Abstract
Context
This mini-review provides an overview of menopausal hormone therapy (HT) and cardiovascular disease (CVD) risk, with a focus on the role of hormone formulation, dose, and route of delivery.
Methods
This summary is based on authors’ knowledge in the field of menopausal HT and supplemented by a PubMed search using the terms “menopause hormone therapy,” “transdermal,” “estradiol,” “conjugated estrogens,” “bioidentical,” “cardiovascular disease,” “lipoproteins,” “glucose,” “progestogens,” “low dose.”
Results
Available evidence indicates that oral unopposed estrogens have a favorable effect on lipoprotein levels, glycemia, insulin, and CVD risk; however, the addition of progestogens blunts the lipid-related effects. The progestogen with the smallest attenuating effect is micronized progesterone. Transdermal estrogens have less effect on coagulation, inflammation, and lipids than oral estrogens and observational studies suggest they pose a lower risk of venous thromboembolism and stroke than oral estrogens. Clinical effects of hormones were not consistently dose dependent.
Conclusions
Although HT continues to have an important role in menopause management, it is not recommended for primary or secondary CVD prevention. Different formulations, doses, and routes of delivery of HT have different effects on cardiometabolic markers and risks of clinical CVD events. However, long-term trials evaluating clinical outcomes with transdermal and other alternate HT regimens are limited.
Keywords: menopause, hormone therapy, estradiol, transdermal, cardiovascular disease
Throughout the late 1990s, menopausal hormone therapy (HT) was one of the most commonly prescribed pharmaceuticals on the market. At that time, prescribing guidelines were primarily based on animal and observational studies, which consistently reported a 40% to 50% reduction in coronary heart disease (CHD) in HT users compared with nonusers (1). Many women were started on HT not only for relief of vasomotor symptoms, but also for its purported cardioprotection and prevention of other chronic diseases. Over the past 2 decades the understanding of HT and cardiovascular disease (CVD) risk has shifted after several pivotal randomized clinical trials (RCTs) in postmenopausal women. The Heart and Estrogen/progestin Replacement Study (HERS) and the Women’s Health Initiative (WHI) trials did not find benefit for primary or secondary CVD prevention and some women, especially those enrolled at older ages and longer intervals since menopause onset, experienced increased CVD events (2-5).
Today, the HT options available to treat vasomotor symptoms extend beyond the regimens studied in the HERS and WHI (conjugated equine estrogens [CEEs] with or without medroxyprogesterone acetate [MPA]), raising the question whether the same risks can be generalized to other forms of HT. Lower-dose formulations are available, as are nonoral routes of administration, specifically transdermal. Also, the Food and Drug Administration has approved several “bioidentical” hormone formulations, such as estradiol and micronized progesterone. While HT remains the most effective option for vasomotor symptoms, there are no large-scale RCTs allowing for head to head comparisons based on formulation, dose, and route of delivery with respect to CVD events. This mini-review provides an overview of HT and cardiovascular risk with respect to formulation, dose, and route of delivery based on the available evidence, including effects on CVD risk factors, biomarkers, intermediate “surrogate” endpoints, and/or clinical CVD events.
Estrogen Alone vs Estrogen ± Progestogens
Effect on CVD risk factors
One reason estrogen was thought to be cardioprotective was its favorable effect on lipid and lipoprotein levels. Many studies found that estrogens lowered low-density lipoprotein cholesterol (LDL-C) and raised high-density lipoprotein cholesterol (HDL-C). Walsh et al. were among the first to study this by formulation and route of delivery, and randomized women with normal lipoproteins in a double-blind crossover trial to oral estradiol (2 mg/day), oral CEE (0.625 mg or 1.25 mg/day), transdermal estradiol (0.1 mg/day), or placebo for 6 weeks. Oral estradiol and CEE lowered LDL-C and raised HDL-C levels by approximately 15%, with higher doses of CEE not augmenting this effect (6) (Table 1). Oral estrogens also increased triglycerides in a dose-dependent manner. Transdermal 17β-estradiol had minimal effect on lipoprotein levels. These data suggested that the first-pass hepatic metabolism of oral estrogens may be a major contributor to changes in lipid and lipoprotein levels.
Table 1.
Mean, median, or percent change in CVD risk factors by different HT formulations, doses, and routes of delivery
| Formulation | LDL–C (mg/dL) | HDL-C (mg/dL) | Triglycerides (mg/dL) | Fasting Glucose (mg/dL) | Fasting Insulin level (pmol/L) | hs-CRP (mg/L) |
|---|---|---|---|---|---|---|
| Walsh et al. (6) (12-week change) | ||||||
| CEE 0.625 mga | –15d | +16d | +24d | — | — | — |
| CEE 1.25 mga | –19d | +18d | +38d | — | — | — |
| Walsh et al. (6) (6-week change) | ||||||
| Oral estradiol 2 mga | –14 | +26d | +24d | — | — | — |
| Transdermal Estradiol patch 0.1 mga | –4 | +10 | +0.1 | — | — | — |
| PEPI (3-year change) (7)c,e | ||||||
| CEE 0.625 mg | –14.5c | 5.6c | 13.7c | –2.8c | –1.7 | — |
| CEE + MPA 10 mg cyclic | –17.7c | 1.6c | 12.7c | –2.7c | 1.3 | — |
| CEE + MPA 2.5 continuous | –16.5c | 1.2c | 11.4c | –2.1c | –3.8 | — |
| CEE + micronized progesterone cyclic | –14.8c | 4.3c | 13.4c | –2.5c | –3.5 | — |
| WHI (1-year change) (8)e | ||||||
| CEE 0.625 mg | –23d | 7.0d | 17.5d | –3.0d | –1.1b,d | 2.2d |
| CEE + MPA 2.5 continuous | –20d | 4.0d | 14.0d | –3.0d | –1.0b,d | 1.1d |
| KEEPS (4-year change) (9)e | ||||||
| CEE 0.45 mg | –4.86d | 3.2d | 13.1d | 1.01 | –7.71 | 15.52d |
| transdermal estradiol 50 µg/day patch | –2.87 | –1.24d | –0.06 | 0.33d | –9.72d | 5.14 |
Abbreviations: LDL-C, low-density lipoprotein cholesterol; HDL-C, high-density lipoprotein cholesterol; hs-CRP, high-sensitivity c-reactive protein; CEE, conjugated equine estrogens; MPA, medroxyprogesterone acetate; PEPI, Postmenopausal Estrogen/Progestin Intervention trial; WHI, Women’s Health Initiative; KEEPS, Kronos Early Estrogen Prevention Study.
a % change from placebo value.
b Units UIU/mL; — data not collected.
c PEPI statistically significant difference between formulations for LDL-C, HDL-C, triglycerides, and fasting glucose.
d Statistically different from placebo.
e PEPI, placebo decreased LDL by 4.1, HDL by 1.2, triglycerides by 3.2, and fasting glucose by 0.5, while increasing fasting insulin by 2.8. WHI, placebo had a null effect with the exception of an increase in fasting insulin. KEEPS, placebo increased HDL by 0.45, triglycerides by 1.66, and hs-CRP by 5.05, while decreased triglycerides by 2 and fasting insulin by 2.9.
The Postmenopausal Estrogen/Progestin Intervention trial further evaluated these findings with respect to the addition of progestogen agents. Healthy menopausal women (n = 875, 45-64 years old) were randomized to CEE alone (0.625 mg/day), CEE (0.625 mg/day) with cyclic MPA (10 mg/day for 12 days/month), continuous MPA (2.5 mg/day), or cyclic micronized progesterone (200 mg/day for 12 days/month), or placebo (7). Over a 3-year follow-up, all HT regimens reduced LDL-C by 14.5 to 17.7 mg/dL and increased triglycerides by 11.4 to 13.7 mg/dL. With respect to HDL-C, differences were seen by formulation in that CEE alone increased HDL by 5.6 mg/dL and CEE plus cyclic micronized progesterone increased HDL-C by 4.1 mg/dL while the addition of MPA blunted this effect (1.2-1.6 mg/dL). All HT regimens also lowered fibrinogen levels with no adverse effect or differences in blood pressure or change in 2-hour insulin in response to a glucose challenge. There was a slight decrease in fasting glucose levels in all treatment groups compared with placebo (Table 1). Although the addition of progestogen blunted the HDL-C effects seen with estrogens, micronized progesterone led to the smallest attenuation.
The WHI evaluated the effect of hormone therapy on CVD biomarkers and lipids in a nested case–control study (n = 1179) (8). After 1 year of use, oral CEE (0.625 mg/day) and oral CEE plus MPA (2.5 mg/day) decreased LDL-C and increased HDL-C and triglycerides. Both formulations also increased high-sensitivity C-reactive protein (hs-CRP) and had a favorable effect on glucose and insulin levels. More recently the Kronos Early Estrogen Prevention Study (KEEPS) randomized 727 healthy women within 3 years of their final menstrual period to oral CEE 0.45 mg/day, transdermal estradiol 50 µg/day patch or placebo, with cyclic oral micronized progesterone 200 mg for 12 days/month (9). The results by route of estrogen administration are summarized below (route of estrogen delivery: oral vs transdermal).
Primary Prevention Randomized Trials
The largest RCTs of HT and primary prevention were the 2 parallel WHI trials of CEE + MPA (EPT trial) and CEE alone (E-alone trial). These trials enrolled 27 347 healthy postmenopausal women with the primary outcome of coronary heart disease, defined as nonfatal myocardial infarction (MI) or coronary death. In the EPT, 16 608 women with an intact uterus were randomized to oral CEE (0.625 mg/day) plus MPA (2.5 mg/day) or placebo with planned 8-year follow-up. The EPT trial was stopped approximately 3 years early (median 5.6 years) due to an unfavorable balance of risks and benefits when used for chronic disease prevention as well as a significant increase in invasive breast cancer (4). After further evaluation, the overall cohort (aged 50-79 years, mean age = 63) had an increased risk of developing CHD by 18%, with a significant increase during the first year of the trial (P = .03, trend for time) (Table 2). The absolute risk of CHD for women on EPT vs placebo was 6 extra cases per 10 000 women per year.
Table 2.
Women’s health initiative estrogen–progestin and estrogen-alone trials, overall cohorts, intervention phasea
| Estrogen–progestin trial | Estrogen-alone trial | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| CEE + MPA | Placebo | CEE | Placebo | |||||||
| Outcome | # events per 10 000 PY | # events per 10 000 PY | Differenceb per 10 000 PY | HR (95% CI) | P | # events per 10 000 PY | # events per 10 000 PY | Differenceb per 10 000 PY | HR (95% CI) | P |
| Cardiovascular disease | ||||||||||
| Coronary heart diseasec | 41 | 35 | 6 | 1.18 (0.95-1.45) | .13 | 55 | 58 | –3 | 0.94 (0.78-1.14) | .53 |
| Myocardial infarction | 35 | 29 | 6 | 1.24 (0.98-1.56) | .07 | 44 | 45 | –1 | 0.97 (0.79-1.21) | .97 |
| Coronary revascularizationd | 42 | 45 | –3 | 0.95 (0.78-1.16) | .64 | 68 | 67 | 1 | 1.00 (0.83-1.19) | .96 |
| Stroke | 33 | 24 | 9 | 1.37 (1.07-1.76) | .01 | 45 | 34 | 11 | 1.35 (1.07-1.70) | .01 |
| Pulmonary embolism | 18 | 9 | 9 | 1.98 (1.36-2.87) | <.001 | 14 | 10 | 4 | 1.35 (0.89-2.05) | .15 |
| Deep vein thrombosis | 25 | 14 | 12 | 1.87 (1.37-2.54) | <.001 | 23 | 15 | 7 | 1.48 (1.06-2.07) | .02 |
| Cardiovascular mortality | 17 | 15 | 2 | 1.08 (0.78-1.48) | .65 | 29 | 28 | 1 | 1.01 (0.78-1.31) | .95 |
| All cardiovascular eventse | 170 | 152 | 19 | 1.13 (1.02-1.25) | .02 | 251 | 224 | 27 | 1.11 (1.01-1.22) | .03 |
| Other related outcomes | ||||||||||
| Diabetes | 72 | 88 | –16 | 0.81 (0.70-0.94) | .005 | 134 | 155 | –21 | 0.86 (0.76-0.98) | .02 |
| All-cause mortality | 52 | 53 | –1 | 0.97 (0.81-1.16) | .76 | 80 | 77 | 3 | 1.03 (0.88-1.21) | .68 |
| Global indexf | 189 | 168 | 20 | 1.12 (1.02-1.24) | .02 | 208 | 204 | 4 | 1.03 (0.93-1.13) | .63 |
Adapted from data in Manson JE, et al. JAMA 2013; 310:1353–68; Manson JE, et al. JAMA 2017; 318:927–938.
Abbreviations: CEE, conjugated equine estrogens; CI, confidence interval; CVD, cardiovascular disease; HR, hazard ratio; MPA, medroxyprogesterone acetate; NA, not applicable (due to hysterectomy); PY, person-years.
a Median length of randomized treatment was 5.6 years for estrogen–progestin and 7.2 years for estrogen alone.
b Difference = # events per 10 000 women per year in the hormone therapy group – # events per 10 000 women per year in the placebo group. Numbers may not add precisely due to rounding error.
c Coronary heart disease is defined as nonfatal myocardial infarction or coronary death.
d Coronary revascularization is defined as coronary artery bypass grafting or percutaneous coronary intervention.
e “All cardiovascular events” is a composite outcome of myocardial infarction, stroke, coronary revascularization, angina, heart failure, carotid artery disease, peripheral vascular disease, venous thromboembolism (pulmonary embolism, deep vein thrombosis), and cardiovascular mortality.
f Global index is a composite outcome of coronary heart disease, stroke, pulmonary embolism, breast cancer, colorectal cancer, endometrial cancer (in the estrogen–progestin trial), hip fracture, and all-cause mortality.
The E-alone trial randomized 10 739 women with a hysterectomy to oral CEE (0.625 mg/day) or placebo. After the EPT trial was stopped, the E-alone trial was allowed to continue, however was also stopped prematurely after a median of 7.2 years due to increased risk of stroke. The E-alone trial was not found to have an increased risk for CHD, with an absolute difference of 3 fewer CHD events for women on E-alone vs placebo per 10 000 women per year (Table 2). Subsequent analyses from the combined WHI trials found that in women closer to the age of natural menopause who start HT, have a lower risk of CHD than women who have further time since menopause. (Table 3)
Table 3.
Health outcomes in the women’s health initiative estrogen–progestin and estrogen-alone trials, according to age at study entry, intervention phasea
| Estrogen-progestin trial | Estrogen-alone trial | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| CEE+MPA | Placebo | CEE | Placebo | |||||||
| Outcome | # events per 10 000 PY | # events per 10 000 PY | Differenceb per 10 000 PY | HR (95% CI) | P | # events per 10 000 PY | # events per 10 000 PY | Differenceb per 10 000 PY | HR (95% CI) | P |
| Coronary heart diseasec | ||||||||||
| 50-59 years | 23 | 17 | 5 | 1.34 (0.82-2.19) | .81 | 17 | 28 | –11 | 0.60 (0.35-1.04) | .08 |
| 60-69 years | 37 | 37 | 0 | 1.01 (0.73-1.39) | 61 | 63 | –3 | 0.95 (0.72-1.24) | ||
| 70-79 years | 82 | 63 | 19 | 1.31 (0.93-1.84) | 97 | 90 | 7 | 1.09 (0.80-1.49) | ||
| Myocardial infarction | ||||||||||
| 50-59 years | 19 | 15 | 4 | 1.32 (0.77-2.25) | .55 | 14 | 25 | –11 | 0.55 (0.31-1.00) | .02 |
| 60-69 years | 33 | 31 | 2 | 1.05 (0.74-1.47) | 46 | 48 | –2 | 0.95 (0.69-1.30) | ||
| 70-79 years | 69 | 47 | 21 | 1.46 (1.00-2.15) | 83 | 69 | 14 | 1.24 (0.88-1.75) | ||
| Stroke | ||||||||||
| 50-59 years | 15 | 10 | 5 | 1.51 (0.81-2.82) | .50 | 16 | 17 | –1 | 0.99 (0.53-1.85) | .77 |
| 60-69 years | 34 | 23 | 11 | 1.45 (1.00-2.11) | 51 | 33 | 18 | 1.55 (1.10-2.16) | ||
| 70-79 years | 63 | 50 | 13 | 1.22 (0.84-1.79) | 77 | 59 | 17 | 1.29 (0.90-1.86) | ||
| All-cause mortality | ||||||||||
| 50-59 years | 21 | 31 | -10 | 0.67 (0.43-1.04) | .20 | 29 | 40 | –11 | 0.70 (0.46-1.09) | .04 |
| 60-69 years | 51 | 47 | 5 | 1.07 (0.81-1.41) | 78 | 77 | 0 | 1.01 (0.79-1.29) | ||
| 70-79 years | 106 | 102 | 3 | 1.03 (0.78-1.36) | 155 | 129 | 26 | 1.21 (0.95-1.56) | ||
Adapted from Manson JE, et al. JAMA 2013;310:1353–68.; Manson JE, et al. JAMA 2017;318:927–938.
Abbreviations: CEE, conjugated equine estrogens; CI, confidence interval; CVD, cardiovascular disease; HR, hazard ratio; MPA, medroxyprogesterone acetate; PY, person-years.
Median length of randomized treatment was 5.6 years for estrogen–progestin and 7.2 years for estrogen alone.
b Difference = # events per 10 000 women per year in the hormone therapy group – # events per 10 000 women per year in the placebo group. Numbers may not add precisely due to rounding error.
c Coronary heart disease is defined as nonfatal myocardial infarction or coronary death.
Secondary Prevention Randomized Trials
The HERS trial was the first large-scale secondary prevention trial and enrolled 2763 women with coronary artery disease (mean age 67 years). Participants were randomized to CEE (0.625 mg/day) plus MPA (2.5 mg/day) or placebo for the primary outcome of nonfatal MI and ischemic heart disease (IHD) death. During the 4-year follow-up, no differences were found for nonfatal MI or death from IHD, despite a decrease in total cholesterol and LDL-C, and an increase in HDL-C in the active treatment group. In a post hoc analysis, there was over a 50% increase in recurrent MI in the HT treatment group during the first year (2). The HERS II trial followed over 90% of the original HERS participants for 3 additional years, finding no evidence of cardioprotective effects with longer duration of follow-up (10).
The Estrogen Replacement and Atherosclerosis (ERA) trial examined the effect of HT on the progression of coronary artery disease by invasive angiography (n = 309, mean age 63.5 years) with coronary stenosis >30% at baseline. After 3 years of treatment there was no change in luminal diameter or atherosclerotic lesions between women randomized to CEE (0.625 mg/day) with or without MPA (2.5 mg continuous or cyclic) and placebo (11). The Women’s Angiographic Vitamin and Estrogen trial randomized women (n = 423, mean age 65 years) with angiographically confirmed coronary stenosis (15-75%) in a 2 × 2 design to CEE (0.625 mg/day) with and without MPA (2.5 mg/day) or placebo, and vitamin C (500 mg) and vitamin E (400 IU) or placebo. No benefit was found and a potential for harm was observed (12).
Formulation of Estrogens: Oral Synthetic vs Bioidentical
After the results of the WHI and the secondary prevention trials, attention focused on whether there was a difference in CVD outcomes when bioidentical HT is used. Oral synthetic estrogens used for HT include CEE, which is a ratio of estrone to 17-β estradiol, equilin and other methylated products. Food and Drug Administration–approved bioidentical estrogens, 17β-estradiol (estradiol) and estrone, are similar in biochemical structure to those produced by women’s ovaries or other tissues in the body. These estrogens are plant-based products that are chemically changed to bioidentical estrogen.
Primary Prevention Studies
The Estrogen and Prevention of Atherosclerosis Trial was a double-blind, placebo-controlled trial using the estrogen formulation of oral estradiol (1 mg/day). Women (n = 199, mean age 62 years) with hyperlipidemia (LDL-C >130 mg/dL) but no coronary artery disease were randomized to unopposed estradiol vs placebo (plus lipid-lowering therapy if LDL-C >160 mg/dL) to determine whether HT could reduce the progression of subclinical atherosclerosis as measured by carotid intima media thickness (CIMT) (13). The estradiol treatment group had less CIMT progression compared to placebo (P = .046), and among those not receiving statin medication, the difference was even larger (P = .002). No differences were seen between the estradiol and placebo groups for the women on lipid-lowering therapy.
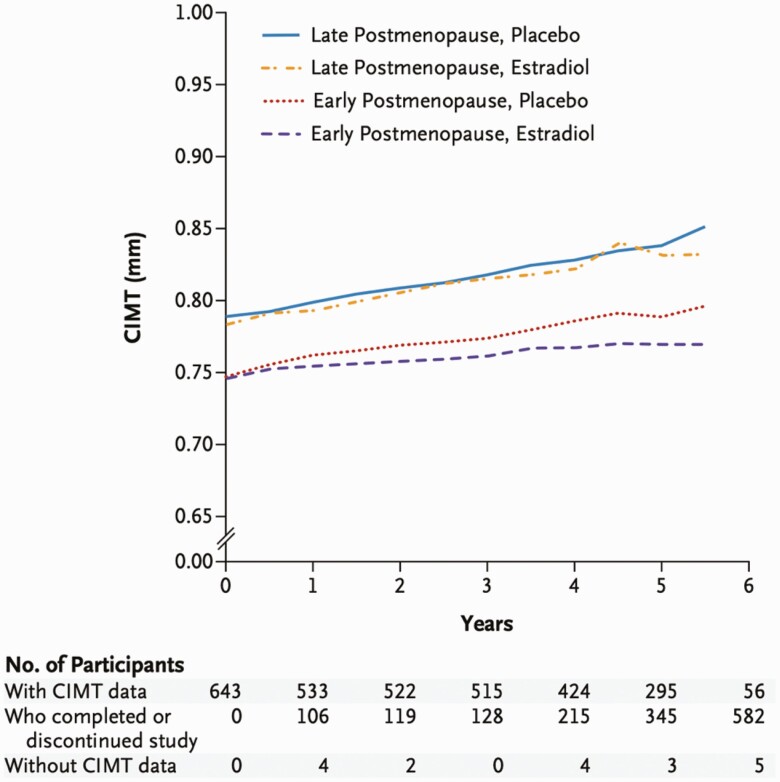
The Early versus Late Intervention Trial with Estradiol also tested the estrogen formulation oral estradiol (1 mg/day) and directly assessed the “timing hypothesis” postulating more favorable effects of HT in early vs later menopause. Healthy women (n = 504) within 6 years (the early cohort, median age 55.4 years) or greater than 10 years since menopause (the late cohort, median age 63.6 years) were assigned to estrogen plus vaginal progesterone gel (45 mg/10 days/month) for women with a uterus vs placebo (14). This trial used intermediate or “surrogate” measures of CVD including CIMT. After 5 years, the early cohort of women randomized to oral estradiol had lower progression of subclinical atherosclerosis as measured by CIMT (P = .008). (Fig. 1) There was no difference in CIMT found in the late cohort (P = .29). Findings from these 2 trials suggest that among menopausal women without established atherosclerosis, oral estradiol may be of benefit as measured by markers of atherosclerosis progression.
Figure 1.
Early versus late intervention trial with estradiol results of CIMT progression. CIMT, carotid intima media thickness. With permission: Hodis et al. NEJM 2016;374(13):1221-1231.
In the open-labeled Danish Osteoporosis Prevention Study (DOPS), women were randomized to oral triphasic estradiol with norethisterone acetate or oral estradiol (2 mg) alone or no treatment (n = 1006, mean age 50 years). Over an 11-year follow-up, there was a significant reduction in a composite CVD endpoint (hazard ratio [HR] 0.48, 95% CI 0.26-0.87) (15). These findings suggested that estradiol, when given early in menopause, may be of benefit for CVD protection. Several important limitations regarding the study method warrant consideration before reaching this conclusion. DOPS was initially designed to evaluate the impact of HT on prevention of fracture, and the composite CVD endpoint was not prespecified in the study design. The composite endpoint included hospitalization for heart failure, not traditionally included in the composite atherosclerotic endpoints of other HT RCTs. Additionally, such open-label design, with no placebo arm, allowed women and their clinicians to be aware of their treatment, which may have resulted in bias, as at the time of DOPS it was perceived that HT prevented heart disease.
Secondary Prevention
The Oestrogen Therapy for Prevention of Reinfarction in Postmenopausal Women was a RCT evaluating secondary prevention of MI using the formulation of estradiol valerate (2 mg/day) vs placebo for 2 years (n = 1017, mean age 62.6 years). In women with a prior MI, estradiol did not reduce risk of future events (relative risk [RR] 0.99, 95% CI 0.70-1.41) or all-cause mortality (RR 0.79, 95% CI 0.50-1.27) (16).
Similar to the ERA trial, the Women’s Estrogen-Progestin Lipid-Lowering Hormone Atherosclerosis Regression Trial (WELL-HART) evaluated the effect of HT on the progression of coronary artery disease by invasive angiography among women with coronary stenosis >30% at baseline. As opposed to ERA, the WELL-HART study (n = 226, mean age 63.5 years) used oral estradiol (1 mg/day), oral estradiol (1 mg/day) with cyclic MPA (5 mg/day for 12 consecutive days) or placebo with a goal to lower the LDL <130 mg/dL through diet and statin therapy. At the end of the 3-year follow-up, there was no effect on progression of atherosclerosis and no evidence of reversal of established coronary artery disease.
Types of Progestogens
Progestogens are necessary in women with a uterus to prevent estrogen-induced endometrial hyperplasia and/or cancer. The most comprehensively studied progestin is MPA due to its combination with CEE in the WHI, HERS, and other large RCTs. However, there are several other types of progestogens used in combination HT, including norethindrone acetate and micronized progesterone (bioidentical). Given the differences in chemical structures, pharmacokinetics, and clinical effects, it is not surprising that different progestogens may have different effects on CVD risk (17).
Primary Prevention
Up until the time of the WHI, observational studies did not find differences in CVD when estrogens were used with or without a progestogen (18). It is now clear from the parallel trials of the WHI (WHI EPT and E-alone trials) that there was an increased risk of CHD in women receiving EPT but not in those receiving CEE alone. These differences have raised questions about whether the type of progestogen in HT is a contributor to adverse CVD outcomes. There are no large-scale RCTs evaluating whether rates of clinical CVD events differ according to the type of progestogen used; therefore, comparisons of formulation are based on observational data.
The 2010 E3N trial was a prospective cohort study of more than 80 000 French women (average age, 54 years) over a 10-year period (19). In this study, the use of micronized progesterone with estrogen was not associated with increased venous thromboembolism (VTE) risk, whereas use of norpregnane progestins was. One limitation is that that dosing, and whether the progestogens were given continuously or sequentially, was not considered. However, the study did suggest that progestogen formulations may have different relationships with VTE risk.
In a multicenter case–control study, the Estrogen and Thromboembolism Risk study evaluated 271 women with a first episode of idiopathic VTE (92 pulmonary embolisms, 63 deep venous thrombosis), with 610 age-matched controls (mean age 62 years) (20). The study evaluated the findings by type of progestogen, finding that the risk of developing an idiopathic venous thromboembolism was not associated with micronized progesterone (odd ratio [OR] 0.7, 95% CI 0.3-1.9) compared with norpregnane derived synthetic progestins (OR 3.9, 95% CI 1.5-10.0).
Secondary Prevention
The ERA and WELL-HART trial enrolled women with established coronary artery disease diagnosed by angiography and used cyclic MPA in women with an intact uterus. Both studies found no differences among the treatment groups in progression of coronary atherosclerosis between women on hormones with MPA vs placebo.
Dose: Low vs High Doses of Estrogen
The doses of 0.625 mg/day CEE used in the WHI and HERS trials is most often referred to as conventional dose CEE, while doses <0.625 mg/day (0.3 mg or 0.45 mg/day) are considered low dose, and >0.625 mg (0.9 mg or 1.25 mg/day) are considered high dose. Across formulations, however, dose equivalency changes: A dose of 0.625 mg/day in oral CEE is thought to be equivalent to 1 mg/day of oral estradiol, which is then equivalent to 0.05/day of transdermal estradiol.
Primary Prevention
The WHI Observational Study (WHI-OS), a longitudinal multicenter prospective study of postmenopausal women (mean age 63.5 years) using various HT regimens, examined the differences between oral low-dose, conventional-dose, and high-dose estrogens controlled for progestogen use in relation to CVD outcomes. While statistical power was limited, women taking oral low-dose HT had suggestive but nonsignificant reductions in CHD, total CVD, and CVD mortality after multivariable adjustment, compared with women who took oral conventional-dose HT (HR 0.82, 95% CI 0.57-1.19, HR 0.86, 95% CI 0.67-1.12, and HR 0.87 95% CI 0.54-1.42, respectively). Another prospective observational study, the Nurses’ Health Study suggested a dose-dependent HT response and risk of stroke in initially healthy women (21, 22). The risk of stroke was statistically increased in women taking CEE 0.625 mg/day or higher with or without a progestin (RR 1.35 95% CI 1.08-1.68 and RR 1.63, 95% CI 1.18–2.26, respectively) compared with HT nonusers, but was not elevated among women taking 0.3 mg/day of CEE.
Secondary Prevention
After the HERS trial, it was established that estrogen should not be used for secondary prevention of CVD among women with established CVD. No additional large-scale RCTs were conducted to evaluate different doses of estrogens on rates of clinical events in the setting of secondary prevention of CVD.
Route of Estrogen Delivery: Oral vs Transdermal
Oral estrogens undergo first-pass hepatic metabolism which results in some adverse changes that may influence CVD risk. Compared with the transdermal route of delivery, hepatic metabolism of oral estrogens increases the production of coagulation factors, C-reactive protein, and triglycerides, and oral estrogens require larger doses as it is more is rapidly converted to estrone in the liver, which results in an increased concentration of estrogen in the liver sinusoids (5, 23-26). To date, there are no large-scale RCTs of transdermal estrogens and clinical CVD events, and available evidence is based on observational studies and smaller RCTs with biomarker or subclinical CVD measures. Despite this, the current HT position statements recommend the use of transdermal estrogens rather than oral estrogens in women who have moderate risk of CVD or an increased risk of VTE, if estrogen is prescribed in these patients (27-29).
Primary Prevention
The KEEPS randomized 727 healthy women within 3 years of their final menstrual period (mean age 52 years) to oral CEE 0.45 mg/day, transdermal estradiol 50 µg/day patch or placebo, with cyclic oral micronized progesterone 200 mg for 12 days/month (9). KEEPS was designed to evaluated the effect of HT on progression of atherosclerosis using preclinical markers including carotid artery CIMT and coronary artery calcification. After 4 years of follow-up, those women who were randomized to oral CEE had greater increases in total and HDL-C, triglycerides, and hs-CRP than women randomized to transdermal estradiol or placebo (9). Transdermal had little effect on fasting glucose after 4 years and the largest decrease in fasting insulin compared with oral CEE or placebo. However, the rate of change in neither CIMT nor coronary artery calcification differed among HT groups at 4 years, suggesting no adverse or beneficial effects of either formulation at these doses on atherosclerosis progression.
In a KEEPS substudy, 467 women had total fat deposition in the heart measured by epicardial and pericardial adipose tissue, which has been associated with CVD events. After 4 years of follow-up there was no differences seen between groups in pericardial adipose tissue; however, compared with placebo, women on oral CEE were less likely to have an increase in epicardial fat (OR 0.62, 95% CI 0.40-0.97) (30). The authors further evaluated the association with pericardial adipose tissue and coronary artery calcification, finding that transdermal estradiol over 4 year follow-up was associated with coronary artery calcification development with an increase in pericardial adipose tissue.
In data from the WHI-OS, with a mean follow-up of 10.4 years, transdermal estradiol compared with oral CEE was associated with a 37% lower risk of CHD; however, this was not statistically significant. In the case–control Estrogen and Thromboembolism Risk study evaluating risk of first VTE with HT, after adjusting for confounders such a body mass index and family history of clots, current use of oral estrogens and transdermal compared with nonusers was 4.2 (95% CI 1.9-8.3) and 0.9 (95% CI 0.4-2.1), respectively. Data from the UK General Practice Research Database case–control study, also found that the risk of stroke was not increased in women using low-dose estrogen transdermal preparation (≤50 mg/day) (31). The UK nested case–control study of VTE risk and use of HT found transdermal estradiol was not associated with an increased risk, across patch, subcutaneous, or gel formulations (OR 0.93, 95% CI 0.87-1.01) (32).
In a recent systematic review and meta-analysis evaluating 15 observational studies (10 case controls and 5 cohort studies) with follow-up of up to 20 years, oral estrogens, but not transdermal estrogens, were associated with an increased risk of VTE (RR 1.63, 95% CI 1.40-1.90), DVT (RR 2.09, 1.35-3.23), and in single case–control study, possibly stroke (RR 1.24, 1.03-1.48) (33). There was no association with pulmonary embolism or MI. In another recent meta-analysis, an increased risk of venous thromboembolism was found with all hormone therapy regimens except for transdermal estrogen (34).
Secondary Prevention
In the Papworth HRT Atherosclerosis study, postmenopausal women (n = 255, mean age 66.7 years) with proven angiographic IHD were randomized to transdermal estradiol (2.5 mg or 80 μg/day) and cyclic transdermal progestin (if there was a uterus present) versus placebo, with the primary end point of angina, MI, or death. Over 4 years of follow-up, the event rates were 15.4 per 100 patients-years in HT group and 11.9 in the controls. No differences were seen between groups, but during the first 2 years of follow-up a higher event rate was seen in the women randomized to estrogen (35).
Summary
Although HT continues to have an important role in menopause management, it is now generally well accepted that HT should not be used for primary or secondary prevention of CVD. Both the WHI and HERS tested oral conjugated estrogens, with and without a synthetic progestin, and there have been no long-term RCTs to assess CVD events with respect to other formulations, doses, or routes of delivery. Available data, with respect to formulation, suggest the use of estrogen alone has a generally favorable effect on lipoproteins, glycemia, insulin, and CVD risk compared with estrogen with progestin. If a progestogen is needed for uterine protection, micronized progesterone has been found to have only a minimal attenuating effect and thus may have advantages over synthetic progestins. Transdermal estrogens also have fewer effects on coagulation and inflammatory biomarkers than oral estrogens, and observational studies suggest they may pose lower risks of VTE and stroke than oral estrogens. HT remains the most effective treatment for menopausal vasomotor symptoms, and current guidelines underscore the need to individualize treatment to maximize benefits and minimize risks. Although additional research is needed, the role of HT formulation, dose, and route of delivery is important to consider in clinical decision making.
Acknowledgments
Financial Support: This work was supported by the National Heart, Lung, and Blood K23HL127262, and the Barbra Streisand Women’s Cardiovascular Research and Education Program, Cedars-Sinai Medical Center, Los Angeles, CA.
Glossary
Abbreviations
- CEE
conjugated equine estrogen
- CHD
coronary heart disease
- CIMT
carotid intima media thickness
- CVD
cardiovascular disease
- DOPS
Danish Osteoporosis Prevention Study
- ERA
Estrogen Replacement and Atherosclerosis
- HDL-C
high-density lipoprotein cholesterol
- HERS
Heart and Estrogen/progestin Replacement Study
- HR
hazard ratio
- HT
hormone therapy
- hs-CRP
high-sensitivity C-reactive protein
- IHD
ischemic heart disease
- KEEPS
Kronos Early Estrogen Prevention Study
- LDL-C
low-density lipoprotein cholesterol
- MI
myocardial infarction
- MPA
medroxyprogesterone acetate
- OR
odds ratio
- PEPI
Postmenopausal Estrogen/Progestin Intervention
- RCT
randomized clinical trials
- RR
relative risk
- VTE
venous thromboembolism
- WELL-HART
Women’s Estrogen-Progestin Lipid-Lowering Hormone Atherosclerosis Regression Trial
- WHI
Women’s Health Initiative
Additional Information
Disclosures: Drs. Shufelt and Manson report no relevant financial relationships.
Data Availability
Data sharing is not applicable to this article as no datasets were generated or analyzed during the current study.
References
- 1. Grodstein F, Stampfer MJ, Manson JE, et al. Postmenopausal estrogen and progestin use and the risk of cardiovascular disease. N Engl J Med. 1996;335(7):453-461. [DOI] [PubMed] [Google Scholar]
- 2. Hulley S, Grady D, Bush T, et al. Randomized trial of estrogen plus progestin for secondary prevention of coronary heart disease in postmenopausal women. Heart and Estrogen/progestin Replacement Study (HERS) Research Group. JAMA. 1998;280(7):605-613. [DOI] [PubMed] [Google Scholar]
- 3. Kannel WB, Hjortland MC, McNamara PM, Gordon T. Menopause and risk of cardiovascular disease: the Framingham study. Ann Intern Med. 1976;85(4):447-452. [DOI] [PubMed] [Google Scholar]
- 4. Rossouw JE, Anderson GL, Prentice RL, et al. ; Writing Group for the Women’s Health Initiative Investigators . Risks and benefits of estrogen plus progestin in healthy postmenopausal women: principal results From the Women’s Health Initiative randomized controlled trial. JAMA. 2002;288(3):321-333. [DOI] [PubMed] [Google Scholar]
- 5. Vickers MR, MacLennan AH, Lawton B, et al. ; WISDOM group . Main morbidities recorded in the women’s international study of long duration oestrogen after menopause (WISDOM): a randomised controlled trial of hormone replacement therapy in postmenopausal women. BMJ. 2007;335(7613):239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Walsh BW, Schiff I, Rosner B, Greenberg L, Ravnikar V, Sacks FM. Effects of postmenopausal estrogen replacement on the concentrations and metabolism of plasma lipoproteins. N Engl J Med. 1991;325(17):1196-1204. [DOI] [PubMed] [Google Scholar]
- 7. Miller VT, LaRosa J, Barnabei V, et al. Effects of estrogen or estrogen/ progestin regimens on heart disease risk factors in postmenopausal women: the Postmenopausal Estrogen/Progestin Interventions (PEPI) trial. JAMA. 1995;273(3):199-208. [PubMed] [Google Scholar]
- 8. Rossouw JE, Cushman M, Greenland P, et al. Inflammatory, lipid, thrombotic, and genetic markers of coronary heart disease risk in the women’s health initiative trials of hormone therapy. Arch Intern Med. 2008;168(20):2245-2253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Harman SM, Black DM, Naftolin F, et al. Arterial imaging outcomes and cardiovascular risk factors in recently menopausal women: a randomized trial. Ann Intern Med. 2014;161(4):249-260. [DOI] [PubMed] [Google Scholar]
- 10. Grady D, Herrington D, Bittner V, et al. ; HERS Research Group . Cardiovascular disease outcomes during 6.8 years of hormone therapy: Heart and Estrogen/progestin Replacement Study follow-up (HERS II). JAMA. 2002;288(1):49-57. [DOI] [PubMed] [Google Scholar]
- 11. Herrington DM, Reboussin DM, Brosnihan KB, et al. Effects of estrogen replacement on the progression of coronary-artery atherosclerosis. N Engl J Med. 2000;343(8):522-529. [DOI] [PubMed] [Google Scholar]
- 12. Waters DD, Alderman EL, Hsia J, et al. Effects of hormone replacement therapy and antioxidant vitamin supplements on coronary atherosclerosis in postmenopausal women: a randomized controlled trial. JAMA. 2002;288(19):2432-2440. [DOI] [PubMed] [Google Scholar]
- 13. Hodis HN, Mack WJ, Lobo RA, et al. ; Estrogen in the Prevention of Atherosclerosis Trial Research Group . Estrogen in the prevention of atherosclerosis. A randomized, double-blind, placebo-controlled trial. Ann Intern Med. 2001;135(11):939-953. [DOI] [PubMed] [Google Scholar]
- 14. Hodis HN, Mack WJ, Henderson VW, et al. ; ELITE Research Group . Vascular effects of early versus late postmenopausal treatment with estradiol. N Engl J Med. 2016;374(13):1221-1231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Schierbeck LL, Rejnmark L, Tofteng CL, et al. Effect of hormone replacement therapy on cardiovascular events in recently postmenopausal women: randomised trial. BMJ. 2012;345:e6409. [DOI] [PubMed] [Google Scholar]
- 16. Oestrogen therapy for prevention of reinfarction in postmenopausal women: a randomised placebo controlled trial. Lancet. 2002;360(9350):2001-2008. [DOI] [PubMed] [Google Scholar]
- 17. Stanczyk FZ, Hapgood JP, Winer S, Mishell DR Jr. Progestogens used in postmenopausal hormone therapy: differences in their pharmacological properties, intracellular actions, and clinical effects. Endocr Rev. 2013;34(2):171-208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Falkeborn M, Persson I, Adami HO, et al. The risk of acute myocardial infarction after oestrogen and oestrogen-progestogen replacement. Br J Obstet Gynaecol. 1992;99(10):821-828. [DOI] [PubMed] [Google Scholar]
- 19. Canonico M, Fournier A, Carcaillon L, et al. Postmenopausal hormone therapy and risk of idiopathic venous thromboembolism: results from the E3N cohort study. Arterioscler Thromb Vasc Biol. 2010;30(2):340-345. [DOI] [PubMed] [Google Scholar]
- 20. Canonico M, Oger E, Plu-Bureau G, et al. ; Estrogen and Thromboembolism Risk (ESTHER) Study Group . Hormone therapy and venous thromboembolism among postmenopausal women: impact of the route of estrogen administration and progestogens: the ESTHER study. Circulation. 2007;115(7):840-845. [DOI] [PubMed] [Google Scholar]
- 21. Grodstein F. Response to comments on ‘A prospective, observational study of postmenopausal hormone therapy and primary prevention of cardiovascular disease’. Maturitas. 2001;38(3):239-241. [DOI] [PubMed] [Google Scholar]
- 22. Grodstein F, Manson JE, Colditz GA, Willett WC, Speizer FE, Stampfer MJ. A prospective, observational study of postmenopausal hormone therapy and primary prevention of cardiovascular disease. Ann Intern Med. 2000;133(12):933-941. [DOI] [PubMed] [Google Scholar]
- 23. Brosnan JF, Sheppard BL, Norris LA. Haemostatic activation in post-menopausal women taking low-dose hormone therapy: less effect with transdermal administration? Thromb Haemost. 2007;97(4):558-565. [PubMed] [Google Scholar]
- 24. Lacut K, Oger E, Le Gal G, et al. ; SARAH Investigators . Differential effects of oral and transdermal postmenopausal estrogen replacement therapies on C-reactive protein. Thromb Haemost. 2003;90(1):124-131. [PubMed] [Google Scholar]
- 25. Post MS, Christella M, Thomassen LG, et al. Effect of oral and transdermal estrogen replacement therapy on hemostatic variables associated with venous thrombosis: a randomized, placebo-controlled study in postmenopausal women. Arterioscler Thromb Vasc Biol. 2003;23(6):1116-1121. [DOI] [PubMed] [Google Scholar]
- 26. Shifren JL, Rifai N, Desindes S, McIlwain M, Doros G, Mazer NA. A comparison of the short-term effects of oral conjugated equine estrogens versus transdermal estradiol on C-reactive protein, other serum markers of inflammation, and other hepatic proteins in naturally menopausal women. J Clin Endocrinol Metab. 2008;93(5):1702-1710. [DOI] [PubMed] [Google Scholar]
- 27. The 2017 hormone therapy position statement of The North American Menopause Society. Menopause. 2017;24(7):728-753. [DOI] [PubMed] [Google Scholar]
- 28. Stuenkel CA, Davis SR, Gompel A, et al. Treatment of symptoms of the menopause: an Endocrine Society Clinical Practice Guideline. J Clin Endocrinol Metab. 2015;100(11):3975-4011. [DOI] [PubMed] [Google Scholar]
- 29. ACOG committee opinion no. 556: Postmenopausal estrogen therapy: route of administration and risk of venous thromboembolism. Obstet Gynecol. 2013;121(4):887-890. [DOI] [PubMed] [Google Scholar]
- 30. El Khoudary SR, Zhao Q, Venugopal V, et al. Effects of hormone therapy on heart fat and coronary artery calcification progression: secondary analysis from the KEEPS trial. J Am Heart Assoc. 2019;8(15):e012763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Renoux C, Dell’aniello S, Garbe E, Suissa S. Transdermal and oral hormone replacement therapy and the risk of stroke: a nested case-control study. BMJ. 2010;340:c2519. [DOI] [PubMed] [Google Scholar]
- 32. Vinogradova Y, Coupland C, Hippisley-Cox J. Use of hormone replacement therapy and risk of venous thromboembolism: nested case-control studies using the QResearch and CPRD databases. BMJ. 2019;364:k4810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Mohammed K, Abu Dabrh AM, Benkhadra K, et al. Oral vs transdermal estrogen therapy and vascular events: a systematic review and meta-analysis. J Clin Endocrinol Metab. 2015;100(11):4012-4020. [DOI] [PubMed] [Google Scholar]
- 34. Marjoribanks J, Farquhar C, Roberts H, Lethaby A, Lee J. Long‐term hormone therapy for perimenopausal and postmenopausal women. Cochrane Database Syst Rev. 2017;1(1):CD004143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Clarke SC, Kelleher J, Lloyd-Jones H, Slack M, Schofiel PM. A study of hormone replacement therapy in postmenopausal women with ischaemic heart disease: the Papworth HRT atherosclerosis study. BJOG. 2002;109(9):1056-1062. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing is not applicable to this article as no datasets were generated or analyzed during the current study.