Abstract
Background:
Exosomes from mesenchymal stromal cells (MSCs) are endosome-derived vesicles that have been shown to enhance functional recovery in rodent models of stroke.
Objective:
Building on these findings, we tested exosomes as a treatment in monkeys with cortical injury.
Methods:
After being trained on a task of fine motor function of the hand, monkeys received a cortical injury to the hand representation in primary motor cortex. Twenty-four hours later and again 14 days after injury, monkeys received exosomes or vehicle control. Recovery of motor function was followed for 12 weeks.
Results:
Compared to monkeys that received vehicle, exosome treated monkeys returned to pre-operative grasp patterns and latency to retrieve a food reward in the first three-five weeks of recovery.
Conclusions:
These results provide evidence that in monkeys exosomes delivered after cortical injury enhance recovery of motor function.
Keywords: Exosomes, cortical injury, recovery, rhesus monkey
1. Introduction
Stroke and resulting cortical damage frequently cause motor dysfunction, especially of the hand, which significantly disrupts activities of daily living. The acute phase of cortical injury is associated with cell death and a build-up of reactive oxygen species (ROS) that stimulate a pro-inflammatory signaling cascade (Crack & Taylor, 2005; Lakhan, Kirchgessner & Hofer, 2009; Margaill, Plotkine, & Lerouet, 2005; Wang, Tang, & Yenari, 2007; Warner, Sheng, & Batinić-Haberle, 2004) which contributes to the development of impairment and limits recovery of function. After this initial phase, inflammation subsides and anti-inflammatory processes predominate with the release of trophic factors that create an environment that facilitates plasticity in cortical areas surrounding the injury. While some degree of recovery usually occurs, full recovery, without compensatory function does not occur in all patients. Hence, there is a critical need for therapeutic interventions to enhance recovery by both limiting inflammation and/or promoting restorative processes and plasticity during recovery with the goal of returning all patients to pre-injury levels of function.
Reduced inflammation and enhanced plasticity following cortical injury have been reported with stem cell based therapies and have been correlated with enhanced recovery. (Lambertsen, Finsen, & Clausen, 2018; Orczykowski et al., 2018; Stonesifer et al., 2017) We and others have shown that cell based therapies reduce inflammation and evoke secretion of growth factors from native parenchymal cells (Chopp & Zhang, 2009; Lakhan, Kirchgessner & Hofer, 2009; Mahmood, Lu, Qu, Goussev, & Chopp, 2005; Stonesifer et al., 2017; Xiong et al., 2011), which enhance synaptogenesis, neurogenesis and axonal sprouting. (Jeong et al., 2014; Liu et al., 2010; Mahmood, Lu, Qu, Goussev, & Chopp, 2005) We have also found that mesenchymal stem cells (MSC), potent inducers of neuroplasticity and recovery, release exosomes containing microRNAs (miRNA) and proteins that appear to alter microglia mediated inflammation and promote both functional recovery and plasticity. (Katakowski et al., 2013; Li et al., 2017; Thomi, Surbek, Haesler, Joerger-Messerli, & Schoeberlein, 2019; Xin et al., 2012)
Exosomes are endosome-derived nano-vesicles that are actively secreted biological containers transporting nucleic acids and proteins between cells. The efficacy of exosomes harvested from mesenchymal stromal cells (MSCs) to reduce inflammation and enhance neural plasticity and functional recovery following cortical injury has been demonstrated in rodent models of stroke. (Chopp & Zhang, 2015; Keller, Sanderson, Stoeck, & Altevogt, 2006; Korkut et al., 2013; Lee, El Andaloussi & Wood, 2012; Li et al., 2017; Morel et al., 2013; Rana, Malinowska, & Zoller, 2013; Simons & Raposo, 2009; Wang, Li, & Feng, 2018; Xin et al., 2012, 2013a,b; Xu & Tahara, 2013; Zhang et al., 2015; Zhang & Chopp, 2016) Building on the work in rodents, we have now begun testing exosomes derived from bone marrow MSCs as a treatment for cortical injury in the non-human primate (NHP). Our monkey model of cortical injury has been used previously to assess potential therapeutics and provides the benefit of using an animal that is capable of fine motor function of the hand which is highly similar to human fine motor function. In addition, we have developed a Non-Human Primate Grasp Assessment Scale that allows us to quantify the precise composite movement of the hands and digits, individual digit action, and finger-thumb pinch in the monkey both prior to injury and during recovery (Pessina et al., 2019).
2. Methods and materials
Ten female rhesus monkeys (Macaca mulatta) (16–26 years, equivalent to approximately 48–78 year old humans) were used in this study. Monkeys were obtained from national primate research facilities or private vendors and had known birth dates and complete health records. Monkeys received medical examinations and magnetic resonance imaging to ensure there was no occult health problems or neurological damage. Monkeys were housed in the Animal Science Center of Boston University School of Medicine which is AAALAC accredited. All procedures were approved by the Boston University Institutional Animal Care and Use Committee.
2.1. Pre-operative training on fine motor function testing
As described in detail previously (Moore, Killiany, Pessina, Moss, & Rosene, 2010; Moore et al., 2016), all monkeys were trained on a task of fine motor function of the hand, the Hand Dexterity Task (HDT), using a testing apparatus that controls, quantifies and video records responses from each hand. Using this apparatus, monkeys were trained on the HDT for a total of 15 days (Monday, Wednesday and Friday each week for 4 weeks). The HDT is a modified version of a Klüver board (Kluver, 1935) and requires precise control of the digits, particularly apposition of the thumb and index finger, to retrieve a small, visible food reward (M&M’s, Mars, Inc) from two different size round wells in a Plexiglas tray. Food rewards were round and approximately 1 cm in diameter. Both wells were 1 cm deep. The large well was 25 mm wide and the small well was 18 mm wide. Time to retrieve the food reward is recorded by a timer that is connected to photocells that are located in the openings on each side of the apparatus. The timer starts when the monkey puts a hand through one of the openings, triggering the photocells to start the timer. The timer stops when the monkey removes his hand. An experimenter records whether or not the reward was successfully retrieved and the response time to retrieve is recorded. The HDT has been used to assess fine motor function of the hand and digits in adult monkeys with and without injury to the hand representation in the motor cortex as well as to compare the performance of middle-aged to young adult rhesus monkeys. (Moore et al., 2012; Moore, Killiany, Pessina, Moss, & Rosene, 2010) Each test day consists of 16 trials for each of the two well sizes as well as for each hand resulting in a total of 32 trials. The order of trials for each hand and well follows a pseudorandom balanced sequence to eliminate any order effects. Monkeys are given 30 seconds to complete a trial. If they do not or would not complete a trial in 30 sec, the trial is terminated and the monkey is given one additional opportunity to complete that trial. After a second failed attempt, a non-response is recorded, the monkey’s difficulties are noted in the study record and the next trial is initiated.
2.2. Hand preference
At the completion of pre-training on the HDT, free choice trials with both sides of the apparatus baited and accessible are administered to determine which hand is “preferred”. This assessment is also compared with the pre-operative acquisition rates for each hand. Based on this assessment, the cortical injury is targeted to the hand representation of the hemisphere controlling the preferred hand to ensure that monkeys are motivated to use the impaired hand during post-operative testing.
2.3. Group assignment and blinding
After the completion of pre-training, the monkeys were randomly assigned in a balanced fashion based on weight and age so that five monkeys were assigned to receive exosomes and five monkeys to receive vehicle control. All study personnel (surgeons, technicians, behavioral testers, etc.) were kept blind to the assignment during surgery and all post-operative in-vivo procedures, testing and terminal brain tissue harvest and processing.
2.4. Electrophysiological mapping of the hand representation in motor cortex
All surgical procedures were carried out under aseptic conditions. Each monkey was sedated with Ketamine (10 mg/kg) and anesthetized with intravenous sodium pentobarbital (15–25 mg/kg) to effect and antibiotics and analgesics were given prior to and during surgery. Many anesthetics are known to be neuroprotective and differentially affect cortical excitability and this could potentially impact cortical mapping, the extent of damage that occurs and recovery of function. However, in the current study, monkeys received one dose of Ketamine (10 mg/kg) approximately 2–3 hours prior to mapping and lesion induction and all monkeys in both the treated and vehicle control groups received the identical dose of Ketamine at the same timing prior to mapping and lesion induction. Hence, even if ketamine had some neuroprotective effect, the dosing and timing prior to lesion induction were identical in both the treatment and control groups. While it is impossible to exclude an interaction of ketamine with exosomes, given the relatively small dose of ketamine we do not believe it could have confounded the results of this study. Overall, all monkeys received the identical anesthetic protocol and were randomly assigned to each experimental group. For our surgical procedure, we use sodium pentobarbital as the anesthesia as it has limited effect on cortical injury and allows for motoric responses to be elicited during the electrical stimulation to map the motor cortex. Heart rate, respiration, temperature, blood oxygenation and muscle tonus were monitored to ensure physiological homeostasis and a safe surgical level of anesthesia. The head was stabilized in a stereotactic apparatus, and a midline incision was made followed by reflection of the temporalis muscle. A bone flap approximately 40 mm in anterior to posterior extent and 35 mm in medial to lateral extent was made centered over the precentral gyrus. The bone was removed in one piece for later replacement. The dura was incised to expose the pre-central sulcus and primary motor cortex.
To create reproducible cortical injury and motor deficits, a calibrated photograph of the pre-central gyrus was taken and printed. The precentral gyrus was then systematically mapped using electrical stimulation delivered through a small monopolar silver ball electrode placed gently on the surface of the pia to evoke movements. A surface electrode was used rather than a sharp electrode that could penetrate the cortex in order to avoid extraneous damage to the motor cortex outside the hand representation. The stimulating electrode was moved across the precentral gyrus systematically in rows with stimulation sites spaced approximately 2 mm apart (anterior to posterior) and separated from the next row by approximately 2 mm (medial to lateral) as shown in Fig. 1. Monopolar stimulus pulses of 250 μsec duration at amplitudes from 2.0 to 3.0 mA were delivered at each site once every 2 seconds first singly and then in a short train of 4 pulses at a rate of 100 Hz. During each stimulation, a trained observer noted muscle movements (eg. distinct movement or twitches of muscle) in specific areas of the digits, hand, forearm or arm, both visually and by palpation. The intensity of the motor response in the hand and digits was graded on a scale of 1 to 3 (barely detectable to maximal). Specific stimulation sites with the lowest threshold and highest motor response were marked on the calibrated photograph creating a cortical surface map of the hand area that was used to guide placement of the lesion (Fig. 1). We have used this mapping methodology for the past twenty years in our laboratory and we have found that the exceedingly short pulses used in our mapping does not produce any detectable damage to the cortex. This is confirmed by postmortem examination of the mapped loci outside of the lesion area where there is no histological evidence of damage to cortex.
Fig. 1.
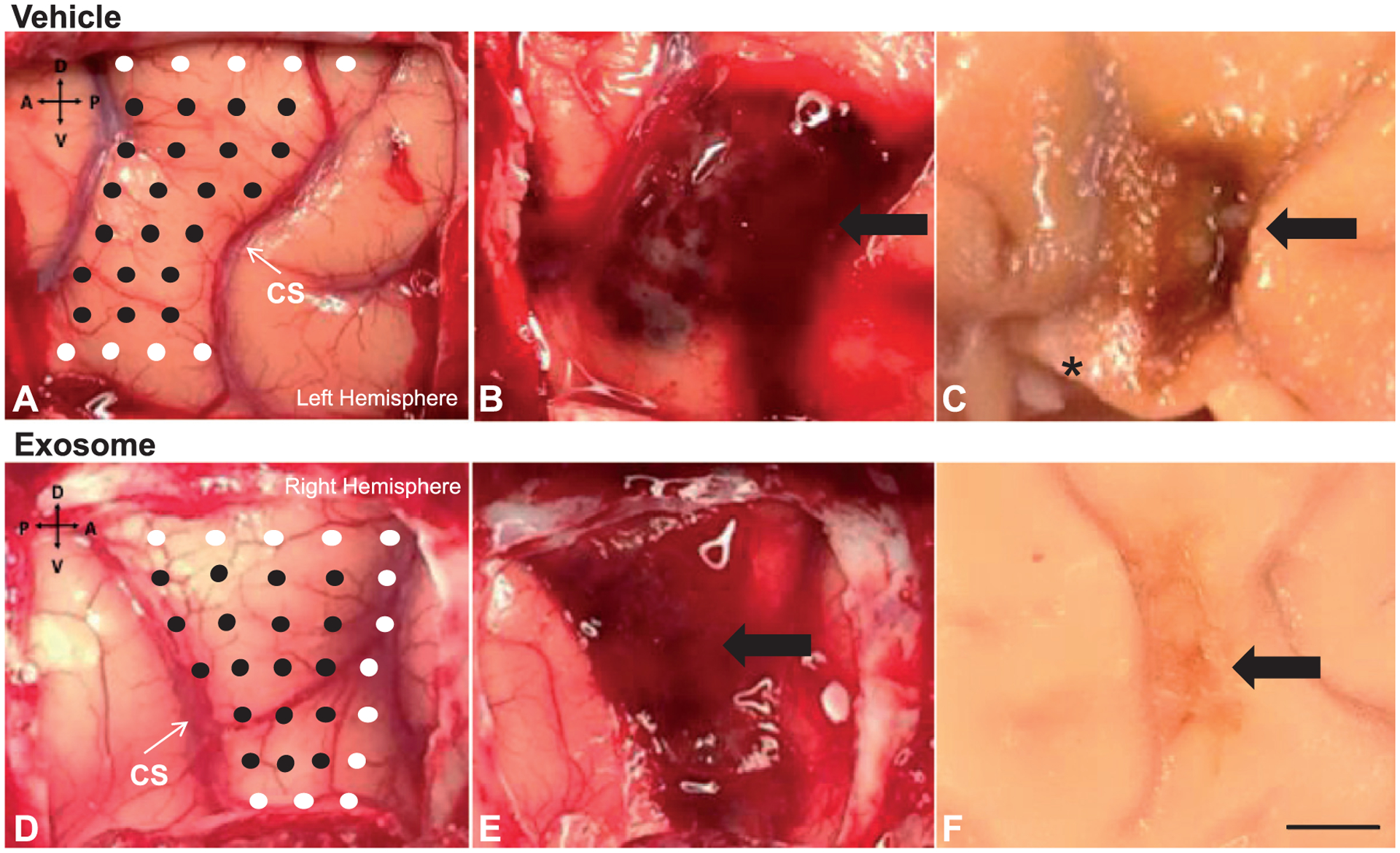
Photographs showing the stimulation sites on the hand representation maps (Panels A&D), the intraoperative lesions (Panels B&E), and final lesion after perfusion (Panels C&F), for one monkey (left hemisphere) that received vehicle control (top row) and one monkey (right hemisphere) that received exosomes (bottom row). On the hand representation maps, the black circles represent stimulation sites that generated a positive response in the hand or digits and the white circles represent stimulation sites that did not generate a positive response. * denotes an area where a brain biopsy was taken during perfusion for a related study. Scale bar = 5mm
2.5. Placement of selective cortical injury
Using the map described above, cortical injury was induced by making a small incision in the pia at the dorsal limit of the mapped representation. A small glass suction pipette was then inserted under the pia and used to bluntly transect the small penetrating arterioles as they enter the underlying cortex. We are careful not to suction or aspirate any cortical tissue; the suction pipette is only used to transect vessels. Suction and irrigation with sterile saline were sufficient to stanch any bleeding and maintain a clear field. Since the hand representation is known to extend down the rostral bank of the central sulcus, the sulcus was then opened down to the fundus along the length of the gyral hand representation by microdissection with a small glass pipette and a blunt periosteal elevator, taking care to leave the somatosensory areas on the caudal bank intact. The pia was then dissected with the glass pipette all the way down to the fundus of the sulcus. The hand area in the sulcus was not electrophysiologically mapped with the electrode to avoid inadvertent damage to the somatosensory areas on the caudal bank of the central sulcus mapping would require prolonged retraction. However, we have verified in terminal experiments the presence of the hand representation on the rostral bank is in alignment with the gyral representation. This pial dissection of penetrating vessels removes the blood supply to the cortex of the hand representation, inducing degeneration of the gray matter that extends down to the border with the underlying white matter without the risk of aspirating white matter. Representative photos of the cortical map and lesion for one treated and one untreated monkey are shown in Fig. 1.
After the lesion was made and all bleeding stopped, the dura was closed, the bone flap was sutured back in place using small burr holes placed in the flap and cranium followed by closing the muscles, fascia and skin in layers. Immediately following surgery, antibiotics and analgesics were administered and monkeys were then returned to their home cage and monitored continuously until fully awake. For the next 3–7 days (or as needed) monkeys were given analgesics and monitored regularly for any signs of infection or complications.
2.6. Exosome preparation and administration
Exosomes were extracted from MSCs harvested from the bone marrow of one young adult monkey. Bone marrow was extracted from the head of the humerus or the iliac crest according to the following protocol. Monkeys were sedated with Ketamine (10 mg/kg, IM) and then anesthetized with sodium pentobarbital (15–25 mg/kg IV). The area over the bone was shaved and sterilized, a sterile drape was placed over the area and a small (1–2 cm) incision was made over the bone site. A Jamshidi Bone Marrow Biopsy Needle (Care Fusion, Vermont Hills, IL) was advanced through the incision into the bone. Once the needle was advanced to the correct position, the lancet was removed and a syringe attached and the sample was extracted. Once the needle was removed, sutures were placed to close the incision. The monkey was treated with Buprenex (0.01–0.03 mg/kg). The extracted bone marrow was mixed with 10–15% anticoagulant citrate dextrose (ACD-A) and shipped on ice via same day delivery from Boston University School of Medicine to Henry Ford Health System.
Once received, bone marrow was centrifuged at 4,000× g for 15 minutes to separate blood components. The buffy coat, which contains mononuclear cells and resides between the lighter plasma fraction and the heavier erythrocyte fraction after centrifugation, was removed by careful pipetting. It was then washed once in culture medium and plated into T75 flasks in alpha-MEM with 20% FBS. Cells were grown to confluence and passaged as necessary. To grow sufficient numbers of exosomes, ten million (10 × 106) cells were seeded into a Quantum incubator (Terumo BCT, Lakewood, CO) and were grown in alpha-MEM containing 10% exosome depleted FBS circulated through fibronectin coated hollow fibers. Media were harvested starting on day three, followed by every other day for 4 days, and then every day for 2 days. Exosomes were isolated from the cell culture media via multistep centrifugation at 4°C, as previously described (Zhang et al., 2015; Zhang et al., 2017), with slight modification. Briefly, the cell culture media were centrifuged at 250× g for 5 minutes, and then the supernatants removed and centrifuged at 3,000× g for 30 minutes, followed by filtration through a 0.22 μm filter (SLMP025SS; EMD Millipore Corporation, Billerica, MA). The resulting media were then centrifuged at 100,000× g for 2 hours to pellet exosomes, which were resuspended in phosphate-buffered saline (PBS) and stored at 4°C.
Exosome concentration was determined on an qNano (Izon, Cambridge, MA) particle counter, and the concentration was adjusted so that each of the treated monkeys received 4 × 1011 particles/kg of body weight in 10 mL of PBS. The exosome doses were administered intravenously 24 hours and again 14 days after cortical injury. The control monkeys received infusions of the PBS vehicle intravenously 24 hours and again 14 days after cortical injury. Dosing concentration and intervals were based on preliminary studies in rodents (Xin et al., 2013a,b). This previous work in rodents indicates that IV and intraarterial (IA) administration produce equivalent results at 24 hours after stroke (Xin et al., 2013a,b; 2017) however, IV administration was chosen, because it is less invasive. The 24 hour interval for the first dose of exosomes (24 hours) was chosen to replicate our rodent work (Xin et al., 2013a,b). However, given that the evolution of recovery after neural injuries is slower in large animals than in rodents (Agoston, 2017) and based on our previous experience with our NHP model, we chose to administer a second dose of exosomes at 14 days later immediately prior to beginning fine motor testing to ensure that we did not miss the therapeutic window.
2.7. Initial cage-side post-operative assessment
Beginning on the day of surgery and continuing for the first 14 days of the post-operative period, prior to beginning formal testing on our motor tasks, the degree of motor impairment in the upper extremity was assessed in each monkey in its home cage using our adapted NHP Upper Extremity Motor Dysfunction Scale (Moore et al., 2016, 2013). This scale assesses impairments in tone, tremor, fine motor function of the hand, strength of the hand, digit flexion as well as movements of the forearm, wrist, arm and shoulder. Each measure is rated on a scale of 0 (no impairment) to 4 (unable or refuses to use impaired limb). This scale was adapted from Zhang et al., 2000 and the National Institutes of Health Stroke Scale (http://www.nihstrokescale.org/).
2.8. Post-operative motor testing
Post-operative re-testing on the HDT began two weeks after surgery and continued for 12 weeks. Testing on the HDT was conducted on Monday, Wednesday and Friday of each week. The schedule was adjusted so that 70% of the trials were given to the impaired hand, while 30% were given to the intact hand. The 30% of trials given to the unimpaired hand provide sufficient rewards to maintain motivation and sufficient data to demonstrate that effects are not due to generalized changes in motivation or overall motor function. Each monkey was given 30 seconds to complete a trial as in pre-operative training. The 70% of the trials that required the use of the impaired hand are similar in nature to constraint-induced therapy used in human rehabilitation which forces use of the impaired limbs. (Corbetta, Sirtori, Castellini, Moja, & Gatti, 2015; Kwakkel et al., 2016; Souza, Conforto, Orsini, Stern, & André, 2015) Testing continued for 12 weeks, the time estimated for monkeys receiving placebo to achieve asymptotic stable performance. The criterion on this task for successful return to pre-operative performance was five consecutive days at or below the pre-operative time to retrieve the food reward.
2.9. Grasp pattern assessment
While some spontaneous recovery does occur after injury to cortical motor areas controlling the hand and digits, full recovery of digit function does not occur in all patients and many aspects of recovery involves the development of compensatory actions. Hence, much of the spontaneous recovery that does occur is compensatory in nature and not a complete return to pre-injury fine motor function (Hylin, Kerr & Holden, 2017). The distinction between complete and compensatory recovery is important for assessing new treatments for stroke and cortical injury as the development of compensatory movements falls short of full functional use and hence limits normal activities of daily living (Levin, Kleim, & Wolf, 2009; Lum et al., 2009). To better assess the topography of motor recovery, we developed a Non-Human Primate Grasp Assessment Scale (GRAS) (Moore et al., 2016, 2013; Pessina, Bowley, Rosene, & Moore, 2019). This instrument allows us to detect and quantify significant impairments in fine motor function of the hand and to document recovery of function of individual digits and the precise finger-thumb pinch used by monkeys to retrieve food morsels and hence to distinguish between compensatory grasp function and a return to pre-injury grasp patterns.
To apply the GRAS to the motor performance of the monkeys, performance on the HDT during pre-operative training and post-operative testing was recorded with fixed placement cameras (Logitech, Newark, CA). A licensed Occupational Therapist (Author - M.A.P.) who has clinical experience in the treatment of patients with upper extremity impairment following stroke, and a trained research technician (Author - B.G.E.B.) analyzed the videotapes using the GRAS. It was adapted from the Eshkol-Wachman Movement Notation (Carr, Shepherd, Nordholm, & Lynne, 1985; Whishaw et al., 2002) and the Fugl-Meyer Motor Assessment scale. (Fugl-Meyer, Jaasko, Leyman, Olsson, & Steglind, 1975) and rates the position of the digits during grasp and the pattern of grasp and release to provide a semi-quantitative measure of maturity of the recovered grasp pattern. The scale includes 8 subdivided hierarchical stages so that a maximum score of 8 reflects normal grasp patterns (functional pinch between thumb and one individual digit) (Pessina, Bowley, Rosene, & Moore, 2019).
2.9.1. Perfusion and tissue processing
At the end of the 12 week post-operative period, monkeys were deeply anesthetized with IV sodium pentobarbital (25 mg/kg to effect) and euthanized by exsanguination during transcardial perfusion of the brain, first for no more than 5 minutes with 4°C Krebs buffer at pH 7.4 and then with 8 liters of 4% paraformaldehyde, pH 7.4 over 10 minutes to completely fix the brain. The skull was opened and the brain was photographed in situ with the photograph aligned to the perspective of the cortical map used to create the lesion. The brain was blocked in situ in the coronal plane to ensure reproducible planes of section during later processing. The brain was removed from the skull, weighed and post-fixed overnight in 4% paraformaldehyde for no more than 18 hours. To eliminate freezing artifact, the brain was then transferred to cryoprotectant solutions of glycerol and buffer and flash frozen at −75°C and stored at −80°C (Rosene, Roy, & Davis, 1986). Frozen blocks were later removed from storage and cut on a sliding microtome into interrupted series of coronal sections (eight series of 30 μm thick sections and one 60 μm thick series, with 300 μm spacing between sections). The 60 μm series was immediately mounted on microscope slides and stained with thionin for lesion reconstruction. Other series were collected in buffer with 15% glycerol, equilibrated overnight at 4°C and stored at −80°C for later histochemical processing (Estrada et al., 2017).
2.9.2. Lesion volume
We determined the lesion volume for nine of the monkeys using the calibrated photograph of the lesion on the surface of the brain acquired after perfusion (see above). The brain of the 10th monkey had been used for a separate study and was therefore not available for lesion reconstruction. The calibrated photograph of each brain was analyzed to determine the surface area of the lesion using the Scale and Measurement tools in Image J. Next, the thionin stained sections through the lesion (Fig. 2) were digitized using a Nikon Microscope equipped with NIS Elements software (Nikon Instruments, Inc, Melville, NY). The depth of the lesion was measured using the extent of gliosis as lesion boundaries on five representative thionin stained sections throughout the lesion from each monkey. Three depth measurement were taken throughout the lesion on each section, resulting in 15 measurements overall for each monkey and these measurements were used to calculate the average depth of each lesion. The total lesion volume was then determined by multiplying the surface area by the average depth.
Fig. 2.
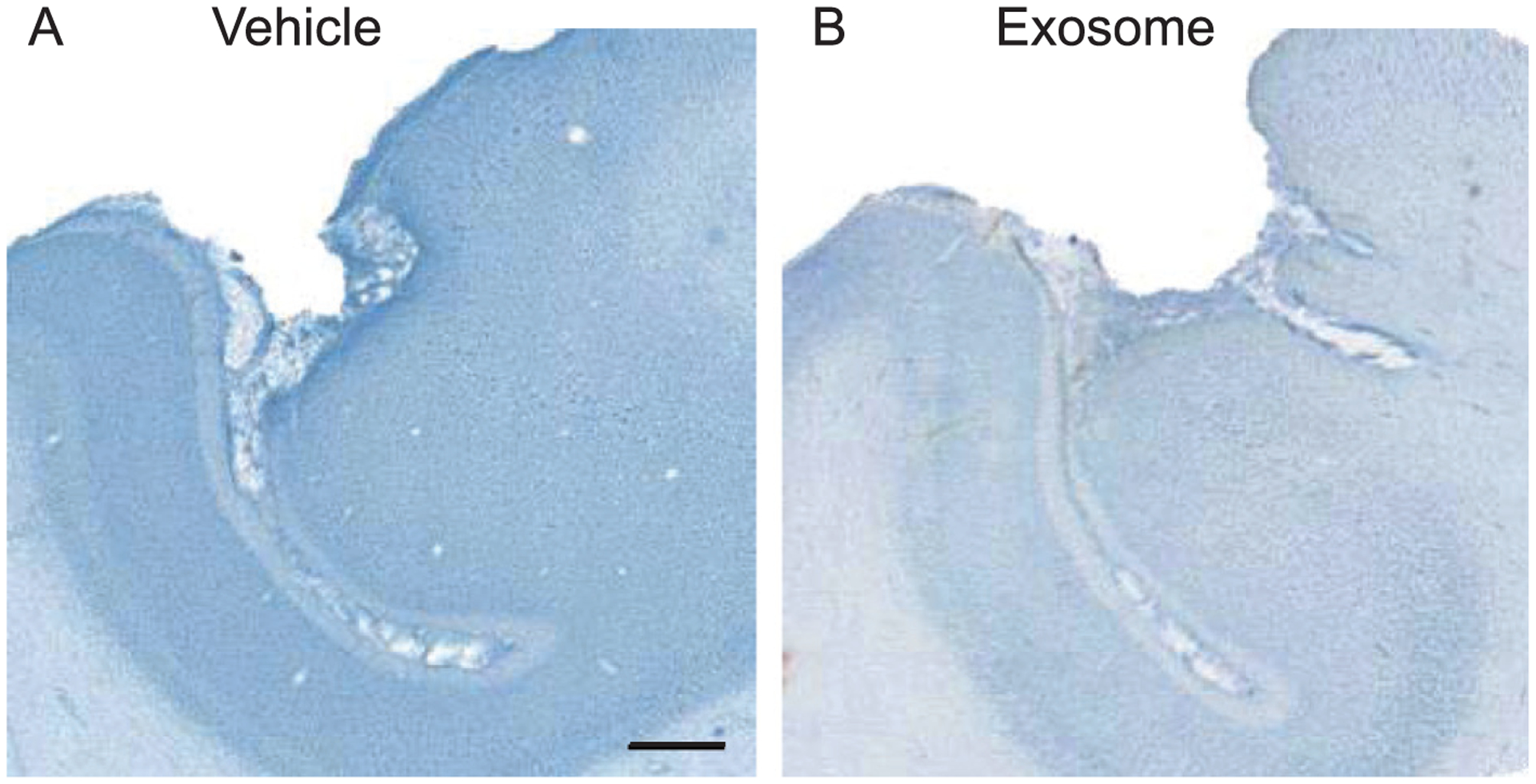
Representative thionin stained sections (60um) from one monkey that received vehicle (A) and one monkey treated with exosomes (B). Scale bar – 1000 um.
3. Results
3.1. Post-operative NHP upper extremity motor dysfunction scale
Beginning on the day of surgery and continuing daily across the 1st two weeks of the post-operative period, the degree of motor impairment in the upper extremity was assessed using our adapted NHP Upper Extremity Motor Dysfunction Scale which assesses impairments in tone, tremor, fine motor function of the hand, strength of the hand, digit flexion as well as movements of the forearm, wrist, arm and shoulder. Each measure is rated on a scale of 0 (no impairment) to 4 (unable or refuses to use impaired limb). The mean rating for each monkey across the 1st two weeks of the post-operative period prior to the commencement of HDT testing was assessed with separate Independent sample Student’s t-tests to compare the recovery scores between groups on measures of fine motor control, strength of the hands and digit flexion (the measures most closely related to grasp function). As shown in Fig. 3, this analysis revealed a significant difference between the groups on the measures of fine motor function and digit flexion (p < 0.05), with a greater degree of recovery in the exosome-treated monkeys.
Fig. 3.
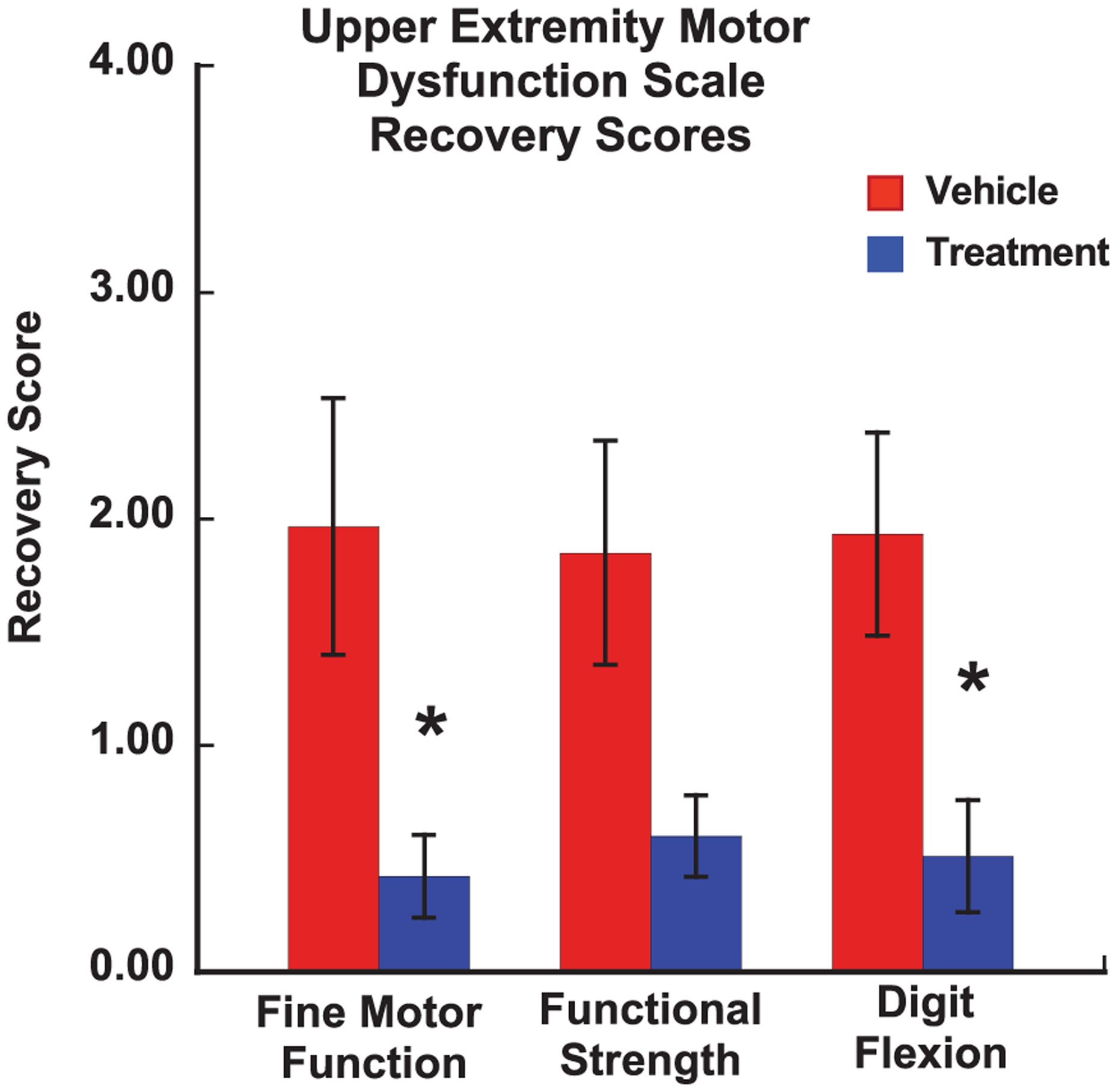
A graph of the mean recovery scores on our Upper Extremity Motor Dysfunction Scale that is used to assess the degree of impairment across the 1st 14 days after surgery (prior to starting formal testing on the HDT). Scores were recorded each day across the 1st two weeks of the post-operative period (prior to beginning formal testing on the HDT). A score of 0 = no impairment and a score of 4 = unable or refuses to use impaired limb. Error bars = Standard Error. * p < 0.05
3.2. Post-operative HDT
Figure 4 shows the mean time to retrieve the food reward each day during the post-operative testing period for each monkey. The dashed line on each graph represents the mean time to retrieve during the pre-operative training. The mean time to retrieve the food reward during the 1st week of post-operative testing (3rd week post-injury) was calculated for each monkey. As shown in Fig. 5A, separate one-way ANOVAs revealed that treated monkeys retrieved the food reward at a faster rate than the untreated monkeys on the large well [F (1, 8) = 5.16, p = 0.05] but not the small well [F (1, 8) = 2.19, p = 0.177] at this early stage in the recovery period.
Fig. 4.
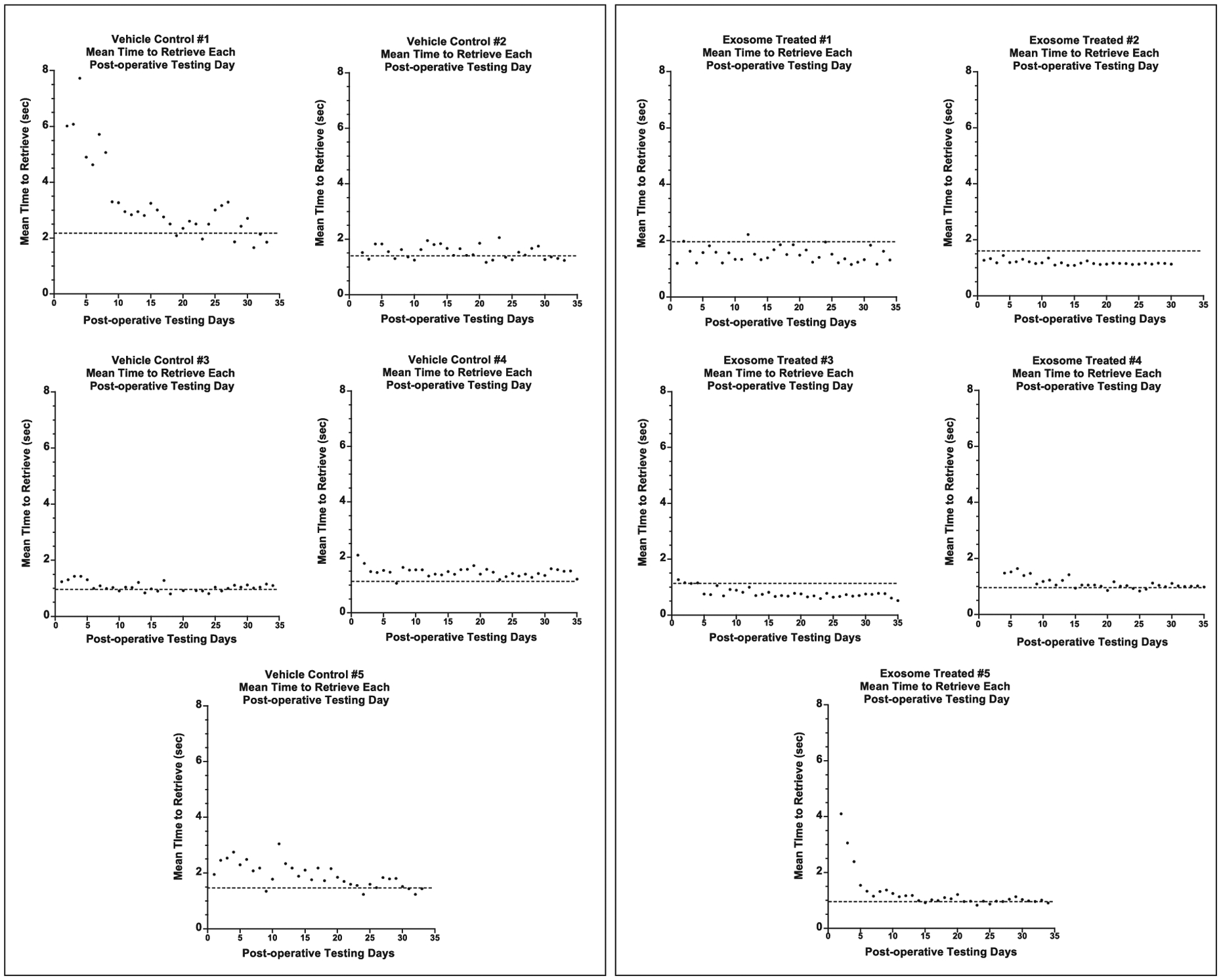
Graphs of the daily mean time to retrieve a food reward across the post-operative testing days. Testing began 14 days after surgery. The dashed line represents the mean time to retrieve the food reward over the last five days of the pre-operative training. Each data point (black dots) represents the mean time to retrieve for each post-operative day.
Fig. 5.
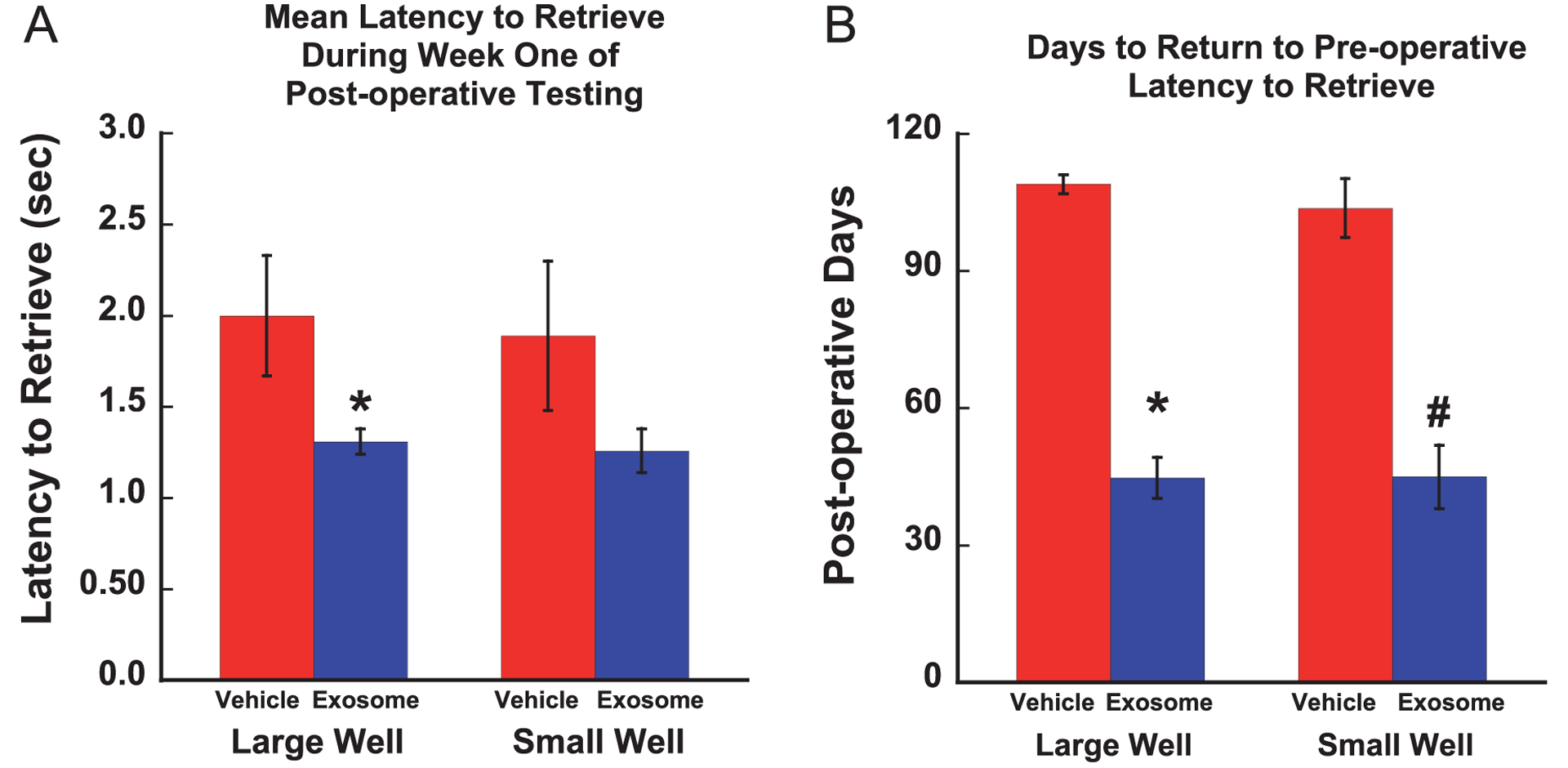
A. Graph of mean latency to retrieve during week one of post-operative testing (* p < 0.05). B. Graph of mean number of post-operative days to return to pre-operative latency to retrieve (*p < 0.0001, #p < 0.0003). Error bars = Standard Error.
As shown in Fig. 5B, separate one-way ANOVAs revealed a significant group difference in the number of days required to return to pre-operative times to retrieve from the large well [F (1, 8) = 170.03, p = 0.0001] and from the small well [F (1, 8) = 38.44, p = 0.0003] with treated monkeys returning to pre-operative time to retrieve the food reward earlier in the recovery period than untreated monkeys. In fact, all 5 treated monkeys returned to pre-operative times between weeks 2 and 5 of post-operative testing (weeks 4–7 post-surgery) while none of the vehicle treated monkeys reached this level of performance, even after 12 weeks of post-operative testing.
3.3. Post-operative grasp assessment
Figure 6 shows the mean grasp rating each day during the post-operative testing period for each monkey (a score of 8 represents a return to pre-operative grasp patterns). In terms of recovery of pre-operative grasp patterns, the treated monkeys demonstrated a greater degree of recovery of grasp in week 1 of post-operative testing (3rd week post-injury) [F (1, 8) = 5.17, p = 0.05] (Fig. 7A) with two treated monkeys demonstrating a complete return to pre-operative grasp function (a score of 8 on the GRAS) while none of the vehicle control monkeys showed this level of recovery during the 1st week of the post-operative period (Fig. 6).
Fig. 6.
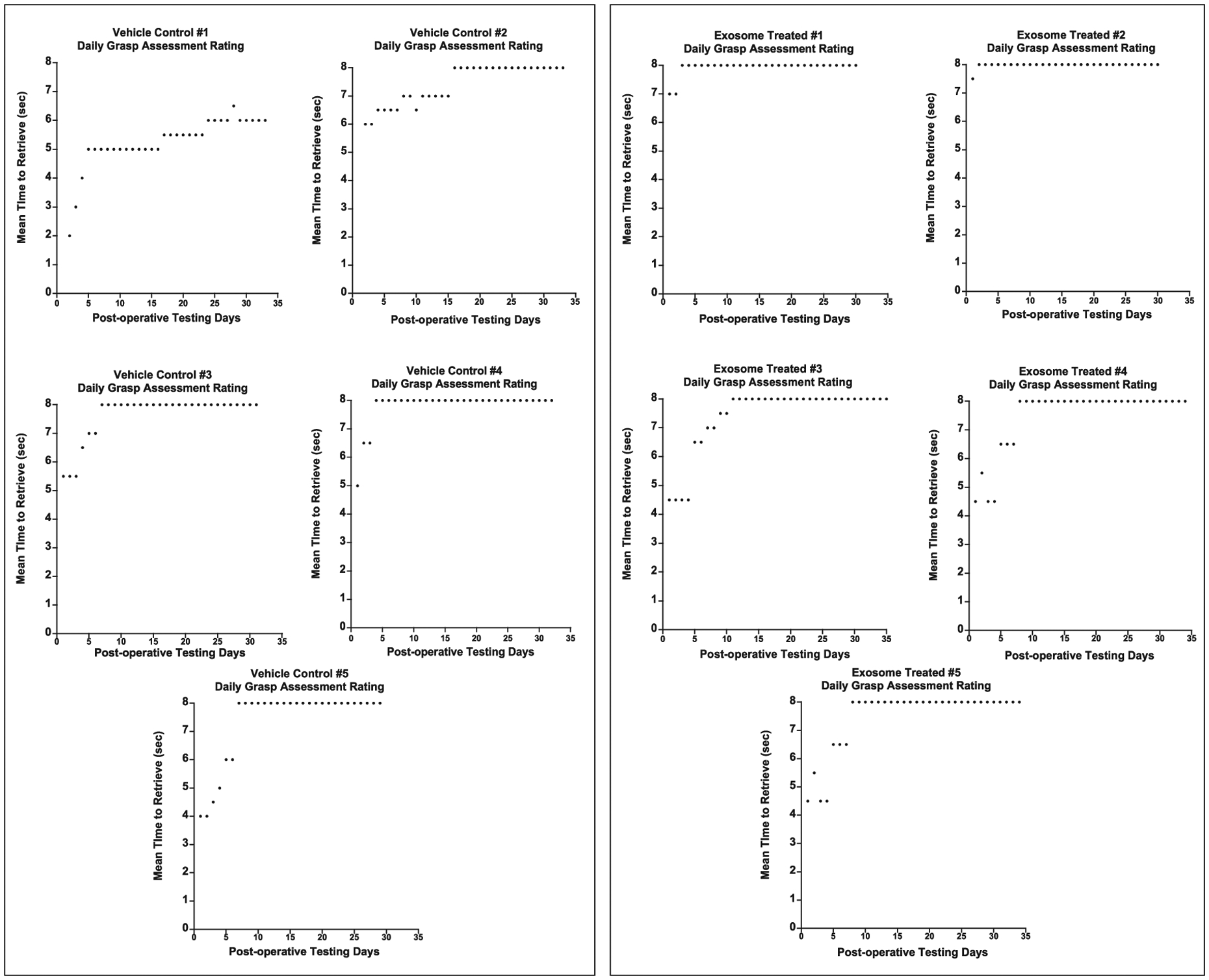
Graphs of the daily mean grasp rating across the post-operative testing days. Testing began 14 days after surgery. A score of 8 represents a normal grasp pattern that was documented during pre-operative training. Each data point (black dots) represents the mean grasp pattern for each post-operative day.
Fig. 7.
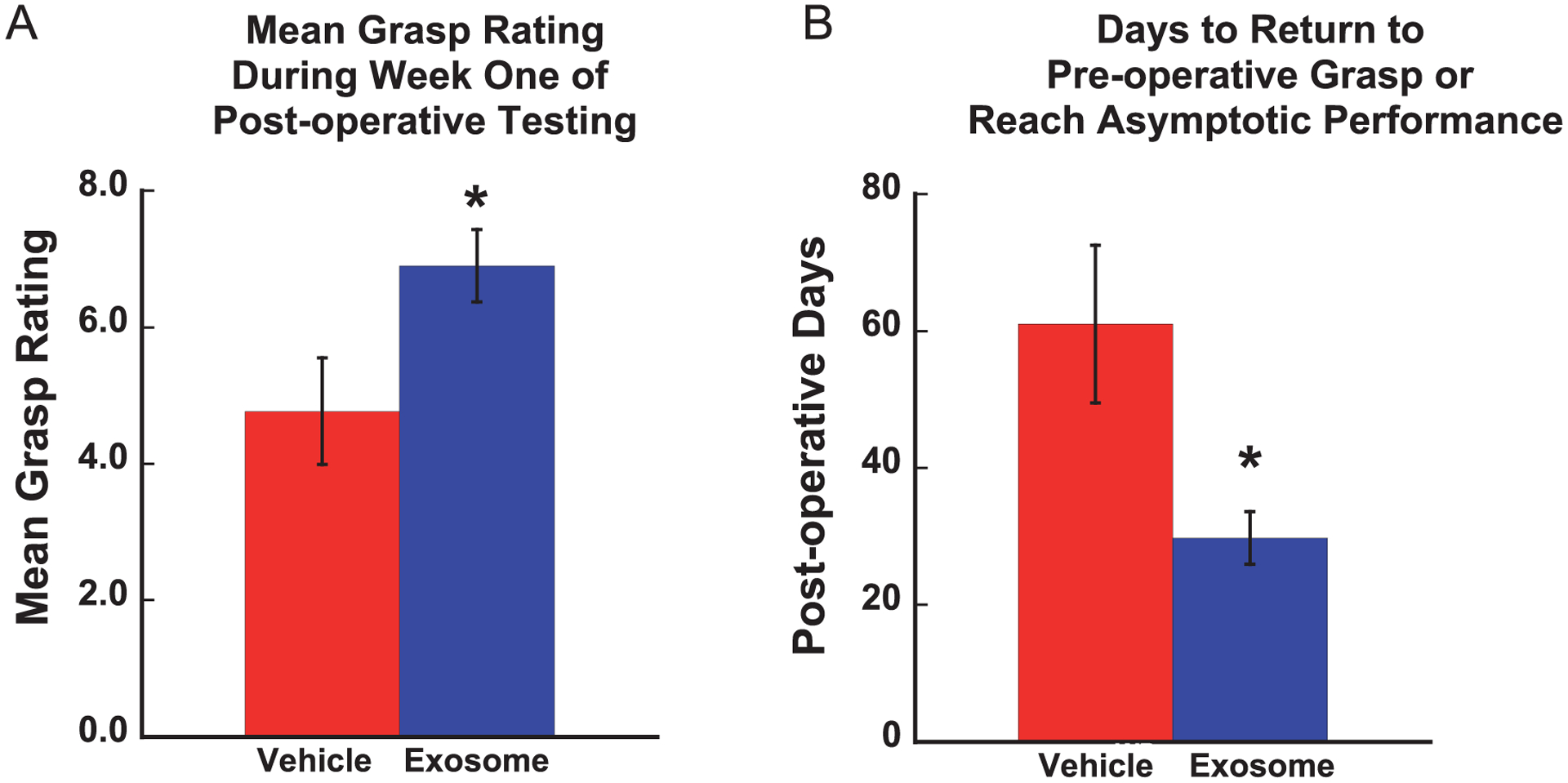
A Graph of mean grasp rating for each group across the post-operative testing days. Testing began 14 days after surgery. A score of 8 represents a return to pre-operative grasp patterns (*p < 0.05). B. Mean number of post-operative days to return to pre-operative grasp patterns or reach asymptotic performance (*p < 0.03). Error bars = Standard Error.
Finally, as shown in Fig. 7B, a one-way ANOVA to compare the mean number of post-operative days to return to pre-operative grasp performance or reach an asymptotic level of compensatory function revealed a significant difference between groups [F (1, 8) = 6.61, p = 0.03] (Fig. 7B). Treated monkeys showed a more complete degree of recovery of grasp pattern earlier in the recovery period than the vehicle control monkeys.
Figure 8 shows representative images of digit use of the impaired hand from one monkey that received vehicle and one monkey that received exosomes, illustrating typical responses at the end of the 12-week evaluation. Panels A&B show compensatory “scooping” involving all fingers of a monkey’s hand in vehicle control group. The fingers work together to retrieve the food reward, a grasp that is referred to as “mass action” of digits and is considered a compensatory grasp pattern. The arrow in panel B shows fingers scooping candy into palm of the hand. Panels C&D shows a precise finger-thumb grasp of a monkey treated with exosomes. This grasp shows isolated digit action and no evidence of “mass action” of the digits and no evidence of compensatory scooping and therefore is representative of recovery of normal function. The arrow in panel D, shows the finger-thumb pinch grasp that is observed during pre-operative testing.
Fig. 8.
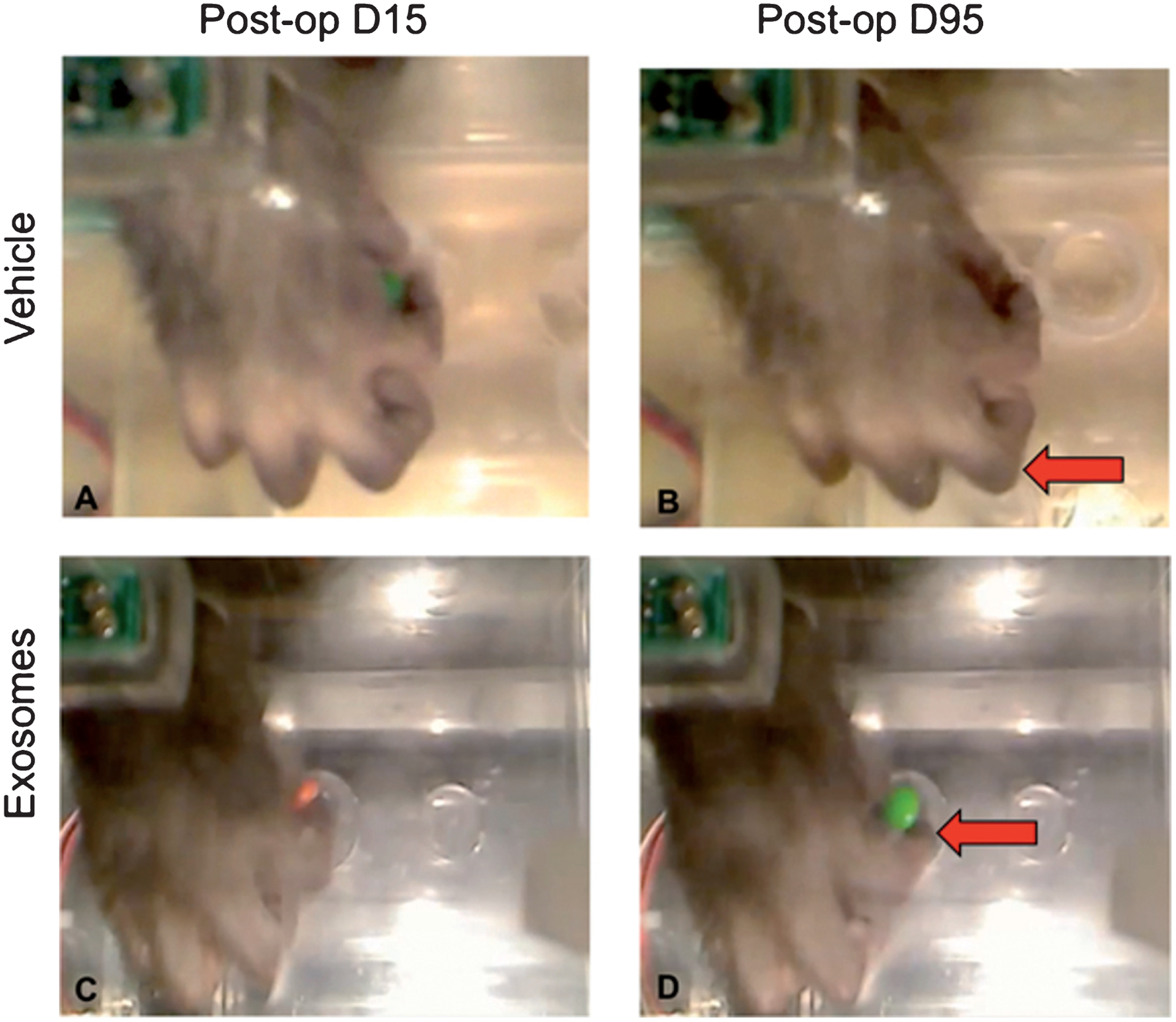
Sequences of post-operative grasp patterns. A&B show compensatory “scooping” involving all fingers of a monkey in vehicle control group. The fingers work together to retrieve the food reward, a grasp that is referred to as “mass action” of digits. Arrow in B shows fingers scooping candy into palm of the hand. C&D shows recovered finger-thumb grasp of a monkey treated with exosomes. This grasp shows isolated digit action (arrow in Panel D) and no evidence of “mass action” of the digits or compensatory scooping.
3.4. Lesion volume
An independent samples Student’s t-test was used to compare the volume of the lesion between the vehicle control and treated animals. Results revealed no significant differences between groups (t = −0.732, p = 0.488; Table 1). Illustrative examples of the lesions in the two groups can be seen in Fig. 2.
Table 1.
| Monkey | Group | Lesion Volume (mm3) |
|---|---|---|
| SM061e | Treated | 44.92 |
| AM332p | Treated | 22.32 |
| AM338p | Treated | 25.32 |
| SM062e | Treated | 81.10 |
| Mean | 43.42 | |
| SD | 27.05 | |
| SE | 13.53 | |
| AM323p | Vehicle Control | 34.03 |
| AM335p | Vehicle Control | 25.81 |
| AM337p | Vehicle Control | 25.83 |
| AM339p | Vehicle Control | 31.27 |
| AM331p | Vehicle Control | 52.18 |
| Mean | 33.83 | |
| SD | 10.86 | |
| SE | 4.86 |
3.5. Qualitative post-mortem assessment of the gross morphology of the lesion
Despite the absence of group differences in lesion volume, as shown in Table 1, qualitative post-mortem inspection of the brains of each monkey after fixation revealed a striking difference in the gross appearance of the lesion in an untreated (Fig. 1C) compared to an exosome-treated monkey (Fig. 1F). The lesion in brains of all vehicle-treated monkeys was demarcated by a prominent accumulation of dark discolored tissue. In contrast, the lesion in brains from the exosome-treated monkeys appeared paler in color and less well-demarcated from the surrounding intact tissue.
4. Discussion
4.1. Exosome treatment facilitates recovery of motor function to pre-injury levels
Cortical damage resulting from stroke or other insults can cause significant impairments of motor function and other behaviors in humans. Though some degree of spontaneous recovery occurs, likely reflecting plasticity in surrounding areas (Carmichael, 2003; Dancause et al., 2005; Nudo, 1999; Ueno et al., 2012; Ward, 2004), to date, there are no effective therapeutic interventions that enhance recovery of function.
In the present study, we demonstrated that administration of MSC derived exosomes in monkeys after cortical injury produces significant functional recovery of fine motor function of the hand with grasp patterns returning to pre-injury levels within the 1st weeks following injury. To our knowledge, this is the first study to demonstrate a return to pre-injury levels of function within the first weeks of recovery in a non-human primate. Specifically, all five monkeys treated with exosomes returned to pre-operative grasp patterns and latency to retrieve a food reward in the first three-five weeks of recovery. These findings are particularly interesting when compared to our previous study showing a positive effect of umbilical derived cells as a treatment for cortical injury (Moore et al., 2013) where three of four treated monkeys returned to pre-operative levels of function though this occurred later in the recovery period (approximately 7–9 weeks post-injury). Therefore, it appears that exosomes, likely the active product of cells, when derived from MSCs and administered alone in a concentrated dose, lead to recovery at an earlier time point in the post-operative period.
It is also of interest to note in this study that while the treated monkeys demonstrated an early, more complete recovery of function, four untreated monkeys also returned to pre-operative grasp patterns, but not pre-operative latencies to retrieve a food reward. In addition, their recovery did not occur until much later in the recovery period. This suggests that their grasp function was still slow and less efficient, and therefore did not represent a full recovery. A return to pre-operative grasp without treatment has not been observed in our NHP model in prior studies with male monkeys without treatment (Moore et al., 2012; Moore et al., 2016; Moore et al., 2013). We hypothesize that this pattern of recovery in the untreated monkeys in this study may be related to sex differences in recovery, often resulting from different levels of estrogen. Therefore, it will be important to further explore exosomes as a treatment in both male and female monkeys.
4.2. Differences in the gross morphology of the lesion in exosome- versus vehicle-treated monkeys
While there was no group difference in overall lesion volume, our qualitative post-mortem assessments of the brains from all monkeys show that, at the gross level, exosome-treatment reduces tissue discoloration of the lesion site, such that it appears more similar to surrounding intact tissue. In contrast, lesions of vehicle-treated monkeys appear to have accumulation of dark tissue material prominently distinguishable from surrounding intact tissue. This difference in lesion appearance was consistent with observations in our earlier study that investigated the efficacy of human umbilical tissue-derived cell (hUTC) therapy in our NHP cortical injury model (Orczykowski et al., 2019) which shows histological evidence of decreased iron accumulation in the lesion area in hUTC treated monkeys, which likely accounts for the reduced tissue discoloration of the lesion observed at the gross level. Follow up analyses of brain tissue from the monkeys in the present study are currently ongoing to assess the histological and cellular changes that underlie the difference in the gross appearance of the lesion between the exosome- and vehicle- treated monkeys. Nevertheless, this striking difference in the appearance of the lesion site between the two groups observed here support an effect of exosome treatment on cortical tissue during recovery from injury.
4.3. Comparison to other studies
The findings in the present study are supported by similar experiments with rodent and swine models of stroke that have demonstrated the significant effect of exosome treatment on recovery of function. (Chen & Chopp, 2018; Venkat, Chen, & Chopp, 2018; Venkat, Chopp, & Chen, 2018; Williams et al., 2018; Xin et al., 2013a,b; Zhang et al., 2016; 2017; Zhang & Chopp, 2016) Specifically, rodents treated with MSC derived exosomes had significantly improved neurological function, spatial learning and performance on motor tasks (Han et al., 2018). In a similar study, using a swine model of traumatic brain injury and hemorrhagic shock, animals treated with exosomes had significantly lower neurological severity scores very early in the recovery period (1st 5 days) (Williams et al., 2018). These findings, taken together with the present findings in an animal model that allows for the distinction between compensatory and complete recovery, provide substantive evidence of the potential of exosomes as a treatment for cortical injury and stroke.
4.4. Potential mechanisms of exosomes as a treatment for injury
In the initial stages following an ischemic event, such as a stroke, the cell death and tissue damage that occur (Anrather & Iadecola, 2016; Brouns & De Deyn, 2009; Graham & Hickey, 2003; Lipton, 1999; Venkat, Chopp, & Chen, 2018) trigger an acute inflammatory response involving recruitment of microglia, astrocytes, and peripheral immune cells to the damaged area (Fumagalli, Perego, Pischiutta, Zanier, & De Simoni, 2015; Kreutzberg, 1996), thus stimulating the release of pro-inflammatory cytokines, proteases, and reactive oxygen species (Patel, Ritzel, McCullough, & Liu, 2013; Venkat, Chopp, & Chen, 2018). At later stages of this cascade, there is a switch to anti-inflammatory mechanisms that promotes repair and plasticity and dampen the pro-inflammatory processes (Fumagalli, Perego, Pischiutta, Zanier, & De Simoni, 2015; Shichita, Ito, & Yoshimura, 2014). MSC derived exosomes contain conglomerates of biologically active material (RNA, miRNA, and proteins) that have the ability to modulate several processes in this ischemic cascade (Burrello et al., 2016) and likely facilitate the shift of microglia from a proinflammatory to anti-inflammatory state earlier in the recovery period (Li et al., 2017; Thomi, Surbek, Haesler, Joerger-Messerli, & Schoeberlein, 2019). Since the treated monkeys in the present study showed a robust recovery within the first weeks of recovery, we hypothesize that MSC derived exosomes did in fact promote the switch from pro- to anti-inflammatory mechanisms early in the recovery period (Zhang et al., 2015, 2016, 2017; Zhang & Chopp, 2016). In support of this hypothesis, there is evidence that MSC exosomes suppress immune activity of peripheral immune cells and subsequently increase the level of anti-inflammatory cytokines (Di Trapani et al., 2016; Phinney et al., 2015; Zhang et al., 2014). Further, studies have demonstrated that exosomes modulate a LPS stimulated murine microglia cell line BV-2 by reducing the release of pro-inflammatory cytokines (especially IL-6 and TNF-alpha), inhibiting the upregulation of cell surface molecules and stimulating the transcription of anti-inflammatory cytokines (Jaimes, Naaldijk, Wenk, Leovsky, & Emmrich, 2017; Li et al., 2017; Thomi, Surbek, Haesler, Joerger-Messerli, & Schoeberlein, 2019). Finally, exosomes significantly reduced pro-inflammatory microglial M1 phenotype cell markers in a rodent model of TBI (Li et al., 2017). Based on these reports in the literature and our recovery data, the effects of exosomes on microglial and other markers of inflammation in the brains from the NHPs in the present study are currently being investigated.
5. Summary
Our results show a significant enhancement of recovery of fine motor function in monkeys treated with exosomes following cortical injury. This recovery represents a more complete recovery of function without evidence of compensatory grasp function. These positive results provide support for the potential value of exosomes as a therapeutic to enhance recovery likely by reducing inflammation and facilitating cortical plasticity following cortical injury in primates. Towards this end, the analysis of the brain tissue from the monkeys in this study is currently ongoing to identify the neural substrates facilitated by exosomes that leads to recovery of function.
Acknowledgments
The authors wish to thank Karen Slater, Reese Edwards, Katelyn Trecartin, Ajay Uprety and Penny Shultz for their assistance with this project.
Sources of funding
NIH-NINDS R21NS102991, NIH-NIA R01AG0 43478, AHA 13SDG16330024 and NIH-NINDS NS 075156. BU-CTSI Grant Number 1UL1TR001430.
References
- Agoston DV (2017). How to translate time? The temporal aspect of human and rodent biology. Frontiers in Neurology, 8, 92. doi: 10.3389/fneur.2017.00092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anrather J, & Iadecola C (2016). Inflammation and stroke: An overview. Neurotherapeutics, 13(4), 661–670. doi: 10.1007/s13311-016-0483-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brouns R, & De Deyn PP (2009). The complexity of neurobiological processes in acute ischemic stroke. Clinical Neurology and Neurosurgery, 111(6), 483–495. doi: 10.1016/j.clineuro.2009.04.001 [DOI] [PubMed] [Google Scholar]
- Burrello J, Monticone S, Gai C, Gomez Y, Kholia S, & Camussi G (2016). Stem cell-derived extracellular vesicles and immune-modulation. Frontiers in Cell and Developmental Biology, 4, 83. doi: 10.3389/fcell.2016.00083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carmichael ST (2003). Plasticity of cortical projections after stroke. Neuroscientist, 9(1), 64–75. [DOI] [PubMed] [Google Scholar]
- Carr JH, Shepherd RB, Nordholm L, & Lynne D (1985). Investigation of a new motor assessment scale for stroke patients. Physical Therapy, 65(2), 175–180. [DOI] [PubMed] [Google Scholar]
- Chen J, & Chopp M (2018). Exosome therapy for stroke. Stroke, 49(5), 1083–1090. doi: 10.1161/strokeaha.117.018292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chopp M, Li Y, & Zhang ZG (2009). Mechanisms underlying improved recovery of neurological function after stroke in the rodent after treatment with neurorestorative cell-based therapies. Stroke, 40(3 Suppl), S143–S145. doi: 10.1161/STROKEAHA.108.533141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chopp M, & Zhang ZG (2015). Emerging potential of exosomes and noncoding microRNAs for the treatment of neurological injury/diseases. Expert Opinion on Emerging Drugs, 20(4), 523–526. doi: 10.1517/14728214.2015.1061993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbetta D, Sirtori V, Castellini G, Moja L, & Gatti R (2015). Constraint-induced movement therapy for upper extremities in people with stroke. Cochrane Database Systematic Reviews (10), CD004433. doi: 10.1002/14651858.CD004433.pub3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crack PJ, & Taylor JM (2005). Reactive oxygen species and the modulation of stroke. Free Radical Biology and Medicine, 38(11), 1433–1444. doi: 10.1016/j.freeradbiomed.2005.01.019 [DOI] [PubMed] [Google Scholar]
- Dancause N, Barbay S, Frost SB, Plautz EJ, Chen D, Zoubina EV, … & Nudo RJ (2005). Extensive cortical rewiring after brain injury. Journal of Neuroscience, 25(44), 10167–10179. doi: 10.1523/JNEUROSCI.3256-05.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Trapani M, Bassi G, Midolo M, Gatti A, Kamga PT, Cassaro A, … & Krampera M (2016). Differential and transferable modulatory effects of mesenchymal stromal cell-derived extracellular vesicles on T, B and NK cell functions. Science Report, 6, 24120. doi: 10.1038/srep24120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Estrada LI, Robinson AA, Amaral AC, Giannaris EL, Heyworth NC, Mortazavi F, … & Rosene DL (2017). Evaluation of long-term cryostorage of brain tissue sections for quantitative histochemistry. Journal of Histochemistry & Cytochemistry, 65(3), 153–171. doi: 10.1369/0022155416686934 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fugl-Meyer AR, Jaasko L, Leyman I, Olsson S, & Steglind S (1975). The post-stroke hemiplegic patient. 1. a method for evaluation of physical performance. Scandinavian Journal of Rehabilitation Medicine, 7(1), 13–31. [PubMed] [Google Scholar]
- Fumagalli S, Perego C, Pischiutta F, Zanier ER, & De Simoni MG (2015). The ischemic environment drives microglia and macrophage function. Frontiers in Neurology, 6, 81. doi: 10.3389/fneur.2015.00081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham SH, & Hickey RW (2003). Cyclooxygenases in central nervous system diseases: A special role for cyclooxygenase 2 in neuronal cell death. Archives of Neurology, 60(4), 628–630. doi: 10.1001/archneur.60.4.628 [DOI] [PubMed] [Google Scholar]
- Han Y, Seyfried D, Meng Y, Yang D, Schultz L, & Chopp M (2018). Multipotent mesenchymal stromal cell-derived exosomes improve functional recovery after experimental intracerebral hemorrhage in the rat. Journal of Neurosurgery, 1–11. doi: 10.3171/2018.2.JNS171475 [DOI] [PubMed] [Google Scholar]
- Hylin MJ, Kerr AL, & Holden R (2017). Understanding the mechanisms of recovery and/or compensation following injury. Neural Plasticity, 2017, 7125057. doi: 10.1155/2017/7125057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaimes Y, Naaldijk Y, Wenk K, Leovsky C, & Emmrich F (2017). Mesenchymal stem cell-derived microvesicles modulate lipopolysaccharides-induced inflammatory responses to microglia cells. Stem Cells, 35(3), 812–823. doi: 10.1002/stem.2541 [DOI] [PubMed] [Google Scholar]
- Jeong CH, Kim SM, Lim JY, Ryu CH, Jun JA, & Jeun SS (2014). Mesenchymal stem cells expressing brain-derived neurotrophic factor enhance endogenous neurogenesis in an ischemic stroke model. BioMed Research International, 2014, 129145. doi: 10.1155/2014/129145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katakowski M, Buller B, Zheng X, Lu Y, Rogers T, Osobamiro O, … & Chopp M (2013). Exosomes from marrow stromal cells expressing miR-146b inhibit glioma growth. Cancer Letters, 335(1), 201–204. doi: 10.1016/j.canlet.2013.02.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller S, Sanderson MP, Stoeck A, & Altevogt P (2006). Exosomes: From biogenesis and secretion to biological function. Immunology Letters, 107(2), 102–108. doi: 10.1016/j.imlet.2006.09.005 [DOI] [PubMed] [Google Scholar]
- Kluver H (1935). An auto-multi-stimulation reaction board for use with sub-human primates. The Journal of Psychology: Interdisciplinary and Applied, 1, 123–127. [Google Scholar]
- Korkut C, Li Y, Koles K, Brewer C, Ashley J, Yoshihara M, & Budnik V (2013). Regulation of postsynaptic retrograde signaling by presynaptic exosome release. Neuron, 77(6), 1039–1046. doi: 10.1016/j.neuron.2013.01.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreutzberg GW (1996). Microglia: A sensor for pathological events in the CNS. Trends in Neurosciences, 19(8), 312–318. [DOI] [PubMed] [Google Scholar]
- Kwakkel G, Winters C, van Wegen EE, Nijland RH, van Kuijk AA, Visser-Meily A, … & Consortium, EXPLICIT-Stroke. (2016). Effects of unilateral upper limb training in two distinct prognostic groups early after stroke: The EXPLICIT-stroke randomized clinical trial. Neurorehabilitation and Neural Repair. doi: 10.1177/1545968315624784 [DOI] [PubMed] [Google Scholar]
- Lakhan SE, Kirchgessner A, & Hofer M (2009). Inflammatory mechanisms in ischemic stroke: Therapeutic approaches. Journal of Translational Medicine, 7, 97. doi: 10.1186/1479-5876-7-97 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambertsen KL, Finsen B, & Clausen BH (2018). Post-stroke inflammation-target or tool for therapy? Acta Neuropathologica. doi: 10.1007/s00401-018-1930-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee Y, El Andaloussi S, & Wood MJ (2012). Exosomes and microvesicles: Extracellular vesicles for genetic information transfer and gene therapy. Human Molecular Genetics, 21(R1), R125–R134. doi: 10.1093/hmg/dds317 [DOI] [PubMed] [Google Scholar]
- Levin MF, Kleim JA, & Wolf SL (2009). What do motor “recovery” and “compensation” mean in patients following stroke? Neurorehabilitation and Neural Repair, 23(4), 313–319. doi: 10.1177/1545968308328727 [DOI] [PubMed] [Google Scholar]
- Li Y, Yang YY, Ren JL, Xu F, Chen FM, & Li A (2017). Exosomes secreted by stem cells from human exfoliated deciduous teeth contribute to functional recovery after traumatic brain injury by shifting microglia M1/M2 polarization in rats. Stem Cell Research & Therapy, 8(1), 198. doi: 10.1186/s13287-017-0648-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipton P (1999). Ischemic cell death in brain neurons. Physiological Reviews, 79(4), 1431–1568. doi: 10.1152/phys-rev.1999.79.4.1431 [DOI] [PubMed] [Google Scholar]
- Liu Z, Li Y, Zhang ZG, Cui X, Cui Y, Lu M, … & Chopp M (2010). Bone marrow stromal cells enhance inter- and intracortical axonal connections after ischemic stroke in adult rats. Journal of Cerebral Blood Flow & Metabolism, 30(7), 1288–1295. doi: 10.1038/jcbfm.2010.8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lum PS, Mulroy S, Amdur RL, Requejo P, Prilutsky BI, & Dromerick AW (2009). Gains in upper extremity function after stroke via recovery or compensation: Potential differential effects on amount of real-world limb use. Topics in Stroke Rehabilitation, 16(4), 237–253. doi: 10.1310/tsr1604-237 [DOI] [PubMed] [Google Scholar]
- Mahmood A, Lu D, Qu C, Goussev A, & Chopp M (2005). Human marrow stromal cell treatment provides long-lasting benefit after traumatic brain injury in rats. Neurosurgery, 57(5), 1026–1031; discussion 1026–1031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Margaill I, Plotkine M, & Lerouet D (2005). Antioxidant strategies in the treatment of stroke. Free Radical Biology and Medicine, 39(4), 429–443. doi: 10.1016/j.freeradbiomed.2005.05.003 [DOI] [PubMed] [Google Scholar]
- Moore TL, Killiany RJ, Pessina MA, Moss MB, & Rosene DL (2010). Assessment of motor function of the hand in aged rhesus monkeys. Somatosensory and Motor Research, 27(3), 121–130. doi: 10.3109/08990220.2010.485963 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore TL, Killiany RJ, Pessina MA, Moss MB, Finklestein SP, & Rosene DL (2012). Recovery from ischemia in the middle-aged brain: A nonhuman primate model. Neurobiology of Aging, 33(3), 619 e619–619 e624. doi: 10.1016/j.neurobiolaging.2011.02.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore TL, Pessina MA, Finklestein SP, Kramer BC, Killiany RJ, & Rosene DL (2013). Recovery of fine motor performance after ischemic damage to motor cortex is facilitated by cell therapy in the rhesus monkey. Somatosensory and Motor Research, 30(4), 185–196. doi: 10.3109/08990220.2013.790806 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore TL, Pessina MA, Finklestein SP, Killiany RJ, Bowley B, Benowitz L, & Rosene DL (2016). Inosine enhances recovery of grasp following cortical injury to the primary motor cortex of the rhesus monkey. Restorative Neurology and Neuroscience, 34(5), 827–848. doi: 10.3233/RNN-160661 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morel L, Regan M, Higashimori H, Ng SK, Esau C, Vidensky S, … & Yang Y, (2013). Neuronal exosomal miRNA-dependent translational regulation of astroglial glutamate transporter GLT1. Journal of Biological Chemistry, 288(10), 7105–7116. doi: 10.1074/jbc.M112.410944 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nudo RJ (1999). Recovery after damage to motor cortical areas. Current Opinion in Neurobiology, 9(6), 740–747. [DOI] [PubMed] [Google Scholar]
- Orczykowski ME, Arndt KR, Palitz LE, Kramer BC, Pessina MA, Oblak AL, … & Moore TL (2018). Cell based therapy enhances activation of ventral premotor cortex to improve recovery following primary motor cortex injury. Experimental Neurology, 305, 13–25. doi: 10.1016/j.expneurol.2018.03.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orczykowski ME, Calderazzo SM, Shobin E, Pessina MA, Oblak AL, Finklestein SP, … & Moore TL (2019). Cell based therapy reduces secondary damage and increases extent of microglial activation following cortical injury. Brain Research, 1717, 147–159. 10.1016/j.brainres.2019.04.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel AR, Ritzel R, McCullough LD, & Liu F (2013). Microglia and ischemic stroke: A double-edged sword. International Journal of Physiology, Pathophysiology and Pharmacology, 5(2), 73–90. [PMC free article] [PubMed] [Google Scholar]
- Pessina MA, Bowley BGE, Rosene DL, & Moore TL (2019). A method for assessing recovery of fine motor function in the rhesus monkey: An adaptation of the fugl-meyer scale and eshkol-wachman movement notation. Somatosensory and Motor Research, 36(1) 69–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phinney DG, Di Giuseppe M, Njah J, Sala E, Shiva S, St Croix CM, … & Ortiz LA (2015). Mesenchymal stem cells use extracellular vesicles to outsource mitophagy and shuttle microRNAs. Nature Communications, 6, 8472. doi: 10.1038/ncomms9472 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rana S, Malinowska K, & Zoller M (2013). Exosomal tumor microRNA modulates premetastatic organ cells. Neoplasia, 15(3), 281–295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosene DL, Roy NJ, & Davis BJ (1986). A cryoprotection method that facilitates cutting frozen sections of whole monkey brains for histological and histochemical processing without freezing artifact. Journal of Histochemistry and Cytochemistry, 34(10), 1301–1315. [DOI] [PubMed] [Google Scholar]
- Shichita T, Ito M, & Yoshimura A (2014). Post-ischemic inflammation regulates neural damage and protection. Frontiers in Cellular Neuroscience, 8, 319. doi: 10.3389/fncel.2014.00319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simons M, & Raposo G (2009). Exosomes–vesicular carriers for intercellular communication. Current Opinion in Cell Biology, 21(4), 575–581. doi: 10.1016/j.ceb.2009.03.007 [DOI] [PubMed] [Google Scholar]
- Souza WC, Conforto AB, Orsini M, Stern A, & André C (2015). Similar effects of two modified constraint-induced therapy protocols on motor impairment, motor function and quality of life in patients with chronic stroke. Neurology International, 7(1), 5430. doi: 10.4081/ni.2015.5430 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stonesifer C, Corey S, Ghanekar S, Diamandis Z, Acosta SA, & Borlongan CV (2017). Stem cell therapy for abrogating stroke-induced neuroinflammation and relevant secondary cell death mechanisms. Progress in Neurobiology, 158, 94–131. doi: 10.1016/j.pneurobio.2017.07.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomi G, Surbek D, Haesler V, Joerger-Messerli M, & Schoeberlein A (2019). Exosomes derived from umbilical cord mesenchymal stem cells reduce microglia-mediated neuroinflammation in perinatal brain injury. Stem Cell Research & Therapy, 10(1), 105. doi: 10.1186/s13287-019-1207-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ueno Y, Chopp M, Zhang L, Buller B, Liu Z, Lehman NL, … & Zhang ZG (2012). Axonal outgrowth and dendritic plasticity in the cortical peri-infarct area after experimental stroke. Stroke. doi: STROKEAHA.111.646224 [pii] 10.1161/STROKEAHA.111.646224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venkat P, Chen J, & Chopp M (2018). Exosome-mediated amplification of endogenous brain repair mechanisms and brain and systemic organ interaction in modulating neurological outcome after stroke. Journal of Cerebral Blood Flow & Metabolism, 38(12), 2165–2178. doi: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venkat P, Chopp M, & Chen J (2018). Cell-based and exosome therapy in diabetic stroke. Stem Cells Translational Medicine, 7(6), 451–455. doi: 10.1002/sctm.18-0014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Q, Tang XN, & Yenari MA (2007). The inflammatory response in stroke. Journal of Neuroimmunology, 184(1–2), 53–68. doi: 10.1016/j.jneuroim.2006.11.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang W, Li Z, & Feng J (2018). The potential role of exosomes in the diagnosis and therapy of ischemic diseases. Cytotherapy, 20(10), 1204–1219. doi: 10.1016/j.jcyt.2018.06.012 [DOI] [PubMed] [Google Scholar]
- Ward NS (2004). Functional reorganization of the cerebral motor system after stroke. Current Opinion in Neurology, 17(6), 725–730. [DOI] [PubMed] [Google Scholar]
- Warner DS, Sheng H, & Batinić-Haberle I (2004). Oxidants, antioxidants and the ischemic brain. Journal of Experimental Biology, 207(Pt 18), 3221–3231. doi: 10.1242/jeb.01022 [DOI] [PubMed] [Google Scholar]
- Whishaw IQ, Suchowersky O, Davis L, Sarna J, Metz GA, & Pellis SM (2002). Impairment of pronation, supination, and body co-ordination in reach-to-grasp tasks in human Parkinson’s disease (PD) reveals homology to deficits in animal models. Behavarioral Brain Research, 133(2), 165–176. [DOI] [PubMed] [Google Scholar]
- Williams AM, Dennahy IS, Bhatti UF, Halaweish I, Xiong Y, Chang P, … & Alam HB (2018). Mesenchymal stem cell-derived exosomes provide neuroprotection and improve long-term neurologic outcomes in a swine model of traumatic brain injury and hemorrhagic shock. Journal of Neurotrauma. doi: 10.1089/neu.2018.5711 [DOI] [PubMed] [Google Scholar]
- Xin H, Li Y, Buller B, Katakowski M, Zhang Y, Wang X, … Chopp M (2012). Exosome-mediated transfer of miR-133b from multipotent mesenchymal stromal cells to neural cells contributes to neurite outgrowth. Stem Cells, 30(7), 1556–1564. doi: 10.1002/stem.1129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xin H, Li Y, Cui Y, Yang JJ, Zhang ZG, & Chopp M (2013a). Systemic administration of exosomes released from mesenchymal stromal cells promote functional recovery and neurovascular plasticity after stroke in rats. Journal of Cerebral Blood Flow & Metabolism, 33(11), 1711–1715. doi: 10.1038/jcbfm.2013.152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xin H, Li Y, Liu Z, Wang X, Shang X, Cui Y, … & Chopp M (2013b). MiR-133b promotes neural plasticity and functional recovery after treatment of stroke with multipotent mesenchymal stromal cells in rats via transfer of exosome-enriched extracellular particles. Stem Cells, 31(12), 2737–2746. doi: 10.1002/stem.1409 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xin H, Wang F, Li Y, Lu QE, Cheung WL, Zhang Y, … & Chopp M (2017). Secondary release of exosomes from astrocytes contributes to the increase in neural plasticity and improvement of functional recovery after stroke in rats treated with exosomes harvested from microRNA 133b-overexpressing multipotent mesenchymal stromal cells. Cell Transplantation, 26(2), 243–257. doi: 10.3727/096368916X693031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiong Y, Zhang Y, Mahmood A, Meng Y, Qu C, & Chopp M (2011). Erythropoietin mediates neurobehavioral recovery and neurovascular remodeling following traumatic brain injury in rats by increasing expression of vascular endothelial growth factor. Translational Stroke Research, 2(4), 619–632. doi: 10.1007/s12975-011-0120-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu D, & Tahara H (2013). The role of exosomes and microRNAs in senescence and aging. Advanced Drug Delivery Reviews, 65(3), 368–375. doi: 10.1016/j.addr.2012.07.010 [DOI] [PubMed] [Google Scholar]
- Zhang Z, Andersen A, Smith C, Grondin R, Gerhardt G, & Gash D (2000). Motor slowing and parkinsonian signs in aging rhesus monkeys mirror human aging. The Journals of Gerontology. Series A, Biological Sciences and Medical Sciences, 55(10), B473–B480. [DOI] [PubMed] [Google Scholar]
- Zhang B, Yin Y, Lai RC, Tan SS, Choo AB, & Lim SK (2014). Mesenchymal stem cells secrete immunologically active exosomes. Stem Cells and Development, 23(11), 1233–1244. doi: 10.1089/scd.2013.0479 [DOI] [PubMed] [Google Scholar]
- Zhang Y, Chopp M, Meng Y, Katakowski M, Xin H, Mahmood A, & Xiong Y (2015). Effect of exosomes derived from multipluripotent mesenchymal stromal cells on functional recovery and neurovascular plasticity in rats after traumatic brain injury. Journal of Neurosurgery, 122(4), 856–867. doi: 10.3171/2014.11.jns14770 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang ZG, & Chopp M (2016). Exosomes in stroke pathogenesis and therapy. Journal of Clinical Investigation, 126(4), 1190–1197. doi: 10.1172/jci81133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Chopp M, Liu XS, Katakowski M, Wang X, Tian X, … & Zhang ZG (2016). Exosomes Derived from Mesenchymal Stromal Cells Promote Axonal Growth of Cortical Neurons. Molecular Neurobiology. doi: 10.1007/s12035-016-9851-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Chopp M, Zhang ZG, Katakowski M, Xin H, Qu C, … & Xiong Y (2017). Systemic administration of cell-free exosomes generated by human bone marrow derived mesenchymal stem cells cultured under 2D and 3D conditions improves functional recovery in rats after traumatic brain injury. Neurochemistry International, 111, 69–81. doi: 10.1016/j.neuint.2016.08.003 [DOI] [PMC free article] [PubMed] [Google Scholar]


