Abstract
Background:
The Center for Disease Control (CDC) recently named childhood abuse histories as a public health risk. Clear links between abuse histories and inflammation exist. However, it remains unknown how abuse histories impact inflammatory trajectories throughout adulthood. Accordingly, this study assessed inflammatory trajectories across three visits among healthy adults with and without abuse histories.
Method:
In this secondary analysis of data from a longitudinal observational study of cancer survivors and noncancer controls, 157 noncancer controls (Mage = 55.8, range = 32 – 83) completed the Childhood Experiences Questionnaire (CTQ), providing data on physical, emotional, and sexual abuse prior to age 18. Cytokines interleukin-6 (IL-6), interleukin 1-beta (IL-1β), and tumor necrosis factor-alpha (TNF-α) were collected at the baseline visit and two follow-up visits approximately one (M months = 11.52, SD = 4.10) and two years (M months = 23.79, SD = 4.40) later. To represent inflammatory changes, cytokine data at each visit were combined into a composite z-score. . Covariates in all analyses included age, biological sex, race, income, body mass index, menopause status, psychological diagnosis history, and medical comorbidities.
Results:
Compared to their nonabused peers, those who had experienced any type of abuse in childhood demonstrated steeper rises in inflammation across time. Inflammation rose more steeply for individuals with physical and emotional abuse histories compared to those without such histories.
Conclusion:
Overall, these data suggest that childhood abuse histories may quicken age-related increases in inflammation, contributing to accelerated aging, morbidity, and early mortality. These findings provide mechanistic insight into why child abuse is a public health risk.
Keywords: abuse, inflammation, childhood trauma, health
1. Introduction
Childhood trauma poses a significant threat to physical and psychological health because it occurs during a developmentally sensitive period. Trauma can dysregulate multiple stress-sensitive systems, including immune function. (Andrea Danese & McEwen, 2012). The Center for Disease Control (CDC) recently named child abuse as a serious public health risk (CDC, 2019). Approximately one in seven children experiences child abuse each year, and the total lifetime economic burden of childhood abuse is approximately $124 billion dollars per year (CDC, 2019).
The biological embedding hypothesis emphasizes that childhood adversity can lead to sustained physiological changes, thus putting an abuse survivor at an increased risk for poor health throughout adulthood (A Danese et al., 2011; Miller, Chen, & Parker, 2011). Childhood adversity is associated with many long-term health problems throughout adulthood, including cardiopulmonary symptoms, chronic pain, obesity, gastrointestinal issues, and lower self-rated health (Sachs-Ericsson, Blazer, Plant, & Arnow, 2005; Wegman & Stetler, 2009). In conjunction with the clearly identified risks to physical health, abuse histories predict depressive, anxiety, post-traumatic stress, and substance use disorders in adulthood (Green et al., 2010; McLaughlin et al., 2010; Scott et al., 2011).
Inflammation increases the risk for many chronic health problems and mortality (Miller et al., 2011). The association between inflammation and childhood abuse histories has been well-documented. For example, people who experienced childhood abuse had greater IL-6 reactivity to acute laboratory stressors compared to people who did not have such histories (Gouin, Glaser, Malarkey, Beversdorf, & Kiecolt-Glaser, 2012). Meta-analytic evidence suggests that adults who were abused as children have higher levels of C-reactive protein (CRP), interleukin-6 (IL-6), and tumor necrosis factor alpha (TNF-α) (Baumeister, Akhtar, Ciufolini, Pariante, & Mondelli, 2016; Tursich et al., 2014). Higher numbers of adverse childhood experiences, including abuse, were associated with increased CRP as well (Iob & Steptoe, 2019).
Despite evidence linking childhood abuse and inflammation, few longitudinal studies have addressed inflammatory trajectories among adults who experienced abuse. Among healthy older adults caring for family members with dementia, those who had experienced childhood abuse had higher IL-6 and TNF-α levels compared with those who were not abused (Kiecolt-Glaser et al., 2011). One longitudinal, prospective study found that adults who experienced abuse in childhood had higher CRP levels at 32 years old compared to those who did not. Childhood abuse histories predicted 10% of adult inflammation (Andrea Danese, Pariante, Caspi, Taylor, & Poulton, 2007). Examining how childhood adversity impacts inflammatory trajectories throughout adulthood will enhance our understanding of how abuse may accelerate aging, morbidity, and mortality (Franceschi et al., 2007).
The current study assessed inflammatory trajectories across three study visits among noncancer controls who participated in a larger longitudinal study. We hypothesized that physical, sexual, and emotional abuse histories would predict steeper increases in their inflammation across the three visits compared to those without such histories.
2. Method
2.1. Participants and procedure
Participants (N = 157) were noncancer controls in a parent longitudinal observational study. Noncancer controls had an initial abnormal breast or colorectal cancer test followed by a benign diagnosis. Table 1 presents the baseline characteristics of the noncancer controls. The sample was 55.8 years old on average, 80% female, and predominately white (83%). The majority were married or in a domestic partnership (65%) and were employed full-time or part-time (60%).
Table 1.
Subject characteristics
| Characteristics/Measure | N | Mean ± SD (median, range) or n (%) |
|---|---|---|
| Age | 157 | 55.8 ± 10.2 (56, 32 – 83) |
| BMI | 157 | 29.0 ± 6.8 (28.1, 17.9 – 55.0) |
| Sex | 157 | |
| Male | 32 (20%) | |
| Female | 125 (80%) | |
| Race | 157 | |
| White | 131 (83%) | |
| Black | 22 (14%) | |
| Asian | 3 (1.9%) | |
| Other | 1 (.6%) | |
| Education | 156 | |
| High school or less | 36 (23%) | |
| Some college | 32 (22%) | |
| College Grade | 46 (29%) | |
| Postgraduate | 42 (27%) | |
| Marital Status | 155 | |
| Single | 16 (10%) | |
| Married/Domestic partner | 101 (65%) | |
| Separated/Divorced/Widowed | 38 (25%) | |
| Employment Status | 155 | |
| Full time/Part time employed | 93 (60%) | |
| Retired | 35 (23%) | |
| Disabled | 13 (8.4%) | |
| Unemployed | 14 (9.0%) | |
| Charlson Comorbidity Index | 157 | .53 ± .93 (0, 0–5) |
| Psychological disorder | 155 | |
| No | 97 (63%) | |
| Yes | 58 (37%) | |
| Female Menopausal Status | 124 | |
| No | 37 (30%) | |
| Yes | 87 (70%) | |
| Race | 157 | |
| White | 131 (83%) | |
| Non-white | 26 (17%) | |
| Income | 155 | |
| <10k | 13 (8%) | |
| 10k to <25k | 15 (10%) | |
| 25k to <50k | 28 (18%) | |
| 50k to <75k | 31 (20%) | |
| 75k to <100k | 20 (13%) | |
| >100k | 28 (18%) | |
| Prefer not to answer | 20 (13%) | |
| ln(TNF-α) | 156 | 2.0 ± .55 (1.97, .64 – 3.56) |
| ln(IL-6) | 154 | .65 ± .88 (.60, −1.08 – 4.84) |
| ln(IL-1β) | 121 | −.47 ± .95 (−.48, −1.90 – 2.10) |
| Serum cytokine z-score | 120 | 0 ± 2.46 (.13, −5.94 – 7.81) |
| History of emotional abuse | 154 | 31 (20%) |
| History of physical abuse | 157 | 28 (18%) |
| History of sexual abuse | 156 | 24 (15%) |
| History of any form of abuse | 153 | 49 (32%) |
Note. SD = standard deviation; BMI = body mass index; ln = log transformed; TNF-α = tumor necrosis factor alpha; IL-6 = interleukin 6; IL-1β = interleukin 1-beta.
At a baseline visit and at two follow-up visits approximately one (M months = 11.52, SD = 4.10) and two years (M months = 23.79, SD = 4.40) later, participants completed questionnaires and provided blood samples. The Ohio State University Institutional Review Board approved the project and all participants provided written informed consent.
2.2. Self-report measures
The Childhood Trauma Questionnaire (CTQ), a widely used 28-item self-report questionnaire, retrospectively assesses abuse and neglect experienced prior to age 18 (Bernstein et al., 1994). The CTQ includes questions related to physical (e.g., “people in my family hit me so hard that is left me with bruises or marks”), emotional (e.g., “people in my family said hurtful or insulting things to me”), and sexual abuse (e.g., “someone tried to touch me in a sexual way or tried to make me touch them”). Participants responded on a 5-point Likert scale ranging from “never true” to “very often true” about childhood experiences. The CTQ demonstrates strong internal consistency and reliability across a range of samples worldwide (Viola et al., 2016).
The high-sensitivity cut-off scores for the CTQ were used to identify individuals reporting emotional (10+), physical (8+), or sexual abuse (8+) respectively (Walker et al., 1999). Additionally, individuals were classified as reporting any abuse if they scored above the threshold on one or more types of abuse. Dichotomous variables were used the four abuse categories (yes/no if someone experienced abuse). Participants completed the CTQ at their second visit.
2.3. Inflammation assays
Fasting blood samples were collected between 7:00 and 9:00 AM to control for diurnal variation. Serum-derived tumor necrosis factor-α (TNF-α), interleukin-6 (IL-6), and interleukin-1β (IL-1β) were measured by using an electrochemiluminescence method with Meso Scale Discovery kits. Sensitivity was .3 pg/mL, .4 pg/mL, and .2 pg/mL for TNF-α, IL-6, and IL-1β, respectively. The intra-assay and inter-assay CVs for TNF-α were 4.32% and 5.30%, respectively; corresponding values were 1.43% and 4.42% for IL-6 and 4.15% and 4.03% for IL-1β. Each participant’s frozen samples were assayed for all inflammatory markers at once, thus using the same controls for all time points for each person. Noncancer controls and cancer patient samples were mixed on the same plate.
2.4. Covariates
All analyses controlled for physical comorbidities, menopause status (yes/no), body mass index (BMI), age, income, psychiatric disorder history, and biological sex. The widely used Charlson comorbidity index provided data on physical comorbidities (Charlson, Szatrowski, Peterson, & Gold, 1994). The measure uses participants’ self-reported health information to assign weights to 19 medical conditions (range, 1 to 37), with greater scores equal to greater comorbidity burden. Psychiatric disorder history was measured using the Structured Clinical Interview for DSM Disorders (SCID) (First, Spitzer, Gibbon, & Williams, 2002).
2.5. Statistical methods
Analysis Sample.
The parent study enrolled a total of 198 control subjects. Abuse history was measured at the second visit, thus participants in the parent study who did not complete the second visit (n=29) were excluded from the analysis sample. An additional 11 participants were excluded who had incomplete cytokine data at all three study visits and thus could not have the z-score composite calculated. Finally, one subject did not have BMI data and was thus excluded. The remaining 157 did not significantly differ from the excluded subjects (n = 41) in terms of age (p = .46), BMI (p = .14), number of comorbidities (p = .96), or biological sex (p = .41). One hundred and twenty participants provided cytokine data at Visit 1, 119 at Visit 2, and 67 at Visit 3. Additionally, there were not significant differences between included and excluded subjects on TNF-α, IL-6, or IL-1 β at visit 1 (p > .20 for all).
Analysis Methods.
Linear mixed effects models were used to test for differences in the trajectories over time of cytokines between subjects who had abuse history and those who did not. Separate models were conducted for each abuse category (emotional, physical, sexual, and any abuse history). Importantly, these models allow participants with incomplete cytokine data at one or two visits to still be included. Fixed effects included visit (categorical), abuse history, and their interaction. Random effects included a subject-specific random intercept, accounting for within-subject correlation, as well as an assay-plate specific random intercept, accounting for the variability between assay plates. Age, BMI, physical comorbidities, and biological sex were adjusted for in all analyses. Serum cytokine values (TNF-α, IL-6, IL-1β) were natural log transformed to better approximate normality of residuals and values more than three standard deviations above the mean were excluded from analysis. To capture overall inflammation, we also created a composite z-score combining the natural log transformed IL-6, TNF-α, and IL-1β variables. This method was previously used as a robust predictor of inflammation (Alfano et al., 2017; Liu et al., 2017). Specifically, each log-transformed cytokine was standardized using the visit 1 mean and standard deviation, and these three z-scores were summed for each subject to create the composite. The z-score composite had a mean of zero and standard deviation of 2.5 at baseline. Thus the increase across visits of +1 units on the z-score composite represents 0.4 SD increase in inflammation. The Kenward-Roger adjustment to the degrees of freedom was used to control Type 1 error rates. A two-sided significance level of α = .05 was used for all tests. All analyses were carried out in SAS version 9.4 (SAS institute, Cary, NC).
3. Results
3.1. Baseline rates of abuse
Twenty percent of the sample had a history of emotional abuse, 18% had a history of physical abuse, 15% a had history of sexual abuse, and 32% of the sample had a history of any form of emotional, physical, or sexual abuse.
3.2. Inflammatory change Based on Abuse History
Table 2 presents the means and standard deviations of the inflammatory composite at each visit. Table 3 presents correlations between all predictors, covariates, and the inflammatory composite variable. Figure 1 shows the inflammatory trajectories by abuse history. Inflammation changed significantly over time for people who experienced emotional abuse (F(2,153) = 3.56, p = .03), physical abuse (F(2,157) = 3.76, p = .03), and any abuse (F(2,152) = 3.57, p = .03), but not sexual abuse (F(2,151) = 1.41, p = .25) compared to those without abuse histories. This pattern of results remained the same when controlling for depressive symptoms, measured by the Center for Epidemiological Studies Depression Scale (Radloff, 1977), rather than a history of a psychological disorder.
Table 2.
Inflammation composite estimated means and standard errors across visits
| Visit 1 | Visit 2 | Visit 3 | |
|---|---|---|---|
| Sexual Abuse | |||
| Had History of sexual abuse | −0.53 (.53) | 0.31 (.57)a | 0.86 (.61)a |
| No history of sexual abuse | −0.36 (.28) | −0.17 (.28) | 0.42 (.31)a,b |
| Physical Abuse | |||
| Had History of physical abuse | −0.49 (.49) | 0.66 (.51)a | 1.19 (.56)a |
| No history of physical abuse | −0.29 (.29) | −0.19 (.29) | 0.40 (.31)a,b |
| Emotional Abuse | |||
| Had History of emotional abuse | −0.68 (.50) | 0.29 (.51)a | 1.16 (.57)a,b |
| No history of emotional abuse | −0.27 (.29) | −0.11 (.29) | 0.37 (.31)a,b |
| Any Abuse | |||
| Had History of any type of abuse | −0.32 (.41) | 0.48 (.41)a | 1.15 (.46)a,b |
| No history of any type of abuse | −0.46 (.30) | −0.37 (.30) | 0.12 (.33)a,b |
Subscripts denote:
significant change from Visit 1
significant change from Visit 2
Table 3.
Correlations between predictors, covariates, and outcomes
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1. Emotional abuse | - | |||||||||||
| 2. Physical abuse | .46** | - | ||||||||||
| 3. Sexual abuse | .40** | .26* | - | |||||||||
| 4. Any abuse | .74** | .65** | .59** | - | ||||||||
| 5. Age | −.06 | −.07 | −.11 | −.12 | - | |||||||
| 6. BMI | .11 | .05 | −.07 | .10 | .01 | - | ||||||
| 7. Comorbidities | .19* | .23* | .06 | .15 | .16 | .08 | - | |||||
| 8. Psych Dx | .31** | .25* | .11 | .29* | −.15 | .09 | .14 | - | ||||
| 9. Race | .01 | −.16 | .08 | −.01 | .24* | −.05 | −.04 | −.01 | - | |||
| 10. Income | −.21* | −.17 | −.01 | −.15 | −.01 | .05 | −.26* | −.12 | .20* | - | ||
| 11. Menopause | −.03 | .07 | −.12 | .03 | .38** | .17 | .05 | −.14 | .15 | −.07 | - | |
| 12. Inflammation | .17 | .18* | .12 | .23* | −.09 | .26* | .02 | .02 | −.06 | −.21* | .03 | - |
Note.
p < .05
p < .001
BMI = body mass index; comorbidities = physical comorbidities measured via the Charlson Comorbidity Index; Psych Dx = history of a psychological diagnosis; Inflammation = inflammatory composite.
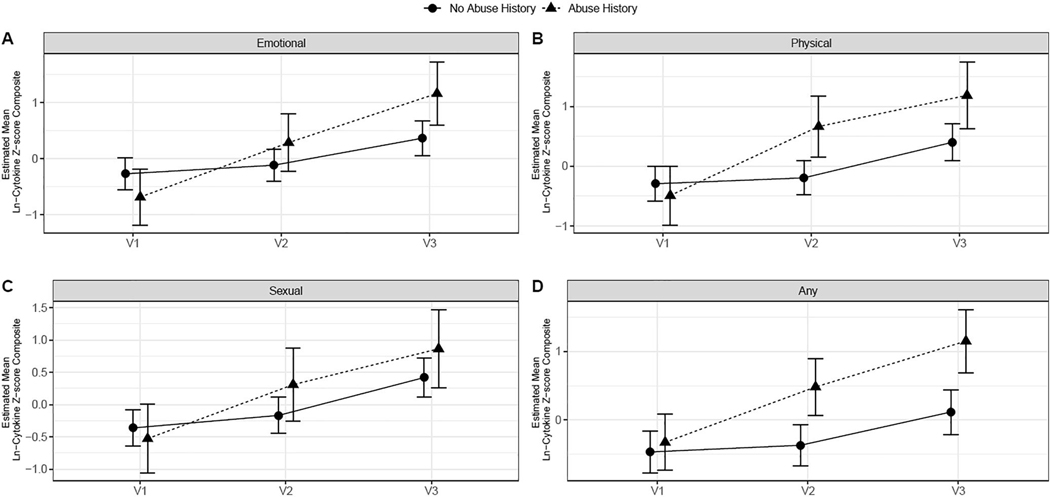
Figure 1.
Inflammatory trajectories based on different types of abuse histories
Individuals with an emotional abuse history had larger increases in inflammation (mean change from baseline = .97 and 1.84 at visits 2 and 3, respectively) compared to those without an emotional abuse history (mean change from baseline = 0.16 and .64 at visits 2 and 3). Similarly, individuals with a physical abuse history had larger increases in inflammation (mean change from baseline = 1.15 and 1.68 at visits 2 and 3) compared to those without physical abuse histories (mean change from baseline = 0.10 and .69 at visits 2 and 3). This pattern held when looking at any type of abuse; participants with any type of abuse history had larger increases in inflammation (mean change from baseline = .80 and 1.47 at visits 2 and 3) compared to those who did not report such a history (mean change from baseline = 0.09 and .58 at visits 2 and 3).
When analyzing the individual cytokines, only IL-6 showed significant differences in trajectories by physical abuse group (F(2,136) = 4.1, p = .02). Trajectories of TNF-α did not significantly differ by any abuse type (p > .24 for all). There were also no significant differences in trajectories of IL-1β by emotional and sexual abuse, though there were trends towards differences between subjects with physical abuse versus no physical abuse (F(2,155) = 2.86, p = .06) and between subjects with any abuse versus no abuse (F(2,146) = 2.85, p = .06).
3.3. Alternative Models
It is plausible that several factors may influence the relationship between abuse histories and inflammation. We therefore tested alternative models examining BMI and psychological disorder history as moderators of how abuse influenced inflammation. There were no significant three way interactions among abuse, visit, and BMI (all ps > .18). Further, no significant three way interactions emerged among abuse, visit, and psychological disorder history (all ps > .07).
Discussion
Consistent with the biological embedding model, this study suggests that childhood abuse has lasting negative effects on inflammation throughout adulthood that worsens over time. Those who experienced any type of abuse during childhood (e.g., physical, emotional, or sexual abuse) had steeper increases in inflammation across the study visits compared to individuals without abuse histories. Participants who experienced emotional and physical abuse experienced steeper increases in inflammation across time compared to participants without abuse histories. In contrast, sexual abuse histories did not predict differential inflammatory trajectories across time in this study. Importantly, sexual abuse history rates were lower than physical or emotional abuse and thus the power to detect differences was much more limited.
This study contributes to a larger body of research examining abuse histories and inflammation in important ways. Other studies of middle-aged and older adults who experienced childhood abuse have reported higher inflammation at a single time point compared to those without abuse histories (Gouin et al., 2012; Hostinar, Lachman, Mroczek, Seeman, & Miller, 2015; Kiecolt-Glaser et al., 2011; Matthews, Chang, Thurston, & Bromberger, 2014; Slopen et al., 2015). However, research on inflammatory trajectories among people who experienced childhood adversity has been limited. Our study addressed this gap and showed how a history of childhood abuse can contribute to steeper increases in inflammation among middle-aged adults across an approximately two-year period. These findings are consistent with a previous longitudinal, prospective study that followed participants up to 32 years old and found that those who experienced childhood abuse had higher inflammation than those without such abuse (Andrea Danese et al., 2007). Our data extends prior literature by highlighting how inflammation increases across time for those who have abuse histories. These findings underscore the importance of examining the long-term biological implications of childhood adversity. These results align with the conserved transcriptional response to adversity, showing that early life adversity primes a heightened inflammatory response to stress in adulthood (Cole, 2019). Steeper inflammatory trajectories among those with abuse histories ultimately may result in accelerated inflammatory aging – or inflammaging – contributing to heightened risk for morbidity and mortality (Franceschi et al., 2007).
The current study has several notable strengths. Inflammation data across three time points allowed us to test trajectories of change based on histories of childhood abuse. Utilizing an inflammatory composite variable demonstrated links between childhood abuse and inflammation across several inflammatory markers (Liu et al., 2017). This study showed that childhood abuse was linked to inflammation after adjusting for several important covariates including BMI, age, physical comorbidities, and biological sex. Limitations include the fact that the participants were not particularly diverse in terms of race and ethnicity. The CTQ uses retrospective reports of abuse. Prospective and retrospective abuse reports generally have low agreement with one another (Baldwin, Reuben, Newbury, & Danese, 2019). Further, prospective reports of abuse previously correlated more strongly with objective markers of health (e.g., inflammation) compared to retrospective abuse reports (Reuben et al., 2016). Findings from the current study therefore should be replicated with prospective reports of abuse. Our findings did not demonstrate baseline differences in inflammation between those with abuse histories versus those without, potentially due to the biases inherent in these retroactive reports of abuse. One further possible explanation for these lack of baseline differences is that the participants in this study had recently had an abnormal breast or colorectal cancer test. Although these diagnoses were benign, these tests could have increased stress among our participants that ultimately influenced inflammation at baseline regardless of their history of abuse. The lack of significant baseline inflammatory differences between those with abuse histories compared to those without warrants further investigation. Additionally, only one aspect of early life stress was assessed in this study. Recent meta-analytic findings have shown a broad range of adverse early life experiences such as neglect, severe illness in childhood, and socioeconomic status can also influence inflammation both cross sectionally and longitudinally (Kuhlman, Horn, Chiang, & Bower, 2019).
Addressing the long-term health effects of childhood adversity is a clear priority of the Center for Disease Control (CDC, 2019). Childhood adversity’s clear links to physical health issues in adulthood – including cardiovascular disease, diabetes, and cancer – highlight the necessity of identifying biological mechanisms underlying this relationship (Fagundes, Glaser, & Kiecolt-Glaser, 2013; Miller et al., 2011). This pattern of results suggests that inflammation may be one longitudinal mechanism linking childhood abuse to poor health in adulthood and underscores the need to address the persistent effects of childhood abuse history in these individuals.
Highlights.
Childhood abuse was recently named a public health risk by the Center for Disease Control
We tested whether childhood abuse histories predicted inflammatory trajectories in adulthood
Participants had blood drawn at three study visits to test for inflammatory markers
Emotional and physical abuse in childhood predicted steeper inflammatory increases
Childhood abuse histories may quicken age-related increases in inflammation
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alfano CM, Peng J, Andridge RR, Lindgren ME, Povoski SP, Lipari AM, . . . Carson WE III (2017). Inflammatory cytokines and comorbidity development in breast cancer survivors versus noncancer controls: evidence for accelerated aging? Journal of Clinical Oncology, 35(2), 149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baldwin JR, Reuben A, Newbury JB, & Danese A. J. J. p. (2019). Agreement between prospective and retrospective measures of childhood maltreatment: a systematic review and meta-analysis. 76(6), 584–593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumeister D, Akhtar R, Ciufolini S, Pariante CM, & Mondelli V. (2016). Childhood trauma and adulthood inflammation: a meta-analysis of peripheral C-reactive protein, interleukin-6 and tumour necrosis factor-α. Molecular psychiatry, 21(5), 642–649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernstein DP, Fink L, Handelsman L, Foote J, Lovejoy M, Wenzel K, . . . Ruggiero J. (1994). Initial reliability and validity of a new retrospective measure of child abuse and neglect. The American Journal of Psychiatry, 151(8), 1132–1136. [DOI] [PubMed] [Google Scholar]
- CDC. (2019). Preventing Child Abuse and Neglect. Retrieved from https://www.cdc.gov/violenceprevention/pdf/CAN-factsheet.pdf
- Charlson M, Szatrowski TP, Peterson J, & Gold J. (1994). Validation of a combined comorbidity index. Journal of clinical Epidemiology, 47(11), 1245–1251. [DOI] [PubMed] [Google Scholar]
- Cole SW (2019). The conserved transcriptional response to adversity. Current Opinion in Behavioral Sciences, 28, 31–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danese A, Caspi A, Williams B, Ambler A, Sugden K, Mika J, . . . Moffitt T. (2011). Biological embedding of stress through inflammation processes in childhood. Molecular psychiatry, 16(3), 244–246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danese A, & McEwen BS (2012). Adverse childhood experiences, allostasis, allostatic load, and age-related disease. Physiology & behavior, 106(1), 29–39. [DOI] [PubMed] [Google Scholar]
- Danese A, Pariante CM, Caspi A, Taylor A, & Poulton R. (2007). Childhood maltreatment predicts adult inflammation in a life-course study. Proceedings of the National Academy of Sciences, 104(4), 1319–1324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fagundes CP, Glaser R, & Kiecolt-Glaser JK (2013). Stressful early life experiences and immune dysregulation across the lifespan. Brain, behavior, and immunity, 27, 8–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, & Williams JB (2002). Structured clinical interview for DSM-IV-TR axis I disorders, research version, patient edition. Retrieved from [Google Scholar]
- Franceschi C, Capri M, Monti D, Giunta S, Olivieri F, Sevini F, . . . Scurti M. (2007). Inflammaging and anti-inflammaging: a systemic perspective on aging and longevity emerged from studies in humans. Mechanisms of Ageing and Development, 128(1), 92–105. [DOI] [PubMed] [Google Scholar]
- Gouin J-P, Glaser R, Malarkey WB, Beversdorf D, & Kiecolt-Glaser JK (2012). Childhood abuse and inflammatory responses to daily stressors. Annals of Behavioral Medicine, 44(2), 287–292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green JG, McLaughlin KA, Berglund PA, Gruber MJ, Sampson NA, Zaslavsky AM, & Kessler RC (2010). Childhood adversities and adult psychiatric disorders in the national comorbidity survey replication I: associations with first onset of DSM-IV disorders. Archives of General Psychiatry, 67(2), 113–123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hostinar CE, Lachman ME, Mroczek DK, Seeman TE, & Miller GE (2015). Additive contributions of childhood adversity and recent stressors to inflammation at midlife: Findings from the MIDUS study. Developmental Psychology, 51(11), 1630–1644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iob E, & Steptoe A. (2019). Adverse childhood experiences, inflammation, and depressive symptoms in later life: a prospective cohort study. The Lancet, 394, S58. [Google Scholar]
- Kiecolt-Glaser JK, Gouin J-P, Weng N. p., Malarkey WB, Beversdorf DQ, & Glaser R. (2011). Childhood adversity heightens the impact of later-life caregiving stress on telomere length and inflammation. Psychosomatic medicine, 73(1), 16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhlman KR, Horn SR, Chiang JJ, & Bower J. (2019). Early life adversity exposure and circulating markers of inflammation in children and adolescents: A systematic review and meta-analysis. Brain, Behavior & Immunity, 86, 30–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu CH, Abrams ND, Carrick DM, Chander P, Dwyer J, Hamlet MR, . . . Tandon P. (2017). Biomarkers of chronic inflammation in disease development and prevention: challenges and opportunities. In: Nature Publishing Group. [DOI] [PubMed] [Google Scholar]
- Matthews KA, Chang Y-F, Thurston RC, & Bromberger JT (2014). Child abuse is related to inflammation in mid-life women: role of obesity. Brain, Behavior & Immunity, 36, 29–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaughlin KA, Green JG, Gruber MJ, Sampson NA, Zaslavsky AM, & Kessler RC (2010). Childhood adversities and adult psychiatric disorders in the national comorbidity survey replication II: associations with persistence of DSM-IV disorders. Archives of General Psychiatry, 67(2), 124–132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller GE, Chen E, & Parker KJ (2011). Psychological stress in childhood and susceptibility to the chronic diseases of aging: Moving toward a model of behavioral and biological mechanisms. Psychological bulletin, 137(6), 959–997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radloff LS (1977). The CES-D scale: A self-report depression scale for research in the general population. Applied psychological measurement, 1(3), 385–401. [Google Scholar]
- Reuben A, Moffitt TE, Caspi A, Belsky DW, Harrington H, Schroeder F, . . . Danese A. (2016). Lest we forget: comparing retrospective and prospective assessments of adverse childhood experiences in the prediction of adult health. Journal of Child Psychology & Psychiatry, 57(10), 1103–1112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sachs-Ericsson N, Blazer D, Plant EA, & Arnow B. (2005). Childhood sexual and physical abuse and the 1-year prevalence of medical problems in the National Comorbidity Survey. Health Psychology, 24(1), 32–40. [DOI] [PubMed] [Google Scholar]
- Scott KM, Von Korff M, Angermeyer MC, Benjet C, Bruffaerts R, De Girolamo G, . . . Posada-Villa J. (2011). Association of childhood adversities and early-onset mental disorders with adult-onset chronic physical conditions. Archives of General Psychiatry, 68(8), 838–844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slopen N, Loucks EB, Appleton AA, Kawachi I, Kubzansky LD, Non AL, . . . Gilman SE (2015). Early origins of inflammation: An examination of prenatal and childhood social adversity in a prospective cohort study. Psychoneuroendocrinology, 51, 403–413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tursich M, Neufeld R, Frewen P, Harricharan S, Kibler J, Rhind S, & Lanius R. (2014). Association of trauma exposure with proinflammatory activity: a transdiagnostic meta-analysis. Translational Psychiatry, 4(7), 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viola TW, Salum GA, Kluwe-Schiavon B, Sanvicente-Vieira B, Levandowski ML, & Grassi-Oliveira R. (2016). The influence of geographical and economic factors in estimates of childhood abuse and neglect using the Childhood Trauma Questionnaire: A worldwide meta-regression analysis. Child Abuse & Neglect, 51, 1–11. [DOI] [PubMed] [Google Scholar]
- Walker EA, Gelfand A, Katon WJ, Koss MP, Von Korff M, Bernstein D, & Russo J. (1999). Adult health status of women with histories of childhood abuse and neglect. The American Journal of Medicine, 107(4), 332–339. [DOI] [PubMed] [Google Scholar]
- Wegman HL, & Stetler C. (2009). A meta-analytic review of the effects of childhood abuse on medical outcomes in adulthood. Psychosomatic medicine, 71(8), 805–812. [DOI] [PubMed] [Google Scholar]