This cohort study assesses the association between COVID-19 and maternal and neonatal outcomes in pregnant women with COVID-19 diagnosis compared with concomitantly enrolled pregnant women without COVID-19 diagnosis.
Key Points
Question
To what extent does COVID-19 in pregnancy alter the risks of adverse maternal and neonatal outcomes compared with pregnant individuals without COVID-19?
Findings
In this multinational cohort study of 2130 pregnant women in 18 countries, women with COVID-19 diagnosis were at increased risk of a composite maternal morbidity and mortality index. Newborns of women with COVID-19 diagnosis had significantly higher severe neonatal morbidity index and severe perinatal morbidity and mortality index compared with newborns of women without COVID-19 diagnosis.
Meaning
This study indicates a consistent association between pregnant individuals with COVID-19 diagnosis and higher rates of adverse outcomes, including maternal mortality, preeclampsia, and preterm birth compared with pregnant individuals without COVID-19 diagnosis.
Abstract
Importance
Detailed information about the association of COVID-19 with outcomes in pregnant individuals compared with not-infected pregnant individuals is much needed.
Objective
To evaluate the risks associated with COVID-19 in pregnancy on maternal and neonatal outcomes compared with not-infected, concomitant pregnant individuals.
Design, Setting, and Participants
In this cohort study that took place from March to October 2020, involving 43 institutions in 18 countries, 2 unmatched, consecutive, not-infected women were concomitantly enrolled immediately after each infected woman was identified, at any stage of pregnancy or delivery, and at the same level of care to minimize bias. Women and neonates were followed up until hospital discharge.
Exposures
COVID-19 in pregnancy determined by laboratory confirmation of COVID-19 and/or radiological pulmonary findings or 2 or more predefined COVID-19 symptoms.
Main Outcomes and Measures
The primary outcome measures were indices of (maternal and severe neonatal/perinatal) morbidity and mortality; the individual components of these indices were secondary outcomes. Models for these outcomes were adjusted for country, month entering study, maternal age, and history of morbidity.
Results
A total of 706 pregnant women with COVID-19 diagnosis and 1424 pregnant women without COVID-19 diagnosis were enrolled, all with broadly similar demographic characteristics (mean [SD] age, 30.2 [6.1] years). Overweight early in pregnancy occurred in 323 women (48.6%) with COVID-19 diagnosis and 554 women (40.2%) without. Women with COVID-19 diagnosis were at higher risk for preeclampsia/eclampsia (relative risk [RR], 1.76; 95% CI, 1.27-2.43), severe infections (RR, 3.38; 95% CI, 1.63-7.01), intensive care unit admission (RR, 5.04; 95% CI, 3.13-8.10), maternal mortality (RR, 22.3; 95% CI, 2.88-172), preterm birth (RR, 1.59; 95% CI, 1.30-1.94), medically indicated preterm birth (RR, 1.97; 95% CI, 1.56-2.51), severe neonatal morbidity index (RR, 2.66; 95% CI, 1.69-4.18), and severe perinatal morbidity and mortality index (RR, 2.14; 95% CI, 1.66-2.75). Fever and shortness of breath for any duration was associated with increased risk of severe maternal complications (RR, 2.56; 95% CI, 1.92-3.40) and neonatal complications (RR, 4.97; 95% CI, 2.11-11.69). Asymptomatic women with COVID-19 diagnosis remained at higher risk only for maternal morbidity (RR, 1.24; 95% CI, 1.00-1.54) and preeclampsia (RR, 1.63; 95% CI, 1.01-2.63). Among women who tested positive (98.1% by real-time polymerase chain reaction), 54 (13%) of their neonates tested positive. Cesarean delivery (RR, 2.15; 95% CI, 1.18-3.91) but not breastfeeding (RR, 1.10; 95% CI, 0.66-1.85) was associated with increased risk for neonatal test positivity.
Conclusions and Relevance
In this multinational cohort study, COVID-19 in pregnancy was associated with consistent and substantial increases in severe maternal morbidity and mortality and neonatal complications when pregnant women with and without COVID-19 diagnosis were compared. The findings should alert pregnant individuals and clinicians to implement strictly all the recommended COVID-19 preventive measures.
Introduction
At the outset of the COVID-19 pandemic, the precise extent of the risks in pregnancy was uncertain, which was affecting pregnant individuals’ mental health.1,2 The lack of clarity arose because, in an early systematic review,3 only 4 studies that involved small numbers compared outcomes between pregnant women with and without COVID-19.4,5,6,7 The question is relevant because of the known deleterious effects of other coronavirus infections in pregnancy (eg, severe acute respiratory syndrome and Middle East respiratory syndrome).8 Therefore, the INTERGROWTH-21st Consortium conducted a prospective, longitudinal, observational study (INTERCOVID), involving 43 hospitals in 18 countries, to assess the association between COVID-19 and maternal and neonatal outcomes in pregnant women with COVID-19 diagnosis, compared with concomitantly enrolled pregnant women without COVID-19 diagnosis.
Methods
Study Design
The Oxford Tropical Research Ethics Committee and all local ethics committees approved the study, which did not interfere with clinical management. Informed consent (oral or written) was obtained from study participants according to local requirements, except when a waiver/exemption of such consent was granted by a local committee. We adhered to the Declaration of Helsinki9 and Good Clinical Practice guidelines. The study protocol, including the laboratory tests used, has been previously published.10
For 8 months from March 2, 2020, we enrolled women 18 years or older at any stage of pregnancy or delivery with the diagnosis of COVID-19 during the present pregnancy based on laboratory confirmation of COVID-19 and/or radiologic pulmonary findings suggestive of COVID-1911 or 2 or more predefined COVID-19 symptoms. A range of different real-time polymerase chain reaction and antibody tests were used at participating institutions (eBox in the Supplement). Two immediately concomitant pregnant women 18 years or older without any of those diagnostic criteria were enrolled per woman with COVID-19 diagnosis to create an unbiased sample of all pregnant women without COVID-19 diagnosis in these institutions. Women were enrolled from 43 institutions in 18 countries (Argentina, Brazil, Egypt, France, Ghana, India, Indonesia, Italy, Japan, Mexico, Nigeria, North Macedonia, Pakistan, Russia, Spain, Switzerland, UK, and the US). Data on race were not collected.
When a woman with a COVID-19 diagnosis was identified antenatally, 2 women without COVID-19 diagnosis of similar gestational age (±2 weeks) receiving standard antenatal care were enrolled that day. If not possible or if those women without COVID-19 diagnosis were lost to follow-up, we enrolled 2 women without COVID-19 diagnosis who delivered immediately after the woman with COVID-19 diagnosis. The same selection strategy was used when a woman with COVID-19 diagnosis was identified at hospital admission and delivery was likely during that admission. If a woman without COVID-19 diagnosis declined participation, the next woman was approached until 2 women without COVID-19 diagnosis were enrolled per woman with COVID-19 diagnosis. We sought confirmation from a biweekly random 10% sample that the 2 women without COVID-19 diagnosis were appropriately chosen; we excluded 5 women who had a COVID-19 diagnosis and the corresponding 10 women without a COVID-19 diagnosis, without such confirmation. Live and stillborn singleton and multiple pregnancies were included, including those with congenital anomalies. However, in keeping with reporting requirements during the pandemic,12 we excluded women/neonates from the final analysis if their data were already published in any comparative study with women without COVID-19 diagnosis.5,13,14,15,16,17,18,19,20,21,22,23
Outcomes
The primary outcomes24 were 3 unweighted indices: (1) maternal morbidity and mortality index including at least 1 of the following pregnancy-related morbidities: third-trimester vaginal bleeding, pregnancy-induced hypertension, preeclampsia/eclampsia/hemolysis, elevated liver enzymes, and low platelet count (HELLP) syndrome, preterm labor, infections requiring antibiotics, or any other pregnancy-related conditions requiring treatment or referral; maternal admission to the intensive care unit (ICU); referral to a higher level of care; or death; (2) severe neonatal morbidity index (SNMI) including at least 3 of the following severe complications: bronchopulmonary dysplasia, hypoxic-ischemic encephalopathy, sepsis, anemia requiring transfusion, patent ductus arteriosus requiring treatment or surgery, intraventricular hemorrhage, and necrotizing enterocolitis or retinopathy of prematurity diagnosed before hospital discharge; and (3) severe perinatal morbidity and mortality index (SPMMI) including fetal death, at least 1 of the severe neonatal conditions listed above, admission to the neonatal ICU (NICU) for 7 days or longer, or neonatal death before hospital discharge. Secondary outcomes were each individual component of the indices described above considered as separate conditions.
Gestational age estimation was based on ultrasonography measurement of fetal crown-rump length (<14 weeks’ gestation) against the international INTERGROWTH-21st standards25 or, if early ultrasonography dating was not carried out, the best obstetric estimate was used based on all clinical and ultrasonography data available at the time of delivery. Newborn weight, length, and head circumference at birth were assessed against the international INTERGROWTH-21st standards.26
Data Management and Statistical Analysis
We used a centrally coordinated data management system developed for the INTERGROWTH-21st Project (MedSciNet).27 Associations between being diagnosed as having COVID-19 and morbidity/mortality indices expressed as binary outcomes were assessed using Poisson models with a log link function and robust standard errors expressed as relative risk (RR) and 95% CI. Associations with number of days in ICU were assessed using negative binomial models with robust standard errors (expressed as an incidence rate ratio and 95% CI). We set statistical significance at P < .05. Models for our primary outcomes were adjusted for country, month entering study, maternal age, and history of maternal morbidity (including diabetes, thyroid, and other endocrine disorders; cardiac disease; hypertension; chronic respiratory disease; kidney disease; malaria; or tuberculosis). Models with preterm birth as an outcome were also adjusted for previous preterm birth. We plotted Kaplan-Meier curves with the percentage of women remaining pregnant by gestational age to compare the distributions between women with and without COVID-19 diagnosis, according to symptom status. We evaluated women with COVID-19 diagnosis for the primary and secondary outcomes using the women without COVID-19 diagnosis as the reference group. We further categorized women with COVID-19 diagnosis as asymptomatic or symptomatic based on type and duration of symptoms, as well as according to past maternal morbidity and normal weight or overweight to explore effect modification. We assessed the association of neonates testing positive for SARS-CoV-2 infection. In separate sensitivity analyses, we adjusted for additional potential confounders, restricted the definition of women with COVID-19 diagnosis to mothers with a positive laboratory test result, and excluded twins. We also performed separate meta-analyses for our primary outcomes of interest using the stratified results to estimate pooled effects and assess heterogeneity by country.
Results
We enrolled 706 women with COVID-19 diagnosis, of which 656 (92.9%) had laboratory/radiological confirmation and 50 (7.1%) had more than 2 symptoms without laboratory confirmation. Of those who tested positive, almost exclusively (640 of 652 [98.1%]) by real-time polymerase chain reaction, 287 (44.0%) were asymptomatic. We also enrolled 1424 women without COVID-19 diagnosis (eFigure in the Supplement). The groups of women with and without diagnosis had similar demographic characteristics. However, 48.6% (n = 323) of the group with COVID-19 diagnosis had overweight early in pregnancy compared with 40.2% (n = 554) of the group without COVID-19 diagnosis. Women with COVID-19 diagnosis had a higher rate of recreational drug use but lower rate of smoking during the index pregnancy, higher rates of previous preterm birth, stillbirth, neonatal death, and preexisting medical conditions (eTable 1 in the Supplement).
During the index pregnancy, women with a COVID-19 diagnosis had higher rates of pregnancy-induced hypertension (RR, 1.46; 95% CI, 1.05-2.02), preeclampsia/eclampsia (RR, 1.76; 95% CI, 1.27-2.43), and infections requiring antibiotics (RR, 3.38; 95% CI, 1.63-7.01), and there was an association with a greater risk of admission to ICU/high-dependency unit (RR, 5.04; 95% CI, 3.13-8.10) and referral to a higher level of care (RR, 6.07; 95% CI, 1.23-30.01). Among all ICU admissions, women with COVID-19 diagnosis stayed 3.73 (95% CI, 2.37-5.86) days longer than women without COVID-19 diagnosis (Table 1).
Table 1. Pregnancy Complications, Perinatal Events, and Neonatal Morbidities Among Women With and Without COVID-19 Diagnosis and Their Newborns.
| Characteristic | No. (%) | Relative risk (95% CI) | |
|---|---|---|---|
| Women with COVID-19 diagnosis (n = 706) | Women without COVID-19 diagnosis (n = 1424) | ||
| Maternal morbidity and mortality indexa | 225 (31.9) | 296 (20.8) | 1.54 (1.33 to 1.78)b |
| Vaginal bleeding | 44 (6.2) | 87 (6.1) | 1.02 (0.72 to 1.46) |
| Pregnancy-induced hypertension | 58 (8.2) | 80 (5.6) | 1.46 (1.05 to 2.02) |
| Preeclampsia/eclampsia/HELLP | 59 (8.4) | 63 (4.4) | 1.76 (1.27 to 2.43)b |
| Hemoglobin level <10 g/dL at >27 wk gestation | 130 (18.4) | 228 (16.0) | 1.15 (0.91 to 1.45) |
| Preterm labor | 52 (7.4) | 88 (6.2) | 1.20 (0.86 to 1.68) |
| Infections requiring antibiotics | 25 (3.6) | 16 (1.1) | 3.38 (1.63 to 7.01) |
| Admitted to ICU | 59 (8.4) | 23 (1.6) | 5.04 (3.13 to 8.10) |
| Time in ICU, mean (SD), d | 7.3 (7.8) | 2.0 (1.7) | 3.73 (2.37 to 5.86)c |
| Referred for higher dependency care | 6 (0.9) | 1 (0.1) | 6.07 (1.23 to 30.01) |
| Maternal death | 11 (1.6) | 1 (0.1) | 22.26 (2.88 to 172.11) |
| Fetal distress | 87 (12.3) | 120 (8.4) | 1.70 (1.06 to 2.75)b |
| Spontaneous initiation of labor | 333 (47.2) | 793 (55.7) | 0.85 (0.77 to 0.93) |
| Induced labor | 157 (22.3) | 320 (22.5) | 0.99 (0.84 to 1.18) |
| Cesarean delivery | 346 (49.0) | 547 (38.4) | 1.28 (1.16 to 1.40)b |
| Prelabor rupture of membranes | 114 (16.1) | 262 (18.4) | 0.87 (0.71 to 1.07) |
| Gestational age at birth, mean (SD), wk | 37.9 (3.3) | 38.5 (3.1) | −0.61 (−0.90 to −0.32)d |
| Preterm birth (<37 wk gestation) | 159 (22.5) | 194 (13.6) | 1.59 (1.30 to 1.94)e |
| Spontaneous preterm birth | 27 (3.8) | 66 (4.6) | 0.81 (0.52 to 1.27) |
| Medically indicated preterm birth | 133 (18.8) | 127 (8.9) | 1.97 (1.56 to 2.51)e |
| Birth weight, mean (SD), kg | 2.96 (0.70) | 3.07 (0.68) | −0.11 (−0.18 to −0.04)d |
| Male | 353 (50.0) | 749 (52.6) | 0.95 (0.87 to 1.04) |
| Female | 353 (50.0) | 675 (47.6) | 1.06 (0.96 to 1.16) |
| Low birth weight (<2500 g) | 145 (20.5) | 181 (12.7) | 1.58 (1.29 to 1.94)b |
| Small for gestational age (<10th centile)f | 97 (13.7) | 181 (12.7) | 1.03 (0.81 to 1.31) |
| Exclusive breastfeeding at discharge | 378 (53.5) | 953 (66.9) | 0.80 (0.74 to 0.87) |
| Any breastfeeding at discharge | 588 (83.3) | 1290 (90.6) | 0.92 (0.88 to 0.96) |
| SNMIg | 44 (6.2) | 33 (2.3) | 2.66 (1.69 to 4.18)b |
| Severe perinatal morbidity and mortality indexh | 120 (17.0) | 113 (7.9) | 2.14 (1.66 to 2.75)b |
Abbreviations: HELLP, hemolysis, elevated liver enzymes, low platelet count; ICU, intensive care unit; SNMI, severe neonatal morbidity index.
SI conversion factors: To convert hemoglobin to grams per liter, multiply by 10.
Maternal morbidity and mortality index includes at least 1 of the following complications during pregnancy: vaginal bleeding, pregnancy-induced hypertension, preeclampsia, eclampsia, HELLP, preterm labor, infections requiring antibiotics or maternal death, admission to ICU, or referral for higher dependency care.
Models adjusted for country, month entering study, maternal age, and history of maternal morbidity (including diabetes, thyroid and other endocrine disorders, cardiac disease, hypertension, chronic respiratory disease, kidney disease, malaria, or tuberculosis).
Incidence rate ratio and 95% CI are reported.
β and 95% CI are reported.
Models for preterm birth adjusted for history of preterm birth, country, month entering study, maternal age, and history of maternal morbidity (including diabetes, thyroid and other endocrine disorders, cardiac disease, hypertension, chronic respiratory disease, kidney disease, malaria, or tuberculosis).
Against the international INTERGROWTH-21st Newborn Size Standards.22
SNMI includes at least 1 of the following morbidities: bronchopulmonary dysplasia, hypoxic-ischemic encephalopathy, sepsis, anemia requiring transfusion, patent ductus arteriosus, intraventricular hemorrhage, necrotizing enterocolitis, or retinopathy of prematurity.
Severe perinatal morbidity and mortality index includes any of the morbidities listed in the SNMI or intrauterine or neonatal death or neonatal ICU stay ≥7 days.
Eleven women (1.6%) with COVID-19 diagnosis died (maternal mortality ratio, 159/10 000 births). Of these, 4 had severe preeclampsia (1 superimposed on chronic hypertension and 1 associated with cardiomyopathy); 3 of these 4 women had respiratory failure that required mechanical ventilation and the fourth woman died of a pulmonary embolism. Five women had worsening respiratory failure antenatally, 2 of whom underwent cesarean delivery and, despite intensive respiratory support, died later. The remaining 2 women developed fever, cough, and breathlessness within 7 days of an uneventful delivery and died shortly after, despite ICU care. In the group of women without COVID-19 diagnosis, there was 1 death due to preexisting liver malignant neoplasm and cirrhosis. Thus, women with COVID-19 diagnosis were 22 times more likely to die (RR, 22.3; 95% CI, 2.88-172), although the CIs were wide owing to the small numbers (Table 1).
Overall, women with COVID-19 diagnosis had a lower rate of spontaneous initiation of labor but higher cesarean delivery rate, reflecting the higher rates of pregnancy complications in this group. They also had higher RRs for preterm birth and fetal distress of 1.59 (95% CI, 1.30-1.94) and 1.70 (95% CI, 1.06-2.75), respectively. Overall, 83% of preterm births (n = 130) in women with COVID-19 diagnosis were medically indicated; hence, the increased risk in this group (RR, 1.97; 95% CI, 1.56-2.51) (Table 1). The leading indications for preterm delivery among women with COVID-19 diagnosis were preeclampsia/eclampsia/HELLP (31 [24.7%]), small for gestational age (24 [15.5%]), and fetal distress (17 [13.2%]). The proportions of spontaneous preterm birth were similar. Women with COVID-19 diagnosis had a higher low birth weight rate (RR, 1.58; 95% CI, 1.29-1.94). The rates of prelabor rupture of membranes were similar in both groups (Table 1). Fully adjusting models of our primary outcomes for all planned variables reduced the sample size owing to missing data, but affected the results minimally (eTable 2 in the Supplement).
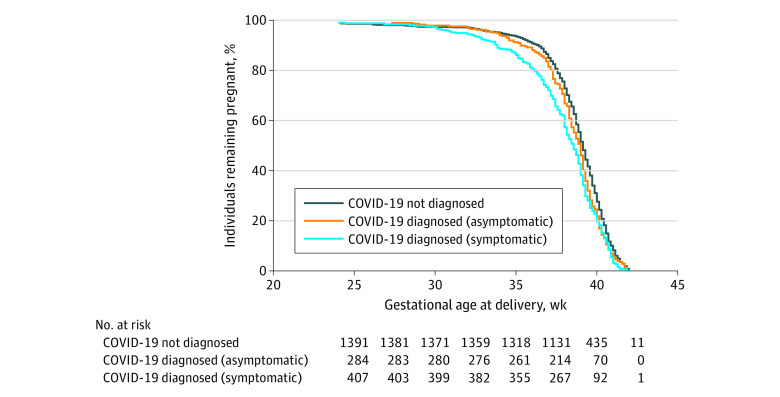
Women with COVID-19 diagnosis delivered earlier than those without COVID-19 diagnosis after approximately 30 weeks’ gestation, with the greatest difference less than 37 weeks’ gestation. The Figure illustrates the probability of remaining pregnant after 25 weeks’ gestation for those women with COVID-19 diagnosis, stratified into those with and without symptoms. Using the log-rank test for trend of survivor curves, we observed a significant downwards trend in the gestational age distributions that progressed from women without COVID-19 diagnosis, to asymptomatic women with COVID-19 diagnosis, to symptomatic women with COVID-19 diagnosis (P < .001 for this trend) (Figure). In regression models, the gestational age at delivery was 0.6 weeks shorter (95% CI, −0.9 to −0.3) in all women with COVID-19 diagnosis and 0.8 weeks shorter (95% CI, −1.2 to −0.5) in symptomatic women with COVID-19 diagnosis than in women without COVID-19 diagnosis.
Figure. Gestational Age at Delivery Among Women With COVID-19 Diagnosis, With and Without Symptoms, and Women Without COVID-19 Diagnosis.
There were 1420 women without COVID-19 diagnosis (dark blue). In the group of women with COVID-19 diagnosis, 417 women were symptomatic (light blue) and 288 women were asymptomatic (orange). There was a significant trend (P < .001) in shorter gestational age at delivery going from women without COVID-19 diagnosis, to asymptomatic women with COVID-19 diagnosis, to symptomatic women with COVID-19 diagnosis (log-rank test for trend of survivor curves). Five women with missing data were excluded from the figure.
The risk of the SNMI among neonates of women with COVID-19 diagnosis was significantly higher (RR, 2.66; 95% CI, 1.69-4.18) than in those of women without COVID-19 diagnosis. The risk of the SPMMI was more than twice as high in the group of women with COVID-19 diagnosis (RR, 2.14; 95% CI, 1.66-2.75) (Table 1).
A total of 416 neonates born to women with COVID-19 diagnosis were tested for SARS-CoV-2 (57.1% [n = 729] of the total mother with COVID-19 diagnosis/neonate dyads); 220 (51.5%) were tested in the first 24 hours after birth and 369 (84.8%) within the first 48 hours. Of these, 54 (12.9%) tested positive. Among test-positive women with test-positive neonates, the cesarean delivery rate was 72.2% (n = 39) and among test-positive women with test-negative neonates was 47.9% (n = 173). The rate in women without COVID-19 diagnosis was 39.4% (n = 568).
In regression models, exploring factors associated with neonatal SARS-CoV-2 positivity that included gestational age at delivery, cesarean delivery, NICU stay 7 days or longer, and exclusive breastfeeding at discharge, only cesarean delivery was independently associated with the risk of a test-positive neonate (RR, 2.15; 95% CI, 1.18-3.91). Reassuringly, there was no association between exclusive breastfeeding and neonatal test positivity (RR, 1.10; 95% CI, 0.66-1.85).
eTable 3 in the Supplement shows the type, number, and prevalence of symptoms reported. Overall, the presence of any symptoms increased the association with adverse outcomes compared with the group of women without COVID-19 diagnosis. Although asymptomatic women with COVID-19 diagnosis had limited risk for most outcomes, there was still an association between the disease and preeclampsia (RR, 1.63; 95% CI, 1.01-2.63). The presence of fever and shortness of breath, separately or in combination with any symptom cluster, was markedly associated with a risk of the 3 summary indices, as well as preterm birth (Table 2).
Table 2. Adjusted Associations for Maternal and Perinatal Outcomes Among Women With and Without COVID-19 Diagnosis According to Symptom Statusa.
| Symptom | No. (%) | RR (95% CI) | ||||
|---|---|---|---|---|---|---|
| MMMIb | SNMIc | SPMMId | Preterm birthe | Preeclampsia/eclampsia/HELLP | ||
| No diagnosis of COVID-19 | 1424 (66.9) | 1 [Reference] | 1 [Reference] | 1 [Reference] | 1 [Reference] | 1 [Reference] |
| COVID-19 | ||||||
| Asymptomatic | 288 (13.5) | 1.24 (1.00-1.54) | 1.42 (0.65-3.08) | 1.08 (0.69-1.69) | 0.99 (0.72-1.36) | 1.63 (1.01-2.63) |
| Any symptom | 418 (19.6) | 1.76 (1.49-2.08) | 3.45 (2.14-5.56) | 3.09 (2.36-4.04) | 2.10 (1.67-2.62) | 2.00 (1.34-2.99) |
| Symptomatic | ||||||
| With diarrhea/vomiting | 48 (2.3) | 1.36 (0.85-2.19) | 4.66 (1.93-11.30) | 2.79 (1.57-4.95) | 2.76 (1.77-4.30) | 0.48 (0.07-3.81) |
| With fever | 199 (9.3) | 1.89 (1.54-2.32) | 4.34 (2.53-7.43) | 3.81 (2.81-5.17) | 2.39 (1.82-3.13) | 1.82 (1.08-3.06) |
| With shortness of breath | 89 (4.2) | 2.46 (1.96-3.08) | 3.88 (1.78-8.49) | 3.86 (2.62-5.67) | 2.88 (2.12-3.89) | 2.72 (1.59-4.64) |
| With fever and shortness of breath | 45 (2.1) | 2.56 (1.92-3.40) | 4.97 (2.11-11.69) | 5.09 (3.30-7.86) | 3.40 (2.38-4.86) | 2.22 (1.06-4.64) |
Abbreviations: HELLP, hemolysis, elevated liver enzymes, low platelet count; MMMI, maternal morbidity and mortality index; RR, relative risk; SNMI, severe neonatal morbidity index; SPMMI, severe perinatal morbidity and mortality index.
All models adjusted for country, month entering study, maternal age, and history of maternal morbidity (including diabetes, thyroid and other endocrine disorders, cardiac disease, hypertension, chronic respiratory disease, kidney disease, malaria, or tuberculosis).
MMMI includes at least 1 of the following complications during pregnancy: vaginal bleeding, pregnancy-induced hypertension, preeclampsia, eclampsia, HELLP, preterm labor, infections requiring antibiotics or maternal death, admission to intensive care unit, or referral for higher dependency care.
SNMI includes at least 1 of the following morbidities: bronchopulmonary dysplasia, hypoxic-ischemic encephalopathy, sepsis, anemia requiring transfusion, patent ductus arteriosus, intraventricular hemorrhage, necrotizing enterocolitis, or retinopathy of prematurity.
SPMMI includes any of the morbidities listed in the SNMI, intrauterine or neonatal death, or neonatal intensive care unit stay ≥7 days.
Models for preterm birth also adjusted for history of preterm birth.
Having shortness of breath, chest pain, and cough with fever was associated with a substantial increase in the risk for severe maternal and neonatal conditions and preterm birth. However, it appears that a longer exposure to symptoms is needed to see an associated increase in risk for preeclampsia, eg, 5 to 10 days of respiratory symptoms (RR, 2.43; 95% CI, 1.29-4.58) (eTable 4 in the Supplement).
Among those with no prepregnancy morbidities and those without overweight at their first antenatal visit, women with COVID-19 diagnosis were still at increased risk of these complications compared with women without COVID-19 diagnosis. However, for maternal outcomes, those women with COVID-19 diagnosis with prepregnancy morbidities had the highest risk in the index pregnancy, suggesting that past morbidities modify the effect of COVID-19 exposure, especially for preeclampsia/eclampsia (RR, 3.29; 95% CI, 2.03-5.33) (Table 3). Women who had overweight at the first antenatal visit and subsequently were diagnosed with COVID-19 had the highest risk for the maternal morbidity and mortality index (RR, 1.81; 95% CI, 1.48-2.21), SNMI (RR, 4.15; 95% CI, 2.15-8.01), and SPMMI and preeclampsia/eclampsia (RR, 2.62; 95% CI, 1.57-4.36), suggesting that overweight status modifies the effect of COVID-19 exposure (Table 3).
Table 3. Adjusted Associations Between Preexisting Maternal Morbidity or Being Overweight Prepregnancy and Maternal and Neonatal Outcomes According to COVID-19 Diagnosisa,b.
| Maternal COVID-19 diagnosis | No. (%) | RR (95% CI) | ||||
|---|---|---|---|---|---|---|
| MMMIc | SNMId | SPMMIe | Preterm birthf | Preeclampsia/eclampsia/HELLP | ||
| Not diagnosed | ||||||
| No past morbidity | 1179 (55.4) | 1 [Reference] | 1 [Reference] | 1 [Reference] | 1 [Reference] | 1 [Reference] |
| Past morbidity | 245 (11.5) | 1.20 (0.92-1.54) | 3.04 (1.48-6.28) | 1.48 (0.95-2.29) | 1.73 (1.26-2.39) | 1.86 (1.11-3.12) |
| Diagnosed | ||||||
| No past morbidity | 547 (25.7) | 1.57 (1.33-1.85) | 4.02 (2.39-6.76) | 2.35 (1.76-3.13) | 1.76 (1.40-2.22) | 1.88 (1.24-2.86) |
| Past morbidity | 159 (7.5) | 1.71 (1.33-2.20) | 1.88 (0.74-4.73) | 2.29 (1.50-3.51) | 1.96 (1.41-2.73) | 3.29 (2.03-5.33) |
| Not diagnosed | ||||||
| Normal weight | 823 (40.3) | 1 [Reference] | 1 [Reference] | 1 [Reference] | 1 [Reference] | 1 [Reference] |
| Overweight | 554 (27.1) | 1.01 (0.81-1.24) | 1.56 (0.76-3.20) | 1.14 (0.78-1.67) | 0.78 (0.59-1.05) | 1.37 (0.82-2.30) |
| Diagnosed | ||||||
| Normal weight | 342 (16.8) | 1.28 (1.03-1.58) | 2.07 (0.99-4.31) | 1.99 (1.38-2.88) | 1.42 (1.07-1.90) | 1.80 (1.06-3.07) |
| Overweight | 323 (15.8) | 1.81 (1.48-2.21) | 4.15 (2.15-8.01) | 2.44 (1.72-3.48) | 1.43 (1.08-1.85) | 2.62 (1.57-4.36) |
Abbreviations: HELLP, Hemolysis, elevated liver enzymes, low platelet count; MMMI, maternal morbidity and mortality index; RR, relative risk; SNMI, severe neonatal morbidity index; SPMMI, severe perinatal morbidity and mortality index.
All models adjusted for country, month entering study, maternal age, and history of maternal morbidity (including diabetes, thyroid and other endocrine disorders, cardiac disease, hypertension, chronic respiratory disease, kidney disease, malaria, or tuberculosis).
Prepregnancy maternal morbidities included at least 1 of the following: diabetes, thyroid and other endocrine disorders, cardiac disease, hypertension, chronic respiratory disease, kidney disease, malaria, or tuberculosis.
MMMI includes at least 1 of the following complications during pregnancy: vaginal bleeding, pregnancy-induced hypertension, preeclampsia, eclampsia, HELLP, preterm labor, infections requiring antibiotics or maternal death, admission to intensive care unit, or referral for higher dependency care.
SNMI includes at least 1 of the following morbidities: bronchopulmonary dysplasia, hypoxic-ischemic encephalopathy, sepsis, anemia requiring transfusion, patent ductus arteriosus, intraventricular hemorrhage, necrotizing enterocolitis, or retinopathy of prematurity.
SPMMI includes any of the morbidities listed in the SNMI, intrauterine or neonatal death, or neonatal intensive care unit stay ≥7 days.
Models for preterm birth also adjusted for history of preterm birth.
We also compared the risk for severe neonatal complications in test-positive and test-negative neonates of women with and without COVID-19 diagnosis, the latter as a reference group. The risks for the SNMI, SPMMI, and NICU stay 7 days or longer were higher in the test-negative neonates of women with COVID-19 diagnosis. However, the test-positive neonates of women with COVID-19 diagnosis had considerably higher risk for the SPMMI and, as expected, a large increased risk for a NICU stay of 7 days or longer (Table 4).
Table 4. Adjusted Associations Between Maternal and Neonatal COVID-19 Diagnosis With Perinatal Morbidity and Mortalitya.
| Maternal and neonatal COVID-19 diagnosis | No. (%) | RR (95% CI) | ||
|---|---|---|---|---|
| SNMIb | SPMMIc | NICU stay ≥7 d | ||
| Not-diagnosed mother and neonate | 1462 (66.7) | 1 [Reference] | 1 [Reference] | 1 [Reference] |
| Diagnosed mother but neonate not tested | 313 (14.3) | 1.40 (0.72-2.70) | 1.68 (1.20-2.37) | 1.02 (0.60-1.83) |
| Diagnosed mother but test-negative neonate | 362 (16.5) | 4.00 (2.29-6.97) | 2.31 (1.69-3.17) | 3.13 (2.10-4.65) |
| Diagnosed mother and test-positive neonate | 54 (2.5) | 4.13 (1.69-10.08) | 3.46 (2.13-5.63) | 6.03 (3.35-10.86) |
Abbreviations: NICU, neonatal intensive care unit; RR, relative risk; SNMI, severe neonatal morbidity index; SPMMI, severe perinatal morbidity and mortality index.
Models adjusted for country, month entering study, maternal age, and history of maternal morbidity (including diabetes, thyroid and other endocrine disorders, cardiac disease, hypertension, chronic respiratory disease, kidney disease, malaria, or tuberculosis).
SNMI includes at least 1 of the following morbidities: bronchopulmonary dysplasia, hypoxic-ischemic encephalopathy, sepsis, anemia requiring transfusion, patent ductus arteriosus, intraventricular hemorrhage, necrotizing enterocolitis, or retinopathy of prematurity.
SPMMI includes any of the morbidities listed in the SNMI, intrauterine or neonatal death, or NICU stay ≥7 days.
In separate sensitivity analyses, adjusting for additional confounders (marital status, overweight, smoking and drug use during pregnancy), restricting mothers with COVID-19 diagnosis to laboratory-confirmed positive results and excluding twins, we found that relative risks for COVID-19–associated maternal and neonatal morbidities were similar to our main results (eTable 2 in the Supplement). Furthermore, we treated each country (all hospitals in a country pooled) as if they were separate studies in a meta-analysis. The pooled estimated RRs (95% CI) were practically identical to the unadjusted and adjusted estimations, except for the SNMI, which was increased from the unadjusted RR of 2.69 (95% CI, 1.72-4.20) to 2.91 (95% CI, 1.76-4.74). As expected, considering the variability of underlying populations and care systems, we identified some heterogeneity of RR estimates across countries in the meta-analysis for the maternal morbidity and mortality index (I2 = 50.0%; P = .02) and SPMMI (I2 = 57.4%; P = .005), although there was no systematic pattern among the countries.
Discussion
We conducted a large-scale, prospective, multinational study to assess the symptoms and associations between COVID-19 in pregnancy and maternal and neonatal outcomes that included, to our knowledge for the first time, immediately concomitant pregnant women without COVID-19 diagnosis from the same populations, carefully enrolled to minimize selection bias.
We demonstrated that women with COVID-19 diagnosis, compared with those without COVID-19 diagnosis, were at substantially increased risk of severe pregnancy complications, including preeclampsia/eclampsia/HELLP syndrome, ICU admission or referral to higher level of care, and infections requiring antibiotics, as well as preterm birth and low birth weight. The risk of maternal mortality was 1.6%, ie, 22 times higher in the group of women with COVID-19 diagnosis. These deaths were concentrated in institutions from less developed regions, implying that when comprehensive ICU services are not fully available, COVID-19 in pregnancy can be lethal. Reassuringly, we also found that asymptomatic women with COVID-19 diagnosis had similar outcomes to women without COVID-19 diagnosis, except for preeclampsia.
Importantly, women with COVID-19 diagnosis, already at high risk of preeclampsia and COVID-19 because of preexisting overweight, diabetes, hypertension, and cardiac and chronic respiratory diseases,28 had almost 4 times greater risk of developing preeclampsia/eclampsia, which could reflect the known association with these comorbidities and/or the acute kidney damage that can occur in patients with COVID-19.29
Our data support reports of an association between COVID-19 and higher rates of preeclampsia/eclampsia/HELLP syndrome,19,30 but it is still uncertain whether COVID-19 manifests in pregnancy with a preeclampsialike syndrome or infection with SARS-CoV-2 results in an increased risk for preeclampsia. Uncertainty persists because the placentas of women with COVID-19, compared with controls, show vascular changes consistent with preeclampsia,31 but the state of systemic inflammation and hypercoagulability found in nonpregnant patients with severe illness and COVID-19 is also a feature of preeclampsia.32
It is known that in nonpregnant patients, distinct subtypes may be predictive of clinical outcomes.33 We found the presence of any COVID-19 symptoms was associated with increased morbidity and mortality. Specifically, severe pregnancy and neonatal complication rates were highest in women if fever and shortness of breath were present, reflecting systemic disease; their presence for 1 to 4 days was associated with severe maternal and neonatal complications. This observation should influence clinical care and referral strategies.
The risks of severe neonatal complications, including NICU stay for 7 days or longer, as well as the summary index of severe neonatal morbidity and its individual components, were also substantially higher in the group of women with COVID-19 diagnosis. The increased neonatal risk remained after adjusting for previous preterm birth and preterm birth in the index pregnancy; thus, a direct effect on the newborn from COVID-19 is likely.
Overall, our results were consistent across morbidities and mostly at an RR near or greater than 2 for maternal and neonatal outcomes, with narrow CIs excluding unity, and above 3 to 4 in several estimates. Sensitivity and stratified analyses confirmed the observed results. They are probably conservative because overall, 41% of women with COVID-19 diagnosis were asymptomatic, a subgroup with a low risk of complications. Hence, higher morbidity and mortality risk should be expected for the general pregnant population, especially in low- to middle-income countries.
We found 12.1% of neonates born to test-positive women also tested positive, a higher figure than in a recent systematic review.34 We speculate whether contamination at the time of cesarean delivery was responsible because the rate in this mother/neonate positive subgroup was 72.2%. Reassuringly, as SARS-CoV-2 has not been isolated from breast milk,35 breastfeeding was not associated with any increase in the rate of test-positive neonates.
Our results mostly reflect COVID-19 diagnosed in the third trimester. Thus, women with COVID-19 diagnosis or whose pregnancy ended earlier in pregnancy are underrepresented either because our study was exclusively hospital based or earlier infection may manifest with mild symptoms, which are either ignored or managed in primary care. Alternatively, most women might have avoided the hospital until late in pregnancy or when in labor. Clearly, the effect of COVID-19 early in pregnancy needs urgently to be studied.
Limitations
Our study has expected limitations. Ideally, we would have collected data prospectively from all pregnancies in the participating institutions, but this was impractical because of their large number of deliveries. There was a small risk of selection bias associated with the reference group of women without COVID-19 diagnosis, despite all efforts to ensure they represented an unbiased sample of the general noninfected pregnant population. The selection of cases with COVID-19 diagnosis was affected by whether routine testing was conducted, awareness of COVID-19 symptoms particularly early in the pandemic, and the availability of test kits. Where universal testing in pregnancy has been introduced, real-time polymerase chain reaction positive rates are 0.5% to 14% in asymptomatic women.36,37 Hence, this group of women without COVID-19 diagnosis may have included small numbers of asymptomatic infected women (a crossover effect when women without COVID-19 diagnosis were enrolled antenatally), which would result in more conservative estimates by reducing the differences between groups. Finally, we acknowledge a risk of reporting bias relating to maternal and neonatal morbidity because women with COVID-19 diagnosis and their newborns may have been more carefully evaluated, tested, and have more events reported than in the sample of women without COVID-19 diagnosis. However, we are reassured that the results reflect a true increased risk because of our careful data monitoring and use of severe morbidity markers.
Conclusions
In summary, in this study, COVID-19 infection during pregnancy was associated with substantial risk of morbidity and mortality in postpartum parents and their infants worldwide, compared with their not-infected pregnant counterparts, especially if the these individuals were symptomatic or have comorbidities. There is an urgent need to follow up with these parents and infants because of possible long-term health effects, including long-term COVID-19.
eBox. Real Time Polymerase Chain Reaction (antigen) tests and antibody tests for SARS-Cov-2 used in the participating centers
eFigure. Enrollment and Covid-19 ‘exposure’ status for women participating in the INTERCOVID Study
eTable 1. Baseline characteristics among women with and without Covid-19 diagnosis
eTable 2. Comparisons of unadjusted and fully adjusted models for maternal and neonatal outcomes
eTable 3. Distribution and duration of symptoms at the time of enrolment among women with Covid-19 diagnosis
eTable 4. Associations between Covid-19 symptom duration and maternal and neonatal outcomes among women with and without COVID-19 diagnosis and their newborns
eAppendix 1. Non-Author Contributors
eAppendix 2. Contributors and Members of the International Study on the Effects of Covid-19 in pregnancy on maternal and newborn outcomes in the INTERGROWTH-21st global network (The INTERCOVID Study)
References
- 1.Wu Y, Zhang C, Liu H, et al. Perinatal depressive and anxiety symptoms of pregnant women during the coronavirus disease 2019 outbreak in China. Am J Obstet Gynecol. 2020;223(2):240.e1-240.e9. doi: 10.1016/j.ajog.2020.05.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Davenport MH, Meyer S, Meah VL, Strynadka MC, Khurana R. Moms are not OK: COVID-19 and maternal mental health. Front Glob Womens Health. Published online June 19, 2020. doi: 10.3389/fgwh.2020.00001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yap M, Debenham L, Kew T, et al. ; PregCOV-19 Consortium . Clinical manifestations, prevalence, risk factors, outcomes, transmission, diagnosis and treatment of COVID-19 in pregnancy and postpartum: a living systematic review protocol. BMJ Open. 2020;10(12):e041868. doi: 10.1136/bmjopen-2020-041868 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Campbell KH, Tornatore JM, Lawrence KE, et al. Prevalence of SARS-CoV-2 among patients admitted for childbirth in southern Connecticut. JAMA. 2020;323(24):2520-2522. doi: 10.1001/jama.2020.8904 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Knight M, Bunch K, Vousden N, et al. ; UK Obstetric Surveillance System SARS-CoV-2 Infection in Pregnancy Collaborative Group . Characteristics and outcomes of pregnant women admitted to hospital with confirmed SARS-CoV-2 infection in UK: national population based cohort study. BMJ. 2020;369:m2107. doi: 10.1136/bmj.m2107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Li N, Han L, Peng M, et al. Maternal and neonatal outcomes of pregnant women with coronavirus disease 2019 (COVID-19) pneumonia: a case-control study. Clin Infect Dis. 2020;71(16):2035-2041. doi: 10.1093/cid/ciaa352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Liao J, He X, Gong Q, Yang L, Zhou C, Li J. Analysis of vaginal delivery outcomes among pregnant women in Wuhan, China during the COVID-19 pandemic. Int J Gynaecol Obstet. 2020;150(1):53-57. doi: 10.1002/ijgo.13188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Alfaraj SH, Al-Tawfiq JA, Memish ZA. Middle East Respiratory Syndrome Coronavirus (MERS-CoV) infection during pregnancy: report of two cases & review of the literature. J Microbiol Immunol Infect. 2019;52(3):501-503. doi: 10.1016/j.jmii.2018.04.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.World Medical Association . World Medical Association Declaration of Helsinki: ethical principles for medical research involving human subjects. JAMA. 2013;310(20):2191-2194. doi: 10.1001/jama.2013.281053 [DOI] [PubMed] [Google Scholar]
- 10.The Global Health Network. INTERCOVID. Accessed April 5, 2021. https://intergrowth21.tghn.org/intercovid/
- 11.Shi H, Han X, Jiang N, et al. Radiological findings from 81 patients with COVID-19 pneumonia in Wuhan, China: a descriptive study. Lancet Infect Dis. 2020;20(4):425-434. doi: 10.1016/S1473-3099(20)30086-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bauchner H, Golub RM, Zylke J. Editorial concern-possible reporting of the same patients with COVID-19 in different reports. JAMA. 2020;323(13):1256. doi: 10.1001/jama.2020.3980 [DOI] [PubMed] [Google Scholar]
- 13.Kayem G, Lecarpentier E, Deruelle P, et al. A snapshot of the Covid-19 pandemic among pregnant women in France. J Gynecol Obstet Hum Reprod. 2020;49(7):101826. doi: 10.1016/j.jogoh.2020.101826 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sentilhes L, De Marcillac F, Jouffrieau C, et al. Coronavirus disease 2019 in pregnancy was associated with maternal morbidity and preterm birth. Am J Obstet Gynecol. 2020;223(6):914.e1-914.e15. doi: 10.1016/j.ajog.2020.06.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Maraschini A, Corsi E, Salvatore MA, Donati S, It OSSC-WG; ItOSS COVID-19 Working Group . Coronavirus and birth in Italy: results of a national population-based cohort study. Ann Ist Super Sanita. 2020;56(3):378-389. [DOI] [PubMed] [Google Scholar]
- 16.Ferrazzi E, Beretta P, Bianchi S, et al. SARS-CoV-2 infection testing at delivery: a clinical and epidemiological priority. J Matern Fetal Neonatal Med. 2020;1-3. doi: 10.1080/14767058.2020.1788532 [DOI] [PubMed] [Google Scholar]
- 17.Collaborative CO; COVIDSurg Collaborative . Mortality and pulmonary complications in patients undergoing surgery with perioperative SARS-CoV-2 infection: an international cohort study. Lancet. 2020;396(10243):27-38. doi: 10.1016/S0140-6736(20)31182-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ochiai D, Kasuga Y, Iida M, Ikenoue S, Tanaka M. Universal screening for SARS-CoV-2 in asymptomatic obstetric patients in Tokyo, Japan. Int J Gynaecol Obstet. 2020;150(2):268-269. doi: 10.1002/ijgo.13252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mendoza M, Garcia-Ruiz I, Maiz N, et al. Pre-eclampsia-like syndrome induced by severe COVID-19: a prospective observational study. BJOG. 2020;127(11):1374-1380. doi: 10.1111/1471-0528.16339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lokken EM, Walker CL, Delaney S, et al. Clinical characteristics of 46 pregnant women with a severe acute respiratory syndrome coronavirus 2 infection in Washington State. Am J Obstet Gynecol. 2020;223(6):911.e1-911.e14. doi: 10.1016/j.ajog.2020.05.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Onwuzurike C, Diouf K, Meadows AR, Nour NM. Racial and ethnic disparities in severity of COVID-19 disease in pregnancy in the United States. Int J Gynaecol Obstet. 2020;151(2):293-295. doi: 10.1002/ijgo.13333 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Goldfarb IT, Diouf K, Barth WH, et al. Universal SARS-CoV-2 testing on admission to the labor and delivery unit: Low prevalence among asymptomatic obstetric patients. Infect Control Hosp Epidemiol. 2020;41(9):1095-1096. doi: 10.1017/ice.2020.255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Docherty AB, Harrison EM, Green CA, et al. ; ISARIC4C investigators . Features of 20 133 UK patients in hospital with covid-19 using the ISARIC WHO Clinical Characterisation Protocol: prospective observational cohort study. BMJ. 2020;369:m1985. doi: 10.1136/bmj.m1985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Villar J, Valladares E, Wojdyla D, et al. ; WHO 2005 global survey on maternal and perinatal health research group . Caesarean delivery rates and pregnancy outcomes: the 2005 WHO global survey on maternal and perinatal health in Latin America. Lancet. 2006;367(9525):1819-1829. doi: 10.1016/S0140-6736(06)68704-7 [DOI] [PubMed] [Google Scholar]
- 25.Papageorghiou AT, Kennedy SH, Salomon LJ, et al. ; International Fetal and Newborn Growth Consortium for the 21st Century (INTERGROWTH-21st) . International standards for early fetal size and pregnancy dating based on ultrasound measurement of crown-rump length in the first trimester of pregnancy. Ultrasound Obstet Gynecol. 2014;44(6):641-648. doi: 10.1002/uog.13448 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Villar J, Cheikh Ismail L, Victora CG, et al. ; International Fetal and Newborn Growth Consortium for the 21st Century (INTERGROWTH-21st) . International standards for newborn weight, length, and head circumference by gestational age and sex: the Newborn Cross-Sectional Study of the INTERGROWTH-21st Project. Lancet. 2014;384(9946):857-868. doi: 10.1016/S0140-6736(14)60932-6 [DOI] [PubMed] [Google Scholar]
- 27.Ohuma EO, Hoch L, Cosgrove C, et al. ; International Fetal and Newborn Growth Consortium for the 21st Century . Managing data for the international, multicentre INTERGROWTH-21st Project. BJOG. 2013;120(suppl 2):64-70, v. doi: 10.1111/1471-0528.12080 [DOI] [PubMed] [Google Scholar]
- 28.Savasi VM, Parisi F, Patanè L, et al. Clinical findings and disease severity in hospitalized pregnant women with coronavirus disease 2019 (COVID-19). Obstet Gynecol. 2020;136(2):252-258. doi: 10.1097/AOG.0000000000003979 [DOI] [PubMed] [Google Scholar]
- 29.Ronco C, Reis T, Husain-Syed F. Management of acute kidney injury in patients with COVID-19. Lancet Respir Med. 2020;8(7):738-742. doi: 10.1016/S2213-2600(20)30229-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Di Mascio D, Khalil A, Saccone G, et al. Outcome of coronavirus spectrum infections (SARS, MERS, COVID-19) during pregnancy: a systematic review and meta-analysis. Am J Obstet Gynecol MFM. 2020;2(2):100107. doi: 10.1016/j.ajogmf.2020.100107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shanes ED, Mithal LB, Otero S, Azad HA, Miller ES, Goldstein JA. Placental pathology in COVID-19. Am J Clin Pathol. 2020;154(1):23-32. doi: 10.1093/ajcp/aqaa089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Narang K, Enninga EAL, Gunaratne MDSK, et al. SARS-CoV-2 infection and COVID-19 during pregnancy: a multidisciplinary review. Mayo Clin Proc. 2020;95(8):1750-1765. doi: 10.1016/j.mayocp.2020.05.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sudre CH, Lee KA, Ni Lochlainn M, et al. Symptom clusters in Covid19: a potential clinical prediction tool from the COVID Symptom study app. medRxiv. Preprint posted online June 16, 2020. doi: 10.1101/2020.06.12.20129056 [DOI]
- 34.Walker KF, O’Donoghue K, Grace N, et al. Maternal transmission of SARS-COV-2 to the neonate, and possible routes for such transmission: a systematic review and critical analysis. BJOG. 2020;127(11):1324-1336. doi: 10.1111/1471-0528.16362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chambers C, Krogstad P, Bertrand K, et al. Evaluation for SARS-CoV-2 in breast milk from 18 infected women. JAMA. 2020;324(13):1347-1348. doi: 10.1001/jama.2020.15580 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sutton D, Fuchs K, D’Alton M, Goffman D. Universal screening for SARS-CoV-2 in women admitted for delivery. N Engl J Med. 2020;382(22):2163-2164. doi: 10.1056/NEJMc2009316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fassett MJ, Lurvey LD, Yasumura L, et al. Universal SARS-Cov-2 screening in women admitted for delivery in a large managed care organization. Am J Perinatol. 2020;37(11):1110-1114. doi: 10.1055/s-0040-1714060 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eBox. Real Time Polymerase Chain Reaction (antigen) tests and antibody tests for SARS-Cov-2 used in the participating centers
eFigure. Enrollment and Covid-19 ‘exposure’ status for women participating in the INTERCOVID Study
eTable 1. Baseline characteristics among women with and without Covid-19 diagnosis
eTable 2. Comparisons of unadjusted and fully adjusted models for maternal and neonatal outcomes
eTable 3. Distribution and duration of symptoms at the time of enrolment among women with Covid-19 diagnosis
eTable 4. Associations between Covid-19 symptom duration and maternal and neonatal outcomes among women with and without COVID-19 diagnosis and their newborns
eAppendix 1. Non-Author Contributors
eAppendix 2. Contributors and Members of the International Study on the Effects of Covid-19 in pregnancy on maternal and newborn outcomes in the INTERGROWTH-21st global network (The INTERCOVID Study)