Abstract
Coprinopsis cinerea lectin 2 (CCL2) is a fucoside-binding lectin from the basidiomycete C. cinerea that is toxic to the bacterivorous nematode Caenorhabditis elegans as well as animal-parasitic and fungivorous nematodes. We expressed CCL2 in Arabidopsis to assess its protective potential toward plant-parasitic nematodes. Our results demonstrate that expression of CCL2 enhances host resistance against the cyst nematode Heterodera schachtii. Surprisingly, CCL2-expressing plants were also more resistant to fungal pathogens including Botrytis cinerea, and the phytopathogenic bacterium Pseudomonas syringae. In addition, CCL2 expression positively affected plant growth indicating that CCL2 has the potential to improve two important agricultural parameters namely biomass production and general disease resistance. The mechanism of the CCL2-mediated enhancement of plant disease resistance depended on fucoside-binding by CCL2 as transgenic plants expressing a mutant version of CCL2 (Y92A), compromised in fucoside-binding, exhibited wild type (WT) disease susceptibility. The protective effect of CCL2 did not seem to be direct as the lectin showed no growth-inhibition toward B. cinerea in in vitro assays. We detected, however, a significantly enhanced transcriptional induction of plant defense genes in CCL2- but not CCL2-Y92A-expressing lines in response to infection with B. cinerea compared to WT plants. This study demonstrates a potential of fungal defense lectins in plant protection beyond their use as toxins.
Keywords: Coprinopsis cinerea lectin 2, Heterodera schachtii, Botrytis cinerea, Pseudomonas syringae, Arabidopsis
Introduction
Plants are exposed to a wide range of biotic stress caused by numerous pathogens and pests. As a consequence, plants evolved a robust multi-layered innate immune system. The first layer, pathogen-associated molecular pattern (PAMP)-triggered immunity (PTI), is activated by the perception of PAMPs such as chitin oligomers or bacterial flagellin via pattern recognition receptors at the cell surface (Jones and Dangl, 2006; Schwessinger and Zipfel, 2008). Many pathogens have evolved effectors (virulence factors) to suppress PTI (Macho and Zipfel, 2015). The second layer of plant immunity, named effector-triggered immunity (ETI), is activated via detection of pathogen effectors by plant resistance proteins (Dangl and Jones, 2001). Plant defense responses are coordinated by hormonal signaling pathways with salicylic acid (SA) and jasmonic acid (JA) playing major roles (Robert-Seilaniantz et al., 2011). A special form of induced plant disease resistance is known as systemic acquired resistance (SAR) which functions as a form of plant immunization. A local inoculation with a potential pathogen or treatment with specific chemical compounds enhances disease resistance of the whole plant against a wide range of pathogens. This is achieved by the local activation of signal transduction pathways that lead to the systemic induction of plant immune responses (Pieterse et al., 2012).
Lectins are proteins that can reversibly bind to carbohydrate epitopes on polysaccharides, glycoproteins, and glycolipids. Most characterized lectins have been isolated from plants, such as the well-known examples ricin and abrin (Sharon and Lis, 2004; Vandenborre et al., 2011). Plant lectins are involved in defense-related functions and their roles in plant response to biotic and abiotic stresses have been well established (Van Damme et al., 2004; Van Holle and Van Damme, 2018). As an example, Nictaba is a lectin from tobacco whose biosynthesis is induced in response to insect herbivory or jasmonate-related compounds. It binds to N-acetylglucosamine (GlcNAc) oligomers and is toxic to phytophagous insects (Delporte et al., 2015). The Nictaba homolog in Arabidopsis is an F-box-Nictaba lectin which possesses a carbohydrate-binding activity toward Gal-GlcNAc (Stefanowicz et al., 2012). Similar to the tobacco homolog, the gene coding for F-box-Nictaba is stress-inducible (Stefanowicz et al., 2016).
Fungi are a valuable source of lectins with novel carbohydrate specificities. The majority of fungal lectins have been discovered from fruiting bodies and sclerotia (82%) and few from microfungi (15%) and yeasts (3%) (Varrot et al., 2013). Fungal lectins have various applications in biomedicine, for instance as diagnostic agents, mitogens, antimicrobial and antiviral agents, immunomodulators, antitumor and antiproliferative agents and other therapeutic applications (Hassan et al., 2015; Singh et al., 2019). There are many reports describing the antimicrobial activity of fungal lectins. For example, Aleuria aurantia lectin showed antifungal activity against Mucor racemosus by specifically binding to L-fucose-containing polysaccharides at the surface of fungal cell walls (Amano et al., 2012). Similarly, Gymnopilus spectabilis and Schizophyllum commune lectins inhibit the growth of Aspergillus niger (Chumkhunthod et al., 2006; Albores et al., 2014). A lectin isolated from fruiting bodies of the mushroom Sparassis latifolia showed antifungal and antibacterial activity (Chandrasekaran et al., 2016). Many fungal lectins also show insecticidal and nematicidal activity (Künzler, 2015; Sabotic et al., 2016). For example, an actinoporin-like lectin from edible mushroom Xerocomus chrysenteron is toxic to the fruit fly Drosophila melanogaster and to aphids (Trigueros et al., 2003; Jaber et al., 2008). Marasmius oreades agglutinin (MOA) has a β-trefoil domain with an additional cysteine-protease domain at the C-terminus. Interestingly, both the glycolipid-binding and enzymatic activities of MOA are required for its toxicity toward Caenorhabditis elegans (Wohlschlager et al., 2011). Coprinopsis cinerea lectin 2 (CCL2) is a β-trefoil dimeric lectin, that shows toxicity toward C. elegans and D. melanogaster (Schubert et al., 2012; Bleuler-Martinez et al., 2017). CCL2 exerts its toxicity by binding to glycoproteins carrying an α1,3-fucosylated N-glycan core at the surface of the C. elegans intestinal epithelium (Schubert et al., 2012; Stutz et al., 2015). Cytoplasmic expression of CCL2 in the fungus, Ashbya gossypii, conferred resistance toward fungivorous nematodes (Tayyrov et al., 2018). Purified CCL2 inhibited larval development of the animal parasitic nematode Haemonchus contortus (Heim et al., 2015).
There are many reports of the potential role of plant lectins in plant immunity. The role of fungal lectins in the regulation of immunity is, however, poorly understood (Künzler, 2018). Similarly, their biotechnological application for plant protection and disease management is largely neglected. This study demonstrates that expression of CCL2 in Arabidopsis plants enhances disease resistance against the sugar beet cyst nematode Heterodera schachtii, three fungal pathogens and the phytopathogenic bacterium Pseudomonas syringae. Enhanced disease resistance appears to be mediated by the carbohydrate-binding ability of CCL2 as a binding-deficient mutant version of the CCL2 protein showed no protective function.
Materials and Methods
Plant Growth Conditions and Quantification of Growth Phenotype
Wild type (WT) Arabidopsis thaliana ecotype Colombia-0 (Col-0) was received from the Nottingham Arabidopsis Stock Centre (Nottingham, United Kingdom). Seeds were sown into Jiffy artificial soil (Jiffy International AS, Kristiansand, Norway). After stratification at 4°C for 3 days, plants were transferred to growth chambers with the following condition: 22.5ı°C day/19°C night temperature and 16 h of light (photon flux density100 μmol m–2 s–1) with 60% relative humidity. For growth quantification rosettes of 4-week-old plants were harvested and carefully cleaned to remove non-plant particles. After recording the fresh weight (FW), the rosettes were incubated at 80°C for 12 h to determine the dry weight (DW). The experiment was repeated three times.
Construction of Plant Expression Vectors
Plasmids directing the expression of 3xFLAG tagged CCL2 or CCL2-Y92A under the control of the CaMV 35S promoter were constructed using the Gateway Cloning Technology (Thermo Fisher Scientific, San Jose, CA, United States). The open reading frames of CCL2 and CCL2-Y92A were PCR-amplified from respective Escherichia coli expression plasmids using gene-specific primers. The PCR was performed using Phusion High-Fidelity DNA Polymerase (New England Biolabs, Ipswich, MA, United States). After agarose gel electrophoresis the PCR products were extracted from the gel with the QIAquick Gel Extraction Kit (Qiagen, Hilden, Germany) and inserted into a pENTR vector (pENTRTM/D-TOPOTM Cloning Kit, Thermo Fisher Scientific). The products were transformed into chemically competent TOP10 E. coli cells. Positive colonies were verified by colony PCR (Biometra, Göttingen, Germany) and DNA sequencing (Eurofins Genomics, Ebersberg, Germany). The entry plasmids were subsequently recombined into the binary Gateway overexpression vector pB2GW7 (Karimi et al., 2002), using LR reaction (GatewayTM LR ClonaseTM II Enzyme mix, Thermo Fisher Scientific). The resulting expression plasmids containing 35S:CCL2-3xFLAG or 35S:CCL2-Y92A-3xFLAG constructs, respectively, were verified by colony PCR and transformed into Agrobacterium tumefaciens strain GV3101 by the freeze-thaw method (Schütze et al., 2009).
Overexpression of CCL2 and CCL2-Y92A in Arabidopsis
Agrobacterium–mediated transformation of Col-0 plants using the floral dip method was performed as previously described (Zhang et al., 2006). Transformed plants were selected by spraying 15 μg mL–1 of Glufosinate-ammonium (Basta®, Bayer CropScience AG, Germany) twice within 2 weeks after sowing. Standard immunoblotting procedures were performed to measure the expression level of recombinant proteins in transgenic lines. Leaf tissue of 4-week-old plants was harvested and frozen in liquid nitrogen. The frozen tissue in 1.5 ml tubes (Eppendorf, Hamburg, Germany) containing two 3 mm glass beads was ground with a mixer mill (Retsch®MM400, Retsch Technology GmbH, Haan, Germany) adjusted at 30 Hz for 3 min. For 30 mg of tissue 90 μL Laemmli buffer (375 mM Tris–HCl, pH 6.8, 37% (v/v) glycerol, 0.06% (w/v) bromophenol blue sodium salt, 12% (w/v) sodium dodecyl sulfate, and 5% (w/v) β-mercaptoethanol) was added. Tubes were incubated for 10 min at 95°C with shaking (1400 rpm). After centrifugation 10 μL of the supernatant was used for SDS-PAGE. The separated proteins were transferred to nitrocellulose membranes (Sigma-Aldrich) with a Mini Trans-Blot® Cell (Bio-Rad Laboratories, Hercules, CA, United States). As a loading control, the membranes were stained with Ponceau S (1% acetic acid, 0.1% (w/v) Ponceau S) for 5 min at room temperature, washed twice with 5% acetic acid and once with water. For immunoblotting, membranes were blocked with 3% milk in TBST buffer (150 mM NaCl, 10 mM Tris, 0.1% (v/v) Triton X-100, pH 7.6). Anti-FLAG primary antibodies (1:1000; monoclonal anti-FLAG M2-Peroxidase (HRP) clone M2, Sigma-Aldrich) were applied for 1 h to detect FLAG-tagged proteins. Pierce ECL Western Blotting Substrate (Thermo Fisher Scientific) and horseradish peroxidase (HRP) were used for blot development. Signals were detected by ImageQuant Las 4000 (GE Healthcare Life Sciences, Marlborough, MA, United States). From 60 transgenic plants two independent lines expressing either CCL2 or CCL2-Y92A at comparable levels in the T3 generation were selected for further experiments.
Construction of Bacterial Expression Vectors
For bacterial expression, cDNAs of CCL2 and CCL2-Y92A, respectively, were inserted between the NdeI and XhoI sites of the bacterial expression vector pET-24a containing a HIS-tag (Novagen, Madison, WI, United States). The ligated products were transformed into TOP10 E. coli competent cells. After colony PCR and sequence verification, the purified plasmids were transformed into E. coli BL21 (DE3) for protein production (Novagen, Madison, WI, United States).
Heterologous Protein Expression and Protein Purification
Bacterial cells were cultured in Luria Bertani (LB) broth at 37°C to an optical density of OD600 = 0.8. Protein production was induced by the addition of 0.5 mM isopropyl β-D-1-thiogalactopyranoside (IPTG) (AppliChem GmbH, Germany). Bacteria were further incubated at 16°C for 18 h. Protein extraction and purification were conducted as previously described (Schubert et al., 2012). HIS-tagged proteins were purified by metal-affinity chromatography using Ni-NTA resins (Qiagen). Protein concentration was estimated by the Pierce BCA Protein Assay Kit (Thermo Fisher Scientific) and the purity of CCL2 and CCL2-Y92A was examined by SDS-PAGE.
In vitro Antifungal Assay
Antifungal activity of CCL2 proteins against Botrytis cinerea strain BMM was tested in 96-well Costar cell culture plates (Corning Incorporated, Corning, NY, United States) in a total volume of 200 μL. Spores of B. cinerea were diluted in 25% PDB medium (Potato Dextrose Broth, Oxoid, Hampshire, United Kingdom) and used at a final density of 1 × 103 spores mL–1. Purified CCL2 proteins in 20 mM Na phosphate buffer pH 6 were added at final concentrations of 0–1000 μg mL–1. After incubation on a shaking platform (80 rpm; 20°C), OD595 was measured using the cell imaging multi-mode plate reader CytationTM 5 (BioTek, Winooski, VT, United States). The absorbance reads were analyzed with Gen5 Image + Software (Version 3.03.14, BioTek). Growth curves were generated by GraphPad Prism version 8.0.2 (GraphPad Software, Inc., La Jolla, CA, United States). Experiments were repeated 3-times.
Heterodera schachtii Infection Assay
Heterodera schachtii infection assays were performed according to Bohlmann and Wieczorek (2015). Transgenic seeds were surface-sterilized (Lindsey et al., 2017), and grown on selective Murashige and Skoog medium (MS, Sigma-Aldrich) containing 3% sucrose and 10 mg L–1 glufosinate-ammonium. Col-0 was grown on plates without glufosinate-ammonium. After 5 days, healthy seedlings were transferred to plates containing a modified 0.2 concentrated Knop medium supplemented with 2% sucrose (Sijmons et al., 1991). Six plates per line with eight plants per plate were incubated in the growth chamber for 7 days. Cysts of H. schachtii were collected from in vitro stock cultures. Hatching of 2nd stage juveniles (J2s) was stimulated by soaking cysts in 3 mM ZnCl2. Prior to inoculation, the J2s were sterilized, and total root length was estimated according to Jürgensen (2001). For infection assays, plants were inoculated with 30 freshly hatched J2s per plant, left in the dark overnight and then transferred into a growth chamber. The nematode infection was assessed 14 dpi (day post-inoculation). The total numbers of females per root cm were calculated and the experiment was repeated three times.
Disease Resistance Tests
Two-independent CCL2 or CCL2-Y92A overexpressing lines and WT were grown under the described conditions. After 4 weeks, four leaves per plant were inoculated with 6 μL droplets of a spore suspension (5 × 104 spores mL–1) of B. cinerea. Plants were covered with a transparent plastic dome to keep high humidity and incubated in the dark. At 3 dpi, the lesion size was measured by Vernier caliper (MarCal 16 ER, Mahr GmbH, Germany). Twenty plants per line were tested, and three independent biological replicates were performed. Fungal hyphae and dead plant tissues were stained in a solution of ethanolic lactophenol Trypan Blue (Hael-Conrad et al., 2015). The samples were analyzed using a Leica DMR microscope with bright-field settings. Colletotrichum higginsianum was grown on oatmeal agar (Condalab S.A., Madrid, Spain) for 7 days at 22°C. Four leaves of 5-week-old Arabidopsis plants were inoculated with 10 μL droplets of 2 × 106 conidia mL–1 suspended in 25% PDB (Oxoid). Droplets of 25% PDB were used as a mock treatment. Plants were covered with a plastic dome to keep humidity and incubated in the growth chamber. Lesions were measured 10 dpi with a digital Vernier caliper (MarCal 16 ER). Ten plants per line were tested and 3 independent biological replicates were performed. Plectosphaerella cucumerina was grown on CM0139 PDA (Potato Dextrose Agar, Oxoid) plates at 25°C. Four leaves of 4-week-old Arabidopsis plants were infected with 10 μL droplets of 5 × 106 spores mL–1 suspended in 25% PDB. The conditions of inoculation were as described for C. higginsianum. Lesion size was measured at 5 dpi. Ten plants per line were tested and three independent biological replicates were performed. P. syringae pv. tomato (Pst) DC3000 was cultured for 16 h at 28°C with shaking (180 rpm) in liquid LB medium (supplied with 50 mg L–1 rifampicin). Bacterial cells were centrifuged at 3000 rpm for 10 min, and the pellet was diluted in 10 mM MgCl2. For basal disease resistance assay, the leaves of 4-week-old plants were syringe-infiltrated with bacterial suspension of Pst DC3000 (105 CFU mL–1). Infiltrated leaves were harvested at 72 hpi (hours post-inoculations) for quantification by qPCR of the oprF gene (Genebank 878442) as a marker of bacterial growth.
Quantification of Fungal and Bacterial Biomass
The fungal biomass was quantified according to Gachon and Saindrenan (2004) with minor modifications. Ten leaf discs were harvested from inoculated leaves and immediately frozen in liquid N2. For each line, three independent biological replicates were performed. Total DNA was isolated using Plant DNA mini Kit (Peqlab/VWR, Darmstadt, Germany). To quantify fungal or bacterial DNA content, the qPCR mixtures were prepared with 12.5 μL of SYBR Green mix (Bioline, London, United Kingdom), 10 μL of DNA (final amount 100 ng), and 0.75 μL of forward and reverse primers (10 μM; Supplementary Table 1). The final volume was 25 μL. The qPCR was conducted with a MIC qPCR machine (Bio Molecular Systems, Australia) using the following conditions: 10 min at 95°C initial denaturation and 40 cycles (95°C for 15 s, 60°C for 1 min and 72°C for 30 s). Specificity of amplification was analyzed by melting point analysis. The level of the fungal Cutinase A gene (Genebank Z69264) or the bacterial oprF gene (Genebank 878442) were normalized against the expG gene (AT4G26410) of Arabidopsis (Czechowski et al., 2005). The 2(–Δ Δ Ct) method was used to analyze the results (Rao et al., 2013).
Systemic Acquired Resistance (SAR)
Three leaves of 4-week-old Col-0 plants were infiltrated with either 500 μg mL–1 of purified CCL2 or purified CCL2-Y92A in 10 mM MgCl2. Infiltration with Pst DC3000 at 106 CFU mL–1 in 10 mM MgCl2 served as positive control. Infiltration with 10 mM MgCl2 served as negative control. After 48 h, three distal leaves were inoculated with Pst DC3000 (105 CFU mL–1). Ten leaf discs were harvested from the distal leaves 3 dpi with a cork borer (discs from different plant leaves) and used for DNA extraction. The level of the bacterial oprF gene (Genbank 878442) was analyzed by qPCR. For transcript levels of SAR defense-related genes after the primary treatments, local leaves were sampled 2 days post-treatment for RNA extraction, cDNA synthesis and qPCR analyses.
Transcript Levels of Defense-Related Genes
Transcript levels of defense-related genes in response to B. cinerea or Pst were analyzed by qPCR. Leaves were ground in liquid N2, and total RNA was extracted with the SpectrumTM Plant Total RNA Kit (Sigma-Aldrich, Saint Louis, MO, United States). The isolated RNA was treated with deoxyribonuclease I enzyme (Sigma-Aldrich) to remove remaining DNA. Two micrograms of purified RNA were used for reverse transcription reactions with the Omniscript Reverse Transcription Kit (Qiagen). The qPCR mixture contained 7.5 μL of SYBR Green (Bioline), 5 μL of cDNA (corresponding to 100 ng RNA), and 0.5 μL of 10 μM forward and reverse primers (Supplementary Table 1). The final volume was completed with DEPC-treated Water (0.1% diethylpyrocarbonate) to 15 μL. The qPCR was done as follows: 10 min at 95°C initial denaturation and 40 cycles (95°C for 15 s, 60°C for 1 min and 72°C for 30 s). Runs were performed on a MIC qPCR machine (Bio Molecular Systems). Transcript levels were normalized against the expG gene (AT4G26410). The analysis was accomplished based on cycle threshold method (2(–Δ Δ Ct); Rao et al., 2013). Three biological replicates were performed for each sample.
Statistical Analysis
Statistical analysis was carried out using GraphPad Prism version 8.0.2 (GraphPad Software, Inc.). One/two-way ANOVA analysis was conducted to identify significant differences among treatments relative to the control. Tukey or Dunnett tests were used for multiple comparisons between the transgenic lines and control. Asterisks indicate statistically significant differences (∗∗∗P ≤ 0.001, ∗∗P ≤ 0.01, ∗P ≤ 0.05) whereas ns (not significant) indicates P > 0.05. The letters a and b signify a between-group difference at the P ≤ 0.05 level.
Results
Expression of CCL2 in Arabidopsis Boosts Plant Growth
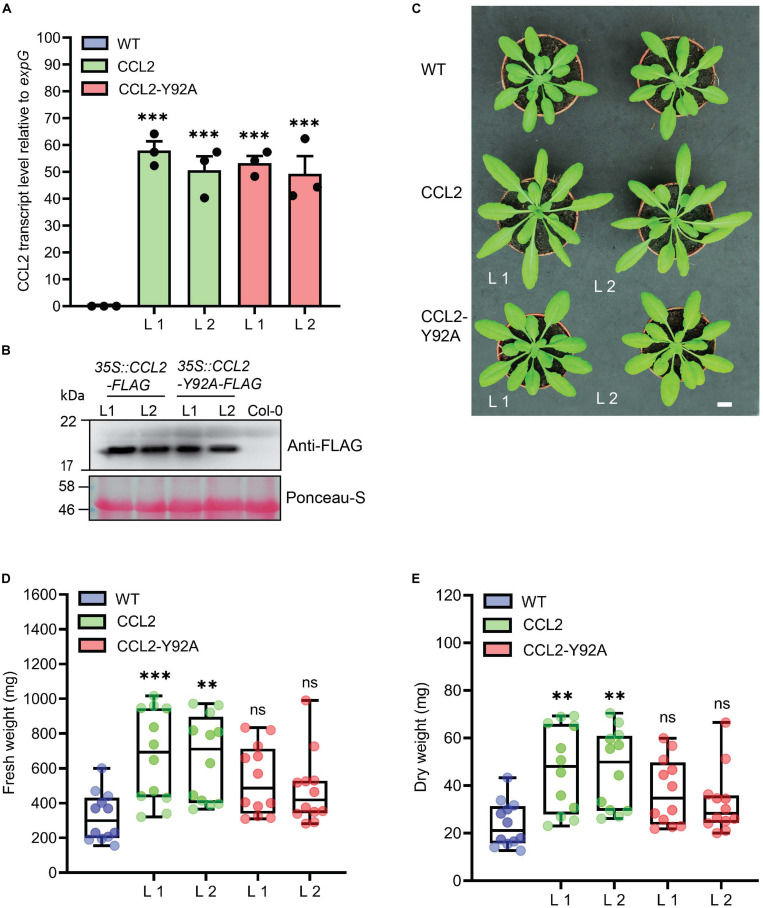
FLAG-tagged CCL2 and a mutated FLAG-tagged CCL2-Y92A version compromised in fucoside-binding were expressed in Arabidopsis (accession Col-0) under the control of the CaMV-35S promoter using the constructs 35S:CCL2-3xFLAG and 35S:CCL2-Y92A-3xFLAG, respectively. Transgenic plants were analyzed by qPCR and immunoblotting to select lines with a comparable expression level of CCL2 or CCL-Y92A, respectively (Figures 1A,B). The transgenic lines grew bigger than WT plants (Figure 1C). Quantification of rosettes of 4-week-old plants indicated that FW and DW were significantly higher in transgenic plants (Figures 1D,E). In CCL2 lines FW and DW of rosettes were 100 and 95% higher than in WT plants, respectively. The differences of FW and DW for CCL2-Y92A lines were not statistically significant compared to the WT plants.
FIGURE 1.
Characterization of CCL2-expressing Arabidopsis lines. (A) qPCR analysis of relative CCL2 and CCL2-Y92A transcript levels in 4-week-old plants. Transcript levels were normalized to expG gene (AT4G26410). Mean values ± SE of three independent experiments. (B) Immunoblot visualizing the expression level of CCL2 and CCL2-Y92A proteins. FLAG-tagged proteins were detected with anti-FLAG antibodies. Ponceau-S stained Rubisco large subunit served as loading control. Size bar = 1 cm (C) Growth phenotype of transgenic lines compared to WT. Two independent lines (L1 and L2) are shown for each construct. (D) Fresh weight (FW) and (E) dry weight (DW) of shoots of 4-week-old plants (n = 12; three independent experiments). Boxplots represent median and 1.5 times the interquartile range. Asterisks show significant differences between transgenic lines compared to the WT (∗∗∗P ≤ 0.001, ∗∗P ≤ 0.01) determined by one-way ANOVA followed by post hoc analysis with Dunnett’s multiple-comparison test.
CCL2 Enhances Disease Resistance Against the Plant-Parasitic Nematode Heterodera schachtii
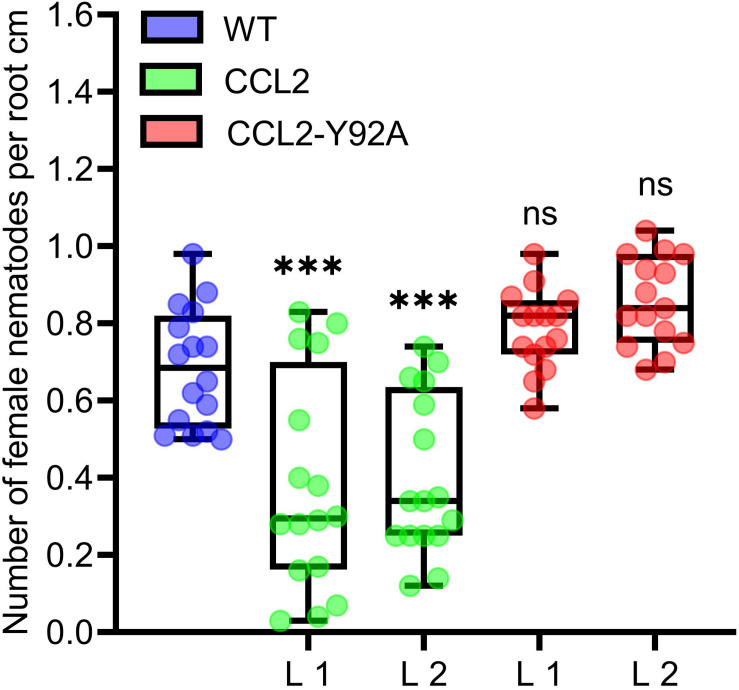
Based on the previous in vitro evidence for nematicidal activity, CCL2 and CCL2-Y92A-expressing Arabidopsis lines were tested with the agronomically important sugar beet cyst nematode H. schachtii. Transgenic lines and WT plants were inoculated with J2 juveniles and the progression of nematode infection was evaluated in roots. The results indicated a protective effect of CCL2. The number of H. schachtii females per cm of root was significantly reduced by 35% in CCL2 lines compared to WT plants (Figure 2). In contrast, CCL2-Y92A-expressing lines showed similar susceptibility as WT plants. Our results indicate that CCL2 expression partially protected Arabidopsis roots from parasitism by H. schachtii and that the protective effect was dependent on carbohydrate-binding activity of CCL2.
FIGURE 2.
Partial resistance of CCL2-expressing plants toward the cyst nematode H. schachtii. 12-day-old Arabidopsis seedlings (WT, CCL2, and CCL2-Y92A lines) were inoculated with 30 freshly hatched juveniles per plant and evaluated 14 dpi for number of female nematodes per root centimeter. Boxplots represent median and 1.5 times the interquartile range (WT and CCL2 lines n = 16; CCL2-Y92A n = 15; three independent experiments). Asterisks above columns indicate statistically significant differences (∗∗∗P ≤ 0.001, ns, not significant) between CCL2 lines and WT plants, analyzed by one-way ANOVA and post hoc analysis with Dunnett’s multiple-comparison test.
CCL2 Enhances Resistance of Arabidopsis Against Fungal Pathogens
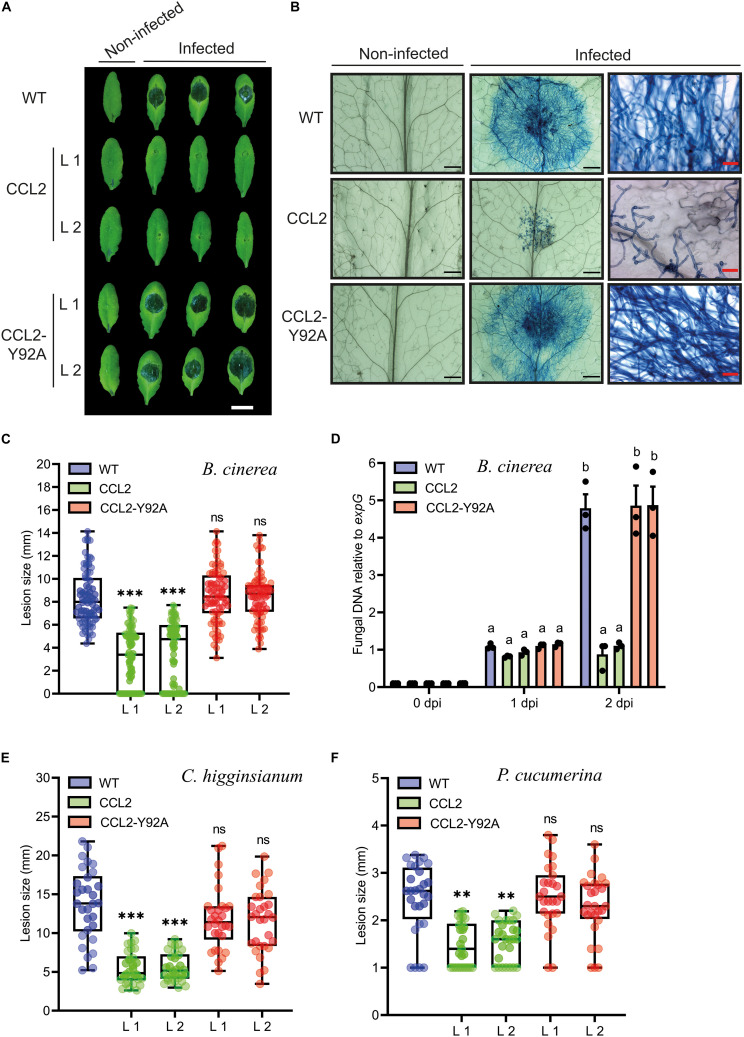
In order to test whether the protective effect of CCL2 was specific for nematodes or more general, the transgenic CCL2 lines and WT plants were inoculated with droplets of a suspension of conidiospores of B. cinerea. The fungal pathogen, known as gray mold, is a necrotrophic plant pathogen that can infect more than 200 plant species, causing losses of agricultural products both pre-and post-harvest (Dean et al., 2012). The lesion size caused by the fungal infection was analyzed at 3 dpi. The pathogen successfully colonized WT and CCL2-Y92A-expressing plants as indicated by the formation of large necrotic lesions spreading from the inoculation site. In contrast, in the two CCL2-expressing lines such lesions were significantly smaller and surrounded by a lighter colored halo (Figure 3A). Trypan Blue staining revealed the growth of fungal hyphae within the infected leaves. In WT and CCL-Y92A-expressing plants, fungal hyphae spread through the leaves whereas the colonization of leaves by the fungus was impaired in CCL2-expressing plants (Figure 3B). Quantitative analysis showed that the lesion size in the CCL2 plants, including the lighter colored halo, was reduced to 54% compared to WT. No significant difference was detected between CCL2-Y92A lines and WT plants (Figure 3C). Quantification of fungal biomass based on qPCR analysis of fungal DNA present in inoculated plants confirmed the results of the macro- and microscopic analysis (Figure 3D). At 2 dpi, the B. cinerea biomass was significantly higher in WT and CCL2-Y92A plants compared to CCL2 plants. These results indicated that expression of CCL2 inhibited colonization of the plant by the fungus in a carbohydrate-binding-dependent manner. In order to test the specificity of the antifungal effect of CCL2, the Arabidopsis CCL2 lines, CCL2-Y92A lines and WT plants were challenged with the fungal pathogens C. higginsianum or P. cucumerina. C. higginsianum is a hemibiotrophic pathogen that globally causes disease in many economically important crops (Yan et al., 2018). Likewise, P. cucumerina is a necrotrophic pathogen that causes diseases in crops worldwide (Sanchez-Vallet et al., 2010). Plant leaves were inoculated with droplets of C. higginsianum spore suspensions. CCL2 lines showed at 10 dpi a significant reduction of lesion size of 60% (L1) and 59% (L2) compared to WT plants (Figure 3E). Similarly, after inoculation with spores of P. cucumerina, the lesions of the CCL2 lines after 5 dpi were 39% (L1) and 36% (L2) smaller than in WT plants (Figure 3F). Plants expressing CCL-Y92A showed WT-like disease resistance to both fungi. Taken together, the results demonstrate that expression of CCL2 partially protected plants against a variety of fungal pathogens, including necrotrophs (B. cinerea and P. cucumerina) and hemibiotrophs (C. higginsianum). The protective effect depended on the ability of CCL2 to bind carbohydrates.
FIGURE 3.
Resistance of CCL2-expressing plants toward fungal pathogens. (A) Necrotic lesions caused by B. cinerea infection on leaves of 4-week-old WT, CCL2- and CCL2-Y92A lines inoculated with 6 μL droplets of a spore suspension (5 × 104 spores mL–1). Plants were photographed 3 dpi. Size bar = 1 cm. (B) Trypan Blue-staining of Arabidopsis leaves 60 hpi. The right-side shows close-up images. Black or red size bares are 1 mm and 50 μm, respectively. (C) Quantification of lesion size at 3 dpi. Boxplots represent median and 1.5 times the interquartile range (n = 80 from three independent experiments). (D) Quantification of fungal DNA by qPCR at 0, 1, and 2 dpi. The fungal Cutinase A gene (Genebank: Z69264) was quantified relative to expG gene (AT4G26410) of Arabidopsis. Bars represent mean values ± SE from three independent experiments. (E) Analysis of lesion size of 5-week-old WT and transgenic CCL2 lines droplet-inoculated with C. higginsianum (10 μL of 2 × 106 spores mL–1 per leaf). Plants were analyzed 10 dpi. (F) Analysis of lesion size of 4-week-old WT and CCL2 lines, droplet-inoculated with P. cucumerina (10 μL of 5 × 106 spores mL–1 per leaf). Plants were analyzed 5 dpi. Boxplots (E,F) represent median and 1.5 times the interquartile range (n = 30 from three independent experiments). The data was analyzed by one-way ANOVA and post hoc analysis by Dunnett’s multiple-comparison test. Asterisks show a statistically significant difference between the CCL2 expressing lines and WT plants (∗∗∗P ≤ 0.001, ∗∗P ≤ 0.01, ns, not significant). The letters a and b signify a between-group difference at the P ≤ 0.05 level.
CCL2 Enhances Transcript Accumulation of Plant Defense Genes Upon Pathogen Inoculation
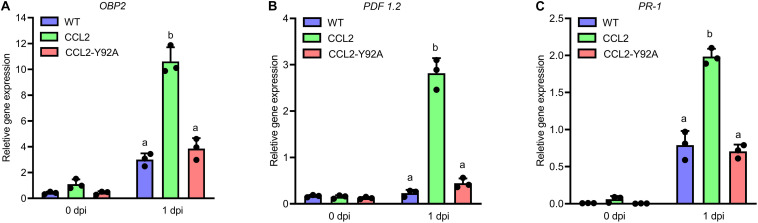
In order to assess whether the fungal growth inhibition by the CCL2 lectin is direct, an in vitro assay for antifungal activity toward B. cinerea was conducted. The purified His-tagged CCL2 proteins (CCL2 and CCL2-Y92A; Supplementary Figure 1) were applied to fungal spores in liquid medium and spore germination and hyphal growth were assessed. No inhibition of fungal growth was detected even at a concentration of 1 mg mL–1 of purified protein (Supplementary Figure 2). Based on these results, we reasoned that CCL2 might have an indirect effect on plant protection via the activation of plant immune responses. The transcript levels of Arabidopsis defense genes in infected WT plants and transgenic lines were assessed by qPCR (Figures 4A–C). Analyzed Arabidopsis defense genes included methyl JA-inducible marker genes (OBP2, AT1G07640), PLANT DEFENSIN (PDF1.2: AT5G44420) and SA-inducible PATHOGENESIS-RELATED PROTEIN-1 (PR-1: AT2G14610). No significant differences in transcript levels between WT and transgenic lines were observed at 0 dpi indicating that CCL2 expression did not directly trigger defense gene expression. However, transcript levels of all three genes were enhanced at 1 dpi in CCL2 lines compared to WT and CCL2-Y92A lines. Induction of OBP2 transcript levels was enhanced 3.5-fold, PDF1.2 transcripts 12-fold and PR-1 transcripts 2.5-fold compared to WT at 1 dpi indicating a priming effect of CCL2 on pathogen-induced expression of these genes. The respective transcript levels were not significantly different between WT and CCL2-Y92A lines. These results suggested that the protective effect of CCL2-expression in Arabidopsis toward fungal pathogens might be achieved by boosting the immune responses of the host plant upon pathogen inoculation.
FIGURE 4.
CCL2 enhances induction of Arabidopsis defense gene expression in response to B. cinerea. Four-week-old Arabidopsis plants (WT, CCL2, and CCL2-Y92A lines) were spray-inoculated with B. cinerea (5 × 105 spores mL–1). Leaves were harvested at 0 and 1 dpi for RNA extraction. Transcript levels of OBP2 (A), PDF1.2 (B), and PR-1 (C) were determined by qPCR. Data were normalized with regard to the Arabidopsis reference gene expG. Data represent mean values ± SE of three independent experiments. The letters a and b signify a between-group difference at the P ≤ 0.05 level. Two-way ANOVA and post hoc analysis by Tukey’s multiple-comparison test were used to calculate significant differences between transgenic lines and WT plants.
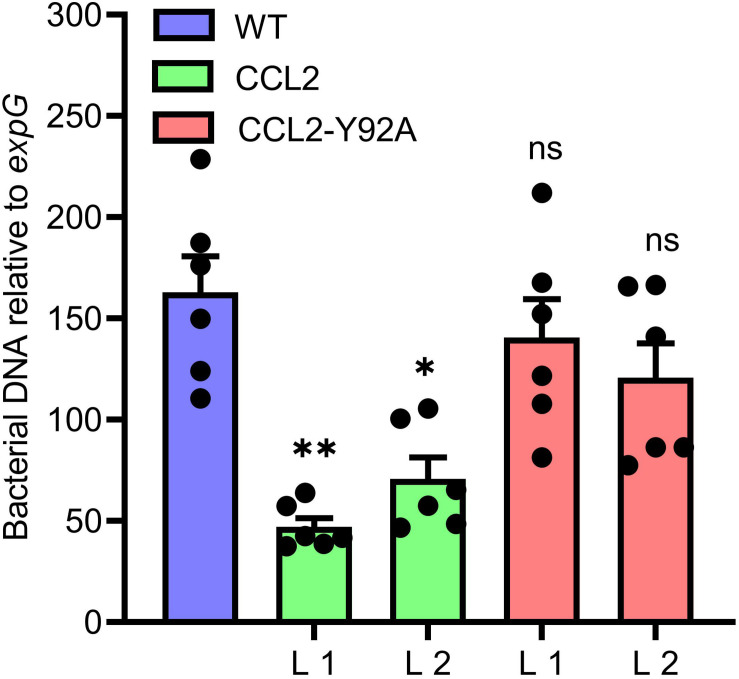
Resistance Against Pseudomonas syringae Is Enhanced in CCL2 Lines
Based on the increased resistance of the CCL2 lines against a variety of fungal plant pathogens, we were interested in assessing the resistance of plants against the bacterial plant pathogen P. syringae pv. tomato (Pst), a hemibiotrophic pathogen that can infect many plant species (Glazebrook, 2005). WT plants and transgenic lines were inoculated with 105 CFU mL–1 of a bacterial suspension. At 3 dpi plant tissues were analyzed by qPCR to quantify bacterial DNA based on the bacterial oprF gene (Ross and Somssich, 2016). The bacterial biomass based on oprF content was significantly reduced by 73 and 57% in the CCL2 lines 1 and 2, respectively, compared to WT (Figure 5). The difference between the CCL2-Y92A lines and WT was not significant. The results indicated that the expression of CCL2 enhanced the resistance toward P. syringae. Similar to the results with fungal pathogens, the protective effect depended on the carbohydrate-binding activity of CCL2 as the mutant version CCL2-Y92A failed to protect plants against P. syringae.
FIGURE 5.
Increased resistance of CCL2-lines toward the bacterial pathogen P. syringae. Growth of virulent Pst DC3000 in WT plants and CCL2 lines was analyzed at 3 dpi. The bacterial oprF gene was quantified by qPCR using DNA extracted from inoculated leaves. Ten leaf discs from six plants were sampled per replicate. The plant expG gene served as reference. Data represent mean values ± SE of three independent experiments (n = 18). Asterisks indicate statistically significant differences (∗P ≤ 0.05, ∗∗P ≤ 0.01, ns: not significant; one-way ANOVA and post hoc analysis with Dunnett’s multiple-comparison test) between transgenic lines and wild type.
Exogenous Application of Purified CCL2 Protein Confers Systemic Acquired Resistance (SAR)
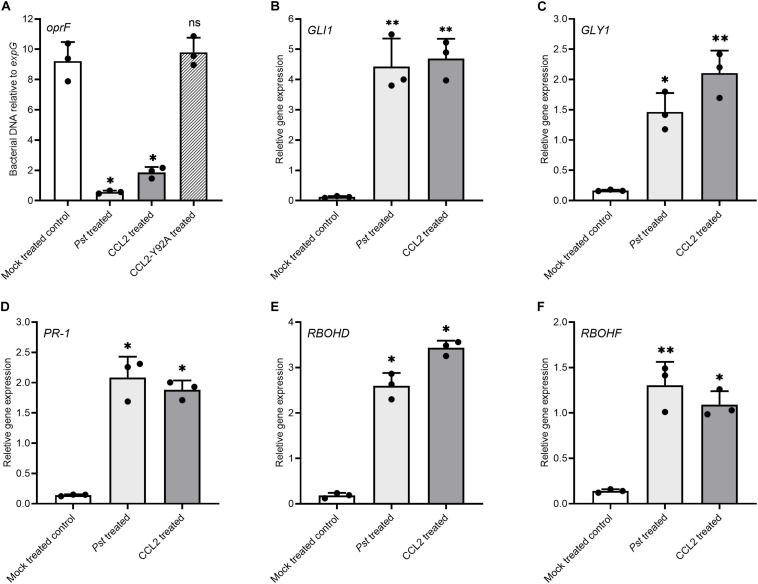
To further support the immune-activating properties of CCL2, the potential of exogenously applied CCL2 for activation of defense gene expression and induction of SAR was analyzed. Purified CCL2 protein (500 μg mL–1) was locally infiltrated into leaves of WT plants and disease resistance toward Pst DC3000 was analyzed in untreated distal leaves. Treatment of local leaves with CCL2 led to an induction of SAR against Pst DC3000 in challenge-inoculated systemic leaves comparable to inoculation of local leaves with Pst (Figure 6A). In contrast, treatment with CCL2-Y92A failed to induce SAR as no significant difference compared to mock treatment was observed. The results suggested that exogenously applied CCL2 protein induced SAR against Pst in a carbohydrate-binding dependent manner. To test the potential of CCL2 for direct activation of defense gene expression, WT plants were infiltrated with purified CCL2 protein (500 μg mL–1) and transcript levels of a number of defense-related genes were analyzed 48 h after treatment: GLI1 (AT1G80460) encoding a glycerol kinase, GLYCEROL-3-PHOSPHATE (G3P) SYNTHESIS GENE GLY1 (AT2G40690), PR-1 (AT2G14610), RESPIRATORY BURST OXIDASE HOMOLOGS D and F (RBOHD: AT5G47910 and RBOHDF: AT1G64060). Similar to treatment with the positive SAR control Pst, treatment with purified CCL2 protein resulted in significant increases compared to mock treatment in transcript abundance of all tested genes (Figures 6B–F). CCL2-treated local leaves of WT plants showed a 39-, 13-, 13-, 19-, and 8-fold increase in transcript levels of GLI1, GLY1, PR-1, RBOHD, or RBOHF, respectively, compared to mock-inoculated plants.
FIGURE 6.
Exogenous application of purified CCL2 induces defense gene expression and SAR toward P. syringae. Three leaves of 4-week-old wild type plants were infiltrated with 10 mM MgCl2 (negative mock control), Pst DC3000 (106 CFU mL–1) as positive SAR control, or 500 μg mL–1 of purified CCL2 or CCL2-Y92A protein, respectively. (A) Three distal leaves were challenge-inoculated with Pst DC3000 (105 CFU mL–1) at 48 h after treatment. Ten leaf discs per treatment were sampled from distal leaves of Ten plants at 3 dpi to quantify by qPCR the abundance of the bacterial oprF gene as a proxy for bacterial biomass. (B–F) Transcript levels relative to expG gene in local leaves 48 h after treatment (B) GLI1 (AT1G80460), (C) GLY1 (AT2G40690), (D) PR-1 (AT2G14610), (E) RBOHD (AT5G47910), and (F) RBOHF (AT1G64060). Asterisks indicate statistically significant differences (∗P ≤ 0.05, ∗∗P ≤ 0.01, ns: not significant; one-way ANOVA and post hoc analysis with Dunnett’s multiple-comparison test) between treatments and mock control. Data represent mean ± SD of three biological replicates.
Discussion
The aim of our research was to test transgenic plants expressing the nematicidal CCL2 lectin of C. cinerea for enhanced disease resistance toward plant-parasitic nematodes. To this end, CCL2 or the carbohydrate binding-compromised mutated version CCL2-Y92A were constitutively expressed in Arabidopsis plants. Surprisingly, transgenic CCL2 lines showed multiple phenotypes. They were not only more resistant than WT against the sugar beet cyst nematode H. schachtii but also showed improved disease resistance toward fungal and bacterial pathogens. In addition, CCL2 expression had a positive effect on plant growth. The multiple phenotypes of CCL2 plants depended on the previously demonstrated carbohydrate-binding activity of CCL2 (Schubert et al., 2012; Bleuler-Martinez et al., 2017) as expression of CCL2-Y92A, a mutated version compromized in carbohydrate binding, did not cause detectable differences compared to WT plants. Unless CCL2 has additional, as of yet undiscovered carbohydrate-binding activities, the observed disease resistance related phenotypes must be the result of binding of CCL2 to α1,3-fucosylated N-glycan cores.
Entomotoxic and nematotoxic activity of fungal lectins has been widely studied (Bleuler-Martinez et al., 2011; Künzler, 2015; Sabotic et al., 2016). Similarly, in vitro antibacterial and antifungal activity of fungal lectins against pathogens have been described (Amano et al., 2012; Albores et al., 2014; Singh et al., 2014; Chandrasekaran et al., 2016; Breitenbach et al., 2018). Transgenic plants expressing plant lectins showed enhanced resistance to phytopathogens and pests (Burrows et al., 1998; Ripoll et al., 2003; Stefanowicz et al., 2016; Van Holle et al., 2016). However, to date no lectins of fungal origin have been expressed in plants for disease protection. CCL2-overexpressing Arabidopsis plants showed significantly reduced susceptibility to the cyst nematode H. schachtii. The protective effect of CCL2 is most probably mediated by its carbohydrate-binding activity as the CCL2-Y92A lines do not show improved resistance against nematodes. H. schachtii is an obligate biotroph taking up the nutrients only after induction of feeding sites within the host root tissue. Hence, it was not possible to directly test in vitro toxic effects of CCL2 on parasite development. It remains, therefore, an open question whether the protective effect of CCL2 is direct via its nematicidal activity and/or indirect via primed induction of plant defenses as shown for other priming-active compounds known to enhance resistance toward e.g., root-knot nematodes (Oka et al., 1999; Cohen et al., 2016).
Coprinopsis cinerea lectin 2 lines were compared to WT and CCL2-Y92A lines more resistant to three fungal pathogens. The failure of CCL2-Y92A to protect plants from infection indicates that the carbohydrate-binding activity of CCL2 is essential for the observed protection. CCL2 did not have a toxic effect on the in vitro growth of B. cinerea. Hence, CCL2 is unlikely to protect plants via direct antifungal activity. As an alternative, we tested whether CCL2 possibly protects plants indirectly via activation of plant defense responses. Transgenically expressed CCL2 had no direct effect on the constitutive expression of defense genes. However, in response to B. cinerea CCL2 plants showed a significantly enhanced accumulation of transcripts of JA-regulated OBP2 and PDF1.2 as well as SA-regulated PR-1 genes compared to WT and CCL2-Y92A lines. The CCL2 lines reacted more strongly in terms of defense gene expression to inoculation with B. cinerea. Boosted activation of defense gene expression in response to pathogens, also called priming, has been demonstrated to enhance general disease resistance (Conrath et al., 2006; Mauch-Mani et al., 2017). It is, therefore, likely that the CCL2-mediated defense priming contributes to the enhanced protection of CCL2-expressing lines against fungal pathogens. Priming typically enhances disease resistance against many different pathogens. In line with this, CCL2 lines were also significantly more resistant to the bacterial pathogen Pst.
Local treatment with purified CCL2 enhanced the disease resistance toward Pst in systemic leaves comparable to primary inoculation with the known SAR inducer Pst. In contrast, treatment with purified CCL2-Y92A had no effect on disease resistance in systemic leaves. Hence, CCL2 activated SAR signaling pathways dependent on its carbohydrate-binding activity. Transcript levels of SAR-related genes were significantly enhanced in local leaves of CCL2-infiltrated WT plants compared to mock-treated plants indicating that CCL2 functions similarly to other defense activating compounds (Tripathi et al., 2019). Transgenically expressed CCL2 did not directly affect defense gene expression as the transgenic CCL2 lines showed WT transcript levels in the absence of a pathogen. In contrast, treatment of plants with purified CCL2 caused enhanced transcript accumulation of defense genes. This contradiction is likely the result of the different concentrations of CCL2 present in the transgenic lines and in plants treated with purified CCL2. Many priming active compounds are known to directly induce immune responses depending on their concentration (Conrath et al., 2006; Mauch-Mani et al., 2017). At lower concentrations they still can protect plants from infection without directly affecting defense gene expression by sensitizing the induction of defense responses upon pathogen attack. The positive effect of CCL2 expression on plant growth was an unexpected finding as plants manipulated for enhanced disease resistance often suffer from fitness costs (Bowling et al., 1994; Mauch et al., 2001). However, priming effects at low concentrations are normally not linked with a growth penalty (Conrath et al., 2006; Mauch-Mani et al., 2017). The positive effect of CCL2 on plant growth also depends on the carbohydrate-binding activity.
Similar to previous findings showing nematicidal activity of CCL2 (Schubert et al., 2012; Bleuler-Martinez et al., 2017), our results confirm the importance of the binding of CCL2 to α1,3-fucosylated N-glycans for its immune-stimulating function as the binding-deficient mutant CCL2-Y92A was not able to protect plants against plant-parasitic nematodes and microbial pathogens. We speculate that CCL2 enhances plant immunity via binding to plant glycoproteins or other glycosylated compounds that are involved in regulation of immunity. Interestingly, a recent study indicated that α1,3-fucosylated N-glycans play an essential role in plant immunity (Zhang et al., 2019). Mutations in a gene involved in the biosynthesis of GDP-L-fucose (SCORD6/MUR1) negatively affected PTI and ETI, including glycosylation of immune receptors. In addition, compromised defenses were also observed in mutants of several fucosyltransferases with specific substrates (O-glycan, N-glycan or DELLA transcriptional repressors; Zhang et al., 2019). These results hinted to a so far unknown plant immunity-related role of L-fucose biosynthesis and fucosylation. Biochemical approaches will be needed to identify the plant targets of CCL2.
Conclusion
Overexpression of CCL2 in Arabidopsis improved plant growth and general disease resistance toward the cyst nematode H. schachtii and various plant pathogens. Protection against H. schachtii is likely based on the direct nematotoxic effect of CCL2. CCL2 did not show direct toxicity toward fungi but primed the expression of JA/SA-related defense genes that are important for plant immunity against microbial pathogens. Thus, CCL2 is postulated to induce resistance against microbial pathogens by binding to fucosylated compounds with a role in plant immunity. In agreement with such a model, the mutant version of CCL2 with abolished carbohydrate-binding lost its protective function. Thus, the fungal lectin CCL2 does not only function as a nematotoxin but has additional roles as a positive regulator of plant immunity.
Accession Numbers
NCBI accession numbers: CCL2 (ACD88750); oprF (878442); Cutinase A (Z69264); PDF 1.2 (AT5G44420); PR-1 (AT2G14610); OBP2 (AT1G07640); GLI1 (AT1G80460); GLY1 (AT2G40690); RBOHD (AT5G47910); RBOHF (AT1G64060); expG (AT4G26410).
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author/s.
Author Contributions
AM: production of CCL2 expressing transgenic Arabidopsis plants, disease resistance tests with B. cinerea and C. higginsianum, heterologous expression of CCL2 in E. coli, antifungal assays, planning projects, analyzing data, and writing of manuscript. ME-S: disease resistance against Pst and SAR experiments. JG: disease resistance tests against P. cucumerina. TA and KW: H. schachtii infection assays. PD: writing of manuscript. MK: provided CCL2 and CCL2-Y92A cDNAs, planning projects, and writing of manuscript. FM: supervising, planning projects, and writing of manuscript. All authors contributed to the article and approved the submitted version.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Footnotes
Funding. AM was supported by a Swiss Government Excellence Scholarship for Foreign Scholars (Swiss State Secretariat for Education, Research, and Innovation). FM and MK were supported by the Swiss National Science Foundation (grant nos 31003A_129696 and 31003A_173097). KW was supported by the Austrian Science Fund (FWF, grant no P 29620-B25).
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpls.2021.657451/full#supplementary-material
CCL2 expression in E. coli and purification.
In vitro antimicrobial assay of bacterially produced CCL2 and CCL2-Y92A against B. cinerea.
Sequences of primers for qPCR. F: Forward primer. R: Reverse primer.
References
- Albores S., Mora P., Bustamante M. J., Cerdeiras M. P., Franco Fraguas L. (2014). Purification and applications of a lectin from the mushroom Gymnopilus spectabilis. Appl. Biochem. Biotechnol. 172 2081–2090. 10.1007/s12010-013-0665-5 [DOI] [PubMed] [Google Scholar]
- Amano K., Katayama H., Saito A., Ando A., Nagata Y. (2012). Aleuria aurantia lectin exhibits antifungal activity against Mucor racemosus. Biosci. Biotechnol. Biochem. 76 967–970. 10.1271/bbb.110982 [DOI] [PubMed] [Google Scholar]
- Bleuler-Martinez S., Butschi A., Garbani M., Walti M. A., Wohlschlager T., Potthoff E., et al. (2011). A lectin-mediated resistance of higher fungi against predators and parasites. Mol. Ecol. 20 3056–3070. 10.1111/j.1365-294X.2011.05093.x [DOI] [PubMed] [Google Scholar]
- Bleuler-Martinez S., Stutz K., Sieber R., Collot M., Mallet J. M., Hengartner M., et al. (2017). Dimerization of the fungal defense lectin CCL2 is essential for its toxicity against nematodes. Glycobiology 27 486–500. 10.1093/glycob/cww113 [DOI] [PubMed] [Google Scholar]
- Bohlmann H., Wieczorek K. (2015). Infection assay of cyst nematodes on Arabidopsis roots. Bio. Protoc. 5:e1596. 10.21769/BioProtoc.1596 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowling S. A., Guo A., Cao H., Gordon A. S., Klessig D. F., Dong X. (1994). A mutation in Arabidopsis that leads to constitutive expression of systemic acquired resistance. Plant Cell 6 1845–1857. 10.1105/tpc.6.12.1845 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breitenbach B., Coelho L. C., Marcelino D., Santos S. P., Felix, De Oliveira W., et al. (2018). Lectins as antimicrobial agents. J. Appl. Microbiol. 125 1238–1252. 10.1111/jam.14055 [DOI] [PubMed] [Google Scholar]
- Burrows P. R., Barker A. D., Newell C. A., Hamilton W. (1998). Plant-derived enzyme inhibitors and lectins for resistance against plant-parasitic nematodes in transgenic crops. Pesticide Sci. 52 176–183. [DOI] [Google Scholar]
- Chandrasekaran G., Lee Y. C., Park H., Wu Y., Shin H. J. (2016). Antibacterial and antifungal activities of lectin extracted from fruiting bodies of the Korean cauliflower medicinal mushroom, Sparassis latifolia (Agaricomycetes). Int. J. Med. Mushrooms. 18 291–299. 10.1615/IntJMedMushrooms.v18.i4.20 [DOI] [PubMed] [Google Scholar]
- Chumkhunthod P., Rodtong S., Lambert S. J., Fordham-Skelton A. P., Rizkallah P. J., Wilkinson M. C., et al. (2006). Purification and characterization of an N-acetyl-D-galactosamine-specific lectin from the edible mushroom Schizophyllum commune. Biochim. Biophys. Acta. 1760 326–332. 10.1016/j.bbagen.2006.01.015 [DOI] [PubMed] [Google Scholar]
- Cohen Y., Vaknin M., Mauch-Mani B. (2016). BABA-induced resistance: milestones along a 55-year journey. Phytoparasitica 44 513–538. 10.1007/s12600-016-0546-x [DOI] [Google Scholar]
- Conrath U., Beckers G. J., Flors V., Garcia-Agustin P., Jakab G., Mauch F., et al. (2006). Priming: getting ready for battle. Mol. Plant. Microbe. Interact. 19 1062–1071. 10.1094/MPMI-19-1062 [DOI] [PubMed] [Google Scholar]
- Czechowski T., Stitt M., Altmann T., Udvardi M. K., Scheible W. R. (2005). Genome-wide identification and testing of superior reference genes for transcript normalization in Arabidopsis. Plant. Physiol. 139 5–17. 10.1104/pp.105.063743 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dangl J. L., Jones J. D. (2001). Plant pathogens and integrated defence responses to infection. Nature 411 826–833. 10.1038/35081161 [DOI] [PubMed] [Google Scholar]
- Dean R., Van Kan J. A., Pretorius Z. A., Hammond-Kosack K. E., Di Pietro A., Spanu P. D., et al. (2012). The Top 10 fungal pathogens in molecular plant pathology. Mol. Plant. Pathol. 13 414–430. 10.1111/j.1364-3703.2011.00783.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delporte A., Van Holle S., Lannoo N., Van Damme E. J. (2015). The tobacco lectin, prototype of the family of Nictaba-related proteins. Curr. Protein Pept. Sci. 16 5–16. 10.2174/1389203716666150213154107 [DOI] [PubMed] [Google Scholar]
- Gachon C., Saindrenan P. (2004). Real-time PCR monitoring of fungal development in Arabidopsis thaliana infected by Alternaria brassicicola and Botrytis cinerea. Plant Physiol. Biochem. 42 367–371. 10.1016/j.plaphy.2004.04.001 [DOI] [PubMed] [Google Scholar]
- Glazebrook J. (2005). Contrasting mechanisms of defense against biotrophic and necrotrophic pathogens. Annu. Rev. Phytopathol. 43 205–227. 10.1146/annurev.phyto.43.040204.135923 [DOI] [PubMed] [Google Scholar]
- Hael-Conrad V., Abou-Mansour E., Diaz-Ricci J. C., Metraux J. P., Serrano M. (2015). The novel elicitor AsES triggers a defense response against Botrytis cinerea in Arabidopsis thaliana. Plant Sci. 241 120–127. 10.1016/j.plantsci.2015.09.025 [DOI] [PubMed] [Google Scholar]
- Hassan M. A., Rouf R., Tiralongo E., May T. W., Tiralongo J. (2015). Mushroom lectins: specificity, structure and bioactivity relevant to human disease. Int. J. Mol. Sci. 16 7802–7838. 10.3390/ijms16047802 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heim C., Hertzberg H., Butschi A., Bleuler-Martinez S., Aebi M., Deplazes P., et al. (2015). Inhibition of Haemonchus contortus larval development by fungal lectins. Parasit Vectors 8:425. 10.1186/s13071-015-1032-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaber K., Cuartero Diaz G., Haubruge E., Francis F. (2008). Investigation of carbohydrate binding property of a fungal lectin from Xerocomus chrysenteron and potential use on Myzus persicae aphid. Commun. Agric. Appl. Biol. Sci. 73 629–638. http://hdl.handle.net/2268/31121 [PubMed] [Google Scholar]
- Jones J. D., Dangl J. L. (2006). The plant immune system. Nature 444 323–329. 10.1038/nature05286 [DOI] [PubMed] [Google Scholar]
- Jürgensen K. (2001). Untersuchungen zum assimilat- und wassertransfer in der interaktion zwischen Arabidopsis thaliana and Heterodera schachtii. PhD, Germany: Agrar- und Ernährungswissenschaftlichen Fakultät, Christian-Albrecht Universität. [Google Scholar]
- Karimi M., Inze D., Depicker A. (2002). GATEWAY vectors for Agrobacterium-mediated plant transformation. Trends Plant Sci. 7 193–195. 10.1016/s1360-1385(02)02251-3 [DOI] [PubMed] [Google Scholar]
- Künzler M. (2015). Hitting the sweet spot-glycans as targets of fungal defense effector proteins. Molecules 20 8144–8167. 10.3390/molecules20058144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Künzler M. (2018). How fungi defend themselves against microbial competitors and animal predators. PLoS Pathog. 14:e1007184. 10.1371/journal.ppat.1007184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindsey B. E., III, Rivero L., Calhoun C. S., Grotewold E., Brkljacic J. (2017). Standardized method for high-throughput sterilization of Arabidopsis seeds. J. Vis. Exp. 2017:e56587. 10.3791/56587 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macho A. P., Zipfel C. (2015). Targeting of plant pattern recognition receptor-triggered immunity by bacterial type-III secretion system effectors. Curr. Opin. Microbiol. 23 14–22. 10.1016/j.mib.2014.10.009 [DOI] [PubMed] [Google Scholar]
- Mauch-Mani B., Baccelli I., Luna E., Flors V. (2017). Defense priming: an adaptive part of induced resistance. Annu. Rev. Plant Biol. 68 485–512. 10.1146/annurev-arplant-042916-041132 [DOI] [PubMed] [Google Scholar]
- Mauch F., Mauch-Mani B., Gaille C., Kull B., Haas D., Reimmann C. (2001). Manipulation of salicylate content in Arabidopsis thaliana by the expression of an engineered bacterial salicylate synthase. Plant J. 25 67–77. 10.1046/j.1365-313x.2001.00940.x [DOI] [PubMed] [Google Scholar]
- Oka Y., Cohen Y., Spiegel Y. (1999). Local and systemic induced resistance to the root-knot nematode in tomato by DL-beta-Amino-n-Butyric Acid. Phytopathol. 89 1138–1143. 10.1094/PHYTO.1999.89.12.1138 [DOI] [PubMed] [Google Scholar]
- Pieterse C. M., Van Der Does D., Zamioudis C., Leon-Reyes A., Van Wees S. C. (2012). Hormonal modulation of plant immunity. Annu. Rev. Cell Dev. Biol. 28 489–521. 10.1146/annurev-cellbio-092910-154055 [DOI] [PubMed] [Google Scholar]
- Rao X., Huang X., Zhou Z., Lin X. (2013). An improvement of the 2^(-delta delta CT) method for quantitative real-time polymerase chain reaction data analysis. Biostat. Bioinforma. Biomath. 3 71–85. [PMC free article] [PubMed] [Google Scholar]
- Ripoll C., Favery B., Lecomte P., Van Damme E., Peumans W., Abad P., et al. (2003). Evaluation of the ability of lectin from snowdrop (Galanthus nivalis) to protect plants against root-knot nematodes. Plant Sci. 164 517–523. 10.1016/S0168-9452(02)00448-X [DOI] [Google Scholar]
- Robert-Seilaniantz A., Grant M., Jones J. D. (2011). Hormone crosstalk in plant disease and defense: more than just jasmonate-salicylate antagonism. Annu. Rev. Phytopathol. 49 317–343. 10.1146/annurev-phyto-073009-114447 [DOI] [PubMed] [Google Scholar]
- Ross A., Somssich I. E. (2016). A DNA-based real-time PCR assay for robust growth quantification of the bacterial pathogen Pseudomonas syringae on Arabidopsis thaliana. Plant Methods 12:48. 10.1186/s13007-016-0149-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabotic J., Ohm R. A., Künzler M. (2016). Entomotoxic and nematotoxic lectins and protease inhibitors from fungal fruiting bodies. Appl. Microbiol. Biotechnol. 100 91–111. 10.1007/s00253-015-7075-2 [DOI] [PubMed] [Google Scholar]
- Sanchez-Vallet A., Ramos B., Bednarek P., Lopez G., Pislewska-Bednarek M., Schulze-Lefert P., et al. (2010). Tryptophan-derived secondary metabolites in Arabidopsis thaliana confer non-host resistance to necrotrophic Plectosphaerella cucumerina fungi. Plant J. 63 115–127. 10.1111/j.1365-313X.2010.04224.x [DOI] [PubMed] [Google Scholar]
- Schubert M., Bleuler-Martinez S., Butschi A., Walti M. A., Egloff P., Stutz K., et al. (2012). Plasticity of the beta-trefoil protein fold in the recognition and control of invertebrate predators and parasites by a fungal defence system. PLoS Pathog. 8:e1002706. 10.1371/journal.ppat.1002706 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schütze K., Harter K., Chaban C. (2009). Bimolecular fluorescence complementation (BiFC) to study protein-protein interactions in living plant cells. Methods Mol. Biol. 479 189–202. 10.1007/978-1-59745-289-2_12 [DOI] [PubMed] [Google Scholar]
- Schwessinger B., Zipfel C. (2008). News from the frontline: recent insights into PAMP-triggered immunity in plants. Curr. Opin. Plant Biol. 11 389–395. 10.1016/j.pbi.2008.06.001 [DOI] [PubMed] [Google Scholar]
- Sharon N., Lis H. (2004). History of lectins: from hemagglutinins to biological recognition molecules. Glycobiology 14 53–62. 10.1093/glycob/cwh122 [DOI] [PubMed] [Google Scholar]
- Sijmons P. C., Grundler F. M. W., Von Mende N., Burrows P. R., Wyss U. (1991). Arabidopsis thaliana as a new model host for plant-parasitic nematodes. Plant J. 1 245–254. 10.1111/j.1365-313X.1991.00245.x [DOI] [Google Scholar]
- Singh R. S., Kaur H. P., Singh J. (2014). Purification and characterization of a mucin specific mycelial lectin from Aspergillus gorakhpurensis: application for mitogenic and antimicrobial activity. PLoS One 9:e109265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh R. S., Walia A. K., Kennedy J. F. (2019). Mushroom lectins in biomedical research and development. Int. J. Biol. Macromol. 151 1340–1350. 10.1016/j.ijbiomac.2019.10.180 [DOI] [PubMed] [Google Scholar]
- Stefanowicz K., Lannoo N., Proost P., Van Damme E. J. (2012). Arabidopsis F-box protein containing a Nictaba-related lectin domain interacts with N-acetyllactosamine structures. FEBS Open Bio. 2 151–158. 10.1016/j.fob.2012.06.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stefanowicz K., Lannoo N., Zhao Y., Eggermont L., Van Hove J., Al Atalah B., et al. (2016). Glycan-binding F-box protein from Arabidopsis thaliana protects plants from Pseudomonas syringae infection. BMC Plant Biol. 16:213. 10.1186/s12870-016-0905-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stutz K., Kaech A., Aebi M., Künzler M., Hengartner M. O. (2015). Disruption of the C. elegans intestinal brush border by the fungal lectin CCL2 phenocopies dietary lectin toxicity in mammals. PLoS One 10:e0129381. 10.1371/journal.pone.0129381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tayyrov A., Schmieder S. S., Bleuler-Martinez S., Plaza D. F., Künzler M. (2018). Toxicity of potential fungal defense proteins towards the fungivorous nematodes Aphelenchus avenae and Bursaphelenchus okinawaensis. Appl. Environ. Microbiol. 84 e2051–e2018. 10.1128/AEM.02051-18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trigueros V., Lougarre A., Ali-Ahmed D., Rahbe Y., Guillot J., Chavant L., et al. (2003). Xerocomus chrysenteron lectin: identification of a new pesticidal protein. Biochim. Bioph. Acta. 1621 292–298. 10.1016/s0304-4165(03)00098-9 [DOI] [PubMed] [Google Scholar]
- Tripathi D., Raikhy G., Kumar D. (2019). Chemical elicitors of systemic acquired resistance–salicylic acid and its functional analogs. Curr. Plant Biol. 17 48–59. 10.1007/s11306-018-1416-y [DOI] [PubMed] [Google Scholar]
- Van Damme E. J., Barre A., Rouge P., Peumans W. J. (2004). Cytoplasmic/nuclear plant lectins: a new story. Trends Plant Sci. 9 484–489. 10.1016/j.tplants.2004.08.003 [DOI] [PubMed] [Google Scholar]
- Van Holle S., Smagghe G., Van Damme E. J. (2016). Overexpression of Nictaba-Like lectin genes from Glycine max confers tolerance toward Pseudomonas syringae infection, aphid infestation and salt stress in transgenic Arabidopsis plants. Front. Plant Sci. 7:1590. 10.3389/fpls.2016.01590 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Holle S., Van Damme E. J. M. (2018). Signaling through plant lectins: modulation of plant immunity and beyond. Biochem. Soc. Trans. 46 217–233. 10.1042/bst20170371 [DOI] [PubMed] [Google Scholar]
- Vandenborre G., Smagghe G., Van Damme E. J. (2011). Plant lectins as defense proteins against phytophagous insects. Phytochemistry 72 1538–1550. 10.1016/j.phytochem.2011.02.024 [DOI] [PubMed] [Google Scholar]
- Varrot A., Basheer S. M., Imberty A. (2013). Fungal lectins: structure, function and potential applications. Curr. Opin. Struct. Biol. 23 678–685. 10.1016/j.sbi.2013.07.007 [DOI] [PubMed] [Google Scholar]
- Wohlschlager T., Butschi A., Zurfluh K., Vonesch S. C., Auf Dem, Keller U., et al. (2011). Nematotoxicity of Marasmius oreades agglutinin (MOA) depends on glycolipid binding and cysteine protease activity. J. Biol. Chem. 286 30337–30343. 10.1074/jbc.M111.258202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan Y., Yuan Q., Tang J., Huang J., Hsiang T., Wei Y., et al. (2018). Colletotrichum higginsianum as a model for understanding host-pathogen interactions: A Review. Int. J. Mol. Sci. 19:2142. 10.3390/ijms19072142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L., Paasch B. C., Chen J., Day B., He S. Y. (2019). An important role of l-fucose biosynthesis and protein fucosylation genes in Arabidopsis immunity. New Phytol. 222 981–994. 10.1111/nph.15639 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X., Henriques R., Lin S. S., Niu Q. W., Chua N. H. (2006). Agrobacterium-mediated transformation of Arabidopsis thaliana using the floral dip method. Nat. Protoc. 1 641–646. 10.1038/nprot.2006.97 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
CCL2 expression in E. coli and purification.
In vitro antimicrobial assay of bacterially produced CCL2 and CCL2-Y92A against B. cinerea.
Sequences of primers for qPCR. F: Forward primer. R: Reverse primer.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author/s.