Abstract
Poor pregnancy outcomes such as recurrent pregnancy loss (RPL) and preeclampsia are associated with impaired decidualization and abnormal trophoblast invasion. Emerging evidence suggests that use of corticosteroids, including prednisolone affects fertility by altering uterine function and may be associated with preeclampsia incidence. In this study, using primary and gestational-age appropriate tissue, we aimed to define the effect of prednisolone on human endometrial stromal fibroblast (hESF) decidualization and determine whether hESF decidualization in the presence of prednisolone would alter hESF regulation of trophoblast function. We found that prednisolone treatment reduced hESF cytokine expression (IL6, IL11, IL18, LIF, and LIFR) but had no effect on hESF expression or secretion of the classic markers of decidualization [prolactin (PRL) and IGFBP1]. Using proteomics we determined that prednisolone altered decidualized hESF protein production, enriching hESF proteins associated with acetylation and mitrochondria. Conditioned media from hESF decidualized in the presence of prednisolone significantly enhanced trophoblast outgrowth and trophoblast mRNA expression of cell motility gene PLCG1 and reduced trophoblast production of PGF. Prednisolone treatment during the menstrual cycle and 1st trimester of pregnancy might alter decidual interactions with other cells, including invasive trophoblast.
Keywords: prednisolone, decidualization, trophoblast, recurrent pregnancy loss, preeclampsia
Introduction
To prepare for embryo implantation and pregnancy, uterine endometrial stromal fibroblast [human(h)ESF] differentiate or “decidualize” in response to progesterone to become decidual cells (Evans et al., 2016). Decidualization is initiated immediately post-ovulation under the control of progesterone and involves the reprogramming of hESF such that different genes are expressed at different stages of differentiation (Popovici et al., 2000). During implantation extravillous trophoblast (EVT) invades into the decidualized endometrium (decidua) and upper third of the myometrium (Lunghi et al., 2007). The decidua produces factors which regulate trophoblast invasion (Dimitriadis et al., 2005; Lunghi et al., 2007; Burton et al., 2010; Menkhorst et al., 2012, 2015, 2019; Pollheimer et al., 2018) and protect the conceptus from the maternal immune system and oxidative stress (Evans et al., 2016; Okada et al., 2018). Poor pregnancy outcomes including recurrent pregnancy loss (RPL) and preeclampsia are associated with impaired decidualization (Founds et al., 2009; Salker et al., 2010; Dimitriadis et al., 2020).
Prednisolone is a corticosteroid which classically acts via the glucocorticoid receptor (GR) and has anti-inflammatory and immuno-modulatory effects (Frolkis et al., 2010). Prednisolone administration during the menstrual cycle and early pregnancy may affect endometrial stromal and trophoblast cells: GR are present in the glandular epithelium and stromal cells of the endometrium and 1st trimester decidua (Henderson et al., 2003) as well as trophoblast cells of the 1st trimester placenta (Yang et al., 2016; Kisanga et al., 2018). Murine models suggest that prednisolone or other corticosteroid administration during early pregnancy affects fertility and pregnancy outcome via actions on the uterus (Matejevic et al., 1995; Li et al., 2018; Kieffer et al., 2020), however, the precise impact of these drugs on endometrial cells specifically is unknown.
Prednisolone is used an off-label therapy for RPL to reduce uterine Natural Killer (uNK) cell numbers (Dimitriadis et al., 2020). The reported efficacy of prednisolone at preventing miscarriage in women with a history of idiopathic miscarriage is highly variable between studies (Tang et al., 2013; Gomaa et al., 2014; Dan et al., 2015; Cooper et al., 2019) however, the most recent systematic review and meta-analysis found that prednisolone administration did not improve miscarriage rates or pregnancy outcome (Woon et al., 2020). Concerningly, there is emerging evidence linking corticosteroid use to preeclampsia incidence in women (Boyd et al., 2015; Bandoli et al., 2017) and dexamethasone treatment in pregnant rats induces the development of PE features (Zhang et al., 2016, 2018), however, most studies investigating the role of prednisolone in RPL are not sufficiently powered to identify rare pregnancy outcomes such as preeclampsia.
We hypothesized that prednisolone treatment may have off-target actions on endometrial stromal fibroblasts, affecting decidualization, decidual regulation of trophoblast function and ultimately the formation of a healthy placenta. In this study, using primary and gestational-age appropriate tissue, we aimed to define the effect of prednisolone on hESF decidualization and determine whether hESF decidualized in the presence of prednisolone would differently regulate trophoblast function.
Materials and Methods
This study was conducted under approvals from The Royal Women’s Hospital and Monash Health Human Research and Ethics Committees (#90317B, #06014C, and #03066B). Written and informed consent was obtained from each patient before surgery. All experiments were performed in accordance with the NHMRC guidelines for ethical conduct in human research.
Endometrial biopsies were collected by dilatation and curettage from women (n = 15 women; age 36.4 ± 1.3 years; range 29–46 years). Women were fertile (n = 2/15), primary infertile (n = 3/15; unable to conceive for ≥12 months), secondary infertile (n = 9/15; unable to conceive for >6–12 months but who have had a previous successful pregnancy), or unknown fertility (n = 1/15; have not attempted to conceive). 4/15 women had polyps, 1/15 had endometriosis, 1/15 had PCOS, 2/15 had menorrhagia and the reminder (7/15) had no obvious endometrial pathology. The women had no hormonal treatment for ≥3 months before tissue collection, however, 2/15 were prescribed prednisolone.
Products of conception were collected from first trimester pregnancies (n = 9; amenorrhea 5–13 weeks) following elective termination of pregnancy by evacuation for psychosocial reasons.
Culture Conditions
All cells were cultured at 37°C in a 5% CO2 humidified culture incubator. hESF were maintained in DMEM/F12 (Gibco, Thermo Fisher Scientific, Inc.) plus 10% charcoal stripped Fetal Bovine serum (FBS; Gibco, Thermo Fisher Scientific, Inc.) and 1% antibiotics (penicillin, streptomycin, amphotericin B; Gibco, Thermo Fisher Scientific, Inc.). Isolated EVTs were maintained in DMEM/F12 containing 10% heat-inactivated FBS (Gibco, Thermo Fisher Scientific, Inc.) and 1% antibiotics.
Decidualization
hESF were isolated as previously described by collagenase digestion and filtration (Menkhorst et al., 2017) which results in a 97% pure stromal cell culture (Dimitriadis et al., 2002). hESF were decidualized as previously described (Menkhorst et al., 2017). Briefly, hESF were treated with estradiol (E, 10–8 M; Sigma) alone or E plus medroxyprogesterone acetate (MPA, 10–7 M; Sigma) in DMEM/F12 containing 2% charcoal stripped FBS and 1% antibiotics for up to 14 days. The media was refreshed every 2–3 days, on a Monday, Wednesday, and Friday. 9/15 cultures (eight secondary infertile, one fertile) were frozen after isolation and subsequently thawed for decidualization, proteomics and trophoblast experiments; 6/15 (two fertile, three primary infertile, and one unknown) were decidualized without being frozen and thawed for decidualization and trophoblast experiments.
Prednisolone Treatment
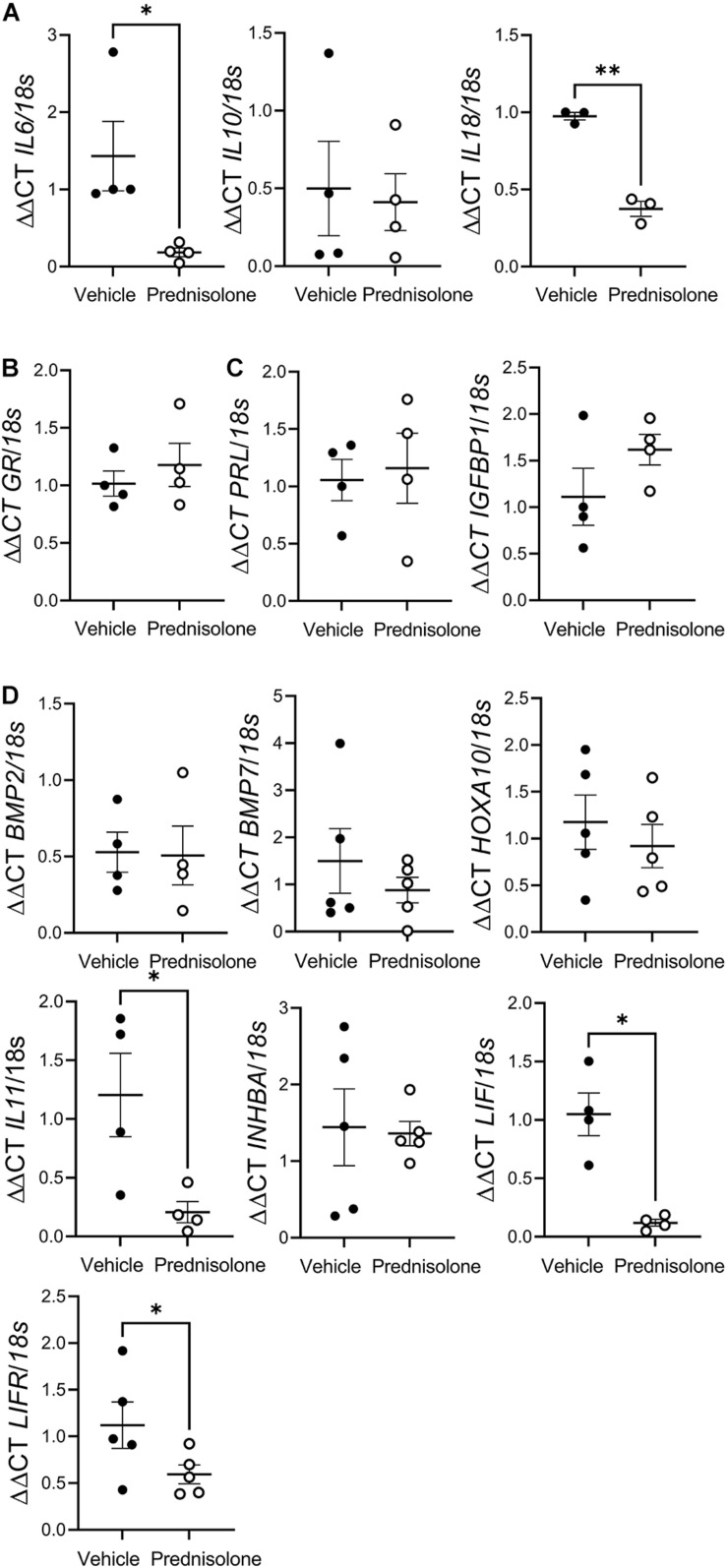
To determine the effect of prednisolone on hESF gene expression, non-decidualized hESF (n = 5 biological replicates) were treated with prednisolone (0.5 μg/ml; Aspen Pharmacare; vehicle control DMSO) for 16 h. To determine the effect of prednisolone treatment during decidualization, hESF (n = 11 biological replicates) undergoing in vitro decidualization as described above were treated with prednisolone (0.5 μg/ml) for 12–13 days. On days 9/10 or 12/13 media that had been incubated with cells for 48 h (conditioned media, CM; added to cells either days 7/8 or 10/11) was collected for prolactin (PRL) or insulin-like growth factor binding protein 1 (IGFBP1) ELISA. The day of collection varied as decidualization treatments were started as soon as the hESF were confluent and media was only changed on Monday, Wednesday, and Friday. On day 13 hESF were washed twice with PBS and cells were cultured for a further 24 h in decidualization treatment (E + MPA) only (prednisolone washout). Cells and CM collected from decidualized hESF on day 14 was pooled and used for trophoblast/outgrowth treatments and day 14 hESF cells (n = 3) were used for gene expression and proteomics analyses. The dose of prednisolone was determined from concentration in plasma following a single 20 mg dose (Wilson et al., 1977) and subsequent trial of various doses (0.05, 0.5, and 5 μg/ml) in vitro. We found 0.5 μg/ml prednisolone effectively suppressed hESF pro-inflammatory cytokine production (Figure 1A).
FIGURE 1.
Prednisolone suppressedpro-inflammatory cytokine production by human endometrial stromalfibroblast (hESF). Prednisolone treatment: (A) inhibitedhESF pro-inflammatory cytokine [interleukin (IL) 6, and IL18] gene expression, but had no effect on IL10 gene expression, paired t-test, n = 3-4/group; (B) had no effect on hESF glucocorticoid receptor (GR) gene expression, paired t-test, n = 4/group; (C) had no effect on classic decidualization makers prolactin (PRL) or insulin-like growth factor binding protein (IGFBP)1 expression; (D) had no effect on decidualization genes bone morphogenic protein (BMP)2, BMP7, homeobox A (HOXA)10 or inhibinβA (INHBA), but inhibited IL11, leukemia inhibitory factor (LIF) and LIF receptor (LIFR) gene expression, paired t-test, n = 4/group. Data presented as mean ± SEM; *P < 0.05.
Treatment of Trophoblast With hESF Conditioned Media
Trophoblast Outgrowth
Trophoblast outgrowth from villous tips (n = 6 biological replicates) was quantified as previously described (Winship et al., 2015; Menkhorst et al., 2019) with slight modification: villous tips were seeded on to neat growth-factor reduced MatrigelTM (Corning) instead of collagen. After 48 h of culture outgrowing villous tips were treated with day 14 hESF CM (FC 50%) for 72 h. The tips were photographed at 48 and 120 h and area of outgrowth quantified using ImageJ at 120 h (normalized to outgrowth at 48 h).
EVT Gene Expression
Trophoblast were isolated as previously described (Menkhorst et al., 2012) and cultured on growth factor reduced MatrigelTM diluted 1:5 in DMEM/F12 to promote differentiation toward the EVT phenotype. EVTs (n = 3 biological replicates) were treated with neat day 14 hESF CM for 16 h before RNA isolation for gene expression analysis.
Prolactin and IGFBP1 ELISA
Prolactin and IGFBP1 secretion by decidualized hESF (days 9/10 and 12/13) was quantified by ELISA of hESF CM as per the manufacturer’s instructions (DuoSet kits #DY682 and #DY871, R&D systems). Briefly, capture antibody was diluted in phosphate buffered saline and used to coat a 96 well microplate overnight at room temperature (RT; 100 μL/well). The following morning the capture antibody was aspirated before the plate was washed three times with wash buffer before non-specific antibody binding was blocked by incubation with reagent diluent (300 μL) for 1 h at RT. The plate was again washed before 100 μL standards or hESF CM was added to the plate and incubated for 2 h at RT. Each standard or sample was assayed in duplicate technical replicates. hESF CM was assayed neat for the PRL ELISA and diluted 1:2 in reagent diluent for the IGFBP1 ELISA. Following a further wash step 100 μl detection antibody was incubated for 2 h at RT. The plate was again washed before 100 μl Streptavidin-HRP complex was incubated for 20 min at RT. The plate was washed a final time before 100 μl of substrate solution was added to each well (incubated for 20 min at RT) and finally minutes 50 μl of Stop solution added to each well. The optical density of each well was immediately determined using a microplate reader (Biostrategy Spectramax PLUS Plate Reader) set to 450 nm.
Gene Expression
RNA extraction and quantitative RT-PCR was performed as previously described (Menkhorst et al., 2020) using Tri Reagent (Sigma-Aldrich) or the RNeasy mini kit (QIAGEN), Superscript III First-Strand Synthesis System (Thermo-Fisher) and Power SYBR Green master mix (Applied Biosystems) on the Veriti 7 fast block real-time qPCR system (Applied Biosystems). A template-free negative control in the presence of primers and RNase-free water only was added for each run and each sample assayed in triplicate technical replicates. Primer sequences are shown in Supplementary Table 1; primers were obtained from Sigma-Aldrich. The qPCR protocol was as follows: 95°C for 10 min and 40 cycles of 95°C for 15 s followed by 60°C for 1 min. Relative expression levels were calculated using comparative cycle threshold method (ΔΔCT) as outlined in the manufacturer’s user manual.
PCR Array: To determine the potential mechanisms by which prednisolone-treated hESF induced trophoblast outgrowth we used a QIAGEN Cell Motility Array (PAHS-1282A) as per the manufacturer’s instructions on EVT treated with hESF CM. RNA was pooled from n = 2 tissues for the array.
Mass Spectrometry
Decidualized hESF (n = 3 biological replicates) cellular proteins following decidualization including treatment with 0.5 μg/ml prednisolone or vehicle control were identified using mass spectrometry. Day 14 cells were lysed and homogenized in ice-cold universal lysis buffer as previously described (Menkhorst et al., 2012).
Full details are provided in the Supplementary Material. Briefly, 3 μg total cellular protein quantified using BCA assay (Pierce) was used for Solid-Phase Protein Preparation followed by LC-MS/MS as previously described (Dagley et al., 2019; Hughes et al., 2019). The analysis of the samples was based on the label-free quantification (LFQ) intensities. Initial analyses and visualization of proteomics data was performed using LFQ-Analyst (Shah et al., 2020). The data was statistically evaluated using Perseus software (version 1.6.7.0). The mass spectrometry proteomics data have been deposited to the ProteomeXchange Consortium via the PRIDE (Perez-Riverol et al., 2019) partner repository with the dataset identifier PXD020543. Assessment of protein function enrichment was performed using DAVID Bioinformatics Resources 6.81 (Huang Da et al., 2009b, a), selecting Homo sapiens as the reference species.
Statistical Analysis
GraphPad Prism 9.02 was used for all statistical analysis. Pairedt-tests and repeated measures ANOVA were used. All data is presented as mean ± SEM. p < 0.05 was considered statistically significant.
Results
Prednisolone Regulated hESF Cytokine Production
To confirm that prednisolone was active in hESF we determined whether prednisolone altered mRNA expression of cytokines known to be regulated by prednisolone. Prednisolone treatment of non-decidualized hESF significantly inhibited mRNA expression of the pro-inflammatory cytokines interleukin (IL) 6 (7.8-fold), and IL18 (2.6-fold), but had no effect on IL10 (Figure 1A) or GR expression (Figure 1B) compared to control.
Prednisolone Had No Effect on the Classical Markers of hESF Decidualization
We determined whether prednisolone could directly regulate genes associated with decidualization: prednisolone had no effect on PRL, IGFBP1 (Figure 1C), bone morphogenic protein (BMP) 2, BMP7, homeobox A (HOXA) 10, or inhibinβA (INHBA) production, but significantly inhibited IL11 (sixfold), Leukemia inhibitory factor (LIF; eightfold), and LIF receptor (LIFR; twofold) mRNA expression (Figure 1D).
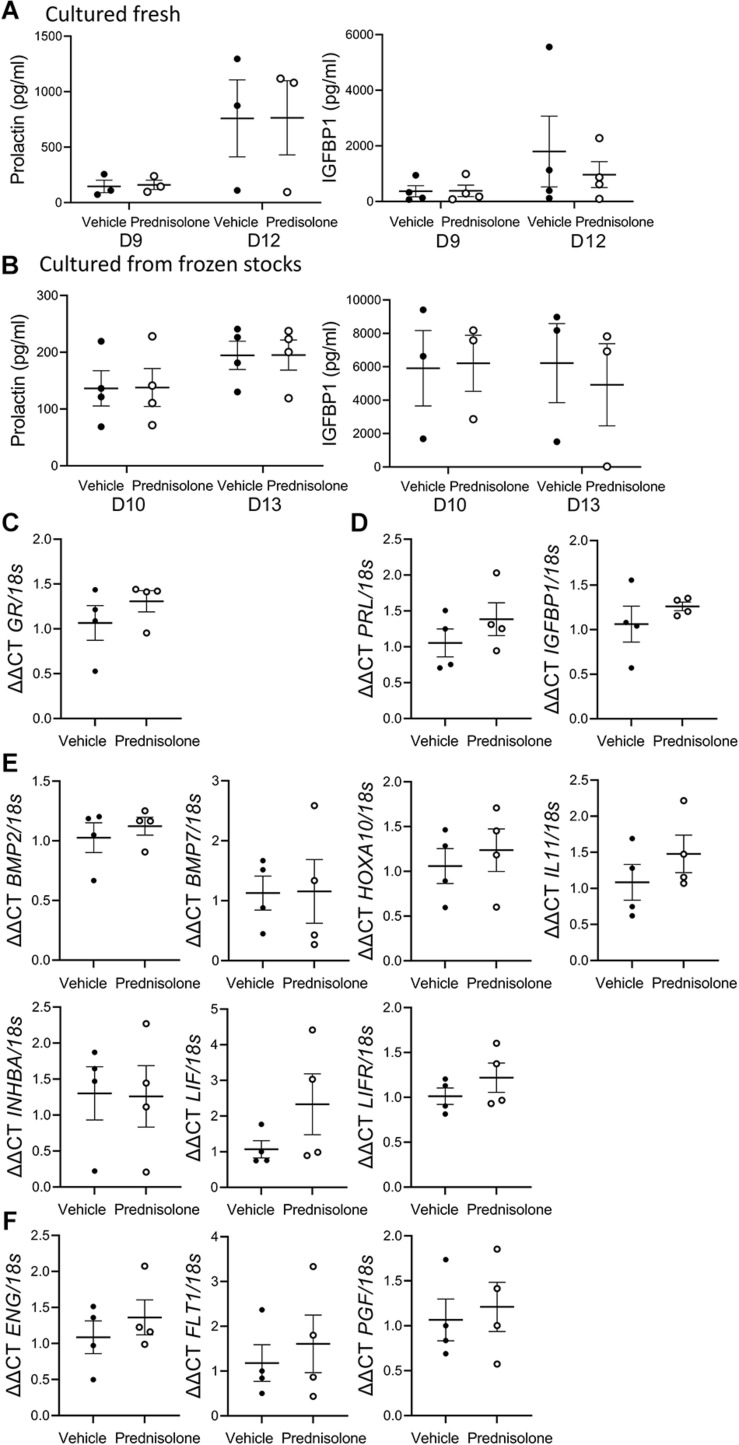
Long-term treatment of hESF with estrogen plus MPA induced decidualization as demonstrated by detectable PRL and IGFBP1 secretion (Figures 2A,B; E alone treatment showed undetectable PRL and IGFBP1 at Day 9/10 and 12/13, data not shown). Addition of 0.5 μg/ml prednisolone to the decidualization treatment showed no significant effect on PRL or IGFBP1 secretion in hESF cultured fresh (Figure 2A; 3/4 primary infertile, 1/4 unknown fertility) or frozen down before seeding for decidualization treatments (Figure 2B; 3/4 secondary infertile, 1/4 fertile).
FIGURE 2.
Prednisolone had no effect on classic decidualization markergene expression or secretion by decidualized human endometrial stromal fibroblast (hESF). Prednisolone treatment: (A,B) had no effect on decidualized hESF secretion of PRL or IGFBP1 from hESF (A) cultured fresh or (B) cultured from frozen stocks, repeated measures ANOVA; n = 3-4/group; (C–F) had no effect on decidualized hESF production of (C) GR; (D) classic decidualization markers PRL or IGFBP1; (E) decidualization genes BMP2, BMP7, HOXA10, IL11, INHBA, LIF or LIFR; (F) preeclampsia-associated genes endoglin (ENG), vascular endothelial growth factor receptor (FLT1) or placental-like growth factor (PGF), paired t-test, n = 4/group; Data presented as mean ± SEM; *P < 0.05.
Decidualized hESF gene expression following long-term treatment with hESF was examined 24 h after prednisolone withdrawal (day 14). There was no effect of prednisolone on decidualized hESF GR (Figure 2C), PRL, IGFBP1 (Figure 2D), BMP2, BMP7, HOXA10, IL11, INHBA, LIF, LIFR (Figure 2E), endoglin (ENG), vascular endothelial growth factor receptor (FLT1), or placental-like growth factor (PGF) production (Figure 2F).
Prednisolone Altered Decidualized hESF Protein Production
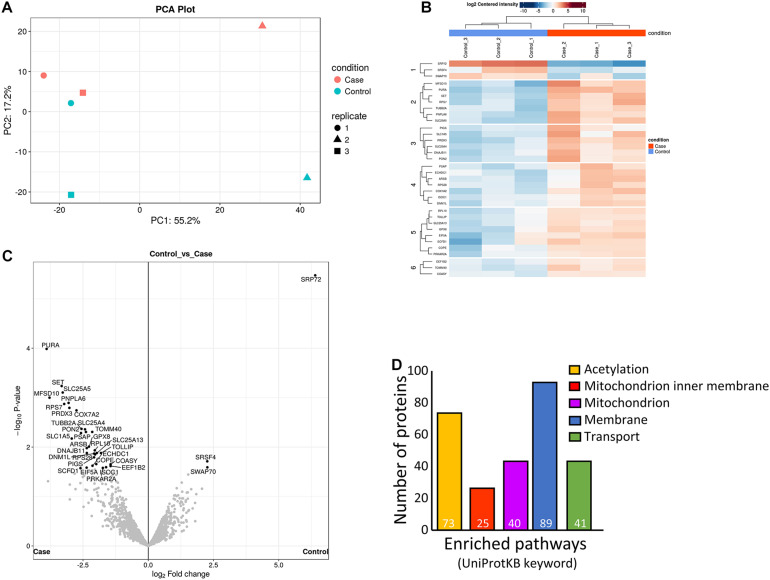
We performed proteomics on hESF cellular protein following in vitro decidualization in the presence of prednisolone (0.5 μg/ml) or vehicle control (DMSO) to identify decidualized hESF proteins regulated by prednisolone. We identified 2,254 individual proteins with >2 peptides in control decidualized hESF by mass spectrometry. We quantitated the production of 1,824 individual proteins between control and prednisolone-treated hESF.
Prednisolone treatment substantially altered decidualized hESF protein production (Figures 3A,B). 176 proteins showed significant fold-changes following prednisolone treatment (Figure 3C), including one down-regulated (Signal recognition particle subunit SRP72) and 175 up-regulated (Supplementary Table 2). Functional clustering analysis (DAVID) identified that hESF decidualized in the presence of prednisolone had enrichment of proteins associated with acetylation (3.4-fold), mitochondrion inner membrane (14.6-fold), mitochondrion (5.7-fold), membrane (1.9-fold), and transport (3.3-fold) (Figure 3D and Supplementary Table 3).
FIGURE 3.
Analysis of differently regulated decidualized human endometrial stromal fibroblast (hESF) proteins following in vitro decidualization in the presence of prednisolone. (A) Principal components analysis. (B) Heat-map. (C) Volcano plot. (D) Enriched pathways. Individual numbers of proteins identified is indicated in white at the base of each bar. Case: prednisolone treated hESF; Control: vehicle control treated hESF.
Trophoblast Outgrowth Was Enhanced by hESF Decidualized in Presence of Prednisolone
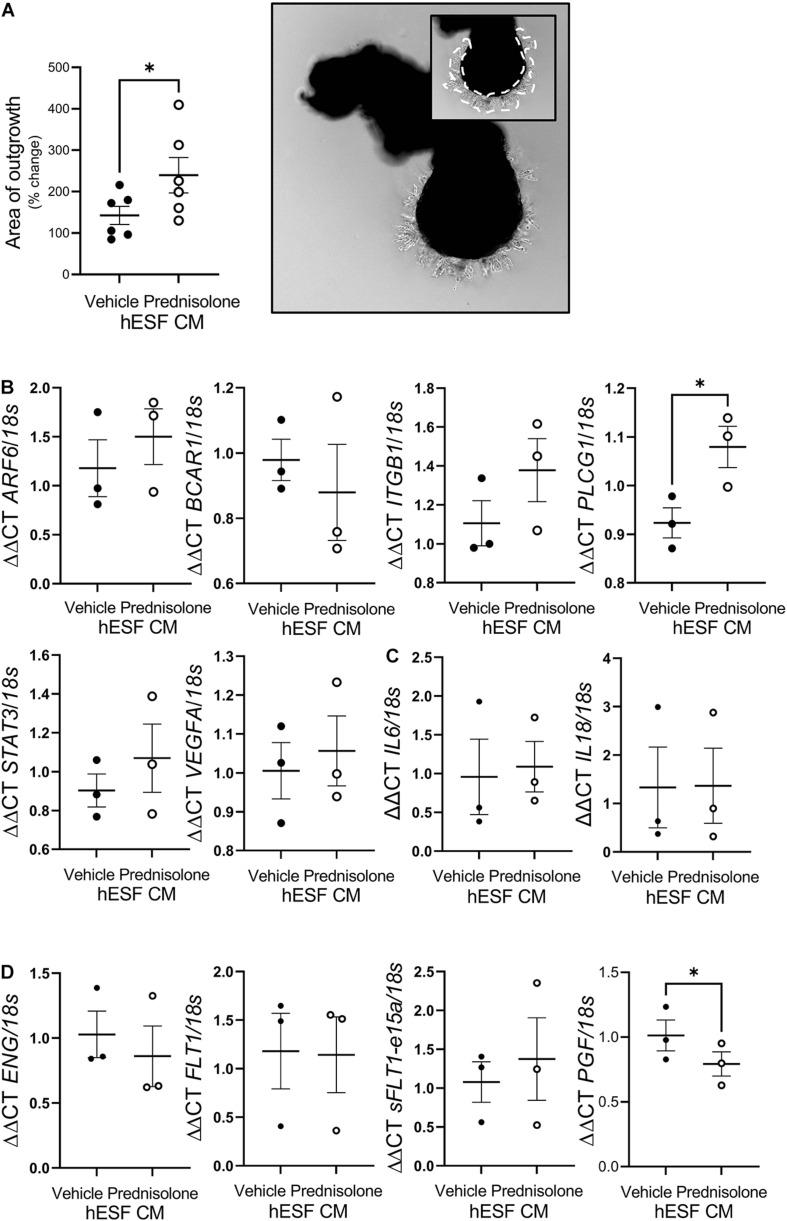
To determine whether decidualization in the presence of prednisolone would impact decidual-trophoblast interactions we determined the effect of hESF CM on EVT outgrowth. EVT outgrowth was significantly enhanced following treatment with CM from hESF decidualized in the presence of prednisolone (1.7-fold, Figure 4A) compared to hESF decidualized in the presence of the vehicle control. The potential mechanism by which CM from hESF decidualized in the presence of prednisolone enhanced EVT outgrowth was investigated by assessing EVT gene expression following treatment with decidualized hESF CM (16 h) using a cell motility array (Supplementary Table 4). Genes highly altered on the array were validated by qPCR (Figure 4B). Phospholipase C, gamma 1 (PLCG1) was significantly increased in EVT exposed to prednisolone-treated decidualized hESF CM (1.2-fold, Figure 3B). To further investigate the effect of CM from hESF decidualized in the presence of prednisolone, we assessed EVT expression of genes associated with inflammation and preeclampsia (Figures 4C,D). Placental-like Growth Factor (PGF) was significantly decreased in EVT exposed to prednisolone-treated decidualized hESF CM (1.3-fold, Figure 4D).
FIGURE 4.
Prednisolone altered human endometrial stromal fibroblast (hESF) regulation of trophoblast function. (A) Extravillous trophoblast (EVT) outgrowth was significantly enhanced by conditioned media (CM) from hESF decidualized in the presence of prednisolone. Image shows representative outgrowth from villous tip. Insert shows area of outgrowth highlighted by dotted line. Paired t-test, n = 6. (B) EVT PLCG1 expression was significantly increased by treatment with CM from hESF decidualized in the presence of prednisolone. Paired t-test, n = 3/group. (C) EVT IL6 and IL18 expression was not altered by treatment with CM from hESF decidualized in the presence of prednisolone. Paired t-test, n = 3/group. (D) EVT PGF expression was significantly inhibited by treatment with CM from hESF decidualized in the presence of prednisolone. Paired t-test, n = 3/group. Data presented as mean ± SEM; *P < 0.05.
Discussion
This is the first study to investigate the effect of prednisolone treatment on decidualization and decidual-trophoblast interactions. Prednisolone treatment during in vitro decidualization did not alter production of the classic decidualization markers PRL or IGFBP1 but altered hESF cytokine gene expression and decidualized hESF cellular protein. Intriguingly, trophoblast-decidual interactions were altered following hESF decidualization in the presence of prednisolone: we found prednisolone treatment enhanced trophoblast outgrowth, elevated EVT PLCG1 production and reduced EVT PGF production.
To our knowledge the direct effect of prednisolone on decidualization in women has never been investigated. Although prednisolone has been shown to downregulate GR production in HeLa cells (Shimojo et al., 1995) here we saw no effect on GR production in non-decidualized or decidualized hESF. Our data suggests that prednisolone suppresses hESF pro-inflammatory cytokine production as has previously been shown in other cell types (Karagiannidis et al., 2004; Andersson et al., 2005; Shmarina et al., 2017). It is interesting that prednisolone suppressed hESF production of IL11, LIF, and LIFR which we previously showed enhanced progesterone-induced decidualization (Dimitriadis et al., 2003; Shyua et al., 2011). Inhibition of IL11 expression by methylprednisolone has previously been show in bronchial epithelium (Chakir et al., 2003), but there is no previous investigation of the effect of prednisolone on LIF production. As IL11 and LIF are only two of many pathways altered during decidualization (Gellersen and Brosens, 2014; Evans et al., 2016) it is unsurprising that suppression of IL11 and LIF did not lead to altered production of PRL or IGFBP1, however, dysregulation of these factors may still impact hESF decidualization. It must be noted that the absolute levels of PRL and IGFBP1 secretion were different between hESF cultured fresh vs. those which were frozen before thawing for culture experiments. As most of the fresh hESF were from women with primary infertility and most of the frozen hESF were from women with secondary infertility this difference could also reflect the clinical characteristics of the women. Regardless, prednisolone had no effect on PRL or IGFBP1 production by hESF.
Since we found no effect of prednisolone treatment on gene expression or secretion of the classical markers of decidualization, we performed mass spectrometry to identify whether hESF proteins were altered by prednisolone treatment during decidualization. Of the 176 hESF proteins significantly regulated by prednisolone, 27 had previously been identified in decidua, including factors which promote decidualization [including GNA11/GNAQ (De Oliveira et al., 2019), CDK6 (Tan et al., 2002), and SCRIB (Whitby et al., 2018; Yuan et al., 2019)], or which are upregulated during decidualization [including CTTN (Paule et al., 2011), CTNNA1 (Patterson et al., 2017), SPTLC2 (Ding et al., 2018), and ALDH1A1 (Tomari et al., 2020)]. Decidualization itself is associated with substantial post-translational modification (Díaz-Gimeno et al., 2014) and here we found that prednisolone stimulated the production of proteins associated with acetylation, however, the effect of altered acetylation in decidualized hESF is unknown. Prednisolone treatment also altered hESF proteins associated with mitochondria, including increased production of factors associated with ATP generation and transport (e.g., UQCRC2, VDAC1/2; ATP5 synthases; NDUF enzymes; SLC25A mitochondrial carrier proteins). Again, the precise effect of altered hESF mitochondrial function is unknown. It is likely that proteins regulated by prednisolone will alter decidual cell interactions with other cells in the uterus, including trophoblast as demonstrated here.
We previously observed that CM from decidualized hESF enhanced trophoblast outgrowth when compared to non-decidualized hESF (Menkhorst et al., 2019). Despite prednisolone having no effect on classic markers of decidualization we observed that CM from hESF decidualized in the presence of prednisolone enhanced trophoblast outgrowth, suggesting that prednisolone altered hESF release of factors which regulate trophoblast function. Future studies are required to elucidate how prednisolone alters hESF CM and thus decidual regulation of trophoblast invasion, however, the data presented here suggests hESF CM may alter trophoblast motility genes including PLCG1. PLCG1 has not previously been identified in trophoblast or the placenta, however, it has well established roles in tumor metastasis where it promotes cell invasion (Kunze et al., 2014; Jang et al., 2018; Tang et al., 2019), potentially via its interactions with MMP2 (Zhang et al., 2019) or EGFR signaling (Jang et al., 2018). The precise effect that increased PLCG1 production by EVTs would have on trophoblast invasion and whether hESF CM regulates other trophoblast functions including viability or proliferation remains to be experimentally determined.
There is emerging evidence linking corticosteroid use to preeclampsia incidence in women (Bandoli et al., 2017) and the development of PE features in rodents (Zhang et al., 2016, 2018). The Danish National Cohort study identified corticosteroid medication use for inflammatory bowel disease had a strong and significant association with preeclampsia (Boyd et al., 2015). Diseases for which prednisolone is a common treatment are also conditions with elevated risk of preeclampsia, including Antiphospholipid syndrome (APS) (when prescribed in addition to aspirin and heparin) (Empson and Al, 2005), chronic kidney disease (10-fold increased risk of preeclampsia) (Wiles et al., 2020) and systemic lupus erythematosus (14% increased risk of preeclampsia) (Chen et al., 2019), however, the contribution of prednisolone vs. the effect of the disease itself to preeclampsia risk has not been established.
The contribution of decidual deficiency in the etiology of preeclampsia is emerging (Garrido-Gómez et al., 2020). Here we found that EVT treated with CM from hESF decidualized in the presence of prednisolone had reduced PGF production. Serum PGF is reduced in early pregnancy serum of women who develop PE and is a biomarker used in the 1st trimester screening test for preterm preeclampsia (Akolekar et al., 2013). In this study hESF also had altered production of factors previously identified to be increased in the decidua of women with preeclampsia, including COL4A1 (Yong et al., 2014, 2015), LNPEP (Yong et al., 2014), TM9SF2 (Garrido-Gomez et al., 2017) and COTL1 (Garrido-Gomez et al., 2017). The impact of prednisolone treatment on the decidua and decidual function could be a novel mechanism by which prednisolone or other corticosteroid use increases preeclampsia risk.
Overall, this study demonstrates that prednisolone alters decidualized hESF and altered decidual-trophoblast interactions. The clinical consequences of these changes are unknown, however, as all available data suggests that corticosteroid administration has no beneficial effect for IVF (Kaye et al., 2017; Mohammadi Yeganeh et al., 2018), RPL (Tang et al., 2013; Cooper et al., 2019; Woon et al., 2020), or repeated implantation failure (Siristatidis et al., 2018) and the emerging evidence that corticosteroid use during pregnancy may be associated with poor obstetrical outcomes (Boyd et al., 2015; Bandoli et al., 2017), off-label use of corticosteroids, in particular prednisolone, during the period of decidualization (secretory phase of the menstrual cycle and the 1st trimester), should be carefully considered.
Data Availability Statement
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found in the article/Supplementary Material.
Ethics Statement
The studies involving human participants were reviewed and approved by Royal Women’s Hospital Human Research Ethics Committee Monash Health Human Research Ethics Committee. The patients/participants provided their written informed consent to participate in this study.
Author Contributions
EG, EM, and ED wrote the main manuscript text. EG prepared Figures 1–4 and Supplementary Tables 2–4. TS prepared Figures 2–4 and Supplementary Tables 2, 3. SV and NW prepared Figure 3 and Supplementary Tables 2, 3. EM prepared Figures 1–4. All authors reviewed the final manuscript.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We are grateful to the women who donated tissue and Emily-Jane Bromley RN, Jeanette Henderson, Judi Hocking RN, Paddy Moore, Philana Nguyen, Luk Rombauts, Lanie Santos, and Beverley Vollenhoven for their contribution to this manuscript.
Funding. This work was supported by the NHMRC (Australia) Project/Program Grant (GNT1098332) and Fellowships (#611827 to EM and #550905 to ED), Rebecca L. Cooper Medical Research Foundation project grant PG2018130 to EM, the University of Melbourne Department of Obstetrics and Gynecology Mid-career Fellowship to EM, and the Victorian Government’s Operational Infrastructure Support. The funders had no involvement in the conduct of research, preparation of the manuscript or the decision to publish.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcell.2021.647496/full#supplementary-material
References
- Akolekar R., Syngelaki A., Poon L., Wright D., Nicholaides K. H. (2013). Competing risks model in early screening for preeclampsia by biophysical and biochemical markers. Fetal Diagn. Ther. 33 8–15. 10.1159/000341264 [DOI] [PubMed] [Google Scholar]
- Andersson A. K., Chaduvula M., Atkinson S. E., Khanolkar-Young S., Jain S., Suneetha L., et al. (2005). Effects of prednisolone treatment on cytokine expression in patients with leprosy type 1 reactions. Infect. Immun. 73 3725–3733. 10.1128/iai.73.6.3725-3733.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bandoli G., Palmsten K., Forbess Smith C. J., Chambers C. D. (2017). A review of systemic corticosteroid use in pregnancy and the risk of select pregnancy and birth outcomes. Rheum. Dis. Clin. North Am. 43 489–502. 10.1016/j.rdc.2017.04.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyd H. A., Basit S., Harpsøe M. C., Wohlfahrt J., Jess T. (2015). Inflammatory bowel disease and risk of adverse pregnancy outcomes. PLoS One 10:e0129567. 10.1371/journal.pone.0129567 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burton G. J., Jauniaux E., Charnock-Jones D. S. (2010). The influence of the intrauterine environment on human placental development. Int. J. Dev. Biol. 54 303–312. 10.1387/ijdb.082764gb [DOI] [PubMed] [Google Scholar]
- Chakir J., Shannon J., Molet S., Fukakusa M., Elias J., Laviolette M., et al. (2003). Airway remodeling-associated mediators in moderate to severe asthma: effect of steroids on TGF-β, IL-11, IL-17, and type I and type III collagen expression. J. Allergy Clin. Immunol. 111 1293–1298. 10.1067/mai.2003.1557 [DOI] [PubMed] [Google Scholar]
- Chen D., Lao M., Cai X., Li H., Zhan Y., Wang X., et al. (2019). Hypertensive disorders of pregnancy associated with adverse pregnant outcomes in patients with systemic lupus erythematosus: a multicenter retrospective study. Clin. Rheumatol. 38 3501–3509. 10.1007/s10067-019-04696-x [DOI] [PubMed] [Google Scholar]
- Cooper S., Laird S. M., Mariee N., Li T. C., Metwally M. (2019). The effect of prednisolone on endometrial uterine NK cell concentrations and pregnancy outcome in women with reproductive failure. a retrospective cohort study. J. Reprod. Immunol. 131 1–6. 10.1016/j.jri.2018.10.001 [DOI] [PubMed] [Google Scholar]
- Dagley L., Infusini G., Larsen R. H., Sandow J. J., Webb A. I. (2019). Universal solid-phase protein preparation for bottom-up and top-down proteomics. J. Proteome Res. 18 2915–2924. 10.1021/acs.jproteome.9b00217 [DOI] [PubMed] [Google Scholar]
- Dan S., Wei W., Yichao S., Hongbo C., Shenmin Y., Jiaxiong W., et al. (2015). Effect of prednisolone administration on patients with unexplained recurrent miscarriage and in routine intracytoplasmic sperm injection: a meta-analysis. Am. J. Reprod. Immunol. 74 89–97. 10.1111/aji.12373 [DOI] [PubMed] [Google Scholar]
- De Oliveira V., Schaefer J., Calder M., Lydon J. P., Demayo F. J., Bhattacharya M., et al. (2019). Uterine Gα(q/11) signaling, in a progesterone-dependent manner, critically regulates the acquisition of uterine receptivity in the female mouse. FASEB J. 33 9374–9387. 10.1096/fj.201900026r [DOI] [PMC free article] [PubMed] [Google Scholar]
- Díaz-Gimeno P., Ruíz-Alonso M., Blesa D., Simón C. (2014). Transcriptomics of the human endometrium. Int. J. Dev. Biol. 58 127–137. 10.1387/ijdb.130340pd [DOI] [PubMed] [Google Scholar]
- Dimitriadis E., Menkhorst E., Saito S., Kutteh W., Brosens J. (2020). Recurrent pregnancy loss. Nat. Rev. Dis. Primers 2020:98. [DOI] [PubMed] [Google Scholar]
- Dimitriadis E., Robb L., Liu Y. X., Enders A. C., Martin H., Stoikos C., et al. (2003). IL-11 and IL-11Ra immunolocalisation at primate implantation sites supports a role for IL-11 in placentation and fetal development. Reprod. Biol. Endocrinol. Biomed. Cent. 1:34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dimitriadis E., Robb L., Salamonsen L. A. (2002). Interleukin 11 advances progesterone-induced decidualization of human endometrial stromal cells. Mol. Hum. Reprod. 8 636–643. 10.1093/molehr/8.7.636 [DOI] [PubMed] [Google Scholar]
- Dimitriadis E., White C. A., Jones R. L., Salamonsen L. A. (2005). Cytokines, chemokines and growth factors in endometrium related to implantation. Hum. Reprod. Update 11 613–630. 10.1093/humupd/dmi023 [DOI] [PubMed] [Google Scholar]
- Ding N.-Z., Qi Q.-R., Gu X.-W., Zuo R.-J., Liu J., Yang Z.-M. (2018). De novo synthesis of sphingolipids is essential for decidualization in mice. Theriogenology 106 227–236. 10.1016/j.theriogenology.2017.09.036 [DOI] [PubMed] [Google Scholar]
- Empson M., Al E. (2005). Prevention of recurrent miscarriage for women with antiphospholipid antibody or lupus anticoagulant. Cochrane Database Syst. Rev. 2:CD002859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans J., Salamonsen L., Winship A., Menkhorst E., Nie G., Gargett C., et al. (2016). Fertile ground: human endometrial programming and lessons in health and disease. Nat. Rev. Endocrinol. 12 654–667. 10.1038/nrendo.2016.116 [DOI] [PubMed] [Google Scholar]
- Frolkis A., Knox C., Lim E., Jewison T., Law V., Hau D. D., et al. (2010). SMPDB: the small molecule pathway database. Nucleic Acids Res. 38 D480–D487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Founds S., Conley Y. P., Lyons-Weiler J. F., Jeyabalan A., Allen Hogge W., Conrad K. P. (2009). Altered global gene expression in first trimester placentas of women destined to develop preeclampsia. Placenta 30 15–24. 10.1016/j.placenta.2008.09.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garrido-Gómez T., Castillo-Marco N., Cordero T., Simón C. (2020). Decidualization resistance in the origin of preeclampsia. Am. J. Obstet. Gynecol. (in press). 10.1016/j.ajog.2020.09.039 [DOI] [PubMed] [Google Scholar]
- Garrido-Gomez T., Dominguez F., Quiñonero A., Diaz-Gimeno P., Kapidzic M., Gormley M., et al. (2017). Defective decidualization during and after severe preeclampsia reveals a possible maternal contribution to the etiology. Proc. Natl. Acad. Sci. 114 E8468–E8477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gellersen B., Brosens J. J. (2014). Cyclic decidualization of the human endometrium in reproductive health and failure. Endocr. Rev. 35 851–905. 10.1210/er.2014-1045 [DOI] [PubMed] [Google Scholar]
- Gomaa M. F., Elkholy A. G., El-Said M. M., Abdel-Salam N. E. (2014). Combined oral prednisolone and heparin versus heparin: the effect on peripheral NK cells and clinical outcome in patients with unexplained recurrent miscarriage. a double-blind placebo randomized controlled trial. Arch. Obstet. Gynaecol. 290 757–762. 10.1007/s00404-014-3262-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henderson T. A., Critchley H. O. D., Saunders P. T. K., Moffett-King A., Groome N. P. (2003). Steroid receptor expression in uterine natural killer cells. Int. J. Clin. Endocrinol. Metab. 88 440–449. 10.1210/jc.2002-021174 [DOI] [PubMed] [Google Scholar]
- Huang Da W., Sherman B. T., Lempicki R. A. (2009a). Bioinformatics enrichment tools: paths toward the comprehensive functional analysis of large gene lists. Nucleic Acids Res. 37 1–13. 10.1093/nar/gkn923 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang Da W., Sherman B. T., Lempicki R. A. (2009b). Systematic and integrative analysis of large gene lists using DAVID bioinformatics resources. Nat. Protoc. 4 44–57. 10.1038/nprot.2008.211 [DOI] [PubMed] [Google Scholar]
- Hughes C., Moggridge S., Müller T., Sorensen P. H., Morin G. B., Krijgsveld J. (2019). Single-pot, solid-phase-enhanced sample preparation for proteomics experiments. Nat. Protoc. 14 68–85. 10.1038/s41596-018-0082-x [DOI] [PubMed] [Google Scholar]
- Jang H.-J., Suh P.-G., Lee Y. J., Shin K. J., Cocco L., Chae Y. C. (2018). PLCγ1: potential arbitrator of cancer progression. Adv. Biol. Regul. 67 179–189. 10.1016/j.jbior.2017.11.003 [DOI] [PubMed] [Google Scholar]
- Karagiannidis C., Akdis M., Holopainen P., Woolley N. J., Hense G., Rückert B., et al. (2004). Glucocorticoids upregulate FOXP3 expression and regulatory T cells in asthma. J. Allergy Clin. Immunol. 114 1425–1433. 10.1016/j.jaci.2004.07.014 [DOI] [PubMed] [Google Scholar]
- Kaye L., Bartels C., Bartolucci A., Engmann L., Nulsen J., Benadiva C. (2017). Old habits die hard: retrospective analysis of outcomes with use of corticosteroids and antibiotics before embryo transfer. Fertil. Steril. 107 1336–1340. 10.1016/j.fertnstert.2017.04.003 [DOI] [PubMed] [Google Scholar]
- Kieffer T. E. C., Chin P. Y., Green E. S., Moldenhauer L. M., Prins J. R., Robertson S. A. (2020). Prednisolone in early pregnancy inhibits regulatory T cell generation and alters fetal and placental development in mice. Mol. Hum. Reprod. 26 340–352. 10.1093/molehr/gaaa019 [DOI] [PubMed] [Google Scholar]
- Kisanga E. P., Tang Z., Guller S., Whirledge S. (2018). Glucocorticoid signaling regulates cell invasion and migration in the human first-trimester trophoblast cell line Sw.71. Am. J. Reprod. Immunol. 80:e12974. 10.1111/aji.12974 [DOI] [PubMed] [Google Scholar]
- Kunze K., Spieker T., Gamerdinger U., Nau K., Berger J., Dreyer T., et al. (2014). A Recurrent activating mutation in cardiac angiosarcomas increases apoptosis resistance and invasiveness of endothelial cells. Cancer Res. 74:6173. 10.1158/0008-5472.can-14-1162 [DOI] [PubMed] [Google Scholar]
- Li Q. N., Li L., Hou G., Wang Z. B., Hou Y., Liu Z. H., et al. (2018). Glucocorticoid exposure affects female fertility by exerting its effect on the uterus but not on the oocyte: lessons from a hypercortisolism mouse model. Hum. Reprod. 33 2285–2294. [DOI] [PubMed] [Google Scholar]
- Lunghi L., Ferretti M., Medici S., Biondi C., Vesce F. (2007). Control of human trophoblast function. Reprod. Biol. Endocrinol. 5:6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matejevic D., Heilmann P., Schuster C., Schoneshofer M., Graf R. (1995). Decidua and placenta in mice after treatment with a synthetic glucocorticoid. Reprod. Fertil. Dev. 7 1551–1555. 10.1071/rd9951551 [DOI] [PubMed] [Google Scholar]
- Menkhorst E., Van Sinderen M., Correia J., Dimitriadis E. (2019). Trophoblast function is altered by decidual factors in gestational-dependant manner. Placenta 80 8–11. 10.1016/j.placenta.2019.03.013 [DOI] [PubMed] [Google Scholar]
- Menkhorst E., Winship A., Van Sinderen M., Dimitriadis E. (2015). Human extravillous trophoblast invasion: intrinsic and extrinsic regulation. Reprod. Fertil. Dev. 28 406–415. 10.1071/rd14208 [DOI] [PubMed] [Google Scholar]
- Menkhorst E., Zhou W., Santos L., Delforce S., So T., Rainczuk K., et al. (2020). Galectin-7 impairs placentation and causes preeclampsia features in mice. Hypertension 76 1185–1194. 10.1161/hypertensionaha.120.15313 [DOI] [PubMed] [Google Scholar]
- Menkhorst E. M., Lane N., Winship A., Li P., Yap J., Meehan K., et al. (2012). Decidual-secreted factors alter invasive trophoblast membrane and secreted proteins implying a role for decidual cell regulation of placentation. PLoS One 7:e31418. 10.1371/journal.pone.0031418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menkhorst E. M., Van Sinderen M. L., Rainczuk K., Cuman C., Winship A., Dimitriadis E. (2017). Invasive trophoblast promote stromal fibroblast decidualization via profilin 1 and ALOX5. Sci. Rep. 7:8690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohammadi Yeganeh L., Moini A., Shiva M., Mirghavam N., Bagheri Lankarani N. (2018). Methylprednisolone for prevention of ovarian hyperstimulation syndrome in patients with polycystic ovarian syndrome undergoing in-vitro fertilisation: a randomised controlled trial. J. Obstet. Gynaecol. 38 241–246. 10.1080/01443615.2017.1346593 [DOI] [PubMed] [Google Scholar]
- Okada H., Tsuzuki T., Murata H. (2018). Decidualization of the human endometrium. Reprod. Med. Biol. 17 220–227. 10.1002/rmb2.12088 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patterson A. L., Pirochta J., Tufano S. Y., Teixeira J. M. (2017). Gain-of-function β-catenin in the uterine mesenchyme leads to impaired implantation and decidualization. J. Endocrinol. 233 119–130. 10.1530/joe-16-0502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paule S., Li Y., Nie G. (2011). Cytoskeletal remodelling proteins identified in fetal-maternal interface in pregnant women and rhesus monkeys. J. Mol. Histol. 42 161–166. 10.1007/s10735-011-9319-5 [DOI] [PubMed] [Google Scholar]
- Perez-Riverol Y., Csordas A., Bai J., Bernal-Llinares M., Hewapathirana S., Inuganti A., et al. (2019). The pride database and related tools and resources in 2019: improving support for quantification data. Nucleic Acids Res. 47 D442–D450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollheimer J., Vondra S., Baltayeva J., Beristain A. G., Knöfler M. (2018). Regulation of placental extravillous trophoblasts by the maternal uterine environment. Front. Immunol. 9:2597. 10.3389/fimmu.2018.02597 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Popovici R. M., Kao L. C., Giudice L. C. (2000). Discovery of new inducible genes in in vitro decidualized human endometrial stromal cells using microarray technology. Endocrinology 141 3510–3515. 10.1210/endo.141.9.7789 [DOI] [PubMed] [Google Scholar]
- Salker M., Teklenburg G., Molokhia M., Lavery S., Trew G., Aojanepong T., et al. (2010). Natural selection of human embryos: impaired decidualization of endometrium disables embryo-maternal interactions and causes recurrent pregnancy loss. PLoS One 5:e10287. 10.1371/journal.pone.0010287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah A. D., Goode R. J. A., Huang C., Powell D. R., Schittenhelm R. B. (2020). LFQ-analyst: an easy-to-use interactive web platform to analyze and visualize label-free proteomics data preprocessed with MaxQuant. J. Proteome Res. 19 204–211. 10.1021/acs.jproteome.9b00496 [DOI] [PubMed] [Google Scholar]
- Shimojo M., Hiroi N., Yakushiji F., Ueshiba H., Yamaguchi N., Miyachi Y. (1995). Differences in down-regulation of glucocorticoid receptor mRNA by cortisol, prednisolone and dexamethasone in HeLa cells. Endocr. J. 42 629–636. 10.1507/endocrj.42.629 [DOI] [PubMed] [Google Scholar]
- Shmarina G., Pukhalsky A., Avakian L., Semykin S., Pukhalskaya D., Alioshkin V. (2017). Steady-state therapy with azithromycin or low-dose prednisolone in paediatric cystic fibrosis patients: inflammatory markers and disease progression. Int. Arch. Allergy Immunol. 172 45–54. 10.1159/000453451 [DOI] [PubMed] [Google Scholar]
- Shyua L. L., Menkhorst E., Yap J., Li P., Dimitriadis E. (2011). Leukemia inhibitory factor enhances endometrial stromal cell decidualization in humans and mice. PLoS One 6:e25288. 10.1371/journal.pone.0025288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siristatidis C., Dafopoulos K., El-Khayat W., Salamalekis G., Anifandis G., Vrantza T., et al. (2018). Administration of prednisolone and low molecular weight heparin in patients with repeated implantation failures: a cohort study. Gynecol. Endocrinol. 34 136–139. 10.1080/09513590.2017.1380182 [DOI] [PubMed] [Google Scholar]
- Tan J., Raja S., Davis M. K., Tawfik O., Dey S. K., Das S. K. (2002). Evidence for coordinated interaction of cyclin D3 with p21 and cdk6 in directing the development of uterine stromal cell decidualization and polyploidy during implantation. Mech. Dev. 111 99–113. 10.1016/s0925-4773(01)00614-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang A.-W., Alfirevic Z., Turner M. A., Drury J. A., Small R., Quenby S. (2013). A feasibility trial of screening women with idiopathic recurrent miscarriage for high uterine natural killer cell density and randomizing to prednisolone or placebo when pregnant. Hum. Reprod. 28 1743–1752. 10.1093/humrep/det117 [DOI] [PubMed] [Google Scholar]
- Tang W., Zhou Y., Sun D., Dong L., Xia J., Yang B. (2019). Oncogenic role of phospholipase C-γ1 in progression of hepatocellular carcinoma. Hepatol. Res. 49 559–569. 10.1111/hepr.13309 [DOI] [PubMed] [Google Scholar]
- Tomari H., Kawamura T., Asanoma K., Egashira K., Kawamura K., Honjo K., et al. (2020). Contribution of senescence in human endometrial stromal cells during proliferative phase to embryo receptivity†. Biol. Reprod. 103 104–113. 10.1093/biolre/ioaa044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitby S., Salamonsen L. A., Evans J. (2018). The endometrial polarity paradox: differential regulation of polarity within secretory-phase human endometrium. Endocrinology 159 506–518. 10.1210/en.2016-1877 [DOI] [PubMed] [Google Scholar]
- Wiles K., Chappell L. C., Lightstone L., Bramham K. (2020). Updates in diagnosis and management of preeclampsia in women with CKD. Clin. J. Am. Soc. Nephrol. 15 1371–1380. 10.2215/cjn.15121219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson C. G., May C. S., Paterson J. W. (1977). Plasma prednisolone levels in man following administration in plain and enteric-coated forms. Br. J. Clin. Pharmacol. 4 351–355. 10.1111/j.1365-2125.1977.tb00723.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winship A., Koga K., Menkhorst E., Van Sinderen M., Rainczuk K., Nagai M., et al. (2015). Interleukin-11 alters placentation and causes preeclampsia features in mice. PNAS 112:15928. 10.1073/pnas.1515076112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woon E. V., Day A., Bracewell-Milnes T., Male V., Johnson M. (2020). Immunotherapy to improve pregnancy outcome in women with abnormal natural killer cell levels/activity and recurrent miscarriage or implantation failure: a systematic review and meta-analysis. J. Reprod. Immunol. 142:103189. 10.1016/j.jri.2020.103189 [DOI] [PubMed] [Google Scholar]
- Yang Q., Wang W., Liu C., Wang Y., Sun K. (2016). Compartmentalized localization of 11β-HSD 1 and 2 at the feto-maternal interface in the first trimester of human pregnancy. Placenta 46 63–71. 10.1016/j.placenta.2016.08.079 [DOI] [PubMed] [Google Scholar]
- Yong H. E. J., Murthi P., Borg A., Kalionis B., Moses E. K., Brennecke S. P., et al. (2014). Increased decidual mRNA expression levels of candidate maternal pre-eclampsia susecptibility genes are associated with clinical severity. Placenta 35 117–124. 10.1016/j.placenta.2013.11.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yong H. E. J., Murthi P., Wong M. H., Kalionis B., Brennecke S. P., Keogh R. J. (2015). Anti-angiogenic collagen fragment arresten is increased from 16 weeks’ gestation in pre-eclamptic plasma. Placenta 36 1300–1309. 10.1016/j.placenta.2015.08.013 [DOI] [PubMed] [Google Scholar]
- Yuan J., Aikawa S., Deng W., Bartos A., Walz G., Grahammer F., et al. (2019). Primary decidual zone formation requires scribble for pregnancy success in mice. Nat. Commun. 10 5425–5425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang D., Liu H., Zeng J., Miao X., Huang W., Chen H., et al. (2016). Glucocorticoid exposure in early placentation induces preeclampsia in rats via interfering trophoblast development. Gen. Comp. Endocrinol. 225 61–70. 10.1016/j.ygcen.2015.09.019 [DOI] [PubMed] [Google Scholar]
- Zhang D., Zeng J., Miao X., Liu H., Ge L., Huang W., et al. (2018). Glucocorticoid exposure induces preeclampsia via dampening 1,25-dihydroxyvitamin D3. Hypertens. Res. 41 104–111. 10.1038/hr.2017.98 [DOI] [PubMed] [Google Scholar]
- Zhang X., Shi G., Gao F., Liu P., Wang H., Tan X. (2019). TSPAN1 upregulates MMP2 to promote pancreatic cancer cell migration and invasion via PLCγ. Oncol. Rep. 41 2117–2125. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found in the article/Supplementary Material.