathi DK, Singh VP, Prasad SM, Chauhan DK, Dubey NK. Silicon nanoparticles (SiNp) alleviate chromium (VI) phytotoxicity in Pisum sativum (L.) seedlings. Plant Physiol Biochem. 2015;96:189–198. doi: 10.1016/j.plaphy.2015.07.026. [DOI] [PubMed] [Google Scholar]
- Tripathy BC, Oelmüller R. Reactive oxygen species generation and signaling in plants. Plant Signal Behav. 2012;7:16–21. doi: 10.4161/psb.22455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tubana BS, Babu T, Datnoff LE. A review of silicon in soils and plants and its role in US agriculture. Soil Sci. 2016;181:1. doi: 10.1097/SS.0000000000000179. [DOI] [Google Scholar]
- Tubaña BS, Heckman JR. Silicon in soils and plants. Cham: Springer; 2015. [Google Scholar]
- Van Bockhaven J, De Vleesschauwer D, Höfte M. Towards establishing broad-spectrum disease resistance in plants: silicon leads the way. J Exp Bot. 2013;64:1281–1293. doi: 10.1093/jxb/ers329. [DOI] [PubMed] [Google Scholar]
- Van Bockhaven J, Steppe K, Bauweraerts I, Kikuchi S, Asano T, Höfte M, De Vleesschauwer D. Primary metabolism plays a central role in moulding silicon-inducible brown spot resistance in rice. Mol Plant Pathol. 2015;16:811–824. doi: 10.1111/mpp.12236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vivancos J, Labbé C, Menzies JG, Bélanger RR. Silicon-mediated resistance of Arabidopsis against powdery mildew involves mechanisms other than the salicylic acid (SA)-dependent defence pathway. Mol Plant Pathol. 2015;16:572–582. doi: 10.1111/mpp.12213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiese J, Wiese H, Schwartz J, Schubert S. Osmotic stress and silicon act additively in enhancing pathogen resistance in barley against barley powdery mildew. J Plant Nutr Soil Sci. 2005;168:269–274. doi: 10.1002/jpln.200420490. [DOI] [Google Scholar]
- Ye M, Song Y, Long J, Wang R, Baerson SR, Pan Z, Zhu-Salzman K, Xie J, Cai K, Luo S, Zeng R. Priming of jasmonate-mediated antiherbivore defense responses in rice by silicon. Proc Natl Acad Sci USA. 2013;38:110. doi: 10.1073/pnas.1305848110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang GL, Dai QG, Zhang HC. Silicon application enhances resistance to sheath blight (Rhizoctonia solani) in rice. J Plant Physiol Mol Biol. 2006;32:600–606. [PubMed] [Google Scholar]
- Zhang H, Zhang D, Chen J, Yang Y, Huang Z, Huang D, Wang XC, Huang R. Tomato stress-responsive factor TSRF1 interacts with ethylene responsive element GCC box and regulates pathogen resistance to Ralstonia solanacearum. Plant Mol Biol. 2004;55:825–834. doi: 10.1007/s11103-004-2140-8. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Shi Y, Gong H, et al. Beneficial effects of silicon on photosynthesis of tomato seedlings under water stress. J Integr Agric. 2018;17:2151–2159. doi: 10.1016/S2095-3119(18)62038-6. [DOI] [Google Scholar]
- Zhu Z, Wei G, Li J, Qian Q, Yu J. Silicon alleviates salt stress and increases antioxidant enzymes activity in leaves of salt-stressed cucumber (Cucumis sativus L.) Plant Sci. 2004;167:527–533. doi: 10.1016/j.plantsci.2004.04.020. [DOI] [Google Scholar]