Abstract
Context
Although observational studies show inverse associations between vitamin D status and body weight/adiposity, there are few large randomized controlled trials (RCTs) investigating this relationship.
Objective
To determine whether vitamin D3 supplementation lowers weight or improves body composition.
Design
The VITamin D and OmegA-3 TriaL (VITAL) was a double-blinded, placebo-controlled RCT including 25 871 US adults. This ancillary study was completed in a sub-cohort that underwent body composition assessments at baseline and 2-year follow-up (89% retention).
Setting
Harvard Clinical and Translational Science Center in Boston.
Participants
771 participants (men ≥ 50 and women ≥ 55 years).
Interventions
2 × 2 factorial design of supplemental vitamin D3 (2000 IU/day) and/or omega-3 fatty acids (1 g/day).
Main Outcome Measures
Endpoints were 2-year changes in weight, body mass index (BMI), waist circumference, and total and/or regional fat and lean tissue measures determined by dual-energy X-ray absorptiometry. Effect modification by clinical variables and total and free 25-hydroxyvitamin D (25[OH]D) levels was explored.
Results
There were no effects of supplemental vitamin D3vs placebo on weight, BMI, or measures of adiposity and lean tissue. Effects did not vary by sex, race/ethnicity, fat mass index, or baseline total or free 25(OH)D levels. Vitamin D3 supplementation did slightly improve body fat percentage in participants with normal BMI at baseline, but not in the overweight or obese (P for interaction = 0.04).
Conclusions
Daily vitamin D3 supplementation vs placebo in the general older population did not improve weight or body composition. Whether supplemental vitamin D3 may benefit individuals with normal BMI warrants further study.
Keywords: vitamin D, body composition, adiposity, lean tissue, weight, body mass index
Obesity is a major public health problem in the United States, affecting one third of older adults and is projected to increase to one half by 2030 (1, 2). Low vitamin D status is also highly prevalent, particularly in obese individuals. According to the National Health and Nutrition Examination Survey (NHANES) 2011-2014, 15% of adults aged ≥60 years and 53% of blacks ≥1 year in the United States have low 25-hydroxyvitamin D (25[OH]D) levels of <50 nmol/L (3). Moreover, older obese women were more likely to be vitamin D insufficient (25[OH]D <40 nmol/L) than older women of healthy weight; there were no differences in 25(OH)D levels among men by weight status (4).
Several observational studies, including both cross-sectional and longitudinal analyses, have shown an association between lower vitamin D status and higher body weight and fat mass (5,6). While obese individuals may have lower 25(OH)D levels due to sequestration of vitamin D in fat and volumetric dilution (7,8), interventional studies have explored effects of vitamin D on weight and adiposity. Lower vitamin D status has also been associated with lower lean tissue mass in the elderly (9-11). Most randomized controlled trials (RCTs), however, found no improvements in measures of body composition, either adiposity or muscle mass, with vitamin D supplementation compared to placebo (12-14). These RCTs were limited by short duration (14-16), small sample size (14,16,17), select patient population (eg, obese, diabetes mellitus type 2) (15-17), or in the setting of weight loss (18). Other trials have been confounded by co-supplementation with calcium (19, 20), limiting attributed effects to vitamin D per se. While the Women’s Health Initiative (WHI) trial found that women randomized to calcium and vitamin D supplementation (400 IU/d) had a small benefit in weight reduction compared to those assigned to placebo (mean difference of −0.13kg) (19), a meta-analysis, including the WHI trial, found that there were no differences in body mass index (BMI), weight, or fat mass in participants who received vitamin D and calcium compared to those who received calcium alone (20). Most studies used weight or BMI as endpoints; weight and BMI are limited as they are composite measures of adipose tissue, lean tissue mass, and bone. In contrast, dual-energy X-ray absorptiometry (DXA) measures fat and lean masses (total and regional) and distribution with good reproducibility and is considered a gold standard for clinical care (21). Finally, some, but not all, studies have suggested that adiposity measures may improve with vitamin D supplementation primarily in individuals with low baseline total vitamin D levels (15,17,18). Because vitamin D primarily circulates bound to vitamin D binding protein and albumin, it is unclear whether free 25(OH)D level may better predict effects of supplemental vitamin D on weight and adiposity and lean tissue measures compared to total 25(OH)D level. In 2011, the Institute of Medicine Committee concluded that large scale and rigorous RCTs are needed to determine the effects of vitamin D on nonskeletal health outcomes, including body composition (22, 23).
The VITamin D and OmegA-3 TriaL (VITAL): Effects on Bone Structure and Architecture is an ancillary study to VITAL in which we tested randomized effects of supplemental vitamin D3 and/or omega-3 fatty acids compared with placebo on bone health and body composition in a sub-cohort of participants at the Harvard Catalyst Clinical and Translational Science Center (CTSC) (24,25). The effects of 2 years of vitamin D3 supplementation of 2000 IU/day compared to placebo on body composition, including measures of adiposity and lean tissue as assessed by DXA, are reported here. We also explored effect modification by sex, race/ethnicity, BMI, fat mass index (FMI), and total and free 25(OH)D levels at baseline and 2-year follow-up.
Materials and Methods
Trial design
The parent VITAL was a 2 × 2 factorial, double-blinded, randomized, placebo-controlled trial that investigated effects of supplemental vitamin D3 supplementation (2000 IU/day) and/or omega-3 fatty acid (1 g/day) on the primary prevention of cancer and cardiovascular disease. Women aged ≥55 years and men aged ≥50 years without prior history of cancer or cardiovascular disease were recruited from 50 US states. For safety, exclusion criteria included hypercalcemia, hypo- or hyperparathyroidism, renal failure, severe liver disease, sarcoidosis or other granulomatous disease, or other serious illness. Participants were required to limit personal supplementation of vitamin D to ≤800 IU daily and calcium to ≤1200 mg daily and to abstain from fish oil supplementation. The trial included a 3-month placebo run-in phase. A total of 25 871 men and women, including 5107 African-Americans/blacks, were treated for a median of 5.3 years (range 3.8-6.1). The trial protocol has been described in detail (26,27), and the primary results of the parent trial have been published (28,29).
A sub-cohort of 1054 VITAL participants from the New England region was enrolled for detailed, in-person phenotyping at the Harvard Catalyst CTSC in Boston, MA, USA. From the CTSC sub-cohort, participants were recruited for the DXA sub-cohort for bone density and body composition assessments by DXA. Eligibility for inclusion in the DXA sub-cohort required no current/recent use of bisphosphonates within 2 years or other osteoporosis medications (ie, denosumab, parathyroid hormone analogs, calcitonin, raloxifene, tamoxifen, or systemic estrogens) within 1 year. No participants were on aromatase inhibitors. Of the 1054 participants in the CTSC sub-cohort, 771 completed blood draw and bone health, body composition, and physical performance assessments at baseline, exceeding enrollment goal of 600 (30). Retention rate at the 2-year follow up was 89% (n = 687). The baseline visits were conducted between January 2012 and March 2014, and the 2-year follow-up visits were completed between October 2014 and July 2016.
This study is included in the Clinical Trials.gov website (NCT01747447). These studies were approved by the Partners Human Research Committees, the Institutional Review Board of Brigham and Women’s Hospital, and patients provided written informed consent.
Measurement of anthropometric variables and body composition
Body weight, height, and waist circumference were measured at baseline and 2 years using standard clinic equipment by trained personnel. Body composition assessments at baseline and 2 years were determined by DXA (Discovery W, APEX Software Version 4.2, Hologic, Bedford, MA, USA) in the Skeletal Health and Osteoporosis Center and Bone Density Unit at Brigham and Women’s Hospital. To distinguish nonosseous from osseous tissues, the Discovery W uses true linear fan-beam acquisition geometry to measure tissue density (31). Adiposity measures include total body fat percentage, FMI, fat mass to lean mass (FM:LM) ratio, visceral adipose tissue (VAT) area, truncal fat mass, and truncal-to-limb fat mass ratio. FMI values were categorized according to sex, age, and race-specific thresholds from the NHANES database (32). Thresholds for overweight by FMI were >6 to 9kg/m2 for males and >9 to 13kg/m2 for females, and thresholds for obesity were >9kg/m2 for males and >13kg/m2 for females (32). The VAT was estimated in a 5 cm wide region across the abdomen at the level of the fourth lumbar vertebrae by subtracting the subcutaneous adipose tissue (measured from the skin line to the outer abdominal muscle wall) from the total adipose tissue (measured within the abdominal muscle walls) (33). Lean tissue measures include lean mass index (LMI; lean mass/height2), appendicular lean mass (ALM), and ALM adjusted for BMI (ALM/BMI). The Foundation for the National Institutes of Health (FNIH) Biomarkers Consortium Sarcopenia Project recommends cut points for ALM/BMI at <0.789 for men and <0.512 for women to define sarcopenia by low lean mass; alternate cut points for ALM are <19.75kg for men and <15.02kg for women (34,35). At our Bone Density Unit, the in vivo precisions [percentage coefficient of variation (CV%)] for total fat mass and total lean mass, based on 60 participants (30 men and 30 women) scanned twice in 2015, were 1.73% and 0.52% for men and 1.44% and 0.83% for women, respectively.
Measurement of blood samples
Fasting blood samples were collected at baseline and year 2 in the same season to control for any seasonal effects on vitamin D levels. Levels of serum total 25(OH)D were assayed by Quest Diagnostics (San Juan Capistrano, CA, USA) using liquid chromatography tandem mass spectrometry. Total 25(OH)D was calibrated to Centers for Disease Control and Prevention standards (36). Free 25(OH)D levels were measured by Future Diagnostics Solutions B.V. (Wijchen, the Netherlands) using a competitive enzyme-linked immunosorbent assay 2-step immunoassay developed in collaboration with DIAsource ImmunoAssays S.A. The CV% for the VITAL samples were 4.2% and 6.3% for total and free 25(OH)D levels, respectively. To minimize potential batch effects, all baseline and year 2 sample pairs were measured at the same time in tandem, blinded to the analyzing labs. Quest Diagnostics performed the serum total 25(OH)D levels at no cost to the trial. Other than the blinded assay analyses, Quest Diagnostics and Future Diagnostics were not involved in the study design, data analysis, or manuscript preparation.
Statistical analysis
Analyses of treatment effects were based on the intent-to-treat principle. This ancillary study was designed a priori to have 80% power to detect differences in weight of 1.70 kg, waist circumference of 1.29 cm, total fat mass of 1.21 kg, truncal fat mass of 0.21 kg, appendicular fat mass of 0.23 kg, total lean mass of 0.60 kg, and ALM of 0.33 kg with a planned sample size of 750 and 10% loss to follow-up (37,38).
Baseline characteristics were compared by treatment assignment to assess whether randomization was balanced among this sub-cohort. Continuous variables were examined for normality. Means (SD) or median (25th, 75th percentiles) are reported as appropriate. T tests and analysis of variance (or the Wilcoxon rank sum and Kruskal-Wallis tests) were used to compare continuous variables across randomized groups. Chi-square tests were used to compare proportions.
The primary analyses were of the main effect of vitamin D on changes in measures of body composition, adjusted for age, sex, and race/ethnicity. Linear regression analysis was used to adjust for these and any imbalance in other key variables at baseline. Percentage changes in variables were computed in regressions using logarithms. Effect modification of the treatment effects according to sex, race/ethnicity, BMI, FMI, and baseline and 2-year total and free 25(OH)D levels (above or below the median) were specified a priori and explored by including interaction terms in the model. In exploratory analyses, we also adjusted for baseline total 25(OH)D level and use of calcium supplements. All statistical analyses were performed using SAS (SAS Institute, Cary, NC, USA). P values < 0.05 were considered statistically significant. As statistical analyses were not adjusted for multiple hypotheses testing, secondary and exploratory end points should be interpreted with caution.
Results
Ancillary study participants
Participants (n = 771) were recruited for DXA scanning for bone mineral density and body composition. Four were excluded from analysis at baseline. Two participants were excluded due to breast implants, which may have uncertain artifactual effects on bone density, fat, and lean mass measurements. One excluded participant had a leg amputation, and the other was unable to tolerate lying down for the scan. Two-year follow-up was completed by 687 participants (89% retention), of whom 1 additional participant was excluded from the analysis due to interval breast implants.
Table 1 shows the baseline characteristics of the DXA sub-cohort. Mean age was 63.8 years; 46.7% were woman and 53.3% were men. At baseline, average BMI was 28.3 kg/m2 with 4 participants underweight (0.5%, BMI < 18.5 kg/m2), 201 normal weight (26.1%, BMI 18.5-<25 kg/m2), 334 overweight (43.4%, BMI 25-<30 kg/m2), and 232 obese (30.0%, BM ≥30 kg/m2). Average FMI was 10.3 kg/m2 with 103 participants with normal FMI (13.4%), 418 overweight (54.6%), and 245 obese (32.0%). According to the FNIH sarcopenia cut-points, 25.3% of men and 25.6% of women had low lean mass by ALM/BMI.
Table 1.
Baseline characteristics according to randomized assignment to vitamin D3 vs placebo groups
| Characteristic | Total (N = 771) | Vitamin D3 Group (N = 388) | Placebo Group (N = 383) | P-value |
|---|---|---|---|---|
| Female sex, n (%) | 360 (46.7%) | 179 (46.1%) | 181 (47.3%) | 0.76 |
| Age, mean (SD) | 63.8 (6.1) | 63.7 (6.0) | 63.9 (6.3) | 0.53 |
| Race or ethnic group,a n (%) | ||||
| Non-Hispanic white | 630 (83.4%) | 317 (82.8%) | 313 (84.1%) | 0.49 |
| Black | 67 (8.9%) | 35 (9.1%) | 32 (8.6%) | |
| Nonblack Hispanic | 26 (3.4%) | 11 (2.9%) | 15 (4.0%) | |
| Asian | 15 (2.0%) | 9 (2.3%) | 6 (1.6%) | |
| Native American or Alaskan native | 5 (0.7%) | 2 (0.5%) | 3 (0.8%) | |
| Other or unknown | 12 (1.6%) | 9 (2.3%) | 3 (0.8%) | |
| Body mass index, mean (SD) kg/m2 | 28.3 (5.1) | 28.3 (5.2) | 28.3 (5.1) | 0.93 |
| Fat mass index, mean (SD) kg/m2 | 10.3 (3.89) | 10.3 (4.04) | 10.3 (3.74) | 0.94 |
| Lean mass index, mean (SD) kg/m2 | 16.5 (2.4) | 16.5 (2.3) | 16.5 (2.5) | 0.97 |
| ALM/BMI, mean (SD) kg/m2 | 0.73 (0.18) | 0.74 (0.18) | 0.72 (0.18) | 0.14 |
| Leisure time physical activity, median (interquartile range) MET, h/week | 21.5 (7.9-37.1) | 21.6 (7.9-37.8) | 21.0 (8.0-36.0) | 0.62 |
| Diabetes history, n (%) | 84 (10.9%) | 44 (11.4%) | 40 (10.4%) | 0.68 |
| Hypertension history, n (%) | 338 (44.0%) | 161 (41.6%) | 177 (46.5%) | 0.18 |
| Use of cholesterol-lowering medications, n (%) | 262 (34.3%) | 132 (34.5%) | 130 (34.2%) | 0.94 |
| Current smoking, n (%) | 48 (6.3%) | 26 (6.8%) | 22 (5.8%) | 0.56 |
| Baseline calcium supplement use,b n (%) | 132 (17.1%) | 69 (17.8%) | 63 (16.4%) | 0.62 |
| Baseline vitamin D supplement use,b n (%) | 326 (42.3%) | 157 (40.5%) | 169 (44.1%) | 0.30 |
| Baseline total 25(OH)D,c mean (SD) nmol/L | 69.1 (22.7) | 67.4 (22.0) | 71.1 (23.2) | 0.03 |
| Baseline free 25(OH)D, mean (SD) pmol/L | 14.6 (4.7) | 14.4 (4.5) | 14.8 (4.8) | 0.21 |
a Race and ethnic groups self-reported by participants.
b Personal calcium supplement intake limited to ≤1200 mg/day, vitamin D intake ≤800 IU/day.
c To convert values of 25(OH)D to ng/mL, multiple by 0.4.
Characteristics were well-balanced between the vitamin D and placebo-treated groups. Leisure-time physical activity was similar in both groups with median metabolic equivalent of task (MET) score of 21.5 MET-h/week. There was no difference in number of participants with a history of diabetes, hypertension, or cholesterol-lowering medication use. Current tobacco and alcohol use were similar between groups.
Despite similar percentages of participants on personal vitamin D supplements at baseline (limited to ≤800 IU/day), the vitamin D–treated group did have a slightly lower baseline total 25(OH)D level of 67.4 nmol/L, compared to 71.1 nmol/L in the placebo-treated group (P = 0.03). Twelve participants (3.7%) had total 25(OH)D levels <30 nmol/L. There was no difference in levels of free 25(OH)D levels at baseline.
Main effects of supplemental vitamin D on body composition measures
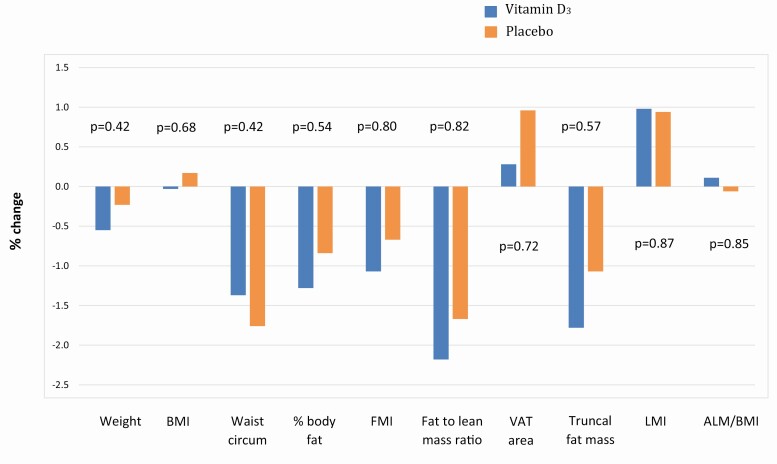
Overall, there were minimal changes (<2%) in weight and measures of body composition in both vitamin D and placebo groups over the 2 years. Daily supplementation with vitamin D3 for 2 years did not have effects on body weight, BMI, waist circumference, total or regional adiposity, or total or regional lean mass, compared to placebo (Fig. 1). There were no significant differences between the vitamin D and the placebo groups in changes in weight (∆0.32%, P = 0.42), BMI (∆0.20%, P = 0.68), waist circumference (∆0.39%, P = 0.42), body fat percentage (∆0.44%, P = 0.54), FMI (∆0.40%, P = 0.80), FM:LM ratio (∆0.53%, P = 0.82), VAT area (∆0.68%, P = 0.72), truncal fat mass (∆0.71%, P = 0.57), truncal-to-limb fat ratio (∆0.37%, P = 0.55), LMI (∆0.04%, P = 0.87), ALM (∆0.04%, P = 0.68), and ALM/BMI (∆0.17%, P = 0.85), adjusted for age, sex, and race/ethnicity.
Figure 1.
Percentage changes over 2 years in body composition measures. Outcomes of adipose and lean tissues were assessed by dual-energy X-ray absorptiometry. Abbreviations: ALM, appendicular lean mass; BMI, body mass index; FMI, fat mass index; LMI, lean mass index; VAT, visceral adipose tissue.
Subgroup analyses
There were no differences in effect of vitamin D3 supplementation vs placebo on weight or body composition by sex (data not shown), race/ethnicity (data not shown), baseline total 25(OH)D level [stratified above and below the median (70 nmol/L) and 50 nmol/L)], or baseline free 25(OH)D level (stratified above and below the median, 14.2 pmol/L), corrected for age, sex, and race/ethnicity (Table 2).
Table 2.
Changes in Body Composition Measures by Intervention, stratified by baseline total and free 25(OH)D levelsa
| Baseline total 25(OH)D level | Baseline free 25(OH)D level | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| <median (70 nmol/L)b | ≥medianb | P for inter-action | <median (14pmol/L)b | ≥medianb | P for inter-action | ||||||
| Vitamin D (n = 213) | Placebo (n = 167) | Vitamin D (n = 174) | Placebo (n = 216) | Vitamin D (n = 203) | Placebo (n = 180) | Vitamin D (n = 184) | Placebo (n = 203) | ||||
| Weight, mean (SD), kg | Baseline | 84.3 (17.1) | 82.9 (17.1) | 79.7 (15.5) | 79.4 (17.6) | 84.2 (17.3) | 83.1 (17.7) | 80.0 (15.3) | 78.9 (17.0) | ||
| % change | −0.26% | −0.55% | −0.90% | 0.03% | 0.22 | −0.35% | −0.63% | −0.76% | 0.14% | 0.19 | |
| BMI, mean (SD), kg/m2 | Baseline | 28.9 (5.6) | 28.6 (5.0) | 27.5 (4.7) | 28.1 (5.1) | 29.0 (5.7) | 29.0 (5.3) | 27.5 (4.6) | 27.7 (4.8) | ||
| % change | 0.21% | −0.23% | −0.33% | 0.47% | 0.19 | 0.12% | −0.21% | −0.19% | 0.50% | 0.25 | |
| Waist circumference, mean (SD), cm | Baseline | 100.3 (14.7) | 99.3 (14.0) | 97.0 (14.8) | 97.6 (16.6) | 100.3 (14.9) | 100.2 (14.8) | 97.1 (14.6) | 96.7 (16.0) | ||
| % change | −0.87% | −1.88% | −1.96% | −1.67% | 0.40 | −0.91% | −1.90% | −1.86% | −1.63% | 0.39 | |
| % body fat, mean (SD) | Baseline | 36.6 (8.7) | 35.8 (8.4) | 35.8 (8.1) | 36.9 (8.7) | 36.9 (8.6) | 37.1 (8.3) | 35.6 (8.2) | 35.8 (8.7) | ||
| % change | −0.89% | −1.03% | −1.78% | −0.70% | 0.31 | −1.06% | −1.28% | −1.54% | −0.44% | 0.19 | |
| FMI, mean (SD), kg/m2 | Baseline | 10.6 (4.3) | 10.2 (3.7) | 9.8 (3.7) | 10.4 (3.8) | 10.7 (4.3) | 10.7 (3.8) | 9.8 (3.7) | 9.9 (3.7) | ||
| % change | −0.61% | −1.23% | −1.68% | −0.25% | 0.37 | −0.81% | −1.47% | −1.38% | 0.09% | 0.27 | |
| Fat-to-lean mass ratio, mean (SD) | Baseline | 0.64 (0.25) | 0.62 (0.23) | 0.62 (0.23) | 0.65 (0.24) | 0.65 (0.25) | 0.65 (0.23) | 0.61 (0.23) | 0.62 (0.24) | ||
| % change | −1.68% | −2.04% | −2.89% | −1.39% | 0.35 | −2.02% | −2.38% | −2.44% | −1.01% | 0.26 | |
| VAT area, mean (SD), cm2 | Baseline | 176 (74) | 170 (68) | 156 (69) | 159 (79) | 176 (75) | 175 (72) | 158 (69) | 155 (75) | ||
| % change | 0.76% | 0.42% | −0.41% | 1.40% | 0.46 | −0.30% | −0.24% | 0.93% | 2.14% | 0.70 | |
| Truncal fat mass, mean (SD), kg | Baseline | 16.1 (6.1) | 15.3 (5.6) | 14.6 (5.5) | 14.9 (5.9) | 16.1 (6.1) | 15.9 (5.8) | 14.7 (5.6) | 14.4 (5.7) | ||
| % change | −1.15% | −1.51% | −2.63% | −0.73% | 0.45 | −1.25% | −1.83% | −2.40% | −0.34% | 0.28 | |
| Truncal-to-limb fat ratio, mean (SD) | Baseline | 1.25 (0.30) | 1.24 (0.29) | 1.20 (0.31) | 1.20 (0.38) | 1.25 (0.31) | 1.22 (0.28) | 1.21 (0.30) | 1.22 (0.39) | ||
| % change | −0.84% | −0.15% | −0.59% | −0.52% | 0.44 | −0.64% | 0.06% | −0.83% | −0.72% | 0.39 | |
| LMI, mean (SD), kg/m2 | Baseline | 16. 7 (2.5) | 16.8 (2.5) | 16.2 (2.2) | 16.2 (2.5) | 16.7 (2.5) | 16.7 (2.6) | 16.2 (2.1) | 16.3 (2.5) | ||
| % change | 1.16% | 0.85% | 0.81% | 1.02% | 0.39 | 1.29% | 0.97% | 0.69% | 0.91% | 0.33 | |
| ALM, mean (SD), kg | Baseline | 21.0 (5.1) | 21.0 (5.4) | 20.0 (4.9) | 19.4 (5.3) | 20.9 (5.1) | 20.5 (5.5) | 20.1 (4.9) | 19.7 (5.3) | ||
| % change | 0.13% | −0.07% | 0.09% | 0.29% | 0.59 | 0.19% | 0.04% | 0.03% | 0.20% | 0.58 | |
| ALM/BMI, mean (SD) | Baseline | 0.74 (0.18) | 0.74 (0.18) | 0.74 (0.18) | 0.70 (0.18) | 0.73 (0.18) | 0.72 (0.18) | 0.74 (0.18) | 0.72 (0.18) | ||
| % change | −0.22% | 0.04% | 0.54% | −0.14% | 0.22 | −0.11% | 0.18% | 0.37% | −0.26% | 0.23 |
a Adjusted for age, sex, and race/ethnicity.
b Nonsignificant P-values comparing effects of vitamin D vs placebo within each sub-group.
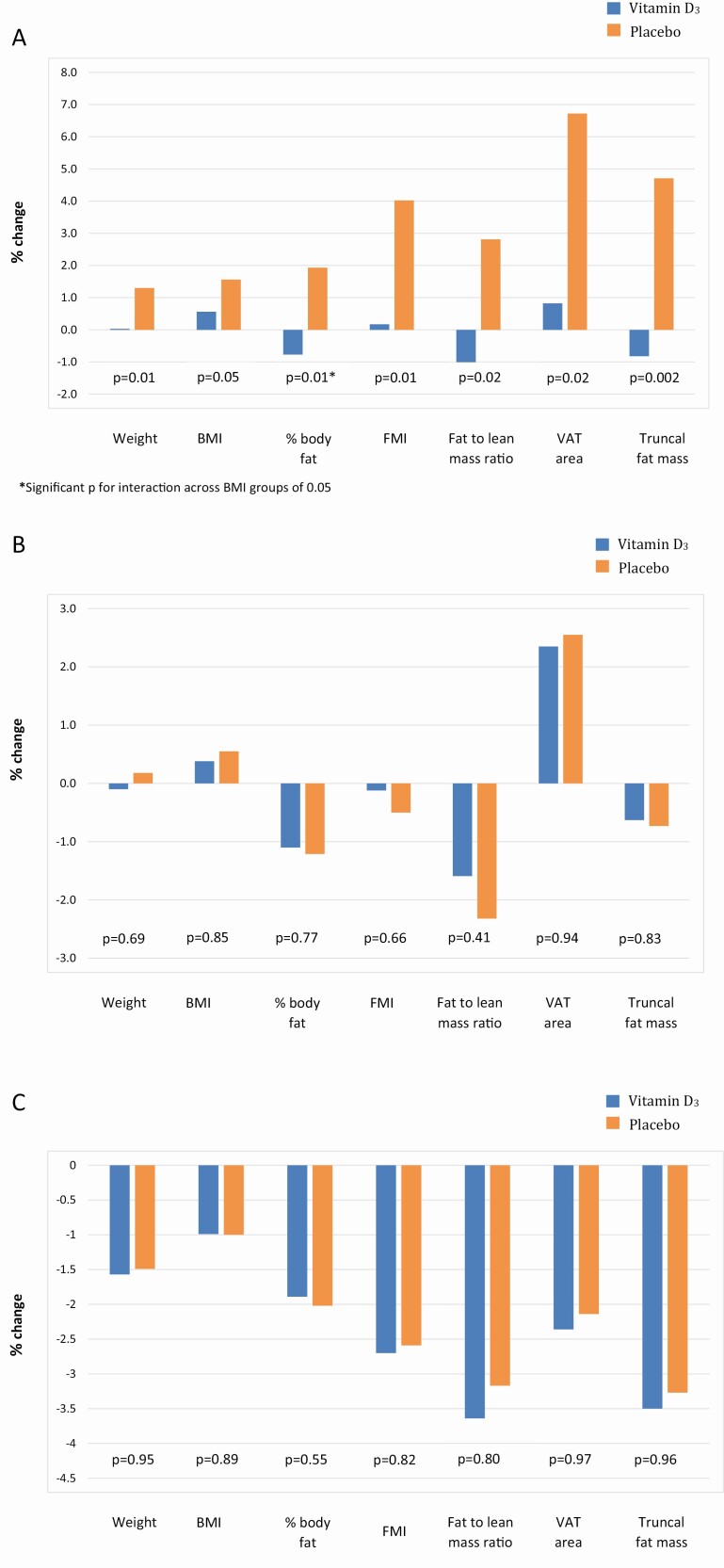
When participants were categorized by BMI as normal (<25 kg/m2), overweight (25-<30kg/m2), or obese (≥30kg/m2), vitamin D3 supplementation, compared to placebo, was associated with improved measures of adiposity in men and women with normal BMI, but not in the overweight or obese groups (Fig. 2). Compared to placebo, normal-weight participants on vitamin D3 supplements had small decreases instead of gains in body fat percentage (−0.77% vs 1.93%, respectively; P = 0.01, P for interaction = 0.05); there was also a trend in the vitamin D group for a reduction in truncal fat mass (−0.82% vs 4.71, P = 0.002, P for interaction = 0.08) and a lower FM:LM ratio (−1.03% vs 2.81%, P = 0.02, P for interaction = 0.05), compared to the placebo group. While the interaction was not significant, in the normal weight participants, those treated with vitamin D vs placebo also had less weight gain (0.03% vs 1.30%, P = 0.01) and less of an increase in FMI (0.17% vs 4.02%, %, P = 0.01) and VAT area (0.82% vs 6.72%, P = 0.02), but there were no differences in these measures among the obese and overweight participants. There were no differences in waist circumference or measures of lean mass between vitamin D and placebo groups across BMI categories. Finally, among participants with normal BMI, there were no differences in clinical characteristics, including sex and racial/ethnic composition, physical activity, and personal use of supplemental vitamin D at baseline, between the vitamin D3– and placebo-treated groups (data not shown).
Figure 2.
Percentage changes over 2 years in body composition measures. (A) In participants with BMI < 25kg/m2, (B) in participants with BMI 25-<30kg/m2, and (C) in participants with BMI > 30kg/ m2. Outcomes of adipose and lean tissues were assessed by dual-energy X-ray absorptiometry. Abbreviations: BMI, body mass index; FMI, fat mass index; VAT, visceral adipose tissue.
When participants were categorized by FMI as normal (<9 kg/m2 for women, <6 kg/m2 for men), overweight (9-13kg/m2 in women, 6-9kg/m2 in men), or obese (>13kg/m2 for women, >9kg/m2 in men), vitamin D3 supplementation had no significant benefit compared to placebo for any FMI category (data not shown).
In the active vitamin D3 treated group, ALM/BMI, a FNIH measure of low lean mass for sarcopenia, improved in participants who had total 25(OH)D level at or above the median (97 nmol/L) after 2 years of supplementation compared to those who had total 25(OH)D below the median at 2 years (0.80% vs −0.58%, P = 0.01)(Table 3). VAT area also decreased in vitamin D3–treated participants who had higher 2-year total 25(OH)D levels (−1.87% vs 2.16%, P = 0.04). No other effect modification by 2-year total 25(OH)D levels was noted for weight or other measures of body composition. Having 2-year free 25(OH)D levels above the median (21 pmol/L) did not affect body composition results.
Table 3.
Changes in body composition measures in the active vitamin D3 group, stratified by 2-year 25(OH)D levels above/below median
| 2-year total 25(OH)D level | 2-year free 25(OH)D level | ||||||
|---|---|---|---|---|---|---|---|
| <median (97 nmol/L) N = 175 | ≥median N = 185 | P-value | <median (21pmol/L) N = 178 | ≥median N = 182 | P-value | ||
| Weight, mean (SD), kg | Baseline | 85.3 (16.7) | 79.0 (16.0) | 87.1 (17.7) | 77.0 (13.9) | ||
| % change | −0.01% | −1.11% | 0.12 | −0.42% | −0.70% | 0.76 | |
| BMI, mean (SD), kg/m2 | Baseline | 29.2 (5.4) | 27.3 (4.9) | 30.0 (5.9) | 26.5 (3.8) | ||
| % change | 0.39% | −0.46% | 0.21 | 0.04% | −0.11% | 0.83 | |
| Waist circumference, mean (SD), cm | Baseline | 101.3 (14.3) | 96.0 (14.9) | 102.8 (15.0) | 94.4 (13.4) | ||
| % change | −0.59% | −2.16% | 0.09 | −1.00% | −1.78% | 0.43 | |
| % Body fat, mean (SD) | Baseline | 36.5 (8.6) | 35.7 (8.3) | 37.8 (9.0) | 34.5 (7.5) | ||
| % change | −0.56% | −2.01% | 0.06 | −1.21% | −1.36% | 0.93 | |
| FMI, mean (SD), kg/m2 | Baseline | 10.7 (4.2) | 9.7 (3.8) | 11.4 (4.7) | 9.0 (2.9) | ||
| % change | −0.10% | −2.14% | 0.17 | −0.91% | −1.29% | 0.92 | |
| Fat to lean mass ratio, mean (SD) | Baseline | 0.64 (0.25) | 0.61 (0.23) | 0.68 (0.27) | 0.58 (0.20) | ||
| % change | −1.33% | −3.12% | 0.15 | −2.23% | −2.20% | 0.83 | |
| VAT area, mean (SD), cm2 | Baseline | 179 (72) | 154 (71) | 190 (76) | 143 (60) | ||
| % change | 2.16% | −1.87% | 0.04 | 0.87% | −0.51% | 0.58 | |
| Truncal fat mass, mean (SD), kg | Baseline | 16.2 (6.1) | 14.4 (5.6) | 17.3 (6.5) | 13.3 (4.5) | ||
| % change | −0.22% | −3.54% | 0.07 | −1.28% | −2.45% | 0.69 | |
| Truncal-to-limb fat ratio, mean (SD) | Baseline | 1.26 (0.31) | 1.20 (0.30) | 1.28 (0.32) | 1.18 (0.28) | ||
| % change | −0.33% | −1.16% | 0.48 | −0.29% | −1.22% | 0.29 | |
| LMI, mean (SD), kg/m2 | Baseline | 16.9 (2.4) | 16.0 (2.3) | 17.0 (2.4) | 15.9 (2.2) | ||
| % change | 1.11% | 0.85% | 0.53 | 1.23% | 0.72% | 0.19 | |
| ALM, mean (SD), kg | Baseline | 21.3 (5.0) | 19.7 (5.0) | 21.2 (5.1) | 19.8 (5.0) | ||
| % change | −0.04% | 0.22% | 0.62 | 0.08% | 0.09% | 0.89 | |
| ALM/BMI, mean (SD) | Baseline | 0.74 (0.18) | 0.73 (0.18) | 0.72 (0.18) | 0.75 (0.18) | ||
| % change | −0.58% | 0.80% | 0.01 | −0.08% | 0.29% | 0.43 |
Results are adjusted for age, sex, and race/ethnicity.
We also performed analyses that additionally adjusted for baseline total 25(OH)D level and use of calcium supplements (data not shown). Results were overall similar between the vitamin D and placebo groups. Vitamin D3 supplementation, compared to placebo, had no effect in non-Hispanic whites but resulted in slight improvement in ALM/BMI in blacks (1.60% vs −0.43%, P = 0.03, P for interaction = 0.04) with these additional adjustments.
Discussion
We found that vitamin D3 supplementation of 2000 IU/day for 2 years did not affect changes in total weight or fat and lean tissue measurements as assessed by DXA in this large RCT. In subgroup analyses, however, vitamin D3 supplementation did improve body fat percentage in participants with BMI in the normal range (<25.0 kg/m2), but not in those in the overweight or obesity categories. In the participants assigned to the active vitamin D3 supplement, there was also a benefit for ALM/BMI, a measure of sarcopenia, and VAT area in those who had 2-year total 25(OH)D level above the median (70 nmol/L). Elevated VAT area is associated with increased risk of diabetes and atherosclerosis (39).
Although observational studies have shown an association between lower vitamin D status and higher body weight and fat mass, vitamin D supplementation did not improve adiposity measures overall in our large ancillary trial, consistent with most prior intervention studies (5,6,20,40). One positive intervention study was a double-blind RCT in 218 postmenopausal women engaged in a weight-loss intervention, which found that supplementation of vitamin D3 resulted in greater weight loss, higher reductions in waist circumference, and greater reduced body fat percentage, in those for whom a total 25(OH)D level of ≥80 nmol/L was achieved (18). Another trial in 77 healthy overweight and obese women found that 1000 IU/day of cholecalciferol for 12 weeks resulted in decreased fat mass, compared to placebo (15). The baseline total 25(OH)D level was low with a mean of 37 nmol/L for the vitamin D group and 47 nmol/L for the placebo group, and a significant reverse correlation was observed between changes in serum total 25(OH)D concentrations and body fat mass. However, in another short-term trial in 52 obese subjects with total 25(OH)D levels <50 nmol/L, 7000 IU/day of vitamin D for 26 weeks did not affect adiposity measures, compared with placebo (17). In our 2-year ancillary study of 771 participants, we found no effects of vitamin D vs placebo on body weight in participants with overweight or obesity, but a small benefit in participants with normal BMI. Furthermore, our baseline mean total 25(OH)D levels were sufficient at 69 nmol/L, which may already be beyond the threshold for which vitamin D may benefit body composition. When we performed subgroup analyses in participants with a baseline total 25(OH)D level below the lower limit of normal (<50 nmol/L), we also found no differences in weight and body composition between the vitamin D3– and placebo-treated groups, though there were only 124 participants with baseline total 25(OH)D levels <50 nmol/L at 2-year follow-up. Differences among interventional trials may be due to differences in age, sex, BMI, and baseline 25(OH)D level; possible interaction with calcium; short duration of trials; and differences in dosing of vitamin D supplementation (13,19).
Notably, other RCTs have found benefits for vitamin D3 supplementation in those with normal BMI. The parent VITAL trial found that vitamin D3 supplementation did not prevent invasive cancer overall but did decrease risk in those with normal BMI (28). Similarly, although randomized vitamin D3 supplementation vs placebo did not lower risk of type 2 diabetes in the Vitamin D and Type 2 Diabetes trial, a benefit was seen in those with BMI < 30 kg/m2 (41). Participants with obesity may require a higher dose of vitamin D supplementation to achieve adequate 25(OH)D levels in circulation due to sequestration of vitamin D in fat and volumetric dilution (7,8). In VITAL, however, there was minimal variation in the change in total 25(OH)D level in response to vitamin D3 supplementation of 2000 IU/day for 2 years across BMI groups (42). Ongoing VITAL analyses are determining whether adiposity affects changes in total and free 25(OH)D levels in response to supplemental vitamin D. Also, hormonal response to vitamin D may be altered in obesity, as parathyroid hormone is suppressed at lower total 25(OH)D levels in the obese compared to the overall population (43). This lower parathyroid hormone level may contribute to a decreased conversion of 25(OH)D to 1,25(OH)2D.
Furthermore, obesity-related differences in vitamin D metabolizing enzymes and receptors in different compartments of adipose tissue have been found that may affect vitamin D actions on a paracrine/autocrine level. Subcutaneous adipose tissue in obese women has been found to have lower CYP2J2 and CYP27B1 expression compared to lean women, and this may result in compromised production of 25(OH)D and 1,25(OH)2D in obese women (44). Differences in physiologic responses to vitamin D in normal-weight vs obese individuals merit further study.
In vitro studies to date have not determined the molecular effects of vitamin D on fat, and results from studies among rodents differ from humans. Several in vitro studies using mice cell lines have shown that 1,25(OH)2D inhibits adipogenesis and differentiation and decreases accumulation of lipids (13). Some but not all rodent studies have found that vitamin D supplementation results in decreased body weight and adiposity (13). In contrast, in vitro studies using human adipocytes have found that vitamin D increases adipocyte differentiation and lipid accumulation (45,46).
Similarly, observational studies have found that low vitamin D status is associated with decreased lean tissue mass in the elderly, while interventional studies have not confirmed causality (9-11,14). Bislev et al treated postmenopausal women with total 25(OH)D levels < 50 nmol/L with 280 IU/day of vitamin D3 or placebo for 3 months and did not find any differences in total lean body mass or appendicular LMI (14). In accordance, we did not find that vitamin D3 supplementation vs placebo improved measures of lean mass or sarcopenia.
A major strength is the size of this study. With 771 participants, this study is one of the largest vitamin D RCTs with data on body composition and allowed for analyses of differences by BMI, FMI, sex, race, and baseline and 2-year 25(OH)D levels. In addition to total 25(OH)D, free vitamin D levels were also examined. Total 25(OH)D assays were calibrated to Centers for Disease Control and Prevention standards, and free 25(OH)D assay was performed by Future Diagnostics. This study had high retention (89%) and the power to detect small changes in weight, total fat mass, and total lean mass. There were also limitations. Although DXA can differentiate regional distributions of fat and lean tissues, it cannot assess visceral, subcutaneous, and intramuscular fat at the tissue-organ level, which require computed tomography and/or magnetic resonance imaging (47). The body composition measures were assessed only at baseline and 2-years of follow-up. Participants were not selected for vitamin D deficiency. Thus, baseline total 25(OH)D levels were generally sufficient in our trial, limiting the ability to determine effects in those with low vitamin D levels. Only 3.7% of our participants had baseline total 25(OH)D levels <30 nmol/L, which is similar to the prevalence found in the 2011-2014 U.S. National Health and Nutrition Examination Survey, indicating that 2.9% of the nationwide population ≥60 years old had total 25(OH)D levels <30 nmol/L (3). Finally, as this study was conducted in older men and women, the results are not generalizable to the younger population.
In summary, in this randomized placebo-controlled trial, daily vitamin D3 supplementation did not improve weight or body composition in the general population of older adults, not selected for vitamin D deficiency or obesity. Whether supplemental vitamin D has benefits for body composition measures in normal-weight individuals warrants further investigation.
Acknowledgments
We thank the trial participants, staff, and investigators. These contents are solely the responsibility of the authors and do not necessarily represent the official views of the National Institute of Arthritis Musculoskeletal and Skin Disease (NIAMS) or NIH. This work was conducted with support from the Harvard Catalyst CTSC (National Center for Research Resources [NCRR] and National Center for Advancing Translational Sciences [NCATS], NIH Award UL1TR001102) and the Office of Dietary Supplements. This study and the parent study are registered at clinicaltrials.gov (NCT01747447, NCT01169259). We also thank Quest Diagnostics, which performed the total 25(OH)D measurements at no additional cost. Pharmavite LLC of Northridge, CA, USA (vitamin D), Pronova BioPharma of Norway (Omacor fish oil), and BASF (Omacor fish oil) donated the study agents, matching placebos, and packaging. We also thank the Centers for Disease Control and Prevention (Drs. Hubert Vesper and Julianne Cook Botelho) for their collaboration on the standardization and calibration of the total 25(OH)D measurements.
Financial Support: This project was supported by grants R01 AR059775, R01 AR070854, and R01 AR060574 (PI, MSL) from National Institute of Arthritis Musculoskeletal and Skin Diseases (NIAMS/NIH). This research was also supported by the VITAL parent grants and U01 CA138962 and R01 CA138962 (PI, JEM, and JEB) from the National Cancer Institute, the National Heart, Lung, and Blood Institute, the Office of Dietary Supplements, the National Institute of Neurological Disorders and Stroke, and the National Center for Complementary and Integrative Health. Dr. Samia Mora was supported by research grants from the National Institute of Diabetes and Digestive and Kidney Diseases (DK112940), National Heart, Lung, and Blood Institute (R01HL134811 and K24 HL136852). Dr. Paulette D. Chandler received support from grant U01 CA138962 from the National Cancer Institute and grant 127524-MRSG-15-012-01-CNE from the American Cancer Society.
Clinical Trial Information: NCT01747447, NCT01704859.
Glossary
Abbreviations:
- 25(OH)D
25-hydroxyvitamin D;
- ALM
appendicular lean mass;
- BMI
body mass index;
- CTSC
Clinical and Translational Science Center;
- CV%
percentage coefficient of variation;
- DXA
dual-energy X-ray absorptiometry;
- FM:LM
fat mass to lean mass;
- FMI
fat mass index;
- FNIH
Foundation for the National Institutes of Health;
- LMI
lean mass index;
- MET
metabolic equivalent of task;
- NHANES
National Health and Nutrition Examination Survey;
- RCT
randomized controlled trial;
- VAT
visceral adipose tissue;
- VITAL
VITamin D and OmegA-3 TriaL;
- WHI
Women’s Health Initiative
Additional Information
Disclosure Summary: Dr. Mora has served as a consultant (modest) for Quest Diagnostics.
Data Availability
Some or all data generated or analyzed during this study are included in this published article or in the data repositories listed in references.
References
- 1. Hales CM, Fryar CD, Carroll MD, Freedman DS, Ogden CL. Trends in obesity and severe obesity prevalence in US youth and adults by sex and age, 2007-2008 to 2015-2016. JAMA. 2018;319(16):1723-1725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Ward ZJ, Bleich SN, Cradock AL, et al. Projected U.S. state-level prevalence of adult obesity and severe obesity. N Engl J Med. 2019;381(25):2440-2450. [DOI] [PubMed] [Google Scholar]
- 3. Herrick KA, Storandt RJ, Afful J, et al. Vitamin D status in the United States, 2011-2014. Am J Clin Nutr. 2019;110(1):150-157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Jun S, Cowan AE, Bhadra A, et al. Older adults with obesity have higher risks of some micronutrient inadequacies and lower overall dietary quality compared to peers with a healthy weight, National Health and Nutrition Examination Surveys (NHANES), 2011–2014. Public Health Nutr. 2020;23(13):2268-2279. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Ceglia L, Nelson J, Ware J, et al. ; Diabetes Prevention Program Research Group . Association between body weight and composition and plasma 25-hydroxyvitamin D level in the Diabetes Prevention Program. Eur J Nutr. 2017;56(1):161-170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Cheng S, Massaro JM, Fox CS, et al. Adiposity, cardiometabolic risk, and vitamin D status: the Framingham Heart Study. Diabetes. 2010;59(1):242-248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Drincic AT, Armas LA, Van Diest EE, Heaney RP. Volumetric dilution, rather than sequestration best explains the low vitamin D status of obesity. Obesity (Silver Spring). 2012;20(7):1444-1448. [DOI] [PubMed] [Google Scholar]
- 8. Wortsman J, Matsuoka LY, Chen TC, Lu Z, Holick MF. Decreased bioavailability of vitamin D in obesity. Am J Clin Nutr. 2000;72(3):690-693. [DOI] [PubMed] [Google Scholar]
- 9. Hirani V, Cumming RG, Naganathan V, et al. Longitudinal associations between vitamin D metabolites and sarcopenia in older Australian men: the concord health and aging in men project. J Gerontol A Biol Sci Med Sci. 2017;73(1):131-138. [DOI] [PubMed] [Google Scholar]
- 10. Scott D, Blizzard L, Fell J, Ding C, Winzenberg T, Jones G. A prospective study of the associations between 25-hydroxy-vitamin D, sarcopenia progression and physical activity in older adults. Clin Endocrinol (Oxf). 2010;73(5):581-587. [DOI] [PubMed] [Google Scholar]
- 11. Tieland M, Brouwer-Brolsma EM, Nienaber-Rousseau C, van Loon LJ, De Groot LC. Low vitamin D status is associated with reduced muscle mass and impaired physical performance in frail elderly people. Eur J Clin Nutr. 2013;67(10):1050-1055. [DOI] [PubMed] [Google Scholar]
- 12. Pathak K, Soares MJ, Calton EK, Zhao Y, Hallett J. Vitamin D supplementation and body weight status: a systematic review and meta-analysis of randomized controlled trials. Obes Rev. 2014;15(6):528-537. [DOI] [PubMed] [Google Scholar]
- 13. Dix CF, Barcley JL, Wright ORL. The role of vitamin D in adipogenesis. Nutr Rev. 2018;76(1):47-59. [DOI] [PubMed] [Google Scholar]
- 14. Bislev LS, Langagergaard Rødbro L, Rolighed L, Sikjaer T, Rejnmark L. Effects of Vitamin D3 supplementation on muscle strength, mass, and physical performance in women with vitamin D insufficiency: a randomized placebo-controlled trial. Calcif Tissue Int. 2018;103(5):483-493. [DOI] [PubMed] [Google Scholar]
- 15. Salehpour A, Hosseinpanah F, Shidfar F, et al. A 12-week double-blind randomized clinical trial of vitamin D3 supplementation on body fat mass in healthy overweight and obese women. Nutr J. 2012;11:78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Karefylakis C, Särnblad S, Ariander A, Ehlersson G, Rask E, Rask P. Effect of Vitamin D supplementation on body composition and cardiorespiratory fitness in overweight men-a randomized controlled trial. Endocrine. 2018;61(3):388-397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Wamberg L, Kampmann U, Stødkilde-Jørgensen H, Rejnmark L, Pedersen SB, Richelsen B. Effects of vitamin D supplementation on body fat accumulation, inflammation, and metabolic risk factors in obese adults with low vitamin D levels - results from a randomized trial. Eur J Intern Med. 2013;24(7):644-649. [DOI] [PubMed] [Google Scholar]
- 18. Mason C, Xiao L, Imayama I, et al. Vitamin D3 supplementation during weight loss: a double-blind randomized controlled trial. Am J Clin Nutr. 2014;99(5):1015-1025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Caan B, Neuhouser M, Aragaki A, et al. Calcium plus vitamin D supplementation and the risk of postmenopausal weight gain. Arch Intern Med. 2007;167(9):893-902. [DOI] [PubMed] [Google Scholar]
- 20. Chandler PD, Wang L, Zhang X, et al. Effect of vitamin D supplementation alone or with calcium on adiposity measures: a systematic review and meta-analysis of randomized controlled trials. Nutr Rev. 2015;73(9):577-593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Haarbo J, Gotfredsen A, Hassager C, Christiansen C. Validation of body composition by dual energy X-ray absorptiometry (DEXA). Clin Physiol. 1991;11(4):331-341. [DOI] [PubMed] [Google Scholar]
- 22. Institute of Medicine. Dietary Reference Intakes for Calcium and Vitamin D. National Academies Press. [PubMed] [Google Scholar]
- 23. Ross AC, Manson JE, Abrams SA, et al. The 2011 dietary reference intakes for calcium and vitamin D: what dietetics practitioners need to know. J Am Diet Assoc. 2011;111(4):524-527. [DOI] [PubMed] [Google Scholar]
- 24. LeBoff MS, Yue AY, Copeland T, Cook NR, Buring JE, Manson JE. VITAL-Bone Health: rationale and design of two ancillary studies evaluating the effects of vitamin D and/or omega-3 fatty acid supplements on incident fractures and bone health outcomes in the VITamin D and OmegA-3 TriaL (VITAL). Contemp Clin Trials. 2015;41:259-268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. LeBoff MS, Chou SH, Murata EM, et al. Effects of supplemental vitamin D on bone health outcomes in women and men in the VITamin D and OmegA-3 TriaL (VITAL). J Bone Miner Res. 2020;35(5):883-893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Manson JE, Bassuk SS, Lee IM, et al. The VITamin D and OmegA-3 TriaL (VITAL): rationale and design of a large randomized controlled trial of vitamin D and marine omega-3 fatty acid supplements for the primary prevention of cancer and cardiovascular disease. Contemp Clin Trials. 2012;33(1):159-171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Bassuk SS, Manson JE, Lee IM, et al. Baseline characteristics of participants in the VITamin D and OmegA-3 TriaL (VITAL). Contemp Clin Trials. 2016;47:235-243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Manson JE, Cook NR, Lee IM, et al. ; VITAL Research Group . Vitamin D supplements and prevention of cancer and cardiovascular disease. N Engl J Med. 2019;380(1):33-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Manson JE, Cook NR, Lee IM, et al. ; VITAL Research Group . Marine n-3 fatty acids and prevention of cardiovascular disease and cancer. N Engl J Med. 2019;380(1):23-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Donlon CM, LeBoff MS, Chou SH, et al. Baseline characteristics of participants in the VITamin D and OmegA-3 triaL (VITAL): effects on bone structure and architecture. Contemp Clin Trials. 2018;67:56-67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Albanese CV, Diessel E, Genant HK. Clinical applications of body composition measurements using DXA. J Clin Densitom. 2003;6(2):75-85. [DOI] [PubMed] [Google Scholar]
- 32. Kelly TL, Wilson KE, Heymsfield SB. Dual energy X-Ray absorptiometry body composition reference values from NHANES. Plos One. 2009;4(9):e7038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Kelly TL, Wilson KE, Ruth CR. Estimating visceral fat by dual-energy x-ray absorptiometry. Patent #7,725,153 (US: Hologic, Inc). 2010. [Google Scholar]
- 34. Studenski SA, Peters KW, Alley DE, et al. The FNIH sarcopenia project: rationale, study description, conference recommendations, and final estimates. J Gerontol A Biol Sci Med Sci. 2014;69(5):547-558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Liu X, Baylin A, Levy PD. Vitamin D deficiency and insufficiency among US adults: prevalence, predictors and clinical implications. Br J Nutr. 2018;119(8):928-936. [DOI] [PubMed] [Google Scholar]
- 36. Luttmann-Gibson H, Mora S, Camargo CA, et al. Serum 25-hydroxyvitamin D in the VITamin D and OmegA-3 TriaL (VITAL): clinical and demographic characteristics associated with baseline and change with randomized vitamin D treatment. Contemp Clin Trials. 2019;87:105854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Sacks FM, Bray GA, Carey VJ, et al. Comparison of weight-loss diets with different compositions of fat, protein, and carbohydrates. N Engl J Med. 2009;360(9):859-873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Tirosh A, de Souza RJ, Sacks F, Bray GA, Smith SR, LeBoff MS. Sex differences in the effects of weight loss diets on bone mineral density and body composition: POUNDS LOST trial. J Clin Endocrinol Metab. 2015;100(6):2463-2471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Direk K, Cecelja M, Astle W, et al. The relationship between DXA-based and anthropometric measures of visceral fat and morbidity in women. BMC Cardiovasc Disord. 2013;13:25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Mathieu SV, Fischer K, Dawson-Hughes B, et al. Association between 25-hydroxyvitamin D status and components of body composition and glucose metabolism in older men and women. Nutrients. 2018;10(12):1826-1838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Pittas AG, Dawson-Hughes B, Sheehan P, et al. ; D2d Research Group . Vitamin D Supplementation and Prevention of Type 2 Diabetes. N Engl J Med. 2019;381(6):520-530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Chou S, Murata E, Yu C, et al. Effect of adiposity on change in total and free 25-hydroxyvitamin D levels in the VITamin D and OmegA-3 TriaL. American Society for Bone and Mineral Research 2019 Annual Meeting, Orlando, FL (MON-722). 2019.
- 43. Shapses SA, Lee EJ, Sukumar D, Durazo-Arvizu R, Schneider SH. The effect of obesity on the relationship between serum parathyroid hormone and 25-hydroxyvitamin D in women. J Clin Endocrinol Metab. 2013;98(5):E886-E890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Wamberg L, Christiansen T, Paulsen SK, et al. Expression of vitamin D-metabolizing enzymes in human adipose tissue: the effect of obesity and diet-induced weight loss. Int J Obes (Lond). 2013;37(5):651-657. [DOI] [PubMed] [Google Scholar]
- 45. Nimitphong H, Holick MF, Fried SK, Lee MJ. 25-hydroxyvitamin D3 and 1,25-dihydroxyvitamin D3 promote the differentiation of human subcutaneous preadipocytes. Plos One. 2012;7(12):e52171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Narvaez CJ, Simmons KM, Brunton J, Salinero A, Chittur SV, Welsh JE. Induction of STEAP4 correlates with 1,25-dihydroxyvitamin D3 stimulation of adipogenesis in mesenchymal progenitor cells derived from human adipose tissue. J Cell Physiol. 2013;228(10):2024-2036. [DOI] [PubMed] [Google Scholar]
- 47. Prado CM, Heymsfield SB. Lean tissue imaging: a new era for nutritional assessment and intervention. JPEN J Parenter Enteral Nutr. 2014;38(8):940-953. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Some or all data generated or analyzed during this study are included in this published article or in the data repositories listed in references.