Abstract
Purpose:
we aimed 1) to evaluate the risk factors associated to the benzodiazepines intake; 2) to assess the impact about the use of long acting injectables antipsychotics (LAIs); 3) to assess the risk in severe and affective disorders and 4) to identify the prescription patterns of use in mental health in a cohort of patients from Spain.
Methods:
735 outpatients from Mental Health were included. Demographic and clinical data were collected. In order to compare the use of benzodiazepines we calculated the daily dose equivalents (mg/day) to diazepam as standard.
Results:
The most commonly prescribed benzodiazepine was clonazepam (33%) and the mean daily dose of diazepam equivalents was 24.9 mg. It was higher in affective disorders (40.35 ± 3.36) and lower in patients using LAIs antipsychotics (17.50 ± 1.39; p = 0.001). Multivariate analysis showed that to be women (OR = 1.559, 95% CI = 1.059–2.295, p = 0.024), the use of drugs (OR = 1.671, 95% CI = 1.127–2.477, p = 0.011) and suffering any affective disorder (OR = 1.542, 95% CI = 1.355–1.826, p = 0.040) increased the risk of benzodiazepine intake. In contrast, the use of LAIs antipsychotics significantly reduced it versus oral antipsychotics (OR = 5.226, 95% CI = 3.185–8.575, p = 0.001).
Conclusions:
benzodiazepines are widely prescribed, mainly clonazepam followed by lorazepam and diazepam. Most of patients used at least one benzodiazepine and the mean daily intake was 25 mg diazepam equivalents. Therefore, benzodiazepines are extensively prescribed and used at higher doses than desirable. These, findings could be useful for clinicians and their practice.
Keywords: benzodiazepines, prescription patterns, diazepam equivalents, mental health
Introduction
The first benzodiazepine was the chlordiazepoxide developed in 1955 followed by the diazepam in 1963.1 Since then, several molecules have been developed constituting a group of drugs exerting its anxiolytic, hypnotic, anticonvulsant and muscle relaxant effects through enhancing the effect of gamma-amynobutyric acid (GABA) at its GABAA receptor.2,3
Despite benzodiazepines being well known for its potential dependence syndrome and their common side effects (sedation, memory loss, falls)4 their prescription and use is widely increasing. In this regard, a 113% increased use of benzodiazepines from 2000 to 2012 has been reported in Spain.5 In this line, a recent survey in Spanish general population showed that the prevalence of use of benzodiazepines was 20.8% sometime and 5.9% daily, always highlighting a higher consumption in women (63%).5 Studies in general population showed that the age is the most consistent predictor of taking benzodiazepines.6,7 Likewise, the consumption of benzodiazepines has been shown to be higher in women than in men in general population.8–10 In contrast, only few studies evaluated the use of benzodiazepines by using a cohort from mental health services.11–13 Unfortunately, most of them focused in particular groups of risk such as substance of abuse users,14 depressed patients15 or whose diagnosed with schizophrenia and panic disorders.16
Therefore, the aim of this study was 1) to evaluate the risk factors associated to the benzodiazepines intake; 2) to assess the impact about the use of long acting injectables antipsychotics (LAIs); 3) to assess the risk in severe and affective disorders and 4) to identify the prescription patterns of use in mental health in a cohort of patients from the Region of Murcia, Spain.
Methods
Study Design
As previously described,17 we designed a cross-sectional study, from 2015 to 2017, based on a representative sample of the adult and non-institutionalized population of Mental Health in the Region of Murcia. A total number of 735 patients, ⩾18 years old, were included if were previously diagnosed with a mental health disorder according to the DSM-V guidelines. Exclusion criteria included institutionalized patients, use of 2 LAIs, intellectual disability or autistic spectrum disorders and patients with missing records or unable to confirm treatment continuation during 1 year. The present study was drawn up following the ‘STrengthening the Reporting of OBservational studies in Epidemiology’ (STROBE) Statement ítems.18
Study Measures
Data collected included demographical information such as age, sex, civil status, tobacco and other drugs use as well as clinical data such as the disorder, its evolution (years), the benzodiazepines dosage as well as concomitant psychiatric medications, including the use of both, oral and long-acting injectable (LAIs) antipsychotics, antidepressants, mood stabilizers and biperiden. In order to compare the use of benzodiazepines we calculated the daily dose equivalents of benzodiazepines (mg/day) by diazepam as standard to compare the corresponding doses as previously published.19
Statistical Analysis and Confounding Factors
All analyses were performed using IBM SPSS Statistics version 21.0 (IBM Corp., Armonk, NY, USA). We expressed quantitative variables as means [ ± standard error media (SEM)] and categorical variables as numbers (percentage). We assessed normality of distributions using histograms and the Shapiro–Wilk test. Sample basal characteristics were analyzed by univariate analysis. Variables associated in the univariate analysis and variables with statistical trend (p < 0.1) were entered as factors in a bivariate logistic regression model to identify the variables independently associated to benzodiazepine intake. Student’s t-test was used to assess differences in diazepam equivalents within groups by sex, use of LAIs or affective disorder. Differences with a p value <0.05 were considered significant.
Results
Sample basal characteristics are shown in Table 1. Patients taking benzodiazepines were more frequently women (70% versus 66% in men; p = 0.006), with shorter disease duration (10.9 ± 0.4 versus 13.0 ± 0.7 in no-users; p = 0.035) and used more frequently drugs (38% versus 22% in non-users; p = 0.001). The 87% and the 67% of patients diagnosed with a general or severe psychiatric disorder, respectively, were prescribed with benzodiazepines. Furthermore, the 81% of patients diagnosed with an affective disorder were prescribed with benzodiazepines while the 64% of patients diagnosed with no-affective disorders took benzodiazepines. Among LAIs users, the rate of benzodiazepines prescription was lower compared to no-LAIs users (62% vs. 86%, respectively).
Table 1. Univariate Anaysis Between Users and Non-Users of Benzodiazepines.
| Total N = 735 |
No BDZ N = 234 |
BDZ N = 501 |
P-value | |||||
| Sex (%) | 0.006 | |||||||
| Women | 325 (44) | 96 (41) | 229 (49) | |||||
| Men | 410 (56) | 138 (58) | 272 (51) | |||||
| Age (y ± SEM) | 41.6 ± 0.5 | 41.7 ± 0.9 | 41.5 ± 0.5 | 0.305 | ||||
| Disorder duration (y ± SEM) | 11.5 ± 0.4 | 13.0 ± 0.7 | 10.9 ± 0.4 | 0.035 | ||||
| Legal status (%) | 0.140 | |||||||
| Single/Divorced/Widow | 563 (77) | 158 (78) | 405 (76) | |||||
| Coupled/Married | 172 (23) | 44 (22) | 128 (24) | |||||
| Tobacco & Drugs (%) | ||||||||
| Tobacco | 325 (44) | 87 (43) | 238 (45) | 0.461 | ||||
| Other drugs | 247 (33) | 44 (22) | 203 (38) | 0.001 | ||||
| Mental Disorder | 0.091 | |||||||
| General Disorders | 203 (28) | 27 (13) | 176 (33) | |||||
| Severe Disorders | 532 (72) | 175 (87) | 357 (67) | |||||
| Affective Disorders | 0.005 | |||||||
| No | 349 (47) | 127 (63) | 222 (42) | |||||
| Yes | 386 (53) | 75 (37) | 311 (58) | |||||
| Use of LAI-antipsychotic | 0.001 | |||||||
| No | 323 (44) | 46 (23) | 277 (52) | |||||
| Yes | 412 (56) | 156 (77) | 256 (48) |
Affective disorders included depressive, anxiety-depressive, anxiety, schizoaffective, bipolar and personality disorders. Non-affective disorders included schizophrenia, psychosis, delusional disorders. BDZ = bendozdiazepines, LAI = long acting injectables antipsychotics, SEM = standard error mean.
We performed a logistic bivariate regression analysis to truly establish the factors independently associated with the intake of benzodiazepines. As shown in Table 2, bivariate logistic regression model showed that women had higher risk of benzodiazepine intake compared to men (OR = 1.559, 95% CI = 1.059–2.295, p = 0.024). Furthermore, the use of drugs (OR = 1.671, 95% CI = 1.127–2.477, p = 0.011) and suffering any affective disorder (OR = 1.542, 95% CI = 1.355–1.826, p = 0.040) increased the risk of benzodiazepines intake. In contrast, the use of LAIs antipsychotics significantly reduced the risk of benzodiazepines intake versus oral antipsychotics (OR = 5.226, 95% CI = 3.185–8.575, p = 0.001).
Table 2. Risk Factors Associated to Benzodiazepine Intake by Bivariate Logistic Regression Model.
| OR | 95% CI | P-value | ||||
| Sex | 0.024 | |||||
| Women | 1.559 | 1.059–2.295 | ||||
| Men | 1 | |||||
| Disorder duration | 0.999 | 0.980–1.018 | 0.894 | |||
| Drugs use | 0.011 | |||||
| No | 1 | |||||
| Yes | 1.671 | 1.127–2.477 | ||||
| Mental Disorder | 0.175 | |||||
| General | 1.433 | 0.852–2.411 | ||||
| Severe | 1 | |||||
| Affective Disorder | 0.040 | |||||
| No | 1 | |||||
| Yes | 1.542 | 1.355–1.826 | ||||
| Use of LAIs | 0.001 | |||||
| No | 5.226 | 3.185–8.575 | ||||
| Yes | 1 | |||||
LAIs = long acting injectable antipsychotics.
Diazepam Equivalents
The mean daily doses of benzodiazepines are shown in Figure 1 as diazepam equivalents. Mean daily dose of diazepam equivalents in our cohort was 24.9 mg (24.90 ± 1.42). As shown in A) student’s t-test showed no statistical differences between the daily dose of women and men (27.41 ± 2.04 and 23.29 ± 1.79, respectively). Nonetheless, as shown in B) and C), statistical differences were found between affective (40.35 ± 3.36) or no-affective disorders (21.98 ± 1.37, χ2[df = 1,734] = 6.06; p = 0.001) as well as when comparing the use of oral (40.25 ± 2.99) versus LAI antipsychotics (17.50 ± 1.39, χ2[df = 1,734] = 7.89; p = 0.001).
Figure 1.
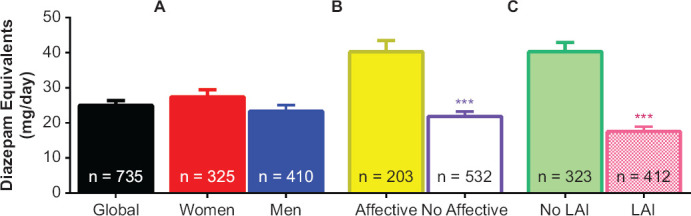
Diazepam Equivalents by (A) Sex, (B) Affective vs No-Affective Disorders and (C) Long-Acting Injectable Use (LAIs)
Global column represents the mean data from the whole cohort of patients. Data are expressed as the mean ± SEM. Student’s t-test revealed statistical differences between affective and no-affective disorders (***p = 0.001) as well as when comparing the use of oral versus LAIs (***p = 0.001) Abv: LAIs = long acting-injectables antipsychotics.
Use of Benzodiazepines by Sex and Mental Disorder
As shown in Table 3, the most commonly prescribed benzodiazepine in both sexes was clonazepam in 33 and 32% of cases, followed by lorazepam (32% in women and 24% in men) and Diazepam (21% in women and 22% in men). Likewise, as shown in Table 4, patients diagnosed with a depressive-anxiety disorder were the more frequently prescribed with benzodiazepines (92%) followed by patients suffering from depression (88%) and schizoaffective disorder (79%). In contrast, a lower rate of patients with bipolar disorder took benzodiazepines (57%). Among psychotic disorders, 65% of patients with schizophrenia took benzodiazepines while only the 48% and 36% of patients with psychosis and Deliroid disorder, respectively. It worth to point out that about 75% of patients diagnosed with a personality disorder were prescribed with benzodiazepines. Most of patients used only one type of benzodiazepine (48%) and the 19% used 2 types. Nonetheless, the 44% and the 24% of patients diagnosed with anxiety-depressive and personality disorders, respectively, used two different benzodiazepines. Once again, clonazepam was the most used in the different disorders (mean 22%, range 4–30) excepting in deliroid, anxiety-depressive and depression disorders which more frequently were prescribed with lorazepam.
Table 3. Use of Benzodiazepines by Sex .
| Total N = 735 |
Women N = 325 |
Men N = 410 |
||||
| BDZs’ users (%) | n = 501 | n = 229 | n = 272 | |||
| 1 | 353 (48) | 159 (69) | 194 (71) | |||
| 2 | 127 (17) | 60 (26) | 67 (25) | |||
| 3 | 21 (3) | 10 (5) | 11 (4) | |||
| BDZs (%) | ||||||
| Alprazolam | 27 (5) | 12 (5) | 15 (6) | |||
| Bromazepam | 14 (3) | 8 (3) | 6 (2) | |||
| Clonazepam | 164 (33) | 76 (33) | 88 (32) | |||
| Clorazepate | 18 (4) | 8 (3) | 10 (4) | |||
| Diazepam | 108 (22) | 48 (21) | 60 (22) | |||
| Flurazepam | 96 (19) | 40 (17) | 56 (21) | |||
| Ketazolam | 10 (2) | 7 (3) | 3 (1) | |||
| Lorazepam | 139 (28) | 74 (32) | 65 (24) | |||
| Lormetazepam | 94 (19) | 48 (21) | 46 (17) | |||
BDZs = benzodiazepines.
Table 4. Use of Benzodiazepines by Mental Disorder.
| Total N = 735 |
Sch N = 277 |
Psy N = 44 |
DelD N = 28 |
SchAff N = 90 |
BPD N = 93 |
PDs N = 112 |
DepD N = 42 |
ADD N = 39 |
Others N = 10 |
|||||||||||
| N benzodiazepines (%) | 501 (68) | 181 (65) | 21 (48) | 10 (36) | 71 (79) | 53 (57) | 83 (74) | 37 (88) | 36 (92) | 9 (90) | ||||||||||
| 1 | 353 (48) | 128 (46) | 18 (41) | 8 (29) | 51 (57) | 38 (38) | 52 (45) | 27 (64) | 22 (56) | 9 (90) | ||||||||||
| 2 | 127 (19) | 44 (16) | 3 (9) | 2 (7) | 16 (18) | 14 (19) | 25 (24) | 9 (21) | 14 (44) | – | ||||||||||
| 3 | 21 (3) | 9 (3) | – | – | 4 (4) | 1 (1) | 6 (5) | 1 (3) | – | – | ||||||||||
| BDZs (%) | ||||||||||||||||||||
| Alprazolam | 27 (4) | 13 (5) | 1 (2) | 1 (4) | – | 1(1) | 7 (6) | 2 (5) | 1 (3) | 1 (10) | ||||||||||
| Bromazepam | 14 (2) | 5 (2) | 1 (2) | – | 2 (2) | 2 (2) | 2 (2) | 2 (5) | – | – | ||||||||||
| Clonazepam | 164 (22) | 59 (21) | 7 (16) | 1 (4) | 27 (30) | 22 (24) | 27 (24) | 10 (24) | 10 (26) | 1 (10) | ||||||||||
| Clorazepate | 18 (2) | 7 (3) | – | 1 (4) | 6 (7) | 1 (1) | 2 (2) | 1 (2) | – | – | ||||||||||
| Diazepam | 108 (15) | 38 (14) | 2 (5) | 1 (4) | 17 (19) | 5 (5) | 24 (21) | 8 (19) | 12 (31) | 1 (10) | ||||||||||
| Flurazepam | 96 (13) | 39 (14) | 4 (9) | 2 (7) | 12 (13) | 10 (11) | 18 (16) | 3 (7) | 8 (21) | – | ||||||||||
| Ketazolam | 10 (1) | 3 (1) | – | – | 2 (2) | 3 (3) | 2 (2) | – | – | – | ||||||||||
| Lorazepam | 139 (19) | 43 (16) | 7 (16) | 5 (18) | 17 (19) | 13 (14) | 23 (21) | 13 (31) | 13 (33) | 5 (50) | ||||||||||
| Lormetazepam | 94 (13) | 36 (13) | 2 (5) | 1 (4) | 12 (13) | 12 (13) | 15 (13) | 9 (21) | 6 (15) | 1 (10) | ||||||||||
Sch = schizophrenia, Psy = psychosis disorder, DelD = delusional disorder, SchAff = schizoaffective disorder, BPD = bipolar disorder, PDs = personality disorder, DepD = depresive disorders, ADD = anxiety-depresive disorder.
Concomitant Treatments
Full treatments are shown as supplementary material (Table 5). Interestingly, antipsychotics are widely used not only in schizophrenia and psychosis (100% and 95%, respectively) but also in personality (66%), bipolar (61%) and schizoaffective (67%) disorders. In this regard, most of patients with schizophrenia were treated with at least 2 different antipsychotics (>67%). Furthermore, antidepressants were extensively used in depression (100%) and anxiety-depressive disorders (64%) but also in personality (49%). Finally, mood stabilizers were often used in bipolar and schizoaffective disorders (74% and 48% respectively).
Table 5. Full Treatments by Mental Disorder .
| Total N = 735 |
Sch N = 277 |
Psy N = 44 |
DelD N = 28 |
SchAff N = 90 |
BPD N = 93 |
PDs N = 112 |
DepD N = 42 |
ADD N = 39 |
Others N = 10 |
|||||||||||
| N benzodiazepines (%) | 501 (68) | 181 (65) | 21 (48) | 7 (25) | 71 (79) | 53 (57) | 83 (74) | 37 (88) | 39 (100) | 9 (90) | ||||||||||
| 1 | 338 (46) | 126 (46) | 17 (39) | 5 (18) | 48 (53) | 34 (35) | 50 (45) | 27 (64) | 22 (56) | 9 (90) | ||||||||||
| 2 | 141 (19) | 46 (17) | 4 (9) | 2 (7) | 18 (20) | 18 (19) | 27 (24) | 9 (21) | 17 (44) | – | ||||||||||
| 3 | 21 (3) | 9 (3) | – | – | 4 (4) | 1 (1) | 6 (5) | 1 (3) | – | – | ||||||||||
| N antipsychotics (%) | 535 (73) | 277 (100) | 42 (95) | 14 (50) | 61 (67) | 57 (61) | 74 (66) | 5 (12) | 2 (5) | 3 (30) | ||||||||||
| 1 | 169 (23) | 45 (16) | 18 (41) | 9 (32) | 13 (14) | 28 (30) | 46 (41) | 5 (12) | 2 (5) | 3 (30) | ||||||||||
| 2 | 284 (39) | 185 (67) | 20 (45) | 4 (14) | 34 (38) | 17 (18) | 24 (21) | – | – | – | ||||||||||
| 3 | 69 (9) | 39 (14) | 4 (9) | 1 (4) | 12 (13) | 9 (9) | 4 (3) | – | – | – | ||||||||||
| 4 | 13 (2) | 8 (3) | – | – | 2 (2) | 3 (3) | – | – | – | – | ||||||||||
| Biperiden (%) | 105 (14) | 67 (24) | 6 (14) | 6 (21) | 14 (16) | 5 (5) | 7 (6) | – | – | -– | ||||||||||
| N antidepressants (%) | 250 (34) | 64 (24) | 10 (22) | 7 (25) | 22 (24) | 21 (22) | 55 (49) | 42 (100) | 25 (64) | 4 (40) | ||||||||||
| 1 | 204 (28) | 53 (19) | 10 (22) | 6 (21) | 18 (20) | 18 (19) | 46 (41) | 29 (69) | 20 (51) | 4 (40) | ||||||||||
| 2 | 46 (6) | 11 (4) | – | 1 (4) | 4 (4) | 3 (3) | 9 (8) | 13 (31) | 5 (13) | – | ||||||||||
| Mood stabilizer (%) | 200 (27) | 39 (14) | 5 (11) | 4 (14) | 43 (48) | 69 (74) | 27 (24) | 8 (19) | 4 (10) | 1 (10) | ||||||||||
| 1 | 178 (24) | 37 (13) | 5 (11) | 4 (14) | 36 (40) | 56 (60) | 27 (24) | 8 (19) | 4 (10) | 1 (10) | ||||||||||
| 2 | 19 (3) | 2 (1) | – | – | 7 (8) | 10 (11) | – | – | – | – | ||||||||||
| 3 | 3 (0.4) | – | – | – | – | 3 (3) | – | – | – | – |
Sch = schizophrenia, Psy = psychosis disorder, DelD = delusional disorder, SchAff = schizoaffective disorder, BPD = bipolar disorder, PDs = personality disorder, DepD=depresive disorders, ADD = anxiety-depresive disorder.
Discussion
Anxiolytics and hypnotics have been one of the most prescribed drugs in Western countries. The 68% of patients in our cohort from mental health took benzodiazepines. The prevalence of benzodiazepine use in general population varies from 13.8% in France,20 between 10 and 25% in the Netherlands,21 and 26.1%in the United Kingdom.22 First, our results demonstrated that the prescription of benzodiazepines was associated to women and to patients who use drugs of abuse. These results are in line with previous findings23,24 suggesting that women could suffer more frequently affective health disorders or symptoms such as insomnia or anxiety. In addition, patients who use drugs may be not aware about the risks of benzodiazepines use. Second, while affective disorders are more likely prescribed with benzodiazepines, the use of LAIs are associated to a lower benzodiazepines intake and lower pharmacy costs.17,25 Although several studies have already examined the influence of these factors either in the context of general population, primary care settings or in some particular mental disorders, the present study is, to the best of our knowledge, the first that investigates this issue using a cohort from mental health.
Diazepam Equivalents
Although our results demonstrated that to be woman is a risk factor to become prescribed with benzodiazepines, our data showed no differences in the dose of diazepam equivalents used by both women and men. This finding is consistent with previous reports using a cohort from mental health.17 In this line, no differences between sexes were found in a cohort of patients diagnosed with schizophrenia.26 In contrast, patients diagnosed with an affective disorder doubled the used dose of diazepam equivalents. Interestingly, patients treated with a LAI used lower diazepam equivalents. This finding was also found in patients diagnosed with psychotic disorders when oral versus LAIs antipsychotic treatments were compared.26,27
Use of Benzodiazepines by Sex and Mental Disorder
In both sexes, clonazepam and lorazepam were the more frequently prescribed benzodiazepines. Similarly, clonazepam and lorazepam were the most frequently prescribed in the different mental disorders. It worth to point out that clonazepam was more prevalent within non-affective disorders while lorazepam was more used in affective disorders. In this regard, clonazepam and lorazepam were also the most used benzodiazepines in several studies.28,29 In contrast, other studies reported diazepam30,31 or bromazepam32 as the most used benzodiazepine. It is important to note that these divergences may occur due to the different groups studied and/or the several drugs that are available in different countries.
Overall, the present study demonstrated that the long-term (1-year) use of benzodiazepines is higher than desirable (>48%). In contrast, scientific evidence recommends the use of benzodiazepine as an adjuvant in treating anxiety, insomnia or depression only during the first four weeks of the treatment. As previously pointed out Smith and Tett,33 clinical guidelines and restriction campaigns contribute to raise awareness of inappropriate use of benzodiazepines. Nonetheless, in Western countries, patients are not treated according to clinical guidelines based on scientific evidence.23 It has been shown that long term use of benzodiazepines (>1 year) increased the risk of abstinence syndrome, accidents, suicide attempt (especially in depressed individuals), reduction of the work capability and increase in the costs of hospitalization.20–22 Therefore, physicians should try to withdrawal benzodiazepines since the first prescription and try to avoid their use in the long-term management of the disease.
Limits
Given our inclusion/exclusion criteria were not restrictive; our cohort provides a high external validity to the western countries, in particular to Spain. Nonetheless, our study may hide some biases. For example, some clinical data, such as tobacco and drugs, are often unrecorded. Moreover, psychotropic medication could be also used in organic diseases such as neuropathic pain, migraine or others. Due to the lack about this information we could have overestimated the use of benzodiazepines in our cohort. In addition, Compliance with the LAIs was guaranteed given the treatment was administered by trained healthcare staff and it could impact in the need of concomitant treatments. Furthermore, considering that the severity of each patient’s condition was not available from hospital records, it is not possible to elucidate whether patients of LAI groups had a worse prognosis versus patients treated with oral antipsychotics. Therefore, more studies including anxiety, anger, depression scales and prescriber habits are needed to truly establish the underlying reasons and risk factors involved in the prescription of benzodiazepines in mental health.
Conclusions
In summary, this is the first study showing the patterns of prescription of benzodiazepines in a cohort from mental health and comparing its dosage in diazepam equivalents. Despite the limitations of our study, we demonstrated that benzodiazepines are widely prescribed, mainly clonazepam followed by lorazepam and diazepam. Most of patients used at least one benzodiazepines and the daily mean intake of benzodiazepines corresponds to 25 mg of diazepam equivalents. Therefore, benzodiazepines are extensively prescribed and used at higher doses than desirable. Further research is needed to clarify the risk factors and reasons to benzodiazepine prescriptions; however, our findings could be useful for clinicians and their practice.
Footnotes
Funding
The authors neither received public or private funding nor grant to carry out this study
Conflict of Interests
None of the authors have conflict of interest.
Ethics Approval
Ethics and methodology issues approval for the study were granted by both the Research Ethic Committee of the Murcia Health Service and by the Ethic Committee of the San Antonio Catholic University of Murcia (UCAM) (CE031914). No identifiable information was retained or is presented in this manuscript.
Key points
1. Benzodiazepines are widely prescribed, mainly clonazepam followed by lorazepam and diazepam
2. To be women and affective disorders are the main risk factors for the prescription of benzodiazepines while the long acting-injectables antipsychotics lower the risk.
Supplementry Material
References
- 1.Wick JY. The history of benzodiazepines. Consult Pharm. 2013;28(9):538–548. doi: 10.4140/TCP.n.2013.538. doi: [DOI] [PubMed] [Google Scholar]
- 2.Schallek W, Horst WD, Schlosser W. Mechanisms of action of benzodiazepines. Adv Pharmacol Chemother. 1979;16:45–87. doi: 10.1016/s1054-3589(08)60242-2. doi: [DOI] [PubMed] [Google Scholar]
- 3.Malcolm RJ. GABA systems, benzodiazepines, and substance dependence. J Clin Psychiatry. 2003;64(Suppl 3):36–40. [PubMed] [Google Scholar]
- 4.Uzun S, Kozumplik O, Jakovljević M, Sedić B. Side effects of treatment with benzodiazepines. Psychiatr Danub. 2010;22(1):90–93. [PubMed] [Google Scholar]
- 5.Agencia Española de Medicamentos y Productos Sanitarios—Utilización de medicamentos ansiolíticos e hipnóticos en España durante el periodo 2000–2012. https://www.actasanitaria.com/wpcontent/uploads/2014/01/ansioliticos_hipnoticos-2000–2012.pdf [Google Scholar]
- 6.Paulose-Ram R, Jonas BS, Orwig D, Safran MA. Prescription psychotropic medication use among the U.S. adult population: results from the third national health and nutrition examination survey, 1988–1994. J Clin Epidemiol. 2004;57(3):309–317. doi: 10.1016/j.jclinepi.2003.05.001. doi: [DOI] [PubMed] [Google Scholar]
- 7.McIntosh B, Clark M, Spry C. Benzodiazepines in Older Adults: a review of clinical effectiveness, cost-effectiveness, and guidelines. Ottawa (ON): canadian agency for drugs and technologies in health; 2011 [cited 2020 Aug 14]. (CADTH Rapid Response Reports). Available from: http://www.ncbi.nlm.nih.gov/books/NBK174561/ [PubMed] [Google Scholar]
- 8.Blanco C, Han B, Jones CM, Johnson K, Compton WM. Prevalence and Correlates of Benzodiazepine Use, Misuse, and Use Disorders Among Adults in the United States. J Clin Psychiatry. 2018;16:79(6):18m12174. doi: 10.4088/JCP.18m12174. doi: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hollingworth SA, Siskind DJ. Anxiolytic, hypnotic and sedative medication use in Australia. Pharmacoepidemiol Drug Saf. 2010;19(3):280–288. doi: 10.1002/pds.1899. [DOI] [PubMed] [Google Scholar]
- 10.Lagnaoui R, Depont F, Fourrier A, Abouelfath A, Bégaud B, Verdoux H, et al. Patterns and correlates of benzodiazepine use in the French general population. Eur J ClinPharmacol. 2004;60(7):523–529. doi: 10.1007/s00228-004-0808-2. [DOI] [PubMed] [Google Scholar]
- 11.Demyttenaere K, Bonnewyn A, Bruffaerts R, De Girolamo G, Gasquet I, Kovess V, Haro JM, Alonso J. Clinical factors influencing the prescription of antidepressants and benzodiazepines: results from the European study of the epidemiology of mental disorders (ESEMeD) J Affect Disord. 2008;110(1–2):84–93. doi: 10.1016/j.jad.2008.01.011. doi: [DOI] [PubMed] [Google Scholar]
- 12.López-Pelayo H, Fàbrega-Ribera M, Garrido-Ocaña JM, Balcells-Oliveró MM, Gual-Solé A. Risk perception in the ongoing prescription of benzodiazepines in mental health and primary care. Adicciones. 2014;26(2):184–186. [PubMed] [Google Scholar]
- 13.Panes A, Fourrier-Réglat A, Verdoux H, Tournier M. Use and misuse of benzodiazepines in patients with psychiatric disorders. Presse Med. 2018;47(10):886–891. doi: 10.1016/j.lpm.2018.10.003. doi: [DOI] [PubMed] [Google Scholar]
- 14.Clark RE, Xie H, Brunette MF. Benzodiazepine prescription practices and substance abuse in persons with severe mental illness. J Clin Psychiatry. 2004;65(2):151–155. doi: 10.4088/jcp.v65n0202. doi: [DOI] [PubMed] [Google Scholar]
- 15.Valenstein M, Taylor KK, Austin K, Kales HC, McCarthy JF, Blow FC. Benzodiazepine use among depressed patients treated in mental health settings. Am J Psychiatry. 2004;161(4):654–661. doi: 10.1176/appi.ajp.161.4.654. doi: [DOI] [PubMed] [Google Scholar]
- 16.Dong M, Zeng LN, Zhang Q, Yang SY, Chen LY, Najoan E, Kallivayalil RA et al. Prescription of antipsychotic and concomitant medications for adult Asian schizophrenia patients: findings of the 2016 research on asian psychotropic prescription patterns (REAP) survey. Asian J Psychiatr. 2019;45:74–80. doi: 10.1016/j.ajp.2019.08.010. doi: [DOI] [PubMed] [Google Scholar]
- 17.García-Carmona JA, Simal-Aguado J, Campos-Navarro MP, Valdivia-Muñoz F, Galindo-Tovar A. Long acting-injectables antipsychotics: analysis of prescription patterns and patient’s characteristics in mental health from a Spanish real-world study. Clin Drug Inv. 2020;40(5):459–468. doi: 10.1007/s40261-020-00913-7. doi: [DOI] [PubMed] [Google Scholar]
- 18.von Elm E, Altman DG, Egger M, Pocock SJ, Gøtzsche PC, Vandenbroucke JP. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. J Clin Epidemiol. 2008;61:344–349. doi: 10.1016/j.jclinepi.2007.11.008. doi. [DOI] [PubMed] [Google Scholar]
- 19.Martínez-Andrés JA, García-Carmona JA. Clozapine, a controversial gold standard antipsychotic for the 21st century: switching to paliperidone palmitate 3-monthly improves the metabolic profile and lowers antipsychotic dose equivalents in a treatment-resistant schizophrenia cohort. Schizophrenia Research. 2019;212:234–236. doi: 10.1016/j.schres.2019.08.001. [DOI] [PubMed] [Google Scholar]
- 20.Bénard-Laribière A, Noize P, Pambrun E, Bazin F, Verdoux H, Tournier M et al. Comorbidities and concurrent medications increasing the risk of adverse drug reactions: prevalence in French benzodiazepine users. Eur J Clin Pharmacol. 2016;72:869–876. doi: 10.1007/s00228-016-2044-y. [DOI] [PubMed] [Google Scholar]
- 21.van Eijk JTM, Bosma H, Jonkers CCM, Lamers F, Muijrers PEM. Prescribing Antidepressants and benzodiazepines in the Netherlands: is chronic physical illness involved. Depress Res Treat. 2010 doi: 10.1155/2010/105931. https://doi.org/10.1155/2010/10593 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kapil V, Green JL, Le Lait C, Wood DM, Dargan PI. Misuse of benzodiazepines and Z-drugs in the UK. Br J Psychiatr. 2014;205:407–408. doi: 10.1192/bjp.bp.114.149252. [DOI] [PubMed] [Google Scholar]
- 23.Agarwal SA, Landon BE. Patterns in Outpatient Benzodiazepine Prescribing in the United States. JAMA Netw Open. 2019;2(1):e187399. doi: 10.1001/jamanetworkopen.2018.7399. doi: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Olfson M, King M, Schoenbaum M. Benzodiazepine use in the United States. JAMA Psychiatry. 2015;72(2):136–142. doi: 10.1001/jamapsychiatry.2014.1763. doi: [DOI] [PubMed] [Google Scholar]
- 25.Pilon D, Tandon N, Lafeuille MH, Kamstra R, Emond B, Lefebvre P, Joshi K. Treatment Patterns, Health Care Resource Utilization, and Spending in Medicaid Beneficiaries Initiating Second-generation Long-acting Injectable Agents Versus Oral Atypical Antipsychotics. Clin Ther. 2017;39(10):1972.e2–1985.e2.. doi: 10.1016/j.clinthera.2017.08.008. doi: [DOI] [PubMed] [Google Scholar]
- 26.Garcia-Carmona JA, Simal-Aguado J, Campos-Navarro P, Valdivia-Muñoz F, Galindo-Tovar A. Evaluation of long-acting injectable antipsychotics with the corresponding oral formulation in a cohort of patients with schizophrenia: a real-world study in Spain. Int Clin Psychopharmacol. 2021;1(36):18–24. doi: 10.1097/YIC.0000000000000339. doi: [DOI] [PubMed] [Google Scholar]
- 27.Marić NP, Petrović SA, Jerotić S, Ristić I, Savić B, Zebić M, Vuković V et al. Maintenance phase treatment of psychotic disorders in outpatients from Serbia—focus on long-term benzodiazepine use. Int J Psychiatry Clin Pract. 2020;24(3):315–321. doi: 10.1080/13651501.2020.1767788.. doi: [DOI] [PubMed] [Google Scholar]
- 28.Comino-Naloto DC, Lopes CF, Barberato-Filho S, Cruz-Lopes L, Del Fiol FS, Bergamaschi C. Prescription of benzodiazepines for adults and older adults from a mental health clinic. Cien Saude Colet. 2016;21(4):1267–1276. doi: 10.1590/1413-81232015214.10292015. doi: [DOI] [PubMed] [Google Scholar]
- 29.Vicente-Sánchez MP, Macías-Saint-Gerons D, de la Fuente-Honrubia C, González-Bermejo D, Montero-Corominas D, Catalá-López F. Trends of use of anxiolytics and hypnotics in Spain from 2000 to 2011. Rev Esp Salud Publica. 2013;87(3):247–255. doi: 10.4321/S11357272013000300004. doi: [DOI] [PubMed] [Google Scholar]
- 30.Noia AS, Secoli SR, Duarte YAO, Lebrão ML, Lieber NSR. Fatores associados ao uso de psicotrópicos em idosos no município de São Paulo. Rev Esc Enferm USP. 2012;46:38–43. doi: 10.1590/s0080-62342012000700006. [DOI] [PubMed] [Google Scholar]
- 31.Netto MUQ, Freitas O, Pereira LRL. Antidepressivos e benzodiazepínicos: estudo sobre o uso racional entre usuários do SUS em Ribeirão Preto. Rev de Ciênc Farm Básica e Aplic. 2012;33(1):77–81. [Google Scholar]
- 32.Alvarenga JM, Loyola Filho AI, Firmo JO, Lima-Costa MF, Uchoa E. Prevalence and sociodemographic characteristics associated with benzodiazepines use among community dwelling older adults: the Bambuí health and aging study (BHAS) Braz J Psychiatry. 2008;30(1):7–11. doi: 10.1590/s1516-44462006005000062. doi: [DOI] [PubMed] [Google Scholar]
- 33.Smith AJ, Tett SE. Improving the use of benzodiazepines—is it possible? A non-systematic review of interventions tried in the last 20 years. BMC Health Serv Res. 2010;10:321. doi: 10.1186/1472-6963-10-321. doi: [DOI] [PMC free article] [PubMed] [Google Scholar]


