Key Points
Question
Do individuals who have clinically recovered from COVID-19 but continue to test positive still transmit SARS-CoV-2?
Findings
In this cohort study of 3648 participants, data were collected during the resumption of the 2020 National Basketball Association season in a closed environment. Individuals who recovered clinically from COVID-19 but continued to test positive did not transmit SARS-CoV-2 to others, despite close proximity with susceptible individuals.
Meaning
US Centers of Disease Control and Prevention guidelines to allow discontinuation of isolation precautions for asymptomatic individuals after 10 days from symptom onset or first reverse-transcription–polymerase chain reaction (RT-PCR) positive test result, without requiring a negative RT-PCR test result, was sufficient to prevent transmission of SARS-CoV-2 in this 2020 cohort.
Abstract
Importance
Clinical data are lacking regarding the risk of viral transmission from individuals who have positive reverse-transcription–polymerase chain reaction (RT-PCR) SARS-CoV-2 test results after recovery from COVID-19.
Objective
To describe case characteristics, including viral dynamics and transmission of infection, for individuals who have clinically recovered from SARS-CoV-2 infection but continued to have positive test results following discontinuation of isolation precautions.
Design, Setting, and Participants
This retrospective cohort study used data collected from June 11, 2020, to October 19, 2020, as part of the National Basketball Association (NBA) closed campus occupational health program in Orlando, Florida, which required daily RT-PCR testing and ad hoc serological testing for SARS-CoV-2 IgG antibodies. Nearly 4000 NBA players, staff, and vendors participated in the NBA’s regular and postseason occupational health program in Orlando. Persistent positive cases were those who recovered from a documented SARS-CoV-2 infection, satisfied US Centers for Disease Control and Prevention criteria for discontinuation of isolation precautions, and had at least 1 postinfection positive RT-PCR test(s) result.
Exposures
Person-days of participation in indoor, unmasked activities that involved direct exposure between persistent positive cases and noninfected individuals.
Main Outcomes and Measures
Transmission of SARS-CoV-2 following interaction with persistent positive individuals, as measured by the number of new COVID-19 cases in the Orlando campus program.
Results
Among 3648 individuals who participated, 36 (1%) were persistent positive cases, most of whom were younger than 30 years (24 [67%]) and male (34 [94%]). Antibodies were detected in 33 individuals (91.7%); all remained asymptomatic following the index persistent positive RT-PCR result. Cycle threshold values for persistent positive RT-PCR test results were typically above the Roche cobas SARS-CoV-2 limit of detection. Cases were monitored for up to 100 days (mean [SD], 51 [23.9] days), during which there were at least 1480 person-days of direct exposure activities, with no transmission events or secondary infections of SARS-CoV-2 detected (0 new cases).
Conclusions and Relevance
In this retrospective cohort study of the 2020 NBA closed campus occupational health program, recovered individuals who continued to test positive for SARS-CoV-2 following discontinuation of isolation were not infectious to others. These findings support time-based US Centers of Disease Control and Prevention recommendations for ending isolation.
This cohort study examines viral dynamics and transmission of infection for National Basketball Association players, staff, and vendors who had clinically recovered from SARS-CoV-2 infection but continued to have positive test results following discontinuation of isolation precautions.
Introduction
Immunocompetent individuals with SARS-CoV-2 infection can remain reverse-transcription–polymerase chain reaction (RT-PCR) positive at high cycle threshold (Ct) counts after recovery. These positive test results may represent low levels of replicating virus or noninfectious viral RNA fragments; however, individuals who remain persistently RT-PCR positive with high Ct values have not been observed to be infectious.1,2,3,4,5,6,7,8,9 While most people with infection show RT-PCR test positivity for a median of approximately 18 to 20 days,10 positive tests have been documented 80 to 105 days after diagnosis.11,12,13 To our knowledge, there is limited evidence to guide clinical decision-making for the question of the extent to which recovered individuals with persistent positive RT-PCR test results are infectious.
Current US Centers for Disease Control and Prevention (CDC) guidance indicates that immunocompetent individuals with improving or resolved symptoms following COVID-19 are no longer infectious 10 days after symptom onset or their first positive RT-PCR test result and may discontinue isolation precautions.14,15 Replication-competent virus is unlikely to be isolated from individuals with mild to moderate COVID-19 beyond 10 days from onset of illness or first positive test result.15 A previous analysis of 790 contacts of 285 recovered patients with persistently positive RT-PCR test results failed to identify secondary transmission of the virus.13 However, to our knowledge, detailed descriptions of frequent longitudinal testing from recovered individuals are lacking; these are needed to understand the time course of viral RNA shedding among individuals who continue to test positive, alongside clinical data regarding the risk of viral transmission.
The possibility of viral transmission from individuals with persistently positive SARS-CoV-2 RT-PCR test results is particularly concerning in certain settings, including competitive sports, as the nature of the activity necessitates close physical contact, often without masking, frequent hand hygiene, and other precautions.16,17 The National Basketball Association (NBA) 2019 to 2020 season was suspended on March 11, 2020, because of the SARS-CoV-2 pandemic. The season was subsequently restarted in July 2020 in Orlando, Florida, during which approximately 4000 individuals underwent frequent (often daily) longitudinal PCR testing in a closed campus over several months. This setting provides an opportunity to understand viral transmission dynamics18 and potential implications of persistent SARS-CoV-2 viral shedding. This study aimed to describe observed testing patterns and viral dynamics for individuals with a COVID-19 diagnosis who clinically recovered but continued to have persistent positive SARS-CoV-2 RT-PCR test results after discontinuation of isolation. We hypothesized that these individuals with persistently positive high Ct RT-PCR results presented a low probability of SARS-CoV-2 transmission.
Methods
From June 11, 2020 to October 19, 2020, data were collected as part of the NBA’s occupational health and safety program to safely resume the 2019 to 2020 basketball season and the 2020 Playoffs in Orlando, Florida, within a closed campus (referred to publicly as the NBA bubble). The NBA Orlando Health and Safety protocols required routine testing for 2 weeks before arrival at the campus, with a mandatory quarantine that ended on receipt of 2 negative PCR test results at least 24 hours apart on arrival. Mandatory testing was performed daily for individuals who were living on campus, while regular testing for local personnel who resided off campus was available before or on each day they worked on campus. This retrospective cohort study was approved by the Advarra institutional review board and followed Strengthening the Reporting of Observational Studies in Epidemiology guidelines for cohort studies. Individuals signed health information authorizations allowing for the collection, storage, and use of health information by NBA for monitoring purposes, including disclosure to medical experts.
Our data set includes longitudinal laboratory tests collected before and during campus activities for all participating players, staff, and vendors. Symptoms, exposure to anyone symptomatic for or positive for COVID-19, history of prior infection, and diagnoses were reported by treating physicians and combined with laboratory data into a deidentified analytic data set. The COVID-19 laboratory diagnostics were performed using assays with emergency use authorization to detect viral RNA via RT-PCR by either Quest Diagnostics or BioReference Laboratories via the Roche cobas19 or the Hologic Panther Fusion system.20 Anterior nasal and oral swabs were combined in a single tube before being assayed. The Roche assay, which was the primary platform used in Orlando and the only assay that provided Ct values, tests against 2 viral RNA targets. Target 1, ORF1, detects SARS-CoV-2, and target 2, the E gene, detects the Sarbecovirus subgenus. For each target, the instrument yields Ct values that serve as proxy measurements for viral RNA concentration. The Roche assay emergency use authorization sets the limits of detection at Ct target 1 (Ct 1) equal to 32.7 and Ct target 2 (Ct 2) equal to 36.4. Cycle threshold values and RNA concentration are inversely associated, such that samples with a Ct 1 value of greater than 32.7 or Ct 2 value of greater than 36.4 are considered outside the limit of detection (max = 40). A positive test result indicates that SARS-CoV-2 RNA was detected and that the Ct values were within the limit of detection, while an inconclusive test result was returned for any sample in which SARS-CoV-2 RNA was detected for target 2 but the Ct values (for target 1 or 2) did not meet the limit of detection. Serological testing for antiviral antibodies was conducted using Siemens Healthineers21 (detects IgG and IgM antibodies to spike protein), Abbott Architect22 (detects IgG antibodies to nucleocapsid protein), Ortho-Clinical Diagnostics23 (detects IgG antibodies to spike protein), or Roche Elecsys24 (detects IgG antibodies to spike protein) SARS-CoV-2 serology assays.
Included individuals participated as an on-campus resident or off-campus/vendor. Those categorized as persistent positive cases either (1) had a documented previous SARS-CoV-2 infection at any time, including before campus arrival, clinically recovered from COVID-19, satisfied CDC criteria for discontinuation of isolation precautions,15 remained asymptomatic after clearing isolation, and had 1 or more subsequent postisolation positive or inconclusive SARS-CoV-2 RT-PCR test results; or (2) did not have a documented previous SARS-CoV-2 infection but had detectable antibodies to SARS-CoV-2, were asymptomatic, and had been testing negative on RT-PCR before a positive or inconclusive RT-PCR result. Ultimately, all persistent positive case participants had a known or suspected SARS-CoV-2 infection before the persistent positive result based on clinician documentation, patient recall of clinical symptoms (eg, fever, cough, muscle aches, vomiting, nausea, sore throat, shortness of breath, headache, chills, diarrhea, fatigue/weakness, abdominal pain, runny nose, and/or loss of taste/smell) and/or laboratory testing (eg, a positive RT-PCR test result or detected antibodies against SARS-CoV-2). Individuals with antibodies to SARS-CoV-2 and without documented prior positive RT-PCR test results were considered recovered if they self-reported previous infection, recognizing that this was not known with absolute certainty.25
Individuals were considered recovered after meeting CDC guidelines for discontinuation of isolation precautions, which changed July 20, 2020, from a test-based strategy that required 2 consecutive negative RT-PCR test results at least 24 hours apart to a time-based strategy that allowed discontinuation after 10 days from symptom onset or first RT-PCR positive test result if asymptomatic.14,15 The NBA Orlando Health and Safety protocols allowed recovered individuals to engage in select indoor activities without masks or physical distancing, including training activities and competitive basketball for players, coaches, and referees, after the postinfection cardiac clearance procedures (required for players).26 Safety protocols required individuals with confirmed initial infection (eg, positive RT-PCR test result without documented prior infection or evidence of antibodies to SARS-CoV-2) to isolate until they met the CDC guidelines for discontinuation. Recovered individuals with persistent positive RT-PCR test results were not required to isolate.
The index date (day 0 of persistent positive phase) was defined as the date of first positive or inconclusive RT-PCR test result after isolation. The follow-up period was defined as the number of days from the earliest to the last recorded test. Duration of participation was defined as the number of days from first to last test on campus in Orlando, excluding tests received before arrival on campus.
Statistical Analysis
Descriptive statistics (mean [SD] and median [range]) were calculated for (1) duration of time from initial infection to the last persistent positive test (if the date of initial infection was known), (2) number of negative test results between positive test results, and (3) mean cycle threshold as reported on Roche platform. Analyses were conducted using SAS (SAS Institute).
Results
Among the 3648 individuals (2915 men [79.9%]; age <30 years, 956 [26.2%]) who participated in the NBA Orlando campus program, 36 individuals (1.0%) met inclusion criteria as a persistent positive case (34 men [94.4%]; age <30 years, 24 [66.7%]; Table 1). Thirty-three (92%) experienced their initial infection before arrival on campus, whereas 3 (8%) received a diagnosis of acute SARS-CoV-2 infection during quarantine before entering the campus. Antibodies were detected in 33 of these individuals (92%) either before or immediately following the first persistent positive RT-PCR result. No persistent positive individuals in this study had required hospitalization for their initial infection, and no cases of reinfection were observed. All remained asymptomatic throughout the remainder of testing.
Table 1. Recovered Persistent/Recurrent Positive Cases of SARS-CoV-2 Infection in the NBA 2020 Orlando, Florida, Campusa.
| Characteristic | Persistent positive cases, No. (%)b |
|---|---|
| No. | 36 |
| Age, y | |
| 18-29 | 24 (66.7) |
| ≥30 | 12 (33.3) |
| Sex | |
| Women | 2 (5.6) |
| Men | 34 (94.4) |
| Antibody testing | |
| Detected, before initial positive RT-PCR result in Orlando | 24 (66.7) |
| Detected, following initial positive RT-PCR result in Orlando | 9 (25.0) |
| Not detected | 1 (2.8) |
| Not available | 2 (5.6) |
| Timing of initial infection | |
| Before arrival on campusc | 33 (91.7) |
| In Orlandod | 3 (8.3) |
| RT-PCR testing cadence in Orlando | |
| Daily testing | 33 (91.7) |
| Periodic testing | 3 (8.3) |
| Participation in Orlando campus, d | |
| ≤30 | 8 (22.2) |
| 31-60 | 16 (44.4) |
| >60 | 12 (33.3) |
| Follow-up (first test to last test), d | |
| ≤30 | 2 (5.6) |
| 31-60 | 10 (27.8) |
| 61-90 | 16 (44.4) |
| >90 | 8 (22.2) |
Abbreviations: NBA, National Basketball Association; RT-PCR, reverse-transcription–polymerase chain reaction.
Recovered persistent/recurrent positive designation based on clinician diagnosis using standardized operational definitions.
Individuals include players, team staff, NBA league staff, and vendors.
Includes individuals with initial infection before arrival in Orlando or detected during quarantine in Orlando, but before entry to campus.
Individual’s initial infection occurred while involved in the Orlando program; these 3 individuals were vendors, 2 of whom lived off campus and 1 with initial infection diagnosed while in quarantine. Their exposure likely occurred in Orlando or immediately before arrival in Orlando.
Nearly all individuals (33 [92%]) were tested daily; 3 (8%) were local vendors who were tested periodically (before campus entrance). Days of participation in testing varied based on arrival date, team elimination date, or other reason for early exit; 28 individuals (78%) were tested for 31 days or more (Table 1). Persistent positive individuals had a mean (range) number of 72 (9-118) days of overall follow-up from their initial infection or first positive test result and participated in the NBA Orlando campus program for up to 100 days (mean, 51 days). Persistent positive individuals continued to have intermittent detectable SARS-CoV-2 RNA for a mean (SD) of 31(11) days (median, 30 days [range, 14-68 days]) from the date of initial infection. Notably, 18 individuals (50%) tested positive on a RT-PCR test at least once 30 days or longer after initial infection. Patterns of persistent positive RT-PCR tests varied greatly among participants (Figure); in many cases, participants experienced several negative test results before testing positive again. For example, participants 4, 12, and 13, all of whom had detectable antibodies, experienced 13, 8, and 48 consecutive negative RT-PCR test results before testing positive again. Participants had a mean (SD) of 44 (23) and maximum of 94 negative RT-PCR test results following their first persistent positive result before departure. For some, these negative tests occurred in long stretches between intermittent positive tests (participants 2, 14, 26, and 33). For others, all RT-PCR test results after the first persistent positive test were negative for SARS-CoV-2 (participants 4, 7, 19, 22, 30, and 36).
Figure. Test Results Among 36 Individuals Who Recovered From COVID-19 With Persistent Positive SARS-CoV-2 Reverse-Transcription–Polymerase Chain Reaction (RT-PCR) Tests.
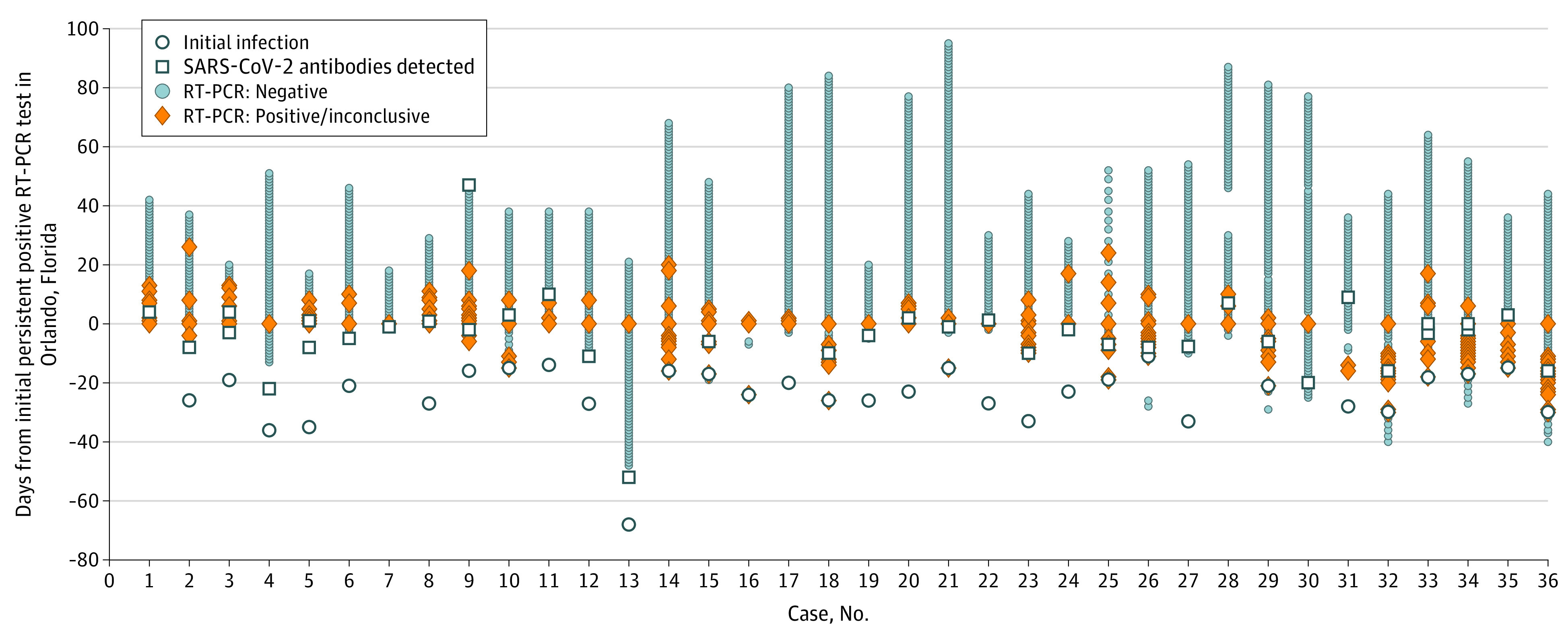
The longitudinal pattern of test results across recovered individuals who continued to test positive differed greatly with some individuals who experienced large stretches of negative test results without any positive test results, while others had more frequent intermittent positive tests. Recovered persistent/recurrent positive designation was based on clinician diagnosis using standardized operational definitions. The index date (day 0) was defined as the date of the first positive or inconclusive RT-PCR test result occurred after isolation had been discontinued. The RT-PCR test results shown were run primarily on the Roche cobas platform; results from the Hologic Panther platform was used before arrival in Orlando, Florida, and in 1 rare occasion in Orlando when the Roche cobas platform was not available (this applies only to case number 25).
Cycle threshold values for persistent positive tests were above the Ct 1 limit of detection (32.7), averaging a target 1 value of 34.1 (range, 30.3–36.7; Table 2; eTable in the Supplement). In contrast, and as expected, the Ct values among individuals with diagnoses of acute infections before or, in the case of vendors, during the program were typically within the limit of detection, as previously reported.18 Participants 1, 7, 28, and 30 did not have a documented initial infection, but all had detectable antibodies before or within 1 week of their first persistent positive test result and Ct target 1 values ranging from 32.3 to 36.4, which was consistent with the other persistent positive individuals. These cases were treated as true infections until subsequent testing demonstrated no increasing viral load that would have been consistent with an active infection.
Table 2. Average Cycle Threshold for Persistent Positive RT-PCR Tests From the Roche Cobasa.
| Roche cobas RT-PCR test resultb | Total No. of tests | No. of tests with reported Ct values | Mean (SD) Ct value | Median (range) Ct value | |
|---|---|---|---|---|---|
| 97 | Ct 1 | 51 | 34.1 (1.7) | 34.5 (30.3-36.7) | |
| Ct 2 | 84 | 36.7 (1.5) | 37.0 (32.2-38.8) | ||
| Inconclusive | 48 | Ct 1 | 2 | 34.3 (3.5) | 34.3 (31.8-36.7) |
| Ct 2 | 48 | 37.5 (0.8) | 37.7 (35.3-38.8) | ||
| Positive | 51 | Ct 1 | 49 | 34.0 (1.7) | 34.5 (30.3-36.5) |
| Ct 2 | 36 | 35.6 (1.6) | 35.7 (32.2-38.7) |
Abbreviations: Ct, cycle threshold; RT-PCR, reverse-transcription–polymerase chain reaction.
Includes RT-PCR test results via the Roche cobas SARS-CoV-2 assay, for which the limits of detection are Ct 1 = 32.7 and Ct 2 = 36.4.
All tests included in this table were run on the Roche cobas platform, which provides Ct values. For the Roche assay, a positive test result indicates SARS-CoV-2 RNA was detected and that Ct values were within the limit of detection, while an inconclusive result was returned for any sample in which SARS-CoV-2 RNA was detected for target 2 but Ct values (target 1 or 2) were outside the limit of detection.
Twenty-nine individuals (81%) with persistent positive test results participated in unmasked activities on campus, such as officiating, coaching, meals, on-court training, and basketball games. Together, they engaged in at least 1480 person-days of indoor, unmasked contact events or situations (approximately 51 days per individual) during the period of persistent positive test results, including 56 person-days for which a positive test result was returned on that day. No transmission events or secondary infections were detected following contact, despite daily RT-PCR testing on the Orlando campus.
Discussion
Multiple strategies have been used to decrease transmission of SARS-CoV-2, including isolation for patients with COVID-19. Determining optimal criteria for discontinuing isolation has been complicated by persistently positive RT-PCR test results after COVID-19 recovery, as well as uncertainty about the extent to which continued viral shedding poses a risk of transmission. In October 2020, the scientific community, including CDC and World Health Organization, began to coalesce around a time-based strategy for discontinuation of isolation rather than using test-based criteria. This recommendation was based on evidence that immunocompetent individuals who continued to test positive were not found to have replication-competent virus 10 days beyond initial diagnosis.15
Our study provides evidence supporting the safety of the time-based approach to discontinuation of isolation by directly observing individuals who remained positive for SARS-CoV-2 RT-PCR after recovery and participated in high-risk, unmasked interactions. Among this group, despite their persistent positive test results, we observed no transmission events during at least 1480 patient-days of indoor, unmasked interactions. The high volume of exposure, combined with the nature of the contact27 and subsequent evident lack of transmission, provides some of the best evidence to date in support of a symptom and time-based strategy. Thus, our results support observations that asymptomatic individuals who have met CDC criteria for discontinuation of isolation, but who have persistent positive RT-PCR test results with high Ct values, do not appear to be infectious to others.
Interpretation of persistent positive test results should include assessing symptoms, Ct values, and antibody tests, if available. Similar to our findings, Liotti et al28 evaluated 176 recovered patients for replicative SARS-CoV-2 RNA on repeated testing; 32 (18%) had repeated positive RT-PCR test results an average of 49 days after initial diagnosis. While all patients initially had replicative virus, only 1 had replicative virus 39 days postdiagnosis; the authors were unable to determine if the patient had relapsed or was reinfected, but this patient was symptomatic during the second episode. Others have also reported COVID-19 symptoms during their second episode,29 while other reports include patients who remain asymptomatic on reinfection.30,31 Thus, additional considerations are required to determine if patients with persistently positive RT-PCR test results are potentially infectious. In addition to supporting a time-based approach to discontinuation of isolation, our study found that RT-PCR Ct values and antibody testing provide useful context, along with clinical history and symptoms, when determining the clinical significance of persistently positive test results in recovered individuals. While antibody testing cannot be used for diagnosing acute infection, it can be used to provide evidence of potential prior infection32 and its timing in an individual who unexpectedly tests positive; however, subsequent PCR tests with Ct values are the key tool for decision-making.
Reverse-transcription PCR Ct values can be informative for decision-making when combined with antibody testing and symptom status. For example, COVID-19 testing was not widely available in the early weeks of the pandemic, and individuals in the NBA Orlando campus were at times unaware that they had a history of a previous SARS-CoV-2 infection. Therefore, if an individual could not recall a prior infection, their first positive test result on campus could have indicated either a new infection or a persistent positive test following recovery from an unknown prior infection. Differentiating between these scenarios was important in determining whether isolation precautions and full contact tracing procedures were required. The presence of antibodies to SARS-CoV-2 before or within 1 to 2 days of the positive test result suggested that a new infection was less likely. In this cohort, the average Ct 1 was 34 for all persistent positive test results. In contrast, individuals with acute infection had lower initial Ct values and decreasing Ct trajectories over subsequent days.18 Thus, asymptomatic individuals with positive antibodies and high Ct 1 values (>32.7) were able to be treated as cases of postinfection persistent positivity rather than an evolving new infection.
Strengths and Limitations
The primary strengths of this study include the availability of detailed epidemiologic and daily quantitative results in a large, closed cohort with measured engagement in regular contact without masking and physical distancing among a subset of individuals. Importantly, this study was able to control for sources of virus exposure because of the nature of the Orlando campus. Further, to our knowledge, this study is one of very few reports of repeated unmasked exposure between recovered SARS-CoV-2 RT-PCR–positive individuals with prolonged viral shedding and COVID-19–negative individuals. In this setting, we did not observe evidence of viral transmission among these individuals. This finding supports the safety of the current CDC guidance for discontinuation of isolation precautions and the underlying hypothesis that individuals are unlikely to have replication-competent virus as a recovered individual, especially if they are asymptomatic and have RT-PCR test results with high Ct values. Previous reports of individuals with persistently positive SARS-CoV-2 RT-PCR test results have largely been among institutionalized or hospitalized populations9,28,33,34,35; this study reports findings among ambulatory immunocompetent individuals who were being tested daily within a closed cohort.
The limitations of this retrospective cohort study include differential loss-to-follow-up among the participants, as NBA staff and team affiliates were more likely than local vendors to have long follow-up after a positive test result, resulting in potential sampling bias. Second, this population was majority male, younger, and healthier than the general population, potentially limiting the generalizability of the findings, especially to immunocompromised individuals; however, biological mechanisms and the likelihood of viral transmission are unlikely to differ greatly. Third, symptoms and their severity were unavailable for infections before arrival at the Orlando campus, limiting the ability to assess the association of symptoms with Ct values or the duration of persistent positivity. Fourth, the date of initial infection was unknown for some individuals, and daily testing stopped at campus exit; these findings may not fully capture the length of time individuals can continue to test positive. Fifth, false-positive or false-negative results should be considered. Last, no individuals in the population were vaccinated, and these data reflect infections with the predominant COVID-19 strains during the study period and may not be applicable to variant strains of SARS-CoV-2.36
Conclusions
Our study suggests that individuals who continue to test positive by RT-PCR for SARS-CoV-2 after meeting CDC criteria for discontinuation of isolation were not infectious to others during regular unmasked exposures. Medical teams allowed these individuals to return to team activity after they fulfilled the CDC criteria in combination with the absence of symptoms and elevated RT-PCR Ct values to indicate that they were no longer infectious. As such, our results support the safety of the time-based CDC public health recommendations regarding discontinuation of isolation precautions. As the pandemic progresses, and particularly if the number of reported reinfection cases increases, interpretation of subsequent positive SARS-CoV-2 RT-PCR test results in recovered individuals will become increasingly challenging. Our results suggest that symptom status and RT-PCR Ct values aid in determining infectiousness among these individuals.
eTable. Longitudinal Ct values of RT-PCR tests from the Roche cobas platform among individuals persistently testing positive after infection with SARS-CoV-2
References
- 1.Vibholm LK, Nielsen SSF, Pahus MH, et al. SARS-CoV-2 persistence is associated with antigen-specific CD8 T-cell responses. EBioMedicine. 2021;64:103230. doi: 10.1016/j.ebiom.2021.103230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wu J, Liu X, Liu J, et al. Coronavirus disease 2019 test results after clinical recovery and hospital discharge among patients in China. JAMA Netw Open. 2020;3(5):e209759. doi: 10.1001/jamanetworkopen.2020.9759 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wu Y, Guo C, Tang L, et al. Prolonged presence of SARS-CoV-2 viral RNA in faecal samples. Lancet Gastroenterol Hepatol. 2020;5(5):434-435. doi: 10.1016/S2468-1253(20)30083-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hartman WR, Hess AS, Connor JP. Persistent viral RNA shedding after COVID-19 symptom resolution in older convalescent plasma donors. Transfusion. 2020;60(10):2189-2191. doi: 10.1111/trf.15927 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhang B, Liu S, Dong Y, et al. Positive rectal swabs in young patients recovered from coronavirus disease 2019 (COVID-19). J Infect. 2020;81(2):e49-e52. doi: 10.1016/j.jinf.2020.04.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Xu K, Chen Y, Yuan J, et al. Factors associated With prolonged viral RNA shedding in patients with coronavirus disease 2019 (COVID-19). Clin Infect Dis. 2020;71(15):799-806. doi: 10.1093/cid/ciaa351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Liu WD, Chang SY, Wang JT, et al. Prolonged virus shedding even after seroconversion in a patient with COVID-19. J Infect. 2020;81(2):318-356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Molina LP, Chow SK, Nickel A, Love JE. Prolonged detection of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) RNA in an obstetric patient with antibody seroconversion. Obstet Gynecol. 2020;136(4):838-841. doi: 10.1097/AOG.0000000000004086 [DOI] [PubMed] [Google Scholar]
- 9.Hong K, Cao W, Liu Z, et al. Prolonged presence of viral nucleic acid in clinically recovered COVID-19 patients was not associated with effective infectiousness. Emerg Microbes Infect. 2020;9(1):2315-2321. doi: 10.1080/22221751.2020.1827983 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Qi L, Yang Y, Jiang D, et al. Factors associated with the duration of viral shedding in adults with COVID-19 outside of Wuhan, China: a retrospective cohort study. Int J Infect Dis. 2020;96:531-537. doi: 10.1016/j.ijid.2020.05.045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rhee C, Kanjilal S, Baker M, Klompas M. Duration of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infectivity: when is it safe to discontinue isolation? Clin Infect Dis. 2020;ciaa1249. doi: 10.1093/cid/ciaa1249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Avanzato VA, Matson MJ, Seifert SN, et al. Case study: prolonged infectious SARS-CoV-2 shedding from an asymptomatic immunocompromised individual with cancer. Cell. 2020;183(7):1901-1912.e9, e1909. doi: 10.1016/j.cell.2020.10.049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Korea Centers for Disease Control and Prevention . Findings from investigation and analysis of re-positive cases. Accessed December 18, 2020. https://www.cdc.go.kr/board/board.es?mid=a30402000000&bid=0030&act=view&list_no=367267&nPage=1external%20icon
- 14.Centers for Disease Control and Prevention . Discontinuation of isolation for persons with COVID-19 not in healthcare settings. Accessed December 18, 2020. https://www.cdc.gov/coronavirus/2019-ncov/hcp/disposition-in-home-patients.html
- 15.Centers for Disease Control and Prevention . Duration of isolation and precautions for adults with COVID-19. Accessed December 18, 2020. https://www.cdc.gov/coronavirus/2019-ncov/hcp/duration-isolation.html#
- 16.Dores H, Cardim N. Return to play after COVID-19: a sport cardiologist’s view. Br J Sports Med. 2020;54(19):1132-1133. doi: 10.1136/bjsports-2020-102482 [DOI] [PubMed] [Google Scholar]
- 17.DiFiori JP, Green G, Meeuwisse W, Putukian M, Solomon GS, Sills A. Return to sport for North American professional sport leagues in the context of COVID-19. Br J Sports Med. 2021;55(8):417-421. doi: 10.1136/bjsports-2020-103227 [DOI] [PubMed] [Google Scholar]
- 18.Kissler SM, Fauver JR, Mack C, et al. . SARS-CoV-2 viral dynamics in acute infections. medRxiv. 2020:2020.2010.2021.20217042.
- 19.Cobas®. Qualitative assay for use on the cobas® 6800/8800 Systems. Accessed December 18, 2020. https://www.fda.gov/media/136049/download
- 20.Panther Fusion® SARS-CoV-2. SARS-CoV-2 assay (Panther Fusion® System). Accessed December 18, 2020. https://www.fda.gov/media/136156/download
- 21.US Food and Drug Administration . Coronavirus (COVID-19) update: FDA authorizes first tests that estimate a patient’s antibodies from past SARS-CoV-2 infection. Accessed December 18, 2020. https://www.fda.gov/news-events/press-announcements/coronavirus-covid-19-update-fda-authorizes-first-tests-estimate-patients-antibodies-past-sars-cov-2
- 22.Abbott. SARS-CoV-2 IgG. Accessed December 18, 2020. https://www.fda.gov/media/137383/download
- 23.Vitros. VITROS immunodiagnostic products anti-SARS-CoV-2 IgG reagent pack. Accessed December 18, 2020. https://www.fda.gov/media/137363/download
- 24.US Food and Drug Administration . Elecsys anti-SARS-CoV-2—FDA. Accessed March 11, 2021. https://www.fda.gov/media/137602/download
- 25.US Centers for Disease Control and Prevention . Using antibody tests for COVID-19. Accessed December 18, 2020. https://www.cdc.gov/coronavirus/2019-ncov/lab/resources/antibody-tests.html#:~:text=In%20general%2C%20a%20positive%20antibody,to%203%20weeks%20after%20infection
- 26.Kim JH, Levine BD, Phelan D, et al. Coronavirus disease 2019 and the athletic heart: emerging perspectives on pathology, risks, and return to play. JAMA Cardiol. 2021;6(2):219-227. doi: 10.1001/jamacardio.2020.5890 [DOI] [PubMed] [Google Scholar]
- 27.Mack CD, Wasserman EB, Perrine CG, et al. ; NFL COVID-19 Advisory and Operational Team . Implementation and evolution of mitigation measures, testing, and contact tracing in the National Football League, August 9-November 21, 2020. MMWR Morb Mortal Wkly Rep. 2021;70(4):130-135. doi: 10.15585/mmwr.mm7004e2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Liotti FM, Menchinelli G, Marchetti S, et al. Assessment of SARS-CoV-2 RNA test results among patients who recovered from COVID-19 with prior negative results. JAMA Intern Med. 2020. Published online November 12, 2020. doi: 10.1001/jamainternmed.2020.7570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tillett RL, Sevinsky JR, Hartley PD, et al. Genomic evidence for reinfection with SARS-CoV-2: a case study. Lancet Infect Dis. 2021;21(1):52-58. doi: 10.1016/S1473-3099(20)30764-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gupta V, Bhoyar RC, Jain A, et al. Asymptomatic reinfection in two healthcare workers from India with genetically distinct SARS-CoV-2. Clin Infect Dis. 2020;ciaa1451. doi: 10.1093/cid/ciaa1451 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.To KK, Hung IF, Ip JD, et al. COVID-19 re-infection by a phylogenetically distinct SARS-coronavirus-2 strain confirmed by whole genome sequencing. Clin Infect Dis. 2020;ciaa1275. doi: 10.1093/cid/ciaa1275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.US Centers for Disease Control and Prevention . Test for past infection. Accessed December 18, 2020. https://www.cdc.gov/coronavirus/2019-ncov/testing/serology-overview.html
- 33.Qiao X-M, Xu X-F, Zi H, et al. Re-positive cases of nucleic acid tests in discharged patients with COVID-19: a follow-up study. Front Med (Lausanne). 2020;7(349):349. doi: 10.3389/fmed.2020.00349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Agarwal V, Venkatakrishnan AJ, Puranik A, et al. Long-term SARS-CoV-2 RNA shedding and its temporal association to IgG seropositivity. Cell Death Discov. 2020;6(1):138. doi: 10.1038/s41420-020-00375-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ridgway JP, Shah NS, Robicsek AA. Prolonged shedding of severe acute respiratory coronavirus virus 2 (SARS-CoV-2) RNA among patients with coronavirus disease 2019 (COVID-19). Infect Control Hosp Epidemiol. 2020;41(10):1235-1236. doi: 10.1017/ice.2020.307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kissler S, Fauver JR, Mack CD, et al. Densely sampled viral trajectories suggest longer duration of acute infection with B.1.1.7 variant relative to non-B.1.1.7 SARS-CoV-2 Accessed March 11, 2021. https://nrs.harvard.edu/URN-3:HUL.INSTREPOS:37366884
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eTable. Longitudinal Ct values of RT-PCR tests from the Roche cobas platform among individuals persistently testing positive after infection with SARS-CoV-2


