Abstract
Background:
Uveal melanoma is an uncommon subtype of this disease with poor prognosis. Agents that have transformed the management of cutaneous melanoma have made minimal inroads in uveal melanoma.
Methods:
We conducted a single-arm, phase II study of pembrolizumab in patients with metastatic uveal melanoma and performed bioinformatics analyses of publically available datasets to characterize the activity of anti-programmed death-1 (PD-1) in this setting, and to understand the mutational and immunologic profile of this disease.
Results:
Five patients received pembrolizumab on this study. The median overall survival was not reached; median progression-free survival was 11.0 months. One patient had a complete response following one dose and two others had prolonged stable disease (20% response rate, 60% clinical benefit rate). Two additional patients had rapidly progressing disease. Notably, the patients who benefited either had no liver metastases or small volume disease whereas rapidly progressing patients had bulky liver involvement. We performed bioinformatics analysis of The Cancer Genome Atlas for uveal melanoma, and confirmed a low mutation burden and low rates of T cell inflammation. Importantly, lack of T cell inflammation strongly correlated with MYC pathway overexpression.
Conclusions:
Anti-PD-1 based therapy may cause clinical benefit in metastatic uveal melanoma, seemingly more often in patients without bulky liver metastases. Lack of mutation burden and T cell infiltration, and MYC overexpression may be factors limiting therapeutic responses.
Keywords: Pembrolizumab, uveal, melanoma, PD-1, ocular
Introduction
Uveal melanoma is an uncommon but aggressive melanocytic neoplasm arising from the choroid, ciliary body, or iris. While radiation and surgery are effective treatment options for a subset of this disease, up to one-third of uveal melanomas will ultimately metastasize.1, 2 In the setting of metastatic disease, treatment options are quite limited and prognosis is poor. Immunotherapies, specifically those targeting programmed death-1 (PD-1) and cytotoxic T lymphocyte antigen-4 (CTLA-4) have transformed the management of cutaneous melanoma. These immunotherapies, however, have demonstrated generally low response rates in retrospective case series, with responses seen in 2–5% of patients.3, 4 Thus, effective therapies for this disease remain urgently needed.
Despite the low response rates in retrospective data, anti-PD-1 based therapy has not been explored in a prospective fashion in uveal melanoma. In addition, retrospective studies may be confounded by poor performance status, hepatic dysfunction, and multiple prior therapies. Thus, the effects of these agents have not been systematically characterized.
To explore this further, we conducted a phase II study of pembrolizumab in metastatic uveal melanoma. To gain further insights into the correlates of response and resistance, we performed bioinformatics analysis of The Cancer Genome Atlas (TCGA) for uveal melanoma.
Methods
Clinical Trial:
We conducted a multicenter, investigator-initiated, phase II study of pembrolizumab in uveal melanoma from 2015 – 2017 (NCT02359851). Patients with metastatic uveal melanoma naïve to PD-1 directed agents and adequate organ function and performance status were eligible. To detect a response rate, using RECIST 1.1, of 20% (null hypothesis 5%), sample size estimates using Simon’s optimal design planned for a stage 1 accrual of 10 patients with an accrual of an additional 19 patients if 1 response occurred in the first 10 patients. The study was opened at two centers, Vanderbilt University Medical Center and University of Chicago.
Bioinformatic Analysis:
The TCGA for uveal and cutaneous melanomas were queried.5 Mutation load was quantified for each sample and compared between uveal and cutaneous melanomas. T cell inflammation signatures were calculated based on RNA sequencing signatures and were quantified as inflamed, intermediate, and non-inflamed as previously published;6, 7 mutation load was then compared between T cell inflamed subsets. MYC gene expression and 21 target genes of MYC transcription were quantified in T cell inflamed subsets to determine whether MYC signatures correlate with T cell inflammation.
Results:
We enrolled 5 patients to the study between May 1, 2015 and January 6, 2017. The study was closed early due to slow accrual. Median age was 63, 3 patients had liver metastases, and 2 had prior therapies (Supplemental Data). Of these 5 patients, one had a complete response (ongoing at 25.5 months) and no partial responses were observed (response rate 20%). One additional patient experienced prolonged stable disease (ongoing at 11 months) with a decrease in tumor diameter approaching a partial response (-23%); an additional patient had stable disease lasting 11 months before progressing (clinical benefit rate 60%). On the other hand, two patients experienced rapid progressive disease and functional decline before even obtaining their first CT scan. Of note, tumors from patients who benefited were confirmed by molecular sequencing to harbor either GNAQ or GNA11 mutations but the patients had either no liver involvement or liver metastases of maximal diameter <1.2cm. By contrast, the two patients lacking clinical benefit had multiple, extensive liver metastases. The median progression-free survival was 11.0 months (Figure 1A) and median overall survival was not reached (Figure 1B; median follow up 11.1 months; range 0.4 to 25.5 months).
Figure 1A.
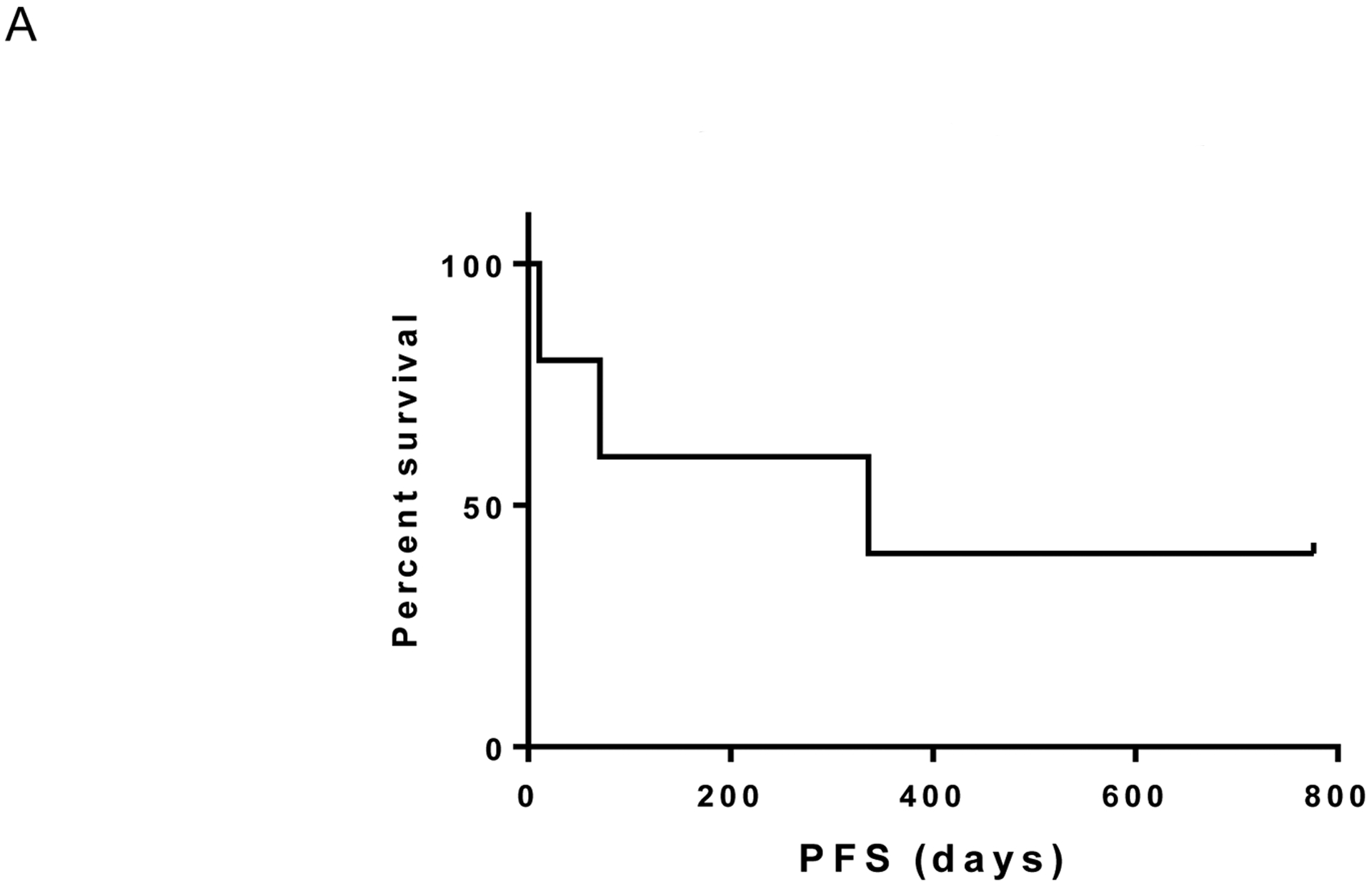
Progression-free survival
Figure 1B:
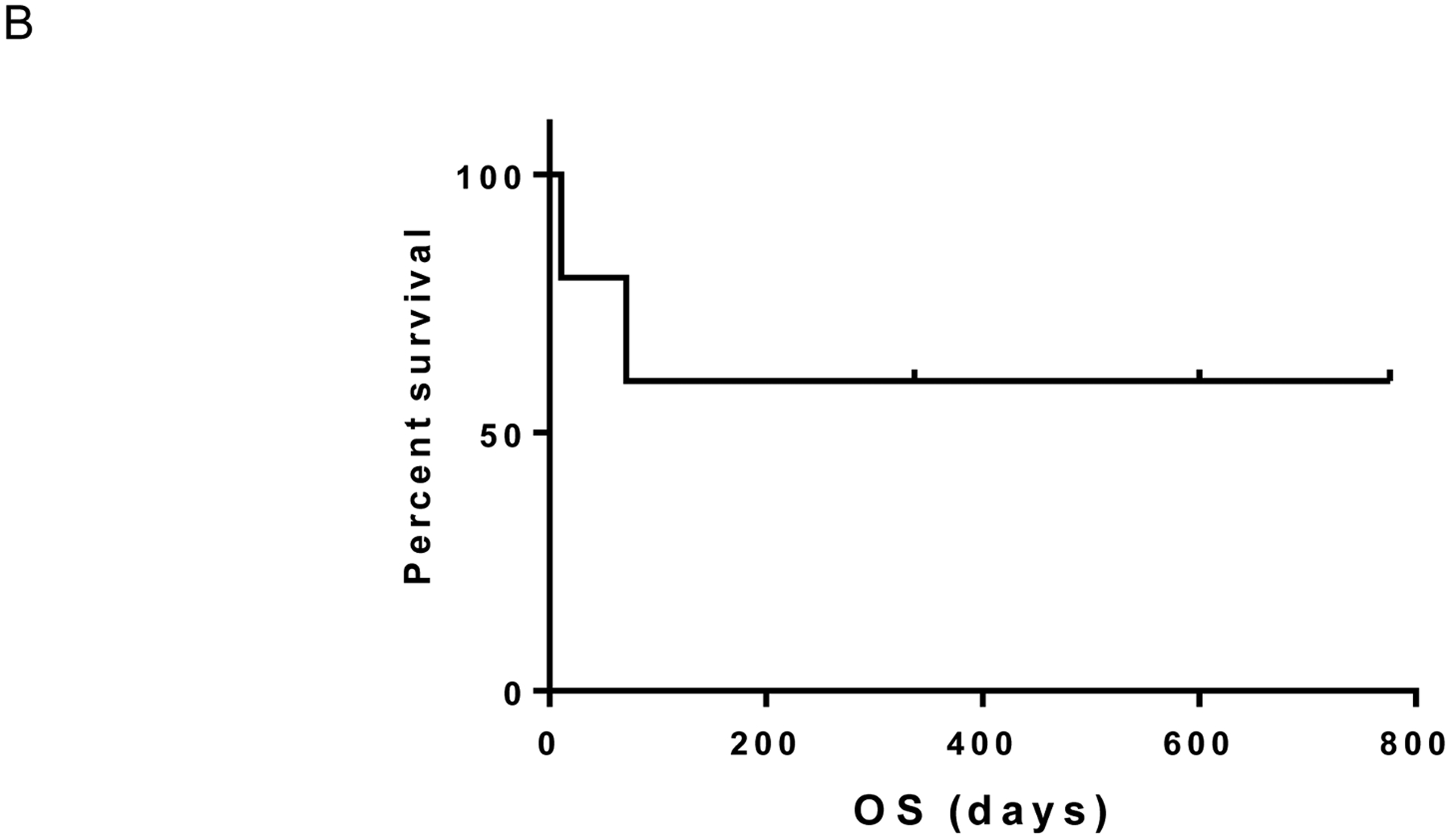
Overall survival
The toxicity profile was consistent with other pembrolizumab studies. The patient who experienced the complete response had fulminant type 1 diabetes (grade 4) that arose following the first dose of pembrolizumab. He stopped treatment based on his side effects and due to the observation of a complete response at his first CT scan. Other toxicities included grade 1 hypothyroidism and rash in one patient each; three patients had no side effects attributed to pembrolizumab. Extremely limited tissue was available for biologic correlates. Two samples, both core biopsies from liver metastases, were able to undergo PD-L1 testing. This revealed absent PD-L1 expression on both samples.
During the study retrospective data became available suggesting an extremely low response rate to PD1 monotherapy.3 We were therefore intrigued by the response and clinical benefit observed in our small series. Given the known association of PD1 response with density of non-synonymous somatic mutations8 and interferon-γ associated gene expression,9 we pursued an analysis of the TCGA stratifying the uveal melanoma cohort by our previously defined T cell-inflamed tumor microenvironment gene signature (with the caveat that TCGA are largely primary tumors rather than metastases).6 Consistent with prior investigations,6, 10 mutational burden was an order of magnitude lower in UM without a significant difference between T cell-inflamed and non-inflamed tumors (Figure 2). We observed that the vast majority of uveal melanomas demonstrated a highly non-T cell-inflamed tumor microenvironment; however a small number were T cell-inflamed (Figure 2 and 3A). Additionally the oncogene MYC, a known mediator of the non-T cell-inflamed tumor microenvironment11, was significantly up-regulated in non-T cell-inflamed uveal melanoma relative to intermediate and T cell-inflamed as evidenced by MYC gene expression (p =0.002) and MYC target molecules expression (p=0.0002; Figure 3A and 3B). These data perhaps suggest MYC directed therapies as rational combination approaches with immunotherapy in UM.
Figure 2. Non-synonymous tumor mutational load comparing skin cutaneous metastatic and uveal melanoma.
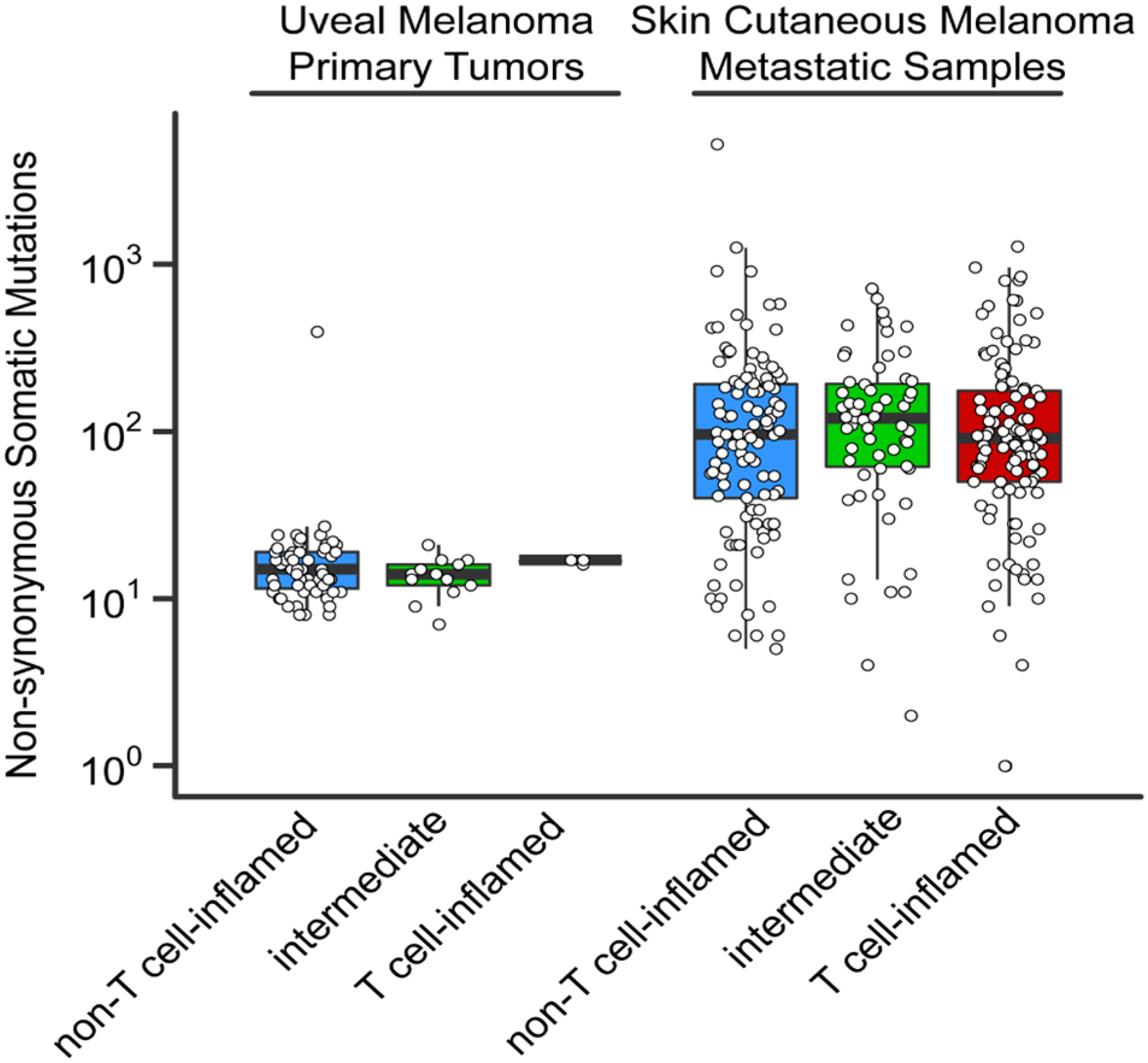
The vertical axis shows the total number of predicted protein-changing somatic mutations (single nucleotide variants and small insertions/deletions). Each data point represents one sample. The non-T cell-inflamed (blue), intermediate (green) and T cell-inflamed (red) tumor group are shown on the horizontal axis.
Figure 3A. MYC gene and target molecule expression by T cell-inflamed gene signature in uveal melanoma.
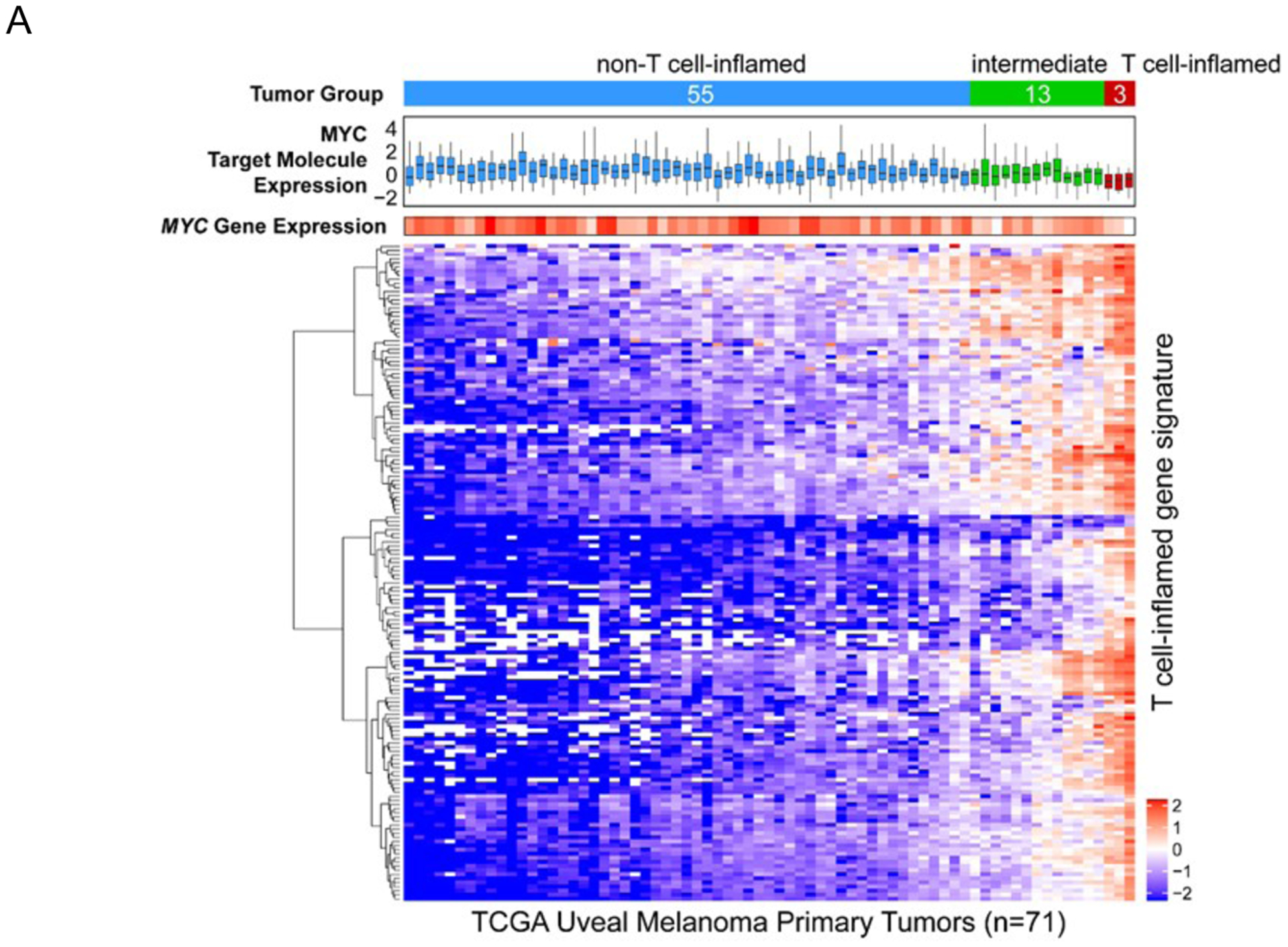
Top to bottom: annotation bar showing the non-T cell-inflamed (blue), intermediate (green) and T cell-inflamed (red) tumor group of uveal melanoma samples from TCGA database; boxplot showing the expression distribution of 21 annotated MYC signaling target molecules (CDC25A, CDK2, ECSIT, FASN, FSTL1, FUT3, GGH, HMGA1, IMPA2, IRS1, JARID2, LAMB2, MAG, MYC, MYH7, PYCR1, RTN2, RUVBL1, SLC16A1, SOX9, TKT) defined in Ingenuity Knowledgebase™ (QIAGEN Inc.) based on experimental evidence; small heatmap panel showing the expression pattern of MYC gene; larger heatmap panel showing the expression pattern of T cell-inflamed gene signature. The color key of the heatmaps are shown to the right corner of the heatmaps, with blue indicating lower expression, and red indicating higher expression.
Figure 3B. MYC gene expression in the non-T cell-inflamed (blue), intermediate (green) and T cell-inflamed (red) tumor group.
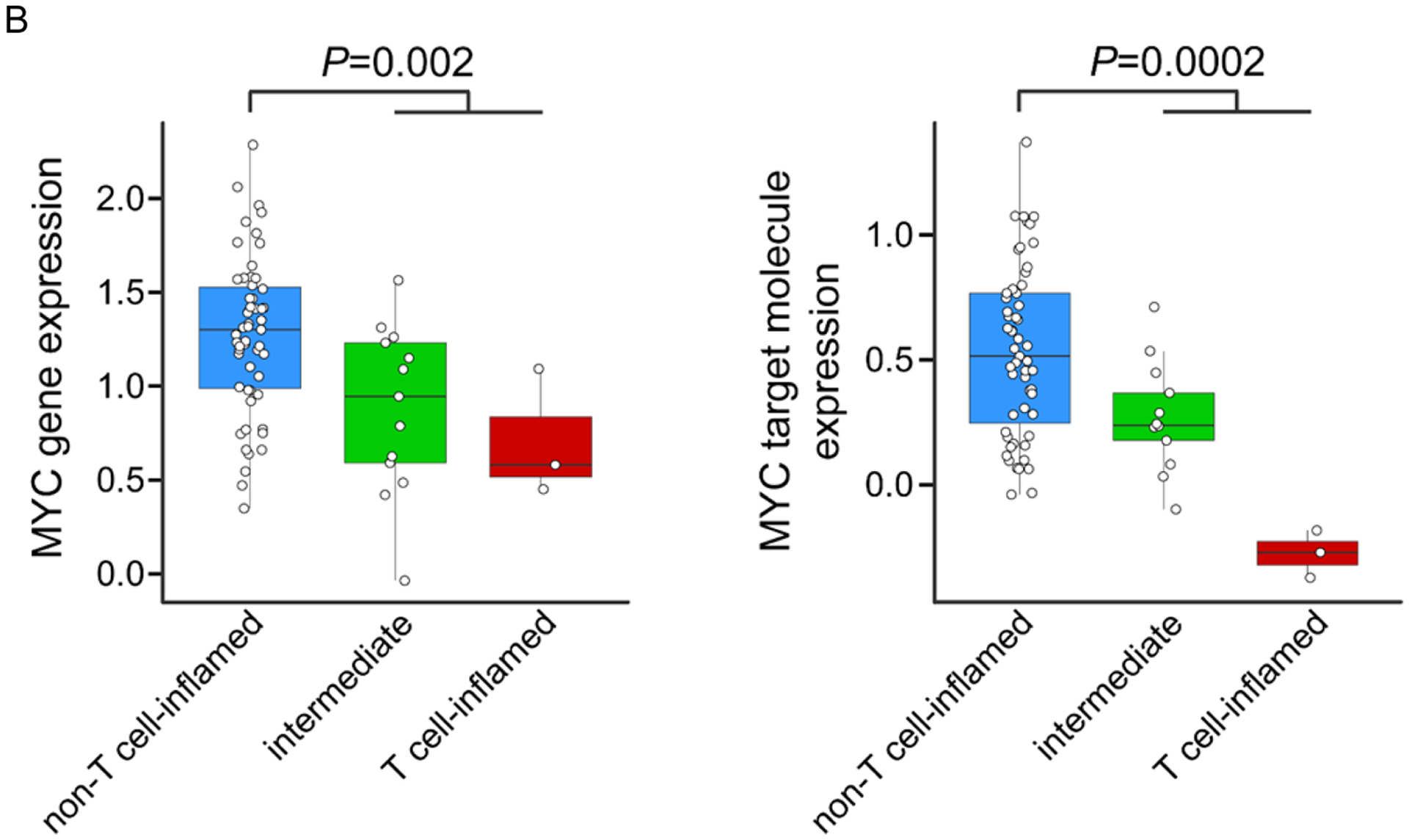
P-value was computed using two-sided Student’s t-test comparing non-T cell-inflamed group with intermediate and T cell-inflamed groups combined due to limited number of samples in the T cell-inflamed tumor group.
Discussion
This small study demonstrated that pembrolizumab can have activity in metastatic uveal melanoma. We learned several valuable lessons from this study. First, anti-PD-1 can produce profound and durable clinical benefit for individual patients with uveal melanoma. Although retrospective data have indicated extremely low response rates, our study suggests that patients with good performance status, adequate organ function and perhaps most importantly, lack of bulky liver metastases, may benefit from therapy. Clearly, however, the small sample size limits generalized conclusions regarding median survival and response rates. Second, accrual to a trial with an approved agent in a rare tumor type is very challenging. Pembrolizumab was approved for cutaneous melanoma in 2014, approximately the same time of our trial’s opening. Between frequent treatment in community practice, exclusion criteria, and subsequent availability of ipilimumab and nivolumab, accrual was a significant challenge, even at institutions that have historically had excellent accrual to uveal melanoma trials. Finally, our experience corroborates other studies that have suggested that patients with both cutaneous and uveal melanoma with bulky liver metastases have poor outcomes and urgently need novel therapeutics.3, 12 Combination clinical trials of hepatic directed therapies in combination checkpoint blockade are underway (ClinicalTrials.gov Identifier: NCT0291341). Additionally epigenetic modifier programs targeting MYC have been proposed13 and have begun to be explored in clinical trials (NCT02959437). Consideration should perhaps be given to future clinical trial designs in UM in order to stratify patients without liver metastases as they may have substantially better outcomes.
Supplementary Material
References:
- 1.Diener-West M, Reynolds SM, Agugliaro DJ, et al. Development of metastatic disease after enrollment in the COMS trials for treatment of choroidal melanoma: Collaborative Ocular Melanoma Study Group Report No. 26. Arch Ophthalmol 2005;123:1639–43. [DOI] [PubMed] [Google Scholar]
- 2.Collaborative Ocular Melanoma Study G. Assessment of metastatic disease status at death in 435 patients with large choroidal melanoma in the Collaborative Ocular Melanoma Study (COMS): COMS report no. 15. Arch Ophthalmol 2001;119:670–6. [DOI] [PubMed] [Google Scholar]
- 3.Algazi AP, Tsai KK, Shoushtari AN, et al. Clinical outcomes in metastatic uveal melanoma treated with PD-1 and PD-L1 antibodies. Cancer 2016;122:3344–3353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Luke JJ, Callahan MK, Postow MA, et al. Clinical activity of ipilimumab for metastatic uveal melanoma: A retrospective review of the Dana-Farber Cancer Institute, Massachusetts General Hospital, Memorial Sloan-Kettering Cancer Center, and University Hospital of Lausanne experience. Cancer 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cancer Genome Atlas Network. Electronic address imo and Cancer Genome Atlas N. Genomic Classification of Cutaneous Melanoma. Cell 2015;161:1681–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Spranger S, Luke JJ, Bao R, et al. Density of immunogenic antigens does not explain the presence or absence of the T-cell-inflamed tumor microenvironment in melanoma. Proc Natl Acad Sci U S A 2016;113:E7759–E7768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Spranger S, Bao R, and Gajewski TF. Melanoma-intrinsic beta-catenin signalling prevents anti-tumour immunity. Nature 2015. [DOI] [PubMed] [Google Scholar]
- 8.Johnson DB, Frampton GM, Rioth MJ, et al. Targeted Next Generation Sequencing Identifies Markers of Response to PD-1 Blockade. Cancer Immunol Res 2016;4:959–967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ayers M, Lunceford J, Nebozhyn M, et al. IFN-gamma-related mRNA profile predicts clinical response to PD-1 blockade. J Clin Invest 2017;127:2930–2940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Robertson AG, Shih J, Yau C, et al. Integrative Analysis Identifies Four Molecular and Clinical Subsets in Uveal Melanoma. Cancer Cell 2017;32:204–220 e15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Spranger S and Gajewski TF. Impact of oncogenic pathways on evasion of antitumour immune responses. Nat Rev Cancer 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tumeh PC, Hellmann MD, Hamid O, et al. Liver Metastasis and Treatment Outcome with Anti-PD-1 Monoclonal Antibody in Patients with Melanoma and NSCLC. Cancer Immunol Res 2017;5:417–424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Topper MJ, Vaz M, Chiappinelli KB, et al. Epigenetic Therapy Ties MYC Depletion to Reversing Immune Evasion and Treating Lung Cancer. Cell 2017;171:1284–1300 e21. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


