Abstract
Successful implementation of automated blood sampling (ABS) into a telemetry instrumented canine cardiovascular model provides simultaneous cardiovascular assessment of novel compounds while collecting multiple blood samples for analysis of drug level, cytokines, and biomarkers. Purpose-bred male Beagle dogs (n = 36) were instrumented with a dual-pressure telemetry transmitter and vascular access port. Modifications to acclimation practices, surgical procedures, and housing were required for implementation of ABS in our established cardiovascular canine telemetry colony. These modifications have increased the use and reproducibility of the model by combining early pharmacokinetic and cardiovascular studies, thus achieving both refinement and reduction from a 3R perspective. In addition, the modified model can shorten timelines and reduce the compound requirement in early stages of drug development. This telemetry–ABS model provides an efficient means to quickly identify potential effects on key cardiovascular parameters in a large animal species and to obtain a more complete pharmacokinetic–pharmacodynamic profile for discovery compounds.
Abbreviations: ABS, automated blood sampling; VAP, vascular access port
Safety pharmacology is an important facet of drug discovery and development. Prior to the first in-human studies, safety pharmacology outcomes are used to predict clinical risk profiles of potential new drugs. Dogs that are chronically instrumented for telemetry are a well-characterized in vivo model for safety pharmacology studies and for screening new compounds for cardiovascular physiologic effects.2 Relatively few blood collections are performed during conscious telemetry studies due to the potential disturbance of cardiovascular data.10 Personnel entering the room cause excitement in dogs, which can mask drug-induced changes in physiologic parameters, such as heart rate and blood pressure.10 The ‘work around’ for the excitation artifact involves obtaining blood samples from a different group of dogs (known as a satellite group) while recording physiologic changes in the first group or repeating the pharmacokinetic study in the telemetry-instrumented dogs. Pharmacokinetic properties of drugs vary due to individual dog variability and can vary across dosing events (for example, batch-to-batch variability). If adverse effects are noted on either study, determining a correlation of occurrences can be difficult especially when involving a single animal. These approaches do not compensate for individual variability, dosing variations between animals, or changing environmental conditions.9
Heart rate and circulating cortisol concentration increase during manual blood sampling as compared with automated blood sampling in macaques17 and swine.12 Automated blood sampling (ABS) is designed to collect blood painlessly from awake and freely moving animals.16 The use of ABS reduces stress from handling and eliminates the repeated venipunctures that are required for manual blood sampling.6 The implementation of ABS allowed us to achieve a complete pharmacokinetic–pharmacodynamic drug assessment in cardiovascular safety telemetry dog studies with no collection-related disturbance of physiologic parameters. Combining the pharmacokinetic and cardiovascular studies by using this method also reduces the need to synthesize more compound and shortens drug development timelines. Another advantage of this approach is the reduction of animal use by eliminating satellite animal groups.19
Our incorporation of ABS into cardiovascular safety telemetry dog studies required changes to the dog acclimation procedure, addition of vascular access ports (VAP) to the surgical protocol, and modifications to the canine housing. This report is an overview of how we successfully incorporated ABS into a colony of telemetry-implanted dogs and the modifications necessary for continued success.
Materials and Methods
Institutional statement.
AbbVie is committed to the internationally accepted standard of the 3Rs (reduction, refinement, replacement) and adheres to the highest standards of animal welfare in the company's research and development programs. Animal studies were approved by AbbVie's Institutional Animal Care and Use Committee. Animal studies were conducted in an AAALAC-accredited program, where veterinary care and oversight were provided to ensure appropriate animal care.
Animals.
Purpose-bred male Beagle dogs (n = 36; age, 8 to 12 mo; Marshall BioResources, North Rose, NY) were deemed healthy according to preoperative blood work and physical exam. Blood work and physicals were performed annually until 4.25 y of age and thereafter were performed biannually until 6 y of age, loss of cardiovascular signal, or exhausted battery life of telemeter device. Dogs were grouped-housed in one-over-one stainless steel dog racks (Allentown, Allentown, PA) except during the study. Daily food rations (Teklad Certified Global Diet 2025, Envigo, Madison, WI) were fed to maintain a normal body condition score. Water was available ad libitum. Animal rooms were maintained on a 12:12-h light:dark cycle, a temperature of 68 to 77 °F [20 to 25 °C], and relative humidity of 30% to 70%. Enrichment was provided by daily positive human interactions and facility-approved toys that were available on a rotational basis to maintain novelty.8
All dogs received a behavior assessment and were acclimated prior to implantation to several aspects of ABS telemetry studies (that is, study-related attire, tethering, dosing techniques). As a result of this acclimation, dogs were familiar with attire and procedures, had minimal stress related to the study, and had a low potential to show procedurally related changes in physiologic parameters.13 Over a 2- to 4-wk period, behavior staff used positive reinforcement to acclimate the dogs. Each dog was incrementally acclimated to wearing an undershirt (Surgi-Sox Torso; DogLeggs, York, PA; Figure 1), a custom dog jacket (Lomir Biomedical, Malone, NY), and a 4-foot tether (Lomir Biomedical). Prior to surgery, dogs were deemed successfully acclimated when no concerns were noted during a 24-h period of wearing the ABS attire.
Figure 1.
The undershirt is made of a breathable stretchy fabric which protects the port site and the slight compression aids to support the needle from dislodging during study. A small hole is cut in the back of the undershirt near the neck, allowing the right-angle extension line to be attached to the tethered line in the jacket. The positioning of the subcutaneous vascular access port is represented by the small blue circular grid area.
Surgical techniques.
Dogs were instrumented simultaneously with a dual-pressure transmitter (model no. TL11M3-D70-PCTP, Data Science International, St Paul, MN) and rounded-tip, tapered 4- to 6-Fr polyurethane catheter (AVA Biomedical, Wilmette, IL) with a medium titanium GridLock port (Grid-CP4-5NC, Access Technologies, Skokie, IL). All surgical implantations were performed using aseptic technique. Dogs were premedicated with 0.05 mg/kg acepromazine maleate (Boehringer Ingelheim, St Joseph, MO), 0.04 mg/kg atropine sulfate (Med-Pharmex, Pomona, CA), and 0.01 mg/kg buprenorphine hydrochloride (Buprenex Injectable 0.3 mg/mL, Rickett Benckiser Pharmaceuticals, Richmond, VA) administered intramuscularly. Immediately before induction, an intravenous catheter was placed, and the dogs received a preoperative intravenous injection of 0.2 mg/kg meloxicam (5 mg/mL Metacam, Boehringer Ingelheim) and 25 mg/kg cefazolin (Westward Pharmaceutical, Eatontown, NJ). Cefazolin dosing was repeated every 2 h perioperatively. Anesthesia was induced by using propofol (3.7 mg/kg IV; Propoflo 28, Abbott Laboratories, North Chicago, IL) until endotracheal intubation could be performed safely. Once dogs were intubated, the lumbosacral space was aseptically prepared for the administration of an epidural containing 0.01 mg/kg buprenorphine and 0.05 mg/kg bupivacaine (Preservative-free 0.5% bupivacaine hydrochloride injection, Hospira, Lake Forest, IL). Sodium chloride 0.9% injectable (Hospira) was used to bring the total volume administered to 0.3 mL/kg. This facilitated the advancement of the epidural anesthetic into the thoracic spinal column, enabling adequate pain management for thoracic procedures.3,23 All dogs were maintained at a surgical plane of anesthesia by using sevoflurane (SevoFlo, Abbott Laboratories) and oxygen for the duration of the procedure. Heated tables were used throughout the induction and implantation surgery to prevent hypothermia. Heart rate, respiratory rate, body temperature, oxygen saturation, and end-tidal carbon dioxide concentration were monitored continuously while dogs were anesthetized and recorded every 10 min by an anesthesia technician. The dogs were shaved and aseptically prepared for surgery. Dogs were maintained on warmed lactated Ringer solution (Hospira) at a flow rate of 10 to 20 mL/kg/h.
Previous publications have described telemetry transmitter5,14,25 and VAP15,24 implantation techniques similar to those we used. A ventral midline incision was made in the abdomen, starting at the xiphoid process extending caudally 1 to 2 cm beyond the umbilicus, and the abdomen accessed. Before opening the thorax, dogs were mechanically ventilated to provide appropriate oxygenation. Inspiratory volume was set at 10 to 15 mL/kg, and respiratory rate was 8 to 15 resp/min. The abdominal incision was extended cranially to a distance of 3 to 4 cm at the xiphoid process. The xiphoid process was partially split by using a pair of heavy scissors and the heart was visualized. The diaphragm was incised vertically from sternum to the musculotendinous junction. The pericardium was grasped and incised to expose the cardiac apex. The pericardial sac was sprayed with 1 to 2 mL of 2% lidocaine (Phoenix, St Joseph, MO) to reduce cardiac irritability. Two nonabsorbable stay sutures were placed on either side of the incised pericardium for retraction, to better visualize the apex of the left ventricle. Another nonabsorbable stay suture was placed in the epicardium, bringing the cardiac apex closer. A 3-0 nonabsorbable purse-string suture was placed in the left ventricular cardiac apex, taking care that no coronary vessels were trapped in the suture. By using a 16-gauge needle, stab incision was made in the wall of the apex of the left ventricle right in the center of purse-string suture, ensuring that the incision extended into the ventricular chamber. The left ventricle pressure catheter tip from the transmitter was passed through the stab incision into the chamber of the left ventricle and secured in place by using the purse-string suture. The diaphragm was closed by using absorbable suture in a continuous pattern, with the left ventricle pressure catheter exiting near the top (animal's ventral surface) of the incision. An approximately 1-cm uninsulated loop was created with the positive ECG lead and sutured to the abdominal side of the diaphragm in direct proximity to the cardiac apex. The partially split xiphoid process was closed with 2-0 nonabsorbable cruciate sutures. A medical aspirator with a 5-Fr red rubber feeding catheter attachment was used to establish negative pressure in the chest.
The dog was weaned off the ventilator and was able to breathe autonomously throughout the remainder of the surgery. The transmitter was secured to the abdominal wall of the peritoneal cavity by using 3 or 4 stay sutures of 2-0 nonabsorbable suture. To establish a lead II configuration, the negative solid-tip ECG lead exited the abdominal cavity and was tunneled subcutaneously to the right jugular furrow. It was advanced into the external jugular vein toward the superior vena cava until acceptable ECG waveform morphology was observed by using Ponemah software (Data Science International). Positioning of the negative solid-tip lead was confirmed by fluoroscopy (BV Pulsera, Philips Healthcare, Amsterdam, Netherlands). Two ligatures using 3-0 nonabsorbable suture were used to secure the negative solid-tip lead in the jugular vein.
An incision was made in the right femoral triangle, and the femoral artery and vein were isolated individually. The pressure catheter was exited out of the abdomen and subcutaneously tunneled to the right femoral area, where it was placed in the right femoral artery by using two 3-0 nonabsorbable ligatures. A rounded-tip, tapered, 4- to 6-Fr polyurethane catheter (AVA Biomedical) prefilled with 0.9% sodium chloride (Hospira) was secured within the right femoral vein by using two 3-0 nonabsorbable ligatures. The venous catheter was subcutaneously tunneled and exteriorized through a small incision off the right dorsal spine, cranial to the last 2 to 3 ribs, and connected to a medium titanium GridLock port (model no. Grid-CP4-5NC, Access Technologies) which was anchored to the underlying musculature by using 2-0 nonabsorbable suture. Incisions were closed appropriately in 2 or 3 layers and staples were used for skin closure. The dead space of the VAP was measured in preparation for future ABS use. The VAP was flushed with 0.9% sodium chloride (Hospira). Taurolidine–citrate catheter solution (Access Technologies) was used for the locking solution.
During recovery, dogs were placed in heated cages and continuously monitored until extubation and return of sternal recumbency. Once ambulatory, dogs were returned to their home cages. Postoperative medications consisted of buprenorphine (0.01 to 0.02 mg/kg SC) twice daily for 3 d, meloxicam (0.01 mg/kg PO; Metacam 1.5 mg/mL oral suspension, Boehringer Ingelheim) once daily for 4 d, and enrofloxacin (5 mg/kg PO; Baytril Taste Tabs, Bayer HealthCare, Shawnee Mission, KS) once daily for 10 d. Skin staples were removed at 14 d after surgery if incisions were adequately healed. Dogs were allowed to recover completely from the surgical procedure. When dogs were not on study, VAP patency was checked monthly by using 0.9% sodium chloride (Hospira) and locked with taurolidine–citrate catheter solution (Access Technologies).
ABS.
ABS units (Culex-L, BASi, West Lafayette, IN) were kept in modified wheeled Metro cabinets (Wilkes-Barre, PA) that permit ABS extension lines to be accessed from either side of the unit (Figure 2). Protocol information and blood collection time points were entered into the ABS software. Sterile tubing kits (BASi) were installed on each unit, and lines were filled with heparinized sodium chloride (10 U/mL;Hospira). Clean technique was used when manipulating all connections. The dead spaces of the extension lines and VAP were programmed into the software to assure appropriate blood withdrawal volumes during study. Blood collection tubes were placed in the refrigerated (4 °C) fraction collector carousel. To prevent coagulation of blood in the extension lines, the software was programmed to administer a small volume of heparinized saline (10U/mL) automatically (known as the Tend function) once the dogs were tethered for study.
Figure 2.
ABS set up for cardiovascular telemetry dog study.
Experimental setup.
Dogs were singly housed in ABS-modified caging for at least 24 h prior to study start, and cardiovascular signals were verified. ABS cabinets were positioned next to the ABS-modified telemetry dog cages on the day of study. Study data were entered into the ABS software, and tubing kits were installed on each ABS unit.
Study attire including a jacket, tether, and swivel (Lomir Biomedical) were prepared (Figure 3). The external line (36-in. polyurethane extension line with female blunt luer, SAI Infusion Technologies, Lake Villa, IL) was connected to the sterile swivel. The opposite end with the female blunt luer was connected to a Clave male adapter plug (Zoetis, Kalamazoo, MI). The internal line (58-in. polyurethane extension line with male luer and silicone plug, SAI Infusion Technologies) was tunneled through the tether with the silicone plug intact to maintain sterility. The silicone plug was then removed cleanly, and the line was connected to the swivel. The tether was attached to the swivel, and the opposite end of the internal line exited into the jacket. The entire extension line was primed by using heparinized saline (10 U/mL).
Figure 3.
Custom dog jacket connected to a 4-ft tether with attached swivel, ready for study use. Extension lines are attached to the swivel. One line is introduced through the tether to the custom dog jacket. The other line is intended for the ABS unit once the dog is set up.
An approximately 3 × 3-in. area over the VAP was shaved, cleared of any excess hair, and finally aseptically prepared. A non-coring 20-gauge, right-angle, 3/4-in. Huber needle extension set (Access Technologies) attached to a 3-mL syringe filled with heparinized saline (10 U/mL) was used to access the port. Patency of the VAP was confirmed. The dog was then placed in the undershirt and jacket tethered to the swivel. The 3-mL syringe was removed from the Huber needle extension set, and the end of the extension set was cleanly connected to the extension line within the jacket. Once the dog was placed in the study cage, the blank plate was removed from the swivel plate inlet and replaced with the swivel for study. The extension line with adapter plug was connected to the tubing kit on the ABS unit. The Tend volume was enabled on the ABS unit, thus automatically administering 200 µL of 10 U/mL heparinized saline every 12 min to prevent blood clot formation in the lines while the dogs were attached to the ABS.
The ABS program was initiated once dosing was complete. During sampling, the 3.5-mL total dead space volume of the VAP and ABS lines, consisting of a mixture of diluted blood and heparinized saline, temporarily collects in the reservoir of the ABS unit. An undiluted blood sample (0.5 mL) was then collected and dispersed into the correct EDTA collection tube (Greiner, Monroe, NC) in the refrigerated carousel. The diluted blood mixture in the reservoir was then returned to the dog, and the Tend function resumed until the next blood sample collection time point. Any remaining diluted blood within the collection lines of the ABS unit was then flushed to the waste bag. Blood was collected automatically as programmed while the dogs maintained their normal behaviors (eating, drinking, sleeping, and playing). During a 24 h study, dogs were checked at least once for clinical issues, needle dislodgement, and machine malfunction. Blood samples were stored in the 4 °C refrigerated carousel of the ABS fraction collector until processed at the end of the study.
At the completion of the study, the VAP was locked with taurolidine–citrate catheter solution, and the Huber needle extension set was removed. A small amount of triple-antibiotic ointment was applied to the needle puncture site and surrounding skin. The ABS tubing kit and all extension lines were disconnected and discarded appropriately. Jackets, tethers, and undershirts were removed from dogs and laundered. Swivels were cleaned and sterilized prior to next use.
Modifications.
Housing.
To maximize room utilization and to minimize dead space between the ABS unit and the dog, existing telemetry cages required modifications. To hold swivels during studies, swivel plate inlets (Figure 4 A) were created in the upper corner of the hinged side of all ECG study rack cage doors (Figure 4 C). On half of the ECG study racks, the doors were reversed, allowing them to open from the opposite direction (Figure 4 D). Blank plates (Figure 4 B) were placed in the inlets when dogs were not on study.
Figure 4.
(A) Swivel plate inlet. The inlet was fabricated to securely house a plate on the hinged side of the cage door. Attaching the tether from this height within the cage allows the dog full ability to move around the cage. (B) Blank plate placed in the swivel plate inlet when dogs are not on study. The small tab was devised for a lock if needed to secure the plate. (C) Swivel in place for study use. The outside pin of the swivel is covered by a removable piece of plastic tubing to reduce the chance of damage from equipment or animals. (D) ECG study rack. Door modified to be opened in the opposite direction. Reduces the length (dead space) of lines from the cage to the ABS unit. Cage door is reversed, and swivel plate inlet is in the upper left area near door hinges.
Acclimation.
The acclimation process was modified by the addition of a mock study after the dogs had completely healed from implantation surgery. This refinement to the original acclimation procedures incorporates all aspects of an actual study to further minimize potential stress responses.13,21 This also allows technicians to determine needle placement for future studies and observe the demeanor of the dogs with complete ABS attire.
VAP.
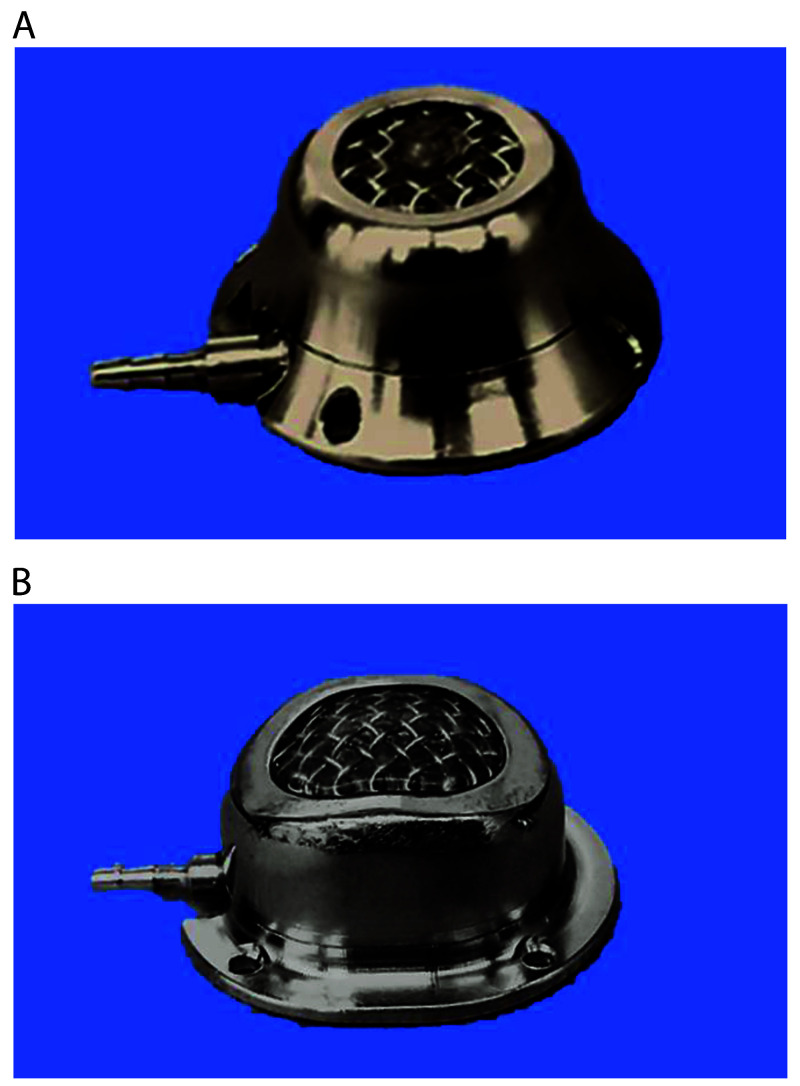
GridLock ports with the ‘sweet spot’ (Access Technologies; Figure 5 A) were used in many of our VAP implanted colonies (rabbits, NHP, pigs). The easily palpable ‘sweet spot’ made the port easily accessible for monthly patency checks, and the peripheral grid worked well for study use. However, if the Huber needle was inadvertently placed in this ‘sweet spot’ instead of in the grid area, the needle was easily dislodged. To address this issue, we switched to the GridLock SwirlPort (Access Technologies; Figure 5 B), which incorporates the grid throughout the entire port.
Figure 5.
(A) GridLock port with the ‘sweet spot’ adaptation. (B) GridLock SwirlPort.
Results
Modifications.
Caging.
Reversing cage doors allowed technical staff full access to dogs and minimized tension of the extension line within the tether and of the extension line from the swivel to the ABS unit. Blank plates were placed in the swivel plate inlet to prevent possible injury of dogs when not being used for ABS collection, thereby allowing caging to be incorporated into normal facility use, if necessary.
Acclimation.
The addition of mock studies for the newly implanted dogs enhanced the overall success of samples by allowing technicians to become familiar with the idiosyncrasies of each dog. The introduction of the ABS units during a mock study allowed the dogs to become familiar with noises from the ABS units before being placed on an actual telemetry study. Introducing the dogs to the machines prior to a study helps to reduce stress and anxiety, which could lead to unwanted variability in physiologic parameters.13
Port selection.
GridLock SwirlPorts incorporate the grid throughout the septum and have been used in our most recently implanted dogs. Among 25 dogs that received the GridLock port with the sweet spot, ABS blood sampling was successful in 82%, whereas in 11 dogs that received the GridLock SwirlPort, ABS sampling was successful in 96%. Septum damage has not yet been noted in these ports.
Study use.
Between 2016 and 2019, the proportion of total telemetry studies that included ABS increased from 29% to 88% of studies conducted in a given year. A total of 36 Beagles were used for 48 ABS studies throughout the 4 y period. Dogs with implanted telemetry were used on ABS studies an average of 5 times annually, with an average service life of 2 y. The percentage of successfully collected blood samples that used ABS during cardiovascular telemetry studies for the years 2016 - 2019 were 79%, 86%, 95%, and 93% respectively).
As an example, a study using dofetilide (PZ0016, Sigma-Aldrich), administered at 0.1 and 0.3 mg/kg PO, produced a dose-dependent prolongation of the QTcV interval (Figure 6 A). ABS was used to collect samples at 0, 0.25, 0.5, 1, 1.5, 2, 4, 6, 8, 12, and 24 h during a 24-h study in dogs with cardiovascular telemetry (n = 4). Figure 6 B shows the pharmacokinetic profile of dofetilide; Cmax values for the oral doses of 0.1 and 0.3 mg/kg were 26 and 85 ng/mL, respectively.
Figure 6.
(A) Effect of dofetilide on the corrected QT interval (Van de Water) in a conscious telemetered dog. (B) Pharmacokinetic profile of dofetilide over 24-h by using ABS.
Needle complications.
The Huber needle length is important when working with the dog VAP model. The Huber needle must be fully inserted beyond the port septum to allow adequate blood flow for ABS. Typically a right-angle Huber extension set is used to limit protrusion from the skin when using a jacketed animal model.24 We found that a slightly longer right angle works best. A 3/4-in. right-angle Huber needle that is fully inserted into the port still protrudes from the skin by approximately 2 to 3 mm (Figure 7), allowing slight movement of the skin without immediate dislodgement of the needle. Inserting the needle of the right-angle Huber extension set at a slight angle into the GridLock port allowed for more secure needle placement during studies. The undershirt made of stretchy bandage-like material helped to keep the needle in the port.
Figure 7.
Right-angle, 20-gauge, 3/4-in. Huber needle extension set placed in port for study. The extra length of Huber needle visible outside the skin over the port allows for the skin to move without immediately dislodging the needle.
Broken Huber needles were observed in 2 dogs during the 4-y period. Both of the broken needles remained in the GridLock ports’ septum and were easily palpable. The dogs were immediately removed from study, and the needles were removed aseptically under anesthesia.
Discussion
ABS from conscious, freely moving animals with surgically implanted vascular catheters16,24 is widely used in rodents7,9,22swine,4,12,18 and nonhuman primates.1,20 ABS has been used in combination with telemetry studies in rodents successfully for years.9,11 Published data of ABS concurrent with cardiovascular telemetry in canine studies is sparse. Traditionally in canine cardiovascular telemetry studies, blood sampling is minimal. However, blood sampling without disturbances that may introduce excitation artifacts is desirable. ABS provides more robust and accurate data, reduced animal use, and improved animal welfare. The implantation of the VAP for ABS use allows a reduction in stress caused by multiple venipunctures and allows the completion of a full pharmacokinetic profile during cardiovascular safety telemetry studies, with no interference to cardiovascular data.24
We have encountered many technical difficulties while working with VAP dogs. Positioning of the skin over an immobilized port was the most difficult issue that we had to overcome. Even when dogs are tethered, they are very mobile and continue normal dog behaviors. Canine skin shifts with each movement, which can create tension between the needle and port. We have observed skin shift of about 2 in. in some dogs, depending on its position (standing or sitting).
Needle movement in the port increases due to the movement of the skin, especially when the dog is highly active. The needle was much more likely to dislodge from the port when inadvertently placed in the ‘sweet spot’ of the GridLock port. This led to increased septum damage due to the needle movement, which appeared to create a slice in the septum. An immediate swelling of the area around the port when accessing the VAP or a firm palpable mass directly over the port was evidence of severe septum damage. Specific care is required when a Huber needle becomes dislodged during a dog ABS study. Needles that were completely out of the skin require an aseptic prep of the port area before placement of a new right-angle, 20-gauge Huber needle extension set prefilled with heparinized saline. The original Huber needle extension set was then removed from the extension line in the tethered jacket and replaced with the new Huber needle extension set by using a clean technique.
When a needle is dislodged, the Tend volume continues to be infused due to lack of resistance. Mild swelling over the port area was apparent if the needle remained in the subcutaneous tissue. This swelling resolved on its own, typically without issue. A Huber needle can bend in a port during study, although not enough to impede blood collection. Bent needles can be difficult to detach from the port at the end of a study. This process requires careful manipulation to reduce risk of trauma to the skin, port damage, or accidental needle breakage. VAP placement is also a key in successful needle securement. If the port is placed too low, the dog can scratch or chew at the Huber needle and manipulate the extension lines from under the jacket. When the port is too high on the back or when the undershirt and jacket are fitted incorrectly, the needle can be pulled out as well.
We have been able to overcome these difficulties throughout the years. We have achieved placement of the port (Figure 1) that is high enough to prevent the dog access and low enough that the jacket and t-shirt are not issues. To combat the skin shift issue, before starting the sterile set-up process, the technician holding the dog raises its forelimbs off of the table in a jumping or stretching motion and then places the dog back in a sitting position. The technician who accesses the port can observe and manipulate the skin to determine the best needle insertion point.
Extending the dog acclimation procedures to include a mock study has further helped to reduce stress in our cardiovascular telemetry dogs. The dogs become accustomed to the additional equipment (that is, Metro cabinets that house the ABS units) and to the operational sounds of the ABS units. This additional acclimation further reduces possible disturbances of physiologic parameters once dogs were placed on actual cardiovascular safety studies.13
In summary, ABS allows completion of a full pharmacokinetic–pharmacodynamic profile and cardiovascular assessment during the same study. This greatly reduces the number of dogs used, shortens study timelines, and considerably reduces compound synthesis and the associated costs. With the successful implementation of ABS, we have established a new standard model for cardiovascular safety studies in our telemetry dogs.
Acknowledgments
We thank AbbVie's Department of Comparative Medicine for their assistance with the maintenance of the dog telemetry colony. We also thank AbbVie's Behavioral Management Team for their contributions to the dog telemetry colony's acclimation and behavior training. Finally, we thank AbbVie's Drug Metabolism, Pharmacokinetics and Bioanalysis department for blood sample analysis. All authors are employees of AbbVie. AbbVie provided the design, study conduct, and financial support for this research. AbbVie participated in the interpretation of data, review, and approval of the publication.
References
- 1.Bentson KL, Miles FP, Astley CA, Smith OA. 1999. A remote-controlled device for long-term blood collection from freely moving, socially housed animals. Behav Res Methods Instrum Comput 31:455–463. 10.3758/BF03200726. [DOI] [PubMed] [Google Scholar]
- 2.Food and Drug Administration, HHS. 2001. International Conference on Harmonisation; guidance on S7A safety pharmacology studies for human pharmaceuticals; availability. Notice. Fed Regist 66:36791–36792. [PubMed] [Google Scholar]
- 3.Freire CD, Torres ML, Fantoni DT, Cavalcanti RL, Noel-Morgan J. 2010. Bupivacaine 0.25% and methylene blue spread with epidural anesthesia in dog. Vet Anaesth Analg 37:63–69. 10.1111/j.1467-2995.2009.00493.x. [DOI] [PubMed] [Google Scholar]
- 4.Gilbert CL, Boulton MI, Forsling ML, Goode JA, McGrath TJ. 1997. Restricting maternal space during parturition in the pig. Effects on oxytocin, vasopressin and cortisol secretion following vagino-cervical stimulation and administration of naloxone. Anim Reprod Sci 46:245–259. 10.1016/S0378-4320(96)01596-5. [DOI] [PubMed] [Google Scholar]
- 5.Henriques TA, Beck TW, Douglas CL, Jones HM, Kremer JJ, Kruzich PJ, Sarazan RD. 2010. Left thoracotomy surgical approach for chronic instrumentation in dogs and monkeys providing high-quality electrocardiogram signals. J Pharmacol Toxicol Methods 62:136–142. 10.1016/j.vascn.2010.05.017. [DOI] [PubMed] [Google Scholar]
- 6.Holmberg A, Pelletier R. [Internet]. 2009. Automated blood sampling and the 3Rs. The National Centre for the Replacement R, and Reduction of Animals in Research. [Cited 13 April 2020]. Available at: http://www.nc3rs.org.uk/news.asp?id=1039 [Google Scholar]
- 7.Hopper LD. 2016. Automated microsampling technologies and enhancements in the 3Rs. ILAR J 57:166–177. 10.1093/ilar/ilw020. [DOI] [PubMed] [Google Scholar]
- 8.Institute for Laboratory Animal Research. 2011. Guide for the care and use of laboratory animals, 8th ed. Washington (DC): National Academies Press. [Google Scholar]
- 9.Kamendi HW, Brott DA, Chen Y, Litwin DC, Lengel DJ, Fonck C, Bui KH, Gorko MA, Bialecki RA. 2010. Combining radio telemetry and automated blood sampling: a novel approach for integrative pharmacology and toxicology studies. J Pharmacol Toxicol Methods 62:30–39. 10.1016/j.vascn.2010.04.014. [DOI] [PubMed] [Google Scholar]
- 10.Klumpp A, Trautmann T, Markert M, Guth B. 2006. Optimizing the experimental environment for dog telemetry studies. J Pharmacol Toxicol Methods 54:141–149. 10.1016/j.vascn.2006.03.010. [DOI] [PubMed] [Google Scholar]
- 11.Litwin DC, Lengel DJ, Kamendi HW, Bialecki RA. 2011. An integrative pharmacological approach to radio telemetry and blood sampling in pharmaceutical drug discovery and safety assessment. Biomed Eng Online 10:1–11. 10.1186/1475-925X-10-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Marchant-Forde JN, Matthews DL, Poletto R, McCain RR, Mann DD, DeGraw RT, Hampsch JM, Peters S, Knipp GT, Kissinger CB. 2012. Plasma cortisol and noradrenalin concentrations in pigs: automated sampling of freely moving pigs housed in the PigTurn® versus manually samples and restrained pigs. Anim Welf 21:197–205. 10.7120/09627286.21.2.197. [DOI] [Google Scholar]
- 13.Meunier LD. 2006. Selection, acclimation, training, and preparation of dogs for the research setting. ILAR J 47:326–347. 10.1093/ilar.47.4.326. [DOI] [PubMed] [Google Scholar]
- 14.Mitchell AZ, Bills AJ, Wittwer GT, Foley CM, Kremer JJ, Chen H, Osinski MA. 2013. Intravenous solid tip ECG lead placement in telemetry implanted dogs: Part 2: High quality telemetry signals yield high sensitivity to drug-induced changes. J Pharmacol Toxicol Methods 68:62–73. 10.1016/j.vascn.2013.04.006. [DOI] [PubMed] [Google Scholar]
- 15.Moroni M, Coolbaugh TV, Mitchell JM, Lombardini E, Moccia KD, Shelton LJ, Nagy V, Whitnall MH. 2011. Vascular access port implantation and serial blood sampling in a Gottingen minipig (Sus scrofa domestica) model of acute radiation injury. J Am Assoc Lab Anim Sci 50:65–72. [PMC free article] [PubMed] [Google Scholar]
- 16.Peters SHJ, Cregor M, Starrett C, Gunaratna G, Kissinger C. 2000. Culex ABS Part I: Automated blood sampling. Curr Sep 18:139–145. [Google Scholar]
- 17.Reinhardt V, Cowley D, Eisele S. 1991. Serum cortisol concentrations of single-housed and isosexually pair-housed adult rhesus macaques. J Exp Anim Sci 34:73–76. [PubMed] [Google Scholar]
- 18.Roth WJ, Kissinger CB, McCain RR, Cooper BR, Marchant-Forde JN, Vreeman RC, Hannou S, Knipp GT. 2013. Assessment of juvenile pigs to serve as human pediatric surrogates for preclinical formulation pharmacokinetic testing. AAPS J 15:763–774. 10.1208/s12248-013-9482-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Russell WMS, Burch RL. 1959. The principles of humane experimental technique. London: Methuen and Company. [Google Scholar]
- 20.Schultze AE, Anderson JM, Kern TG, Justus RW, Lee HY, Zieske LR, Goodson RJ, Florey SH. 2015. Longitudinal studies of cardiac troponin I concentrations in serum from male cynomolgus monkeys: resting values and effects of oral and intravenous dosing on biologic variability. Vet Clin Pathol 44:465–471. 10.1111/vcp.12272. [DOI] [PubMed] [Google Scholar]
- 21.Slaughter MR, Birmingham JM, Patel B. 2002. Extended acclimatization is required to eliminate stress effects of periodic blood-sampling procedures on vasoactive hormones and blood volume in beagle dogs. Lab Anim 36:403–410. 10.1258/002367702320389062. [DOI] [PubMed] [Google Scholar]
- 22.Soto M, Pham R, Almon V, Wagner M, Primack R, Ponce M, Meyer J, James CA, Salyers KL, Retter MW. 2014. Evaluation of matrix microsampling methods for therapeutic drug candidate quantification in discovery-stage rodent pharmacokinetic studies. Bioanalysis 6:2135–2146. 10.4155/bio.14.184. [DOI] [PubMed] [Google Scholar]
- 23.Steagall PVM, Simon BT, Teixeira Neto FJ, Luna SPL. 2017. An update on drugs used for lumbosacral epidural anesthesia and analgesia in dogs. Front Vet Sci 4:1–12. 10.3389/fvets.2017.00068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Swindle MM, Nolan T, Jacobson A, Wolf P, Dalton MJ, Smith AC. 2005. Vascular access port (VAP) usage in large animal species. Contemp Top Lab Anim Sci 44:7–17. [PubMed] [Google Scholar]
- 25.Walisser JA, Mitchell AZ, Bills AJ, Sharma AK, Latimer K, Taschwer M, Osinski MA. 2013. Intravenous solid tip lead placement in telemetry implanted dogs. Part 1: Surgical methods, signal quality, and pathological endpoints. J Pharmacol Toxicol Methods 68:52–61. 10.1016/j.vascn.2013.04.001. [DOI] [PubMed] [Google Scholar]