Abstract
Aerobic exercise (AE) has recently received increasing attention in the prevention of Alzheimer’s disease (AD). There is some evidence that it can improve neurocognitive function in elderly individuals. However, the mechanism of these improvements is not completely understood. In this prospective clinical trial, thirty amnestic mild cognitive impairment participants were enrolled into two groups and underwent 12 months of intervention. One group (n = 15) performed AE training (8M/7F, age = 66.4 years), whereas the other (n = 15) performed stretch training (8M/7F, age = 66.1 years) as a control intervention. Both groups performed 25–30 minutes training, 3 times per week. Frequency and duration were gradually increased over time. Twelve-month AE training improved cardiorespiratory fitness (p = 0.04) and memory function (p = 0.004). Cerebral blood flow (CBF) was measured at pre- and post-training using pseudo-continuous-arterial-spin-labeling MRI. Relative to the stretch group, the AE group displayed a training-related increase in CBF in the anterior cingulate cortex (p = 0.016). Furthermore, across individuals, the extent of memory improvement was associated with CBF increases in anterior cingulate cortex and adjacent prefrontal cortex (voxel-wise p < 0.05). In contrast, AE resulted in a decrease in CBF of the posterior cingulate cortex, when compared to the stretch group (p = 0.01). These results suggest that salutary effects of AE in AD may be mediated by redistribution of blood flow and neural activity in AD-sensitive regions of brain.
Keywords: Aerobic exercise training, Alzheimer’s disease, amnestic mild cognitive impairment, anterior cingulate cortex, cerebral blood flow, posterior cingulate cortex, stretch training
INTRODUCTION
Amnestic mild cognitive impairment (aMCI) is a precursor stage to the development of Alzheimer’s disease (AD). Clinical trials based on anti-amyloid strategies to prevent the decline from aMCI to AD have largely failed [1] and have led many investigators to search for other alternatives. Aerobic exercise (AE) is a low-cost, potentially effective approach in AD prevention.
AE has been shown to improve cardiorespiratory fitness and neurocognitive function in elderly with [2, 3] and without [4-6] aMCI. However, the mechanism associated with these benefits is not fully understood. Several cross-sectional studies have been conducted to investigate the relationship between brain structure/function and fitness [7-9]. For example, Colcombe et al. showed that brain tissue density was better preserved in high-fit compared to low-fit elderly [10]. Studies have also demonstrated an association between white matter structural integrity and AE [8]. Greater brain functional activity to a spatial processing task was observed in the prefrontal and parietal cortices in highly fit compared to less fit elderly [11]. Researchers have also begun to investigate the effect of short-term (e.g., several weeks to months) AE on the brain [12-14]. Pereira et al. showed that three-month exercise promoted neurogenesis in the dentate gyrus of the hippocampal formation in mice and humans [12]. However, van der Kleij et al. showed that sixteen-week AE did not have an effect on cerebral blood flow (CBF) in mild to moderate AD patients [13]. A plausible reason for the discrepancy in the literature is that the intervention period in most prior studies was limited in length, due to potential challenges in participant retention and adherence to the protocol, as well as the high costs. In particular, to our best knowledge, no studies have investigated brain perfusion changes in aMCI participants following a longer period (12 months) of AE.
The purpose of the present study is to assess AE-related improvement in terms of brain perfusion in aMCI. We measured resting CBF before and after 12 months of AE training using a non-invasive Pseudo-Continuous-Arterial-Spin-Labeling (pCASL) MRI technique. We hypothesized that 12 months of AE will yield meaningful changes in brain perfusion associated with improvement in neurocognitive function. To evaluate if the observed brain changes are specific to AE, we recruited an aMCI group of participants that only performed stretch training for 12 months. Regional CBF results were compared between two time-points and across groups. Changes in CBF were compared to changes in cognitive function. Finally, to confirm cross-sectional CBF differences between aMCI and cognitively normal elderly individuals, a group of healthy age-matched control participants were recruited, who received pCASL MRI at the initial time point but did not undergo longitudinal follow-up. The aims of the present study were to assess if 12 months of AE training would yield improvement in brain perfusion in aMCI.
METHODS
Participants
This is a prospective study registered as a clinical trial: NCT01146717, Aerobic Exercise Training in Mild Cognitive Impairment Study (AETMCI), URL: https://www.nia.nih.gov/alzheimers/clinical-trials/aerobic-exercise-training-mild-cognitive-impairment. Recruitment was conducted between June 2010 and June 2014, and data collection was completed by September 2016. This study was approved by the Institutional Review Board of the University of Texas Southwestern Medical Center and Texas Health Presbyterian Hospital Dallas, and was performed in accordance with the guidelines of the Declaration of Helsinki and Belmont Report. All participants gave informed written consent before being enrolled into the study. There are no conflicts of interest to report. Participants in this study are a subgroup of participants from the study performed by Tarumi et al. [15].
Recruitment of aMCI participants
Recruitment was conducted in the Dallas-Fort Worth metropolitan area using community-based advertisements and through the University of Texas Southwestern Medical Center Alzheimer’s Disease Center. A telephone interview was first conducted to identify potential aMCI participants who 1) had memory concerns, 2) did not exercise regularly, and 3) were aged 55–80 years. These individuals were invited to visit the clinic for further screening.
Diagnosis of aMCI
The diagnosis of aMCI was based on Petersen criteria [16], as modified by the Alzheimer’s Disease Neuroimaging Initiative project (http://adni-info.org). Specifically, aMCI participants met the following criteria: a global Clinical Dementia Rating scale of 0.5, with a score of 0.5 in the memory category, in addition to memory impairment as indicated by education-adjusted scores on the Logical Memory (LM) subtest of the Wechsler Memory Scale-Revised, and a Mini-Mental State Exam (MMSE) score between 24 and 30.
Neuropsychological function
The LM subtest of the Wechsler Memory Scale-Revised (WMS-R) is a commonly used test of verbal episodic memory. During this test, participants were orally presented with a paragraph of conceptually organized information in a story format. Subjects are asked to recall as much information as possible immediately after the paragraph is read to them, and then again following a 20 min delay.
The Delis Kaplan executive function system (D-KEFS) comprises verbal and nonverbal executive function measures with each of the tests designed to stand alone. The Color-Word Interference Test primarily measures the ability to inhibit an overlearned verbal response (i.e., reading the printed words) to generate the conflicting response of naming the dissonant ink colors in which the words are printed. Also, an inhibition/switching condition evaluates both inhibition and cognitive flexibility.
The California verbal learning test (CVLT) is a verbal 16-item list-learning task that serves as a measure of verbal learning and episodic memory. The standardized total score, which reflects the number of words learned across the 5 learning trials, are reported (Table 2).
Table 2.
Fitness and cognitive results before and after intervention for stretch and exercise groups (mean ± S.D.)
| Stretch | p | Exercise | p | |||
|---|---|---|---|---|---|---|
| Pre | Post | Pre | Post | |||
| VO2 max (ml/kg/min) | 25.5 ± 5.9 | 24.7 ± 5.9 | 0.68 | 23.5 ± 6.2 | 25.6 ± 7.1 | 0.04 |
| Logical Memory delayed recall | 8.9 ± 2.2 | 11.1 ± 4.2 | 0.09 | 9.4 ± 3.1 | 13.8 ± 2.6 | 0.004 |
| Delis Kaplan executive function system (DKEFS) | ||||||
| Word interference test - Color naming | 10.9 ± 1.9 | 11.1 ± 2.4 | 0.66 | 8.9 ± 2.5 | 10.5 ± 1.6 | 0.007 |
| Word Interference test - inhibition | 11.1 ± 2.1 | 12.0 ± 1.8 | 0.22 | 10.6 ± 1.5 | 11.3 ± 1.5 | 0.02 |
| California verbal learning test – total t-score | 45.2 ± 11.9 | 46.1 ± 9.8 | 0.66 | 46.6 ± 9.48 | 52.6 ± 10.6 | 0.08 |
| Boston naming test | 27.9 ± 2.0 | 27.6 ± 2.06 | 0.65 | 27.9 ± 1.5 | 27.6 ± 2.3 | 0.56 |
| Clock drawing test 1 | 12.4 ± 1.3 | 12.8 ± 1.3 | 0.37 | 12.8 ± 1.3 | 13.5 0.8 | 0.56 |
p, Two tailed paired T-test comparison of Pre and Post intervention data.
The Boston Naming test is comprised of 60 line drawings of objects of graded difficulty, ranging from common objects (e.g., a tree) to less familiar objects such as an abacus. Items are presented one at a time, and the participant is required to say the name of each pictured object. If the participant fails to provide the correct response within 20 s, the examiner provides a categorical or phonemic cue, and responses are recorded.
The Clock drawing test requires that the participant draw the face of a clock and then draw the hands to indicate a particular time. This single test may be sensitive to dementia because it involves many cognitive areas that can be affected by dementia, including executive function, visuospatial abilities, motor programming, attention, and concentration.
Exclusion criteria
Participants were excluded if they had any of the following condition(s): diagnosis of AD or other type of dementia, major neurological, vascular, or psychiatric disorders including depression, history of a clinical diagnosis of B12 deficiency or hypothyroidism (stable treatment for at least 3 months is allowed), or chronic inflammatory diseases including lupus, rheumatoid arthritis, and polymyalgia rheumatica. In addition, participation in regular exercise within the last 2 years, body mass index ≥35 kg/m2, sleep disorders including clinically diagnosed or self-reported sleep apnea, uncontrolled hypertension, diabetes, and/or a history of smoking within the past 2 years were considered as meeting the exclusion criteria. Prior to being enrolled into the study, each aMCI participant wore an accelerometer for 1 week (Actical, Philips Respironics, USA), and those who spent >90 min of moderate-to-vigorous physical activity (>4.0 metabolic equivalents, METs) per week were also excluded. For a list of all exclusion criteria, please refer to clinical trial: NCT01146717.
Study protocol
Figure 1 shows a flowchart of patient screening, attrition, and enrollment. Of the final cohort enrolled into the trial, each aMCI participant was randomly assigned to one of the two study arms, aerobic exercise versus stretching and toning group, using randomization and blinding procedures. Clinical, neuropsychological, cardiorespiratory and imaging measures were collected before and after the 12-month training period. Relevant to this study, usable CBF data were available from 30 aMCI participants, with 15 in the AE group and 15 in the stretch training group (Fig. 1). Unless otherwise specified, further reports of the data are based on this cohort of participants. Participants in this study are a subgroup of participants reported in the study by Tarumi et al. [15].
Fig. 1.
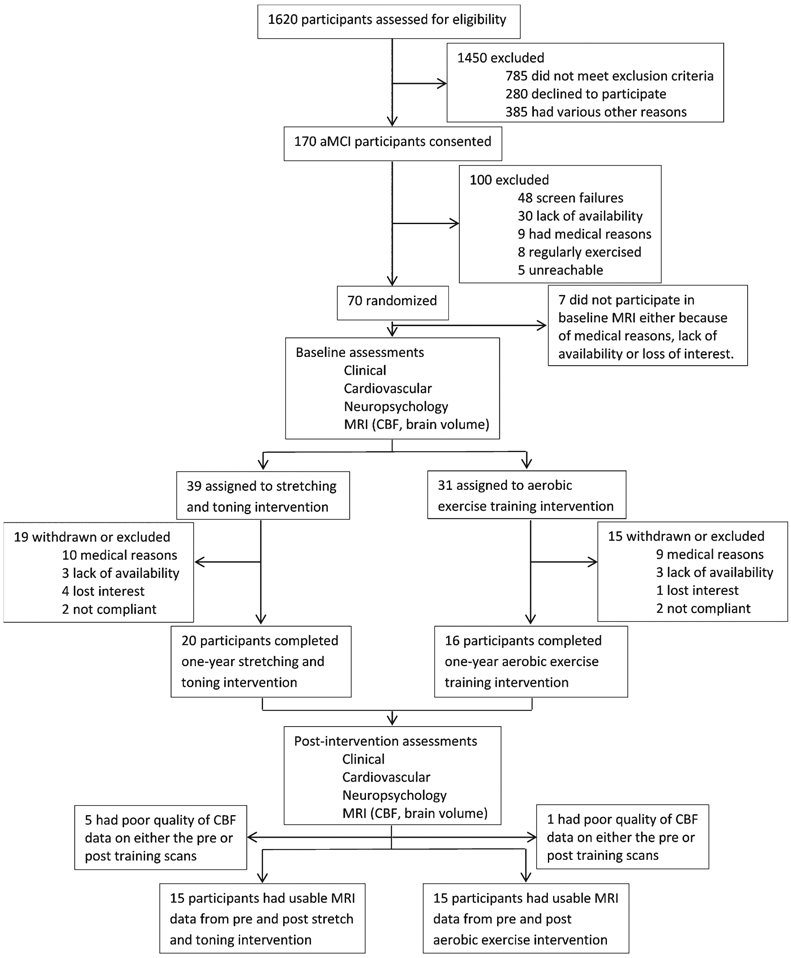
Flowchart of participant screening, exclusion, and enrollment numbers.
Recruitment of healthy age-matched cognitively normal participants
Additionally, for cross-sectional comparison, 20 healthy age-matched cognitively normal (CN) participants were recruited.
Randomization and blinding
A randomized, single-blind, placebo-controlled trial design (Aerobic exercise versus stretching and toning groups) was used in this study. SAS V9.2 was used to generate the stratified, randomization lists using a blocking factor of 4. Patients were stratified by age (55–70 and 71–80) and sex (men and women). Investigators conducting the analysis were blinded to treatment assignment. Participants were instructed to maintain their normal daily activities aside from the assigned interventions and were instructed not to disclose their group assignment or to discuss their interventions during outcome measurements or meeting with other participants.
Inclusion criteria for cognitively normal participants
For CN participants, the criteria were: age 55–80 years, did not exercise regularly, a global CDR scale of 0 with a score of 0 in the memory category, intact memory function as indicated by education-adjusted scores on the LM subtest of the Wechsler Memory Scale Revised, and a MMSE score between 26 and 30.
Aerobic exercise training regimen
The AE group was instructed to perform moderate to vigorous AE. The dose and intensity of the AE was based on each individual’s fitness level, assessed by the maximal oxygen uptake (VO2 max) measured during a treadmill test [17]. Dose and intensity were progressively increased as participants adapted to previous workloads. Specifically, the program started with a frequency of 3 exercise sessions per week for 25–30 min per session at the intensity of 75–85% of maximal heart rate that was measured during the VO2 max test at baseline. At week 11, participants started alternating between 3 and 4 exercise sessions per week for 30–35 min per session. During the weeks in which they performed 3 exercise sessions per week, a high intensity exercise session was introduced which consisted of 30 min of walking at the intensity of 85–90% of maximal heart rate (e.g., brisk uphill walking). After week 26, participants performed 4–5 exercise sessions per week for 30–40 min, including two high intensity sessions. Each exercise session included a 5 min warm-up and a 5 min cool-down. Any mode of aerobic exercise was allowed as long as they maintained the prescribed training dose and intensity, as monitored by changes in heart rate during each of the exercise sessions. All participants were provided with a heart rate monitor (Polar RS400, Polar 201 Electro, USA). This AE program meets the national physical activity guidelines for older adults [18] and has been used in our previous studies that showed significant improvement of cardiorespiratory fitness in sedentary individuals older than 65 years of age [19].
Stretch training regimen
Stretch training was used as active control to keep participants engaged with the same level of attention received from the investigators as those in the AE group. The stretch group performed a stretch and balance routine that focused on the upper and lower body. Participants were trained to maintain their heart rate below 50% of maximal heart rate during each session. The frequency and duration of the Stretch training program was the same as the AE program. At week 19, we introduced a second set of full body stretches that are more advanced than the previous set. At week 26, we introduced a set of low resistance theraband exercises that focused on strengthening the upper and lower body.
In both the AE and Stretch training programs, each participant was trained and supervised by a research assistant with a background in exercise physiology. Training for the first several weeks occurred at the Presbyterian Hospital in Dallas where adverse events can be safely handled, until they could comfortably exercise by themselves at home. AE and stretch training was performed at the convenience of the participants and not in groups. There was no social aspect to the training; however, this was not specifically controlled for. During the study period, they were asked to perform assigned intervention on top of their regular physical activities.
Assessment of adherence to intervention
To ensure adherence to each program, participants were required to make a training log in addition to heart rate monitoring. Each month, participants visited the clinic to download heart rate data and review their training log together with an exercise physiologist to ensure implementation of the prescribed training programs. When adherence to training programs was not met with the prescribed intensity, duration, and frequencies, in-person and/or telephone meetings were held to solve the issues and encourage participants to continue the program. The total amount of exercise performed by the AE group over 12 months was calculated by the training impulse (TRIMP) score, which is calculated by multiplying the duration of exercise session by the average heart rate achieved during each session weighted for exercise intensity [20].
Adverse events
During the study, 10 adverse events occurred. During VO2 max testing, 4 had arrhythmia, 1 had foot pain, and 1 had pain in the mouth caused by wearing the mouthpiece. During aerobic exercise training, 1 fell from the treadmill, 2 had ankle pain, and 1 had knee pain. The number of participants who experienced adverse events was not different between the Stretch and AE groups.
MRI experiment
MRI experiments were performed on a 3 Tesla MRI scanner using an 8-channel head coil (Philips Healthcare, Best, Netherlands). All participants were requested to refrain from consuming caffeine and alcohol for 8 h prior to the MRI scans. A body coil was used for RF transmission. Foam padding was placed around the head to minimize motion during MRI scan acquisition. The MRI protocol consisted, among other sequences, of a T1-weighted magnetization-prepared rapid acquisition of gradient echo sequence (T1-MPRAGE) and a pCASL sequence [21]. The scan parameters of the T1-MPRAGE sequence were as follows; TR/TE/TI = 8.1/3.7/1100 ms, shot interval 2100 ms, flip angle = 12°, voxel size 1 × 1 × 1 mm3, number of slices 160, sagittal slice orientation and duration 3 min 57 s. PCASL MRI is the recommended method in clinic by the ISMRM perfusion study group and the European consortium for ASL in dementia [22]. Scan parameters of the pCASL sequence were: field of view (FOV) = 240 × 240 mm2, matrix = 80 × 80, 29 axial slices, thickness = 5 mm, TR/TE = 4260/14 ms, labeling duration = 1.65 s, postlabeling delay = 1.525 s, single-shot echo-planar-imaging (EPI), 40 pairs of label and control images, 2D readout without background suppression, duration 6 min. The post-labeling delay was slightly shorter than that recommended in the “ASL whitepaper” [22] as the study started in 2010, 5 years before the white paper was published.
CBF and brain volume quantification
CBF maps were generated from the pCASL MRI images using the Johns Hopkins University’s cloud based ASL analysis software, ASL-MRICloud (https://braingps.mricloud.org/asl) [23]. ASL scripts on the cloud server were written in Matlab 2013 and SPM12. The analysis procedure followed recommendations in the ASL white paper [22].
The ASL-MRICloud data processing used the following procedures. Motion correction was performed and the difference between control and label image pairs (control – label) was calculated. Quantification of CBF in physiological units (ml/100 g/min) was based on a kinetic model [22]:
| (1) |
where SASL is the signal difference between the control and label images from the pCASL acquisition; λ is the brain/blood partition coefficient, assumed to be 0.9ml/g; w is the post-labeling delay time (1525 msec); T1blood is the longitudinal relaxation time of blood and was set at 1650 ms; α is the labeling efficiency, assumed to be 0.85; M0 reflects the signal intensity of spins at equilibrium magnetization and was estimated from the control image after accounting for the T1 correction; and τ is the label duration (1650 ms). The CBF map was co-registered to the T1-weighted MPRAGE image by means of a 12-parameter affine transformation.
The T1 data were then normalized to Montreal Neurologic Institute (MNI) template, in which 19 brain atlases were used to transform the individual image to the template and the warped images were automatically segmented into 289 brain regions [24]. Use of multiple atlases reduces errors produced by individual atlas-based image registration. Brain volumes were then extracted from the brain regions of interest mentioned below.
The region-of-interest (ROI) analysis focused on seven major brain cortical regions: occipital, temporal, parietal, and frontal lobe, anterior cingulate cortex (ACC), posterior cingulate cortex (PCC), and the hippocampus. We focused on ACC and PCC because previous literature has suggested potential effect of exercise on these structures [4, 5, 25]. The hippocampus was included because of its key role in episodic memory [26], aMCI, and AD [27, 28]. The spatial transformation was also applied to the CBF map to normalize it to the MNI space, which allowed voxel-wise analysis. The ROI and voxel-wise comparisons of CBF used relative values (r.v.) (each voxel normalized against whole-brain value). Use of relative CBF values has been shown to be more sensitive in detecting regional differences when whole-brain CBF does not manifest a difference (verified in our data) [29].
Cardiovascular assessment
Participants underwent cardiovascular assessments at baseline and after completion of the 12 months of AE or stretch training. Maximal oxygen uptake (VO2 max) test was performed to assess cardiovascular fitness. VO2 max test measures the maximum amount of oxygen a person can utilize during intense exercise. It is an index of an individual’s fitness level. VO2 max was assessed using a modified Astrand-Saltin protocol on a treadmill [17]. The treadmill grade was increased by 2% every 2 min during testing until exhaustion while participants walked or jogged at a fixed speed. VO2 was measured during the second minute of each stage using the Douglas bag method. Also, the breath-by-breath VO2, VCO2, respiratory exchange ratio (RER), and ventilation were continuously monitored using an online computer system. Gas fractions were analyzed by mass spectrometry (Marquette MGA 1100) and ventilatory volume was measured by a Tissot spirometer. Blood pressure, 12-lead electrocardiogram, and heart rate were monitored continuously during exercise testing to assess cardiovascular responses. The peak VO2 was defined as the highest VO2 measured from a > 30 s Douglas bag during the last stage of testing. The criteria to confirm that peak VO2 was achieved included an increase in VO2 < 150 ml despite increasing work rate of 2% grade, a RER > 1.1, and heart rate < 5 beats/min of age-predicted maximal values. In all cases, at least two of these criteria were achieved, confirming the identification of peak VO2 based on the American College of Sports Medicine guidelines [18]. Our previous studies show that by using these methods, peak VO2 can be measured reliably in sedentary older adults [19].
Amyloid-PET image acquisition and processing
In a sub-set of the participants (9 aMCI participants in the aerobic exercise group and 6 aMCI participants in the stretch group, out of the 30 participants), we performed amyloid PET imaging to confirm their amyloid positivity. PET scanning was added to the study protocol a year after trial initiation upon obtaining supplemental funding. All participants on whom we acquired amyloid PET data were part of the 30 participants on whom we acquired ASL data. Amyloid PET (Siemens ECAT HR scanner) was performed with an intravenous bolus injection of 10-mCi 18F-florbetapir [30]. At 30 min post-injection, participants were positioned on an imaging table with the head being secured with Velcro straps and foam wedges to minimize movements. At 50 min post-injection, 2 frames of 5 min PET emission scan and a 7 min transmission scan were acquired in 3-dimensional mode using the following parameters: matrix size = 128 × 128, resolution = 5 × 5 mm, slice thickness = 2.42 mm, and field of view = 58.3 cm.
Each participant’s PET image was spatially normalized to a florbetapir uptake template (2 × 2 × 2 mm3 voxels) using SPM8 (Wellcome Department of Cognitive Neurology, London, UK) and in-house MATLAB (Mathworks Inc., Sherborn, MA) scripts, and visually inspected for registration quality. Standardized uptake value ratio (SUVR) was calculated using the mean cerebellar uptake as a reference [31]. The mean cortical SUVR was calculated as an average of the posterior and anterior cingulate, precuneus, temporal, dorsolateral prefrontal, orbital frontal, parietal, and occipital SUVRs [32]. A threshold of mean cortical SUVR of 1.06 was used to determine amyloid positivity [33].
Statistical analysis
Univariate analysis of pre- and post-intervention data was conducted using a paired t-test and between-group comparison was performed using a two-sample t-test. Repeated measures ANOVA were performed to assess the group-by-time interaction effect in pre- and post-intervention between AE and stretch training groups. A p value of 0.05 or less was considered significant. Voxel-wise correlation of CBF change with memory change was performed using regression analysis in SPM. Voxels with a Family-Wise-Error (FWE) corrected threshold of p < 0.05 are considered significant.
RESULTS
Characteristics of aMCI participants at baseline
Demographic, cardiorespiratory, and neuropsychological characteristics of the aMCI participants in the exercise and stretch groups were not different prior to starting the training intervention. Participant age, gender, education, measurement of body fat using body mass index, cognitive function measured with the MMSE and Clinical Dementia Rating are displayed in Table 1. Amyloid PET data revealed that all of the MCI participants (exercise and stretch groups) tested for amyloid were amyloid-positive. This confirms that the MCI participants recruited were likely of impairment due to AD pathology [34]. There was not a difference in mean cortical SUVR between the two groups at baseline (1.17 ± 0.05 in exercise group versus 1.27 ± 0.15 in stretch group, mean ± S.D., p = 0.22, Cohen’s d effect size, d = 0.89, T = 1.38).
Table 1.
Baseline (Pre-training) characteristics of amnestic mild cognitive impairment participants in the exercise and stretch groups (mean ± S.D.)
| Stretch | Exercise | p | |
|---|---|---|---|
| Age (y) | 66.1 ± 7.2 | 66.4 ± 6.6 | 0.92 |
| Gender (M/F) | 8/7 | 8/7 | - |
| Education (y) | 15.7 ± 2.0 | 16.5 ± 1.9 | 0.34 |
| Body mass index | 27.6 ± 4.3 | 26.3 ± 4.4 | 0.41 |
| Mini-Mental State Exam | 29.2 ± 1.0 | 29.2 ± 0.9 | >0.99 |
| Clinical Dementia Rating | 0.5 ± 0.0 | 0.5 ± 0.0 | >0.99 |
Effect of aerobic exercise on fitness level
VO2 max was found to be similar (p = 0.4) in the exercise and stretch groups before training. However, the exercise group showed a significant (p = 0.04, d = 0.32, T = 2.26)) increase in VO2 max when comparing post-training to pre-training, while the stretch group did not show a difference (p = 0.68) (Table 2). Repeated measures ANOVA revealed a group-by-time interaction effect (p = 0.035, partial eta squared effect size = 0.16, F = 4.93) on VO2 max. These data demonstrate that 12-month AE training can significantly improve fitness level in aMCI. The average compliance to exercise training programs was 69%, which is calculated by the ratio of prescribed exercise sessions over the actually completed exercise sessions in which participants achieved the target heart rate. The TRIMP scores showed substantial individual variability and were not correlated with changes in VO2 max. TRIMP scores are reported in the supplementary material of our earlier paper published by Tarumi et al. on this randomized controlled trial [15].
Effect of aerobic exercise on memory in aMCI
LM scores in the AE group improved significantly (p = 0.004, d = 1.54, T = 3.71) from pre-training levels, whereas the stretch group did not show a significant change (p = 0.09) (Table 2). LM was chosen as the primary measure of episodic memory for this project because of its brevity and widespread use in the diagnosis and characterization of patients with MCI.
Effect of aerobic exercise on brain perfusion
All CBF data reported in this paper are relative values, relative to mean whole-brain CBF. We report a significant (p = 0.016, partial eta squared effect size = 0.19, F = 6.62) group-by-time interaction effect in CBF in the ACC, wherein the AE group showed a training-related increase in CBF relative to the stretch group. When comparing pre- and post-training ACC CBF values within each group, the exercise group showed an increase in post-training ACC CBF compared to pre-training (Post-Pre CBF change was 0.061 ± 0.160 (5.9%), mean ± S.D.), while the stretch group showed a decrease in CBF (Post-Pre CBF change = −0.108 ± 0.187 (9.9%)) (Table 3).
Table 3.
rCBF in regions of interest before and after intervention for stretch and exercise groups (mean ± S.D.)
| Stretch | Exercise | |||||
|---|---|---|---|---|---|---|
| Pre | Post | p | Pre | Post | P | |
| ACC | 1.09 ± 0.17 | 0.98 ± 0.19 | 0.049 | 1.04 ± 0.15 | 1.10 ± 0.17 | 0.17 |
| PCC | 1.53 ± 0.11 | 1.57 ± 0.11 | 0.34 | 1.56 ± 0.08 | 1.47 ± 0.12 | 0.007 |
| Hippocampus | 1.50 ± 0.29 | 1.52 ± 0.25 | 0.83 | 1.44 ± 0.20 | 1.56 ± 0.21 | 0.045 |
| Frontal lobe | 1.13 ± 0.11 | 1.06 ± 0.11 | 0.049 | 1.13 ± 0.12 | 1.16 ± 0.10 | 0.59 |
| Parietal lobe | 1.22 ± 0.15 | 1.20 ± 0.13 | 0.72 | 1.22 ± 0.12 | 1.20 ± 0.13 | 0.54 |
| Temporal lobe | 1.19 ± 0.10 | 1.19 ± 0.09 | 0.95 | 1.15 ± 0.09 | 1.17 ± 0.10 | 0.37 |
| Occipital lobe | 1.36 ± 0.12 | 1.41 ± 0.19 | 0.38 | 1.35 ± 0.10 | 1.36 ± 0.11 | 0.85 |
p, Two tailed paired T-test comparison of Pre and Post intervention data. rCBF, regional cerebral blood flow; ACC, anterior cingulate cortex; PCC, posterior cingulate cortex.
We also report a significant (p = 0.01, partial eta squared effect size = 0.21, F = 7.63) group-by-time interaction effect in CBF in the PCC, but the direction of the effect was opposite to that in ACC. The AE group showed a training-related decrease in PCC CBF relative to the stretch group. Specifically, the exercise group showed Post-Pre PCC CBF change of −0.098 ± 0.116 (6.3%), whereas the stretch group showed a Post-Pre PCC CBF change of 0.038 ± 0.145 (2.5%). Supplementary Figure 1 displays the group-by-time interaction effect in CBF in the ACC and PCC.
We also report a significant (p = 0.045, d = 0.58, T = 2.20) increase in CBF in the hippocampus in the AE group after one year, which was not observed in the Stretch group (Table 3). The AE group showed a hippocampal CBF increase of 0.122 ± 0.21 (8.5%). However, we did not observe a group-by-time interaction effect in CBF in the hippocampus.
The other ROIs (occipital, temporal, parietal, frontal, and hippocampus) did not show significant differences (based on the group-by-time interaction effect) between exercise and stretch groups.
Relationship between CBF and memory function in aMCI
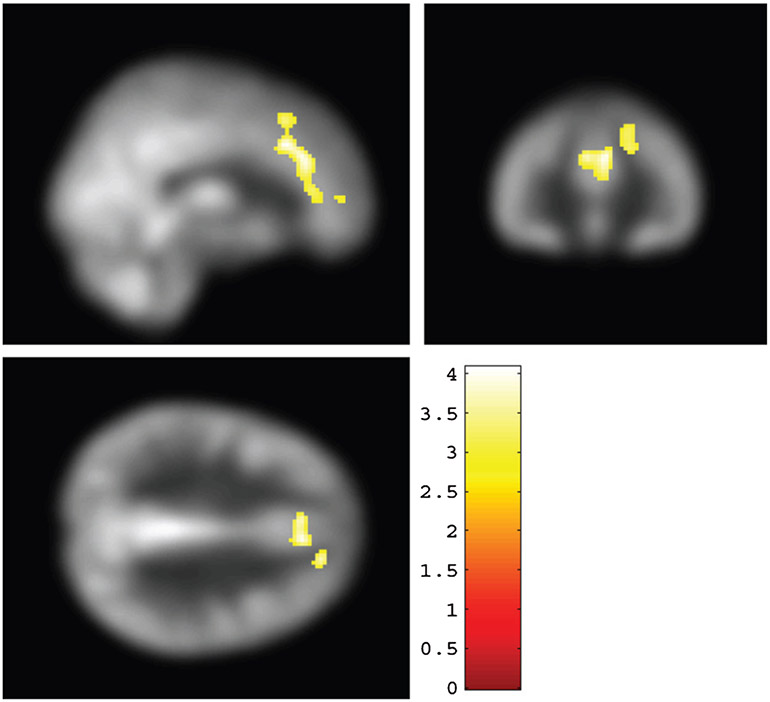
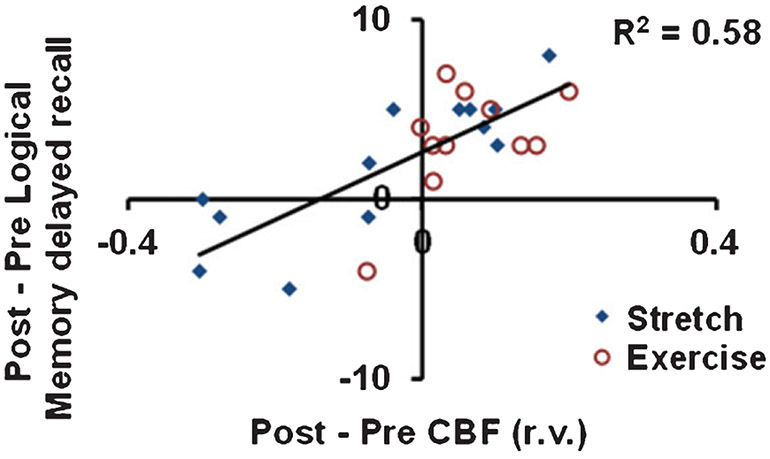
In order to explore the relationship between episodic memory function and CBF, we conducted a voxel-wise analysis between Post-Pre changes in LM and Post-Pre changes in CBF across aMCI participants. aMCI participants from both AE and stretch groups were included in this analysis. Figure 2 shows brain areas where CBF increases were significantly associated with improvements in LM delayed recall scores (family-wise-error corrected, FWE, p < 0.05). These areas included ACC (BA 32), cingulate gyrus, middle, medial, and superior frontal gyrus. All regions, coordinates, and T-values are listed in Supplementary Table 1. Figure 3 shows a scatter plot between CBF and LM delayed recall score changes in these regions.
Fig. 2.
Voxel-wise regression results between changes in Logical Memory (LM) delayed recall scores and changes in cerebral blood flow (CBF) before and after training. Improvement in LM delayed recall scores positively correlated with an increase in CBF in anterior cingulate cortex and medial frontal gyrus (BA6) (colored voxels). The significant voxels (family-wise-error corrected p < 0.05) were overlaid on CBF map in the MNI template space.
Fig. 3.
Scatter plot between changes in cerebral blood flow (CBF, post-intervention minus pre-intervention) relative values (r.v.) and changes in Logical Memory delayed recall score in the clusters depicted in Fig. 2. Red circles indicate aerobic exercise participants. Blue diamonds indicate stretching participants.
Cross-sectional comparison of CBF between aMCI and elderly controls
To assess cross-sectional CBF differences between aMCI and CN elderly control participants, we investigated CBF abnormalities in the aMCI participants (combining both exercise and stretch groups at their pre-training time-point) relative to age-matched CN elderly. Of the seven regions mentioned above, PCC CBF (1.530 ± 0.145, N = 63), but not ACC, in aMCI participants were found to be significantly lower by 4.6% (p = 0.05, d = 0.52, T = 2.03) than that in CN (1.603 ± 0.136, N = 20). Relative CBF from all seven ROIs from aMCI and CN and the statistical significance of comparison between groups is provided in Supplementary Table 3. PCC CBF was not different (p = 0.366, d = 0.35, T = 0.92) between aMCI participants in the AE and stretch groups. No other brain regions investigated showed a CBF difference between aMCI and CN.
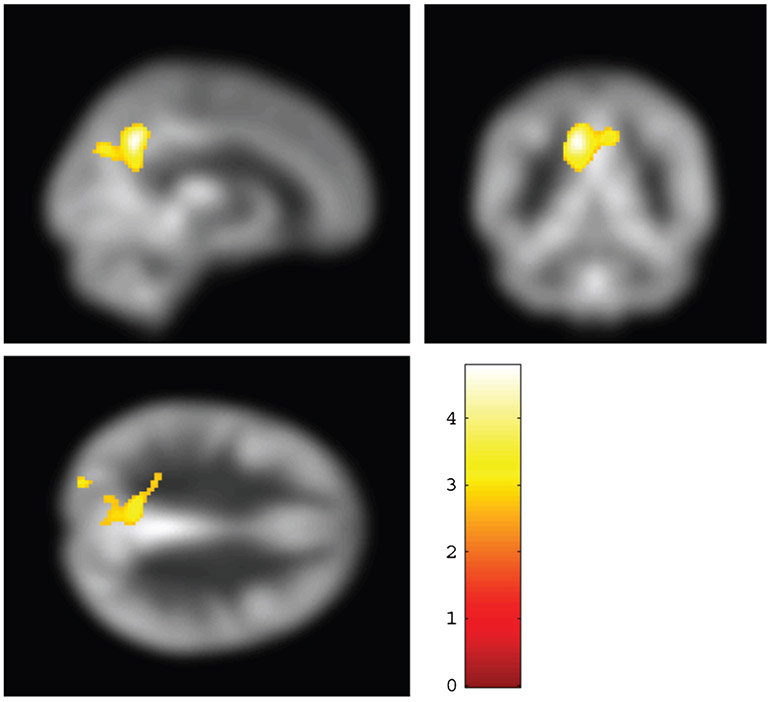
We further performed a voxel-wise CBF comparison between aMCI and CN. A lower CBF in the aMCI participants relative to controls was observed in PCC and precuneus (Fig. 4). All regions, coordinates, and T-values are listed in Supplementary Table 2.
Fig. 4.
Voxel-wise two sample t-test results of significant differences in cerebral blood flow (CBF) between mild cognitive impairment (N = 63) and elderly control participants (N = 20). Increases in CBF were seen in precuneus (colored voxels). The significant voxels (family-wise-error corrected p < 0.05) were overlaid on CBF map in the MNI template space.
Effect of 12 months of aerobic exercise on brain volume
To investigate whether physiological changes in CBF were accompanied by anatomic changes, we compared brain volume of above-mentioned regions before and after interventions. No differences in brain volume in the 7 ROIs were found between aMCI and CN, or between the two aMCI subgroups (exercise compared to stretch) (p > 0.05 for all comparisons). There was not a group-by-time interaction effect on brain volume in any of the ROIs. That is, none of the ROIs showed any difference in brain volume change between the exercise and stretch groups.
Summary
To summarize, the AE group showed a significant increase in VO2 max post-training compared to pre-training, while the stretch group did not show a difference (Table 2). LM scores in the AE group improved significantly from pre-training levels, whereas the stretch group did not show a significant change (Table 2). We report a significant group-by-time interaction effect in CBF in the ACC, wherein the AE group showed a training-related increase in CBF relative to the stretch group. A training-related increase in CBF in the hippocampus was observed only in the Aerobic Exercise group and not in the Stretch group. We also report a significant group-by-time interaction effect in CBF in the PCC, but the direction of the effect was opposite to that in ACC. The AE group showed a training-related decrease in PCC CBF relative to the stretch group. A voxel-wise correlation of CBF change after intervention (exercise and stretch groups combined) with memory function change showed a significant positive correlation. Brain areas where CBF increases were significantly associated with improvements in memory function included ACC (BA 32), cingulate gyrus, middle, medial, and superior frontal gyrus (Fig. 2). Comparison of resting CBF in aMCI participants to that in age-matched control participants revealed that CBF was significantly lower in the aMCI participants relative to controls in the PCC and precuneus (Fig. 4). Finally, none of the ROIs showed any difference in brain volume change between the exercise and stretch groups.
DISCUSSION
AE is a cost-effective approach with a potential to slow age-related cognitive decline and has received increasing interest from the scientific field and general public. The current work is the first to measure effects of AE on brain perfusion in aMCI participants after 12 months of intervention. We report that AE resulted in a significant increase in CBF in the ACC as confirmed by ROI analysis. The AE group also showed an improvement in memory function. Voxel-wise correlation of change in CBF with change in logical memory in all aMCI participants (AE and stretch groups) showed that CBF increase in anterior brain regions such as ACC, middle, medial, and superior frontal were correlated with improvement in logical memory. To increase the power of the correlation analysis, we have included data from both AE and stretch groups. This result suggests that increase in CBF in these frontal regions helped support memory function. In contrast, posterior brain regions such as PCC revealed a decrease in CBF following AE. These results suggest a posterior-to-anterior redistribution of brain perfusion, presumably accompanied by a similar shift in the underlying neural activity occurred following AE.
Our observation of CBF increase in the ACC is consistent with several prior reports. Increase in ACC volume was reported in elderly participants who performed 6 months of AE training compared to an elderly group that performed stretching and toning [7]. Another 6-month long AE study in MCI participants reported that exercise dose was associated with increase in ACC volume, with participants in the exercise group reporting an improvement in verbal and executive memory [35]. CBF in the ACC was higher in a group of cognitively normal elderly individuals after 12 weeks of AE training relative to a non-AE control group [4]. CBF in left ACC was increased after 6 months of exercise training in patients with coronary artery disease [25]. On the other hand, Alfini et al. reported in MCI participants a decrease in CBF in the left ACC after a 12-week exercise intervention, with an improvement in working memory and verbal fluency [14]. Difference in the MCI study group such as mean age (80.5), average MMSE score (24.9), and duration of exercise intervention (12 weeks) are the probable reasons for discrepancy in ACC CBF reported by Alfini et al. It is difficult to assess the mechanism of CBF reduction in the ACC associated with cognitive function improvement in the lower functioning, older group of MCI studied by Alfini et al. compared to the MCI group from this study. The study by Chapman et al. recruited older adults with similar mean age (64 years) and reported similar results with increase in ACC CBF and improvement in memory function in the aerobic exercise group, as reported here. Maass et al. performed a 3-month long aerobic exercise intervention in older adults and reported increasing fitness levels were associated with increasing hippocampal perfusion and volume that were positively related to changes in memory function [36]. They reported that adults between the ages of 60 and 70 years tended toward increases in perfusion whereas older individuals tended toward decreases. We did not make this observation in our cohort which was supported by literature reports of exercise induced CBF increases in adults older than 70 years [5, 37]. This result may explain the difference in CBF reported by Alfini et al. and those reported in this paper. One source of variability causing perfusion decrease in response to exercise in older individuals may be amyloid deposition that can occur in 20% of apparently healthy older adults and is reported to increase with age [38], which may negatively impact neuronal metabolism and plasticity. Another possibility is that stress related increase in cortisol with age [39] negatively impacts neuronal health and leads to cognitive decline [40], effects of which may not be fully reversed by shorter term aerobic exercise intervention.
The increase in CBF in the ACC after training was found to correlate positively with increase in episodic memory. The ACC has been reported to be involved in episodic memory tasks [41-43]. ACC along with neighboring frontal lobe structures represent a critical node in memory [44, 45]. These regions are involved in monitoring of memory [46] as well as allocation of attention supporting memory [44]. Thus, it appears that AE may improve memory by augmenting ACC CBF. These results and previous reports of improvement in ACC function suggest that increases in ACC CBF may be the mechanism by which AE induces improvement in memory function, presumably through altered neural activity. These findings also support that ASL perfusion is a potential biomarker of exercise-induced improvement in aMCI memory function. Recent reports in literature have also suggested the use of ASL perfusion as a biomarker to predict cognitive function in elderly [47], for early diagnosis and disease tracking in AD [48] and to predict cognitive decline, conversion to dementia [49]. ASL perfusion in the hippocampus of adult APOE ε4 carriers with genetic risk for AD was shown to be higher by leading a sedentary lifestyle, suggesting a CBF regulatory response to compensate for metabolic alterations in dementia risk [50]. Unfortunately, APOE data was not available for all MCI participants from this study and we could not assess if APOE ε4 status may have contributed to the CBF changes observed in this study. These reports suggest that CBF is a good marker of exercise, physical activity, and an active or sedentary lifestyle.
PCC is implicated in aging and AD and is characterized by high levels of amyloid deposition, reduced metabolic rate, diminished resting-state connectivity and CBF [51-53], an observation confirmed by our cross-sectional results. A significant finding of the present study is that 12 months of AE training was not able to reverse the decline in PCC CBF in aMCI. In fact, PCC CBF was further decreased after 12 months of AE training. PCC is a key region in the default-mode-network (DMN) and highly sensitive to age-related cognitive decline, AD, and dementia [54-57]. Our results suggest that once this key DMN region is affected in aMCI, AE cannot halt further decline. Instead, the brain seems to shift its neural activity anteriorly and uses regions such as ACC and prefrontal cortex to compensate for reduced activity in PCC. Increased activation in prefrontal cortices in light of declining efficiency of posterior processing regions has previously been reported in studies focusing on episodic memory [58, 59] and has been interpreted as reflecting a process of compensatory scaffolding [60] whereby the over-activation in frontal cortices is thought to compensate for decline in function in posterior processing regions [60-62].
We also reported that one year of aerobic exercise training led to an increase in CBF in the hippocampus; the stretch group, however, did not show any change in hippocampal CBF. This finding of aerobic exercise related increase in CBF in the hippocampus has been reported in literature [4, 36].
One limitation of the present study is that sample size is modest, as both the exercise and stretch groups had only 15 aMCI participants. This is primarily due to the particular cohort we aimed to recruit and the long intervention period (1 year) used in this study. Our participants were patients with cognitive impairment, inclusion criteria were relatively stringent, and that we had multiple experimental components such as neurocognitive assessment, cardiorespiratory fitness evaluation, and MRI. Only participants who had pre- and post-intervention data from all measures were included in the final analyses. Therefore, despite screening a total of 1,620 participants, only 30 met all inclusion criteria and yielded complete data.
CBF results were not corrected for multiple comparisons despite obtaining data from 7 regions of interest. So, the exploratory nature of the CBF results must be noted. The rationale for including CBF results is to show an evidence of CBF change and its association with memory function improvement.
It should be noted that we have strict inclusion and exclusion criteria; we recruited aMCI participants who did not exercise regularly with minimal cardiovascular risk factors, e.g., without diabetes, obesity, and uncontrolled hypertension. These criteria may help exclude confounding effects of vascular disease and lead to a very pure sample of early AD in whom we evaluated the effects of AE and stretch training for 12 months. This could be considered as a positive feature of the study. Future studies, however, are needed to confirm whether perfusion changes observed in the present study can be extended to MCI patients with more cardiovascular risk factors.
Another limitation is that we did not assess benefits of training regimens mid-way into the training. Thus, we were not able to asses a dose-based response of this training. This study also did not perform further follow-up of participants to assess if beneficial effects of AE were sustained longer than one year to assess maintenance of treatment benefits. A positive of this study is that this was a home-based training regimen, i.e., participants were allowed to train on their own. This type of training regimen has the advantage that it can be scaled to a larger population with the use of fewer resources.
Brain atrophy in participants with aMCI could cause errors in CBF calculation based on normal anatomical atlases, because the atrophied region is stretched during normalization. After 12 months, the atrophy patterns could well change in participants with aMCI. However, we did not see a significant difference in brain volume in aMCI participants after 12 months. A 10% or greater atrophy may cause a significant effect on the CBF calculation (Note: PCASL CBF measurement has a coefficient of variation of about 5%). Finally, we did not find significant changes in brain volume between the AE and stretching groups and were not able to replicate the brain volume increase observed by Colcombe et al. [7].
Another limitation is that APOE genotype data was available on a sub-set of aMCI participants only. Of the 30 aMCI participants, APOE genotype data was available in 14 participants only. Of these 14 participants, 4 participants were APOE ε4 positive. There were 2 participants that were APOE ε4 positive in each group (stretch, AE)
In conclusion, aerobic exercise resulted in an alteration in cerebral perfusion, in that CBF in PCC showed a reduction following AE whereas CBF in ACC manifested a CBF increase. This CBF increase was positively correlated with increase in logical memory, suggesting a potential link between changes in brain and cognitive function.
Supplementary Material
ACKNOWLEDGMENTS
We would like to thank our funding source: National Institute on Aging - R01 AG033106
Footnotes
Authors’ disclosures available online (https://www.j-alz.com/manuscript-disclosures/19-0977r3).
SUPPLEMENTARY MATERIAL
The supplementary material is available in the electronic version of this article: https://dx.doi.org/10.3233/JAD-190977.
REFERENCES
- [1].Mullane K, Williams M (2013) Alzheimer’s therapeutics: continued clinical failures question the validity of the amyloid hypothesis-but what lies beyond? Biochem Pharmacol 85, 289–305. [DOI] [PubMed] [Google Scholar]
- [2].Baker LD, Frank LL, Foster-Schubert K, Green PS, Wilkinson CW, McTiernan A, Plymate SR, Fishel MA, Watson GS, Cholerton BA, Duncan GE, Mehta PD, Craft S (2010) Effects of aerobic exercise on mild cognitive impairment: a controlled trial. Arch Neurol 67, 71–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Varela S, Ayan C, Cancela JM, Martin V (2012) Effects of two different intensities of aerobic exercise on elderly people with mild cognitive impairment: a randomized pilot study. Clin Rehabil 26, 442–450. [DOI] [PubMed] [Google Scholar]
- [4].Chapman SB, Aslan S, Spence JS, Defina LF, Keebler MW, Didehbani N, Lu H (2013) Shorter term aerobic exercise improves brain, cognition, and cardiovascular fitness in aging. Front Aging Neurosci 5, 75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Thomas BP, Yezhuvath US, Tseng BY, Liu P, Levine BD, Zhang R, Lu H (2013) Life-long aerobic exercise preserved baseline cerebral blood flow but reduced vascular reactivity to CO2. J Magn Reson Imaging 38, 1177–1183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Colcombe S, Kramer AF (2003) Fitness effects on the cognitive function of older adults: a meta-analytic study. Psychol Sci 14, 125–130. [DOI] [PubMed] [Google Scholar]
- [7].Colcombe SJ, Erickson KI, Scalf PE, Kim JS, Prakash R, McAuley E, Elavsky S, Marquez DX, Hu L, Kramer AF (2006) Aerobic exercise training increases brain volume in aging humans. J Gerontol A Biol Sci Med Sci 61, 1166–1170. [DOI] [PubMed] [Google Scholar]
- [8].Tseng BY, Gundapuneedi T, Khan MA, Diaz-Arrastia R, Levine BD, Lu H, Huang H, Zhang R (2013) White matter integrity in physically fit older adults. Neuroimage 82, 510–516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Dougherty RJ, Boots EA, Lindheimer JB, Stegner AJ, Van Riper S, Edwards DF, Gallagher CL, Carlsson CM, Rowley HA, Bendlin BB, Asthana S, Hermann BP, Sager MA, Johnson SC, Okonkwo OC, Cook DB (2019) Fitness, independent of physical activity is associated with cerebral blood flow in adults at risk for Alzheimer’s disease. Brain Imaging Behav, doi: 10.1007/s11682-019-00068-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Colcombe SJ, Erickson KI, Raz N, Webb AG, Cohen NJ, McAuley E, Kramer AF (2003) Aerobic fitness reduces brain tissue loss in aging humans. J Gerontol A Biol Sci Med Sci 58, 176–180. [DOI] [PubMed] [Google Scholar]
- [11].Colcombe SJ, Kramer AF, Erickson KI, Scalf P, McAuley E, Cohen NJ, Webb A, Jerome GJ, Marquez DX, Elavsky S (2004) Cardiovascular fitness, cortical plasticity, and aging. Proc Natl Acad Sci U S A 101, 3316–3321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Pereira AC, Huddleston DE, Brickman AM, Sosunov AA, Hen R, McKhann GM, Sloan R, Gage FH, Brown TR, Small SA (2007) An in vivo correlate of exercise-induced neurogenesis in the adult dentate gyrus. Proc Natl Acad Sci U S A 104, 5638–5643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].van der Kleij LA, Petersen ET, Siebner HR, Hendrikse J, Frederiksen KS, Sobol NA, Hasselbalch SG, Garde E (2018) The effect of physical exercise on cerebral blood flow in Alzheimer’s disease. Neuroimage Clin 20, 650–654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Alfini AJ, Weiss LR, Nielson KA, Verber MD, Smith JC (2019) Resting cerebral blood flow after exercise training in mild cognitive impairment. J Alzheimers Dis 67, 671–684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Tarumi T, Rossetti H, Thomas BP, Harris T, Tseng BY, Turner M, Wang C, German Z, Martin-Cook K, Stowe AM, Womack KB, Mathews D, Kerwin DR, Hynan L, Diaz-Arrastia R, Lu H, Munro Cullum C, Zhang R (2019) Exercise training in amnestic mild cognitive impairment: a one-year randomized controlled trial. J Alzheimers Dis 71, 421–433. [DOI] [PubMed] [Google Scholar]
- [16].Petersen RC, Doody R, Kurz A, Mohs RC, Morris JC, Rabins PV, Ritchie K, Rossor M, Thal L, Winblad B (2001) Current concepts in mild cognitive impairment. Arch Neurol 58, 1985–1992. [DOI] [PubMed] [Google Scholar]
- [17].Levine BD, Stray-Gundersen J (1997) “Living high-training low”: effect of moderate-altitude acclimatization with low-altitude training on performance. J Appl Physiol (1985) 83, 102–112. [DOI] [PubMed] [Google Scholar]
- [18].American College of Sports Medicine (2018) ACSM’s guidelines for exercise testing and prescription, Wolters Kluwer, Philadelphia. [DOI] [PubMed] [Google Scholar]
- [19].Fujimoto N, Prasad A, Hastings JL, Arbab-Zadeh A, Bhella PS, Shibata S, Palmer D, Levine BD (2010) Cardiovascular effects of 1 year of progressive and vigorous exercise training in previously sedentary individuals older than 65 years of age. Circulation 122, 1797–1805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Iwasaki K, Zhang R, Zuckerman JH, Levine BD (2003) Dose-response relationship of the cardiovascular adaptation to endurance training in healthy adults: how much training for what benefit? J Appl Physiol (1985) 95, 1575–1583. [DOI] [PubMed] [Google Scholar]
- [21].Dai W, Garcia D, de Bazelaire C, Alsop DC (2008) Continuous flow-driven inversion for arterial spin labeling using pulsed radio frequency and gradient fields. Magn Reson Med 60, 1488–1497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Alsop DC, Detre JA, Golay X, Gunther M, Hendrikse J, Hernandez-Garcia L, Lu H, MacIntosh BJ, Parkes LM, Smits M, van Osch MJ, Wang DJ, Wong EC, Zaharchuk G (2015) Recommended implementation of arterial spin-labeled perfusion MRI for clinical applications: A consensus of the ISMRM perfusion study group and the European consortium for ASL in dementia. Magn Reson Med 73, 102–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Li Y, Liu P, Li Y, Fan H, Su P, Peng SL, Park DC, Rodrigue KM, Jiang H, Faria AV, Ceritoglu C, Miller M, Mori S, Lu H (2018) ASL-MRICloud: An online tool for the processing of ASL MRI data. NMR Biomed 32, e4051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Mori S, Wu D, Ceritoglu C, Li Y, Kolasny A, Vaillant MA, Faria AV, Oishi K, Miller MI (2016) MRICloud: delivering high-throughput MRI neuroinformatics as cloud-based software as a service. Comput Sci Eng 18, 21–35. [Google Scholar]
- [25].MacIntosh BJ, Swardfager W, Crane DE, Ranepura N, Saleem M, Oh PI, Stefanovic B, Herrmann N, Lanctot KL (2014) Cardiopulmonary fitness correlates with regional cerebral grey matter perfusion and density in men with coronary artery disease. PLoS One 9, e91251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Preston AR, Eichenbaum H (2013) Interplay of hippocampus and prefrontal cortex in memory. Curr Biol 23, R764–773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Stoub TR, deToledo-Morrell L, Stebbins GT, Leurgans S, Bennett DA, Shah RC (2006) Hippocampal disconnection contributes to memory dysfunction in individuals at risk for Alzheimer’s disease. Proc Natl Acad Sci U S A 103, 10041–10045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].West MJ, Coleman PD, Flood DG, Troncoso JC (1994) Differences in the pattern of hippocampal neuronal loss in normal ageing and Alzheimer’s disease. Lancet 344, 769–772. [DOI] [PubMed] [Google Scholar]
- [29].Aslan S, Lu H (2010) On the sensitivity of ASL MRI in detecting regional differences in cerebral blood flow. Magn Reson Imaging 28, 928–935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Joshi AD, Pontecorvo MJ, Clark CM, Carpenter AP, Jennings DL, Sadowsky CH, Adler LP, Kovnat KD, Seibyl JP, Arora A, Saha K, Burns JD, Lowrey MJ, Mintun MA, Skovronsky DM, Florbetapir FSI (2012) Performance characteristics of amyloid PET with florbetapir F 18 in patients with alzheimer’s disease and cognitively normal subjects. J Nucl Med 53, 378–384. [DOI] [PubMed] [Google Scholar]
- [31].Bullich S, Villemagne VL, Catafau AM, Jovalekic A, Koglin N, Rowe CC, De Santi S (2017) Optimal reference region to measure longitudinal amyloid-beta change with (18)F-Florbetaben PET. J Nucl Med 58, 1300–1306. [DOI] [PubMed] [Google Scholar]
- [32].Tarumi T, Harris TS, Hill C, German Z, Riley J, Turner M, Womack KB, Kerwin DR, Monson NL, Stowe AM, Mathews D, Cullum CM, Zhang R (2015) Amyloid burden and sleep blood pressure in amnestic mild cognitive impairment. Neurology 85, 1922–1929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Bilgel M, An Y, Zhou Y, Wong DF, Prince JL, Ferrucci L, Resnick SM (2016) Individual estimates of age at detectable amyloid onset for risk factor assessment. Alzheimers Dement 12, 373–379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Wolk DA, Price JC, Saxton JA, Snitz BE, James JA, Lopez OL, Aizenstein HJ, Cohen AD, Weissfeld LA, Mathis CA, Klunk WE, De-Kosky ST (2009) Amyloid imaging in mild cognitive impairment subtypes. Ann Neurol 65, 557–568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Anderson-Hanley C, Barcelos NM, Zimmerman EA, Gillen RW, Dunnam M, Cohen BD, Yerokhin V, Miller KE, Hayes DJ, Arciero PJ, Maloney M, Kramer AF (2018) The Aerobic and Cognitive Exercise Study (ACES) for community-dwelling older adults with or at-risk for mild cognitive impairment (MCI): neuropsychological, neurobiological and neuroimaging outcomes of a randomized clinical trial. Front Aging Neurosci 10, 76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Maass A, Duzel S, Goerke M, Becke A, Sobieray U, Neumann K, Lovden M, Lindenberger U, Backman L, Braun-Dullaeus R, Ahrens D, Heinze HJ, Muller NG, Duzel E (2015) Vascular hippocampal plasticity after aerobic exercise in older adults. Mol Psychiatry 20, 585–593. [DOI] [PubMed] [Google Scholar]
- [37].Burdette JH, Laurienti PJ, Espeland MA, Morgan A, Telesford Q, Vechlekar CD, Hayasaka S, Jennings JM, Katula JA, Kraft RA, Rejeski WJ (2010) Using network science to evaluate exercise-associated brain changes in older adults. Front Aging Neurosci 2, 23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Yamada M, Tsukagoshi H, Otomo E, Hayakawa M (1988) Systemic amyloid deposition in old age and dementia of Alzheimer type: the relationship of brain amyloid to other amyloid. Acta Neuropathol 77, 136–141. [DOI] [PubMed] [Google Scholar]
- [39].Van Cauter E, Leproult R, Kupfer DJ (1996) Effects of gender and age on the levels and circadian rhythmicity of plasma cortisol. J Clin Endocrinol Metab 81, 2468–2473. [DOI] [PubMed] [Google Scholar]
- [40].McEwen BS, Sapolsky RM (1995) Stress and cognitive function. Curr Opin Neurobiol 5, 205–216. [DOI] [PubMed] [Google Scholar]
- [41].de Chastelaine M, Friedman D, Cycowicz YM (2007) The development of control processes supporting source memory discrimination as revealed by event-related potentials. J Cogn Neurosci 19, 1286–1301. [DOI] [PubMed] [Google Scholar]
- [42].Kompus K, Hugdahl K, Ohman A, Marklund P, Nyberg L (2009) Distinct control networks for cognition and emotion in the prefrontal cortex. Neurosci Lett 467, 76–80. [DOI] [PubMed] [Google Scholar]
- [43].Fleck MS, Daselaar SM, Dobbins IG, Cabeza R (2006) Role of prefrontal and anterior cingulate regions in decision-making processes shared by memory and nonmemory tasks. Cereb Cortex 16, 1623–1630. [DOI] [PubMed] [Google Scholar]
- [44].Kondo H, Morishita M, Osaka N, Osaka M, Fukuyama H, Shibasaki H (2004) Functional roles of the cingulo-frontal network in performance on working memory. Neuroimage 21, 2–14. [DOI] [PubMed] [Google Scholar]
- [45].Engstrom M, Landtblom AM, Karlsson T (2013) Brain and effort: brain activation and effort-related working memory in healthy participants and patients with working memory deficits. Front Hum Neurosci 7, 140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Risius UM, Staniloiu A, Piefke M, Maderwald S, Schulte FP, Brand M, Markowitsch HJ (2013) Retrieval, monitoring, and control processes: a 7 tesla FMRI approach to memory accuracy. Front Behav Neurosci 7, 24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].De Vis JB, Peng SL, Chen X, Li Y, Liu P, Sur S, Rodrigue KM, Park DC, Lu H (2018) Arterial-spin-labeling (ASL) perfusion MRI predicts cognitive function in elderly individuals: A 4-year longitudinal study. J Magn Reson Imaging 48, 449–458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Wang Z, Das SR, Xie SX, Arnold SE, Detre JA, Wolk DA, Alzheimer’s Disease Neuroimaging Initiative (2013) Arterial spin labeled MRI in prodromal Alzheimer’s disease: A multi-site study. Neuroimage Clin 2, 630–636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Chao LL, Buckley ST, Kornak J, Schuff N, Madison C, Yaffe K, Miller BL, Kramer JH, Weiner MW (2010) ASL perfusion MRI predicts cognitive decline and conversion from MCI to dementia. Alzheimer Dis Assoc Disord 24, 19–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Zlatar ZZ, Wierenga CE, Bangen KJ, Liu TT, Jak AJ (2014) Increased hippocampal blood flow in sedentary older adults at genetic risk for Alzheimer’s disease. J Alzheimers Dis 41, 809–817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Bai F, Watson DR, Yu H, Shi Y, Yuan Y, Zhang Z (2009) Abnormal resting-state functional connectivity of posterior cingulate cortex in amnestic type mild cognitive impairment. Brain Res 1302, 167–174. [DOI] [PubMed] [Google Scholar]
- [52].Dai W, Lopez OL, Carmichael OT, Becker JT, Kuller LH, Gach HM (2009) Mild cognitive impairment and alzheimer disease: patterns of altered cerebral blood flow at MR imaging. Radiology 250, 856–866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Chen JJ, Rosas HD, Salat DH (2011) Age-associated reductions in cerebral blood flow are independent from regional atrophy. Neuroimage 55, 468–478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Lustig C, Snyder AZ, Bhakta M, O’Brien KC, McAvoy M, Raichle ME, Morris JC, Buckner RL (2003) Functional deactivations: change with age and dementia of the Alzheimer type. Proc Natl Acad Sci U S A 100, 14504–14509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Greicius MD, Srivastava G, Reiss AL, Menon V (2004) Default-mode network activity distinguishes Alzheimer’s disease from healthy aging: evidence from functional MRI. Proc Natl Acad Sci U S A 101, 4637–4642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Alsop DC, Detre JA, Grossman M (2000) Assessment of cerebral blood flow in Alzheimer’s disease by spin-labeled magnetic resonance imaging. Ann Neurol 47, 93–100. [PubMed] [Google Scholar]
- [57].Srinivasa RN, Rossetti HC, Gupta MK, Rosenberg RN, Weiner MF, Peshock RM, McColl RW, Hynan LS, Lucarelli RT, King KS (2016) Cardiovascular risk factors associated with smaller brain volumes in regions identified as early predictors of cognitive decline. Radiology 278, 198–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Grady CL, McIntosh AR, Horwitz B, Maisog JM, Ungerleider LG, Mentis MJ, Pietrini P, Schapiro MB, Haxby JV (1995) Age-related reductions in human recognition memory due to impaired encoding. Science 269, 218–221. [DOI] [PubMed] [Google Scholar]
- [59].Gutchess AH, Welsh RC, Hedden T, Bangert A, Minear M, Liu LL, Park DC (2005) Aging and the neural correlates of successful picture encoding: frontal activations compensate for decreased medial-temporal activity. J Cogn Neurosci 17, 84–96. [DOI] [PubMed] [Google Scholar]
- [60].Park DC, Reuter-Lorenz P (2009) The adaptive brain: aging and neurocognitive scaffolding. Annu Rev Psychol 60, 173–196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Davis SW, Dennis NA, Daselaar SM, Fleck MS, Cabeza R (2008) Que PASA? The posterior-anterior shift in aging. Cereb Cortex 18, 1201–1209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Zhang H, Lee A, Qiu A (2017) A posterior-to-anterior shift of brain functional dynamics in aging. Brain Struct Funct 222, 3665–3676. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.