Abstract
Context
Combining a sodium-glucose cotransporter 2 inhibitor with a xanthine oxidase inhibitor (XOI) and a urate transporter 1 (URAT1) inhibitor may enhance serum uric acid (sUA) lowering. However, concerns exist regarding high urinary UA (uUA) excretion rates and subsequent crystallization in renal tubules.
Objective
To assess whether dapagliflozin added to verinurad, a selective URAT1 inhibitor, and febuxostat, an XOI, increases uUA excretion.
Design
Randomized, placebo-controlled, 2-way crossover study (NCT03316131).
Patients
Adults with asymptomatic hyperuricemia.
Interventions
Subjects (N = 36) were randomized to oral once-daily 9 mg verinurad plus 80 mg febuxostat plus 10 mg dapagliflozin for 7 days and 7 days of oral once-daily 9 mg verinurad plus 80 mg febuxostat plus placebo with an intervening 7- to 21-day washout period.
Main Outcome Measure
Difference in peak uUA excretion between groups from baseline to day 7. Secondary outcomes included changes in sUA levels and 24-h uUA excretion.
Results
Both regimens lowered mean peak uUA excretion (least squares mean changes from baseline: −12.9 mg/h [95% confidence interval (CI): −21.0 to −4.7], dapagliflozin; −13.2 mg/h [95% CI −21.3 to –5.0], placebo). sUA concentrations were lower with dapagliflozin (mean treatment difference –62.3 µmol/L [95% CI −82.8 to −41.8]). Dapagliflozin did not impact verinurad pharmacokinetics, its main metabolites, or febuxostat or fasting plasma glucose levels vs verinurad plus febuxostat. There were no clinically relevant changes in safety parameters.
Conclusions
Dapagliflozin further reduced sUA without influencing uUA excretion, suggesting that its combination with verinurad and febuxostat at the doses tested does not adversely affect kidney function.
Clinical trial registration number
Keywords: dapagliflozin, febuxostat, hyperuricemia, uric acid, verinurad, xanthine oxidase
Hyperuricemia, defined as serum uric acid (sUA) ≥6.8 mg/dL (≥401.2 µmol/L) by American College of Rheumatology guidelines, is associated with gout, kidney stones, chronic kidney disease (CKD), and major cardiovascular events (1,2). The prevalence of hyperuricemia exceeds 20% in most Western countries and recent studies suggest rising international trends (2-4). Lowering of sUA levels is key for the effective management of gout (5-9). Furthermore, an emerging body of evidence suggests that the lowering of sUA levels may also confer substantial benefits to patients with kidney disease by reducing the rate of disease progression and the risk of kidney failure (10). In general, studies have shown that the greater the reduction in sUA levels, the greater the clinical benefit, suggesting that an intensive uric acid (UA)-lowering strategy is preferable for maximum reno- and cardioprotective benefits (10,11).
The preferred UA-lowering strategy and the optimal thresholds for UA reduction in cardiorenal disease remain to be determined. Febuxostat, a potent selective nonpurine analog xanthine oxidase inhibitor (XOI), is effective in lowering sUA levels in the treatment of gout with greater efficacy than allopurinol (5,12,13). Urate transporter 1 (URAT1) inhibitors, acting in the proximal renal tubule, provide additional therapeutic options to further lower UA levels beyond xanthine oxidase inhibition. Lesinurad, when combined with an XOI, is effective in lowering sUA levels in clinical trials (6-8). However, concerns have been raised when using URAT1 inhibitors as monotherapy regarding their impact on kidney function, owing to elevations in serum creatinine, partly attributed to increased urinary excretion rates of UA in tubules (14). Verinurad, a newer and more specific URAT1 inhibitor with an extended-release profile, is highly effective in lowering UA levels without adversely increasing peak urinary UA (uUA) excretion when combined with an XOI (15). An intensive UA-lowering strategy that combines an XOI with verinurad may therefore confer additional clinical benefits owing to greater lowering of sUA levels in at-risk patients.
Sodium-glucose cotransporter 2 (SGLT2) inhibitors, such as dapagliflozin, have emerged as a new treatment paradigm for improving clinical outcomes in patients with CKD and heart failure. Clinical studies have shown that SGLT2 inhibitors lower sUA concentrations, possibly by increasing urinary excretion rates of UA (16-18). Therefore, combining an SGLT2 inhibitor with an XOI and URAT1 inhibitor may confer additional clinical benefits from intensive sUA lowering but may inadvertently increase urinary excretion rates of UA thereby increasing the risk of tubular damage and UA nephrolithiasis. To explore this hypothesis, we evaluated the effect of adding dapagliflozin to verinurad and febuxostat on uUA excretion and sUA levels in subjects with asymptomatic hyperuricemia (the QUARTZ study; NCT03316131).
Methods
Study design
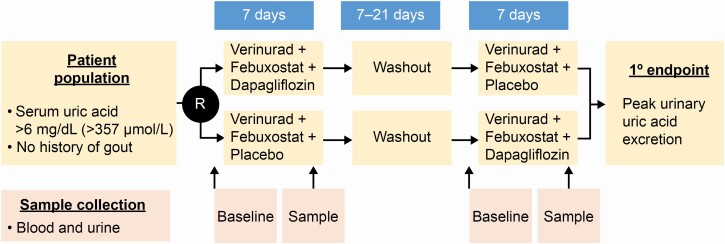
This was a phase 2, randomized, placebo-controlled, 2-way crossover study (Fig. 1). Subjects with asymptomatic hyperuricemia were randomized 1:1 to receive oral once-daily 9 mg verinurad plus 80 mg febuxostat plus 10 mg dapagliflozin (dapagliflozin group) for 7 days, and then cross over to once-daily 9 mg verinurad plus 80 mg febuxostat plus placebo (placebo group) for 7 days, with an intervening 7- to 21-day washout period, or the same treatment regimens in the reverse order.
Figure 1.
Study design. Abbreviations: 1o, primary; R, randomization.
For each treatment regimen, kidney function was assessed at baseline with measurements of serum creatinine and estimated glomerular filtration rates (eGFR; mL/min/1.73 m2). Urinary excretion of UA was determined at baseline and at day 7 from hourly collections for 12 h, followed by a single 12-h collection. Baseline collection on day −1 was started at a time to coincide with that of scheduled daily dosing during the study.
Eligibility
Eligible patients were aged between 18 and 65 years, had asymptomatic high sUA (>6.0 mg/dL [>354 μmol/L] at screening), body mass index between 18 and 35 kg/m2, and weighed between 50 and ≤150 kg. Patients were excluded if they had a history or presence of gastrointestinal, hepatic, or renal disease or any other condition known to interfere with absorption, distribution, metabolism, or excretion of drugs; had an eGFR <45 mL/min/1.73 m2 at screening; type 2 diabetes mellitus; history of diabetic ketoacidosis or hyperosmolar nonketotic coma; or history of gout; or were receiving ongoing treatment with an SGLT2 inhibitor, URAT1 inhibitor, and/or an XOI.
Outcome measures
The primary objective was to evaluate the effects of intensive UA-lowering therapy with verinurad, febuxostat, and dapagliflozin on urinary excretion of UA by comparing the difference between baseline and day 7 in peak uUA excretion during the dapagliflozin treatment period with that during the placebo treatment period. Urine for these analyses was collected hourly for the first 12 h after dosing and then a single pooled 12- to 24-h collection. Secondary outcomes assessed the effects of verinurad, febuxostat, and dapagliflozin on sUA levels; pharmacokinetic (PK) profiles of verinurad and its main metabolites (M1 and M8), febuxostat, and dapagliflozin; and assessed renal and general safety and tolerability profiles of an intensive UA-lowering therapy. The study also evaluated the effect of intensive UA lowering on several additional measures of renal function and UA metabolism, including serum creatinine and cystatin C; blood urea nitrogen, sodium, and potassium; urinary creatinine; cystatin C; and sodium.
Serial blood samples for plasma PK and serum pharmacodynamic analyses were collected predose and up to 24 h postdose on day 7 of each treatment period. The analysis of verinurad, M1, M8, febuxostat, and dapagliflozin was performed by Covance Bioanalytical Services LLC (Indianapolis, Indiana, USA) using methods validated according to the US Food and Drug Administration Bioanalytical Method Validation Guidance for Industry (19). The determination of dapagliflozin and febuxostat was performed using methodologies similar to those in previous studies (20,21). PK parameters for verinurad, M1, M8, and febuxostat were calculated using Phoenix WinNonlin, Version 6.4 (Pharsight Corporation, Mountain View, California, USA); these parameters included time to maximum observed plasma concentration (tmax), maximum observed plasma concentration (Cmax), and area under the plasma concentration–time curve over the dosing interval (AUCτ).
Additional exploratory objectives assessed the effect of each treatment period on fasting plasma glucose, urinary glucose excretion, and hematocrit and examined the relationship between plasma levels of dapagliflozin, verinurad, and febuxostat on plasma sUA levels and urinary excretion rate. The effects of the dapagliflozin vs placebo treatment periods on fractional excretion of UA (FEUA) (%) were compared at the end of each treatment period in a post hoc analysis. FEUA was calculated using the following equation (22,23):
Plasma UA and creatinine measurements correspond to the morning plasma samples (collected predose at baseline and day 7 of each treatment period), whereas uUA and creatinine measurements correspond to the 24-h amount in urine/24-h urine volume.
Statistical analysis
Safety analyses were carried out for all patients who received at least 1 dose of study medication and for whom any safety data were available (safety analysis set). Pharmacodynamic analyses were carried out for all randomized patients without sufficient missingness in urine voids (pharmacodynamic analysis set) and PK analyses included all randomized patients for whom at least 1 PK parameter for at least 1 analyte could be evaluated and who had no major protocol deviations impacting the PK analyses (PK analysis set).
The study sample size was calculated based on the results of a phase 2 study evaluating verinurad in combination with febuxostat in adults with gout (24). It was assumed that the within-patient standard deviation (SD) for the between-treatment differences in uUA was 0.32. The study planned to recruit 24 subjects based on an expected SD of 0.32 for the between-treatment difference in UA excretion in urine per hour and the expectation that 20 patients would complete the study. However, at an interim review of study data, the sample size was increased to 36 to achieve the required statistical precision due to incomplete urine sample collection in 11 of the first 24 subjects.
The primary endpoint and secondary sUA outcomes were evaluated using a linear mixed-effects analysis of variance model with fixed-effect terms for sequence, period, and treatment. Patients nested within sequence were assumed to be a random effect. FEUA was evaluated using a similar model. Geometric means and associated 95% confidence intervals (CI), along with ratios of individual patient values, were calculated. Other variables were evaluated using descriptive statistics.
Results
A total of 36 subjects were enrolled and randomized at 2 study centers. The average age was 42.3 years (SD 12.0), the majority were male (97.2%), and 47.2% were Black. Mean eGFR was 84.5 mL/min/1.73 m2 (SD 14.7), and mean sUA levels at baseline (day −1 of each treatment period) were 430.1 µmol/L (7.3 mg/dL) and 398.7 µmol/L (6.8 mg/dL) in the dapagliflozin and placebo periods, respectively. The remaining baseline characteristics are presented in Table 1.
Table 1.
Baseline demographics
| Demographics | Total Subjects (N = 36) |
|---|---|
| Age, years | |
| Mean (SD) | 42.3 (12.0) |
| Median (range) | 41.0 (20.0-63.0) |
| Sex, n (%) | |
| Female | 1 (2.8) |
| Male | 35 (97.2) |
| Race, n (%) | |
| White | 14 (38.9) |
| Black or African American | 17 (47.2) |
| Asian | 2 (5.6) |
| Other | 3 (8.4) |
| Body mass index, kg/m2 | |
| Mean (SD) | 27.9 (3.0) |
| eGFR, mL/min/1.73 m2 | |
| Mean (SD) | 84.5 (14.7) |
| Serum uric acid, µmol/L | |
| Mean (SD) on day −1 of the dapagliflozina period | 430.1 (65.0) |
| Mean (SD) on day −1 of the placebo period | 398.7 (53.3) |
| Serum creatinine, µmol/L | |
| Mean (SD) on day −1 of the dapagliflozina period | 94.4 (14.2) |
| Mean (SD) on day −1 of the placebo period | 95.5 (14.4) |
| Serum sodium, mmol/L | |
| Mean (SD) on day −1 of the dapagliflozina period | 139.0 (3.0) |
| Mean (SD) on day −1 of the placebo period | 139.0 (3.0) |
| Fasting plasma glucose, mmol/L | |
| Mean (SD) on day −1 of the dapagliflozina period | 5.3 (0.7) |
| Mean (SD) on day −1 of the placebo period | 5.2 (0.7) |
| FEUA, % | |
| Mean (95% CI) on day −1 of the dapagliflozinb period | 4.9 (4.4-5.3) |
| Mean (95% CI) on day −1 of the placebob period | 4.9 (4.5-5.3) |
Abbreviations: CI, confidence interval; eGFR, estimated glomerular filtration rate; SD, standard deviation.
aN = 35.
bN = 25.
Excretion rates of uUA and levels of sUA
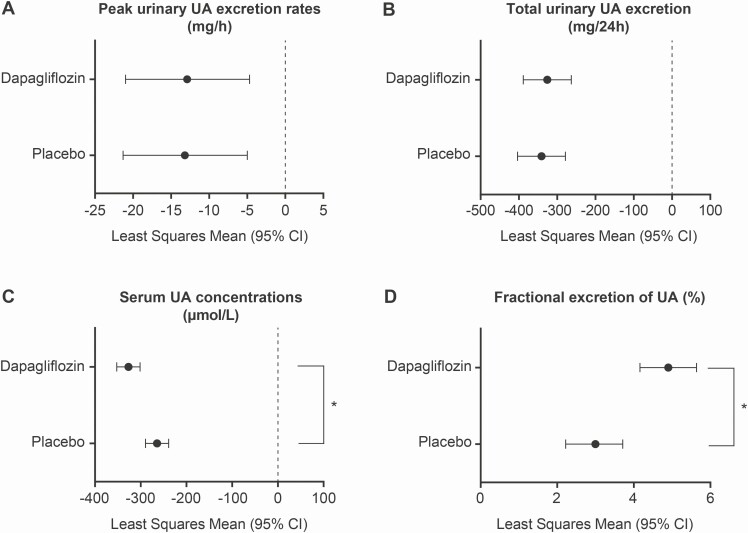
Hourly urine collection was insufficient to determine peak uUA excretion, total uUA excretion, and FEUA in 11 of the 36 subjects enrolled, as samples were not collected hourly in accordance with the study protocol. Evaluations of these parameters were therefore conducted for the 25 remaining participants. Peak uUA excretion was assessed as the maximum UA excreted as measured in milligrams of UA excreted in urine within 1-h intervals for the first 8 h after dosing. Mean peak UA excretion was similar for both treatment periods at baseline (47.3 mg [SD 13.0] before dapagliflozin-containing and 52.2 mg [SD 18.5] before placebo-containing treatment started) and then lower on day 7 of both treatment periods compared with baseline, with least squares mean (LSM) differences in UA excretion rates of −12.9 mg/h (95% CI −21.0 to −4.7) in the dapagliflozin period and −13.2 mg/h (95% CI −21.3 to –5.0) in the placebo period (Fig. 2A). Both treatments had a similar effect, with no statistically significant mean difference between groups (0.3 mg/h [95% CI −8.7 to 9.2]).
Figure 2.
uUA excretion rates and sUA concentrations. (A) Peak uUA excretion rates (mg/h) for each treatment showing differences between baseline and day 7. (B) Total uUA excretion in the 24 h following treatment administration (mean change from baseline at day 7). (C) sUA concentrations after treatment administration on day 7. (D) Fractional excretion of UA (%) for each treatment showing change from baseline at day 7. Mean peak UA excretion was determined as UA (mg) excreted within 1-h intervals for the first 8 h after dosing. Peak and total uUA excretion rates and fractional excretion of UA were assessed for 25 participants. Abbreviations: CI, confidence interval; sUA, serum uric acid; UA, uric acid; uUA, urinary uric acid. *P < 0.05.
The amount of uUA excreted over 24 h was determined in 25 participants and was decreased from baseline to day 7 in both treatment groups. The LSM was −325.9 mg/day (95% CI −388.6 to −263.3) in the dapagliflozin period and −341.0 mg/day (95% CI −403.6 to −278.4) in the placebo period. Both treatments had a similar effect, with no statistically significant mean difference between treatments (mean difference 15.1 mg/day [95% CI −46.5 to 76.7]; Fig. 2B).
After 7 days of treatment, mean sUA concentrations were significantly lower in the dapagliflozin period (n = 35) than in the placebo period (n = 36). The LSM was −327.2 µmol/L (95% CI −352.6 to −301.8) in the dapagliflozin group and −264.9 µmol/L (95% CI −290.1 to −239.6) in the placebo group. The sUA reduction in the dapagliflozin period was significantly larger than in the placebo period, with a mean difference of −62.3 µmol/L (95% CI −82.8 to −41.8) between treatments; the maximum effect on sUA concentrations was observed at 12 h postdose (Fig. 2C).
The FEUA was assessed in 25 participants and increased from baseline to day 7 in both treatment periods; the LSM was 4.9% (95% CI 4.16 to 5.64) and 3.0% (95% CI 2.2 to 3.7) in the dapagliflozin and placebo periods, respectively (Fig. 2D). The FEUA increase in the dapagliflozin period was higher than in the placebo period, with a statistically significant mean difference of 1.94% (95% CI 1.19 to 2.68).
PKs of verinurad, febuxostat, and dapagliflozin
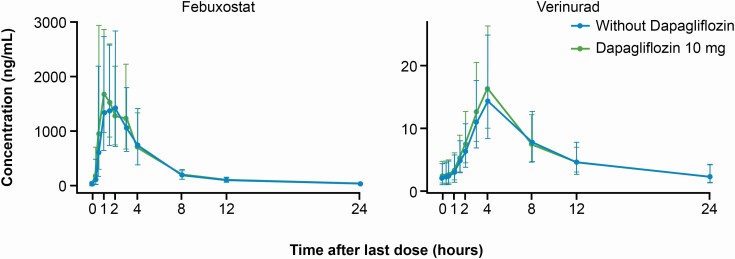
Verinurad was absorbed slowly after administration of the 8-h extended-release capsule formulation, with the Cmax observed at 4 h postdose (Fig. 3). The 2 main metabolites of verinurad, M1 and M8, followed the PK of verinurad (Table 2).
Figure 3.
Geometric mean (standard deviation) verinurad or febuxostat plasma concentrations over time with or without dapagliflozin. Error bars represent standard deviation.
Table 2.
Plasma pharmacokinetic parameters for verinurad, its metabolites M1 and M8, and febuxostat after daily oral administration of verinurad and febuxostat with dapagliflozin or placebo
| Verinurad | M1 | M8 | Febuxostat | Dapagliflozin | |||||
|---|---|---|---|---|---|---|---|---|---|
| Dapagliflozin (n = 35) | Placebo (n = 36) | Dapagliflozin (n = 35) | Placebo (n = 36) | Dapagliflozin (n = 35) | Placebo (n = 36) | Dapagliflozin (n = 35) | Placebo (n = 36) | Dapagliflozin (n = 35) | |
| Cmax, ng/mL | |||||||||
| Geomean (GCV%) | 17.52 (45.87) | 15.26 (52.48) | 25.28 (41.55) | 25.61 (57.24) | 18.45 (35.45) | 18.42 (44.36) | 2448 (41.37) | 2462 (47.88) | 142.6 (24.12) |
| GMR (90% CI) | 1.14 (1.03-1.25) | 0.97 (0.88-1.07) | 0.99 (0.91-1.08) | 1.00 (0.89-1.11) | — | ||||
| AUCτ, h∙ng/mL | |||||||||
| Geomean (GCV%) | 149.0 (37.84) | 140.9 (44.90) | 212.6 (42.78) | 221.1 (49.13) | 174.1 (34.59) | 176.3 (36.81) | 8145 (31.63) | 7907 (35.50) | 512.8 (21.51) |
| GMR (90% CI) | 1.06 (1.00-1.13) | 0.96 (0.90-1.02) | 0.99 (0.94-1.04) | 1.03 (0.99-1.07) | — | ||||
| tmax, h | |||||||||
| Median (min-max) | 4.00 (2.00-8.00) | 4.00 (3.00-8.08) | 4.00 (2.00-8.00) | 4.00 (3.00-4.03) | 4.00 (3.00-8.02) | 4.00 (3.00-8.08) | 1.50 (0.48-4.00) | 1.52 (0.50-4.03) | 1.00 (0.50-2.00) |
Geomean, GMR, and CI values are shown in their original units of measurement.
Abbreviations: AUCτ, area under the plasma concentration-time curve over the dosing interval; CI, confidence interval; Cmax, maximum plasma concentration; GCV, geometric coefficient of variation; Geomean, geometric mean; GMR, ratio of geometric means; M, metabolite; tmax, time to maximum plasma concentration.
Febuxostat was rapidly absorbed and maximum plasma concentrations were achieved at a median of 1.5 h (Fig. 3). Addition of dapagliflozin did not alter the PKs of verinurad or febuxostat (Table 2). Peak plasma levels of dapagliflozin typically occurred 1-h postdose.
Additional pharmacodynamic parameters
Compared with the addition of placebo, 7 days of dapagliflozin treatment added to verinurad plus febuxostat had no significant effect on serum creatinine, cystatin C, blood urea nitrogen, sodium, or potassium (Table 3). The addition of dapagliflozin to verinurad plus febuxostat also had no significant effect on the amount of creatinine, cystatin C, or sodium excreted in urine over 24 h on day 7 of treatment vs the addition of placebo (Table 4).
Table 3.
Serum and urinary concentrations of biomarkers at baseline and day 7, with change from baseline after 7 days of treatment with dapagliflozin vs placebo
| Treatment Period | sUA (µmol/L) | Peak uUA excretion (mg) | Creatinine (µmol/L) | Cystatin C (mg/L) | Blood Urea Nitrogen (mmol/L) | Sodium (mmol/L) | Potassium (mmol/L) |
|---|---|---|---|---|---|---|---|
| Baseline, mean (SD) | |||||||
| Dapagliflozin period | 430.1 (65.0) | 47.3 (13.0) | 94.4 (14.2) | 1.0 (0.2) | 5.2 (1.3) | 139 (3.0) | 4.3 (0.3) |
| Placebo period | 398.7 (53.3) | 52.2 (18.5) | 95.5 (14.4) | 0.9 (0.2) | 5.1 (1.9) | 139 (3.0) | 4.3 (0.3) |
| Day 7, mean (SD) | |||||||
| Dapagliflozin period | 103.5 (39.6) | 34.6 (13.0) | 91.9 (12.3) | 1.0 (0.2) | 4.9 (0.9) | 139 (2.0) | 4.1 (0.3) |
| Placebo period | 133.8 (57.5) | 39.3 (20.4) | 90.5 (11.8) | 1.0 (0.1) | 4.7 (0.9) | 139 (2.0) | 4.2 (0.3) |
| Change from baseline, LSM (95% CI) | |||||||
| Dapagliflozin period | −327.16 (−352.56 to –301.76) | −12.87 (−21.03 to −4.70) | −2.511 (−5.809 to 0.787) | 0.022 (−0.006to 0.051) | −0.268 (−0.740 to 0.205) | 0.720 (−0.245 to 1.686) | −0.146 (−0.261to –0.031) |
| Placebo period | −264.85 (−290.06 to −239.64) | −13.15 (−21.31 to −4.98) | −5.034 (−8.294 to −1.774) | 0.020 (−0.008 to 0.048) | −0.397 (−0.862 to 0.069) | −0.028 (−0.979 to 0.923) | −0.114 (−0.227 to −0.001) |
| Mean difference (95% CI) | −62.31 (−82.84to −41.78) | 0.28 (−8.67 to 9.22) | 2.523 (−0.935 to 5.981) | 0.003 (−0.037 to 0.043) | 0.129 (−0.539 to 0.797) | 0.748 (−0.535 to 2.031) | −0.032 (−0.194 to 0.129) |
Abbreviations: CI, confidence interval; LSM, least squares mean; SD, standard deviation; sUA, serum uric acid; uUA, urinary uric acid.
Table 4.
Effect of the addition of dapagliflozin vs placebo on time-matched changes in pharmacodynamic parameters from baseline to day 7
| Parameter | Change from Baseline at Day 7 LSM (95% CI) | Mean Difference Between Treatments (95% CI) |
|---|---|---|
| Creatinine in urine,a mmol/L | ||
| Dapagliflozin period (n = 25) | 0.5 (−0.6 to 1.6) | 0.6 (−0.5 to 1.8) |
| Placebo period (n = 25) | −0.1 (−1.2 to 1.0) | |
| Cystatin C in urine, mg | ||
| Dapagliflozin period (n = 25) | 2.4 (0.6 to 4.3) | 1.1 (−0.7 to 2.9) |
| Placebo period (n = 25) | 1.3 (−0.5 to 3.2) | |
| Sodium in urine, mmol | ||
| Dapagliflozin period (n = 25) | −0.9 (−2.7 to 0.8) | 0.1 (−1.4 to 1.6) |
| Placebo period (n = 25) | −1.0 (−2.8 to 0.7) | |
| FPG, mmol/L | ||
| Dapagliflozin period (n = 16) | −0.4 (−0.5 to −0.3) | −0.007 (−0.155 to 0.142) |
| Placebo period (n = 16) | −0.4 (−0.5 to −0.3) | |
| Hematocrit ratio | ||
| Dapagliflozin period (n = 35) | 0.003 (−0.004 to 0.011) | 0.005 (−0.005 to 0.015) |
| Placebo period (n = 36) | −0.002 (−0.009 to 0.006) |
Abbreviations: CI, confidence interval; FPG, fasting plasma glucose; LSM, least squares mean.
aTime-matched change from baseline.
Exploratory outcomes
Fasting plasma glucose values on day 7 were unchanged by the addition of dapagliflozin to the UA-lowering regimen compared with the addition of placebo (Table 4). All patients had undetectable levels of glucose in urine at all assessments at baseline in both treatment periods. Glucose was detected in 3 patients during the placebo period on day 7; these 3 patients had not developed diabetes. Therefore, significance of the findings of glucose on the 3 patients during the placebo period on day 7 is unknown. All patients had glucose in their urine on day 7 of the dapagliflozin period. Hematocrit ratios were similar on day 7 in both treatment groups (Table 4).
Safety
In total, 35 patients were exposed to verinurad plus febuxostat plus dapagliflozin, and 36 were exposed to verinurad plus febuxostat plus placebo across 2 treatment periods. Overall, 10 subjects each had ≥1 adverse event (AE) across both treatment periods, with 16 events occurring in total (dapagliflozin period, 7 events; placebo period, 9 events). All AEs were mild in intensity; no serious AEs or deaths were reported.
The most common AEs were gastrointestinal related, occurring in 13.9% (n = 5) of all patients across both treatment periods, including diarrhea (5.6%; n = 2), flatulence (5.6%; n = 2), and nausea (2.8%; n = 1). In the dapagliflozin period, gastrointestinal-related AEs occurred in 5.7% (n = 2) of patients, with diarrhea and flatulence observed in 2.9% (n = 1) of patients. In the placebo period, 8.3% (n = 3) of patients experienced gastrointestinal-related AEs, including diarrhea, flatulence, and nausea (2.8% [n = 1] each). A total of 5 AEs were considered to be related to the study drugs (dapagliflozin period, n = 1; placebo period, n = 4). No clinically relevant changes were seen in laboratory safety measures or vital signs, and no significant elevations in creatinine were reported.
Discussion and Conclusions
The present crossover trial demonstrated that the addition of dapagliflozin to a combination of verinurad plus febuxostat did not increase urinary excretion rates of UA among patients with hyperuricemia. Neither the peak urinary excretion of UA nor the total daily urinary excretion of UA differed significantly at the end of each treatment period. Importantly, however, the addition of dapagliflozin led to a marked and significant lowering of sUA compared with verinurad plus febuxostat alone, and this was achieved without any detectable increase in total uUA excretion rates. Dapagliflozin further enhanced the UA-lowering effect of verinurad and febuxostat by decreasing sUA to below the target level specified by American College of Rheumatology guidelines for the management of gout (≥6.0 mg/dL [≥354 µmol/L]) in all patients (2). Therefore, the SGLT2 inhibitor dapagliflozin directly enhances the UA-lowering capacity of a verinurad and febuxostat combination without adversely impacting UA excretion.
Lowering UA below thresholds specified in clinical guidelines is a key goal in the treatment of gout and may also confer additional cardiorenal protective benefits beyond gouty arthritis (5-11,25). The use of SGLT2 inhibitors is likely to increase in clinical practice given the positive impact on cardiorenal outcomes in recent clinical trials (26-31). Consequently, it is likely that many patients may be treated with both an SGLT2 inhibitor and combinations of a uricosuric agent and an XOI. Whether these combinations can lead to downstream benefits or risks has not been previously explored. This current study provides evidence that the addition of dapagliflozin to verinurad plus febuxostat is a well-tolerated combination that leads to a marked reduction in sUA concentrations but does not significantly increase either peak or total UA excretion. This combination is therefore unlikely to increase UA crystallization and damage kidney function.
A novel finding from this trial was the observation that treatment with the dapagliflozin combination led to a significant and clinically important reduction in sUA levels compared with placebo. Importantly, this further reduction in sUA levels occurred in the context of near maximal dose of a URAT1 inhibitor and XOI. The net reduction in sUA levels was in the order of 62 µmol/L (~1 mg/dL), a 23.5% reduction as compared with placebo, strengthening the argument for combining an SGLT2 inhibitor with existing urate-lowering therapy to achieve desired sUA targets and potentially better clinical outcomes.
Previous studies suggest that SGLT2 inhibitor monotherapy may reduce sUA levels by a uricosuric effect that is likely the result of glycosuria, possibly by suppressing activity of the facilitative hexose/urate transporter protein glucose transporter 9b (SLC2A9 isoform a) (16,26,32). In this study, while we did observe a modest increase in the fractional excretion of UA, there was no effect whatsoever on peak or total uUA excretion following treatment with dapagliflozin. These results would strongly suggest that treatment with dapagliflozin reduced the production of UA, which in turn reduced the filtered load of UA in the kidney. This is evidenced by a reduction in both the total and peak rates of uUA excretion despite a modest increase in the percentage of the filtered UA that is excreted from the tubule.
Genetic and pharmacological studies in mice have shown that URAT1 is required for the acute uricosuric action of SGLT2 inhibition (22). In line with the effect of URAT1 inhibition in mice, we did not observe any effect on total uUA excretion when the SGLT2 inhibitor dapagliflozin was added to the URAT1 inhibitor verinurad. The renal handling of UA was affected by dapagliflozin as indicated by the increased FEUA, but the lack of effect on peak and 24-h uUA excretion argues for reduced UA production. Our findings suggest that dapagliflozin treatment reduces purine metabolism or reduces cell death/turnover. Reductions in plasma alanine aminotransferase and aspartate aminotransferase levels, as well as other markers of cell death such as cytokeratin-18, have been observed after treatment with dapagliflozin (33). A metabolomic analysis of samples from the EFFECT II trial (ClinicalTrial.gov identifier: NCT02279407), in which patients with type 2 diabetes and nonalcoholic fatty liver disease received dapagliflozin monotherapy 10 mg/day or placebo, adds further support to this hypothesis. At 12 weeks of follow-up, dapagliflozin-treated patients had reduced plasma urate levels with concomitantly reduced levels of the xanthine metabolite, theophylline, which is upstream of urate in purine degradation (34).
The similar tmax, Cmax, and AUCτ values with and without concomitant use of dapagliflozin demonstrated that this SGLT2 inhibitor had no effect on the PKs of verinurad and its metabolites or febuxostat. Treatment exposure, including the tmax of verinurad, M1 and M8, may have been underestimated, as a postdose blood sample was taken at 4 h, but not between 4- and 8-h postdose. However, this is unlikely to have affected findings regarding the lack of drug-drug interactions with dapagliflozin. Furthermore, no additional safety or tolerability concerns were identified when the 3 agents were utilized together.
In keeping with its observed mechanism of action, treatment with dapagliflozin resulted in glucose in the urine of patients who received the dapagliflozin-containing regimen. However, analysis also showed the presence of glucose in the urine of 3 patients after, but not before, treatment with the placebo-containing regimen on day 7. Although these patients did not have diabetes, they may have had some degree of impaired glucose tolerance. The significance of these findings is currently unknown. It is possible that the combination of verinurad and febuxostat has a minor effect on glucose excretion.
A key limitation of this study was that the original study sample size planned for this trial was reduced due to incomplete urine sample collection in a fraction of patients, whereby collection of urine samples was not conducted according to protocol guidance. To address this, the investigators increased the number of subjects enrolled to ensure adequate statistical power, but it is possible that patients who were excluded may have introduced bias. In addition, while we closely monitored uUA excretion, we did not measure markers of proximal tubular function, urine flow, or pH in this study. This precludes us from making any firm conclusions on the risk of tubular damage and the longer-term risks of UA nephrolithiasis. Finally, the study participants were largely male, and therefore our results are primarily generalizable to men. A key strength of this study was the frequency of urine and blood sampling, which enhanced the reliability of the data and allowed for a detailed assessment of uUA excretion over time.
The addition of an SGLT2 inhibitor, dapagliflozin, to a combination of verinurad plus febuxostat led to significant and substantial lowering of sUA without a significant increase in urinary excretion of UA. These findings suggest that combining dapagliflozin with a verinurad-based intensive UA-lowering strategy can further reduce sUA levels without adversely affecting kidney function. Given the potential adverse consequences of hyperuricemia on clinical outcomes and the likely substantial benefits of SGLT2 inhibitors in high-risk cardiorenal populations, strategies that combine an SGLT2 inhibitor with an intensive UA-lowering strategy may lead to greater clinical benefits.
Acknowledgments
The authors would like to thank the patients, their families, and all investigators involved in this study. We would also like to thank Jenny Jonasson for her assistance with developing the manuscript. Medical writing support was provided by Minal Kotecha, PhD, and editorial support was provided by Bethany King, BSc (Hons), both of Core, Knutsford, UK, supported by AstraZeneca according to Good Publication Practice guidelines (http://annals.org/aim/article/2424869/good-publication-practice-communicating-company-sponsored-medical-research-gpp3). The Sponsor was involved in the study design, collection, analysis, and interpretation of data, as well as data checking of information provided in the manuscript. However, ultimate responsibility for opinions, conclusions, and data interpretation lies with the authors.
Financial Support: This study was supported by AstraZeneca.
Author contributions: Study design: EJ, FE. Data collection: DH, RG, ND, EJ. Data analysis and interpretation: AGS, SJ, ND, JO, EJ, JP, FE. All authors critically reviewed the manuscript, approved the final version, and accept accountability for the overall work.
Glossary
Abbreviations
- AE
adverse event
- AUCτ
area under the plasma concentration–time curve over the dosing interval
- CI
confidence interval
- CKD
chronic kidney disease
- Cmax
maximum observed plasma concentration
- eGFR
estimated glomerular filtration rates
- FEUA
fractional excretion of UA
- LSM
least squares mean
- M
metabolites
- PK
pharmacokinetic
- SD
standard deviation
- SGLT2
sodium-glucose cotransporter 2
- sUA
serum uric acid
- tmax
time to maximum observed plasma concentration
- UA
uric acid
- URAT1
urate transporter 1
- uUA
urinary UA
- XOI
xanthine oxidase inhibitor
Additional Information
Disclosure Summary : FE, JO, SJ, ND, JP, and EJ are employees and shareholders of AstraZeneca. RG and DH are employees of Parexel International. AGS is supported by grants from the Health Research Board (HRA-2013-PHR-437 and HRA-2014-PHR-685), Midwest Kidney Disease Research and Education Foundation (MKid). He has served as consultant to AstraZeneca, Grünenthal, and Menarini.
Data Availability
Restrictions apply to the availability of data generated or analyzed during this study to preserve patient confidentiality or because they were used under license. The corresponding author will on request detail the restrictions and any conditions under which access to some data may be provided. Data underlying the findings described in this manuscript may be obtained in accordance with AstraZeneca’s data-sharing policy described at https://astrazenecagroup-dt.pharmacm.com/DT/Home (see http://www.icmje.org/recommendations/browse/publishing-and-editorial-issues/clinical-trial-registration.html).
References
- 1. Johnson RJ, Bakris GL, Borghi C, et al. Hyperuricemia, acute and chronic kidney disease, hypertension, and cardiovascular disease: report of a Scientific Workshop Organized by the National Kidney Foundation. Am J Kidney Dis. 2018;71(6): 851-865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Bove M, Cicero AF, Veronesi M, Borghi C. An evidence-based review on urate-lowering treatments: implications for optimal treatment of chronic hyperuricemia. Vasc Health Risk Manag. 2017;13:23-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Zhu Y, Pandya BJ, Choi HK. Prevalence of gout and hyperuricemia in the US general population: the National Health and Nutrition Examination Survey 2007-2008. Arthritis Rheum. 2011;63(10):3136-3141. [DOI] [PubMed] [Google Scholar]
- 4. Kumar A U A, Browne LD, Li X, et al. Temporal trends in hyperuricaemia in the Irish health system from 2006-2014: a cohort study. PLOS ONE. 2018;13(5):e0198197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Schumacher HR Jr, Becker MA, Wortmann RL, et al. Effects of febuxostat versus allopurinol and placebo in reducing serum urate in subjects with hyperuricemia and gout: a 28-week, phase III, randomized, double-blind, parallel-group trial. Arthritis Rheum. 2008;59(11):1540-1548. [DOI] [PubMed] [Google Scholar]
- 6. Saag KG, Fitz-Patrick D, Kopicko J, et al. Lesinurad combined with allopurinol: a randomized, double-blind, placebo-controlled study in gout patients with an inadequate response to standard-of-care allopurinol (a US-based study). Arthritis Rheumatol. 2017;69(1):203-212. [DOI] [PubMed] [Google Scholar]
- 7. Bardin T, Keenan RT, Khanna PP, et al. Lesinurad in combination with allopurinol: a randomised, double-blind, placebo-controlled study in patients with gout with inadequate response to standard of care (the multinational CLEAR 2 study). Ann Rheum Dis. 2017;76(5):811-820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Dalbeth N, Jones G, Terkeltaub R, et al. Lesinurad, a selective uric acid reabsorption inhibitor, in combination with febuxostat in patients with tophaceous gout: findings of a phase III clinical trial. Arthritis Rheumatol. 2017;69(9):1903-1913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Perez-Ruiz F, Calabozo M, Pijoan JI, Herrero-Beites AM, Ruibal A. Effect of urate-lowering therapy on the velocity of size reduction of tophi in chronic gout. Arthritis Rheum. 2002;47(4):356-360. [DOI] [PubMed] [Google Scholar]
- 10. Su X, Xu B, Yan B, Qiao X, Wang L. Effects of uric acid-lowering therapy in patients with chronic kidney disease: a meta-analysis. PLOS ONE. 2017;12(11):e0187550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Miao Y, Ottenbros SA, Laverman GD, et al. Effect of a reduction in uric acid on renal outcomes during losartan treatment: a post hoc analysis of the reduction of endpoints in non-insulin-dependent diabetes mellitus with the Angiotensin II Antagonist Losartan Trial. Hypertension. 2011;58(1):2-7. [DOI] [PubMed] [Google Scholar]
- 12. Becker MA, Schumacher HR Jr, Wortmann RL, et al. Febuxostat compared with allopurinol in patients with hyperuricemia and gout. N Engl J Med. 2005;353(23):2450-2461. [DOI] [PubMed] [Google Scholar]
- 13. Becker MA, Schumacher HR, Espinoza LR, et al. The urate-lowering efficacy and safety of febuxostat in the treatment of the hyperuricemia of gout: the CONFIRMS trial. Arthritis Res Ther. 2010;12(2):R63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Terkeltaub R, Saag KG, Goldfarb DS, et al. Integrated safety studies of the urate reabsorption inhibitor lesinurad in treatment of gout. Rheumatology (Oxford). 2019;58(1):61-69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Shiramoto M, Liu S, Shen Z, et al. Verinurad combined with febuxostat in Japanese adults with gout or asymptomatic hyperuricaemia: a phase 2a, open-label study. Rheumatology (Oxford). 2018;57(9):1602-1610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Chino Y, Samukawa Y, Sakai S, et al. SGLT2 inhibitor lowers serum uric acid through alteration of uric acid transport activity in renal tubule by increased glycosuria. Biopharm Drug Dispos. 2014;35(7):391-404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Lytvyn Y, Škrtić M, Yang GK, Yip PM, Perkins BA, Cherney DZ. Glycosuria-mediated urinary uric acid excretion in patients with uncomplicated type 1 diabetes mellitus. Am J Physiol Renal Physiol. 2015;308(2):F77-F83. [DOI] [PubMed] [Google Scholar]
- 18. Vallon V, Thomson SC. Targeting renal glucose reabsorption to treat hyperglycaemia: the pleiotropic effects of SGLT2 inhibition. Diabetologia. 2017;60(2):215-225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. US Food and Drug Administration. Bioanalytical method validation guidance for industry. 2018. https://www.fda.gov/files/drugs/published/Bioanalytical-Method-Validation-Guidance-for-Industry.pdf. Accessed November 13, 2019. [Google Scholar]
- 20. Ji QC, Xu X, Ma E, et al. Selective reaction monitoring of negative electrospray ionization acetate adduct ions for the bioanalysis of dapagliflozin in clinical studies. Anal Chem. 2015;87(6):3247-3254. [DOI] [PubMed] [Google Scholar]
- 21. Hall J, Gillen M, Yang X, Shen Z. Pharmacokinetics, pharmacodynamics, and tolerability of concomitant administration of verinurad and febuxostat in healthy male volunteers. Clin Pharmacol Drug Dev. 2019;8(2):179-187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Novikov A, Fu Y, Huang W, et al. SGLT2 inhibition and renal urate excretion: role of luminal glucose, GLUT9, and URAT1. Am J Physiol Renal Physiol. 2019;316(1):F173-F185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Narang RK, Vincent Z, Phipps-Green A, Stamp LK, Merriman TR, Dalbeth N. Population-specific factors associated with fractional excretion of uric acid. Arthritis Res Ther. 2019;21(1):234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Fleischmann R, Winkle P, Hall J, et al. Pharmacodynamic and pharmacokinetic effects and safety of verinurad in combination with febuxostat in adults with gout: a phase IIa, open-label study. RMD Open. 2018;4(1):e000647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Wortmann RL, Macdonald PA, Hunt B, Jackson RL. Effect of prophylaxis on gout flares after the initiation of urate-lowering therapy: analysis of data from three phase III trials. Clin Ther. 2010;32(14):2386-2397. [DOI] [PubMed] [Google Scholar]
- 26. Bailey CJ. Uric acid and the cardio-renal effects of SGLT2 inhibitors. Diabetes Obes Metab. 2019;21(6):1291-1298. [DOI] [PubMed] [Google Scholar]
- 27. McMurray JJV, Solomon SD, Inzucchi SE, et al. ; DAPA-HF Trial Committees and Investigators . Dapagliflozin in patients with heart failure and reduced ejection fraction. N Engl J Med. 2019;381(21):1995-2008. [DOI] [PubMed] [Google Scholar]
- 28. Rosenstock J, Aggarwal N, Polidori D, et al. ; Canagliflozin DIA 2001 Study Group . Dose-ranging effects of canagliflozin, a sodium-glucose cotransporter 2 inhibitor, as add-on to metformin in subjects with type 2 diabetes. Diabetes Care. 2012;35(6):1232-1238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Zinman B, Wanner C, Lachin JM, et al. ; EMPA-REG OUTCOME Investigators . Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med. 2015;373(22):2117-2128. [DOI] [PubMed] [Google Scholar]
- 30. Wanner C, Inzucchi SE, Lachin JM, et al. ; EMPA-REG OUTCOME Investigators . Empagliflozin and progression of kidney disease in type 2 diabetes. N Engl J Med. 2016;375(4):323-334. [DOI] [PubMed] [Google Scholar]
- 31. Zhao Y, Xu L, Tian D, et al. Effects of sodium-glucose co-transporter 2 (SGLT2) inhibitors on serum uric acid level: a meta-analysis of randomized controlled trials. Diabetes Obes Metab. 2018;20(2):458-462. [DOI] [PubMed] [Google Scholar]
- 32. van Bommel EJ, Muskiet MH, Tonneijck L, Kramer MH, Nieuwdorp M, van Raalte DH. SGLT2 Inhibition in the diabetic kidney-from mechanisms to clinical outcome. Clin J Am Soc Nephrol. 2017;12(4):700-710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Eriksson JW, Lundkvist P, Jansson PA, et al. Effects of dapagliflozin and n-3 carboxylic acids on non-alcoholic fatty liver disease in people with type 2 diabetes: a double-blind randomised placebo-controlled study. Diabetologia. 2018;61(9):1923-1934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Mulder S, Hammarstedt A, Nagaraj SB, et al. A metabolomics-based molecular pathway analysis of how the sodium-glucose co-transporter-2 inhibitor dapagliflozin may slow kidney function decline in patients with diabetes. Diabetes Obes Metab. 2020;22(7):1157-1166. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Restrictions apply to the availability of data generated or analyzed during this study to preserve patient confidentiality or because they were used under license. The corresponding author will on request detail the restrictions and any conditions under which access to some data may be provided. Data underlying the findings described in this manuscript may be obtained in accordance with AstraZeneca’s data-sharing policy described at https://astrazenecagroup-dt.pharmacm.com/DT/Home (see http://www.icmje.org/recommendations/browse/publishing-and-editorial-issues/clinical-trial-registration.html).