Abstract
Introduction
Obesity and type 2 Diabetes (T2D) are both associated with greater bone mineral density (BMD) but increased risk of fractures. The effect of the combination of both conditions on bone metabolism, microarchitecture, and strength in the obese population remains unknown.
Methods
Data from 112 obese men were collected. Bone turnover and biochemical markers were measured by enzyme-linked immunosorbent assay, body composition and BMD at all sites were assessed by dual energy X-ray absorptiometry, whereas bone microarchitecture and strength (stiffness and failure load) were measured by high-resolution peripheral computed tomography. Data were compared among metabolically healthy obese (MHO) and metabolically unhealthy obese (MUHO) with and without T2D and between obese without and with T2D.
Results
Compared to MHO and MUHO without T2D, MUHO with T2D had significantly lower levels of osteocalcin ((7.49 ± 3.0 and 6.03 ± 2.47 vs 4.24 ± 2.72 ng/mL, respectively, P = 0.003) and C-terminal telopeptide of type I collagen (CTx) (0.28 ± 0.10 and 0.29 ± 0.13 vs 0.21 ± 0.15 ng/mL, respectively, P = 0.02). Dividing our subjects simply into those with and without T2D showed that obese men with T2D had significantly lower levels of osteocalcin (P = 0.003) and CTx (P = 0.005), greater trabecular separation at the tibia and radius (P = 0.03 and P = 0.04, respectively), and lower tibial failure load and stiffness (both P = 0.04), relative to obese men without T2D.
Conclusion
In men, the combination of obesity and T2D is associated with reduced bone turnover and poorer trabecular bone microarchitecture and bone strength compared to those who are obese but without T2D, suggesting worse bone disease.
Keywords: obesity, diabetes mellitus, bone microarchitecture, osteoporosis
Type 2 diabetes mellitus (T2D) is associated with normal or better than normal bone mineral density (BMD) but increased risk of fractures (1-3). Similar to T2D, obesity is associated with increased risk of fractures despite normal or higher BMD (4,5), phenomenon often described under the name of “obesity paradox” (6), although this has not been confirmed by some investigators (7). However, whether there is a distinct subgroup in the obese population particularly at risk for alteration in bone metabolism that predisposes them to fractures remains unknown.
BMD testing and the Fracture Risk Assessment (FRAX) score might not be accurate predictors of fracture risk in the diabetic population. Schwartz et al demonstrated that in people with T2D, the risk for fractures was higher for a given T-score or FRAX score, thus suggesting that factors other than BMD may impact bone quality in this population (2). Low bone turnover, increased accumulation of advanced glycation end products in bone and increased bone marrow adiposity are identified contributing factors to the development of bone fragility in patients with diabetes (8). Also, in some patients, an increased risk for falls due to hypoglycemic events (especially among those on insulin), or those who developed complications due to long-standing diabetes mellitus, which may negatively impact physical balance also add to the high risk for falls and fractures among these patients (8,9).
The greater mechanical load on the bone of individuals with obesity (10), together with the hyperestrogenemia due the elevated aromatase activity in the expanded adipose tissue (11-13), are postulated to account for the elevated BMD in this population. On the other hand, increased levels of pro-inflammatory cytokines and parathyroid hormone, and the lower 25-OH vitamin D may negatively influence the skeletal health in subjects with obesity (14) and could expose them to greater risk for fractures as suggested by certain studies (15). As obesity is a risk factor for the development of T2D and insulin resistance (16) and the prevalence of T2D increases with increasing body mass index (BMI) (17), it would be difficult to separate the effect of one over the other on bone. On the other hand, it is also possible that the combination of both could result in worse bone disease.
Recent studies suggested the existence of a phenotype variability among individuals with obesity and propose to divide these subjects into 2 main categories: the metabolically healthy obese (MHO) and the metabolically unhealthy obese (MUHO) (18), which includes patients with T2D. MHO are those subjects who, despite being obese, have none of the metabolic syndrome components nor have previous diagnosis of cardiovascular disease (18) and represent 10% to 30% of obese individuals (19). They have greater subcutaneous adipose tissue mass, lower visceral adipose tissue (VAT) mass and adipocytes size, lower grade of inflammation, and greater insulin sensitivity compared to their metabolically unhealthy counterparts (19). All these metabolic dysfunctions, which are present in the MUHO subset, may have an effect on bone, which is different from the one observed in MHO (15,20).
In this study, we evaluated if there is subgroup of obese subjects who could be at even greater risk for bone fragility due to altered bone metabolism, which may result in poorer bone quality. We hypothesize that among obese subjects, the coexistence with T2D would result in worse bone quality. Thus, our objectives are (i) to evaluate the bone turnover markers, bone microarchitecture and bone strength as assessed finite element derived-parameters in subjects who MHO and MUHO without and with T2D and (ii) to evaluate the bone turnover markers, bone microarchitecture, and bone strength as assessed finite element derived-parameters in in the general population of obese subjects with and without T2D.
Materials and Methods
Study design and study population
This is a substudy using the baseline data from a clinical trial in male veteran participants who volunteered to be screened for the study evaluating the effect of aromatase inhibitors and weight loss in obese men with hypogonadism (NCT: 03490513) between May 2018 and October 2019. Inclusion criteria in the primary study include men between 35 and 65 years old with BMI of 35 kg/m2 or more who have an average fasting total T level from 2 measurements taken between 8 to 10 am on 2 separate days within 1 month of less than 300 ng/dL, with luteinizing hormone of less than 9.0 mIU/L, E2 of 14 pg/mL or more and with symptoms consistent with androgen deficiency as assessed by the quantitative Androgen Deficiency in Aging Male questionnaire. Since the primary study includes lifestyle intervention to promote weight loss by dietary behavioral modification and supervised exercise program in addition to either aromatase inhibitors or placebo, those with cardiopulmonary disease [eg, recent myocardial infarction or myocardial infarction defined as myocardial infarction within 6 months at the time of study entry, unstable angina, stroke] or unstable disease (eg, New York Heart Association Class III or IV congestive heart failure), severe pulmonary disease requiring steroid pills, or the use of supplemental oxygen (that would contraindicate exercise or dietary restriction) were excluded from participation. Other exclusion criteria include (i) clinical/biochemical evidence of pituitary or hypothalamic disease; (ii) drugs affecting gonadal hormone levels, production, and action or bone metabolism (bisphosphonates, teriparatide, denosumab, glucocorticoids, phenytoin); (iii) diseases affecting bone metabolism (eg, hyperparathyroidism, untreated hyperthyroidism, osteomalacia, chronic liver disease, significant renal failure, hypercortisolism, malabsorption, immobilization, Paget’s disease); (iv) prostate carcinoma or elevated serum prostate-specific antigen > 4 ng/mL; (v) hematocrit more than 50%; (vi) untreated severe obstructive sleep apnea; (vii) history of deep vein thrombosis or pulmonary embolism; (viii) severe lower urinary tract or prostate symptoms with International Prostate Symptom Score above 19; (ix) excessive alcohol or substance abuse; (x) unstable weight (ie, ±2 kg) in the last 3 months; (xi) condition that could prevent from completing the study; and (xii) a screening BMD T-score of less than −2.0 at the spine, femoral neck, or total femur; history of osteoporosis; or fragility fracture; (xiii) diabetes mellitus with a fasting blood glucose of more than 160 mg/dL and/or hemoglobin A1c (A1C) more than 9.5%.
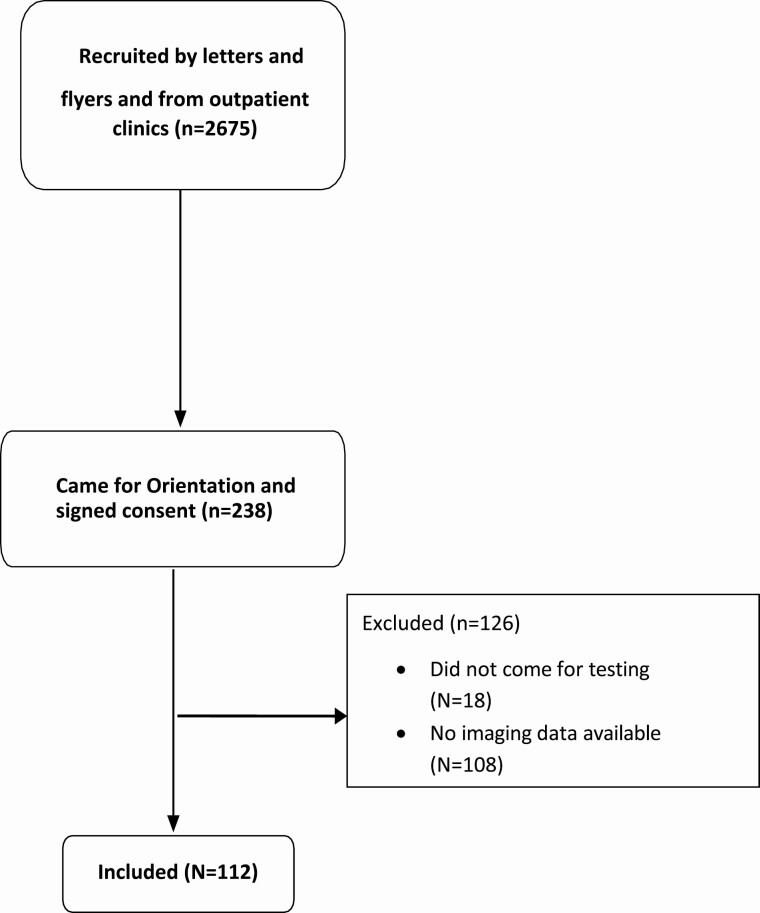
Veterans (n = 2675) were recruited by letters and flyers and direct approach from outpatient clinics; 296 came for orientation while 238 men consented to be screened for the study (Fig. 1). Out of those who were screened, the baseline data of 112 consecutive men who were able to provide the outcomes of interest were included in the analysis. Screening tests included a fasting blood test between 8:00 and 10:00 am for measurement of serum testosterone, estradiol, luteinizing hormone, follicle-stimulating hormone, sex hormone binding globulin (SHBG), hematocrit, lipid profile [which included triglycerides and high-density lipoprotein (HDL)], liver enzymes, fasting glucose, glycated hemoglobin (A1C), prostate-specific antigen, thyroid hormones, parathyroid hormone, cortisol, prolactin, insulin-like growth factor-1, and 25-OH vitamin D. Serum testosterone was measured twice at least 1 day apart. Serum samples were stored at −80°C in our laboratory until analysis. Blood pressure was measured using a standard sphygmomanometer.
Figure 1.
Flow diagram of recruitment.
All participants provided written informed consent in accordance with the guidelines in the Declaration of Helsinki for the ethical treatment of human subjects. The protocol was approved by the Baylor College of Medicine Institutional Review Board and conducted at the Michael E. DeBakey VA Medical Center (Houston, TX, USA).
Type 2 diabetes mellitus
The presence of T2D was ascertained from diagnosis in the chart, the intake of medication for T2D, A1C values ≥6.5% and fasting blood glucose of ≥126 mg/dL. Definition of T2D was made if at least of 1 these rules is present.
Metabolically healthy obese and metabolically unhealthy obese
MHO was established when obese subjects with obesity had none of the metabolic syndrome components and no previous diagnosis of cardiovascular disease as previously described by van Vlet-Ostaptchouk (18). Briefly, 4 clinical measurements were used to identify components of metabolic syndrome in our population of obese subjects based on the Third Report of the National Cholesterol Education Program Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (NCEP ATP III) definition: (i) elevated fasting blood glucose levels ≥110 mg/dL or diagnosis of T2D or use of medication for T2D; (ii) decreased HDL-cholesterol levels ≤40 mg/dL or intake of medications aimed to increase HDL-cholesterol levels; (iii) hypertriglyceridemia defined as ≥150 mg/dL or intake of medication aimed to reduce triglycerides levels; and (iv) elevated blood pressure, defined as systolic blood pressure ≥140 mmHg or diastolic blood pressure ≥90 mmHg or antihypertensive drug treatment (21). Notably, since our population included only subjects affected by obesity, a less strict criteria for the diagnosis of elevated blood pressure was applied as cut-off, as previously done by van Vlet-Ostaptchouk (18).
Body mass index and body composition
Body weight and height were measured by standard weighing scale and stadiometer, respectively. BMI (kg/m2) was calculated by dividing the weight (in kilograms) by height (in meters) squared.
Measurement of body composition was performed by dual-energy X-ray absorptiometry (DXA; Hologic-Discovery; Enhanced Whole Body 11.2 software version; Hologic Inc, Bedford, MA, USA). Images were analyzed according to manufacturer’s instructions. The coefficient of variation (CV) for fat mass and lean mass measurements in our center is 1.5% (22). Visceral adipose tissue volume (g/cm3) was calculated from the DXA body composition scan using APEX software (version 5.5.2; Hologic Inc., Bedford, MA, USA) as previously described (23).
Bone mineral density, geometry, and microarchitecture
Areal BMD (aBMD) of the lumbar spine and proximal femur (which included regions of interest as the total hip and the femoral neck) was measured by DXA. The CVs at our center are ~1.1% for the lumbar spine and ~1.2% for the proximal femur (22).
Volumetric BMD (vBMD), bone microarchitecture, geometry, and mechanical properties were assessed by high-resolution peripheral quantitative computed tomography (HR-pQCT) (Xtreme CTII, Scanco Medical AG, Brüttisellen, Switzerland) at the nondominant distal radius and tibia (if the patient had prior surgeries or experienced previous fractures on the nondominant extremity the contralateral arm/leg was scanned). Assessment procedure and settings are the same previously described. In brief, after performing a scout view, a reference line was placed on the endplate of radius and tibia. The first slice was acquired 9.0 and 22.0 mm proximal to the reference line for the radius and tibia, respectively. If motion artifacts occurred, scans were repeated up to 3 times to improve the image quality. To extract the mineralized bone phase, a low-pass Gaussian filter (sigma 0.8, support 1.0) was used. We applied a fixed threshold to extract the trabecular bone (320 mg HA/cm3) and cortical bone (450 mg HA/cm3). Bone microarchitecture was assessed in the trabecular and cortical regions using voxel-based measurements. Parameters of interest were total area (mm2), total volumetric BMD (mg HA/cm3), trabecular volumetric BMD (Tb.vBMD; mg HA/cm3), trabecular thickness (mm), and trabecular separation (Tb.Sp; mm), cortical BMD (mgHA/cm3), cortical perimeter (mm), cortical thickness (mm), and cortical porosity (unit free). These parameters were read directly from the machine.
Micro-finite element analysis of radius and tibia, represented by failure load (f.load; kN) and stiffness (kN/mm), were performed creating micro finite element models that accurately describe the actual bone microarchitecture in detail, automatically converting each voxel of bone tissue into an equally sized brick element, as previously described (23). Cortical and trabecular bone elements were assigned a Young’s modulus of 20 and 17 GPa, respectively, and a Poisson’s ratio (24). The micro-finite element analysis consists of a compression test simulation in which a load in the longitudinal direction is applied at one end, while the other end is fully constrained, to simulate a fall from standing height on an outstretched hand (25). The f.load was calculated using the criterion developed by Pistoia et al, which was demonstrated to well predict experimental f.load measured by loading cadaver forearms (25). Stiffness is the extent to which an object resists stress deformation resulting from an applied force. The CVs for the different parameters measured by HR-pQCT are as follows: 0.2% to 2.5% for geometry, 0.6% to 1.7% for BMD, 0.7% to 2.4% for trabecular bone compartment parameters, and 1.1% to 1.3% for cortical thickness, while cortical porosity was high at 11.0% to 13.3% (26).
Biochemical measurements
Blood samples were collected in the morning between 8 and 10 am after participants had been fasting for 10 to 12 h. Serum total testosterone and estradiol were measured by liquid chromatography/mass spectroscopy by LabCorp laboratory (Burlington, NC, USA) total testosterone intra-assay CVs are 7.4%, 6.1%, 9.0%, 2.3%, and 0.9% at 0.65, 4.3, 48, 118, and 832 ng/dL, respectively. Inter-assay CVs are 8.9%, 6.9%, 4.0%, 3.6%, and 3.5% at 0.69, 4.3, 45, 117, and 841 ng/dL, respectively. The detection range is 0.5 to 2,000 ng/dL (23). Estradiol assay sensitivity is 0.23 to 405 pg/mL, intra-assay CV is 1.4% to 11.8% and inter-assay CV is 4.8% to 10.8% (27). SHBG was measured with electrochemiluminescence immunoassay by LabCorp laboratory (Burlington, NC, USA);The following were measured by the clinical laboratory at the Michael E. DeBakey VA Medical Center: serum cortisol by UniCel DxI 800 Auto-analyzer (Beckman Coulter, Brena, CA, USA), A1C was measured by high-pressure liquid chromatography using Tosoh Automated Glycohemoglobin Analyzer HLC-723G8. (Tosoh Bioscience, Inc. South San Francisco, CA, USA); CV < 2%, triglycerides by fluorometric assay, and HDL by colorimetric assay by UNICEL DxC (Beckman Coulter, Inc., Brea, CA, USA). Detection limits for these measurements are 5 to 135 mg/dL (0.13-3.5nmol/L) for HDL, 10 to 1000 mg/dL (0.1-11.3 mmol/L) for triglycerides; CVs < 10% for all measurements (23). The following were measured using enzyme-linked immunosorbent assay kits: osteocalcin (Alpco Diagnostics, Salem, NH, USA), CTx (Crosslaps; Immunodiagnostic Systems Inc., Gaithersburg, MD, USA), sclerostin (high sensitivity; Quidel Corporation, San Diego, CA, USA), adiponectin and leptin (EMD Millipore Corporation, St. Louis, MO, USA), and interleukin 6 (IL-6; Quantikine; R&D Systems Inc., Minneapolis, MN, USA); CVs < 10% for all measurements. The free estradiol index was calculated as the molar ratio of total estradiol to total SHBG (pmol/nmol). SHBG was used to calculate free testosterone and free estradiol as previously described by Sowers et al (28).
Statistical analysis
Data are presented as means ± SD in tables, text, and Figure 2 and as means ± SE in Figure 3. Analyses of variance with adjustment for covariates as age and free hormones for bone parameters were performed to compare the effect of obesity without T2D vs obesity with T2D and MHO vs MUHO with or without T2D on multiple variables. Post hoc analyses with multiple-range testing using the Hayter-Fisher method. This requires significance (P ≤ 0.05) on analysis of variance with post-hoc comparison between groups of the 3 groups using Fisher’s least significant difference method; this procedure maintains familywise error rates for n = 3 groups (29,30). Simple correlation analysis was performed to identify the variables that correlated with tibial and radius f.load and stiffness. Multiple regression analysis was performed to identify the best predictors of tibial and radius f.load and stiffness in the group of obese without T2D and obese with T2D. Coefficient of correlation is expressed in percentage. The variables included in the multiple regression analysis were those that have significant or have borderline significant correlation with f.load and stiffness in both tibia and radius. Data were managed using Excel 2013 (Microsoft, Redmond, WA, USA) and analyzed using SAS version 9.3 (SAS Institute, Inc., Cary, NC, USA). A P < 0.05 was considered statistically significant. Standardized beta values and standard errors for standardized beta were computed manually. A P ≤ 0.05 was considered statistically significant. Outliers were <1% and were excluded from the analysis.
Figure 2.
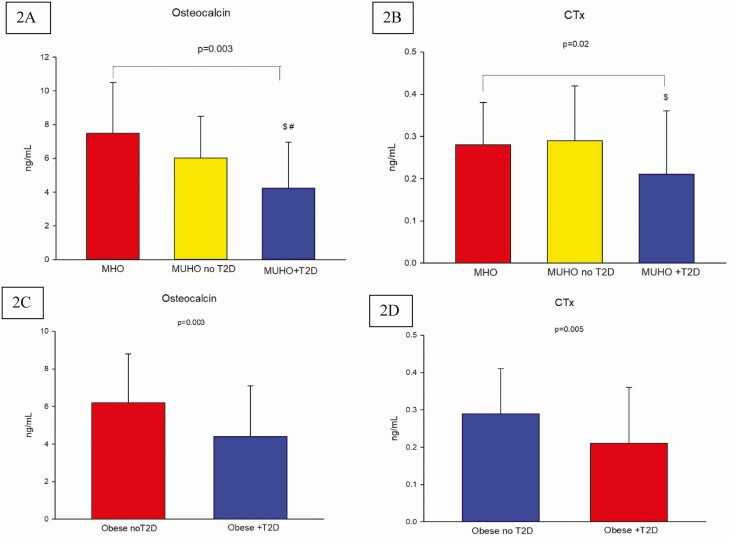
Osteocalcin and C-terminal telopeptide of type 1 collagen (CTx) serum levels in metabolically healthy obese (MHO), metabolically unhealthy obese without T2D (MUHO no T2D) and MUHO with T2D (MUHO + T2D); error bars represent SD. post-hoc analysis for Osteocalcin showed P-value < 0.05 for the comparison between MUHO with T2D vs MHO$ and vs MUHO without T2D (A); post-hoc analysis for CTx showed P-value < 0.05 for the comparison between MUHO with T2D vs MUHO without T2D. $P < 0.05 for comparison of MUHO with T2D to MHO; #P < 0.05 for comparison of MUHO with T2D to MUHO without T2D (B). Comparison of osteocalcin levels between obese subjects without and with T2D showed a P-value 0.003 (C), and CTx levels between obese subjects without and with T2D showed P-value of 0.0005 (D).
Figure 3.
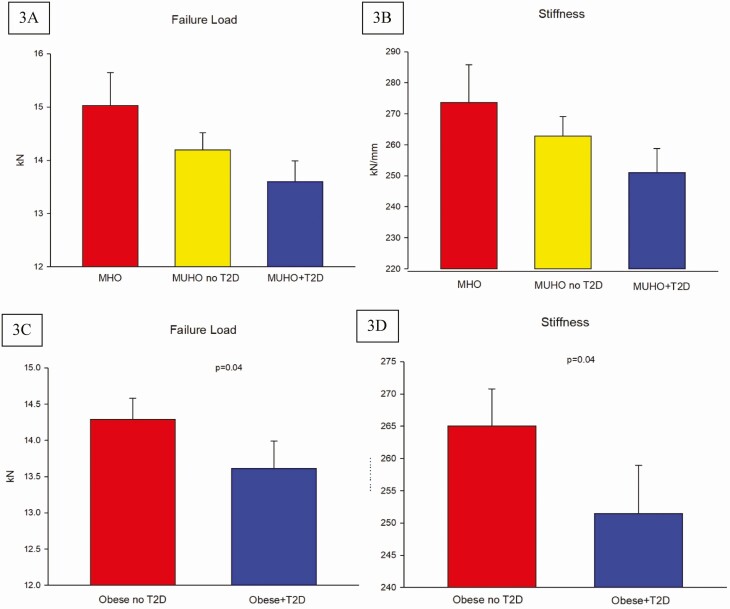
Tibial bone failure load (F.load) (A) and stiffness (B) in metabolically healthy obese (MHO), metabolically unhealthy obese without T2D (MUHO no T2D) and MUHO with T2D (MUHO + T2D); error bars represent SE. Values were adjusted for age (years), free testosterone (nmol), and free estradiol (pmol). P = 0.09 between groups. Considering progression in metabolic condition (3 groups) as linear, trend analyses show statistically significant decrease in F.load and stiffness (both P = 0.03). Tibial bone failure load (F.load) (C) and stiffness (D) in obese without T2D (Obese no T2D) and obese with T2D (Obese + T2D); error bars represent SE. Values are adjusted for age (years), free testosterone (nmol) and free estradiol (pmol).
Results
Out of 238 men screened, the data of 112 men with obesity who were able to provide the outcomes of interest were included in the analysis. Table 1 shows the clinical features of the men who were not included compared to those who were included in the study. There were no differences in characteristics between those not included and those included except for lower testosterone level in the included group, which was part of the inclusion criteria in the parent study. Those with testosterone level close to 300 ng/dL were screened the second time.
Table 1.
Clinical characteristics of the subjects not included and included in the study
| Not included (N = 126) | Included (N = 112) | P-value | |
|---|---|---|---|
| Age (years) | 52.3 ± 8.1 | 50.7 ± 7.9 | 0.10 |
| BMI (kg/m2) | 39.1 ± 5.3 | 37.9 ± 5.9 | 0.08 |
| Total testosterone (ng/dL) | 399.21 ± 105.75 | 318.79 ± 129.59 | 0.0001 |
| Estradiol (pg/mL) | 29.32 ± 9.19 | 28.46 ± 11.35 | 0.65 |
| Calcium (mg/dL) | 9.19 ± 0.34 | 9.24 ± 0.37 | 0.39 |
| Vitamin D (ng/mL) | 24.23 ± 10.06 | 23.75 ± 9.76 | 0.77 |
| Total cholesterol (mg/dL) | 180.25 ± 41.61 | 177.66 ± 40.33 | 0.70 |
| LDL cholesterol (mg/dL) | 112.64 ± 39.24 | 109.59 ± 37.30 | 0.62 |
| Triglycerides (mg/dL) | 148.84 ± 118.19 | 164.46 ± 138.19 | 0.47 |
| HDL cholesterol (mg/dL) | 42.98 ± 15.78 | 40.6 ± 17.15 | 0.38 |
Results are presented as mean ± SD.
Abbreviations: LDL, low-density lipoprotein.
Forty-two obese men had T2D, and 70 men had no T2D. Among our population of obese men, 15 (16%) were MHO and 97 were MUHO. Among those who are MUHO, 56 did not have diabetes. The characteristics of our subjects divided according to the metabolic status and the presence of T2D (ie, MHO, MUHO without T2D, and MUHO with T2D) are shown in Table 2. The men with diabetes were diagnosed with the disease for as short at 3 months to as much as 19 years at study entry. Of the 42 subjects who have diabetes; 7 were on diet and exercise only; 32 were on metformin either alone or in combination with insulin, glucagon-like peptide 1 agonist, dipeptidyl peptidase 4 inhibitors, sulfonylurea, and one in combination with a sodium-glucose co-transporter-2 inhibitor (empagliflozin). There were 15 subjects on insulin; 14 of them in combination with any of the previously discussed oral agents or a glucagon-like peptide 1 agonist.
Table 2.
Baseline characteristics of the participants according to metabolic profile
| MHO | MUHO without T2D | MUHO with T2D | P-value | |
|---|---|---|---|---|
| Age (year) | 47.1 ± 9.3 | 50.1 ± 7.1 | 53.7 ± 7.9 | 0.01 |
| BMI (kg/m2) | 39.4 ± 4.7 | 40.6 ± 5.9 | 39.1 ± 4.1 | 0.37 |
| Hemoglobin A1C (%) | 5.7 ± 0.5 | 5.8 ± 0.4 | 7.8 ± 1.5 | <0.001 |
| Cortisol am (µg/dL) | 8.6 ± 4.3 | 9.0 ± 3.9 | 10.0 ± 4.1 | 0.40 |
| SHBG (nM/L) | 33.8 ± 9.5 | 33.5 ± 13.9 | 27.7 ± 8.8 | 0.05 |
| Testosterone (ng/dL) | 362.5 ± 130.7 | 317.1 ± 115.0 | 284.7 ± 113.4 | 0.08 |
| Free testosterone (nmol) | 37.4 ± 8.8 | 34.7 ± 13.8 | 37.8 ± 15.0 | 0.52 |
| Estradiol (pg/dL) | 30.5 ± 6.9 | 29.4 ± 11.3 | 26.5 ± 12.6 | 0.36 |
| Free estradiol (pmol) | 3.7 ± 1.8 | 3.6 ± 1.9 | 3.8 ± 2.2 | 0.82 |
Results are presented as mean ± standard deviation, P values in bold are significant
Abbreviations: MHO, metabolically healthy obese; MUHO, metabolically unhealthy obese; T2D, type 2 diabetes mellitus.
Subjects who are MUHO with T2D are significantly older than those who are MUHO without T2D and MHO (53.7 ± 7.9 vs 50.1 ± 7.1 and 47.1 ± 9.3 years old respectively, P = 0.01). As expected A1C level in those who are MUHO with T2D is significantly higher compared to the MUHO without T2D and MHO groups (7.8 ± 1.5 vs 5.8 ± 0.4 and 5.7 ± 0.5 %, respectively, P < 0.001). SHBG level is lower in MUHO with T2D compared to the MUHO without T2D and MHO groups (27.7 ± 8.8 vs 33.5 ± 13.9 and 33.8 ± 9.5 nM/L, respectively, P = 0.05). BMI, cortisol, free testosterone, estradiol, and free estradiol were comparable among the 3 groups (all P- values > 0.5). Serum levels of testosterone in our study population were 310.9 ± 117.76 ng/dL and aligned with those levels found in obese population (31).
Body composition and serum adipokines (leptin, adiponectin and IL-6)
Results of body composition and biochemical profile are shown in Table 3. Parameters of body composition were comparable between the 3 groups. There were also no significant differences in serum leptin and IL-6 levels between the groups; however, men in the MUHO without T2D group had significantly higher levels of adiponectin compared to MHO and MUHO with T2D groups (15.2 ±± 7.6 vs 11.2 ± 4.7 and 11.9 ± 5.7 ng/mL, respectively, P = <0.05).
Table 3.
Body composition and adipokines of the participants according to metabolic profile
| MHO | MUHO without T2D | MUHO with T2D | P-value | |
|---|---|---|---|---|
| Fat mass (kg) | 51.8 ± 15.0 | 51.1 ± 12.4 | 49.1 ± 11.5 | 0.91 |
| Lean mass (kg) | 72.2 ± 6.6 | 72.5 ± 7.8 | 72.1 ± 7.1 | 0.78 |
| BMC (kg) | 2.8 ± 0.4 | 2.8 ± 0.4 | 2.8 ± 0.4 | 0.48 |
| FFM (kg) | 75.0 ± 6.8 | 75.3 ± 8.1 | 74.9 ± 7.3 | 0.76 |
| Total mass (kg) | 126.8 ± 17.4 | 126.4 ± 17.9 | 124.0 ± 15.7 | 0.91 |
| Total body fat (%) | 40.3 ± 5.9 | 39.9 ± 4.9 | 39.2 ± 4.8 | 0.79 |
| VAT volume (cm3) | 1054.6 ± 394.1 | 1316.0 ± 416.1 | 1349.5 ± 347.4 | 0.21 |
| Leptin (ng/mL) | 38.8 ± 17.1 | 44.4 ± 22.4 | 37.4 ± 20.8 | 0.33 |
| Adiponectin (ng/mL) | 11.2 ± 4.7 | 15.2 ± 7.6 | 11.9 ± 5.7 | <0.05 |
| IL-6 (pg/mL) | 4.3 ± 2.2 | 4.4 ± 2.1 | 3.6 ± 1.3 | 0.25 |
Results are presented as mean ± standard deviation. P values for body composition parameters are adjusted for age, free testosterone and free estradiol; P values for Leptin, Adiponectin, and IL-6 (Interleukin-6) are unadjusted. P-values in bold are significant.
Abbreviations: BMC, bone mineral content; FFM, fat free mass; IL-6, interleukin 6; MHO, metabolically healthy obese; MUHO, metabolically unhealthy obese; T2D, type 2 diabetes mellitus; VAT, visceral adipose tissue.
BMD and bone turnover markers
aBMD by DXA at all sites and serum levels of bone turnover markers are presented in Table 4. Values of aBMD at all sites were comparable among the groups. However, compared to both MHO and MUHO without T2D, osteocalcin level was significantly lower in the MUHO with T2D group (7.49 ± 3.0 and 6.03 ± 2.47 vs 4.24 ± 2.72 ng/mL, respectively, P = 0.003) (Fig. 2A). Similarly, compared to MHO and MUHO without T2D, MUHO with T2D had lower CTx level (0.28 ± 0.10 and 0.29 ± 0.13 ng/mL vs 0.21 ± 0.15, respectively, P = 0.02). However, post-hoc analysis for CTx showed that between-group difference was only significant between MUHO with T2D vs MUHO without T2D (P < 0.05) but not with MHO (Fig. 2B). Sclerostin levels were not significantly different among the groups.
Table 4.
Bone mineral density and bone turnover markers of the participants according to metabolic profile
| MHO | MUHO without T2D | MUHO with T2D | P-value | |
|---|---|---|---|---|
| Lumbar spine aBMD (g/cm2) | 1.10±/0.09 | 1.12 ± 0.16 | 1.13 ± 0.15 | 0.54 |
| Total hip aBMD (g/cm2) | 1.15 ± 0.11 | 1.13 ± 0.13 | 1.12 ± 0.11 | 0.79 |
| Femoral neck aBMD (g/cm2) | 1.03 ± 0.15 | 0.97 ± 0.14 | 0.95 ± 0.13 | 0.54 |
| Osteocalcin (ng/mL) | 7.49 ± 3.0 | 6.03 ± 2.47 | 4.24 ± 2.72 | 0.003 |
| CTx (ng/mL) | 0.28 ± 0.10 | 0.29 ± 0.13 | 0.21 ± 0.15 | 0.02 |
| Sclerostin (ng/mL) | 0.59 ± 0.21 | 0.65 ± 0.188 | 0.65 ± 0.18 | 0.66 |
Results are presented as mean ± SD. P-values for aBMD of spine, total hip, and femoral neck are adjusted for age, free testosterone ,and free estradiol. P values for sclerostin, CTx, and osteocalcin are unadjusted. P-values in bold are significant.
Abbreviations: CTx, C-terminal telopeptide of type I collagen; MHO, metabolically healthy obese; MUHO, metabolically unhealthy obese, T2D, type 1 diabetes mellitus.
Bone microarchitecture and bone strength
Parameters of bone microarchitecture and strength are shown in Table 5. None of these parameters were significantly different among the groups at the tibia. Although Tb.Sp. at the tibia appears to be greater in the MUHO with T2D group compared to the other 2 groups, the difference was not significant (P = 0.07). There was a stepladder decrease in f.load and stiffness at the tibia depending on the degree of metabolic derangement. Both f.load and stiffness were lowest in men with MUHO and T2D, highest in the MHO group and intermediate between the groups in MUHO without T2D; but the differences among the groups were not statistically significant (P = 0.09 for both f.load and stiffness) (Fig. 3). Meanwhile, parameters of bone microarchitecture and bone strength at the radius were comparable among the groups (Table 5).
Table 5.
Tibial bone microarchitecture, failure load, and stiffness of the participants according to metabolic profile
| MHO | MUHO without T2D | MUHO with T2D | P-value | |
|---|---|---|---|---|
| Tibia | ||||
| Total area (mm2) | 911.9 ± 119.4 | 865.7 ± 175.2 | 896.7 ± 141.2 | 0.44 |
| Total vBMD (mg HA/cm3) | 336.0 ± 43.1 | 343.2/ 53.8 | 321.3 ± 49.5 | 0.26 |
| Tb. vBMD (mg HA/cm3) | 193.0 ± 37.4 | 191.8 ± 47.8 | 175.8 ± 33.3 | 0.17 |
| Tb. Th (mm) | 0.27 ± 0.02 | 0.26 ± 0.02 | 0.26 ± 0.02 | 0.49 |
| Tb. Sp (mm) | 0.63 ± 0.09 | 0.61 ± 0.11 | 0.66 ± 0.10 | 0.07 |
| Ct. vBMD (mg HA/cm3) | 904.8 ± 40.0 | 887.7 ± 106.2 | 888.3 ± 51.3 | 0.87 |
| Ct. Pm (mm) | 118.2 ± 8.1 | 115.0 ± 12.2 | 118.1 ± 10.4 | 0.52 |
| Ct. Th(mm) | 1.8 ± 0.2 | 1.9 ± 0.4 | 1.9 ± 0.4 | 0.43 |
| Ct. Po | 0.02 ± 0.01 | 0.03 ± 0.01 | 0.03 ± 0.02 | 0.99 |
| F.load (kN) | 15.0 ± 3.1 | 14.2 ± 2.0 | 13.6 ± 2.3 | 0.09 |
| Stiffness (kN/mm | 273.6 ± 62.3 | 262.8 ± 39.5 | 251.1 ± 44.5 | 0.09 |
| Radius | ||||
| Total area (mm2) | 395.0 ± 59.3 | 406.9 ± 77.6 | 400.2 ± 68.1 | 0.27 |
| Total vBMD (mg HA/cm3) | 324.9 ± 46.2 | 326.8 ± 61.2 | 314.0 ± 49.3 | 0.59 |
| Tb. vBMD (mg HA/cm3) | 191.5 ± 39.3 | 184.5 ± 36.7 | 174.4 ± 32.9 | 0.21 |
| Tb. Th (mm) | 0.25 ± 0.02 | 0.24 ± 0.02 | 0.24 ± 0.02 | 0.53 |
| Tb. Sp(mm) | 0.58 ± 0.07 | 0.59 ± 0.09 | 0.62 ± 0.10 | 0.13 |
| Ct. vBMD (mg HA/cm3) | 866.2 ± 24.8 | 874.4 ± 52.7 | 871.6 ± 54.1 | 0.50 |
| Ct. Pm(mm) | 85.9 ± 6.9 | 86.4 ± 8.7 | 86.4 ± 8.4 | 0.55 |
| Ct. Th (mm) | 1.10 ± 0.14 | 1.16 ± 0.21 | 1.11 ± 0.17 | 0.25 |
| Ct. Po | 0.01 ± 0.004 | 0.01 ± 0.005 | 0.01 ± 0.005 | 0.22 |
| F.load (kN) | 5.2 ± 1.3 | 5.2 ± 1.2 | 5.0 ± 1.1 | 0.27 |
| Stiffness (kN/mm) | 96.8 ± 23.4 | 96.8 ± 22.8 | 91.8 ± 20.1 | 0.23 |
Results are presented as mean ± SD. All P-values are adjusted for age, free testosterone (nmol) and free estradiol (pmol). Bold P-values are significant.
Abbreviations: Ct., cortical; f.load, failure load; MHO, metabolically healthy obese; MUHO, metabolically unhealthy obese; Pm, perimeter; Po, porosity; Sp, spacing; T2D, type 2 diabetes mellitus; Tb., trabecular; Th, thickness; vBMD, volumetric bone mineral density.
Effect of T2D on the bone of obese men
To further evaluate the impact of T2D alone on the bone of obese men irrespective of co-existing metabolic disorders other than diabetes, we simply divided our subjects into 2 groups, ie, those without and with T2D. There were no significant differences noted in the aBMD at all sites between those without and with T2D as shown in Table 6. However, osteocalcin level was significantly lower in the group with T2D compared to those without T2D (4.4 ± 2.7 and 6.2 ± 2.6ng/mL respectively, P = 0.003, Fig. 2C). Similarly, CTx levels were lower in those with T2D compared to those without T2D (0.21 ± 0.15 and 0.29 ± 0.12ng/mL respectively, P = 0.005, Fig. 2D). Sclerostin levels were similar between the two groups.
Table 6.
Bone mineral density and bone turnover markers of the participants according to the presence of type 2 diabetes mellitus
| No type 2 diabetes mellitus (n = 71) | Type 2 diabetes mellitus (n = 42) | P-value | |
|---|---|---|---|
| Lumbar spine aBMD (g/cm2) | 1.12 ± 0.15 | 1.13 ± 0.15 | 0.27 |
| Total hip aBMD (g/cm2) | 1.13 ± 0.13 | 1.12 ± 0.11 | 0.51 |
| Femoral neck aBMD (g/cm2) | 0.98 ± 0.14 | 0.95 ± 0.13 | 0.44 |
| Osteocalcin (ng/mL) | 6.2 ± 2.6 | 4.4 ± 2.7 | 0.003 |
| CTx (ng/mL) | 0.29 ± 0.12 | 0.21 ± 0.15 | 0.005 |
| Sclerostin (ng/mL) | 0.64 ± 0.19 | 0.65 ± 0.17 | 0.76 |
Analysis of covariance. Results are presented as mean ± SD. P-values for bone mineral density are adjusted for age, free testosterone (nmol), and free estradiol (pmol); P values for sclerostin, CTx, and osteocalcin are unadjusted. Bold P-values are significant.
Abbreviations: aBMD, areal bone mineral density; CTx: C-terminal telopeptide of type I collagen; MHO, metabolically healthy obese; MUHO, metabolically unhealthy obese.
Analyses of parameters of bone microarchitecture and strength between those without and with T2D as shown in Table 7 showed significantly greater Tb.Sp at both tibia and radius in men with T2D (P = 0.03 and P = 0.04, respectively). At the tibia, f.load and stiffness were significantly lower in men with T2D compared to those without T2D (P = 0.04 for both) (Table 7, Fig. 3C and D). Although tibial Tb.vBMD and radius Tb.vBMD are lower in men with T2D compared to those without T2D (P = 0.06 and P = 0.08, respectively), the difference did not reach statistical significance. Moreover, the stiffness is lower at the radius in the group of obese with T2D compared to nondiabetic obese, but the difference was not statistically significant (P = 0.09).
Table 7.
Bone microarchitecture, failure load, and stiffness of the participants according to the presence of type 2 diabetes mellitus
| No type 2 diabetes mellitus (n = 71) | Type 2 diabetes mellitus (n = 42) | P-value | |
|---|---|---|---|
| Tibia | |||
| Total area (mm2) | 875.9 ± 164.8 | 8 897.5 ± 139.3 | 0.67 |
| Total vBMD (mg HA/cm3) | 341.7 ± 51.4 | 321.8 ± 48.9 | 0.16 |
| Tb. vBMD (mg HA/cm3) | 192.1 ± 45. | 4 176.8 ± 33.4 | 0.06 |
| Tb. Th (mm) | 0.26 ± 0.02 | 0.26 ± 0.02 | 0.83 |
| Tb. Sp (mm) | 0.62 ± 0.10 | 0.65 ± 0.10 | 0.03 |
| Ct. vBMD (mg HA/cm3) | 890.7 ± 48.5 | 900.3 ± 51.9 | 0.75 |
| Ct. Pm (mm) | 115.7 ± 11.4 | 118.1 ± 10.2 | 0.96 |
| Ct. Th (mm) | 1.92 ± 0.3 | 1.87 ± 0.4 | 0.93 |
| Ct. Po | 0.03 ± 0.01 | 0.03 ± 0.02 | 0.90 |
| F.load (kN) | 14.29 ± 2.25 | 13.61 ± 2.26 | 0.04 |
| Stiffness (kN/mm) | 265.0 ± 44.73 | 251.43 ± 43.93 | 0.04 |
| Radius | |||
| Total area (mm2) | 404.4 ± 73.9 | 400.1 ± 67.2 | 0.10 |
| Total vBMD (mg HA/cm3) | 326.4 ± 58.1 | 315.6 ± 49.7 | 0.43 |
| Tb. vBMD (mg HA/cm3) | 185.9 ± 37.0 | 175.8 ± 33.7 | 0.08 |
| Tb. Th (mm) | 0.24 ± 0.02 | 0.24 ± 0.02 | 0.27 |
| Tb. Sp (mm) | 0.58 ± 0.08 | 0.61 ± 0.10 | 0.04 |
| Ct. vBMD (mg HA/cm3) | 872.7 ± 48.2 | 872.2 ± 53.6 | 0.65 |
| Ct. Pm (mm) | 86.5 ± 8.1 | 86.1 ± 8.8 | 0.28 |
| Ct. Th (mm) | 1.15 ± 0.2 | 1.11 ± 0.2 | 0.34 |
| Ct. Po | 0.01 ± 0.005 | 0.01 ± 0.005 | 0.13 |
| F.load (kN) | 5.24 ± 1.22 | 4.98 ± 1.10 | 0.10 |
| Stiffness (kN/mm) | 96.72 ± 22.74 | 91.80 ± 20.06 | 0.09 |
Analysis of covariance. Results are presented as mean ± SD. P-value adjusted for age, free testosterone (nmol) and free estradiol (pmol).
Abbreviations: Ct., cortical; f.load, failure load; MHO, metabolically healthy obese; MUHO, metabolically unhealthy obese; Pm, perimeter; Po, porosity; Sp, spacing; Th, thickness; Tb., trabecular; vBMD, volumetric bone mineral density.
Predictors of bone strength
Simple correlation analysis was performed to identify variables that correlate with bone strength (ie, f.load and stiffness) in the entire population of obese men regardless of the presence of T2D and their metabolic phenotype. As shown in Table 8, lean mass, fat free mass (FFM), aBMD at all sites, free testosterone, and sclerostin were found to be positively associated with both tibial f.load and stiffness. On the contrary, percentage total body fat and cortisol negatively correlated with the f.load, while percentage total body fat, VAT, and cortisol negatively correlated negatively stiffness at the tibia.
Table 8.
Simple correlation analysis between the different variables with failure load and stiffness at the tibia
| Tibia FAILURE LOAD (kN) | Tibia stiffness (kN/mm) | |||
|---|---|---|---|---|
| r | P-value | r | P-value | |
| Age (year) | −0.13 | 0.21 | −0.14 | 0.17 |
| BMI (kg/m2) | 0.06 | 0.60 | 0.04 | 0.69 |
| Fat mass (Kg) | −0.06 | 0.58 | −0.07 | 0.51 |
| Total mass (Kg) | 0.16 | 0.13 | 0.14 | 0.17 |
| Total body fat (%) | −0.28 | 0.006 | −0.28 | 0.006 |
| VAT volume (cm3) | −0.19 | 0.07 | −0.20 | 0.05 |
| Lean mass (Kg) | 0.44 | <0.001 | 0.41 | <0.001 |
| FFM (Kg) | 0.46 | <0.001 | 0.43 | <0.001 |
| Lumbar spine aBMD (g/cm2) | 0.55 | <0.001 | 0.54 | <0.001 |
| Femoral neck aBMD (g/cm2) | 0.49 | <0.001 | 0.49 | <0.001 |
| Total hip aBMD (g/cm2) | 0.53 | <0.001 | 0.53 | <0.001 |
| Hemoglobin A1C (%) | −0.15 | 0.13 | −0.16 | 0.11 |
| Cortisol (µg/dL) | −0.30 | 0.003 | −0.29 | 0.004 |
| Osteocalcin (ng/mL) | −0.05 | 0.65 | −0.04 | 0.73 |
| CTx (ng/mL) | −0.16 | 0.13 | −0.15 | 0.15 |
| Sclerostin (ng/mL) | 0.28 | 0.008 | 0.28 | 0.01 |
| Leptin (ng/mL) | −0.05 | 0.62 | −0.05 | 0.63 |
| Adiponectin (ng/mL) | −0.11 | 0.31 | −0.11 | 0.30 |
| IL-6 (pg/mL) | −0.11 | 0.37 | −0.12 | 0.33 |
| Free testosterone (nmol) | 0.30 | 0.003 | 0.30 | 0.003 |
| Free estradiol (pmol) | 0.18 | 0.07 | 0.20 | 0.06 |
Underlined P-values are borderline significance; bold P-values are significant.
Abbreviations: aBMD, areal bone mineral density; BMI, body mass index; CTx, C-terminal telopeptide of type I collagen; FFM, fat free mass; IL-6, interleukin 6; VAT, visceral adipose tissue.
Multiple regression analysis was performed to identify the best independent predictors for tibial f.load and stiffness for the 2 group of obese without T2D and obese with T2D (Table 9). Free testosterone, FFM, femoral neck aBMD, and lumbar spine aBMD were found to be independent predictors of tibial f.load (R2 = 51.5, P < 0.001), while free testosterone, femoral neck aBMD, and lumbar spine aBMD were found to be independent predictors of stiffness at the tibia in the group of obese without T2D (R2 = 50.6, P < 0.001). Analysis in the group of obese with T2D showed that percentage total body fat, VAT volume, lumbar spine aBMD, and total hip aBMD were found to be the independent predictors for tibial f.load (R2 = 90.8, P < 0.001). Meanwhile, percentage total body fat, VAT volume, total hip aBMD, and free estradiol were found to be independent predictors for bone stiffness at the tibia in the same group (R2 = 92.0, P < 0.001).
Table 9.
Multiple regression analysis for predictors of tibial failure load and stiffness
| Parameters | R 2 | STB | SE | P | P (model) |
|---|---|---|---|---|---|
| Obese men without type 2 diabetes mellitus | |||||
| F. load (kN) | <0.001 | ||||
| Free T (nmol) | 51.5 | 0.44 | 0.11 | <0.001 | |
| Fat free mass (Kg) | 0.20 | 0.10 | <0.04 | ||
| Femoral neck BMD (g/cm2) | 0.18 | 0.09 | <0.05 | ||
| Lumbar spine BMD (g/cm2) | 0.43 | 0.11 | <0.001 | ||
| Stiffness (kN/mm) | <0.001 | ||||
| Free T (nmol) | 50.6 | 0.36 | 0.11 | 0.003 | |
| Femoral neck BMD (g/cm2) | 0.21 | 0.09 | 0.03 | ||
| Lumbar spine BMD (g/cm2) | 0.45 | 0.11 | <0.001 | ||
| Obese men with type 2 diabetes mellitus | |||||
| F. load (kN) | <0.001 | ||||
| Total body fat (%) | 90.8 | −0.65 | 0.10 | <0.001 | |
| VAT volume (cm3) | 0.37 | 0.09 | <0.001 | ||
| Total hip aBMD (g/cm2) | 0.51 | 0.08 | <0.001 | ||
| Lumbar spine BMD (g/cm2) | 0.23 | 0.09 | 0.02 | ||
| Stiffness (kN/mm) | <0.001 | ||||
| Total body fat (%) | 92.0 | −0.84 | 0.08 | <0.001 | |
| VAT volume (cm3) | 0.49 | 0.08 | <0.001 | ||
| Total hip aBMD (g/cm2) | 0.51 | 0.09 | <0.001 | ||
| Free estradiol (pmol) | 0.19 | 0.08 | 0.005 |
Values excluded in the analysis of variance were excluded in the multiple regression analysis. R2 is expressed in percentage values; STB is unitless and expresses the change in outcome SD per unit change in predictor SD. All significant variables from simple correlation analysis were included as possible predictors. for the step-wise multiple regression analysis, borderline significance values were included.
Abbreviations: aBMD, areal bone mineral density; F.load, failure load; STB, standardized beta; VAT, visceral adipose tissue.
Variables that correlated with f.load and stiffness at the radius are presented in the supplemental material (Table S1) (32). Briefly, lean mass, FFM, aBMD at all sites, sclerostin, and free testosterone positively correlated with both f.load and stiffness, whereas total body mass, percentage total body fat, and CTx negatively correlated with f.load and stiffness at the radius. Independent predictors of f.load and stiffness at the radius from multiple regression analysis are presented in Table S2 (32). Percentage total body fat and aBMD at the hip were the 2 independent predictors of radial f.load and bone stiffness in the group of obese subjects without T2D. Differently, in the group of obese subjects suffering from T2D, lumbar spine aBMD was the best independent predictor for f.load, while lumbar spine aBMD and sclerostin (ng/mL) were the independent predictors of bone stiffness.
Discussion
Our study demonstrates that MUHO with T2D have suppressed bone turnover (as shown by lower osteocalcin and CTx levels) compared to those who are MHO and MUHO without T2D. There appears to be a progressive reduction in osteocalcin levels with progression of metabolic disorders, worse for those who have T2D. Similar to our observation in osteocalcin levels, there was also a progressive reduction in both f.load and stiffness with progression in metabolic conditions and lowest in those with T2D, although between-group differences were not statistically significant. Further analyses to examine the effect of T2D alone exclusive of other co-existing metabolic disorders showed that the presence of T2D in the general population of obese men is associated with suppression of bone turnover, poorer microarchitecture, and reduced bone strength suggesting the active role of diabetes in altering bone phenotype in obese men. To our knowledge, this is the first study comparing the bone microarchitecture and biomechanical properties in obese men without and with metabolic impairment and obese men without and with T2D, results of which further illustrate the detrimental effect of the derangement in glucose homeostasis on bone quality over and above that of obesity.
Several studies demonstrated the existence of subgroups of subjects with different cardiometabolic risk within the population of people with obesity (33,34). Likewise, there also appears to be a subset of obese subjects who are prone to fractures compared to others perhaps accounting for the inconsistent relationship between fractures and obesity with some showing an increase in risk while others showing none (5-7). It is possible that the differences in findings among these studies is due to differences in the demographics or underlying conditions inherent in the population investigation. Colleluori et al demonstrated that hypogonadal men with T2D have lower serum bone turnover markers compared to hypogonadal men without diabetes (27). In the same study, hypogonadal men with T2D showed higher aBMD at the total hip and higher total volumetric density but smaller bone size at the 38% tibia (evaluated by HR-pQCT), yet suggesting a possible effect of diabetes on bone phenotype. The impact of T2D on bone is again demonstrated by our findings in this study among men with obesity. The lower bone turnover in obese men with T2D compared to other obese men even those who are metabolically unhealthy but without T2D highlights the detrimental effect of T2D on bone metabolism. Moreover, levels of osteocalcin, a marker of bone formation, appears to correlate with the presence of co-existing metabolic abnormality and lowest if T2D is present. Although others report lower bone turnover in obese compared to nonobese individuals (35), the additional impact of T2D on bone turnover in the obese population is not well-defined. Results from this study suggest that T2D may account at least in part for the reduced bone turnover in obese subjects observed in prior studies. Reduced bone remodeling results in inability of the bone to repair micro damages (36), which when propagated lead to bone fracture (37). In diabetics, the increasing duration of diabetes lead to accumulation of advanced glycation end products in the collagen matrix of bone (8), resulting in impaired bone material strength, reduced ability to deform, and brittleness of bones (38). Also, hyperglycemia, which has been shown as toxic to the osteoblasts (39), can lead to reduced bone formation. Moreover, long-term diabetics suffer from visual and neuropathic complications, which can lead to increased risk for falls and fractures (8).
In agreement with the results from previous studies (40,41), we observed lower Tb.vBMD and higher Tb.Sp in the diabetic group, both at the tibia and the radius suggesting reduced trabecular bone mass and impaired trabecular microarchitecture in these patients. Similar to findings by previous investigators, we did not observe any differences in cortical bone microarchitecture (42). Nevertheless, men with T2D had lower f.load and bone stiffness at the tibia suggesting poor bone quality, which likely contributes to the increased limb fractures in these subjects (1,43). These findings are concordant with the findings by Samelson et al in the Framingham HR-pQCT study who demonstrated that subjects with T2D had nonsignificantly lower finite element analysis–estimated f.load at the tibia compared to nondiabetics (42). On the other hand, our patients had additional potential reason for poor bone quality (ie, obesity), which if also associated with altered bone metabolism could theoretically result in an even greater risk for skeletal fragility.
The interplay between high BMI and bone is complex and controversial. Although mechanical stimulation on the skeleton in obese subjects may promote bone formation to accommodate the greater load thus yielding higher BMD (44,45), obesity is also associated with metabolic alterations, which may in turn be harmful for the bone. Indeed, obesity is associated with increased levels of proinflammatory cytokines including tumor necrosis factor alpha, interleukin-1, and IL-6, which are proven to have detrimental effects on the bone (46). Moreover, obesity is traditionally associated with increased levels of leptin and decreased levels of adiponectin (47), 2 adipocyte-derived cytokines that exert different effects on bone metabolism. Overproduction of leptin has been associated with reduced bone mass in a mouse model (48), whereas adiponectin has been found to inhibit osteoclastogenesis, reduce bone resorption, and increase bone mass in mice (49). Although we did not observe any significant difference in IL-6, adiponectin, and leptin levels in the 3 metabolic groups, it is possible that the circulating levels of these adipocytokines do not reflect local concentrations in the bone microenvironment. Thus, obesity by itself could have harmful effects on the skeleton, but the concomitant presence of T2D may make the bones even weaker and may explain why in our group of obese patients, those with T2D have poorer bone strength than those without T2D.
Although obese subjects have a greater amount of total body fat than lean individuals, it is the distribution of the adipose tissue in the body that determines the metabolic alterations found in obesity (50). A predominant truncal fat distribution with fat accumulation in critical organs like the liver and muscle, and adipocytes size are all factors that concur to the development of metabolic abnormalities present in obese individuals with insulin resistance and T2D (19). In our study, we did not find significant differences in VAT volume in the different metabolic group classifications or according to the presence of T2D, but we demonstrated that adiposity measures such VAT volume and percentage of body fat, negatively correlated with both f.load and stiffness at the tibia in obese diabetics and are among the predictors of these bone strength parameters. The relationship between bone density and adiposity has been investigated in many studies that showed that increased adiposity is negatively associated with BMD in children and adolescents (51) as well as in obese adults (52). However, to our knowledge, no study has evaluated the effect of adiposity on bone strength in patients who have both T2D and obesity.
Despite the significantly lower levels of osteocalcin in MUHO with T2D, we did not observe significant differences in bone microarchitectural features in these subjects compared to MHO and MUHO without T2D. Although bone strength appears to be lowest in MUHO with T2D compared to the other groups, the difference was not significant. This lack of statistical significance is likely from the low sample size. On the other hand, grouping our subjects into those with and without T2D clearly showed the impact of T2D on bone. The graded reduction in osteocalcin and bone strength parameters (f.load and stiffness) at the tibia according to the presence of metabolic abnormalities with the greatest decrease in those with T2D suggest the worse impact of T2D among the metabolic factors present. However, MUHO with T2D subjects were significantly older than the other 2 groups. It is possible that MHO and MUHO without T2D may only reflect transient metabolic states that will deteriorate with aging and persistent obesity.
Our study has limitations and include the lack of a nonobese control group, the relatively low sample size and the cross-sectional design, which does not allow us to establish a cause-effect relation between the outcomes investigated. In particular, the low sample size in the MHO group limits the strength of our conclusion on the effect of T2D on the bone health of obese men. However, even 15 subjects is able to determine the mean bone turnover of both osteocalcin and CTx with an accuracy (SE) of ±0.77 ng/mL and ±0.026 ng/mL, respectively. In addition, since our study population only consists of men, our findings cannot be applied to the population of obese women with certainty.
To conclude, the combination of T2D and obesity in men is associated with a reduction in bone turnover and poorer bone quality compared to those with obesity but without T2D. It is possible that, in the population of obese subjects, a healthy bone metabolism is maintained if the development of T2D is prevented, a concept that deserves further investigation.
Acknowledgments
We thank the participants for their cooperation and Marco Marcelli, MD, and Vittoria Russo, MS, for their help in the collection of data. The contents of this manuscript do not represent the views of the US Department of Veterans Affairs or the United States Government.
Financial Support : The findings reported in this article are the result of work supported by funding from the National institute of Health (5R01HD093047-03) and the use of facilities at the Michael E. DeBakey VA Medical Center and at the Center for Translational Research on Inflammatory Diseases (CTRID).
Clinical Trial Registration: www.ClinicalTrials.gov; Identifier: NCT03490513.
Additional Information
Disclosure: The authors have nothing to disclose.
Data Availability
The data sets generated during and/or analyzed during the current study are not publicly available but are available from the corresponding author on reasonable request.
References
- 1. de Liefde II, van der Klift M, de Laet CEDH, van Daele PLA, Hofman A, Pols HAP. Bone mineral density and fracture risk in type-2 diabetes mellitus: the Rotterdam Study. Osteoporos Int. 2005;16(12):1713-1720. [DOI] [PubMed] [Google Scholar]
- 2. Schwartz AV, Vittinghoff E, Bauer DC, et al. ; Study of Osteoporotic Fractures (SOF) Research Group; Osteoporotic Fractures in Men (MrOS) Research Group; Health, Aging, and Body Composition (Health ABC) Research Group . Association of BMD and FRAX score with risk of fracture in older adults with type 2 diabetes. JAMA. 2011;305(21):2184-2192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Jiajue R, Qi X, Jiang Y, et al. Incident fracture risk in type 2 diabetic postmenopausal women in mainland China: Peking Vertebral Fracture study. Calcif Tissue Int. 2019;105(5):466-475. [DOI] [PubMed] [Google Scholar]
- 4. Gnudi S, Sitta E, Lisi L. Relationship of body mass index with main limb fragility fractures in postmenopausal women. J Bone Miner Metab. 2009;27(4):479-484. [DOI] [PubMed] [Google Scholar]
- 5. Johansson H, Kanis JA, Odén A, et al. A meta-analysis of the association of fracture risk and body mass index in women. J Bone Miner Res. 2014;29(1):223-233. [DOI] [PubMed] [Google Scholar]
- 6. Fassio A, Idolazzi L, Rossini M, et al. The obesity paradox and osteoporosis. Eat Weight Disord. 2018;23(3):293-302. [DOI] [PubMed] [Google Scholar]
- 7. Tang X, Liu G, Kang J, et al. Obesity and risk of hip fracture in adults: a meta-analysis of prospective cohort studies. PloS One. 2013;8(4):e55077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Napoli N, Chandran M, Pierroz DD, Abrahamsen B, Schwartz AV, Ferrari SL; IOF Bone and Diabetes Working Group . Mechanisms of diabetes mellitus-induced bone fragility. Nat Rev Endocrinol. 2017;13:208-219. [DOI] [PubMed] [Google Scholar]
- 9. Napoli N, Strotmeyer ES, Ensrud KE, et al. Fracture risk in diabetic elderly men: the MrOS study. Diabetologia. 2014;57(10):2057-2065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. De Laet C, Kanis JA, Odén A, et al. Body mass index as a predictor of fracture risk: a meta-analysis. Osteoporos Int. 2005;16(11):1330-1338. [DOI] [PubMed] [Google Scholar]
- 11. Schneider G, Kirschner MA, Berkowitz R, Ertel NH. Increased estrogen production in obese men. J Clin Endocrinol Metab. 1979;48(4):633-638. [DOI] [PubMed] [Google Scholar]
- 12. Falahati-Nini A, Riggs BL, Atkinson EJ, O’Fallon WM, Eastell R, Khosla S. Relative contributions of testosterone and estrogen in regulating bone resorption and formation in normal elderly men. J Clin Invest. 2000;106(12):1553-1560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Napoli N, Faccio R, Shrestha V, Bucchieri S, Rini GB, Armamento-Villareal R. Estrogen metabolism modulates bone density in men. Calcif Tissue Int. 2007;80(4):227-232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Gonnelli S, Caffarelli C, Nuti R. Obesity and fracture risk. Clin Cases Miner Bone Metab. 2014;11(1):9-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Loureiro LM, Lessa S, Mendes R, Pereira S, Saboya CJ, Ramalho A. Does the metabolically healthy obese phenotype protect adults with class III obesity from biochemical alterations related to bone metabolism? Nutrients 2019;11(9):2125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Al-Goblan AS, Al-Alfi MA, Khan MZ. Mechanism linking diabetes mellitus and obesity. Diabetes Metab Syndr Obes. 2014;7:587-591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Bays HE, Chapman RH, Grandy S; SHIELD Investigators’ Group . The relationship of body mass index to diabetes mellitus, hypertension and dyslipidaemia: comparison of data from two national surveys. Int J Clin Pract. 2007;61(5):737-747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. van Vliet-Ostaptchouk JV, Nuotio ML, Slagter SN, et al. The prevalence of metabolic syndrome and metabolically healthy obesity in Europe: a collaborative analysis of ten large cohort studies. BMC Endocr Disord. 2014;14:9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Goossens GH. The metabolic phenotype in obesity: fat mass, body fat distribution, and adipose tissue function. Obes Facts. 2017;10(3):207-215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Mirzababaei A, Mirzaei K, Khorrami-Nezhad L, Maghbooli Z, Keshavarz SA. Metabolically healthy/unhealthy components may modify bone mineral density in obese people. Arch Osteoporos. 2017;12(1):95. [DOI] [PubMed] [Google Scholar]
- 21. Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults. Treatment of high blood cholesterol in, executive summary of the third report of the National Cholesterol Education Program (NCEP) expert panel on detection, evaluation, and treatment of high blood cholesterol in adults (adult treatment panel III). JAMA 2001;285(19):2486-2497. [DOI] [PubMed] [Google Scholar]
- 22. Aguirre LE, Colleluori G, Fowler KE, et al. High aromatase activity in hypogonadal men is associated with higher spine bone mineral density, increased truncal fat and reduced lean mass. Eur J Endocrinol. 2015;173(2):167-174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Colleluori G, Chen R, Turin CG, et al. Aromatase inhibitors plus weight loss improves the hormonal profile of obese hypogonadal men without causing major side effects. Front Endocrinol (Lausanne). 2020;11:277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Vilayphiou N, Boutroy S, Szulc P, et al. Finite element analysis performed on radius and tibia HR-pQCT images and fragility fractures at all sites in men. J Bone Miner Res. 2011;26(5):965-973. [DOI] [PubMed] [Google Scholar]
- 25. Pistoia W, van Rietbergen B, Lochmüller EM, Lill CA, Eckstein F, Rüegsegger P. Estimation of distal radius failure load with micro-finite element analysis models based on three-dimensional peripheral quantitative computed tomography images. Bone. 2002;30(6):842-848. [DOI] [PubMed] [Google Scholar]
- 26. Chiba K, Okazaki N, Kurogi A, et al. Precision of second-generation high-resolution peripheral quantitative computed tomography: intra- and intertester reproducibilities and factors involved in the reproducibility of cortical porosity. J Clin Densitom. 2018;21(2):295-302. [DOI] [PubMed] [Google Scholar]
- 27. Colleluori G, Aguirre L, Dorin R, et al. Hypogonadal men with type 2 diabetes mellitus have smaller bone size and lower bone turnover. Bone. 2017;99:14-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Sowers MR, Randolph J Jr, Jannausch M, Lasley B, Jackson E, McConnell D. Levels of sex steroid and cardiovascular disease measures in premenopausal and hormone-treated women at midlife: implications for the “timing hypothesis”. Arch Intern Med. 2008;168(19):2146-2153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Hayter A. The maximum familywise error rate of Fisher’s least significant difference test. J Am Stat Assoc. 1986;12:1000-1004. [Google Scholar]
- 30. Villareal DT, Aguirre L, Gurney AB, et al. Aerobic or resistance exercise, or both, in dieting obese older adults. N Engl J Med. 2017;376(20):1943-1955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Kelly DM, Jones TH. Testosterone and obesity. Obes Rev. 2015;16(7):581-606. [DOI] [PubMed] [Google Scholar]
- 32. Supplemental Tables: S1 Simple Correlation Analysis Between the Different Variables with Failure Load and Stiffness at the Radius. Table S2: Multiple Regression Analysis. of Predictors of Radius Failure Load and Stiffness Obese Men without Type 2 Diabetes. https://datadryad.org/stash/share/-D4JgarKULGKZ0bNqQEl1jbeariyQqYCYGOwLqSGvbY [Google Scholar]
- 33. Bouchonville M, Armamento-Villareal R, Shah K, et al. Weight loss, exercise or both and cardiometabolic risk factors in obese older adults: results of a randomized controlled trial. Int J Obes (Lond). 2014;38(3):423-431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Aronne LJ, Isoldi KK. Overweight and obesity: key components of cardiometabolic risk. Clin Cornerstone. 2007;8(3):29-37. [DOI] [PubMed] [Google Scholar]
- 35. Viljakainen H, Ivaska KK, Paldánius P, et al. Suppressed bone turnover in obesity: a link to energy metabolism? A case-control study. J Clin Endocrinol Metab. 2014;99(6):2155-2163. [DOI] [PubMed] [Google Scholar]
- 36. Mashiba T, Hirano T, Turner CH, Forwood MR, Johnston CC, Burr DB. Suppressed bone turnover by bisphosphonates increases microdamage accumulation and reduces some biomechanical properties in dog rib. J Bone Miner Res. 2000;15(4):613-620. [DOI] [PubMed] [Google Scholar]
- 37. Fonseca H, Moreira-Gonçalves D, Coriolano HJ, Duarte JA. Bone quality: the determinants of bone strength and fragility. Sports Med. 2014;44(1):37-53. [DOI] [PubMed] [Google Scholar]
- 38. Farr JN, Khosla S. Determinants of bone strength and quality in diabetes mellitus in humans. Bone. 2016;82:28-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Cunha JS, Ferreira VM, Maquigussa E, Naves MA, Boim MA. Effects of high glucose and high insulin concentrations on osteoblast function in vitro. Cell Tissue Res. 2014;358(1):249-256. [DOI] [PubMed] [Google Scholar]
- 40. Shah VN, Sippl R, Joshee P, et al. Trabecular bone quality is lower in adults with type 1 diabetes and is negatively associated with insulin resistance. Osteoporos Int. 2018;29(3):733-739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Ho-Pham LT, Nguyen TV. Association between trabecular bone score and type 2 diabetes: a quantitative update of evidence. Osteoporos Int. 2019;30(10):2079-2085. [DOI] [PubMed] [Google Scholar]
- 42. Samelson EJ, Demissie S, Cupples LA, et al. Diabetes and deficits in cortical bone density, microarchitecture, and bone size: framingham HR-pQCT Study. J Bone Miner Res. 2018;33(1):54-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Liu J, Cao L, Qian YW, et al. The association between risk of limb fracture and type 2 diabetes mellitus. Oncotarget. 2018;9(58):31302-31310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Iwaniec UT, Turner RT. Influence of body weight on bone mass, architecture and turnover. J Endocrinol. 2016;230(3):R115-R130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Aguirre LE, Colleluori G, Dorin R, et al. Hypogonadal men with higher body mass index have higher bone density and better bone quality but reduced muscle density. Calcif Tissue Int. 2017;101(6):602-611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Legrand E, Chappard D, Pascaretti C, et al. Trabecular bone microarchitecture, bone mineral density, and vertebral fractures in male osteoporosis. J Bone Miner Res. 2000;15(1):13-19. [DOI] [PubMed] [Google Scholar]
- 47. Vega GL, Grundy SM. Metabolic risk susceptibility in men is partially related to adiponectin/leptin ratio. J Obes. 2013;2013:409679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Elefteriou F, Takeda S, Ebihara K, et al. Serum leptin level is a regulator of bone mass. Proc Natl Acad Sci U S A. 2004;101(9):3258-3263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Oshima K, Nampei A, Matsuda M, et al. Adiponectin increases bone mass by suppressing osteoclast and activating osteoblast. Biochem Biophys Res Commun. 2005;331(2):520-526. [DOI] [PubMed] [Google Scholar]
- 50. Longo M, Zatterale F, Naderi J, et al. Adipose tissue dysfunction as determinant of obesity-associated metabolic complications. Int J Mol Sci 2019;20(9). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Gállego Suárez C, Singer BH, Gebremariam A, Lee JM, Singer K. The relationship between adiposity and bone density in U.S. children and adolescents. PloS One. 2017;12(7):e0181587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Aguirre L, Napoli N, Waters D, Qualls C, Villareal DT, Armamento-Villareal R. Increasing adiposity is associated with higher adipokine levels and lower bone mineral density in obese older adults. J Clin Endocrinol Metab. 2014;99(9):3290-3297. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data sets generated during and/or analyzed during the current study are not publicly available but are available from the corresponding author on reasonable request.