Abstract
Background and Aim:
The impact of household income, a surrogate of socioeconomic status, on hospital readmission rates for patients with decompensated cirrhosis has not been well characterized.
Methods:
The Nationwide Readmission Database from 2012 to 2014 was used to study the association of lower median household income on 30-, 90-, and 180-day hospital readmission rates for patients with decompensated cirrhosis.
Results:
From the 42 679 001 hospital admissions contained in the sample, there were 82 598 patients with decompensated cirrhosis who survived a hospital admission in the first 6 months of the year. During a uniform 6-month follow-up period, 25 914 (31.4%), 39 928 (48.3%), and 47 496 (57.5%) patients were readmitted at 30, 90, and 180 days, respectively. After controlling for demographic and clinical confounders, patients residing in the three lowest income quartiles were significantly more likely to be readmitted at 30 days than those in the fourth quartile (first quartile, odds ratio [OR] 1.32 [95% confidence interval, CI, 1.17–1.47, P < 0.01]; second quartile, OR 1.25 [95% CI 1.13–1.38, P < 0.01]; and third quartile, OR 1.08 [95% CI 0.97–1.20, P = 0.07]). The association between lower socioeconomic status and the higher risk of readmissions persisted at 90 days (first quartile, OR 1.21 [95% CI 1.14–1.30, P < 0.01]) and 180 days (first quartile, OR 1.32 [95% CI 1.20–1.44, P < 0.01]).
Conclusion:
Patients with decompensated cirrhosis residing in the lowest income quartile had a 32% higher odds of hospital readmissions at 30, 90, and 180 days compared with those in the highest income quartile.
Keywords: Cirrhosis, NRD, Readmission, Socioeconomic status
Introduction
There are an estimated 5.5 million people (2.2% of the population) living in the USA with chronic liver disease.1–3 Ongoing liver inflammation and fibrosis leads to the development of cirrhosis, a disease state characterized by hepatic decompensation and repeated hospital admissions. Reported 30-day readmission rates for patients with decompensated cirrhosis range from 30% to 40%, resulting in a substantial economic burden.4–6 Annual estimated costs for a patient with decompensated cirrhosis are over $60 000 (USD) with the majority of costs related to inpatient care and repeated hospitalizations.7 For example, hepatic encephalopathy (HE) is responsible for over 100 000 discharges per year in the USA at an average cost of $17 812 per admission.8 Given the economic impact of frequent hospital readmissions as well as its negative effect on health-care-related quality of life, attention has been devoted to predicting and ultimately reducing readmissions in this population.9
Many patient-level factors have been associated with higher readmission rates including disease severity (model of end-stage liver disease [MELD] score and hyponatremia), number of discharge medications, and specific decompensating events such as HE.4–6,10 In addition, there is a growing literature demonstrating that socioeconomic status (SES) is a predictor of hospital readmissions in numerous patient populations.11–14 It has been suggested that SES also impacts readmission rates in cirrhosis, with Medicaid status being associated with higher readmission rates in one single-center study.6 However, the relationship between household income, a surrogate marker of SES, and readmission rates in decompensated cirrhosis has not yet been examined in a large, heterogeneous, generalizable nationwide sample.
The primary aim of our study was to use a nationally representative database to examine the relationship between household income and 30-, 90-, and 180-day hospital readmission rates in patients with decompensated cirrhosis. Based on existing literature in non-liver patient populations, we hypothesized that patients with lower household income would have higher readmission rates.11–13,15,16
Methods
The study was conducted and presented in accordance with the STrengthening the Reporting of OBservational studies in Epidemiology (STROBE) statement.17 A waiver of consent for this study was obtained from the Review Ethics Board of the University of Manitoba.
Dataset.
The Nationwide Readmission Database (NRD) sample from the 2012–2014 calendar year was used for this analysis. The NRD is an administrative database covering all inpatient admissions from 22 states in the USA and is produced by the US Government via the Agency for Healthcare Research and Quality (AHRQ).18 The NRD is powered to track individual patients throughout the calendar year linking all inpatient admissions in order to study readmissions using a complex survey design allowing for national-level projections. Further information on the weighting scheme and database design is available from the AHRQ.
Cohort selection.
All patients > 18 years of age with a diagnosis of decompensated cirrhosis who were admitted in the first 6 months of 2012–2014 were included in the analysis. Decompensated cirrhosis was defined according to algorithm #2 described by Goldberg et al., which has a 91.4% positive predictive value of identifying patients with decompensated cirrhosis.19 The list of International Classification of Disease ninth revision codes used in the cohort selection is published in Appendix S1. We only included patients who had at least one decompensating event related to cirrhosis: hepatorenal syndrome (HRS), peritonitis, esophageal varices with or without bleeding, ascites, or HE. Patients who underwent liver transplantation (50.5×) were excluded from the analysis. Patients who survived their index hospitalization were followed for readmissions with a uniform follow-up time of 6 months. Thus, each patient had a total of 6 months’ follow-up. The primary outcome was readmission to hospital for any reason by 30, 90, and 180 days during the follow-up period. A patient selection flow diagram is displayed in Figure 1.
Figure 1.
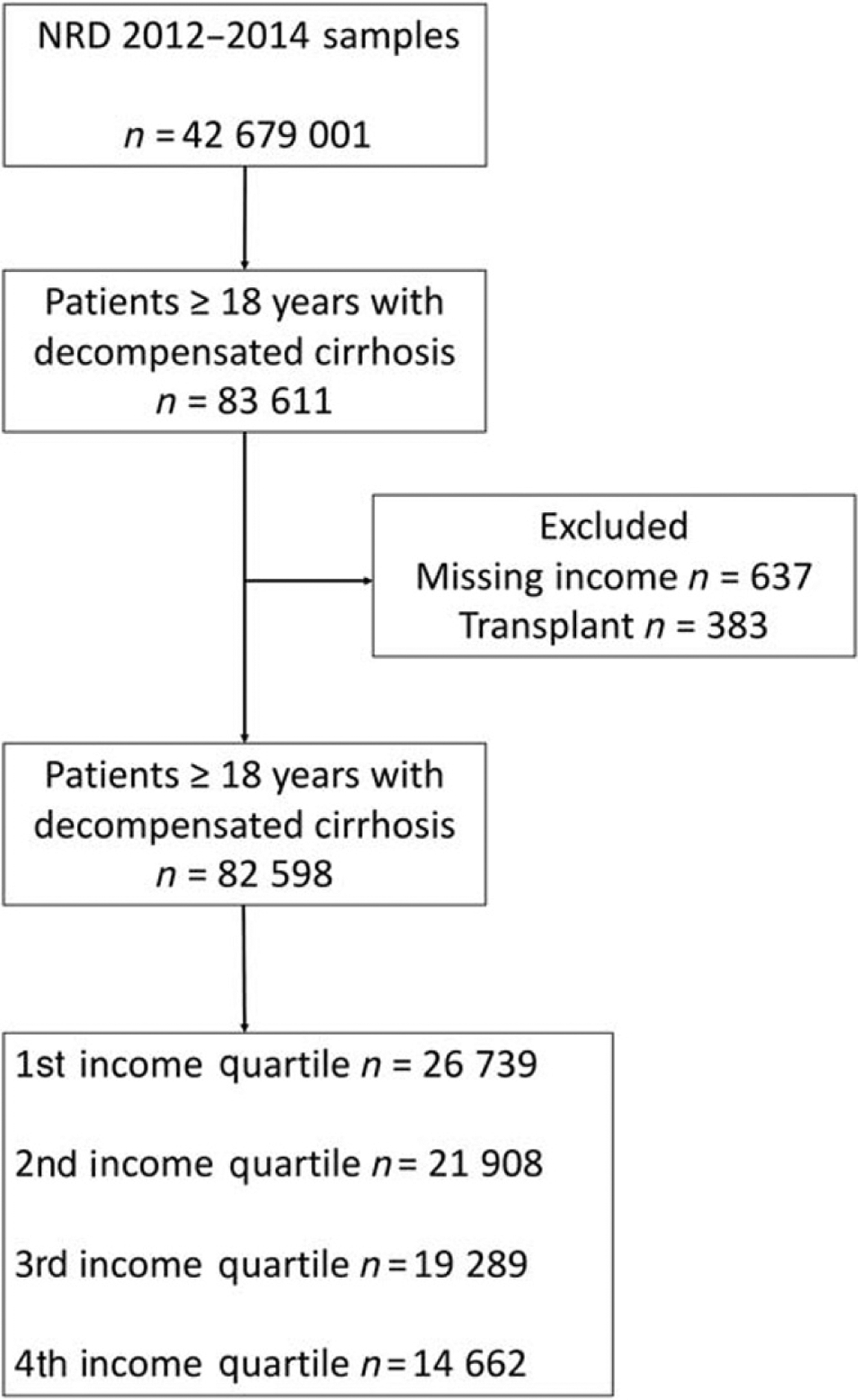
Patient flow diagram. NRD, Nationwide Readmission Database.
Patient characteristics.
The following demographic information was obtained for each patient: age, gender, discharge location from index hospitalization (home, skilled nursing facility, hospice care, and other), median household income quartile by zip code, hospital teaching status, and health insurance coverage (yes vs no). We also obtained the presence of Do Not Resuscitate (DNR) status as well as the presence of hepatocellular carcinoma (HCC) for each patient. We obtained the 29 Elixhauser comorbidity indices for each patient. By definition, all patients in our cohort had liver disease, so this comorbidity was removed from all analyses. We also removed the codes for HRS from the Elixhauser definition of renal failure.
Income status.
The median household income quartile by zip code for each patient was obtained from the NRD. The AHRQ only provides the quartile of income rather than actual individual household income in order to prevent identification of patients. For 2012–2014, the income levels (USD) for each of the quartiles were approximately: first ($0–$37 999), second ($38 000–$47 999), third ($48 000–$63 999), and fourth (> $64 000). Unfortunately, the NRD does not provide other measures of SES such as education level obtained or access to health-care services. Income level data were missing from approximately 2% of patients, so these patients were excluded from the analysis.
Statistical analysis.
SAS v9.4 (SAS Institute, Cary, NC, USA) was used for all statistical analyses. Complex survey procedures were utilized throughout the analysis in order to preserve the integrity of the NRD and to calculate accurate confidence intervals (CIs). All statistical tests were performed with a two-sided alpha level of 0.05. Categorical variables were analyzed using chi-squared analysis while continuous variables were interrogated using the Wilcoxon rank sum or t-test depending on the normality of the distributions. Where appropriate, 95% CIs are displayed. A multivariate logistic regression model predicting the risk of hospital readmission was created. Variables used in the model were selected a priori and included age, gender, zip code income quartile, discharge location from index hospitalization, hyponatremia, DNR status, ascites, HRS, peritonitis, HE, esophageal varices, HCC, and the Elixhauser comorbidity indices. A pre-specific subgroup analysis utilizing a stratified logistic regression model for cause of cirrhosis was also performed.
Results
From the 42 679 001 hospital admissions contained in the 2012–2014 NRD samples, there were 82 598 (0.18%) patients with decompensated cirrhosis who survived a hospital admission in the first 6 months of the year and had income data available. The baseline characteristics for all income quartiles are displayed in Table 1. Patients in the highest income quartile compared with the lowest quartile were older (57.7 years [SD 10.9] vs 56.0 years [SD 10.5]; P < 0.01), more likely to have insurance coverage (90.0% vs 86.0%; P < 0.01) as well as a co-existent diagnoses of HE (22.5% vs 17.6%; P < 0.01), presence of varices/variceal bleeding (41.9% vs 40.4%; P < 0.01), HRS (8.7% vs 6.9%; P = 0.01), hyponatremia (26.1% vs 21.8%; P = 0.01), HCC (10.2% vs 8.5%; P = 0.01), and have a DNR status (9.8% vs 7.6%; P = 0.01).
Table 1.
Baseline demographic characteristics in patients with decompensated cirrhosis admitted by income quartiles
| 1st income quartile (n = 26 730) |
2nd income quartile (n = 21 908) |
3rd income quartile (n = 19 289) |
4th income quartile (n = 14 662) |
P-value | |
|---|---|---|---|---|---|
| Age in years, mean (SD) | 56.0 (10.5) | 56.4 (10.2) | 56.9 (10.0) | 57.7 (10.9) | < 0.01 |
| Readmission at 30 days, n (%) | 8611 (32.2) | 6810 (31.1) | 5928 (30.7) | 4565 (31.1) | < 0.01 |
| Readmission at 90 days, n (%) | 13 301 (49.8] | 10 487 (47.9) | 9126 (47.3) | 7014 (47.8) | < 0.01 |
| Readmission at 180 days, n (%) | 15 761 (59.1) | 12 524 (57.3) | 10 919 (56.3) | 8292 (56.7) | < 0.01 |
| Length of stay in days, median (IQR) | 5 (3–8) | 4 (3–8) | 5 (3–8) | 5 (3–9) | 0.83 |
| Female gender, n (%) | 9377 (35.1) | 7778 (35.6) | 6716 (35.0) | 5064 (34.8) | 0.64 |
| Insurance coverage, n (%) | 22 976 (86.0) | 18 842 (86.5) | 16 487 (87.5) | 13 173 (90.0) | < 0.01 |
| State resident, n (%) | 26 039 (97.4) | 21 504 (98.1) | 18 913 (98.1) | 14 321 (97.7) | 0.12 |
| Varices/variceal bleed, n (%) | 10 786 (40.4) | 9148 (41.8) | 8262 (42.4) | 6225 (41.9) | < 0.01 |
| Ascites, n (%) | 18 513 (69.3) | 14 879 (68.2) | 12 921 (66.4) | 10 087 (68.6) | < 0.01 |
| Hepatic encephalopathy, n (%) | 4556 (17.6) | 4243 (20.8) | 4038 (22.4) | 3149 (22.5) | < 0.01 |
| Peritonitis, n (%) | 435 (1.7) | 384 (1.8) | 319 (1.6) | 223 (1.5) | 0.37 |
| Hepatorenal syndrome, n (%) | 1849 (6.9) | 1675 (7.6) | 1514 (7.9) | 1280 (8.7) | < 0.01 |
| Hyponatremia, n (%) | 5717 (21.8) | 5209 (24.2) | 4679 (24.5) | 3820 (26.1) | < 0.01 |
| Hepatocellular carcinoma, n (%) | 2225 (8.5) | 1957 (8.7) | 1828 (9.1) | 1595 (10.2) | < 0.01 |
| Do Not Resuscitate status, n (%) | 2138 (7.6) | 2040 (8.9) | 1826 (9.1) | 1520 (9.8) | < 0.01 |
| Teaching hospital, n (%) | 16 849 (65.0) | 12 458 (60.0) | 11 266 (61.5) | 9220 (65.2) | < 0.01 |
| Hepatitis B or C, n (%) | 19 368 (71.5) | 14 923 (65.6) | 12 633 (62.7) | 8968 (58.9) | < 0.01 |
| Alcohol use, n (%) | 14 269 (53.6) | 11 353 (51.7) | 9831 (50.9) | 7041 (48.5) | < 0.01 |
Income levels for first quartile: $0–$37 999; second quartile: $38 000–$47 999; third quartile: $48 000–$63 999; and fourth quartile: $64 000 and above. IQR, interquartile range.
>During a uniform 6-month follow-up period, 25 914 (31.4%) patients were readmitted by 30 days, 39 928 (48.3%) patients were readmitted by 90 days, and 47 496 (57.5%) patients were readmitted by 180 days. The number of index admissions was as follows: first quartile (26 739), second quartile (21 908), third quartile (19 289), and fourth quartile (14 662). The majority of patients who were readmitted had multiple readmissions during the follow-up period with a total of 118 013 subsequent admissions with the following breakdown: first quartile (40 800), second quartile (31 078), third quartile (26 139), and fourth quartile (19 996). The number of readmissions ranged from 0 to 13, with a median number of 2 (interquartile range 1–3). The median time to first readmission for all patients was 26 days (interquartile range 10–63 days). Patients in the first quartile were more likely to have multiple readmissions compared with those in the fourth (highest) income quartile (36.0% vs 32.7%; P < 0.01).
The results of multivariable analysis predicting the outcome of readmission to hospital for any reason are displayed in Table 2. After controlling for all variables in the model, patients residing in the first (odds ratio [OR] 1.32 [95% CI 1.17–1.47, P < 0.01]) and second (OR 1.25 [95% CI 1.13–1.38, P < 0.01]) income quartiles were significantly more likely to be readmitted than those in the highest fourth income quartile by 30 days (Fig. 2). By 90 days, similar findings of inequality were found (first quartile, OR 1.21 [95% CI 1.14–1.30, P < 0.01]; second quartile, OR 1.19 [95% CI 1.12–1.28, P < 0.01]; and third quartile, OR 1.08 [95% CI 1.02–1.15, P < 0.01]). These disparities persisted to 180-day readmission rates, with patients in the lowest quartile having a 32% higher risk of hospital readmission at 180 days compared with those in the highest quartile (first quartile, OR 1.32 [95% CI 1.20–1.44, P < 0.01]; second quartile, OR 1.30 [95% CI 1.19–1.41, P < 0.01]; and third quartile, OR 1.14 [95% CI 1.05–1.24, P < 0.01]).
Table 2.
Results of multivariate analysis predicting readmission to hospital in patients with decompensated cirrhosis
| 30-day readmission | 90-day readmission | 180-day readmission | ||||
|---|---|---|---|---|---|---|
| Odds ratio (95% CI) | P-value | Odds ratio (95% CI) | P-value | Odds ratio (95% CI) | P-value | |
| Age, per 10-year increase | 1.08 (1.05–1.10) | < 0.01 | 1.11 (1.09–1.13) | < 0.01 | 1.12 (1.10–1.14) | < 0.01 |
| Female gender | 0.79 (0.74–0.85) | < 0.01 | 0.70 (0.66–0.74) | < 0.01 | 0.78 (0.74–0.82) | < 0.01 |
| Insurance coverage | ||||||
| Private | 1.0 | — | 1.0 | — | 1.0 | — |
| Medicare | 1.09 (0.99–1.21) | 0.09 | 1.08 (0.99–1.17) | 0.06 | 1.08 (0.99–1.18) | 0.06 |
| Medicaid | 1.74 (1.56–1.94) | < 0.01 | 1.79 (1.69–1.89) | < 0.01 | 1.81 (1.66–1.97) | < 0.01 |
| Other | 1.12 (0.99–1.26) | 0.07 | 1.14 (1.08–1.20) | < 0.01 | 1.16 (1.05–1.28) | < 0.01 |
| 1st zip code income quartile | 1.32 (1.17–1.47) | < 0.01 | 1.21 (1.14–1.30) | < 0.01 | 1.32 (1.20–1.44) | < 0.01 |
| 2nd zip code income quartile | 1.25 (1.13–1.38) | < 0.01 | 1.19 (1.12–1.28) | < 0.01 | 1.30 (1.19–1.41) | < 0.01 |
| 3rd zip code income quartile | 1.08 (0.97–1.20) | 0.15 | 1.08 (1.02–1.15) | < 0.01 | 1.14 (1.05–1.24) | < 0.01 |
| 4th zip code income quartile | 1.0 | — | — | — | 1.0 | — |
| Discharge location | ||||||
| Home | 1.0 | — | 1.0 | — | 1.0 | — |
| Skilled nursing facility | 0.89 (0.81–0.97) | 0.01 | 0.86 (0.81–0.91) | < 0.01 | 0.77 (0.71–0.83) | < 0.01 |
| Hospice/home care | 1.09 (1.00–1.19) | 0.04 | 1.05 (0.99–1.12) | 0.09 | 0.97 (0.90–1.05) | 0.50 |
| Other | 1.61 (1.41–1.83) | < 0.01 | 1.36 (1.24–1.48) | < 0.01 | 1.33 (1.19–1.49) | < 0.01 |
| State resident | 1.14 (0.90–1.45) | 0.28 | 1.35 (1.15–1.58) | < 0.01 | 1.28 (1.02–1.61) | 0.03 |
| Varices/variceal bleed | 4.60 (4.27–4.93) | < 0.01 | 5.66 (5.23–6.12) | < 0.01 | 6.83 (6.12–7.55) | < 0.01 |
| Ascites | 82.8 (71.1–96.5) | < 0.01 | 86.5 (78.7–95.9) | < 0.01 | 60.6 (53.7–68.3) | < 0.01 |
| Hepatic encephalopathy | 1.31 (1.16–1.48) | < 0.01 | 1.37 (1.26–1.50) | < 0.01 | 1.31 (1.17–1.47) | < 0.01 |
| Spontaneous bacterial peritonitis | 0.87 (0.69–1.10) | 0.25 | 0.90 (0.78–1.05) | < 0.01 | 1.01 (0.83–1.23) | 0.94 |
| Hepatorenal syndrome | 0.94 (0.83–1.06) | 0.31 | 0.81 (0.74–0.88) | < 0.01 | 0.73 (0.65–0.82) | < 0.01 |
| Hyponatremia | 1.30 (1.20–1.40) | < 0.01 | 1.20 (1.14–1.27) | < 0.01 | 1.15 (1.08–1.23) | < 0.01 |
| Hepatocellular carcinoma | 3.19 (2.74–3.72) | < 0.01 | 5.94 (5.11–6.89) | < 0.01 | 3.19 (2.78–3.65) | < 0.01 |
| Do Not Resuscitate status | 0.40 (0.35–0.45) | < 0.01 | 0.38 (0.35–0.42) | < 0.01 | 0.36 (0.32–0.40) | < 0.01 |
| Alcohol use | 1.66 (1.52–1.81) | < 0.01 | 1.80 (1.68–1.92) | < 0.01 | 1.93 (1.80–2.08) | < 0.01 |
| Rural hospital | 0.76 (0.67–0.87) | < 0.01 | 0.78 (0.72–0.84) | < 0.01 | 0.79 (0.71–0.88) | < 0.01 |
| Teaching hospital | 1.07 (0.98–1.17) | 0.11 | 1.18 (1.09–1.26) | < 0.01 | 1.09 (1.01–1.17) | 0.04 |
All variables present in the model are displayed except for the Elixhauser comorbidity indices, which are displayed in Table S1. Area under the curve of model is 0.91. CI, confidence interval.
Figure 2.
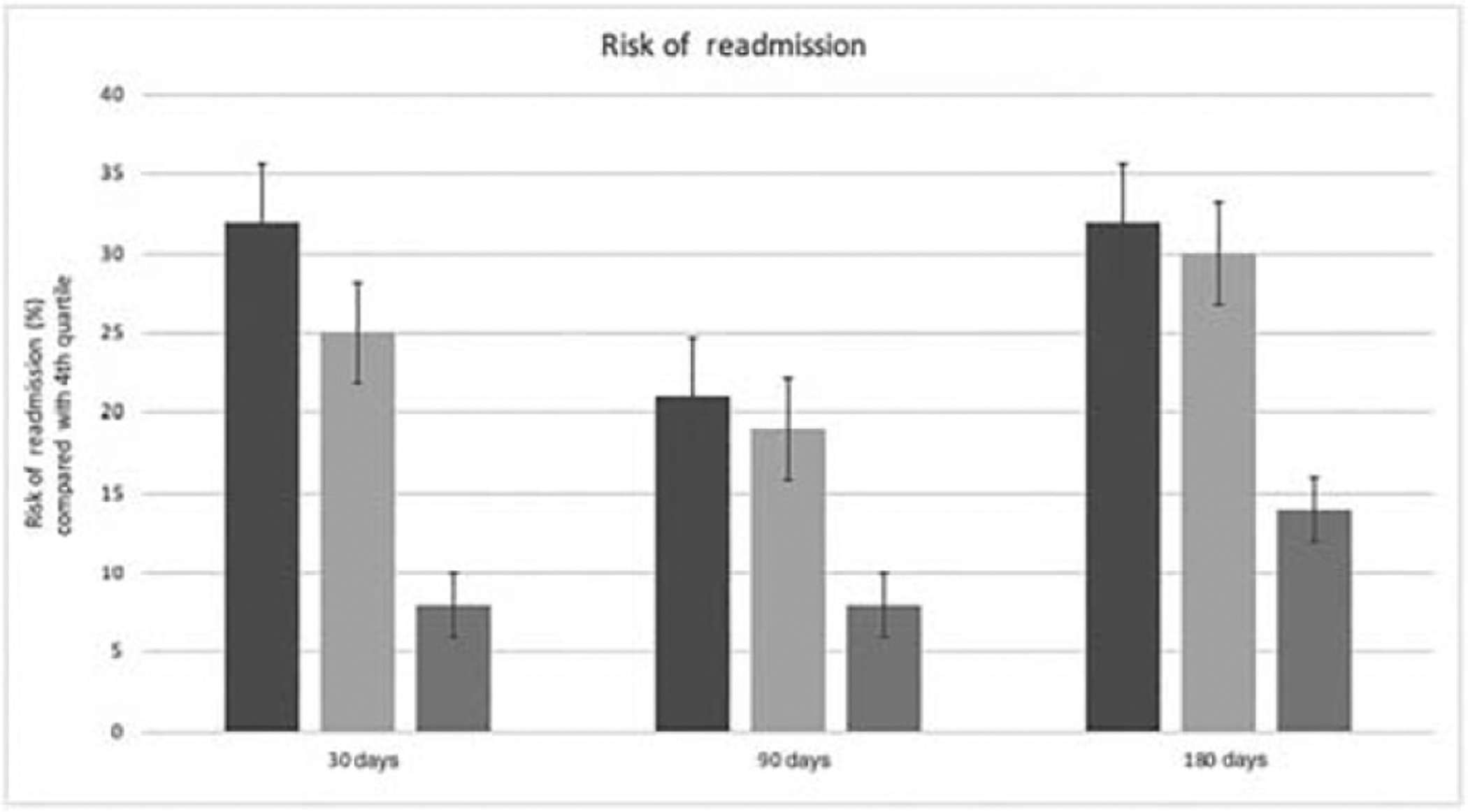
Risk of readmission by income quartile. ■, First quartile; ■, second quartile; ■, third quartile.
The overall c-statistic of the model was 0.91. The Cochran–Armitage test for trend for the OR of zip code income quartiles had a P-value of < 0.01 for both 30- and 180-day readmission rates. Stratified logistic regression modeling for patients at 180 days with viral hepatitis demonstrated similar conclusions as the main results (fourth quartile, OR 0.62 [95% CI 0.56–0.70]; third quartile, OR 0.74 [95% CI 0.67–0.80]; and second quartile, OR 0.90 [95% CI 0.83–0.98]). The non-viral hepatitis subgroup patients did not see a statistically significant difference for income (fourth quartile, OR 0.90 [95% CI 0.79–0.99]; third quartile, OR 1.05 [95% CI 0.94–1.15]; and second quartile, OR 1.07 [95% CI 0.97–1.19]).
Discussion
In this large, nationally representative study of patients with decompensated cirrhosis, we found that lower median household zip code income, a surrogate of SES, was associated with a higher odds of hospital readmission. The risk of hospital readmission decreased as household wealth increased in a linear fashion.
Our findings build on earlier work by Singal et al. who found that Medicaid status and number of address changes in the preceding year (two markers of SES) were associated with higher 30-day readmission rates in cirrhosis.6 While the majority of the literature to date has focused on disease-related predictors of readmission such as MELD score or presence of HE, there is increasing recognition that patient and system factors also play a role.5–7,10,20,21 A three-pronged model of risk factors for readmission in this population has been suggested, and our study lends support to the importance of SES as a key patient-level variable in predicting readmission. Moreover, this is consistent with prior work in other areas of medicine looking at specific disease populations such as congestive heart failure and pneumonia where it has been shown that low income and financial assets, lower education level, and Medicaid enrollment have all been associated with higher hospital readmission rates.11–14,22 To our knowledge, this is the largest nationally representative study to confirm this association in patients with decompensated cirrhosis.
There are likely multiple mechanisms why lower household income impacts readmission rates in cirrhotic patients. There is evidence that lower income groups have more comorbidities and higher general health-care utilization along with being affected by social factors such as lack of appropriate housing, overcrowding, poor nutrition, and decreased access to preventative health-care measures.23–25 In addition, Arbaje et al. have described the importance of post-discharge environmental (PDE) factors in readmission, which include characteristics of the home and caregiving environments. Factors such as having a usual source of health care, reliable access to care, unmet functional needs, and lack of self-management ability are especially important in conditions such as cirrhosis that require a high intensity of care.11 It is possible that the interaction between lower income and unfavorable PDE factors in many of these patients makes them especially vulnerable to frequent hospital readmissions.
Furthermore, there is considerable literature to suggest that health literacy is an important predictor of health-care outcomes. Health literacy is a skill set by which patients are able to obtain, understand, and process information in order to function in a health-care environment and make health-care decisions.26–28 Prior work has shown that lower health literacy is associated with poorer health-care knowledge, worse control of chronic diseases, poorer medication compliance, increased hospital admission, and worse outcomes.28–32 Poverty has been shown to be associated with lower health literacy, as have age, gender, marital status, education level, home language, and time spent in the USA.33 While health literacy has not been extensively studied in patients with cirrhosis, there is some work to suggest that patients have significant gaps in knowledge of their disease process, which may contribute to worse outcomes.34,35 It is possible that poor health literacy in groups with lower SES can lead to increased readmission rates in those residing in the lowest income quartile.
Identifying income as a predictor of readmission in cirrhosis is important for several reasons. Firstly, from a patient care perspective, improved ability to predict patients at high readmission risk can help clinicians tailor their care and post-discharge planning. For example, Mumtaz et al. have developed a validated model to stratify patients with end-stage liver disease into low, medium, and high risk of readmission based on 11 clinical risk factors.36 Moreover, given the burden of disease of cirrhosis, significant attention has recently been focused on quality improvement initiatives to decrease readmission in this population.20,21 A better understanding of factors driving readmission can help tailor interventions to specific patient populations, resulting in lower readmission costs and improved patient outcomes.34,37,38 Secondly, a great deal of emphasis has been placed on readmission as a quality of care marker in US hospitals, with financial penalties frequently levied against institutions with high readmission rates.7,21,39 However, as Goodwin et al. have pointed out, this may be misguided.40 Our study supports the notion that certain factors influencing readmission in patients with cirrhosis are non-modifiable patient-level and community-level factors. Therefore, readmission does not simply reflect quality of care received in hospital and may not be an ideal target to which to tie financial incentives. It may be that hospitals caring for lower income/SES cirrhotic patients and who may have higher readmission rates might actually be those in greatest need of additional funding and improved community supports.
The strength of our study lies primarily in its size and generalizability. By using a large, national dataset that captures all types of hospitals, we are able to provide strong evidence that our results apply to a broad population within the USA. Furthermore, the use of the NRD allowed us to capture readmissions to separate hospitals within the same state. This is important, as one study found that up to one-quarter of readmissions for cirrhosis were to different hospitals.21 However, our study also has limitations, and its results must be interpreted in the context of the cross-sectional study design. All retrospective database studies are inherently vulnerable to residual confounding, unmeasured confounders, coding, and misclassification errors. In addition, the NRD, when used to study readmissions, contains inherent selection bias as patients who die or are treated at a hospital in a different state are not captured as readmissions. Given the limits of the data gathered by the NRD, we were unable to comment on granular, patient-level data such as MELD score or medication usage, which have been shown to be strongly tied to readmission risk; however, we attempted to control for “sickness” by incorporating the Elixhauser comorbidity index.6,10,20,21 Furthermore, although median zip code income is widely used a surrogate of SES, it is imperfect and fails to capture other components of SES that are relevant to health care. Other groups have explored the concept of medically underserved areas as a more complete representation of SES.39 This encompasses percentage of individuals living below the poverty line, primary care physicians per capita, infant mortality rate, and percentage of the population over the age of 65 and has been shown to be associated with increased mortality in sepsis.39 Other facets of SES not captured in our study that have been associated with readmission in cirrhosis include education level, total assets, labor force status, Medicaid enrollment, and PDE factors.6,11–13 Further studies evaluating a more complete picture of SES and PDE will help better guide interventions to reduced readmissions in cirrhosis and optimally care for this vulnerable population.
Conclusion
Lower median household zip code income is strongly associated with increased hospital readmission in patients with decompensated cirrhosis across the USA. The factors driving these findings warrant further study in order to improve patient care and reduce the burden of repeated hospitalizations in this population.
Supplementary Material
Data S1. Supporting information.
Footnotes
Declaration of conflict of interest: No authors disclose any conflicts of interest.
Supporting information
Additional supporting information may be found online in the Supporting Information section at the end of the article.
References
- 1.Peery AF, Crockett SD, Murphy CC et al. Burden and cost of gastrointestinal, liver, and pancreatic diseases in the United States: update 2018. Gastroenterology 2019; 156: 254–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Udompap P, Kim D, Kim WR. Current and future burden of chronic nonmalignant liver disease. Clin. Gastroenterol. Hepatol 2015; 13: 2031–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kochanek KD, Murphy SL, Xu J. National vital statistics reports Deaths: final data for 2014. 2016; 65 (4). [PubMed] [Google Scholar]
- 4.Tapper EB, Halbert B, Mellinger J. Rates of and reasons for hospital readmissions in patients with cirrhosis: a multistate population-based cohort study. Clin. Gastroenterol. Hepatol 2016; 14: 1181–8. [DOI] [PubMed] [Google Scholar]
- 5.Volk ML, Tocco RS, Bazick J, Rakoski MO, Lok AS. Hospital re-admissions among patients with decompensated cirrhosis. Am. J. Gastroenterol 2012; 107: 247–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Singal AG, Rahimi RS, Clark C et al. An automated model using electronic medical record data identifies patients with cirrhosis at high risk for readmission. Clin. Gastroenterol. Hepatol 2013; 11: 1335–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Desai AP, Reau N. The burden of rehospitalization for patients with liver cirrhosis. Hosp. Pract 2016; 44: 60–9. [DOI] [PubMed] [Google Scholar]
- 8.Stepanova M, Mishra A, Venkatesan C, Younossi ZM. In-hospital mortality and economic burden associated with hepatic encephalopathy in the United States from 2005 to 2009. Clin. Gastroenterol. Hepatol 2012; 10: 1034–41. [DOI] [PubMed] [Google Scholar]
- 9.Arguedas MR, DeLawrence TG, McGuire BM. Influence of hepatic encephalopathy on health-related quality of life in patients with cirrhosis. Dig. Dis. Sci 2003; 48: 1622–6. [DOI] [PubMed] [Google Scholar]
- 10.Planas R, Ballest B, lvarez MA et al. Natural history of decompensated hepatitis C virus-related cirrhosis. A study of 200 patients. J. Hepatol 2004; 40: 823–30. [DOI] [PubMed] [Google Scholar]
- 11.Arbaje AI, Wolff JL, Yu Q, Powe NR, Anderson GF, Boult C. Postdischarge environmental and socioeconomic factors and the likelihood of early hospital readmission among community-dwelling medicare beneficiaries. Gerontology 2008; 48: 495–504. [DOI] [PubMed] [Google Scholar]
- 12.Coleman EA, Min S, Chomiak A, Kramer AM. Posthospital care transitions: patterns, complications, and risk identification. Health Serv. Res 2004; 39: 1449–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Barnett ML, Hsu J, McWilliams JM. Patient characteristics and differences in hospital readmission rates. JAMA Intern. Med 2015; 175: 1803–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Calvillo-King L, Arnold D, Eubank KJ et al. Impact of social factors on risk of readmission or mortality in pneumonia and heart failure: systematic review. J. Gen. Intern. Med 2013; 28: 269–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Medbery RL, Gillespie TW, Liu Y et al. Socioeconomic factors are associated with readmission after lobectomy for early stage lung cancer. Ann. Thorac. Surg 2016; 102: 1660–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schnegelsberg A, Mackenhauer J, Nibro HL, Dreyer P, Koch K, Kirkegaard H. Impact of socioeconomic status on mortality and unplanned readmission in septic intensive care unit patients. Acta Anaesthesiol. Scand 2016; 60: 465–75. [DOI] [PubMed] [Google Scholar]
- 17.Vandenbroucke JP, von Elm E, Altman DG et al. Strengthening the Reporting of Observational Studies in Epidemiology (STROBE): explanation and elaboration. Epidemiology 2007; 18: 805–35. [DOI] [PubMed] [Google Scholar]
- 18.AHRQ. National Readmission Database—Agency for Healthcare Research and Quality Accessed June 1, 2017. Available from: https://www.hcup-us.ahrq.gov/nrdoverview.jsp
- 19.Goldberg D, Lewis J, Halpern S, Weiner M, Lo Re V III. Validation of three coding algorithms to identify patients with end-stage liver disease in an administrative database. Pharmacoepidemiol. Drug Saf 2012; 21: 765–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tapper EB, Volk M. Strategies to reduce 30-day readmissions in patients with cirrhosis. Curr. Gastroenterol. Rep 2017; 19: 1–7. [DOI] [PubMed] [Google Scholar]
- 21.Tapper EB, Finkelstein D, Mittleman MA, Piatkowski G, Chang M, Lai M. A quality improvement initiative reduces 30-day rate of readmission for patients with cirrhosis. Clin. Gastroenterol. Hepatol 2016; 14: 753–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Weissman JS, Stern RS, Epstein AM, Stern RS, Epstein AM. Prospective study in four Massachusetts hospitals socioeconomic status and other social factors on readmission: a hospitals prospective study in four Massachusetts 2017; 31(2): 163–72. [PubMed] [Google Scholar]
- 23.Droomers M, Westert GP. Do lower socioeconomic groups use more health services, because they suffer from more illnesses? Eur. J. Public Health 2004; 14: 311–3. [DOI] [PubMed] [Google Scholar]
- 24.Murray CJL, Kulkarni SC, Michaud C et al. Eight Americas: investigating mortality disparities across races, counties, and race-counties in the United States. PLoS Med 2006; 3: 1513–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Baker MG, Barnard LT, Kvalsvig A et al. Increasing incidence of serious infectious diseases and inequalities in New Zealand: a national epidemiological study. Lancet 2012; 379: 1112–9. [DOI] [PubMed] [Google Scholar]
- 26.Health literacy: report of the Council on Scientific Affairs. Ad Hoc Committee on Health Literacy for the Council on Scientific Affairs, American Medical Association. JAMA 1999; 281: 552–7. [PubMed] [Google Scholar]
- 27.Cajita MI, Cajita TR, Han H-R. Health literacy and heart failure. J. Cardiovasc. Nurs 2016; 31: 121–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schillinger D, Grumbach K, Piette J et al. Association of health literacy. JAMA 2002; 288: 475–82. [DOI] [PubMed] [Google Scholar]
- 29.Gazmararian JA, Williams MV, Peel JBD. Health literacy and knowledge of chronic disease. Patient Educ. Couns 2003; 51: 267–75. [DOI] [PubMed] [Google Scholar]
- 30.Persell SD, Osborn CY, Richard R, Skripkauskas S, Wolf MS. Limited health literacy is a barrier to medication reconciliation in ambulatory care. J. Gen. Intern. Med 2007; 22: 1523–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Baker DW, Parker RM, Williams MV, Clark WS. Health literacy and the risk of hospital admission. J. Gen. Intern. Med 1998; 13: 791–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Berkman ND, Sheridan SL, Donahue KE, Halpern DJ, Crotty K. Low health literacy and health outcomes: an updated systematic review. Ann. Intern. Med 2011; 155: 97–107. [DOI] [PubMed] [Google Scholar]
- 33.Martin LT, Ruder T, Escarce JJ et al. Developing predictive models of health literacy. J. Gen. Intern. Med 2009; 24: 1211–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Beg S, Curtis S, Shariff M. Patient education and its effect on self-management in cirrhosis. Eur. J. Gastroenterol. Hepatol 2016; 28: 582–7. [DOI] [PubMed] [Google Scholar]
- 35.Dahl TF, Cowie BC, Biggs B-A, Leder K, MacLachlan JH, Marshall C. Health literacy in patients with chronic hepatitis B attending a tertiary hospital in Melbourne: a questionnaire based survey. BMC Infect. Dis 2014; 14: 537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mumtaz K, Issak A, Porter K et al. Validation of risk score in predicting early readmissions in decompensated cirrhotic patients: a model based on the administrative database. Hepatology 2019; 70: 630–9. [DOI] [PubMed] [Google Scholar]
- 37.Kanwal F, Asch SM, Kramer JR, Cao Y, Asrani S, El-serag HB. Early outpatient follow-up and 30-day outcomes in patients hospitalized with cirrhosis. Hepatology 2016; 64: 569–81. [DOI] [PubMed] [Google Scholar]
- 38.Jack BW, Chetty VK, Anthony D et al. A reengineered hospital discharge program to decrease rehospitalization: a randomized trial. Ann. Intern. Med 2009; 150: 178–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Joynt KE, Jha AK. Thirty-day readmissions—truth and consequences. N. Engl. J. Med 2012; 366: 1366–9. [DOI] [PubMed] [Google Scholar]
- 40.Goodwin AJ, Nadig NR, McElligott JT, Simpson KN, Ford DW. Where you live matters: the impact of place of residence on severe sepsis incidence and mortality. Chest 2016; 150: 829–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data S1. Supporting information.


