Summary
Background
The Burden of non-communicable disease (NCDs) has continued to rise globally, particularly in low- and middle-income countries. In Turkey, NCDs account for 89% of all deaths, with nearly one in five deaths occurring before age 70. This study investigates the number of NCD deaths that could be prevented if Turkey met national and international targets for major modifiable NCD risk factors.
Methods
Preventable deaths were estimated using the World Health Organization (WHO) ‘Preventable Risk Integrated ModEl’ (PRIME), by combining: 1) Baseline exposure data for risk factors, referenced from national surveillance and cohort studies; 2) Aetiological associations from published meta-analyses; and 3) Demographic and mortality statistics obtained from the Turkish Statistical Institute (TurkStat). Confidence intervals were estimated using Monte Carlo simulations.
Findings
If Turkey met its NCD risk factor targets for reducing tobacco and salt consumption by 30%, and physical inactivity by 10% in 2017, an estimated 19,859 deaths (95%CI: 12,802 to 26,609) could have been prevented. Approximately two thirds of these preventable deaths were in men, and one in three were in adults below 75 years. A 30% relative reduction in the consumption of alcohol, tobacco, and salt, as well as physical inactivity, would prevent 180 (107 to 259); 4,786 (3,679 to 5,836); 13,112 (5,819 to 19,952); and 7,124 (5,053 to 9,212) deaths, respectively.
Interpretation
Among major modifiable NCD risk factors, population-level reductions in salt intake and physical inactivity present the greatest opportunity for reducing NCD mortality in Turkey. These findings can help Turkey prioritise interventions to meet the Sustainable Development Goal target of reducing NCD mortality by one third, by 2030.
1. Research in Context
1.1. Evidence before this study
WHO Europe's ‘NCD Prime’ model (originally, PRIME; the ‘Preventable Risk Integrated ModEl’) is a means of informing the formulation of robust data-driven NCD prevention and control strategies at the national-level. PRIME can be used to model the impact of a range of counterfactual policy scenarios, allowing policymakers to prioritise interventions that offer the greatest impact of achieving policy objectives. We searched PubMed for "Preventable Risk Integrated ModEl" OR "NCD Prime" on 20th July without restrictions for language or date. Nine studies have used the tool to date. However, all studies were based on high-income populations. We searched PubMed, Google Scholar, and medRxiv for studies that estimated the health impact of policies for the prevention and control of NCDs in Turkey using any modelling approach, using various keywords for NCDs, policy interventions and targets, and quantitative methods. Only one study (2014) met the search criteria. This study found that reducing baseline population-level salt intake by 30% was associated with 378,439 life years gained in 2010, using the IMPACT CHD policy modelling tool.
1.2. Added value of this study
We modelled the impact of reducing the major modifiable risk factors for NCDs (consumption of tobacco, alcohol, salt, and physical inactivity) in the national population of Turkey. In addition to quantifying the number of deaths that would be averted or delayed by meeting the current national policy targets, we provide a comparative analysis of the contribution from each risk factor. Our models suggest that population-level policies for reducing salt consumption present the greatest opportunity for reducing NCD mortality in Turkey. We provide further perspective by estimating counterfactual scenarios of population impact stemming from progressive reductions in the prevalence of each risk factor by 10%, 20% and 30%.
1.3. Implications of all the available evidence
Modest reductions in population-level salt intake in Turkey are associated with large population health gains in terms of preventable deaths. Conversely, even large reductions in alcohol consumption yield only a negligible population impact, owing to low baseline prevalence of alcohol consumption. Our matrix-based approach to modelling the impact of different risk factor targets can be extended to other low- and middle-income countries to prioritise the development of evidence-based, nationally-tailored policy targets for the prevention and control of NCDs, in concert with current WHO guidance.
2. Introduction
Non-communicable diseases (NCDs) account for over 70% of global mortality, two thirds of disability-adjusted life years (DALYs), and 15 million premature deaths every year. Low- and middle-income countries (LMICs) bear a disproportionate NCD burden, with a 1.5 times higher risk of premature mortality than high-income countries [1,2,3,4].
Turkey is an upper-middle income country with a large proportion of young people [5,6]. NCDs account for 89% of all deaths, and nearly one in five occur in under 70-year olds [6]. The high social and economic costs of NCDs, compounded by an imminent expansion of Turkey's elderly population, has prompted the government to develop the Multisectoral Action Plan of Turkey for Noncommunicable Diseases 2017–2025 [5].
Turkey's NCD action plan outlines a number of national targets that are based on the global aspirations chartered in the UN Political Declaration on NCDs [7]. This 2011 document advanced the ‘4 × 4’ framing of strategies against NCDs, focusing on tobacco, alcohol, diet, and physical inactivity as key risk factors for cardiovascular disease, chronic respiratory disease, cancers, and diabetes - the four conditions responsible for 80% of global NCD deaths. Signatories committed to developing national targets based on their unique domestic situations.
Turkey's intersectoral NCD action plan directly adopts the global targets for relative reductions of 30% for tobacco use, a 30% relative reduction for salt intake, and a 10% relative reduction in physical inactivity. Rather than adopting the target of a 10% relative reduction in the harmful use of alcohol, Turkey opted to target ‘halting the rise in harmful alcohol consumption’.[6]
Whilst the government has access to reliable epidemiological data on the burden of NCDs, like many LMICs, Turkey did not base the selection of its national targets on modelling the relative impact that would be expected under differing thresholds, for example the number of preventable deaths from reducing salt intake by 20% vs 30%. The intersectoral plan does not itemise estimates for the number of potential deaths averted from intervening in each risk factor, or estimate how many deaths might be averted if all of its risk factor targets were met.
Using the WHO Euro-endorsed ‘PRIME’ NCD mortality modelling tool, we aimed to estimate the number of deaths that would be averted if Turkey fully met its tobacco, alcohol, salt, and physical inactivity targets. We also aimed to quantify the relative contribution made by each risk factor, and to model the impact associated with targeting different risk factor reduction thresholds: 10%, 20%, and 30%. Understanding the relative contribution made by different risk factors and thresholds can help to inform the prioritisation of NCD policy options as Turkey strives to meet its SDG commitment to reduce premature NCD deaths by one third by 2030.
Turkey has the highest salt consumption in Europe, and even with a 30% reduction in current consumption levels, mean salt intake would still remain at 138% of WHO recommended levels. As a secondary aim, we set out to model the number of deaths that would be averted if the population met the Turkish- and WHO-recommended limit of 5 g salt/person/day.
Finally, a high proportion of the population does not consume fruit and vegetables on a daily basis. IHME data suggest that inadequate fruit and vegetable consumption is a leading cause of morbidity and mortality [8]. As a tertiary objective we set out to model the impact of increasing the proportion of the population consuming at least one portion of fruit and vegetables by 10%, 20%, and 30%.
3. Method
3.1. Modelling tool
To quantify the impact of modifying Turkey`s population-level NCD risk factor prevalence we used the ‘Preventable Risk Integrated ModEl’ (PRIME), an openly-available policy scenario modelling tool developed by researchers at the WHO Collaborating Centre on Population Approaches for NCD Prevention at the University of Oxford [9]. Based on a given country's demographic profile, burden of NCDs, and distribution of risk factors, PRIME estimates the impact of counterfactual scenarios using relative risk estimates from published meta-analyses. PRIME calculates how many NCD deaths would have occurred based on the counterfactual risk factor distribution, with reference to the baseline data. The tool has been used to model scenarios in numerous studies from Australia, Canada, Portugal, and the UK [10,11,12,13,14,15,16,17,18,19,20]. Further details are available in the Appendix, and in a 2014 methodological article[21] and the WHO Europe NCD Prime handbook [22].
3.2. Data sources
PRIME requires four sets of input data; the structure of the population aged >15 year in five-year bands, data on mortality for 24 ICD-10 coded NCDs (Appendix 1), the baseline distribution of behavioural risk factors by sex and age in five-year bands; and data on the counterfactual distribution of behavioural risk factors. Ideally, baseline data (age and sex-specific means and standard deviations, and the proportion of non-consumers for risk factors that are not normally distributed) should be all obtained for the same year, however, in reality this is often not possible.
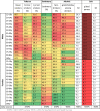
We used the latest available data (2017) for Turkey's population structure (Supplementary Table 1) and ICD-10 mortality statistics from the Turkish Statistical Institute (Supplementary Table 2). The baseline distribution of behavioural risk factors was derived from three nationally representative studies; the 2017 ‘STEPwise approach to Surveillance’ (STEPS) survey - jointly conducted by WHO Europe and the Ministry of Health of Turkey [23], the 2010 Turkey Nutrition and Health Survey (TNHS) [24], and the 2012 SALTURK II study which assessed 24-hour urinary sodium excretion (SALTURK) [25].Briefly, the 2017 STEPS survey, used random multistage cluster sampling methods to survey NCD risk factors, ensuring representativeness and equal distribution according to age and sex [23]. The TNHS sample was designed as a weighted, multi-stage, stratified cluster sample which provides nationally-representative estimates, including the 5 Turkish regions, 12 Nomenclature of Territorial Units for Statistics (NUTS-1) regions, and the 7 metropolitans [24]. The Twelve NUTS-1 regions were developed during EU compliance processes and constitute the first tier of the Statistical Region Classification System which are used as official national statistics. Data from the SALTURK II study was designed to be representative of the adult population in urban and rural areas of 4 major Turkish cities (Ankara, Istanbul, Izmir, and Konya) which account for one-third of Turkey's population and reflect a diversity of dietary practices. Table 1 shows a heat map of risk factor distributions in Turkey's population used for the main analysis, highlighting the demographic groups at highest risk.
Table 1.
Baseline population-level risk factor data and sources.
 |
3.3. Tobacco
Data on the prevalence of tobacco consumption (never-, former-, and current- smokers) were taken from the STEPS 2017 survey. Individuals currently using any tobacco products were considered current smokers; those who reported using tobacco products in the past, but not presently, were classified as former smokers; individuals reporting “no” to both questions were assigned as never-smokers [23].
3.4. Physical inactivity
The proportions of each demographic band considered ‘sedentary’ were obtained from the Global Physical Activity Questionnaire (GPAQ) included in the 2017 STEPS survey [23].
3.5. Alcohol
We obtained data on the percentages of non-drinkers in each demographic band (those consuming <1 g of ethanol per day) from the 2017 STEPS survey. The latest available data on mean grams/ethanol/day and the associated standard deviations (SD) were referenced from the 2010 TNHS survey as these data were not presented in the STEPS survey. Owing to the aggregation of data from two different instruments, we conducted a sensitivity analysis for alcohol using contemporaneous data from the 2010 THNS survey (Table 2).
Table. 2.
Alternate salt and alcohol baseline data used in the sensitivity analyses.
| Salt | Alcohol | |||
|---|---|---|---|---|
| g/day/person | SD | Non-consumers (%) | ||
| Males | 15–19y | 15.7 | 5.5 | 97.8 |
| 20–24y | 15.7 | 5.5 | 96.8 | |
| 25–29y | 15.7 | 5.5 | 96.0 | |
| 30–34y | 15.7 | 5.5 | 94.7 | |
| 35–39y | 15.7 | 5.5 | 94.2 | |
| 40–44y | 15.7 | 5.5 | 93.9 | |
| 45–49y | 15.7 | 5.5 | 91.7 | |
| 50–54y | 15.7 | 5.5 | 91.9 | |
| 55–59y | 15.7 | 5.5 | 92.4 | |
| 60–64y | 15.7 | 5.5 | 96.0 | |
| 65–69y | 15.7 | 5.5 | 99.4 | |
| 70–74y | 15.7 | 5.5 | 99.1 | |
| 75–79y | 15.7 | 5.5 | 100.0 | |
| 80–84y | 15.7 | 5.5 | 96.0 | |
| ≥85y | 15.7 | 5.5 | 93.3 | |
| Females | 15–19 | 14 | 5.2 | 99.8 |
| 20–24 | 14 | 5.2 | 99.5 | |
| 25–29 | 14 | 5.2 | 99.5 | |
| 30–34 | 14 | 5.2 | 99.3 | |
| 35–39 | 14 | 5.2 | 99.2 | |
| 40–44 | 14 | 5.2 | 99.3 | |
| 45–49 | 14 | 5.2 | 98.6 | |
| 50–54 | 14 | 5.2 | 99.1 | |
| 55–59 | 14 | 5.2 | 99.0 | |
| 60–64 | 14 | 5.2 | 99.6 | |
| 65–69 | 14 | 5.2 | 100.0 | |
| 70–74 | 14 | 5.2 | 100.0 | |
| 75–79 | 14 | 5.2 | 100.0 | |
| 80–84 | 14 | 5.2 | 100.0 | |
| 85+ | 14 | 5.2 | 100.0 | |
| Source | SALTURK | TNHS | ||
SALTURK = SALTURK II study; TNHS = 2010 Turkey Nutrition and Health Survey
3.6. Salt
The 2017 STEPS survey provides population-level mean values for each demographic band based on biometric measurements in over 3,000 individuals, albeit without the corresponding standard deviations. For the main analysis we referenced standard deviations from the 2010 THNS survey. The latest available estimates providing both means and SDs were from the 2012 SALTURK II study which assessed dietary intake and 24 h urinary sodium excretion from 464 participants. This study found considerably higher mean daily salt intake per person (14.8 g vs 9.9 g from STEPS) but is commonly used in government reports, including the NCD action plan [5]. Therefore, we used the SALTURK data in a second sensitivity analysis (Table 2).
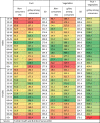
3.7. Fruit and vegetables
The latest available data on the percentage of non-consumers (eating less than one 106 g portion/day), and mean grams/day consumed by those eating ≥1 portion/day with associated SDs were from the 2010 THNS study. WHO recommends that individuals consume >400 g fruit and vegetables/day [26]. Table 3 shows that with the exception of women aged >85 years, all groups that consume >1 portion of fruit and vegetables are meeting this target, however 60% of the Turkey`s population consumes less than one portion of fruit per day, and approximately a quarter consume less than one portion of vegetables per day.
Table. 3.
Fruit and vegetables baseline data.
 |
4. Counterfactual scenarios
4.1. Main analysis: attainment of the multisectoral action plan targets
Using PRIME, we modelled how many NCD deaths would have been observed in Turkey in 2017 if the action plan targets were achieved. As per the PRIME manual, we amended proportions and means but maintained baseline standard deviation values [22]. We modelled a 30% relative reduction in mean salt intake for each demographic band; a 30% relative reduction in the proportion of ‘current smokers’ (down to a floor of 0%) with a concomitant rise in the proportion of ‘former-smokers’ for each demographic band; a 10% relative reduction in the proportion of the population considered ‘sedentary’ for each demographic band; and no change in alcohol consumption. We modelled the number of deaths associated with the change in each risk factor, and overall. We used Monte Carlo simulation — a statistical procedure for compiling uncertainty around deterministic point estimates in scenarios where more than one risk factor is altered — with 5,000 iterations to estimate 95% confidence intervals (95%CI) around point estimates of the preventable deaths.
4.2. Sensitivity analysis: alternative baseline data for salt intake
Based on the observation that contemporary salt intake is contested, we performed two sensitivity analyses using alternate baseline data for salt intake, based on the sensitivity analysis for the national targets we used. Given that baseline data for salt are uncertain and the alternate data source shows higher levels of consumption than the Turkish government projects, we performed two sensitivity analyses. In the first, we use the (higher) SALTURK survey data for mean salt intake and standard deviations. In the second we reduced the STEPS baseline levels by 25%, and used the same standard deviations from THNS. Estimates for both scenarios are provided in Appendix 1.
4.3. Estimating the impact of different policy intervention levels
To quantify the relative contributions of intervening in each risk factor and to model the impact associated with different potential targets, we performed three further Monte Carlo simulations for each risk factor - plus fruit & vegetables and the two alternative data sources for salt and alcohol intake - with relative reductions of 10%, 20%, and 30% within each demographic band.
5. Meeting the WHO recommended limit for salt intake
We used the two different data sources for salt intake (SALTURK & STEPS/TNHS) to model the impact of attaining the WHO recommended level of 5/g/person/day. All of the counterfactual scenarios are summarised in Table 4.
Table. 4.
Summary of the counterfactual scenarios.
| Counterfactual scenario | Baseline risk factor equation | Data sources |
|---|---|---|
| 1.1 Multisectoral Action Plan targets | Current smokers*0.7 | STEPS |
| Salt intake*0.7 | STEPS & TNHS | |
| Physical inactivity*0.9 | STEPS | |
| Alcohol*1 (no change) | STEPS & TNHS | |
| 1.2 Sensitivity analysis: Multisectoral Action Plan targets with alternative salt data | Current smokers*0.7 | STEPS |
| Salt intake*0.7 | SALTURK | |
| Physical inactivity*0.9 | STEPS | |
| Alcohol*1 (no change) | STEPS & TNHS | |
| 1.3 Sensitivity analysis: Multisectoral Action Plan targets with alternative salt data | Current smokers*0.7 | STEPS |
| Salt intake*0.7 | STEPS*0.75 &THNS | |
| Physical inactivity*0.9 | STEPS | |
| Alcohol*1 (no change) | STEPS & TNHS | |
| 2. Estimating the impact of different policy intervention levels | Current smokers*0.9, 0.8, 0.7 | STEPS |
| Physical inactivity*0.9, 0.8, 0.7 | STEPS | |
| Alcohol consumption*0.9, 0.8, 0.7 | STEPS & TNHS | |
| Alcohol consumption*0.9, 0.8, 0.7 | TNHS | |
| Salt intake*0.9, 0.8, 0.7 | STEPS & TNHS | |
| Salt intake*0.9, 0.8, 0.7 | SALTURK | |
| Fruit & Vegetable non-consumption*0.9, 0.8, 0.7 | TNHS | |
| 3. Meeting the recommended 5g/day limit for salt | Salt intake reduced to 5g for each demographic group | STEPS & TNHS |
| Salt intake reduced to 5g for each demographic group | SALTURK | |
| Salt intake reduced to 5g for each demographic group | STEPS*0.75 & THNS |
6. Findings
6.1. Attainment of the NCD action plan targets
If the Turkey goals of reducing tobacco and salt consumption by 30%, and physical inactivity by 10% were met, PRIME estimates that 19,859 fewer deaths would have been observed in 2017 (95%CI: 12,802 to 26,609) (Table 5). Approximately two thirds (64.5%) of averted deaths were observed in males, and 34.5% in adults below 75 years. Of the four main NCDs, 0.1% of the reduction in deaths were from diabetes (20/19,859); 2.5% (490/19,859) from COPD; 14.4% (2854/19,859) from averted cancers; and 85.2% from cardiovascular disease (16,917/19,859). Our sensitivity analysis that used a lower baseline for 2017 salt consumption (25% lower than the STEPS values) estimated that 9,899 (4,282 to 15,439) deaths could be averted if salt consumption was reduced by 30%.
Table 5.
Deaths averted if the Multisectoral Action Plan targets were met in 2017.
|
Deaths averted (95%CI) |
|||
| Main analysis | With alternate salt data | ||
| Tobacco (30% reduction) | 4,786 (3,679 to 5,836) | ||
| Physical inactivity (10% reduction) | 2,383 (1,653 to 3,073) | ||
| Alcohol (status quo) | 0 | ||
| Salt (30% reduction) | 13,112 (5,819 to 19,952)⁎ | 18,550 (8,205 to 28,196)⁎⁎ | 9,899 (4,282 to 15,439)⁎⁎⁎ |
| Total | 19,859 (12,802 to 26,609) | 26,035 (15,226 to 36,661)⁎⁎ | 16,773 (11,045 to 22,119)⁎⁎⁎ |
Using data from the STEPS and TNHS studies
Using data from the SALTURK study [cf = 11 men, 9.8 women
Using lower baseline values (STEPS x 75%)
6.2. Estimating the impact of different policy intervention levels
Table 6 shows how many deaths would be averted if the baseline prevalence of each risk factor was reduced by 10%, 20%, and 30%. The estimated population health impact of salt reduction is an order of magnitude higher than tobacco and physical inactivity, and two orders of magnitude higher than alcohol reduction at the 20–30% level.
Table 6.
Preventable deaths associated with progressive reductions in risk factor prevalence.
| Deaths averted (95%CI) | |||
|---|---|---|---|
| Risk factor | 10% relative reduction | 20% relative reduction | 30% relative reduction |
| % current tobacco users | 1,590 (1,227 to 1,922) | 3,186 (2,470 to 3,881) | 4,786 (3,679 to 5,836) |
| % physically inactive | 2,383 (1,653 to 3,073) | 4,749 (3,311 to 6,169) | 7,124 (5,053 to 9,212) |
| g/day alcohol consumed by drinkers* | 56 (-13 to 124) | 116 (48 to 189) | 180 (107 to 259) |
| g/day alcohol consumed by drinkers** | 87 (-15 to 189) | 117 (15 to 221) | 117 (2 to 230) |
| g/day salt intake† | 4,556 (1,946 to 7,064) | 8,909 (3,958 to 13,770) | 13,112 (5,819 to 19,952) |
| g/day salt intake 2†† | 6,408 (2,774 to 9,692) | 12,560 (5,497 to 18,911) | 18,550 (8,205 to 28,196) |
| Fruit & Vegetable non-consumption* | 1,321 (946 to 1,691) | 2,633 (1,885 to 3,372) | 3,959 (2,813 to 5,094) |
Alcohol*: using data from STEPS 2017
Alcohol**: using data from THNS 2010
Salt †: using g/day consumption from STEPS 2017
Salt ††: using g/day consumption from SALTURK
Fruit & Veg*: The proportion of the population consuming <1 portion of fruit or vegetables
6.3. Meeting the WHO recommended limit for salt intake
Reducing current salt consumption from 11.0 g for men, and 8.7 g for women (STEPS data) to the WHO-recommended limit of 5 g/day was associated with 20,628 fewer deaths in 2017 (95%CI 9,202 to 30,881). Alternative estimates using the SALTURK data (15.7 g/day for men and 14.0 g/day for women) put the reduction at 37,933 fewer deaths (17,249 to 54,087). Assuming that baseline salt intake was 25% lower than the STEPS estimates, 10,607 deaths would have been averted (4,592 to 16,314).
7. Discussion
7.1. Summary of findings
Using the PRIME modelling tool, we estimate that achieving Turkey's Multisectoral Action Plan targets for alcohol, physical inactivity, salt, and tobacco would have prevented or delayed 19,859 deaths in 2017 (95%CI: 12,802 to 26,609). Half of these averted deaths were associated with one risk factor - reducing salt intake by 30%.
In modelling the impact of different target thresholds, we found that like-for-like reductions in salt consumption were associated with the largest proportion of averted deaths. Conversely, even large reductions in alcohol intake yielded relatively modest gains in terms of deaths averted due to the high prevalence of abstinence in the Turkish population. Reducing tobacco use and physical inactivity led to moderate numbers of averted deaths with approximately 1,500–2,500 deaths averted for every 10% reduction in each risk factor.
7.2. Strengths and limitations
We used reliable demographic and epidemiological baseline data from the Turkish Statistical Institute. We also used nationally representative survey data for all risk factors, including the use of internationally comparable WHO STEPS data. Unfortunately, mean values and corresponding standard deviations were not always reported in the same survey for each risk factor, and our use of multiple datasets and different years is a major limitation. Using salt as an example, 2017 population means were extracted from STEPS (2017), but age-banded SDs were referenced from THNS (2010). To mitigate this limitation, we presented alternative estimates for alcohol and salt in separate sensitivity analyses.
We based our analysis on Turkey's own national targets and provide a new approach for multiple scenario modelling that can be used in other settings. We also used a validated open-access modelling tool that is endorsed by WHO Europe. PRIME has its own limitations, documented comprehensively elsewhere [21,22], and these weaknesses have been incorporated into our own study. The most pertinent are the dependence on risk estimates from published meta-analyses that may not be directly generalisable to the Turkish population. PRIME also assumes a linear dose-response association between risk factor consumption and mortality. A final limitation of PRIME is that the output is not particularly intuitive; it presents the number of deaths that would have been observed for a given year (2017 in our case) for a given counterfactual scenario, rather than a recurring annual number of lives saved. As such, we cannot quantify exactly how many lives would be saved if the same counterfactual scenarios were achieved in 2021, or indeed how many lives would be saved by 2030. A constraint of large population-based surveys of lifestyle factors such as diet and physical activity are that they primarily capture self-reported exposures rather than objective measurements. Measurement error and self-reported biases would serve to underestimate the true aetiological associations between these lifestyle factors and mortality. This limitation, however, would potentially serve to render our findings more conservative [27]. Nevertheless, our analyses provide the first robust estimate of the scale of mortality relating to each behavioural risk factor in this population. As the PRIME model is not equipped for cost-effectiveness analyses, future research could further support policymakers by exploring the tradeoffs around different NCD prevention and control strategies, incorporating the economic costs of delivering various interventions.
7.3. Wider literature
The Sustainable Development Goals frame the overarching international health and development agenda for the next decade. These goals place a strong emphasis on NCD prevention and control [28,29], including a commitment to reduce premature mortality by a third by 2030 [30]. The prevention and control of NCDs should be a key priority in Turkey as NCDs account for 89% of all deaths (higher than the global average of 70%) [6]. This could be owing to the comparatively high intake of salt in Turkey as our analysis has revealed that population-level reductions in salt intake present the greatest opportunity for reducing NCD mortality in Turkey. Accordingly, the most recent 2017 Global Burden of Disease analysis showed that unhealthy diets were the leading cause of death globally, in particular, high sodium intakes were attributable for 3 million deaths and 70 million DALYs [32]. Additionally, the high social and economic costs of NCDs, compounded by an imminent increase in Turkey's elderly population, has prompted the government to develop the Multisectoral Action Plan of Turkey for Noncommunicable Diseases 2017–2025.Turkey's NCD Multisectoral Action Plan is aligned with this commitment, however the specific targets designed to realise this aspiration have not been based on policy scenario modelling. Turkey is not alone in this respect, and there is a general paucity of published studies exploring the different combinations of policy options that contribute to achieving national policy objectives.
Several studies have used PRIME to model the health impact of shifting the baseline distribution of specific NCD risk factors, including sugar-sweetened beverages [12,17,18]; fruit, vegetables, salt, fibre, and fats [11], and alcohol [14]. As far as we are aware, only one study has modelled the impact of achieving national NCD targets [12]. This Portuguese paper found that achieving national targets for salt, sugar, and trans-fatty acid consumption would avert 248 premature deaths in 2016. We found that salt reduction was associated with the largest reduction in deaths for Turkey. A separate 2014 modelling study found that reducing 2010 baseline salt intake by 30% was associated with 378,439 life years gained, using IMPACT CHD Policy (beta) [31]. As PRIME does not provide estimates for the mean age of death we are unable to convert the 13,000 salt-related averted deaths into Years of Life Lost or Gained (YLL/YLG), however we note that sodium is a leading cause of premature NCD mortality [32], and was responsible for more NCD deaths than alcohol use, physical inactivity or air pollution in 2017. WHO currently recommends a national daily intake of 5/salt per person [33]. Whilst Turkey has made great strides in recent years [34], mean consumption is currently double this amount according to conservative estimates from the 2017 WHO STEPS data. The available evidence suggests that salt reduction should be a top priority for the Turkey's national NCD strategy. We note that this analysis has only considered the absolute number of deaths associated with each risk factor, but PRIME enables users to interrogate the sociodemographic patterning of deaths, which is important for equity considerations.
Many countries don't have reliable data on salt consumption and other risk factors [31]. Conflicting, patchy, and absent data on the baseline distribution NCD risk factors can obfuscate effective action. The WHO STEPS surveys have helped in this regard, but there is still much room to improve the standardisation of reporting and publication of data in the public domain, as we found with this and previous studies [35].
8. Conclusion
Achieving the behavioural risk factor targets in the Multisectoral Action Plan of Turkey for Noncommunicable Diseases 2017–2025 would have averted approximately 20,000 deaths in 2017. Among major modifiable NCD risk factors, population-level reductions in salt intake and physical inactivity present the greatest opportunity for reducing NCD mortality in Turkey, and over half of preventable deaths could be averted by reducing population-level salt consumption. Our analysis suggests that the most important risk factors for reducing NCD deaths, in order of priority are: 1) Salt intake, 2) Physical inactivity, 3) Tobacco use, 4) Fruit and vegetables; and 5) Alcohol consumption. These findings using the WHO PRIME modelling tool can help Turkey and other low- and middle-income countries prioritise interventions to meet the Sustainable Development Goal target of reducing NCD mortality by one third, by 2030.
9. Disclaimer
The authors alone are responsible for the views expressed in this article and those views do not necessarily represent the views, decisions or policies of the institutions with which they are affiliated. The opinions in this paper do not reflect the position of the World Health Organization.
Declaration of Competing Interest
All authors have nothing to disclose.
Acknowledgements
The authors would like to thank and acknowledge the generous contribution of Professor Peter Scarborough (University of Oxford, United Kingdom) for developing the PRIME model as an open-source tool and making it available via the WHO Regional Office for Europe.
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.lanepe.2020.100018.
Appendix 1: ICD-10 codes used by PRIME
-
•
I60-I69: Cerebrovascular diseases
-
•
I20-I25: Ischaemic heart diseases
-
•
C00-C14: Lip, oral cavity and pharynx
-
•
C15: Oesophagus
-
•
C16: Stomach
-
•
C34: Bronchus and lung
-
•
C25: Pancreas
-
•
C18-20: Colorectum
-
•
C50: Breast
-
•
C54.1: Endometrium
C23: Gallbladder
-
•
C64: Kidney
-
•
I10-I15: Hypertensive disease
-
•
E11,E14: Diabetes
-
•
C67: Bladder cancer
-
•
C22: Liver cancer
-
•
C53: Cervix cancer
-
•
J40-J44: Chronic obstructive pulmonary disease
-
•
K70, K74: Liver disease
-
•
I50: Heart failure
-
•
I71: Aortic aneurysm
-
•
I26: Pulmonary embolism
-
•
I05-09: Rheumatic heart disease
-
•
N18: Chronic renal failure
Appendix B. Supplementary materials
References
- 1.Roth GA, Abate D, Abate KH, Abay SM, Abbafati C, Abbasi N, Abbastabar H, Abd-Allah F, Abdela J, Abdelalim A, Abdollahpour I. Global, regional, and national age-sex-specific mortality for 282 causes of death in 195 countries and territories, 1980–2017: a systematic analysis for the global burden of disease study 2017. Lancet North Am Ed. 2018;392(10159):1736–1788. doi: 10.1016/S0140-6736(18)32203-7. Nov 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kyu HH, Abate D, Abate KH, Abay SM, Abbafati C, Abbasi N, Abbastabar H, Abd-Allah F, Abdela J, Abdelalim A, Abdollahpour I. Global, regional, and national disability-adjusted life-years (DALYs) for 359 diseases and injuries and healthy life expectancy (HALE) for 195 countries and territories, 1990–2017: a systematic analysis for the global burden of disease study 2017. Lancet North Am Ed. 2018;392(10159):1859–1922. doi: 10.1016/S0140-6736(18)32335-3. Nov 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.WHO. Factsheet. NCDs: https://www.who.int/news-room/fact-sheets/detail/noncommunicable-diseases
- 4.Allen L, Cobiac L, Townsend N. Quantifying the global distribution of premature mortality from non-communicable diseases. J Public Health. 2017;39(4):698–703. doi: 10.1093/pubmed/fdx008. Dec 1. [DOI] [PubMed] [Google Scholar]
- 5.Republic of Turkey Ministry of Health. Multisectoral action plan of Turkey for noncommunicable diseases, 2017–2025. Department of chronic diseases, elderly health and disabled people of the public health institution, ministry of health of Turkey. Ankara, 2017. Available at: https://www.euro.who.int/en/countries/turkey/publications/multisectoral-action-plan-of-turkey-for-noncommunicable-diseases-2017-2025-english-and-turkish [Accessed 06.07.2020].
- 6.WHO NCD progress monitor. Turkey. WHO, 2020. Available at: https://www.who.int/publications/i/item/ncd-progress-monitor-2020 [Accessed 06.07.2020].
- 7.UN General Assembly. Resolution 66/2: political declaration on NCDS. September 2011.
- 8.Institute for Health Metrics and Evaluation . IHME, University of Washington; Seattle, WA: 2015. GBD compare.http://vizhub.healthdata.org/gbd-compare Available from. [Accessed 06.07.2020] [Google Scholar]
- 9.WHO NCD Action Portal. NCDprime - Modelling the impact of national policies on noncommunicable disease (NCD) mortality using PRIME: a policy scenario modelling tool. Available at:https://www.knowledge-action-portal.com/en/content/ncdprime-modelling-impact-national-policies-noncommunicable-disease-ncd-mortality-using [Accessed 07.07.2020]
- 10.Alston L, Peterson KL, Jacobs JP, Allender S, Nichols M. Quantifying the role of modifiable risk factors in the differences in cardiovascular disease mortality rates between metropolitan and rural populations in Australia: a macrosimulation modelling study. BMJ Open. 2017;7(11) doi: 10.1136/bmjopen-2017-018307. Nov 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bélanger M, Poirier M, Jbilou J, Scarborough P. Modelling the impact of compliance with dietary recommendations on cancer and cardiovascular disease mortality in Canada. Public Health. 2014;128(3):222–230. doi: 10.1016/j.puhe.2013.11.003. Mar 1. [DOI] [PubMed] [Google Scholar]
- 12.Goiana-da-Silva F, Severo M, Cruz e Silva D, João Gregório M, Allen LN, Muc M, Nunes A, Torres D, Miraldo M, Ashrafian H, Rito A, Wickramasinghe K, Breda J, Darzi A, Araújo F, Lopes C. Projected impact of the Portuguese sugar sweetened beverage tax on obesity incidence across different age groups: A modelling study. PLoS Med. 2020 doi: 10.1371/journal.pmed.1003036. March 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Goiana-da-Silva F, Cruz-e-Silva D, Allen L, Gregório MJ, Severo M, Nogueira PJ, Nunes AM, Graça P, Lopes C, Miraldo M, Breda J. Modelling impacts of food industry co-regulation on noncommunicable disease mortality, Portugal. WHO Bull. 2019;97:7. doi: 10.2471/BLT.18.220566. Jul 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nichols M, Scarborough P, Allender S, Rayner M. What is the optimal level of population alcohol consumption for chronic disease prevention in England? Modelling the impact of changes in average consumption levels. BMJ Open. 2012;2(3) doi: 10.1136/bmjopen-2012-000957. Jan 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Scarborough P, Morgan RD, Webster P, Rayner M. Differences in coronary heart disease, stroke and cancer mortality rates between England, Wales, Scotland and Northern Ireland: the role of diet and nutrition. BMJ Open. 2011;1(1) doi: 10.1136/bmjopen-2011-000263. Jan 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Briggs AD, Kehlbacher A, Tiffin R, Garnett T, Rayner M, Scarborough P. Assessing the impact on chronic disease of incorporating the societal cost of greenhouse gases into the price of food: an econometric and comparative risk assessment modelling study. BMJ Open. 2013;3(10) doi: 10.1136/bmjopen-2013-003543. Oct 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Briggs AD, Mytton OT, Kehlbacher A, Tiffin R, Rayner M, Scarborough P. Overall and income specific effect on prevalence of overweight and obesity of 20% sugar sweetened drink tax in UK: econometric and comparative risk assessment modelling study. BMJ. 2013;347:f6189. doi: 10.1136/bmj.f6189. Oct 31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Briggs AD, Mytton OT, Madden D, O'Shea D, Rayner M, Scarborough P. The potential impact on obesity of a 10% tax on sugar-sweetened beverages in Ireland, an effect assessment modelling study. BMC Public Health. 2013;13(1):860. doi: 10.1186/1471-2458-13-860. Dec 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nnoaham KE, Sacks G, Rayner M, Mytton O, Gray A. Modelling income group differences in the health and economic impacts of targeted food taxes and subsidies. Int J Epidemiol. 2009;38(5):1324–1333. doi: 10.1093/ije/dyp214. Oct 1. [DOI] [PubMed] [Google Scholar]
- 20.Scarborough P, Allender S, Clarke D, Wickramasinghe K, Rayner M. Modelling the health impact of environmentally sustainable dietary scenarios in the UK. Eur J Clin Nutr. 2012;66(6):710–715. doi: 10.1038/ejcn.2012.34. Jun. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Scarborough P, Harrington RA, Mizdrak A, Zhou LM, Doherty A. The preventable risk integrated ModEl and its use to estimate the health impact of public health policy scenarios. Scientifica. 2014 doi: 10.1155/2014/748750. 2014 Jan 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.WHO Europe: Modelling the impact of national policies on noncommunicable disease (NCD) mortality using PRIME: a policy scenario modelling tool. Available at:https://www.euro.who.int/__data/assets/pdf_file/0014/411251/PRIME-a-policy-scenario-modelling-tool.pdf. [Accessed 07.07.2020]
- 23.WHO Europe and Republic of Turkey Ministry of Health . In: National household health survey – prevalence of noncommunicable disease risk factors in Turkey 2017 (STEPS) Üner S, Balcılar M, Ergüder T, editors. World Health Organization Country Office in Turkey; Ankara: 2018. [Google Scholar]
- 24.Güler S, Budakoğlu İ, Besler T, Pekcan G, Türkyılmaz AS, Çıngı H, Buzgan T, Zengin N, Dilmen U, Tosun N. Methodology of national Turkey nutrition and health survey (TNHS) 2010. Med J Islamic World Acad Sci. 1565;109:1–23. 2014 Jan. [Google Scholar]
- 25.Erdem Y, Akpolat T, Derici Ü, Şengül Ş, Ertürk Ş, Ulusoy Ş, Altun B, Arıcı M. Dietary sources of high sodium intake in Turkey: SALTURK II. Nutrients. 2017;9(9):933. doi: 10.3390/nu9090933. Sep. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.World Health Organization. Healthy diet: key facts. Updated 29 April 2020. Available at:https://www.who.int/news-room/fact-sheets/detail/healthy-diet. [Accessed 13.07.2020].
- 27.Celis-Morales CA, Perez-Bravo F, Ibañez L, Salas C, Bailey ME, Gill JM. Objective vs. self-reported physical activity and sedentary time: effects of measurement method on relationships with risk biomarkers. PLoS One. 2012;7(5):e36345. doi: 10.1371/journal.pone.0036345. May 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nugent R, Bertram MY, Jan S, Niessen LW, Sassi F, Jamison DT, Pier EG, Beaglehole R. Investing in non-communicable disease prevention and management to advance the sustainable development goals. Lancet North Am Ed. 2018;391(10134):2029–2035. doi: 10.1016/S0140-6736(18)30667-6. May 19. [DOI] [PubMed] [Google Scholar]
- 29.WHO Global Coordination Mechanism on the Prevention and Control of NCDs. NCD and the Sustainable Development Goals. 2018. Available at:https://www.who.int/global-coordination-mechanism/ncd-themes/sustainable-development-goals/en/ [Accessed 20/07/2020].
- 30.UN General Assembly. Transforming our world: the 2030 Agenda for Sustainable development, A/RES/70/1. New York. 2015 Oct 21.
- 31.Mason H, Shoaibi A, Ghandour R, O'Flaherty M, Capewell S, Khatib R, Jabr S, Unal B, Sözmen K, Arfa C, Aissi W. A cost effectiveness analysis of salt reduction policies to reduce coronary heart disease in four Eastern Mediterranean countries. PLoS One. 2014;9(1):e84445. doi: 10.1371/journal.pone.0084445. Jan 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Afshin A, Sur PJ, Fay KA, Cornaby L, Ferrara G, Salama JS, Mullany EC, Abate KH, Abbafati C, Abebe Z, Afarideh M. Health effects of dietary risks in 195 countries, 1990–2017: a systematic analysis for the global burden of disease study 2017. Lancet North Am Ed. 2019;393(10184):1958–1972. doi: 10.1016/S0140-6736(19)30041-8. May 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.WHO. Guideline: Sodium intake for adults and children. 2012. Available at: https://www.who.int/nutrition/publications/guidelines/sodium_intake_printversion.pdf (Accessed 16/06/2020). [PubMed]
- 34.WHO Europe. Progress in reducing salt consumption in Turkey. 2013. Available at:https://www.euro.who.int/en/health-topics/disease-prevention/nutrition/news/news/2013/04/progress-in-reducing-salt-consumption-in-turkey. (Accessed 16/06/2020).
- 35.Allen L, Williams J, Townsend N, Mikkelsen B, Roberts N, Foster C, Wickramasinghe K. Socioeconomic status and non-communicable disease behavioural risk factors in low-income and lower-middle-income countries: a systematic review. The Lancet Glob Health. 2017;5(3):e277–e289. doi: 10.1016/S2214-109X(17)30058-X. Mar 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


