Abstract
The recent development of gold nanoparticles as diagnostic tools is burgeoning, especially in the cancer community with a focus on theranostic applications to both cancer diagnosis and treatment. Gold nanoparticles have also demonstrated great potential for use in diagnostic and therapeutic approaches in ophthalmology. Although many ophthalmic imaging modalities are available, there is still a considerable unmet need, in particular for ophthalmic molecular imaging for the early detection of eye disease before morphological changes are more grossly visible. An understanding of how gold nanoparticles are leveraged in other fields could inform new ways they could be utilized in ophthalmology. In this paper, we review current ophthalmic imaging techniques and then identify optical coherence tomography (OCT) and photoacoustic imaging (PAI) as the most promising technologies amenable to the use of gold nanoparticles for molecular imaging. Within this context, the development of gold nanoparticles as OCT and PAI contrast agents are reviewed, with the most recent developments described in detail.
Gold nanoparticles are promising OCT and PAI contrast agents for eye imaging because of their high light scattering/absorption from SPR
1. Gold nanoparticles in medicine
Gold has a long history in medicine. Between the 17th–19th centuries, it was found to have therapeutic benefits in the treatment of fevers, syphilis, tuberculosis, rheumatoid arthritis, and discoid lupus erythematosus.1 As an element, gold has a number of properties that make it useful for in vivo applications, such as its corrosion resistance and stability in physiological environments. In nanoparticle form, gold exhibits a high degree of surface plasmon resonance, which leads to strong electromagnetic fields on the particle surface, enhanced radiative properties such as absorption and scattering, as well as the rapid conversion of absorbed light into heat through non-radiative processes.2
1.1. Recent advances of gold nanoparticles
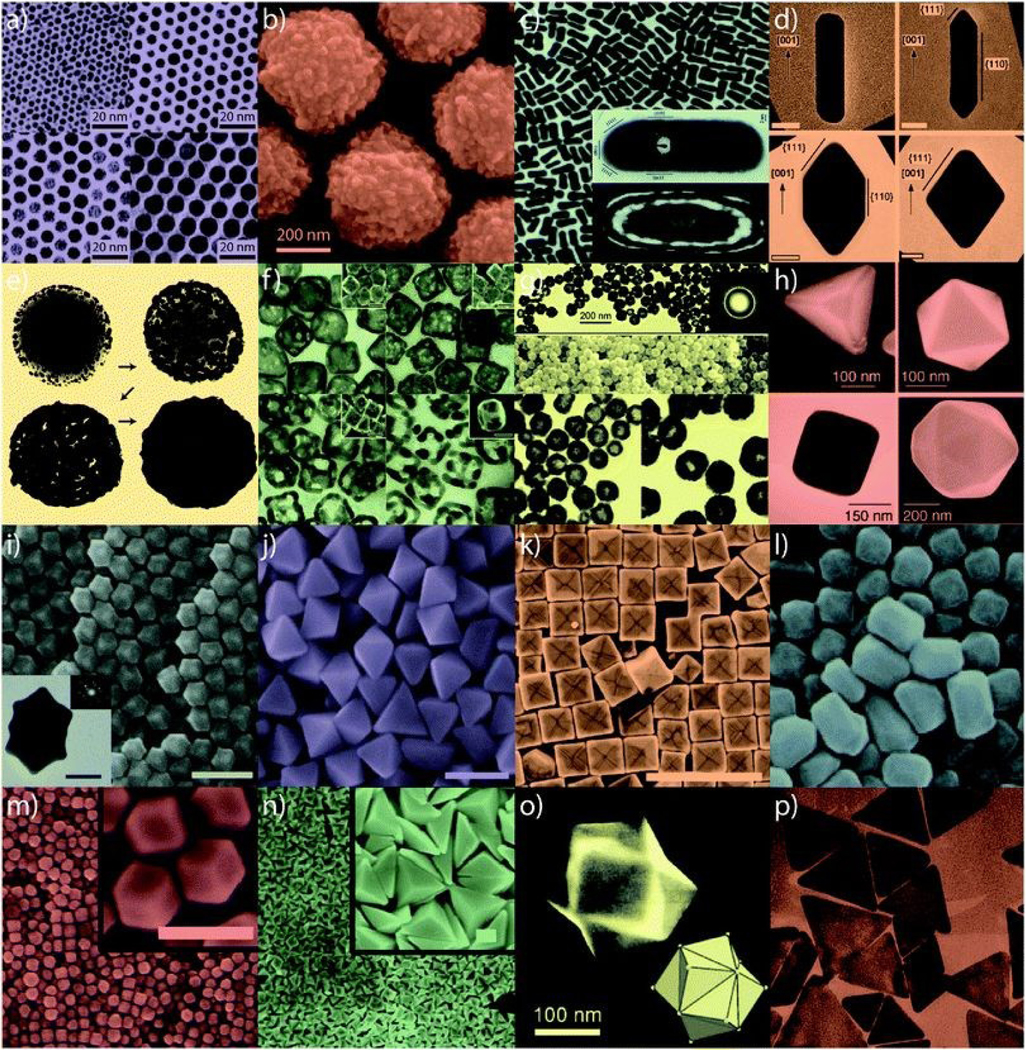
The absorption and scattering light wavelength of gold nanoparticles can be tuned by changing their morphology. Efforts have been made to synthesize various shapes of gold nanoparticles to push the absorption/scattering peak to the near infrared window so that gold nanoparticles in deep tissues can still receive incident light energy. Gold nanoparticles with various shapes have been reported over the past two decades (Figure 1), including nanosphere,3,4 nanorods,3,5,6 nanoplates,7 nanodisks,8 nanoshells,9 nanoprisms,10 nanocages,6,11 nanostars,12,13 nanovesicles,14 and nano bipyramids.3 The absorption peak of gold nanoparticles can be tuned within a wide spectral range in 520 – 1415 nm by varying the size and shape of the gold nanomaterial,15,3,16 along with the development of advanced synthesis methods.
Figure 1.
Various gold nanoparticles developed in the past two decades. (a) Small nanospheres, (b) large nanospheres, (c) nanorods, (d) sharpened nanorods, (e) nanoshells, (f) nanocages/frames, (g) hollow nanospheres, (h) tetrahedra/octahedra/cubes/icosahedra, (i) rhombic dodecahedra, (j) octahedra, (k) concave nanocubes, (l) tetrahexahedra, (m) rhombic dodecahedra, (n) obtuse triangular bipyramids, (o) trisoctahedra, and (p) nanoprisms. Reproduced from ref. 177 with permission from the Royal Society of Chemistry, copyright [2020].
Simply structured gold nanospheres and nanorods are usually grown through reduction of metal ions onto surfaces of gold seeds. The growth direction can be preferentially tuned by adjusting the surface energy of a crystal facet with different surface coatings and thus form complex structures.17 Recently, ascorbic acid was used as a shape controller to form gold nanorods with high aspect ratios ranging from 8.5 to 15.6.18 By replacing the original surface coating (citrate) on gold nanoparticles and nanorods with polyvinylpyrrolidone, steric effects were introduced and the reactivity of the nano gold decreased.19 The decreased activity enabled controllable growth of copper chalcogenides on surface of nano gold and formed noble metal-semiconductor core-shell hybrid nanocomposites.
Complicated structured gold nanomaterials could also be synthesized via reduction of metal ions onto polymeric templates. Song et al. made a branched nanoporous gold nanoshell by reducing Au3+ on self-assembled redox-reactive amphiphilic deblock polymeric nanospheres.20 After the polymeric templates were washed away with organic solvents, branched gold nanoshells were produced. The shell thickness was increased with prolonged reaction duration. The porosity, number of branches, and branch sizes were also tunable by changing the injection speed of Au3+ into the solution, reaction time, added amount of Au3+, and the density of the reducing polymers.
While the use of surfactants for nano gold synthesis can raise concerns for toxicity, advances in novel surfactant-free approaches may address these concerns. For instance, Zhou et al. described the development of dopamine-coated hyperbranched gold nanoparticles.21 Dopamine not only reduced Au3+ into Au but also self-polymerized and formed a polydopamine coating on gold nanoparticles, which they reported to increase light absorption and photothermal stability of the nano gold. The particle size could be tuned from 45 −250 nm by changing feeding ratio of Au3+ and dopamine. Han et al. also synthesized a polydopamine-coated gold nanostar by a seed-mediated surfactant-free method and extended its theranostic potentials via different surface modifications.22 Other than polydopamine, the reductive nature of Fe3O4 was also used to initiate the reduction of gold cations and deposition of a gold seed on the surface Fe3O4 NPs and then grow the gold seed to the desired size through a seed-mediated process.23 The size of the Fe3O4 and gold nanoparticles in the heterodimers can be controlled independently.
In addition to the nucleation/growth method, metal-deposition approaches can also produce gold nanomaterials.24 Unlike the nucleation/growth method, products of metal-deposition approaches may have bare gold surface without any coatings, which could minimize toxicity of the gold nanomaterials. For example, monodispersed gold nanodisks were fabricated via a nanoimprint lithography method.8 The thickness and diameter of the nanodisks can be independently controlled by changing the deposition time and deposition angle. These changes can tune optical absorption peaks to within the near infrared window. Moreover, stacked nanodisks with a smaller disk on top of a larger one could produce dual light absorption peaks.
Gold nanoparticles already have an excellent track record in medicine. They have been shown to be capable of delivering drugs that have antiangiogenic, radiosensitizing, and photothermal effects, which make these nanoparticles promising therapeutic tools for the treatment of various diseases. In the July of 2019, the U.S. Food and Drug Administration (FDA) granted an orally administrating gold nanoparticle-based drug (CNM-Au8, Clene Nanomedicine, Inc.) to treat amyotrophic lateral sclerosis (ALS). Additionally, there are multiple clinical trials using gold nanoparticles to treat diseases such as atherosclerosis, glioblastoma, ad type 1 diabetes respectively.25
1.2. Safety of gold nanoparticles
The safety of gold nanoparticles-based contrast agents is usually characterized by direct toxicity to cells/tissue, including necrosis or apoptosis, oxidative stress, genetic alteration, and activation of local or systematic inflammatory/immunological responses. The in vivo toxicity of gold nanoparticles can be influenced by multiple factors, including route of administration, particle size and shape, dose of administration, surface coating, and surface charge.
The routes to deliver gold nanoparticles include intravenous (IV), Intraperitoneal (IP), subcutaneous (SC), intraocular and intra-muscular injections, topical and oral administration, etc. Among them, IV injection is most used preclinically and clinically to deliver gold nanoparticle-contrast agents to the targeted body sites, because other administration routes often lead to retention of the gold nanoparticles in the local lymph nodes. Most studies have suggested IV administered gold nanoparticles are safe to animals because they do not cause loss of weight or appetite, do not increase white blood cells, do not increase mortality rates, and do not notably change animal behavior.26,27 Recently, Song et al. injected 10 pM gold nanodisks intravenously in a mouse model of retinopathy, and assessed the in vivo toxicity of the nanoparticles to the mouse retina and neural system with histology analysis, TUNEL assay and ERG recording after 5 weeks of administration.28 These measurements revealed no signs of inflammation and damage of the retinal layers, did not affect apoptotic activity throughout the retina and left ERG amplitudes unchanged, suggesting that IV injected gold naondisks did not cause any clinically significant toxicity to experimental animals.
Increasing the administered dose of gold nanoparticles does not increase gold concentration in blood, but increases the gold content in solid organs proportionally.29 Lasagna-Reeves and coworkers show that after repeated IV injection of gold nanoparticles in mice, the animals did not exhibit a change in their survival, behavior, weight, organ morphology, tissue biology, blood biochemistry, or experience any sub-acute physiological damage,29 suggesting no clinically significant toxicity. Maltzahn et al. injected gold nanorods with a daily dose of 0.5 mg kg−1, and did not observe any notable toxicity of the animal after weeklong administration.30 Khlebtsov and coworkers have thoroughly studied the dose-related toxicity of PEGylated gold nanoshell to rats by IV injection at 75, 150, 225, and 300 μg kg−1.26 Organ analysis after 15 days of injection shows that administration of 75 and 150 μg kg−1 gold nanoshells increased the B lymphocyte and T suppressor cell amount whereas decreased the T helper cell population. Injections of 225, and 300 μg kg−1 gold nanoshells did not change the level of white blood cells, but did result in a decrease in immune complexes in the blood, macroscopic changes in the liver and spleen, as well as historical modifications of the liver cells.
The size of gold nanoparticles plays an essential role in their biodistribution, accumulation, clearance and in vivo toxicity. IV administered gold nanoparticles of all sizes mainly accumulate in the liver and spleen.31 Excretion of these accumulated nanoparticles can take several months. The long accumulation time may cause inflammation of these organs, although no evidence of short-period toxicity can be observed.26 Depending on the size, gold nanoparticles can also accumulate in other organs in different levels. 20 nm gold nanoparticles delivered through IV injection accumulate less in the liver and spleen, but do present at higher levels in the heart, kidney and intestines.32 Gold nanoparticles of the same size can also pass the blood-retinal barrier and distribute in all retinal layers without causing any cytotoxicity.33 Gold nanoparticles with a hydrodynamic diameter smaller than 10 nm can penetrate the blood brain barrier34,35 and accumulate in a broad range of organs including liver, spleen, testis, lung, blood, and brain after 24 hours of injection.35 However, nanoparticles that are smaller than 5.5 nm can be excreted by kidney.36 The cutoff size of efficient renal clearance for positively charged gold nanoparticles can be as large as 8 nm.37 Smaller gold nanoparticles that are 1~2 nm in diameter exhibit higher cellular toxicity because they can bind to cellular biopolymers irreversibly.38,39 In addition, they can be translocated through the air/blood barrier of the respiratory tract and trapped by the lung.31
The surface chemistry of gold nanoparticles can significantly affect their biodistribution and toxicity. Replacing cetrimonium bromide molecules, which are toxic and often present on the surface of as-synthesized gold nanostructures with an anti-fouling agent such as polyethylene glycol (PEG), can dramatically extend the blood circulation time and reduce the cytotoxicity of gold nanoparticles.40 Due to its hydrophilic nature, PEG can protect gold nanoparticles from nonspecific binding by plasma proteins and reduce their uptake by reticuloendothelial system. Gold nanoparticles modified with high molecular weight (> 5000 Da) PEG are reported to be more stable and less toxic than their counterparts coated with low molecular weight (< 5000 Da) PEG.32,41
1.3. Gold nanoparticles in ophthalmology
Gold nanoparticles have been investigated in ophthalmology for the enhancement of imaging and treatment of diseases such as corneal and choroidal neovascularization, retinal angiogenesis, and neovascular age-related macular degeneration.42–48 There has been particular interest in Understanding their toxicity, distribution, and pharmacokinetics. In one study, the in vitro toxicity of gold nanospheres, nanorods, and nanocubes with different sizes to adult retinal pigment epithelial cell line 19 (ARPE-19) were investigated. Gold nanocubes (50 nm) and nanospheres ( 50 and 100 nm) were compatible to ARPE-19 when the particles’ incubation concentration was as high as 5 mg/mL.49 Gold nanospheres exhibited increased toxicity with decreased diameter for the same mass concentrations, but this size-dependent toxicity was negated when the nanospheres’ surface concentration was controlled. The lethal dose of gold nanospheres for killing 50% of the ARPE-19 was 23 cm2/mL. Another recent in vitro study showed that gold nanoparticles with 3–5 nm size were non-toxic to but significantly inhibited the migration of vascular endothelial growth factor (VEGF)-induced choroid-retina endothelial (RF/6A) cells when the particle concentration was 0.004 mg/mL.42 An in vivo study showed that gold nanoparticles with a size of 20 nm could across the blood-retina barrier and caused no retinal toxicity when intravenously injected at a dose of 1g/Kg.33 While most studies have concluded that gold nanoparticles are compatible with the eye,25 there have also been conflicting reports on their toxicity. For example, in one study, 20 nm gold nanoparticle was found to negatively affect cell survival, oxidative stress, and glia and microglial activity and were toxic to ex vivo retinal tissue.50 Details such as manufacturing process and surface modification are known to be crucial factors that affect toxicity of biomaterials, and further work is merited to reconcile the conflicting reports on 20 nm diameter gold nanoparticles.
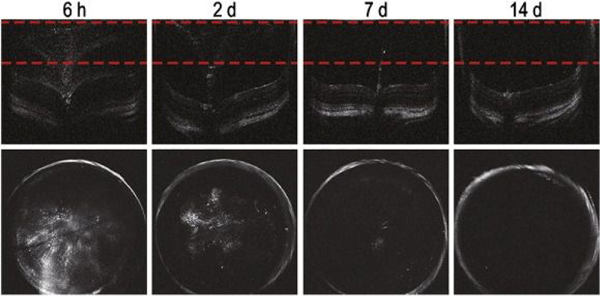
Administration routes of gold nanoparticles to the eye that have been explored include intravenous,33 intravitreal,28 and topical.51 In one study, after being intravenously injected into 6 mice, small gold nanoparticles (20 nm) passed through the blood-retinal barrier and reached all the retinal layers but large ones (100 nm) did not. However, the percent injected dose per gram (%ID/g) in the eye in this study were not reported. Also unclear is the systemic toxicity of small gold nanoparticles at an injection dose of 1 g/Kg and the clearance of nanoparticles from the eye and the body. In other work, intravitreally injected gold nanodisks (160 nm in diameter and 20 nm in thickness) in mice were gradually cleared within 14 days (Figure 2),28 which is a promising result because gold nanoparticles are inert and non-biodegradable. Topically administrated gold nanoparticles (approximately 20 nm) have been shown to penetrate the cornea of guinea pigs.51 However, the particles’ distribution throughout the rest of the globe and their clearance were not discussed. A phase 2 clinical trial VISIONARY-MS (NCT03536559) is now undergoing to test the safety and efficacy of CNM-Au8 in remyelinating nerve cells related to vision. Nevertheless, further studies are still needed to understand the pharmacokinetics of gold nanoparticles in the eye. More information about the toxicity, biodistribution, and clearance of gold nanoparticles in the eye is available in a review published in 2018.25
Figure 2.
Intravitreally injected gold nanodisks were completely cleared from mice eye within 14 days. After intravitreal injections of gold nanodisks (160 nm) at a concentration of 10 pM, the eyes of mice were evaluated at 6 h, 2 days, 7 days, and 14 days. Obtained cross-sectional OCT images (upper) were processed to produce projectional images (lower) from the marked area between the red dotted lines. Reprinted from [28], Copyright (2020), with permission from Elsevier.
Improved understanding of the unique optical properties, tunable surface plasmon resonance, and biocompatibility of gold nanoparticles could help to advance their use in ophthalmic imaging. Next, we will give an overview of ophthalmic imaging modalities with a focus on potential ophthalmic molecular imaging techniques—Optical Coherence Tomography (OCT) and Photoacoustic Imaging (PAI), and consider the frontiers where gold nanoparticles have the potential to advance these fields.
2. Overview of ophthalmic imaging
Conventional ophthalmic imaging involves white light for anterior and posterior segment photography through slit lamp and fundus cameras. Intravenously administered fluorescein is used as an adjunct to fundus imaging using blue light illumination to detect time-dependent vascular abnormalities such as microaneurysms, leakage, pooling, and window defects in retinal circulation. Indocyanine Green (ICG) imaging involves the use of longer wavelengths of light to image the deeper, choroidal vasculature. Both fluorescein and ICG are familiar contrast agents to provide additional, spatio-temporal information about the eye as important adjuncts to conventional, en face photography. Scanning laser ophthalmoscope (SLO) cameras use laser light to create a composite image of the retina with the potential for extremely wide fields of view, and can be used in conjunction with fluorescein and ICG. Ultrasound technology provides relatively low resolution, but provides important cross-sectional information about the eye which is particularly important in cases of media opacities like corneal opacities, cataracts, or vitreous hemorrhage. While critically important to clinical practice, these imaging modalities are widely used, have been known and understood for many years, and are outside the scope of this review.
Despite the versatility of ophthalmic imaging techniques, traditional optical imaging and ultrasound imaging are not yet adept at detecting early pathologies of eye diseases before morphological changes are visible. Molecular imaging can play an important role in providing this early pathological information by visualizing functional and molecular changes in the eye.52, 53 By 2014, imaging modalities that have been adapted for ophthalmic molecular imaging purposes include optical imaging, radionuclide imaging, and magnetic resonance imaging (MRI).54 The features of these imaging modalities are summarized in Table 1. 17,54,55,56,57,58,59 The most promising molecular imaging modalities for the eye continue to be optical in nature given the transparency of the cornea, lens, and retina, and because of the high spatial resolution they allow of ocular structures as well as their lower cost and safety compared to radionuclide imaging and MRI. Among the optical molecular imaging modalities, fluorescence-based imaging has garnered attention to detect early pathological molecular events in retinal diseases with the aid of contrast agents in the form of molecules, nanoparticles, and microparticles.60–63 Still, novel imaging modalities and contrast agents are needed to provide cellular and molecular-level and functional information beyond what is possibly with current technologies.
Table 1.
Features of imaging modalities currently used in ophthalmic molecular imaging.
| Modality | Depth | Resolution | Speed | Sensitivity | Cost | Safety | Applications | Clinical use |
|---|---|---|---|---|---|---|---|---|
| Optical imaging | ||||||||
| Fluorescence mediated tomography | <1 cm | 2–3 mm | s-min | 109−10−12 M | $ | Good | Blood-retinal barrier studies | No |
| Scanning laser ophthalmoscope | ~330 μm | ~5–200 μm | s | Not determined | $ | Good | Apoptosis & cell imaging; Ocular immune response | Yes |
| Multispectral imaging | <460 nm | ~0.5–10 μm | min-h | Not determined | $ | Good | Retinal oxygen homeostasis; Optic nerve head imaging; β-amyloid retinal plaques imaging | Yes |
| Optical coherence tomography | ~2–3 mm | ~1 μm | s | 10−10–10−11 M | $ | Good | Corneal imaging; Cell imaging; Choroidal neovasculature imaging; Drug and other molecules tracking | Yes |
| In vivo confocal scanning microscopy | <300 μm | ~ 4 μm | min | Not determined | $ | Good | Cellular imaging of the cornea; Ocular immune response | Yes |
| Multiphoton excitation fluorescence microscopy | <1 mm | ~10–1000 μm | s-min | Not determined | $ | Good | Cellular imaging of the cornea; Intra-operative monitoring; Trabecular meshwork imaging | Clinically translatable |
| Photoacoustic imaging | ~5–6 cm | ~5–1000 μm | s-min | Not determined | $ | Nano-pulsed laser | Ocular tumor detection; Choroidal neovasculature imaging | Clinically translatable |
| Radionuclide imaging | ||||||||
| Single-photon emission computed tomography | Limitless | ~1–10 mm | min | 10−10–10−11 M | $$ | Ionizing radiation | Detection of uveal melanoma; Neural visual cortex response | Yes |
| Positron emission tomography | Limitless | ~1–5 mm | s-min | 10−11–10−12 M | $$$ | Ionizing radiation | Neural visual cortex response | Yes |
| Magnetic Resonance Imaging (MRI) | Limitless | ~500 μm | min-h | 10−3–10−5 M | $$$ | Strong magnet | Oxygenation studies; Blood-retinal barrier studies; Ion activity in the eye; Ocular drug delivery studies | Yes |
Among the optical imaging modalities, optical coherence tomography (OCT) shows the highest promise in terms of tissue penetration depth and spatial resolution. OCT was first developed around three decades ago and has since revolutionized the diagnosis and treatment of a wide spectrum of ophthalmologic disease.64 Considered an optical, high resolution version of ultrasonography, OCT both captures high resolution images in cross section, collecting line images in the z-plane, and then reconstructs the collected signals into a 3D image. The difference is that OCT sends out light with a broad spectral bandwidth and detects the tissue-backscattered light instead of sound. Most OCT devices use near infrared light which could sacrifice the imaging resolution but allows a higher tissue penetration depth (several millimeters) compared to visible light.65 Like ultrasound imaging, OCT can capture structural information of eyes without any contrast agents. It has been implemented ubiquitously in eye clinics to diagnose and manage a variety of eye conditions and diseases such as age-related macular degeneration (AMD), including choroidal neovascularization (CNV), central serous chorioretinopathy (CSC), and polypoidal choroidal vasculopathy (PCV), glaucoma, and diabetic macular edema by enabling exquisitely detailed cross-sectional imaging of the cellular layers and vascular of the retina.66,67 The emergence of OCT has revolutionized the practice of ophthalmology, providing images of the macula and optic nerve that were once only possible with histological sections of post-mortem or post-enucleation tissue.68
More recently, optical coherence tomography angiography (OCTA) has emerged as a high-resolution and non-invasive modality based on OCT, has become a revolutionary method in the imaging of the vascular system in different retinal and choroidal layers. With the three-dimensional retinal structural information by OCTA, new associations linking vascular damage and glaucoma have been found, which could benefit early diagnosis of glaucoma and prevent vision loss for glaucoma patients.69 It also has shown great promise in detecting ocular tumors.70
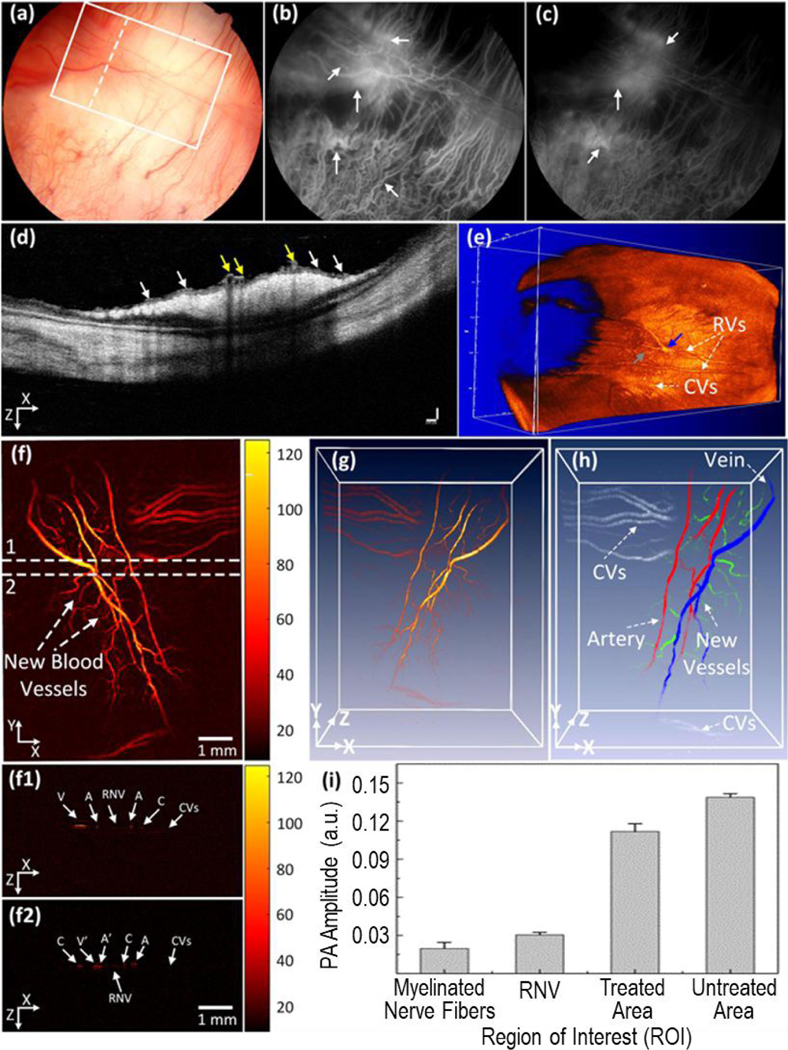
Despite these advances of OCT, issues with tissue penetration depth and spatial resolution at the molecular level remain. Photoacoustic imaging (PAI) has the potential to further increase the tissue penetration depth with high spatial resolution. PAI is based on the photoacoustic effect: when photons from a pulsed nanosecond-long laser is absorbed by a tissue or material, the energy is converted to thermoelastic expansion and then generates pressure waves (ultrasound), which can then be detected with an ultrasonic transducer and then converted into photoacoustic images.71 PAI combines the benefits of optical and ultrasound imaging into a single modality that functions as a “light-in sound-out” technique. It detects optical absorption which is independent of that detected by conventional OCT (optical scattering) or ultrasound (ultrasound waves). PAI is relatively insensitive to light scattering, which allows PAI to image deeper tissues than OCT.72 The combined use of light and sound gives PAI unique scalability of its spatial resolution (as low as 5 μm) and depth penetration (up to 5–6 cm) across both optical and ultrasonic dimensions.15,73 Moreover, the eye has certain endogenous PAI contrast agents, such as melanin and hemoglobin. For instance, PAI can detect distribution of melanin in uveal tissue, including information about melanin distribution within pigmented ocular tumors. Hemoglobin exists in the microvasculature of the uveal tract, superficial microvessels of retina and conjunctiva, and pathologic neovascularization in, for example, neoplasms. Therefore, PAI has great potential in ocular angiography. It has been shown to visualize the blood distribution of large animal eyes and quantify the chorioretinal oxygen gradients in rabbit eyes.74, 75 PAI has also been reported to image individual red blood cells traveling along the iris capillaries and detect the hemoglobin oxygen saturation in the iris microvasculature.76 PAI usually simultaneously produces ultrasound images that provides visualization of anatomic structures and a PAI image that shows molecular information with the aid of contrast agents. PAI has also been jointly used with OCT to visualize retinal neovascularization and to monitor dynamic changes in retinal vein occlusions in living rabbits (Figure 3).77
Figure 3.
Multimodal imaging of retinal neovascularization. (a) Color fundus image of the retina. White rectangle shows the selected scanning region of photoacoustic microscope images and whited dotted line show the scanning line position (OCT). (b, c) Fluorescein angiography image at early phase and late phase. White arrows show the position of retinal neovascularization. (d) B-scan OCT image acquired along the dotted line in (a). White arrows indicate the position of retinal neovascularization (RNVs), whereas yellow arrows represent the location of treated retinal vessels. (e) 3D rendering OCT image. Blue arrow shows the treated areas with higher contrast in comparison with the background. Gray arrow depicts the location of the artery. (f) Corresponding photoacoustic microscopic images after laser irradiation at day 28. It shows clearly the structure of individual retinal blood vessels including RNVs, choroidal vessels (CVs), retinal vessels (RVs), and capillaries. (f1 and f2) B-scan images from upper and lower dotted lines in (f). (g) 3D rendering photoacoustic microscopic images. (h) Segmentation image of retinal vessels for RNV identification. Pseudo-color blue, red, white, and green indicate the position of vein, artery, choroidal vessels and RNV, respectively. (i) Comparison of maximum PA signals at different positions on rabbit retinal vessel after treatment (*p < 0.001 and N = 4). The PA signal slightly decreased after laser treatment. Reprinted from [77]. Copyright (2019) Springer Nature.
OCT and PAI are likely the most promising ophthalmic molecular imaging modalities available today for visualizing deep ocular tissue with high spatial resolution. However, OCT and PAI can provide only anatomic images with very limited molecular information without exogenous contrast agents. Suboptimal OCT contrast between normal and diseased tissues limits the application of label-free OCT, especially in the detection of tumor margins. Similarly, lesions without morphological changes and endogenous photoacoustic contrast are not detectable with PAI. Thus, the performance of OCT and PAI can be further extended and provide more pathologic molecular information with the aid of exogenous contrast agents.56,78
The integration of OCT/OCTA, PAI and fluorescence microscopy/angiography (FM/FA) in a multimodal imaging system can provide unique advantages for ophthalmic anatomy and pathophysiology imaging. FA is currently the gold standard for evaluating and managing retinal vasculature-related diseases, but it requires the injection of fluorescein or ICG as a contrast agent. OCT allows high-contrast imaging of different retinal layers, but has difficulty in distinguishing normal and blood vessels and neovascularization. In contrast, PAI is very sensitive in detecting microvasculature and irregular vascular networks by neovascularization. Song and co-workers developed a multimodality retinal imaging platform that integrates PA microscopy, OCT and FA.79 The system provides high-resolution and complementary-contrast in vivo images of rat’s retina. Very recently, Zhang et al. designed a multimodal imaging system that combines OCT, PAI and FM to study vascular endothelial growth factor (VEGF)-induced retinal neovascularization (RNV) in the live rabbit eyes.80 This multimodality imaging system allows simultaneous visualization of RNV using OCT and PAI and detection of the leakage of neovascularization using FM in both albino and pigmented rabbits.
While recent developments in contrast agents have made substantial progress in molecular imaging outside of the eye,18 ophthalmic molecular imaging lags relatively behind. Gold nanoparticles show great potential in OCT and PAI as contrast agents in ophthalmology.65–67 We will next review the recent advances in gold nanoparticles as OCT and PAI contrast agents in ophthalmic molecular imaging. Related research outside of ophthalmic molecular imaging will also be reviewed to foster potential application of gold nanoparticles in ophthalmology.
3. Recent progress on gold nanoparticles-based OCT and PAI contrast agents
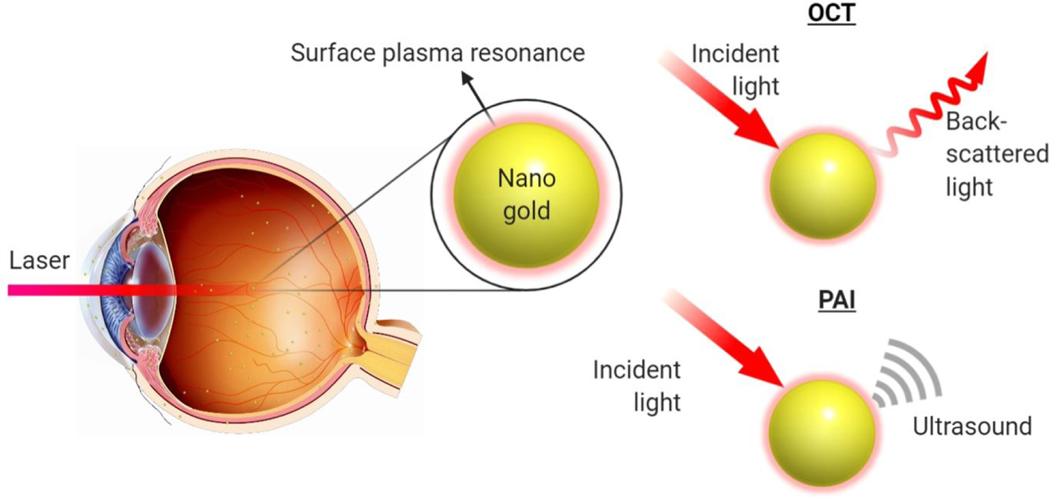
Gold nanoparticles are promising candidates as ophthalmic imaging contrast agents due to their inertness, accessibility to the entire eye, and clearance behavior from the eye. Moreover, gold nanoparticles exhibits surface plasmon resonance (SPR), where free charges on the particle surfaces oscillate in response to an applied electromagnetic field.15 The SPR effect confers gold nanoparticles with strong and tunable optical scattering and absorption characteristics, which contribute to OCT and PAI contrast respectively (Figure 4). Because OCT detects the reflectivity and/or back-scattered light of imaged objects, the strong scattering property of gold nanoparticles enhances backscattering and results in a strong OCT signal. The SPR effect of gold nanoparticles also change the intensity of backscattered light at different wavelengths, leading to spectral signature signals on top of scattering signals.84 On the other hand, PAI detects ultrasound waves generated by the photoacoustic effect after light absorption.71 Plasmonic gold nanoparticles can effectively and rapidly convert absorbed energy into heat, acoustic waves.85 Therefore, gold nanomaterials are promising OCT and PAI contrast agents for ocular imaging (Figure 4).
Figure 4.
Schematic illustration of gold nanoparticles functioning as OCT and PAI contrast agents in the eye. Gold nanoparticles can be administrated to both anterior and posterior chambers and tissues of the eye. When stimulated with incident light, the gold nanoparticles can produce back-scattered light signal which can be detected by OCT camera and form OCT images or ultrasound signal which can be detected by a PAI transducer and form PAI images.
The optical properties of gold nanoparticles can be tuned by changing their shape. There have been many gold nanomaterials reported over the past two decades, including nanosphere,3,4 nanorods,3,5,6 nanoplates,7 nanodisks,8 nanoshells,9 nanoprisms,10 nanocages,6,11 nanostars,12,13 nanovesicles,14 and nano bipyramids.3 The development of nano gold synthesis and versatility of morphology have greatly promoted its potential in medical imaging applications. In recent years, gold nanoparticles have started to garnered particular attention as contrast agents in ophthalmic molecular imaging. Here, literature reports of gold nanoparticles in ophthalmic molecular imaging will be reviewed. We summarize the most recent developments of gold nanoparticles as OCT and PAI contrast agents outside the ophthalmic imaging field, with the hope that this work may find application in ophthalmic molecular imaging in the future. This review focuses specifically on OCT and PAI due to their potential in conjunction with gold nanoparticles for ophthalmic molecular imaging with high spatial resolution and deep tissue penetration.
3.1. Potential of gold nanoparticles for OCT contrast agents in ophthalmic molecular imaging
Depending on the contrast mechanisms of OCT, the OCT contrast agents can be classified into five categories: backscattering enhancers, spectral contrast agents, signal-modulation agents, dark contrast agents, and depolarizing agents. Gold nanoparticles are probably the most investigated materials as scattering-enhancement OCT contrast agents due to their highly tunable SPR properties. The investigation of gold nanoparticles as OCT contrast agents are relatively new and we found 26 articles on this topic. Among these articles, six explored their application to ophthalmic molecular imaging. This relatively high portion of efforts in ophthalmic imaging can be attributed to the fact that OCT is widely used in ophthalmology. Table 2 lists the reported OCT contrast mechanisms of different types of gold nanoparticles and their applications in ophthalmic molecular imaging.
Table 2.
OCT contrast mechanisms associated with gold nanoparticles with different shape and their applications in ophthalmic molecular imaging.
| Type of particles | OCT contrast mechanism | Applications in ophthalmic molecular imaging |
|---|---|---|
| Gold nanorods | Backscattering enhancement 95, 96, 97 | Imaging ocular surface squamous neoplasia 95 Cornea imaging 96 Tracking cells in retinal vessels 97 |
| Spectral contrast 99,100, 101,102, 103, 104 | -- | |
| Signal-modulation (PT-OCT) 112, 113 | Imaging choroidal neovascularization 112, 113 | |
| Dark contrast (Depolarization) 114 | -- | |
| Gold nanoshells | Backscattering enhancement 86–90, 91, 92 | -- |
| Gold nanodisks | Backscattering enhancement 28, 98 | Therapeutic gold nanoparticle tracking 28 |
| Gold nanoprisms | Backscattering enhancement 10 | -- |
| Biosensing 108, 109, 110 | Monitoring ozone penetration into cornea 108 | |
| Gold nanobipyramds | Spectral contrast 73,105 | -- |
Gold nanoparticles as backscattering enhancers.
By changing the size and shape of gold nanoparticles, one can tune the plasmonic resonance peak, ratio of scattering to absorption and even the plasmonic spectral bandwidth. Many gold nanoparticles, including gold nanoshells, gold nanostars, gold nanodisks and gold nanoprisms have shown promising scattering-enhancing effect and increase the OCT signals significantly.
Gold nanoshells, for example, are probably one of the earliest types of gold nanoparticles being investigated as OCT contrast agents,86–90 due to their high scattering cross section91 and clinical safety.88 Hu et al. demonstrated recently that gold nanoshells with a silica core diameter of 200 ± 10 nm and a shell thickness of 30 nm can be used can be used as cardiovascular OCT contrast agents for individual cell detection.92 Gold nanoshells exhibit a broad extinction band covering both visible and near infrared spectra and a high scattering cross section the OCT operating wavelength of 1320 nm. After incubating Hela cells for 24 h with the gold nanoshell, the cells exhibited significantly enhanced backscattering contrast within the cardiovascular phantom.
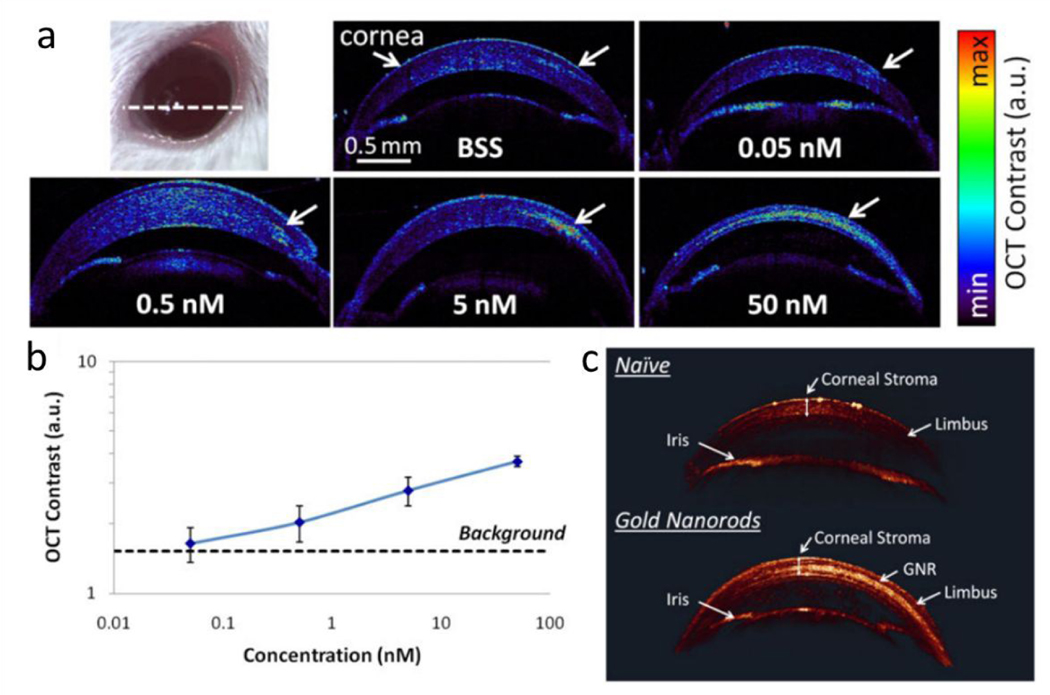
The first application of gold nanorods as OCT contrast agent to enhance the backscattering of light or OCT intensity signals was reported by Troutman et al., who found gold nanorods with plasmon resonant wavelengths overlapping the OCT source (815~965 nm) yield a signal to noise ratio of 4.5 dB.93 Huang et al. recently showed that by delivering gold nanorods to embryos during ex vivo culture, the OCT intensity, image contrast and penetration depth of embryos can all be enhanced, which allow better study of the process of embryogenesis.94 Gold nanorods have been investigated in ophthalmic molecular imaging. In 2013, an ex vivo study used gold nanorods modified with anti-glucose transporter-1 antibodies to enhance the OCT scattering signal of epithelial atypia.95 It proposed the use of a commercially available OCT system to evaluate molecular histopathology of ocular surface squamous neoplasia using functionalized gold nanorods. While gold nanorods have functioned well to increase OCT scattering signals, the selection of molecular markers needs to be optimized. In 2015, gold nanorods with a size of 13 nm in diameter and 45 nm in length were injected into the anterior chamber and cornea of mouse eyes (Figure 5).96 The OCT signal intensity of the gold nanorods in the anterior chamber of mice eye was concentration-dependent (Figure 5a, b). A dose of 30 nM gold nanorods increased OCT contrast by almost 50-fold compared to 0 nM controls. The limit of detection of the gold nanorods in anterior chamber of the mouse eye was estimated to be 120 pM. In 2016, Sen et al. coated gold nanorods with anti-mouse CD45 and then used the coated gold nanorods to label mouse leukocytes.97 After intravenous injection, a single cell that was labeled with gold nanorods could be detected within retinal vessels under OCT.
Figure 5.
Gold nanorods increased corneal OCT contrast. (a) OCT images of cross-sectional slide (white dashed line on the eye photo) through mice corneas injected with 5 μL of gold nanorods at various concentrations from 50 nM to 0 nM. (b) Quantification of the OCT contrast was observed to increase with the GNR concentration, with the lowest detectable concentration being 0.5 nM. The error bars represent standard deviation of the OCT signal. (c) 3D rendering of OCT images show a much brighter OCT signal from the corneal stroma injected with 10 μL of GNR at 50 nM (lower eye) than naïve corneal stroma (upper eye). The corneal structures (corneal stroma, limbus and iris) exhibit very consistent OCT signal intensities across both the naïve and injected eyes. Reproduced from ref. 96 with permission from John Wiley and Sons, copyright [2020].
Gold nanoplates, including gold nanodisks and gold nanoprisms, have been the subject of increased investigations as OCT contrast agents in recent years due to their high scattering coefficient. In a simulation study, Song et al. shows that gold nanodisks with a diameter of 160 nm and thickness of 20 nm exhibit a resonant wavelength at 830 nm and a scattering cross section that is almost 5-fold higher than their absorption cross section.28 These nanodisks were fabricated using a nanoimprint lithography method and injected intraocularly to study their inhibitory effect on retinal neovascularization. Strong OCT signals of gold nanodisks can be visualized in the eye immediately after intravitreal injection of gold nanodisks with a concentration as low as of 0.1 pM. The same group later reported a physical synthesis method to fabricate stacked gold nanodisks of two different diameters, which exhibit two plasmonic peaks and can be used as bimodal imaging agents for both OCT and PAI.98 Si et al. recently synthesized gold nanoprisms which have strong scattering in the second near infrared optical window (1100~1400 nm),10 allowing the contrast agents to be imaged in deeper tissue. The authors demonstrated that by intravenous injection of PEGylated gold nanoprisms, the signal-to-noise ratio of tumor microvasculature in an OCT image can be dramatically enhanced, resulting in significantly improved contrast of OCT angiogram.
Gold nanoparticles as spectral contrast agents.
In spite of the contrast-enhancement effects of gold nanostructures, their potential application as backscattering-enhancing, positive OCT contrast agents is limited by the fact that human tissue also exhibits high backscattering, making the signals difficult to distinguish from each other. Spectral contrast is a unique signature provided only by certain types of nanoparticles, allowing the nanoparticle contrast agents to be specifically detected within tissue. The first OCT spectral contrast approaches were reported using a dual band method with gold nanorods as contrast agents.99,100 Gold nanorods were used as spectral contrast agents because of their highly tunable SPR effects in a wide spectral range and narrow plasmon linewidth. The resonance peak of gold nanorods is easily adjustable by changing the size and aspect ratio of the nanoparticles. These optical properties make gold nanorods ideal candidates for OCT spectral imaging and multiplexing.
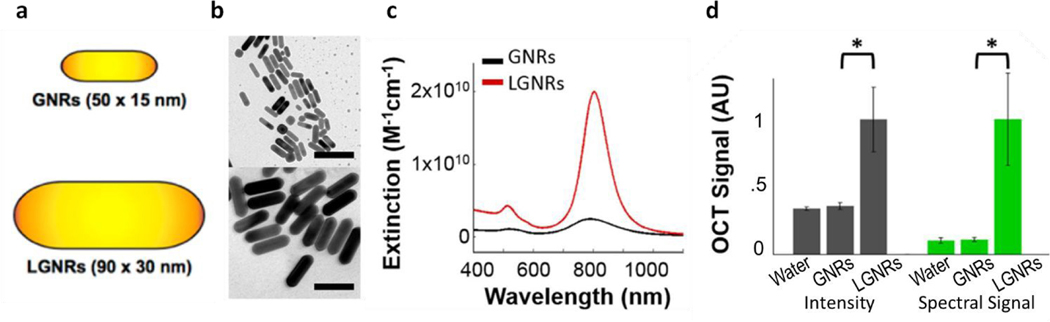
Liba and SoRelle et al. have recently further improved the performance of spectral contrast agents by using large gold nanorods (LGNRs).101,102 Compared to regular gold nanorods, LGNRs have significantly higher scattering cross sections due to their larger size, producing stronger OCT backscattered and spectral signals. Both their length and width are approximately two times greater than those of regular gold nanorods (Figure 6a, b). Each large gold nanorod exhibits 30-fold greater OCT intensity and 110-fold greater spectral contrast signals, respectively, compared to control regular gold nanorods (Figure 6c, d). Two types of large gold nanorods with different resonance peaks, 815 nm and 925 nm, can be simultaneously imaged by OCT with the dual band spectral contrast method. These large gold nanorods were then further implemented as OCT spectral contrast agents to detect circulating tumor cells103 and track the migration of tumor-associated macrophages spatiotemporally in a mouse model of glioblastoma.104
Figure 6.
Comparison of gold nanorods (GNR) and large gold nanorods (LGNR) as OCT contrast agents. (a) Schematic illustration of the dimensions of GNR and LGNR. (b) TEM images of GNR and LGNR. Scale bar: 100 nm. Figures reprinted with permission from [102]. Copyright (2015) American Chemical Society. (c) LGNRs exhibit 8-fold greater total extinction than GNRs at equal nanoparticle concentration. (d) OCT intensity and spectral signals of GNR and LGNR. The OCT intensity and spectral signal of each LGNR particle are 30- and 110-fold greater than each GNR particle, respectively. Figures reprinted with permission from [101]. Copyright (2016) Springer Nature.
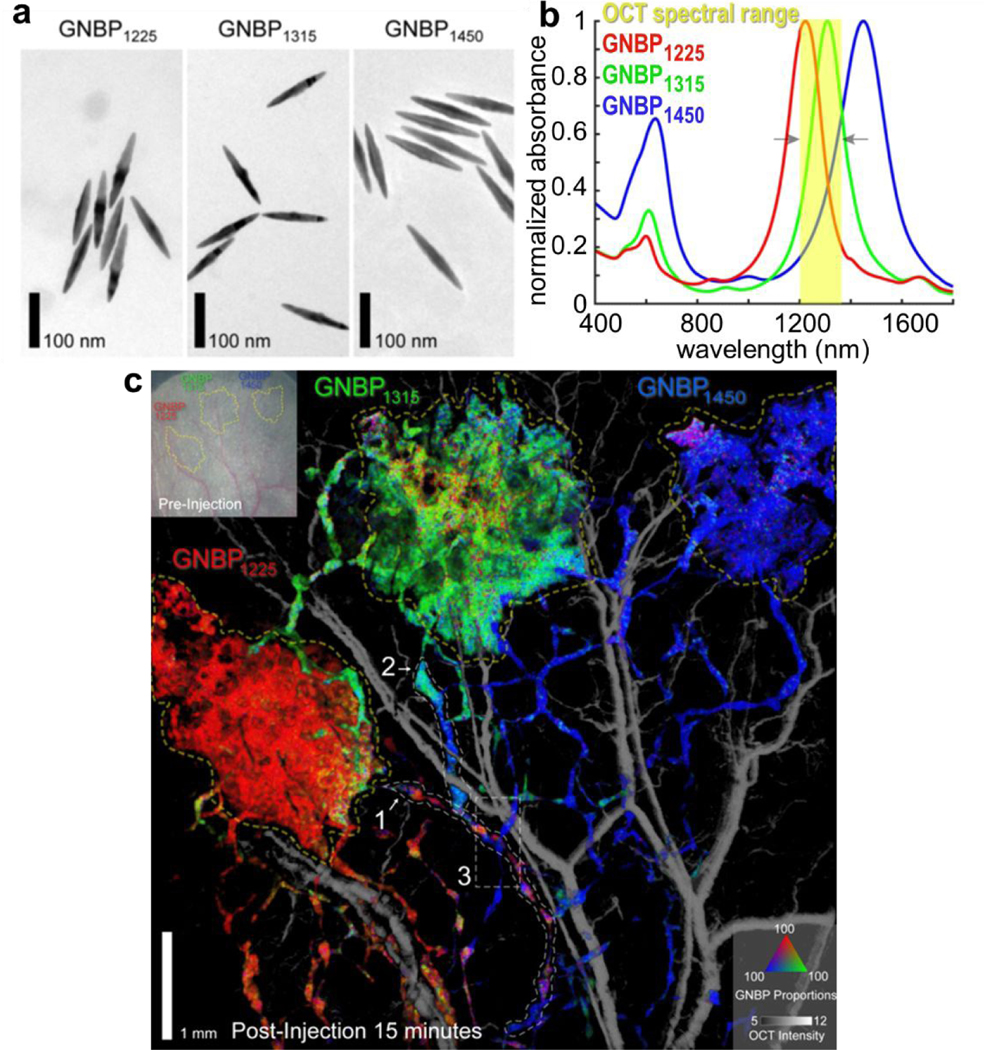
Very recently, Si and Yuan et al. demonstrated for the first time that gold nanobipyramids can be used as OCT spectral contrast agents for multiplexing in the second near infrared window-II (1100~1400 nm) (Figure 7).73,105 The plasmon resonance gold nanobipyramids can be tuned to wavelengths in the range of 1100~1400 nm (NIR-II window). Gold nanobipyramids with different aspect ratios can be used as OCT spectral contrast agents for multiplexed imaging of tissue with greater depth. Compared to large gold nanorods, gold nanobipyramids have narrower plasmon resonance linewidth in the NIR-II spectral window, making them ideal contrast agents for multiplexing studies in deeper tissue.
Figure 7.
Gold nanobipyramids (GNBP) as spectral OCT contrast agents in the NIR-II window. (a) TEM images of GNBPs with three different resonance peaks at 1225, 1315 and 1450 nm, respectively. (b) The Vis-NIR spectra of GNBP1225, GNBP1315 and GNBP1450. (c) Spectral contrast OCT image of a mouse ear after subcutaneous injections of GNBP1225, GNBP1315 and GNBP1450. The spectral contrast signals of GNBP1225, GNBP1315 and GNBP1450 are shown in red, green and blue colors. Figures reprinted with permission from [105]. Copyright (2020) American Chemical Society.
Gold nanoparticles as signal-modulation agents.
Another promising mechanism for enhancing contrast in OCT is through the design of dynamic contrast agents and systems that can modulate OCT signals over time. OCT signals can be modulated by contrast agents through light, magnetic field, ultrasound, and biological environments. Dynamic OCT contrast is measured either by the variation of OCT signals during the time of modulation or the signal difference before and after modulation. In recent years, quite a few novel nanoparticle design and dynamic OCT contrast techniques have emerged.106,107
When optical properties (spectrum or backscattering) of contrast agents are responsive to different biological environments, including different biomolecule concentrations and pH, their OCT signals can be modulated dynamically, which in effect gives them the ability to sense changes in biological environments. For instance, gold nanoprisms can be used as a sensing agent for ozone, exposure to which gradually rounds the corner of gold nanoprisms and turns them into circular gold nanodisks. The transition from gold nanoprisms to gold nanodisks blue shifts the nanoparticles’ plasmon resonance. Employing this unique property, Jiang et al.108 used gold nanoprisms with a resonance peak of 1050 nm as a molecular sensing probe to image the penetration of ozone into the anterior chamber of an isolated crucian carp eye, with an OCT system equipped with central laser spectral wavelength of 830 nm. Upon exposure to ozone, the resonance peak of gold nanoprisms shifts from 1050 nm to 829 nm, which overlaps with the central wavelength of OCT spectral source, leading to higher OCT intensity signals. A nanohybrid material of gold nanoprisms/Polyaniline core-shell (GNPs@PANI) structures was designed to rapidly sense the pH change in biological environment using OCT.109 At different pH, the hybrid nanomaterial exhibits different optical density or scattering coefficient at the OCT central wavelength 830 nm. Kim et al. assembled gold nanoclusters with acid-cleavable linkers and applied these gold nanoclusters to detect dysplastic tissue with OCT.110 The gold nanoclusters disassemble themselves into individual gold nanoparticles under mildly acidic conditions, resulting in diminished OCT intensity and increased OCT Doppler frequency.
Photothermal OCT of gold nanorods have been used in ophthalmic molecular imaging. Photothermal OCT (PT-OCT) is a technique that heats a small region of interest of tissue with laser and light-absorbing contrast agents, and detects the resulting variation of local refractive index using phase-sensitive OCT.111 Lapierre-Landry et al.112 and Gordon et al.113 have demonstrated gold nanorods as PT-OCT contrast agents that can be specifically detected in the retina of laser-induced choroidal neovascularization mouse models after systematic injections. The former study shows that the PT-OCT signals in the laser-induced choroidal neovascularization lesion are associated with non-targeted gold nanorods passively accumulated in the eye, whereas the latter study applies PT-OCT to detect antibody-conjugated gold nanorods that are targeting to the laser-induced choroidal neovascularization lesion. The later study also investigated the effect of antiangiogenic treatments in the retina of a laser-induced choroidal neovascularization mouse model using targeted gold nanorods as PT-OCT contrast agents. Notable reduction of PT-OCT signals is detected in the laser-induced choroidal neovascularization lesion after intravitreal delivery of neutralizing monoclonal anti-vascular endothelial growth factor antibodies.
Gold nanoparticles as dark and depolarizing contrast agents.
Dark contrast agents or negative contrast agents reduce OCT signals. They can reduce the OCT intensity or the polarization of OCT beam. Lippok et al. reported that scattering cross-section of gold nanorods is highly polarization-dependent in the vicinity of its longitudinal resonance.114 Such a property allows gold nanorods to depolarize their backscattered light strongly. The depolarization signature produced by GNRs can be exploited as a source of OCT contrast. A limitation of dark contrast agents is that they can lose their contrast in tissue with greater depth where OCT signal is significantly attenuated.115
3.2. Potential of gold nanoparticles for PAI contrast agents in ophthalmic molecular imaging
Although PAI is a new imaging modality, investigation into photoacoustic contrast agents is burgeoning and shows great potential in molecular, cellular, vasculature, and medical imaging. Ideal photoacoustic contrast agents have high optical absorption cross section, efficient conversion of light to heat, low thermal resistance, and production of pressure transients.116,117 A great variety of PAI contrast agents have been developed during this period, which include gold-based nanomaterials, silver nanoplates, CuS nanoparticles, transition metal dichalcogenides nanoparticles (TiS2, MoS2,WS2, WSe2, MoSe2, and Bi2Se3), upconversion nanomaterials, SWNTs, graphene, SiC nanomaterials, hybrid inorganic nanoparticles, semiconductor polymer nanoparticles, melanin nanoparticles, and dye-embedded nanoparticles.15,17,118 Gold-based nanoparticles are probably one of the most reported photoacoustic contrast agents due to their high optical absorption and tunable absorption peak within a wide spectral range in 520 – 1415 nm.15,3 The PAI signal intensity of gold nanoparticles can also be improved by increasing their aggregation. For example, the PAI signal of gold nanoparticles encapsulated within liposome microbubbles were significantly increased due to the gold nanoparticles aggregation upon ultrasound triggering.119
The applications of gold nanoparticle-based PAI contrast agents are broad. While searching the database PubMed with keywords: ‘nano’ AND ‘gold’ AND ‘photoacoustic’, 90 articles are found related to the development of gold nanoparticles as PAI contrast agents. These articles can be classified into 7 topics: cancer theranostic application, cancer imaging, brain imaging, other molecular imaging, imaging signal optimizing, biocompatibility, and other applications. Table 3 lists the distribution of these articles in different topics and years. It is obvious that the development of gold-based photoacoustic contrast agents mainly focused on cancer diagnosis and treatment: 51 out of the 90 articles are about cancer with 8 articles on cancer imaging and 43 on cancer theranostics. Noticeably, 2 of these 51 articles are used in ophthalmic molecular imaging to visualize eye tumors, lower than the fraction (6/26) of gold nanoparticles as OCT contrast agents in ophthalmology. This is likely due to the shorter history of PAI than OCT especially in ophthalmic imaging and the current lack of commercially available ophthalmic PAI systems.
Table 3.
Topics of published nano gold-based photoacoustic contrast agents. The literature was searched through PubMed with keywords “‘nano’ AND ‘gold’ AND ‘photoacoustic’”. Review and irrelevant articles were excluded.
| Topics | 2007 | 2009 | 2010 | 2011 | 2012 | 2013 | 2014 | 2015 | 2016 | 2017 | 2018 | 2019 | 2020 | Total |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cancer imaging | 2125,130 | 1129 | 1126 | 1127 | 1128 | 2121,178 | 8 | |||||||
| Cancer theranostics | 4131–133,179 | 1134 | 2135,136 | 6137–142 | 1019,20,120,143–147,164,180 | 421,148,149,165 | 1222,23,160,161,150–152,154–156,158,159 | 3157,162,163, | 42 | |||||
| Signal optimizing | 1181 | 1182 | 1183 | 2179,184 | 3185–187 | 2188,189 | 3190–192 | 298,193 | 218,24 | 1119 | 18 | |||
| Other imaging | 2194,195 | 1196 | 1197 | 1198 | 1180 | 3153,170,178 | 1171 | 10 | ||||||
| Biocompatibility | 1195 | 1199 | 2185,200 | 4 | ||||||||||
| Brain imaging | 1201 | 1202 | 2 | |||||||||||
| Other applications | 1203 | 1204 | 1205 | 1173 | 2172,175 | 6 | ||||||||
| Total | 1 | 5 | 2 | 3 | 5 | 7 | 8 | 6 | 9 | 13 | 12 | 16 | 3 | 90 |
Photoacoustic gold nanomaterials in ophthalmology.
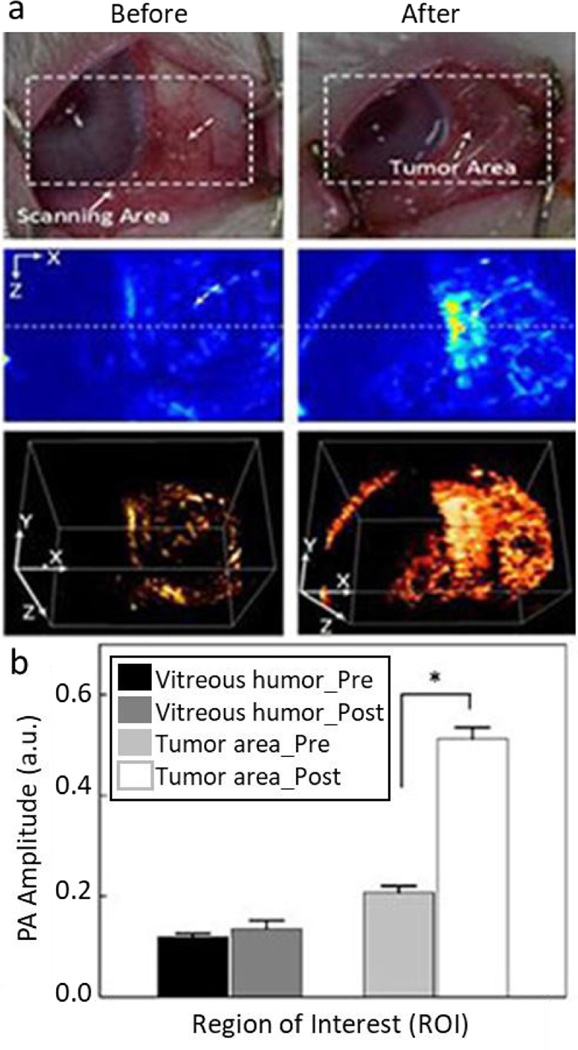
Reports on gold nanoparticles-based PAI contrast agents in ophthalmic molecular imaging emerged in 2017. The reported gold nanoparticles include gold nanospheres77, 120 and nanocages121. One application of gold nanoparticle in ophthalmic imaging is the imaging of ocular tumors. In 2017, Kim et al. applied doxorubicin-conjugated fucoidan-encapsulated gold nanoparticles to image ocular tumors with PAI and treat the tumor.120 The PAI contrast of the tumor regions was increased after gold nanoparticles were injected directly into the neoplasm (Figure 8). The nanoparticles were capped with fucoidan and then loaded with chemotherapeutic agent doxorubicin. Synergistic chemotherapy and photothermal therapy significantly decreased cancer cell viability. The therapeutic efficacy was laser energy and nanoparticle concentration dependent. With a 532-nm laser irradiation, the treatment was effective when irradiance and concentration were 0.11 W/cm2 and 200 μg/ml, respectively; while there was no effective treatment when the irradiance was 0.06 W/cm2, irrespective of nanoparticles concentration. In 2018, Raveendran et al. injected gold nanocages into the iris of ex vivo porcine eyes and studied their PAI contrast.121 A potential application of the PAI signal of these nanocages is early diagnosis of uveal melanoma. Combined with ultrasound imaging, uveal melanoma-targeting gold nanocages have the potential to more precisely localize uveal melanomas in the eye.
Figure 8.
PAI of rabbit eye tumors injected with doxorubicin-conjugated fucoidan-encapsulated gold nanoparticles. (a) Photographs (top row), photoacoustic images (middle row), and 3D reconstruction of photoacoustic images (bottom row) of rabbit eye tumor were compared before and after Dox-Fu@AuNPs injection. (b) Photoacoustic signals from different positions in the rabbit eye tumor were compared before and after injection of the gold nanoparticles (*p < 0.05 compared with pre-injection). Reproduced from ref. 120 with permission from Oncotarget, copyright [2020].
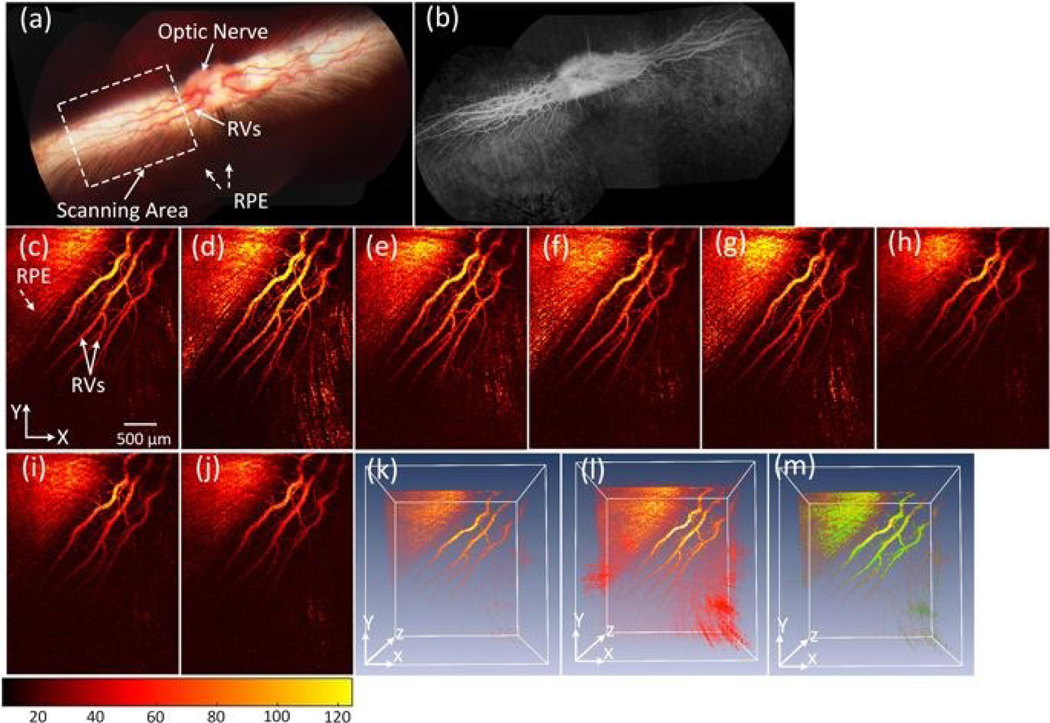
Another application of gold nanoparticles in ophthalmic imaging is vasculature visualization. In 2019, Nguyen et al. intravenously injected polyethylene glycol (PEG)-capped gold nanoparticles with a size of 20 nm into rabbits.122 These gold nanoparticles exhibited high OCT and PAI contrast. When the gold nanoparticles circulated into the retinal and choroidal vessels in living rabbits, the photoacoustic signal of the blood vessels was enhanced by up to 82%. The injected gold nanoparticles enabled detection of individual blood vessels by photoacoustic microscopy (Figure 9). The gold nanoparticles also enabled the visualization of retinal neovascularization and monitoring of the dynamic changes which occur as a result of retinal vein occlusion in living rabbits.122
Figure 9.
PA imaging of retinal vessels injected with PEG-capped gold nanoparticles (20 nm) at a concentration of 5 mg/mL in pigmented rabbits. (a) Color fundus image of the retina, showing retinal vessels (RVs), optic nerve, and retinal pigment epithelium (RPE). The white dotted rectangle shows the scanning area. (b) Fluorescein angiography (FA) image. (c-j) Corresponding MIP PA images of retinal vessels before and after injection of the gold nanoparticles. (k) and (l) 3D visualization of the PAM data pre- and post-injections, respectively. The 3D rendering image after injection showing retina with high contrast. In addition, retinal and choroidal vessels are located at different depths. (m) Subtraction and overlay 3D PA image. Pseudo green color reveals the signal enhancement after the gold nanoparticles administration. Reprinted from [122]. Copyright (2019) Springer Nature.
While gold nanoparticles have shown potential in imaging ocular tumors and vasculature, its potential for ophthalmic imaging should be considered within the context of laser safety provided by the American National Standards Institute (ANSI) Z136.1–2005. Laser exposure can damage the retina by three mechanisms: 1) photothermal damage due to protein denaturation induced by temperature increases along with light absorption by melanin in the retinal pigment epithelium, which occurs when exposure durations are longer than ~20 μs; 2) thermoacoustic damage due to various nonlinear mechanisms such as laser-induced breakdown and self-focusing, which occurs for pulses shorter than ~1 ns; and 3) photochemical damage likely due to a photo-oxidative insult to the photoreceptors and to lipofuscin pigments in the RPE, which occurs at short visible wavelengths (<600 nm) when exposure duration is longer than ~1 s.123 While PAI uses nanosecond pulsed laser with wavelength in the near infrared window, exposure to PAI lasers could damage the retina via photothermal and thermoacoustic mechanisms. Unlike OCT (though it also uses laser), PAI is new and the laser of PAI systems needs further investigation for safety regulation for ophthalmology applications. Therefore, while evaluating gold nanoparticles as ophthalmic PAI contrast agents, it is critical to choose the incident laser with suitable power and wavelength and also to take the exposure duration and the size of exposed retinal area into consideration. Of note, photoacoustic microscopy (PAM) imaging down to 1% of the ANSI safety limit has been achieved.124
Current research on the use of gold nanoparticles as PAI contrast agents in ophthalmic molecular imaging are new and relatively sparse. The paucity of research in this area to date, however, does not reflect on the potential of gold nanoparticle-based PAI contrast agents in ophthalmology. Instead, it is a sign of an opportunity to apply lessons learned about how gold nanoparticle-based PAI contrast agents have played roles as important theranostic tools in other area of medicine. To learn further from research areas outside ophthalmic molecular imaging, we will next analyze the trend of research interests in gold nanoparticles as PAI contrast agents and review the most recent research on this subject over the past three years.
Photoacoustic gold nanomaterials as theranostic tools for cancer.
The most promising area to bridge gold nanoparticles into ophthalmology may be ocular oncology. Gold nanoparticle-based theranostic photoacoustic contrast agents showed success in treating and imaging eye tumors in 2017.120 Early investigation (before 2014) focused on cancer imaging only (Table 3). During this period, gold nanomaterials have been reported to visualize lymphatic systems,125,126 ovarian cancer,127 gastric cancer,128 melanomas,129 and subcutaneous tumor130 with PAI. In 2018, gold nanomaterials were used to image uveal melanoma.121 Starting from 2013, studies on gold-based photoacoustic contrast agents were advanced to the development of theranostic photoacoustic contrast agents for cancer treatment.131,132,141–145,133–140 This area was getting more and more attention, as reports over the past 3 years (28 publications) have more than doubled compared to those before 2017 (13 publications) (Table 3).
Gold nanoparticles are applicable to cancer treatment in three distinct ways: (1) they have intrinsic photothermal and photodynamic effects, which can kill cancer cells with increased temperature and oxidative stress;146,147,148,149,150,151,152,153,154 (2) they can be loaded with and then deliver anti-cancer drugs or siRNA that can kill cancer cells;155,156,157 and (3) they can enhance the radiotherapy of radiosensitizers.145,158,159–161. Regardless of the mechanism, the photoacoustic signal of gold nanoparticles allows them to be tracked within light-reachable tissues with PAI. For examples, Zhu et al. used porous gold nanovesicles as a carrier for an immune inhibitor and an anticancer polymeric prodrug.162 The gold nanovesicles were modified with poly(ethylene glycol) (PEG) and dual pH/GSH-responsive polyprodrug poly(SN38-co-4-vinylpyridine) and showed intense PA signal in the NIR-II window. These gold nanovesicles with enhanced PA imaging could guide and track chemo-immunotherapy in vivo and inhibited the growth of both primary tumors and metastatic tumors.
The photoacoustic signal of gold nanoparticles can be multiplexed with other imaging modalities to gain more diagnostic information deep within tissues. For example, gold nanoclusters were conjugated with indocyanine green and used as a multifunctional theranostic reagent with the capacities for PAI, computed tomography, and near-infrared fluorescence imaging.163 Titanium carbide and molybdenum disulfide nanosheets were respectively coated with gold and then used for photoacoustic and computed tomography dual-modal imaging as well as photothermal, photodynamic, and radio therapies for cancer treatment.160,161 Ju et al. designed a gold-Fe2C Janus nanoparticle of 12 nm for triple-modal imaging-guided photothermal therapy to precisely treat cancer.164 Liu et al. reported a gold nanowreath (branched nanoring) enclosed in a silica shell whose surface was covered by positively charged polymers and negatively charged magnetic iron oxide nanoparticles, consecutively via a layer-by-layer method.165 The branches, small junctions, and central holes in the gold nanowreath was likely to generate strong plasmon coupling and therefore increase the PAI and photothermal effects. This nanoparticle was responsive to the relatively high glutathione concentration within tumors, leading to disassembly of the magnetic iron oxide nanoparticles and increase in MRI contrast.
Photoacoustic gold nanomaterials in cell tracking.
Gold nanomaterials are great candidates for molecular and cellular labeling by virtue of the fact that they can be synthesized to a variety of sizes. Once labeled with gold nanomaterials, the molecules or cells can be indirectly visualized and monitored via imaging methods. PAI is an emerging imaging modality based on ultrasound imaging. As ultrasound imaging has been demonstrated for real-time cell delivery guidance,166,167 PAI also showed great potential for guiding cell delivery and imaging the cells post-injection when the cells were labeled with photoacoustic contrast agents.168,169 Unlike ultrasound imaging, cells usually produce no PA signal and therefore are invisible under PAI. Therefore, the PA contrast agents are crucial for cell tracking with PAI. Donnelly recently used gold nanospheres to label mesenchymal stem cells and injected these cells into rat spinal cords with PAI-guidance injection accurately and precisely.170
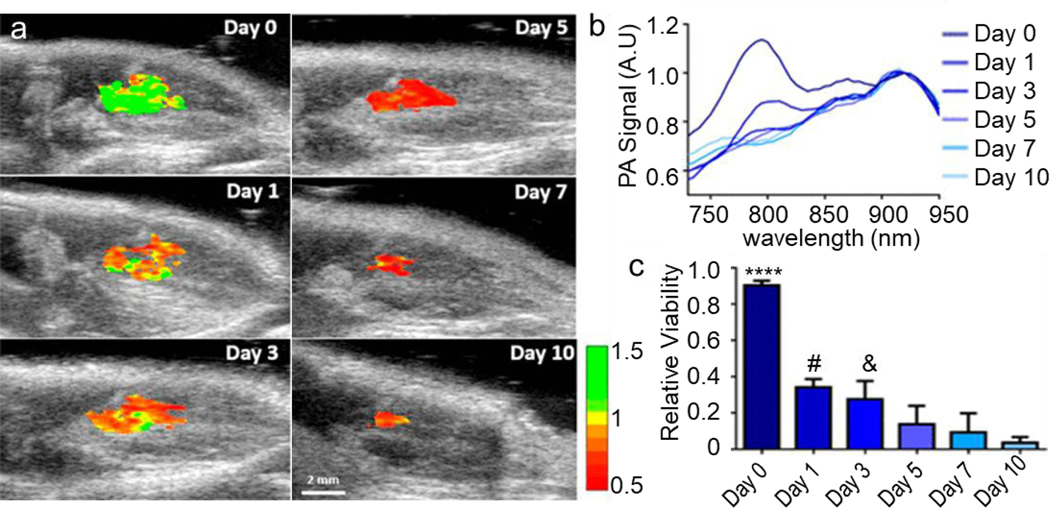
A long-standing question about nanoparticles-based contrast agents for cell tracking is their inability to distinguish live versus dead cells. Very recently, Dhada et al. used a reactive oxygen species (ROS) sensitive NIR dye IR775c-conjugated gold nanorods to track stem cell location and assessing the cell viability in vivo (Figure 10a).171 The PA signal of the IR775c (at 790 nm) was ROS-dependent and therefore cell death-dependent, while the PA signal of the gold nanorods (at 910 n) were independent on the cell viability (Figure 10b). Hence, the in vivo cell viability could be detected by comparing the PA signals of IR775c to gold nanorods (Figure 10c).
Figure 10.
Detection of IR775c-conjugated gold nanorods-labeled cell viability in vivo. (a) Ratiometric imaging of stem cell viability in vivo created with photoacoustic images of day 0, 1, 3, 5, 7, and 10 at 795 and 920 nm. (b) Photoacoustic spectra changes over time; the IR775c peak (790 nm) gradually decreases over time, while the gold nanorods peak (910 nm) was stable. (c) Quantification of relative viability showed 92% viable cells on day 0 to 5% viable by day 10, which is similar to in vitro measurements of stem cells incubated with doxorubicin. **** p < 0.0001 compared to all days. # p <.05 compared to day 7 and 10. & p < 0.05 compared to day 10. Adapted with permission from [171]. Copyright (2019) American Chemical Society.
Photoacoustic gold nanomaterials in other biomedical applications.
Gold nanomaterials or their hybrids also show great potential in eradicating bacteria through the photothermal effect. Zhi et al. conjugated gold nanostars with cis-aconitic anhydride-modified anti-H. pylori polyclonal antibodies to remove H. pylori in the stomach.172 The cis-aconitic anhydride was acid sensitive and could protect binding ability of antibodies on the gold nanostars to bind with H. pylori in the low pH environment of the stomach. Upon NIR laser irradiation, the photoacoustic and photothermal effects of gold nanostars could track and kill H. pylori in the stomach. This pH-sensitivity could protect the microbiome in the gut where the pH is higher than that in the stomach. The gold nanostars were orally administrated and could be excreted out of gut within 7 days in mice.
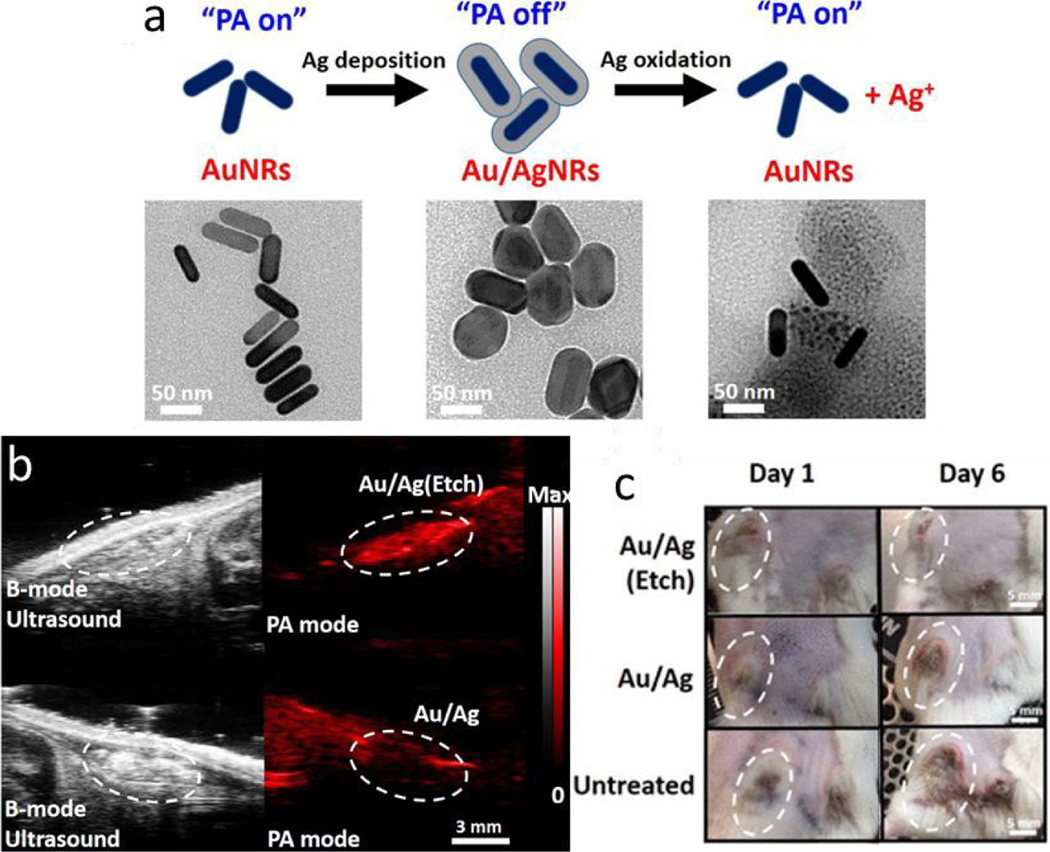
A silver-coated gold nanorod was developed as a theranostic agent for bacterial infection.173 Silver ions can be released from the hybrid nanoparticle upon addition of silver etchant. Upon release of silver ions, the enclosed gold nanorod was exposed and its NIR light absorption recovered, which resulted in increased PAI signals (Figure 11a). The PAI signal could be used to sense the trigger (etchant) for the release of silver ions from the silver-gold hybrid nanoparticles in vivo (Figure 11b). The released silver ions exhibited a strong bactericidal effect on both Gram-positive methicillin-resistant Staphylococcus aureus (MRSA) and Gram-negative Escherichia coli (Figure 11c).
Figure 11.
Dynamic photoacoustic signal and bacterial killing effect of silver coated gold nanoparticles. (a) Schematic of the photoacoustic signal (PA) changes associated with the silver coating and releasing. Representative TEM images show gold nanorods, silver coated gold nanorods, and etched silver coated gold nanorods. (b) Ultrasound and photoacoustic images of subcutaneously injected silver-gold hybrid nanoparticles with and without the addition of etchant, which triggered the release of silver ions. The PAI signal was significantly higher when the silver ions were released. (c) Representative photographs of the MRSA-infected wound from mice in three different treatment groups. The hybrid nanoparticles with released silver ions (Au/Ag (Etch)) showed a strong bactericidal efficacy in vivo. Figures reprinted with permission from [173]. Copyright (2018) American Chemical Society.
The PA signal increase of silver-coated gold nanorods as the silver coating was etched away can also be used to sense triggers for etching such as reactive oxygen species. Mantri et al. used this phenomenon and PAI to detect reactive oxygen and nitrogen species (RONS) in cell lines and murine models.174 The silver coating on gold nanorods was doped with iodide to lower the reduction potential of silver and increased detection sensitivity of H2O2 and ONOO− 1000- and 100000-fold respectively. The RONS detection limit was optimized by tuning the silver coating thickness and iodide-to-silver ratio.
Gold nanoparticles have also been used to track and deliver drugs for systemic lupus erythematosus (SLE) treatment. Xu et al. investigated the potential of gold nanocages in murine systemic lupus erythematosus (SLE) treatment.175 The role of gold nanocages was as drug carriers for liver X receptor agonist T0901317, which could promote the phagocytic clearance of apoptotic cells and treat SLE. The photoacoustic signal of the gold nanocage have the potential to track the drug T0901317.
To summarize, gold nanoparticles have been used for the imaging of ocular tumors and vasculature with PAI in pre-clinical models. While applications of photoacoustic gold nanoparticles in ophthalmology are sparse, lessons can be learned from such studies outside ophthalmology. First, when conjugated with targeting moieties, gold nanoparticles can bind targets such as specific proteins, cell organelles, and specific cell types to make them detectable with PAI. This feature can be used to image molecules of interest in the eye to better understand the pathological mechanisms for eye diseases, which could lead to more sensitive diagnoses for eye diseases. Second, gold nanoparticles have been used in monitoring therapeutic tools (either the gold nanoparticles themselves, loaded therapeutic molecules, or therapeutic cells labeled with gold nanoparticles) for cancer, bacterial infection, and systemic lupus erythematosus in living animals with PAI. This feature may benefit the field of ophthalmology as drug delivery, tumor prohibition, and infection treatment are also necessary treatment for the eye. Drug delivery through gold nanoparticles could greatly increase treatment efficacy by prolonging drug retention in the eye. This could be of benefit in the treatment of both ocular infections and tumors. Tracking the therapeutic systems in the eye might renew our understanding for the ophthalmic treatment. Nevertheless, the application of photoacoustic gold nanoparticles in ophthalmology as well as other areas merits further attention and investigation. To this end, a better understanding for systematic biodistribution, toxicity, and clearance of the gold nanoparticles as well as laser safety of PAI is needed to translate photoacoustic gold nanoparticles into clinical practice in ophthalmology.
3.3. Gold nanoparticles as OCT/PAI multimodal imaging agents
Because light absorption and scattering can both be enhanced by the localized surface plasmon resonance (LSPR) of gold nanoparticles, gold nanoparticles have recently been explored as bimodal imaging contrast agents for OCT and PAI. Wi and co-workers fabricated stacked gold nanodisks comprised of two nanodisk layers: the top layer with a diameter of 80 nm and bottom layer with a diameter of 180 nm, which allow light absorption and scattering at two different wavelengths at 630 and 850 nm, respectively.98 The gold nanodisks can thus be used as dual modal contrast agent for OCT with a central wavelength of 850 nm and PAI with a laser source operated at 630. The strong absorption and scattering of gold nanodisks at 630 and 850 nm greatly amplified both the PA and OCT signals and enhanced the contrast of PA and OCT images. More recently, Nguyen et al. reported on multimodal PA and OCT imaging of choroidal and retinal vessels in live rabbit eyes using colloidal gold nanoparticles as contrast agents.176 They fabricated ultrapure gold nanoparticles (AuNPs) using femtosecond laser ablation, and functionalized the surface of the AuNPs with PEG. The AuNP-PEG, with a dimension of ~20 nm, enhanced PA and OCT signals of the retinal and choroidal vessels in living rabbit eyes by 82% and 45%, respectively.
Conclusion & perspective
There is an unmet need in ophthalmology for new imaging modalities to visualize deep tissues in the eye with high spatial resolution, which can provide molecular-level, pathophysiological markers of diseases before any morphological or structural abnormalities are detectable. OCT and PAI are promising imaging techniques to meet this need. OCT can image tissues with a penetration depth of several millimeters and a spatial resolution of several micrometers. OCT has been widely used in ophthalmology to capture structural information of eyes without any contrast agents. With a suitable exogenous contrast agent, OCT is capable of providing even more powerful, molecular-level information, but no such agent is currently available. Gold nanomaterials have shown promise as an answer to this clinical need. PAI can image tissues as deep as 5–6 cm with a spatial resolution as low as 5 μm, and provide information in three dimensions. It can simultaneously produce ultrasound images that visualize ocular anatomy and PAI images that show molecular information if suitable contrast agents are utilized. Endogenous contrast agents present in the eye are hemoglobin and melanin. Hence, PAI can be used to perform angiography and the visualize the distribution of melanin in the eye. Nevertheless, to date, PAI has not yet found clinical utility in ophthalmology the way OCT has. One barrier is the ability to keep laser exposure under the maximum permissible exposures for ocular safety (ANSI 2005) while incorporating the use of photoacoustic contrast agents for ophthalmic imaging.
Gold nanoparticles have shown great promise as contrast agents for molecular imaging of the eye. First, gold nanoparticles are inherently inert in biological and physiological conditions, which means they do not decompose or produce toxic products in the body. Gold nanoparticles can be administrated to the eye intravenously, topically, and intraocularly. Intravenously administrated gold nanoparticles can pass through the blood-retinal barrier and reach all retinal layers if they are small enough. Intravitreally injected gold nanoparticles have been demonstrated to be gradually cleared from the eye after administration. The retention of topically administrated gold nanoparticles has been shown to last for 30 days with frequent applications. These results indicate the potential of gold nanoparticles for therapeutic and diagnostic uses in ophthalmology. Nevertheless, further work is merited to understand more about the biocompatibility, biodistribution, clearance, and pharmacokinetics of various gold nanoparticles in the eye.
Second, gold nanoparticles also have unique optical properties due to their intrinsic surface plasmon resonance, which result in their characteristically high and tunable absorption and scattering peaks. The high scattering of gold nanoparticles makes them excellent backscattering enhancers for OCT. The SPR effect-dependent scattering peak also confers unique spectral OCT signals to gold nanoparticles. Gold nanoparticles also exhibit a photoacoustic effect which can produce sound waves that can be detect by ultrasound transducers to produce photoacoustic images. Enormous efforts have been put into creation of gold nanoparticles with special shapes so that their absorption/scattering peaks lie in the second near-infrared window, which allows for deeper tissue penetration.
With the ongoing advancements in the synthesis, modification, and imaging contrast of gold nanoparticles, gold nanoparticles hold great promise in ophthalmic imaging for both diagnosis and therapy. For diagnosis, gold nanoparticles with OCT and PAI can enable angiography in deeper ocular tissues with higher sensitivity. Gold nanoparticles can potentially be used to identify diseases such as ocular tumors, infectious diseases, and bacterial diseases after being conjugated with ligands that target to specific loci. For the therapy, gold nanoparticles can track therapeutics and their effects in treating ocular diseases. Gold nanoparticles themselves can be used as therapeutics in ophthalmology due to their photothermal effect and have been reported to have antiangiogenic effects in the eye. Additionally, gold nanoparticles have intrinsic photothermal effects, which can be used to kill cancer cells, bacteria, and viruses. They can also load and deliver therapeutic molecules into the eye. Research in other fields have shown promising results when gold nanoparticles are used in photothermal therapy, photodynamic therapy, chemotherapy, radiotherapy, and/or gene therapy through specifically engineered particle designs. OCT and PAI using gold nanoparticles allow for the monitoring of their movement as well as indirect information about where treatment effects are occurring.
Gold nanoparticles have the potential to change the face of what is possible in ophthalmology by providing new understanding of molecular-level pathophysiology and enabling new therapeutic modalities. Similar to other types of nanoparticles, translation of gold nanoparticles into clinical use is relatively rare compared to the number of pre-clinical research articles on the subject. To accelerate the translation of nanoparticles, the authors believe it is essential for the nanoparticle researchers to take a collaborative approach and reach out to clinicians in order to identify and solve unmet problems together. With a concerted, interdisciplinary effort between nanomaterial scientists and clinicians, gold nanoparticles may well find further applications in medicine and in particular clinical ophthalmology toward the improvement of patient outcomes.
Acknowledgements
This work was supported by the Matilda Ziegler Foundation, departmental core grants to Stanford from the National Eye Institute (P30-EY026877), Research to Prevent Blindness, Discovery Innovation Fund 2019, Damon Runyon Cancer Research Foundation (DFS# 06–13), Claire Giannini Fund, the Susan G. Komen Breast Cancer Foundation (SAB15–00003), the Mary Kay Foundation (017–14), the Skippy Frank Foundation, the Donald E. and Delia B. Baxter Foundation, and a seed grant from the Center for Cancer Nanotechnology Excellence and Translation (CCNE-T; NIH-NCI U54CA151459). A.d.l.Z is a Chan Zuckerberg Biohub investigator and a Pew-Stewart Scholar for Cancer Research supported by The Pew Charitable Trusts and The Alexander and Margaret Stewart Trust.
Footnotes
Conflicts of interest
There are no conflicts to declare.
Publisher's Disclaimer: Accepted Manuscripts are published online shortly after acceptance, before technical editing, formatting and proof reading. Using this free service, authors can make their results available to the community, in citable form, before we publish the edited article. We will replace this Accepted Manuscript with the edited and formatted Advance Article as soon as it is available.
Please note that technical editing may introduce minor changes to the text and/or graphics, which may alter content. The journal’s standard Terms & Conditions and the Ethical guidelines still apply. In no event shall the Royal Society of Chemistry be held responsible for any errors or omissions in this Accepted Manuscript or any consequences arising from the use of any information it contains.
References
- 1.Su S and V Jokerst J, in Hybrid Nanomaterials, CRC Press, 2017, pp. 355–392. [Google Scholar]
- 2.Huang X and El-Sayed MA, J. Adv. Res, 2010, 1, 13–28. [Google Scholar]
- 3.Si P, Shevidi S, Yuan E, Yuan K, Lautman Z, Jeffrey SS, Sledge GW and de la Zerda A, Nano Lett., 2019, 20, 101–108. [DOI] [PubMed] [Google Scholar]
- 4.Liu X, Atwater M, Wang J and Huo Q, Colloids Surfaces B Biointerfaces, 2007, 58, 3–7. [DOI] [PubMed] [Google Scholar]
- 5.Lee KS and El-Sayed MA, J. Phys. Chem. B, 2005, 109, 20331–20338. [DOI] [PubMed] [Google Scholar]
- 6.Cho EC, Kim C, Zhou F, Cobley CM, Song KH, Chen J, Li ZY, Wang LV and Xia Y, J. Phys. Chem. C, 2009, 113, 9023–9028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Seong Ah C, Ju Yun Y, Ju Park H, Kim W-J, Han Ha D and Soo Yun W, Chem. Mater, 2005, 17, 5558–5561. [Google Scholar]
- 8.Wi JS, Park J, Kang H, Jung D, Lee SW and Lee TG, ACS Nano, 2017, 11, 6225–6232. [DOI] [PubMed] [Google Scholar]
- 9.Melancon MP, Lu W, Yang Z, Zhang R, Cheng Z, Elliot AM, Stafford J, Olson T, Zhang JZ and Li C, Mol. Cancer Ther, 2008, 7, 1730–1739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Si P, Yuan E, Liba O, Winetraub Y, Yousefi S, Daniel SoRelle E, William Yecies D, Dutta R and de la Zerda A, ACS Nano, 2018, 12, 11986–11994. [DOI] [PubMed] [Google Scholar]
- 11.Chen J, Saeki F, Wiley BJ, Cang H, Cobb MJ, Li ZY, Au L, Zhang H, Kimmey MB, Li X and Xia Y, Nano Lett., 2005, 5, 473–477. [DOI] [PubMed] [Google Scholar]
- 12.Kim C, Song HM, Cai X, Yao J, Wei A and Wang LV, J. Mater. Chem, 2011, 21, 2841–2844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hao E, Bailey RC, Schatz GC, Hupp JT and Li S, Nano Lett., 2004, 4, 327–330. [Google Scholar]
- 14.Huang P, Lin J, Li W, Rong P, Wang Z, Wang S, Wang X, Sun X, Aronova M, Niu G, Leapman RD, Nie Z and Chen X, Angew. Chemie - Int. Ed, 2013, 52, 13958–13964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Li W and Chen X, Nanomedicine, 2015, 10, 299–320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yun-Sheng Chen SE, Zhao Yang, Yoon Soon Joon, Gambhir Sanjiv Sam, Nat. Nanotechnol, 2019, 14, 465–472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Smith BR and Gambhir SS, Chem. Rev, 2017, 117, 901–986. [DOI] [PubMed] [Google Scholar]
- 18.Li P, Wu Y, Li D, Su X, Luo C, Wang Y, Hu J, Li G, Jiang H and Zhang W, Nanoscale Res Lett, 2018, 13, 313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhu H, Wang Y, Chen C, Ma M, Zeng J, Li S, Xia Y and Gao M, ACS Nano, 2017, 11, 8273–8281. [DOI] [PubMed] [Google Scholar]
- 20.Song J, Yang X, Yang Z, Lin L, Liu Y, Zhou Z, Shen Z, Yu G, Dai Y, Jacobson O, Munasinghe J, Yung B, Teng GJ and Chen X, ACS Nano, 2017, 11, 6102–6113. [DOI] [PubMed] [Google Scholar]
- 21.Zhou J, Jiang Y, Hou S, Upputuri PK, Wu D, Li J, Wang P, Zhen X, Pramanik M, Pu K and Duan H, ACS Nano, 2018, 12, 2643–2651. [DOI] [PubMed] [Google Scholar]
- 22.Han X, Xu Y, Li Y, Zhao X, Zhang Y, Min H, Qi Y, Anderson GJ, You L, Zhao Y and Nie G, ACS Nano, 2019, 13, 4379–4391. [DOI] [PubMed] [Google Scholar]
- 23.Zeng J, Gong M, Wang D, Li M, Xu W, Li Z, Li S, Zhang D, Yan Z and Yin Y, Nano Lett., 2019, 19, 3011–3018. [DOI] [PubMed] [Google Scholar]
- 24.Yi C, Su MN, Dongare PD, Chakraborty D, Cai YY, Marolf DM, Kress RN, Ostovar B, Tauzin LJ, Wen F, Chang WS, Jones MR, Sader JE, Halas NJ and Link S, Nano Lett., 2018, 18, 3494–3501. [DOI] [PubMed] [Google Scholar]
- 25.Masse F, Ouellette M, Lamoureux G and Boisselier E, Med. Res. Rev, 2019, 39, 302–327. [DOI] [PubMed] [Google Scholar]
- 26.Khlebtsov N and Dykman L, Chem. Soc. Rev, 2011, 40, 1647–1671. [DOI] [PubMed] [Google Scholar]
- 27.Dreaden EC, Alkilany AM, Huang X, Murphy CJ and El-Sayed MA, Chem. Soc. Rev, 2012, 41, 2740–2779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Song HB, Wi JS, Jo DH, Kim JH, Lee SW, Lee TG and Kim JH, Nanomedicine Nanotechnology, Biol. Med, 2017, 13, 1901–1911. [DOI] [PubMed] [Google Scholar]
- 29.Lasagna-Reeves C, Gonzalez-Romero D, Barria MA, Olmedo I, Clos A, Sadagopa Ramanujam VM, Urayama A, Vergara L, Kogan MJ and Soto C, Biochem. Biophys. Res. Commun, 2010, 393, 649–655. [DOI] [PubMed] [Google Scholar]
- 30.von Maltzahn G, Park J-H, Agrawal A, Bandaru NK, Das SK, Sailor MJ and Bhatia SN, Cancer Res., 2009, 69, 3892–3900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Boisselier E and Astruc D, Chem. Soc. Rev, 2009, 38, 1759. [DOI] [PubMed] [Google Scholar]
- 32.Zhang G, Yang Z, Lu W, Zhang R, Huang Q, Tian M, Li L, Liang D and Li C, Biomaterials, 2009, 30, 1928–1936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kim JH, Kim JH, Kim KW, Kim MH and Yu YS, Nanotechnology, 2009, 20, 505101. [DOI] [PubMed] [Google Scholar]
- 34.Sonavane G, Tomoda K and Makino K, Colloids Surfaces B Biointerfaces, DOI: 10.1016/j.colsurfb.2008.07.004. [DOI] [PubMed] [Google Scholar]
- 35.De Jong WH, Hagens WI, Krystek P, Burger MC, Sips AJAM and Geertsma RE, Biomaterials, 2008, 29, 1912–1919. [DOI] [PubMed] [Google Scholar]
- 36.Soo Choi H, Liu W, Misra P, Tanaka E, Zimmer JP, Itty Ipe B, Bawendi MG and Frangioni JV, Nat. Biotechnol, 2007, 25, 1165–1170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Longmire M, Choyke PL and Kobayashi H, Nanomedicine, 2008, 3, 703–717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Dykman L and Khlebtsov N, Chem. Soc. Rev, 2012, 41, 2256–2282. [DOI] [PubMed] [Google Scholar]
- 39.Pan Y, Leifert A, Ruau D, Neuss S, Bornemann J, Schmid G, Brandau W, Simon U and Jahnen-Dechent W, Small, 2009, 5, 2067–2076. [DOI] [PubMed] [Google Scholar]
- 40.Niidome T, Yamagata M, Okamoto Y, Akiyama Y, Takahashi H, Kawano T, Katayama Y and Niidome Y, J. Control. Release, 2006, 114, 343–347. [DOI] [PubMed] [Google Scholar]
- 41.Zhai J, Hinton TM, Waddington LJ, Fong C, Tran N, Mulet X, Drummond CJ and Muir BW, Langmuir, 2015, 31, 10871–10880. [DOI] [PubMed] [Google Scholar]
- 42.Chan CM, Hsiao CY, Li HJ, Fang JY, Chang DC and Hung CF, Int. J. Mol. Sci, DOI: 10.3390/ijms21010109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ngwa W, Makrigiorgos GM and Berbeco RI, Phys. Med. Biol, 2012, 57, 6371–6380. [DOI] [PubMed] [Google Scholar]
- 44.Shen N, Zhang R, Zhang HR, Luo HY, Shen W, Gao X, Guo DZ and Shen J, Int. J. Ophthalmol, 2018, 11, 1269–1276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Cho WK, Kang S, Choi H and Rho CR, Cornea, 2015, 34, 456–459. [DOI] [PubMed] [Google Scholar]
- 46.Roh YJ, Rho CR, Cho WK and Kang S, Investig. Ophthalmol. Vis. Sci, 2016, 57, 6561–6567. [DOI] [PubMed] [Google Scholar]
- 47.Liu X, Wang S, Wang X, Liang J and Zhang Y, Chem. Biol. Drug Des, 2017, 90, 653–664. [DOI] [PubMed] [Google Scholar]
- 48.Wang Y, Xia R, Hu H and Peng T, J. Photochem. Photobiol. B Biol, 2018, 187, 180–183. [DOI] [PubMed] [Google Scholar]
- 49.Karakoçak BB, Raliya R, Davis JT, Chavalmane S, Wang WN, Ravi N and Biswas P, Toxicol. Vitr, 2016, 37, 61–69. [DOI] [PubMed] [Google Scholar]
- 50.Söderstjerna E, Bauer P, Cedervall T, Abdshill H, Johansson F and Johansson UE, PLoS One, DOI: 10.1371/journal.pone.0105359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Azharuddin M, Sahana S, Datta H and Dasgupta AK, J. Nanosci. Nanotechnol, 2014, 14, 5669–5675. [DOI] [PubMed] [Google Scholar]
- 52.van Velthoven MEJ, Faber DJ, Verbraak FD, van Leeuwen TG and de Smet MD, Prog. Retin. Eye Res, 2007, 26, 57–77. [DOI] [PubMed] [Google Scholar]
- 53.and Francisco AW Robles E, Wilson Christy, Grant Gerald, Nat Photonics, 2011, 5, 744–747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ramos de Carvalho JE, Verbraak FD, Aalders MC, van Noorden CJ and Schlingemann RO, Surv. Ophthalmol, 2014, 59, 393–413. [DOI] [PubMed] [Google Scholar]
- 55.James ML and Gambhir SS, Physiol. Rev, 2012, 92, 897–965. [DOI] [PubMed] [Google Scholar]
- 56.Si P, Honkala A, de la Zerda A and Smith BR, Trends Cancer, 2020, 6, 205–222. [DOI] [PubMed] [Google Scholar]
- 57.Pan ZW and Shen HL, IEEE Trans. Image Process, 2019, 28, 1783–1797. [DOI] [PubMed] [Google Scholar]
- 58.M. R. and Neil Lagali PF, Peebo Beatrice Bourghardt, Germundsson Johan, Edén Ulla, Danyali Reza, in Confocal Laser Microscopy - Principles and Applications in Medicine, Biology, and the Food Sciences References, 2013, pp. 51–80. [Google Scholar]
- 59.Bille JF, Ed., High Resolution Imaging in Microscopy and Ophthalmology, Springer Nature; Switzerland AG, 2019. [PubMed] [Google Scholar]
- 60.Parviz M, Esfandiari H, Behnaz N, Fatemeh J, Sima A and Javadi M, Kalantarion Mohammad Ali, J. Ophthalmic Vis. Res, 2017, 12, 270–274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Capozzi ME, Gordon AY, Penn JS and Jayagopal A, J. Ocul. Pharmacol. Ther, 2013, 29, 275–286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Xie F, Luo W, Zhang Z and Sun D, J. Ophthalmol, DOI: 10.1155/2012/429387. [DOI] [Google Scholar]
- 63.Russmann C and Amiji M, J. Ophthalmic Vis. Res, 2017, 12, 129–131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Huang D, a Swanson E, Lin CP, Schuman JS, Stinson WG, Chang W, Hee MR, Flotire T, Gregory K, a Puliafito C and Fujimoto JG, 1991, 1–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Shu X, Beckmann L and Zhang HF, J. Biomed. Opt, 2017, 22, 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Coffey AM, Hutton EK, Combe L, Bhindi P, Gertig D and Constable PA, Clin. Exp. Optom, DOI: 10.1111/cxo.13068. [DOI] [PubMed] [Google Scholar]
- 67.Li DQ and Choudhry N, Curr. Opin. Ophthalmol, 2020, 31, 199–206. [DOI] [PubMed] [Google Scholar]
- 68.Davila JR and Mruthyunjaya P, F1000Research. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Bekkers A, Borren N, Ederveen V, Fokkinga E, De DA, Luisa S, Klein S, Van Walsum T and Stalmans I, Acta Ophthalmol., 2020, 1–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Naseripour M, Ghasemi Falavarjani K, Mirshahi R and Sedaghat A, Eye, DOI: 10.1038/s41433-020-0819-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Moore C, Chen F, Wang J and Jokerst JV, Adv. Drug Deliv. Rev, DOI: 10.1016/j.addr.2019.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Silverman RH, Kong F, Lloyd HO and Chen YC, Photons Plus Ultrasound Imaging Sens. 2010, 2010, 7564, 75640Y. [Google Scholar]
- 73.Si P, Shevidi S, Yuan E, Yuan K, Lautman Z, Jeffrey SS, Sledge GW and de la Zerda A, bioRxiv, 2019, 656744. [DOI] [PubMed] [Google Scholar]
- 74.de la Zerda A, Paulus YM, Teed R, Bodapati S, Dollberg Y, Khuri-Yakub BT, Blumenkranz MS, Moshfeghi DM and Gambhir SS, Opt. Lett, 2010, 35, 270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Hariri A, Wang J, Kim Y, Jhunjhunwala A, Chao DL and Jokerst JV, J. Biomed. Opt, 2018, 23, 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.and Hu L. V. W. Song, Rao Bin, Maslov Konstantin, Opt Lett., 2010, 35, 1–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Nguyen VP, Li Y, Zhang W, Wang X and Paulus YM, Sci. Rep, 2019, 9, 1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Wu Y, Ali MRK, Chen K, Fang N and El-Sayed MA, Nano Today, 2019, 24, 120–140. [Google Scholar]
- 79.Song W, Wei Q, Liu T, Kuai D, Burke JM, Jiao S and Zhang HF, J. Biomed. Opt, DOI: 10.1117/1.jbo.17.6.061206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Zhang W, Li Y, Nguyen VP, Huang Z, Liu Z, Wang X and Paulus YM, Light Sci. Appl, 2018, 7, 103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Wang P, Kim T, Harada M, Contag C, Huang X and Smith BR, Nanoscale Horizons, 2020, 5, 628–653. [DOI] [PubMed] [Google Scholar]
- 82.Ehlerding EB, Grodzinski P, Cai W and Liu CH, ACS Nano, 2018, 12, 2106–2121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Chapman S, Dobrovolskaia M, Farahani K, Goodwin A, Joshi A, Lee H, Meade T, Pomper M, Ptak K, Rao J, Singh R, Sridhar S, Stern S, Wang A, Weaver JB, Woloschak G and Yang L, Nano Today, 2013, 8, 454–460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Cang H, Sun T, Li Z-Y, Chen J, Wiley BJ, Xia Y and Li X, Opt. Lett, 2005, 30, 3048–3050. [DOI] [PubMed] [Google Scholar]
- 85.Zharov VP, Nat. Photonics, 2011, 5, 110–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Agrawal A, Huang S, Wei Haw Lin A, Lee M-H, Barton JK, Drezek RA and Pfefer TJ, J. Biomed. Opt, 2006, 11, 041121. [DOI] [PubMed] [Google Scholar]
- 87.Gobin AM, Lee MH, Halas NJ, James WD, Drezek RA and West JL, Nano Lett., 2007, 7 VN-re, 1929–1934. [DOI] [PubMed] [Google Scholar]
- 88.Kah JCY, Olivo M, Chow TH, Song KS, Koh KZY, Mhaisalkar S and Sheppard CJR, J. Biomed. Opt, 2009, 14, 054015. [DOI] [PubMed] [Google Scholar]
- 89.Zagaynova EV, Shirmanova MV, Kirillin MY, Khlebtsov BN, Orlova AG, Balalaeva IV, Sirotkina MA, Bugrova ML, Agrba PD and Kamensky VA, Phys. Med. Biol, 2008, 53, 4995–5009. [DOI] [PubMed] [Google Scholar]
- 90.Kirillin M, Shirmanova M, Sirotkina M, Bugrova M, Khlebtsov B and Zagaynova E, J. Biomed. Opt, 2009, 14, 021017. [DOI] [PubMed] [Google Scholar]
- 91.Jain PK, Lee KS, El-Sayed IH and El-Sayed MA, J. Phys. Chem. B, 2006, 110, 7238–7248. [DOI] [PubMed] [Google Scholar]
- 92.Hu J, Sanz-Rodríguez F, Rivero F, Rodríguez EM, Torres RA, Ortgies DH, Solé JG, Alfonso F and Jaque D, Nano Res., 2018, 11, 676–685. [Google Scholar]
- 93.Troutman TS, Barton JK and Romanowski M, Opt. Lett, 2007, 32, 1438–1440. [DOI] [PubMed] [Google Scholar]
- 94.Huang Y, Li M, Huang D, Qiu Q, Lin W, Liu J, Yang W, Yao Y, Yan G, Qu N, Tuchin VV, Fan S, Liu G, Zhao Q and Chen X, Small, 2019, 15, 1902346. [DOI] [PubMed] [Google Scholar]
- 95.Prabhulkar S, Matthews J, Rawal S and Awdeh RM, Investig. Ophthalmol. Vis. Sci, 2013, 54, 1192–1200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.de la Zerda A, Prabhulkar S, Perez VL, Ruggeri M, Paranjape AS, Habte F, Gambhir SS and Awdeh RM, Clin. Exp. Ophthalmol, 2015, 43, 358–366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Sen D, SoRelle ED, Liba O, Dalal R, Paulus YM, Kim T-W, Moshfeghi DM and de la Zerda A, J. Biomed. Opt, 2016, 21, 066002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Wi JS, Park J, Kang H, Jung D, Lee SW and Lee TG, ACS Nano, 2017, 11, 6225–6232. [DOI] [PubMed] [Google Scholar]
- 99.Al Rawashdeh W, Weyand T, Kray S, Lenz M, Buchkremer A, Spöler F, Simon U, Möller M, Kiessling F and Lederle W, J. Nanoparticle Res, DOI: 10.1007/s11051-015-2949-x. [DOI] [Google Scholar]
- 100.Zhang M, Ma L and Yu P, Biomed. Opt. Express, 2014, 5, 167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Liba O, SoRelle ED, Sen D and de la Zerda A, Sci. Rep, 2016, 6, 23337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.SoRelle ED, Liba O, Hussain Z, Gambhir M and De La Zerda A, Langmuir, 2015, 31, 12339–12347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Dutta R, Liba O, Sorelle ED, Winetraub Y, Ramani VC, Jeffrey SS, Sledge GW and De La Zerda A, Nano Lett., DOI: 10.1021/acs.nanolett.8b05005. [DOI] [PubMed] [Google Scholar]
- 104.Sorelle ED, Yecies DW, Liba O, Bennett FC, Graef CM, Dutta R, Mitra S, Joubert LM, Cheshier S, Grant GA and De La Zerda A, ACS Nano, 2019, 13, 7985–7995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Yuan E, Si P, Shevidi S and de la Zerda A, bioRxiv, 2019, 846170. [Google Scholar]
- 106.Lu GJ, Chou L, Malounda D, Patel AK, Welsbie DS, Chao DL, Ramalingam T and Shapiro MG, ACS Nano, 2020, acsnano.9b08432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Hu J, Gorsak T, Martín Rodríguez E, Calle D, Muñoz-Ortiz T, Jaque D, Fernández N, Cussó L, Rivero F, Aguilar Torres R, García Solé J, Mertelj A, Makovec D, Desco M, Lisjak D, Alfonso F, Sanz-Rodríguez F and Ortgies DH, ChemPhotoChem, 2019, 3, 529–539. [Google Scholar]
- 108.Jiang X, Tang P, Gao P, Zhang YS, Yi C and Zhou J, Anal. Chem, 2017, 89, 2561–2568. [DOI] [PubMed] [Google Scholar]
- 109.Tang P, Jiang X, Wang Y, Chen H, Zhang YS, Gao P, Wang H, Li X and Zhou J, DOI: 10.1021/acs.analchem.7b01623. [DOI] [Google Scholar]
- 110.Kim CS, Ingato D, Wilder-Smith P, Chen Z and Kwon YJ, Nano Converg., 2018, 5, 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Mattison SP, Kim W, Park J and Applegate BE, Curr. Mol. Imaging, 2014, 3, 88–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Lapierre-Landry M, Gordon AY, Penn JS and Skala MC, Sci. Rep, 2017, 7, 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Gordon AY, Lapierre-Landry M, Skala MC and Penn JS, Transl. Vis. Sci. Technol, DOI: 10.1167/tvst.8.3.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Lippok N, Villiger M, Albanese A, Meijer EFJ, Chung K, Padera TP, Bhatia SN and Bouma BE, Nat. Photonics, 2017, 11, 583–588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Marin R, Lifante J, Besteiro LV, Wang Z, Govorov AO, Rivero F, Alfonso F, Sanz-Rodríguez F and Jaque D, Adv. Healthc. Mater, 2020, 9, 1901627. [DOI] [PubMed] [Google Scholar]
- 116.Yang X, Stein EW, Ashkenazi S and Wang LV, Wiley Interdiscip. Rev. Nanomedicine Nanobiotechnology, 2009, 1, 360–368. [DOI] [PubMed] [Google Scholar]
- 117.Chen YS, Frey W, Kim S, Kruizinga P, Homan K and Emelianov S, Nano Lett., 2011, 11, 348–354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Chen F, Zhao ER, Hu T, Shi Y, Sirbuly DJ and Jokerst JV, Nanoscale Adv., DOI: 10.1039/c9na00237e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Meng Z, Zhou X, She J, Zhang Y, Feng L and Liu Z, Nano Lett., 2019, 19, 8109–8117. [DOI] [PubMed] [Google Scholar]
- 120.Kim H, Nguyen VP, Manivasagan P, Jung MJ, Kim SW, Oh J and Kang HW, Oncotarget, 2017, 8, 113719–113733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Raveendran S, Lim HT, Maekawa T, Vadakke Matham M and Sakthi Kumar D, Nanoscale, 2018, 10, 13959–13968. [DOI] [PubMed] [Google Scholar]
- 122.Nguyen VP, Li Y, Qian W, Liu B, Tian C, Zhang W, Huang Z, Ponduri A, Tarnowski M, Wang X and Paulus YM, Sci. Rep, 2019, 9, 1–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Delori FC, Webb RH and Sliney DH, J. Opt. Soc. Am. A, 2007, 24, 1250. [DOI] [PubMed] [Google Scholar]
- 124.Zhang W, Li Y, Nguyen VP, Derouin K, Xia X, Paulus YM and Wang X, J. Biomed. Opt, 2020, 25, 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Song KH, Kim C, Cobley CM, Xia Y and V Wang L, Nano Lett., 2009, 9, 183–188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Cai X, Li W, Kim C-H, Yuan Y, V Wang L and Xia Y, ACS Nano, 2011, 5, 9658–9667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.V Jokerst J, Cole AJ, Van de Sompel D and Gambhir SS, ACS Nano, 2012, 6, 10366–10377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Wang C, Bao C, Liang S, Fu H, Wang K, Deng M, Liao Q and Cui D, Nanoscale Res. Lett, 2014, 9, 264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Kim C, Cho EC, Chen J, Song KH, Au L, Favazza C, Zhang Q, Cobley CM, Gao F, Xia Y and V Wang L, ACS Nano, 2010, 4, 4559–4564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Mallidi S, Larson T, Tam J, Joshi PP, Karpiouk A, Sokolov K and Emelianov S, Nano Lett., 2009, 9, 2825–2831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Lin J, Wang S, Huang P, Wang Z, Chen S, Niu G, Li W, He J, Cui D, Lu G, Chen X and Nie Z, ACS Nano, 2013, 7, 5320–5329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Ohulchanskyy TY, Kopwitthaya A, Jeon M, Guo M, Law W-C, Furlani EP, Kim C and Prasad PN, Nanomedicine, 2013, 9, 1192–1202. [DOI] [PubMed] [Google Scholar]
- 133.Yasun E, Kang H, Erdal H, Cansiz S, Ocsoy I, Huang Y-F and Tan W, Interface Focus, 2013, 3, 20130006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Li Y, Wen T, Zhao R, Liu X, Ji T, Wang H, Shi X, Shi J, Wei J, Zhao Y, Wu X and Nie G, ACS Nano, 2014, 8, 11529–11542. [DOI] [PubMed] [Google Scholar]
- 135.Song J, Yang X, Jacobson O, Lin L, Huang P, Niu G, Ma Q and Chen X, ACS Nano, 2015, 9, 9199–9209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Liang S, Li C, Zhang C, Chen Y, Xu L, Bao C, Wang X, Liu G, Zhang F and Cui D, Theranostics, 2015, 5, 970–984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Li L, Fu S, Chen C, Wang X, Fu C, Wang S, Guo W, Yu X, Zhang X, Liu Z, Qiu J and Liu H, ACS Nano, 2016, 10, 7094–7105. [DOI] [PubMed] [Google Scholar]
- 138.Yang Y, Zhang J, Xia F, Zhang C, Qian Q, Zhi X, Yue C, Sun R, Cheng S, Fang S, Jin W, Yang Y and Cui D, Nanoscale Res. Lett, 2016, 11, 285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Han J, Zhang J, Yang M, Cui D and de la Fuente JM, Nanoscale, 2016, 8, 492–499. [DOI] [PubMed] [Google Scholar]
- 140.Liu Y, Yang M, Zhang J, Zhi X, Li C, Zhang C, Pan F, Wang K, Yang Y, Martinez J Fuentea de la and Cui D, ACS Nano, 2016, 10, 2375–2385. [DOI] [PubMed] [Google Scholar]
- 141.Zhang L, Su H, Cai J, Cheng D, Ma Y, Zhang J, Zhou C, Liu S, Shi H, Zhang Y and Zhang C, ACS Nano, 2016, 10, 10404–10417. [DOI] [PubMed] [Google Scholar]
- 142.Gao S, Zhang L, Wang G, Yang K, Chen M, Tian R, Ma Q and Zhu L, Biomaterials, 2016, 79, 36–45. [DOI] [PubMed] [Google Scholar]
- 143.Fang S, Lin J, Li C, Huang P, Hou W, Zhang C, Liu J, Huang S, Luo Y, Fan W, Cui D, Xu Y and Li Z, Small, DOI: 10.1002/smll.201602580. [DOI] [Google Scholar]
- 144.Liu Y, Zhi X, Yang M, Zhang J, Lin L, Zhao X, Hou W, Zhang C, Zhang Q, Pan F, Alfranca G, Yang Y, de la Fuente JM, Ni J and Cui D, Theranostics, 2017, 7, 1650–1662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Min KH, Kim Y-H, Wang Z, Kim J, Kim JS, Kim SH, Kim K, Kwon IC, Kiesewetter DO and Chen X, Theranostics, 2017, 7, 4240–4254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Liu Y, Wang Z, Liu Y, Zhu G, Jacobson O, Fu X, Bai R, Lin X, Lu N, Yang X, Fan W, Song J, Wang Z, Yu G, Zhang F, Kalish H, Niu G, Nie Z and Chen X, ACS Nano, 2017, 11, 10539–10548. [DOI] [PubMed] [Google Scholar]
- 147.Yu Z, Wang M, Pan W, Wang H, Li N and Tang B, Chem Sci, 2017, 8, 4896–4903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Xu C, Feng Q, Yang H, Wang G, Huang L, Bai Q, Zhang C, Wang Y, Chen Y, Cheng Q, Chen M, Han Y, Yu Z, Lesniak MS and Cheng Y, Adv Sci, 2018, 5, 1800382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Ma Z, Han K, Dai X and Han H, ACS Nano, 2018, 12, 6252–6262. [DOI] [PubMed] [Google Scholar]
- 150.Huang L, Xu C, Xu P, Qin Y, Chen M, Feng Q, Pan J, Cheng Q, Liang F, Wen X, Wang Y, Shi Y and Cheng Y, Nanotheranostics, 2019, 3, 41–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Liu B, Cao W, Cheng J, Fan S, Pan S, Wang L, Niu J, Pan Y, Liu Y, Sun X, Ma L, Song J, Ni J and Cui D, Cancer Biol Med, 2019, 16, 756–770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Kong C, Hao M, Chen X, Zhao X, Wang Y, Li J, Gao Y, Zhang H, Yang B and Jiang J, Biomater Sci, 2019, 7, 2559–2570. [DOI] [PubMed] [Google Scholar]
- 153.Cao W, Gao W, Liu Z, Hao W, Li X, Sun Y, Tong L and Tang B, Anal. Chem, 2018, 90, 9125–9131. [DOI] [PubMed] [Google Scholar]
- 154.Xia F, Niu J, Hong Y, Li C, Cao W, Wang L, Hou W, Liu Y and Cui D, Acta Biomater., 2019, 89, 289–299. [DOI] [PubMed] [Google Scholar]
- 155.Liu B, Cao W, Qiao G, Yao S, Pan S, Wang L, Yue C, Ma L, Liu Y and Cui D, Acta Biomater., 2019, 99, 307–319. [DOI] [PubMed] [Google Scholar]
- 156.Deng X, Liang S, Cai X, Huang S, Cheng Z, Shi Y, Pang M, Ma P and Lin J, Nano Lett., 2019, 19, 6772–6780. [DOI] [PubMed] [Google Scholar]
- 157.Tan H, Hou N, Liu Y, Liu B, Cao W, Zheng D, Li W, Liu Y, Xu B, Wang Z and Cui D, Nanomedicine, 2020, 102192. [DOI] [PubMed] [Google Scholar]
- 158.Xu X, Chong Y, Liu X, Fu H, Yu C, Huang J and Zhang Z, Acta Biomater., 2019, 84, 328–338. [DOI] [PubMed] [Google Scholar]
- 159.Huang Q, Zhang S, Zhang H, Han Y, Liu H, Ren F, Sun Q, Li Z and Gao M, ACS Nano, 2019, 13, 1342–1353. [DOI] [PubMed] [Google Scholar]
- 160.Tang W, Dong Z, Zhang R, Yi X, Yang K, Jin M, Yuan C, Xiao Z, Liu Z and Cheng L, ACS Nano, 2019, 13, 284–294. [DOI] [PubMed] [Google Scholar]
- 161.Liu T, Shen S, Huang Y, Zhang X, Lai Z, Tran TH, Liu Z and Cheng L, Nanoscale, 2019, 11, 22788–22795. [DOI] [PubMed] [Google Scholar]
- 162.Zhu R, Su L, Dai J, Li ZW, Bai S, Li Q, Chen X, Song J and Yang H, ACS Nano, DOI: 10.1021/acsnano.9b07984. [DOI] [PubMed] [Google Scholar]
- 163.Dan Q, Hu D, Ge Y, Zhang S, Li S, Gao D, Luo W, Ma T, Liu X, Zheng H, Li Y and Sheng Z, Biomater Sci, 2020, 8, 973–987. [DOI] [PubMed] [Google Scholar]
- 164.Ju Y, Zhang H, Yu J, Tong S, Tian N, Wang Z, Wang X, Su X, Chu X, Lin J, Ding Y, Li G, Sheng F and Hou Y, ACS Nano, 2017, 11, 9239–9248. [DOI] [PubMed] [Google Scholar]
- 165.Liu Y, Yang Z, Huang X, Yu G, Wang S, Zhou Z, Shen Z, Fan W, Liu Y, Davisson M, Kalish H, Niu G, Nie Z and Chen X, ACS Nano, 2018, 12, 8129–8137. [DOI] [PubMed] [Google Scholar]
- 166.Chen F, Zhao ER, Hableel G, Hu T, Kim T, Li J, Gonzalez-Pech NI, Cheng DJ, Lemaster JE, Xie Y, Grassian VH, Sen GL and Jokerst JV, ACS Nano, DOI: 10.1021/acsnano.9b00653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Chen F, Ma M, Wang J, Wang F, Chern S-X, Zhao ER, Jhunjhunwala A, Darmadi S, Chen H and Jokerst JV, Nanoscale, DOI: 10.1039/c6nr08177k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Lemaster JE, Chen F, Kim T, Hariri A and Jokerst JV, ACS Appl. Nano Mater, DOI: 10.1021/acsanm.8b00063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Kim T, Lemaster JE, Chen F, Li J and Jokerst JV, ACS Nano, DOI: 10.1021/acsnano.7b03519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Donnelly EM, Kubelick KP, Dumani DS and Emelianov SY, Nano Lett., 2018, 18, 6625–6632. [DOI] [PubMed] [Google Scholar]
- 171.Dhada KS, Hernandez DS and Suggs LJ, ACS Nano, 2019, 13, 7791–7799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Zhi X, Liu Y, Lin L, Yang M, Zhang L, Zhang L, Liu Y, Alfranca G, Ma L, Zhang Q, Fu H, Conde J, Ding X, Chen D, Ni J, Song J and Cui D, Nanomedicine, 2019, 20, 102019. [DOI] [PubMed] [Google Scholar]
- 173.Kim T, Zhang Q, Li J, Zhang L and V Jokerst J, ACS Nano, 2018, 12, 5615–5625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Mantri Y, Davidi B, Lemaster JE, Hariri A and Jokerst JV, Nanoscale, 2020, 12, 10511–10520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Xu N, Li J, Gao Y, Zhou N, Ma Q, Wu M, Zhang Y, Sun X, Xie J, Shen G, Yang M, Tu Q, Xu X, Zhu J and Tao J, Biomaterials, 2019, 197, 380–392. [DOI] [PubMed] [Google Scholar]
- 176.Nguyen VP, Li Y, Qian W, Liu B, Tian C, Zhang W, Huang Z, Ponduri A, Tarnowski M, Wang X and Paulus YM, Sci. Rep, 2019, 9, 1–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Dreaden EC, Alkilany AM, Huang X, Murphy CJ and El-Sayed MA, Chem. Soc. Rev, 2012, 41, 2740–2779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Armanetti P, Pocovi-Martinez S, Flori A, Avigo C, Cassano D, Menichetti L and Voliani V, Nanomedicine, 2018, 14, 1787–1795. [DOI] [PubMed] [Google Scholar]
- 179.Chen P-J, Hu S-H, Fan C-T, Li M-L, Chen Y-Y, Chen S-Y and Liu D-M, Chem. Commun. (Camb), 2013, 49, 892–894. [DOI] [PubMed] [Google Scholar]
- 180.Galanzha EI, Weingold R, Nedosekin DA, Sarimollaoglu M, Nolan J, Harrington W, Kuchyanov AS, Parkhomenko RG, Watanabe F, Nima Z, Biris AS, Plekhanov AI, Stockman MI and Zharov VP, Nat Commun, 2017, 8, 15528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Chen Y-S, Frey W, Kim S, Homan K, Kruizinga P, Sokolov K and Emelianov S, Opt. Express, 2010, 18, 8867–8878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Chen Y-SS, Frey W, Kim S, Kruizinga P, Homan K and Emelianov S, Nano Lett., 2011, 11, 348–354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Jung Y, Guan G, Wei C-W, Reif R, Gao X, O’Donnell M and Wang RK, J. Biomed. Opt, 2012, 17, 16015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Liu X, Lee C, Law W-C, Zhu D, Liu M, Jeon M, Kim J, Prasad PN, Kim C and Swihart MT, Nano Lett., 2013, 13, 4333–4339. [DOI] [PubMed] [Google Scholar]
- 185.Cheng K, Kothapalli S-R, Liu H, Koh AL, V Jokerst J, Jiang H, Yang M, Li J, Levi J, Wu JC, Gambhir SS and Cheng Z, J. Am. Chem. Soc, 2014, 136, 3560–3571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Hu J, Zhu X, Li H, Zhao Z, Chi X, Huang G, Huang D, Liu G, Wang X and Gao J, Theranostics, 2014, 4, 534–545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Hannah A, Luke G, Wilson K, Homan K and Emelianov S, ACS Nano, 2014, 8, 250–259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Moon H, Kumar D, Kim H, Sim C, Chang J-H, Kim J-M, Kim H and Lim D-K, ACS Nano, 2015, 9, 2711–2719. [DOI] [PubMed] [Google Scholar]
- 189.Li J, Arnal B, Wei C-W, Shang J, Nguyen T-M, O’Donnell M and Gao X, ACS Nano, 2015, 9, 1964–1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Park J, Kang H, Kim YH, Lee S-W, Lee TG and Wi J-S, Nanoscale, 2016, 8, 15514–15520. [DOI] [PubMed] [Google Scholar]
- 191.Comenge J, Fragueiro O, Sharkey J, Taylor A, Held M, Burton NC, Park BK, Wilm B, Murray P, Brust M and Levy R, ACS Nano, 2016, 10, 7106–7116. [DOI] [PubMed] [Google Scholar]
- 192.Masim FCP, Liu H-L, Porta M, Yonezawa T, Balcytis A, Juodkazis S, Hsu W-H and Hatanaka K, Opt. Express, 2016, 24, 14781–14792. [DOI] [PubMed] [Google Scholar]
- 193.Masim FCP, Hsu WH, Liu HL, Yonezawa T, Balcytis A, Juodkazis S and Hatanaka K, Opt. Express, 2017, 25, 19497–19507. [DOI] [PubMed] [Google Scholar]
- 194.Wang B, Yantsen E, Larson T, Karpiouk AB, Sethuraman S, Su JL, Sokolov K and Emelianov SY, Nano Lett., 2009, 9, 2212–2217. [DOI] [PubMed] [Google Scholar]
- 195.Green DE, Longtin JP and Sitharaman B, ACS Nano, 2009, 3, 2065–2072. [DOI] [PubMed] [Google Scholar]
- 196.V Jokerst J, Thangaraj M, Kempen PJ, Sinclair R and Gambhir SS, ACS Nano, 2012, 6, 5920–5930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Cui H, Hong C, Ying A, Yang X and Ren S, ACS Nano, 2013, 7, 7805–7811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Yasmin Z, Khachatryan E, Lee Y-H, Maswadi S, Glickman R and Nash KL, Biosens. Bioelectron, 2015, 64, 676–682. [DOI] [PubMed] [Google Scholar]
- 199.Ungureanu C, Kroes R, Petersen W, Groothuis TAM, Ungureanu F, Janssen H, van Leeuwen FWB, Kooyman RPH, Manohar S and van Leeuwen TG, Nano Lett., 2011, 11, 1887–1894. [DOI] [PubMed] [Google Scholar]
- 200.Black KCL, Wang Y, Luehmann HP, Cai X, Xing W, Pang B, Zhao Y, Cutler CS, V Wang L, Liu Y and Xia Y, ACS Nano, 2014, 8, 4385–4394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Yang X, Skrabalak SE, Li Z-Y, Xia Y and V Wang L, Nano Lett., 2007, 7, 3798–3802. [DOI] [PubMed] [Google Scholar]
- 202.Wang P-H, Liu H-L, Hsu P-H, Lin C-Y, Wang C-RC, Chen P-Y, Wei K-C, Yen T-C and Li M-L, J. Biomed. Opt, 2012, 17, 61222. [DOI] [PubMed] [Google Scholar]
- 203.Galanzha EI, Shashkov E, Sarimollaoglu M, Beenken KE, Basnakian AG, Shirtliff ME, Kim J-W, Smeltzer MS and Zharov VP, PLoS One, 2012, 7, e45557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Foster SR, Galanzha EI, Totten DC, Benes H, Shmookler Reis RJ and Zharov VP, J. Biophotonics, 2014, 7, 465–473. [DOI] [PubMed] [Google Scholar]
- 205.Song K, Huang P, Yi C, Ning B, Hu S, Nie L, Chen X and Nie Z, ACS Nano, 2015, 9, 12344–12348. [DOI] [PMC free article] [PubMed] [Google Scholar]