Abstract
Context
Standard glucocorticoid therapy in congenital adrenal hyperplasia (CAH) regularly fails to control androgen excess, causing glucocorticoid overexposure and poor health outcomes.
Objective
We investigated whether modified-release hydrocortisone (MR-HC), which mimics physiologic cortisol secretion, could improve disease control.
Methods
A 6-month, randomized, phase 3 study was conducted of MR-HC vs standard glucocorticoid, followed by a single-arm MR-HC extension study. Primary outcomes were change in 24-hour SD score (SDS) of androgen precursor 17-hydroxyprogesterone (17OHP) for phase 3, and efficacy, safety and tolerability of MR-HC for the extension study.
Results
The phase 3 study recruited 122 adult CAH patients. Although the study failed its primary outcome at 6 months, there was evidence of better biochemical control on MR-HC, with lower 17OHP SDS at 4 (P = .007) and 12 (P = .019) weeks, and between 07:00h to 15:00h (P = .044) at 6 months. The percentage of patients with controlled 09:00h serum 17OHP (< 1200 ng/dL) was 52% at baseline, at 6 months 91% for MR-HC and 71% for standard therapy (P = .002), and 80% for MR-HC at 18 months’ extension. The median daily hydrocortisone dose was 25 mg at baseline, at 6 months 31 mg for standard therapy, and 30 mg for MR-HC, and after 18 months 20 mg MR-HC. Three adrenal crises occurred in phase 3, none on MR-HC and 4 in the extension study. MR-HC resulted in patient-reported benefit including menses restoration in 8 patients (1 on standard therapy), and 3 patient and 4 partner pregnancies (none on standard therapy).
Conclusion
MR-HC improved biochemical disease control in adults with reduction in steroid dose over time and patient-reported benefit.
Keywords: congenital adrenal hyperplasia, 21-hydroxylase deficiency, glucocorticoid, hydrocortisone, adrenal insufficiency
Classic congenital adrenal hyperplasia, due to 21-hydroxylase deficiency (21-OHD-CAH), is a genetic disorder of steroidogenesis affecting approximately 1:15 000 live births (1). Lack of 21-hydroxylase causes cortisol deficiency and a counter-regulatory increase in pituitary adrenocorticotropin (ACTH) secretion, which drives overproduction of adrenal androgens, and adrenal hyperplasia. Patients with 21-OHD-CAH have 2 major problems: adrenal insufficiency and androgen excess. Adrenal insufficiency causes life-threatening adrenal crises (1-3), while androgen excess causes atypical genitalia in 46,XX neonates, promotes abnormal growth, short stature, and precocious puberty, and in adulthood, virilization of women and infertility in both sexes (4). Treatment aims to replace cortisol, and, where necessary, aldosterone. Supraphysiologic doses of glucocorticoids are typically needed to suppress ACTH and adrenal androgens. Management involves balancing glucocorticoid doses to avoid both glucocorticoid deficiency, risking adrenal crisis, and iatrogenic glucocorticoid excess, leading to short stature, obesity, hypertension, osteoporosis, and an adverse metabolic profile (1-3, 5-7). Patients with 21-OHD-CAH have increased mortality (8, 9) and poor health outcomes (10, 11) because current therapy fails to control adrenal androgen excess resulting in glucocorticoid overtreatment (5, 12).
Cortisol has a circadian rhythm with a nadir on going to sleep, increasing during early morning hours, peaking on waking, then decreasing through the day (13). In 21-OHD-CAH, absent cortisol overnight results in excess early-morning ACTH, which in turn drives excess generation of adrenal androgens. The adrenal androgen precursors, 17-hydroxyprogesterone (17OHP) and androstenedione are used for monitoring. Current glucocorticoids used in the treatment of 21-OHD-CAH are immediate-release preparations such as hydrocortisone, prednisolone, prednisone, and dexamethasone, which fail to mimic the early-morning cortisol rise (10, 11). There is consensus that hydrocortisone should be used in children to avoid risk of growth suppression with long-acting glucocorticoids (14); however, in adults, there is no agreement on which glucocorticoid to use. Patients often take glucocorticoids later in the evening to achieve biochemical control, when cortisol is normally low, resulting in metabolically adverse consequences (15). Despite different treatment regimens, optimal biochemical control, defined as 17OHP below 3 times the upper limit of normal (< 1200 ng/mL, 36 nmol/L) and androstenedione within the reference range, is achieved in only approximately 40% of patients (10, 11).
Hydrocortisone infusions mimicking the cortisol circadian rhythm have demonstrated improved biochemical control of 21-OHD-CAH (16, 17). A modified-release formulation of hydrocortisone (MR-HC), with a delayed-release action, given twice daily, simulates the overnight increase of cortisol (18, 19), and improved 21-OHD-CAH disease control in a phase 2 study (19). We now report findings from a phase 3 study of MR-HC vs standard glucocorticoid therapy followed by a single-arm efficacy and safety extension study in adults with 21-OHD-CAH.
Materials and Methods
Patients
Patients were recruited from 10 centers (7 countries) from February 2016 to January 2018. Patients had classic 21-OHD-CAH diagnosed in childhood, adequate mineralocorticoid replacement with renin less than 2 times the upper limit of normal, and were on stable glucocorticoid therapy over the preceding 6 months. Exclusion criteria included use of medication interfering with glucocorticoid metabolism, bilateral adrenalectomy, and night-shift work. The study protocols for the phase 3 extension study were approved by local ethics/institutional review boards and the Medicines and Healthcare Products Regulatory Agency (NCT03062280, Eudract 2015-005448-32). The trials were performed in accordance with the principles of the Declaration of Helsinki.
Trial Design
Patients, stratified by baseline glucocorticoid treatment (Table 1), were randomly assigned by an interactive web response system to receive MR-HC (Chronocort Diurnal Ltd UK) or to continue on standard therapy and after 6 months were offered MR-HC in the extension study. MR-HC was prescribed as 5-, 10-, or 20-mg capsules, and the initial dose was the hydrocortisone dose equivalent to their baseline therapy, with approximately one-third of the daily dose taken at 07:00h and two-thirds of the daily dose taken at 23:00h. At 4 and 12 weeks, dose titrations were made for both treatment groups, using identical rules, following centralized advice by 2 independent physicians blinded to all data except 24-hour hormone profiles and an investigator-completed adrenal insufficiency checklist. The blinded titrators considered morning and/or evening dose adjustments of either MR-HC or standard glucocorticoid using 17OHP/androstenedione measurements from the 24-hour profile and adrenal insufficiency symptom questionnaire (Table 2) results using the following algorithm:
Table 1.
Patient baseline characteristics
| Characteristic | Modified-release hydrocortisone group | Standard glucocorticoid group | Safety extension study |
|---|---|---|---|
| No. | 61 | 61 | 91 |
| Age, y, median (range) | 35 (19-61) | 40 (19-68) | 35 (20-67) |
| Female sex, No. (%) | 42 (68.9) | 36 (59) | 62 (68.1) |
| Salt-wasting, No. (%) | 49 (80) | 51 (84) | 77(85) |
| BMI, median (range) | 27.8 (18.0-43.7) | 27.0 (19.7-36.8) | 28.3 (18.0-43.7) |
| Fludrocortisone use, No. (%) | 52 (85) | 52 (85) | 77 (85) |
| Fludrocortisone mcg/d, median (range) | 100 (25-400) | 100 (25-400) | 100 (25-500) |
| Good disease control, No. (%)a,b | 20 (37.7) | 32 (61.5) | 52 (50.0) |
| Prestudy glucocorticoid treatment | |||
| Hydrocortisone, No. (%) | 36 (59.0) | 39 (63.9) | – |
| Prednisolone, No. (%) | 21 (34.4) | 22 (36.1) | – |
| Dexamethasone, No. (%) | 5 (8.2) | 5 (8.2) | – |
| Prednisone, No. (%) | 3 (4.9) | 2 (3.3) | – |
Abbreviation: BMI, body mass index.
a Good disease control defined as 09:00-hour 17-hydroxyprogesterone less than 1200 ng/dL.
b Patients meeting the criteria for the efficacy analysis.
Table 2.
Adrenal insufficiency checklist
This questionnaire should be used to determine whether symptoms of underreplacement or overreplacement of glucocorticoids have occurred in the preceding 4 weeks. Please ask participant: Have you experienced any of the following symptoms more than once per week in the last 4 weeks? Date of assessment (mm/dd/yyyy).
| Symptoms | Y/N | If Yes, do you believe this to be related to under or over replacement of glucocorticoid? Please state over/under | Any clinically significant findings? Y/N |
|---|---|---|---|
| Sudden weight loss | |||
| Sudden weight gain | |||
| Lack of appetite | |||
| Nausea | |||
| Vomiting | |||
| Headache | |||
| Blurred vision | |||
| Fatigue | |||
| Weakness | |||
| Dizziness | |||
| Lightheadedness | |||
| Syncope (sudden loss of consciousness) | |||
| Sleeping difficulties | |||
| Increased acne | |||
| Other | |||
| If yes to other, please specify: |
Abbreviations: N, no; Y, yes.
the 5 samples between 01:00 to 09:00h were considered to reflect glucocorticoids given in the evening/night time;
the 5 samples between 11:00 and 19:00h to reflect glucocorticoids given in the morning;
if 3 or more of these 5 samples were out of range, dose adjustments would be made, unless the adrenal insufficiency symptom questionnaire was in conflict with the biochemical findings; and
if 17OHP and androstenedione were inconsistent then the androstenedione results were to take precedence.
Target ranges for titration were the optimal range for 17OHP and reference range for androstenedione as follows:
17OHP 40 to 1200 ng/dL (range, 1.2-36.4 nmol/L);
androstenedione (males) 40 to 150 ng/dL (range, 1.4-5.2 nmol/L); and
androstenedione (females) 30 to 200 ng/dL (range, 1.0-7.0 nmol/L).
Local investigators and patients were aware of the trial-group assignment but were otherwise blinded. After 6 months, patients who had enrolled in the phase 3 and the previous phase 2 studies were invited to enroll in the extension study. The initial dose of MR-HC was the hydrocortisone dose equivalent to their dose at time of enrollment in the extension study. Dose titration was performed by the local investigators according to hormone results and symptoms of overreplacement or underreplacement. Hydrocortisone stress dosing and fludrocortisone dose adjustment occurred as medically indicated (20).
Trial Procedures
In the phase 3 study, 17OHP and androstenedione were measured at baseline, 4, 12, and 24 weeks every 2 hours from 15:00h to 15:00h, and in the extension study, blood was drawn at 09:00h and 13:00h. Hormones were measured by high-performance liquid chromatography–tandem mass spectrometry (Q2 Solutions). Additional assessments included body composition, dual-energy x-ray absorptiometry (DEXA) scanning, metabolic bloodwork (C-terminal cross-link telopeptide, fasting osteocalcin, high- sensitivity C-reactive protein, fasting glucose, fasting insulin, glycated hemoglobin A1c, plasma renin activity, total testosterone), and quality of life (QoL) assessments (Medical Outcome Short Form Health Survey Form 36 [SF-36], Global Fatigue Index, Standardized Health Questionnaire [EQ-5D]).
Outcome Measures
The primary outcome in the phase 3 study was the change from baseline to 24 weeks in the mean of the 24-hour 17OHP SD scores (SDS). Natural log transformation was performed to approximate a normal distribution. For each 2-hourly value of log 17OHP, the number of SDs from the midpoint of the natural logarithm of the reference range (males 40-220 ng/dL [1.2-6.7nmol/L], females 40-285 ng/dL [1.2-8.6 nmol/L]) was calculated, unsigned to provide equal weight to values above or below the midpoint. Secondary outcomes included serum androstenedione, safety (specifically stress dosing and adrenal crises) (21), and changes in weight, body mass index, waist circumference, body composition, and blood pressure. Exploratory end points included the primary outcome at 4 and 12 weeks. Post hoc analyses included percentage of patients with good disease control defined as 09:00h for 17OHP less than 1200 ng/dL (< 36 nmol/L) and for androstenedione below the reference range upper limit as previously defined (10, 11); areas under the curve (AUCs) of 17OHP and androstenedione; and 17OHP variability expressed as the ratio of arithmetic range of concentrations over 24 hours at 24 weeks to baseline. In the extension study, the primary end point was MR-HC safety assessed longitudinally using signs and symptoms of adrenal insufficiency or overtreatment; use of sick day rules; and adverse events including adrenal crises. The secondary end points included MR-HC long-term efficacy measurements such as daily dose of hydrocortisone and disease control assessed via 17OHP and androstenedione. Because patients had differing exposure to MR-HC before the study start, assessment was made against a pre–MR-HC baseline.
Statistical Analysis
The trial was designed to have greater than 95% power, at a 2-sided α of 5%, to detect a difference between treatment groups in the primary outcome consistent with the phase 2 study results. The primary outcome was compared between treatment groups within an analysis of covariance linear model with prestudy treatment category and baseline mean SDS as covariates. The same model was used for secondary and exploratory analyses of SDS and the area under the 24-hour profiles curve (AUC). Variability (amplitude) within a 24-hour hormone profile was defined as the maximum value divided by the minimum value. The change in the amplitude between baseline and 24 weeks, expressed as a ratio, was compared between treatment groups using a Wilcoxon test. The proportion of patients with good disease control at 24 weeks (09:00h 17OHP < 1200 ng/dL) was compared between treatment groups using a logistic model, adjusting for good disease control at baseline. All P values are 2-tailed and all CIs are 95% 2-sided. Because all participants in the extension trial received MR-HC, there were no formal treatment comparisons, but summaries over time were produced for safety and efficacy parameters.
Safety
Adverse events were recorded, including adverse events of special interest such as adrenal crisis. Events of therapeutic benefit such as restoration of menstruation have been recorded using the MedDRA term “unexpected therapeutic benefit” as recommended by regulators.
Results
Patients
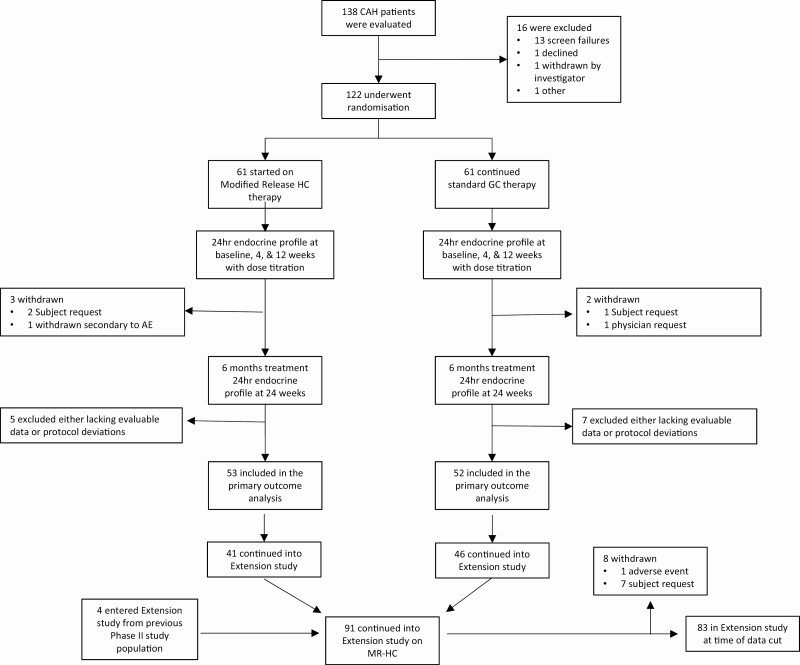
In the phase 3 study, 138 patients were screened, 122 were randomly assigned, 117 completed the study, and 105 met the criteria for efficacy analysis (Fig. 1). Between 3 and 25 patients were recruited from each European center, with 8 from the US center. Overall, the 2 treatment groups were balanced (see Table 1), but the number of patients with good baseline disease control was higher in the standard group. At baseline, 84% of patients were taking standard glucocorticoids after 18:00h, and 84% of patients were diagnosed as salt-wasting (see Table 1). Five patients (3 MR-HC, 2 standard) had experienced an adrenal crisis in the preceding year. In the extension study, 91 patients received at least one dose of MR-HC and 83 were participating at the time of this data cut (see Fig. 1). Safety data are presented up to 2 years and biochemical up to 18 months. Patients who chose not to participate in the extension study did so predominantly from one center because of logistical reasons (n = 13). Withdrawals were primarily for practical reasons, including desire for pregnancy.
Figure 1.
Patient screening, randomization treatment, and follow-up. The safety population included all randomly assigned patients who received at least one dose of trial treatment. *Patients could have more than one reason for study exclusion and withdrawn patients are included in patients excluded.
Biochemical Disease Control
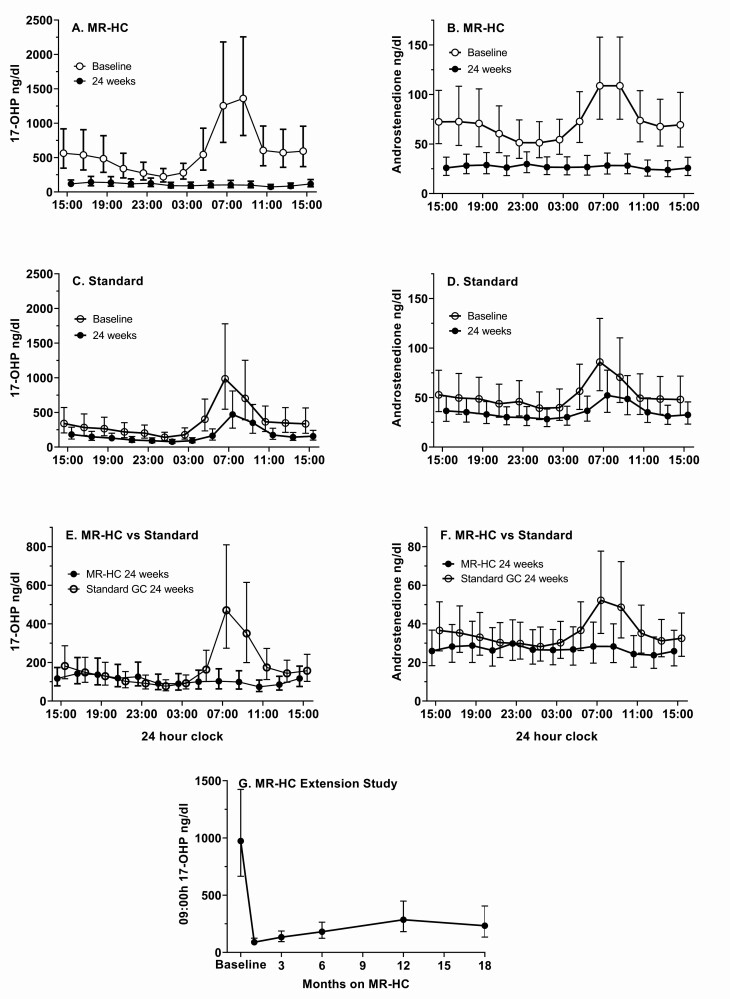
In phase 3, both groups achieved better hormonal control at 24 weeks compared to baseline reflected by a negative change in the 17OHP SDS 24-hour profile, therefore the trial failed its primary outcome (P = .55) (Table 3). There was no statistical difference between any of the groups, and removing any of the groups from the analysis did not change the primary outcome. The change from baseline was greater in MR-HC group at 4 (P = .007) and 12 (P = .019) weeks. At 24 weeks, a greater reduction in the 17OHP SDS profile was observed in the MR-HC group compared to the standard group between 07:00h and 15:00h (P = .044), and a reduction in the 17OHP AUC occurred in both groups, with a greater reduction in the MR-HC group (P = .025). At 24 weeks, compared to the standard group, good disease control (17OHP < 1200 ng/dL at 09:00h) was achieved more often in the MR-HC group (90.6% vs 71.2%, P = .002) and 17OHP variability (amplitude) over 24 hours was reduced in the MR-HC group (P < .001). At baseline the group randomly assigned to continue on standard treatment was better controlled than the MR-HC group 32/52 (62%) vs 20/53 (38%), and after 24 weeks 28 of 33 (85%) of those not under control at baseline were controlled in the MR-HC group, with 10 and 20 (50%) in the standard treatment group. The androstenedione SDS profile and change in AUC was similar to that for 17OHP. The pattern of biochemical control displayed in the 24-hour profiles differed between the 2 groups (Fig. 2). At 24 weeks, the MR-HC group 24-hour profile flattened and normalized both for 17OHP (Fig. 2A) and androstenedione (Fig. 2B), whereas the pathological morning increase in adrenal androgen precursors persisted in the standard group (Fig. 2C and 2D). Thus, a difference between the 2 groups at week 24 was observed during the morning hours, but not throughout the day (Fig. 2E and 2F). In the extension study, the number of patients in good disease control for 17OHP at 09:00h was 80% at 18 months vs 52% at baseline (Fig. 2G, Tables 1 and 3) and for androstenedione 96% vs 45% at baseline. In the phase 3 study, it did not make a difference to the analysis whether good control was measured at either 0700h or 0900h, so we used 0900h in the extension study because this is when many clinics measure hormones.
Table 3.
Study outcomes and disease-relevant clinical eventsa
| MR-HC group | Standard group | Comparison between groups | |
|---|---|---|---|
| Biochemical outcomes from phase 3 | N = 53 | N = 52 | Treatment effectb (95% CI), Pc |
| Baseline natural log 17OHP SDS profile | 1.25 ± 0.73 | 1.03 ± 0.82 | |
| Change from baseline in natural log 17OHP SDS profile | |||
| 24-h profile at 4 wks | –0.37 ± 0.63 | –0.07 ± 0.42 | –0.26 (–0.46 to –0.07), P = .007 |
| 24-h profile at 12 wks | –0.52 ± 0.85 | –0.10 ± 0.67 | –0.30 (–0.54 to –0.05), P = .019 |
| Primary end point: 24-h profile at 24 wks | –0.40 ± 0.85 | –0.17 ± 0.78 | –0.07 (–0.30 to 0.16), P = .55 |
| 07:00h-15:00h profile at 24 wks | –0.69 ± 0.96 | –0.21 ± 0.79 | –0.29 (–0.56 to –0.01), P = .044 |
| Baseline natural log 17OHP 24-h AUC | 65.2 ± 38.5 | 54.0 ± 39.2 | |
| Change from baseline in natural log 17OHP 24-h AUC | |||
| 24-h profile at 4 wks | –23.9 ± 27.7 | –6.1 ± 19.3 | –16.6 (–25.5 to –7.8), P < .001 |
| 24-h profile at 12 wks | –35.5 ± 35.3 | –13.5 ± 28.5 | –17.8 (–29.0 to –6.6), P = .002 |
| 24-h profile at 24 wks | –37.7 ± 42.6 | –17.8 ± 29.0 | –13.8 (–25.8 to –1.8), P = .025 |
| Amplitude ratio of 17OHPd: median (95% nonparametric CI]) | 0.36 [0.24, 0.65] | 0.92 [0.77, 1.37] | 0.38 (0.24, 0.61), P < .001 |
| Baseline natural log androstenedione 24-h AUC | 21.4 ± 30.4 | 13.9 ± 32.2 | |
| Change from baseline in natural log androstenedione 24-h AUC | |||
| 24-h profile at 4 wks | –12.5 ± 22.2 | –3.1 ± 11.3 | –8.9 (–15.6 to –2.1), P = .011 |
| 24-h profile at 12 wks | –20.6 ± 23.8 | –8.0 ± 15.1 | –10.9 (–18.3 to –3.5), P = .004 |
| 24-h profile at 24 wks | –22.9 ± 26.9 | –9.3 ± 20.4 | –10.5 (–18.7 to –2.3), P = .013 |
| Disease-relevant clinical events phase 3 study | N = 61 | N = 61 | |
| Adrenal crises, No. of patients (%) | 0 (0) | 3 (5.8) | NA |
| Stress dosing, No. of patients (%) | 26 (49.1) | 36 (69.2) | NA |
| Restoration of menses, No. of patients (%) | 4 (7.5) | 1 (1.9) | NA |
| Partner pregnancy (%) | 2 (3.8) | 0 (0) | NA |
| Biochemical outcomes from extension study | (N = 50) | ||
| Good disease controle (17OHP) at 18 mos, No. of patients (%) | 40 (80.0) | – | NA |
| 17OHP suppressed, No. of patients (%)↓ | 2 (4.0) | – | NA |
| Disease-relevant clinical events phase 3 study | (N = 91) | ||
| Adrenal crises, No. of patients (%) | 4 (4.4) | – | NA |
| Stress dosing, No. of patients (%) | 72 (79.1) | – | NA |
| Restoration of menses, No. of patients | 4 (4.4) | – | NA |
| Patient pregnancy | 3 (3.3) | – | NA |
| Partner pregnancy | 2 (2.2) | – | NA |
↓Suppressed 17OHP defined as undetectable.
Abbreviations: 17OHP, 17-hydroxyprogesterone; AUC, area under the curve; GC, glucocorticoid; MR-HC, modified-release hydrocortisone; NA, not available; SDS, SD score.
a Plus-minus values are means ± SD.
b Treatment effect is defined as least-squares mean difference (MR-HC – standard GC) for SDS profiles and 24-hour AUC adjusted for baseline value and prebaseline therapy, as the ratio MR-HC to standard GC for amplitude ratio, and as the odds ratio MR-HC vs standard GC for good disease control adjusted for baseline disease control status.
c CIs and P values were obtained from an analysis of covariance model for SDS profiles and 24-hour AUC, by the Hodges-Lehmann, and Wilcoxon methods, respectively, for amplitude ratio, and from a logistic model for good disease control.
d Amplitude is defined as the maximum divided by the minimum over the 24-hour assessment period. The ratio is the amplitude at 24 weeks divided by the amplitude at baseline.
e Good disease control defined as 09:00h 17OHP less than 1200 ng/dL.
Figure 2.
Twenty-four–hour endocrine profiles for 17-hydroxyprogesterone (17OHP) and androstenedione at week 24 vs baseline (geometric mean ± 95% CIs, patients meeting the criteria for the efficacy analysis) and 09:00hrs 17OHP during the extension study. A, At week 24, the 17OHP 24-hour profile for patients receiving modified-release hydrocortisone (MR-HC) was flat, and the morning rise in 17OHP observed at baseline was no longer present. B, Similar results were observed for androstenedione. Patients in the standard glucocorticoid group had improvement in hormonal control with glucocorticoid dose adjustments according to the protocol, but the pattern of hormone secretion did not change: C, 17OHP, and D, androstenedione, profiles continued to display a morning increase. At week 24, the MR-HC vs standard groups differed during the morning hours but not throughout the 24 hours for E, 17OHP, and F, androstenedione. G, During the extension study, the geometric mean 09:00h 17OHP fell from baseline into the optimal range and remained there despite a reduction in MR-HC daily dose.
Daily Glucocorticoid and Mineralocorticoid Dose
At 24 weeks, the MR-HC group increased from a median dose of 25 mg to 30 mg and standard glucocorticoid from 25 mg to 31 mg hydrocortisone dose equivalent (Table 4). Overall and by type of glucocorticoid at baseline, the MR-HC and standard groups were receiving similar glucocorticoid doses, but more patients required uptitrations in the standard group (31 vs 28) and more required downtitrations in MR-HC group (13 vs 3). The dose of fludrocortisone was changed in 3 patients (2 MR-HC and 1 standard). In the extension study, patients were downtitrated and the median dose at 18 months was 20 mg (see Table 4).
Table 4.
Glucocorticoid doses at baseline, 24 weeks, and during the extension study
| Dose | MR-HC group | Standard glucocorticoid groupa | ||||
|---|---|---|---|---|---|---|
| Baseline | 24 wks | Baseline | 24 wks | |||
| All (hydrocortisone dose equivalents) b | ||||||
| Median daily dose, mg | 25.0 | 30.0 | 25.0 | 31.3 | ||
| Range | 15-50 | 10-65 | 12.5-80 | 12.5-80 | ||
| Median dose/BSA, mg/m2/d | 13.6 | 15.8 | 14.4 | 17.0 | ||
| On hydrocortisone at baseline | ||||||
| Median daily dose, mg | 20.0 | 25.0 | 23.75 | 25.0 | ||
| Range | 12.5-40 | 10-65 | 12.5-35 | 15-55 | ||
| Median dose/BSA, mg/m2/d | 12.0 | 15.1 | 12.3 | 14.5 | ||
| On prednis(ol)one at baseline | ||||||
| Median daily dose, mg | 30 | 27.5 | 26.6 | 32.8 | ||
| Median dose/BSA, mg/m2/d | 16.7 | 16.5 | 15.7 | 18.5 | ||
| Range | 12.5-50 | 15-50 | 12.5-50 | 12.5-50 | ||
| On dexamethasone at baseline | ||||||
| Median daily dose, mg | 30 | 30 | 40 | 40 | ||
| Range | 29.6-38 | 30-45 | 20-80 | 33.5-80 | ||
| Median dose/BSA, mg/m2/d | 17.3 | 17.3 | 17.5 | 20.6 | ||
| MR-HC Safety Extension Study | ||||||
| Time from study start | 0-4 wks | 4-12 wks | 12-24 wks | 6-12 mos | 12-18 mos | 18-24 mos |
| MR-HC | MR-HC | MR-HC | MR-HC | MR-HC | MR-HC | |
| Median daily dose, mg, MR-HC | 30 | 26.0 | 25.0 | 20.3 | 20.1 | 20.0 |
| Range | 10-55 | 10-55 | 10-55 | 10-50.3 | 10-50.3 | 7.3-55 |
| Median dose, mg/m2/d, MR-HC | 15.8 | 15.0 | 13.5 | 12.5 | 11.7 | 11.1 |
| No. of patients with data available | 91 | 91 | 88 | 87 | 74 | 50 |
Abbreviations: BSA, body surface area; MR-HC, modified-release hydrocortisone.
a Standard glucocorticoid group are patients who continued on their conventional prestudy glucocorticoid treatment.
b Conversion factors established in endocrinology were used: Prednisone/prednisolone dose was multiplied by 5, and dexamethasone dose was multiplied by 80 (11). This dexamethasone conversion was used up to a maximum starting dose of MR-HC 30 mg, split as 20 mg at night and 10 mg in the morning.
There was no statistical difference between participants on the different standard treatment regimens at baseline, and removing any of the groups from the analysis did not change the primary outcome. In the prednisolone/prednisone at baseline patient group, the hydrocortisone dose on MR-HC fell from a median of 30 mg at baseline to 27.5 mg at 24 weeks and for those who continued on prednisolone, the hydrocortisone dose equivalent increased from 26.6 mg to 32.8 mg. There were relatively few patients on dexamethasone at baseline (n = 10) and they were split into those continuing dexamethasone (n = 5) and those who transferred to MR-HC (n = 5). For the patients who were on dexamethasone at baseline, the median dose in hydrocortisone equivalents of dexamethasone and MR-HC did not change from baseline to 24 weeks.
Secondary Outcomes of Interest
No differences between the 2 treatment groups were seen for fat mass, lean mass, or bone mineral density by DEXA; bone markers (serum C-terminal telopeptide and fasting osteocalcin); laboratory assessments of interest including high-sensitivity C-reactive protein, fasting glucose, fasting insulin, homeostatic model assessment of insulin resistance, glycated hemoglobin A1c, total testosterone, and plasma renin activity (Table 5). No significant changes in QoL were seen although responses for the majority of domains improved (Table 6).
Table 5.
Secondary outcomes and vital signs at baseline and change at 24 weeks and at 2 years in the extension study
| Outcome | MR-HC group baseline and change at 24 wks | Standard glucocorticoid group baseline and change at 24 wks | Extension study MR-HC baseline and change at 18-24 mos | |||
|---|---|---|---|---|---|---|
| Safety set | N = 61 | N = 61 | N = 50 | |||
| Baseline | Change | Baseline | Change | Baselinea | Change | |
| Weight, kg | 75.5 (18.5) | 0.87 (3.7) | 74.6 (13.2) | 1.0 (2.7) | 75.6 (16.1) | –0.28 (4.8) |
| Body mass index, kg/m2 | 28.5 (6.4) | 0.3 (1.5) | 27.7 (4.3) | 0.4 (1.0) | 28.8 (5.7) | –0.08 (2.0) |
| Waist circumference, cm | 90.9 (16.3) | 0.2 (5.4) | 90.5 (11.8) | 1.0 (5.6) | 91.5 (14.8) | 0.69(5.7) |
| Systolic blood pressure, mm Hg | 120.9 (13.6) | -1.8 (11.4) | 120.2 (14.4) | 0.5 (11.2) | 120.4 (13.9) | –3.1 (10.4) |
| Diastolic blood pressure, mm Hg | 71.1 (10.6) | -0.5 (9.2) | 70.6 (11.0) | 0.2 (9.2) | 70.6 (10.8) | –0.4 (9.4) |
| Efficacy evaluable set | N = 53 | N = 52 | N = 50 | |||
| Fat mass, kg | 29.535 (11.7) | –0.575 (3.3) | 26.163 (10.3) | 0.445 (2.5) | 27.943 (11.5) | –0.718 (4.8) |
| Lean mass, kg | 46.975 (9.3) | 0.640 (2.3) | 45.468 (9.1) | 0.234 (1.4) | 45.819 (9.3) | –0.079 (3.4) |
| Bone mineral density, g/cm2 | 1.126 (0.1) | –0.001 (0.0) | 1.111 (0.1) | –0.008 (0.0) | 1.094 (0.092) | 0.001 (0.04) |
| C-terminal cross-linked telopeptide, ng/L | 570 (257) | 9.3 (161) | 590 (260) | –23.3 (120) | 540.1 (252) | –52.3(176) |
| Fasting osteocalcin, μg/L | 19.93 (8.4) | –0.6 (8.1) | 21.51 (10.0) | –2.1 (6.3) | 19.49 (7.9) | 4.4 (8.2) |
| hsCRP, mg/L | 1.38 (1.3) | 0.54 (2.6) | 2.04 (4.4) | 0.20 (7.5) | 1.78 (5.0) | 2.3 (6.2) |
| Fasting glucose, mg/dL | 92.1 (8.5) | 9.9 (10.2) | 90.1 (9.9) | 1.8 (8.8) | 91.7 (7.8) | 2.6 (9.8) |
| Fasting insulin, mIU/L | 12.6 (6.3) | 1.9 (6.8) | 11.7 (5.6) | 3.0 (7.4) | 13.0 (5.9) | –2.1 (6.3) |
| HOMA-IR | 2.894 (1.6) | 0.914 (2.1) | 2.59 (1.3) | 0.77 (1.8) | – | – |
| HbA1c (%) | 5.16 (0.28) | 0.02 (0.26) | 5.18 (0.43) | –0.02 (0.28) | 5.17 (0.31) | 0.12 (0.23) |
| Plasma renin activity, ng/mL/h | 3.5 (2.5) | –1.0 (2.4) | 2.9 (2.4) | 0.4 (2.5) | 3.2 (3.5) | –0.2 (0.9) |
| Total testosterone women, ng/dL | 33 (33.4) | -21 (127.8) | 32 (52.9) | –9 (30.4) | 147 (263.8) | –13 (63.4) |
| Total testosterone men, ng/dL | 453 (320.9) | –26 (284.1) | 481 (142.6) | –36 (135.8) | 271 (305.9) | 77 (177.1) |
Mean (SD).
Abbreviations: HbA1c, glycated hemoglobin A1c; HOMA-IR, homeostatic model assessment of insulin resistance; hsCRP, high-sensitivity C-reactive protein; MR-HC, modified-release hydrocortisone.
a Baseline in extension study is pre–MR-HC.
Table 6.
Quality of life assessments at 24 weeks (phase 3 study) and at 12 and 18 months (extension study)
| Parameter | Phase 3 study | Safety extension study | ||
|---|---|---|---|---|
| MR-HC groupN = 53 | Standard groupN = 52 | 12 mosN = 73 | 18 mosN = 51 | |
| SF-36 absolute change from baseline by domaina | ||||
| T score: bodily painb | NA | NA | NA | NA |
| T score: general health perceptions | 0.79 (7.54) | –1.88 (5.97) | 1.43 (8.76) | 2.11 (5.66) |
| T score: mental health | 0.86 (7.32) | 0.35 (7.81) | 1.49 (9.44) | 1.33 (6.89) |
| T score: physical functioning | 1.16 (6.43) | -0.52 (4.27) | 0.41 (4.38) | 0.28 (4.25) |
| T score: role emotional | 0.99 (9.95) | -0.34 (9.21) | 1.38 (11.65) | 0.48 (8.50) |
| T score: role physical | 1.91 (8.33) | 0.50 (6.68) | 1.42 (7.53) | 0.81 (9.24) |
| T score: social functioning | 2.18 (9.25) | 0.87 (6.86) | 2.54 (9.00) | 0.89 (8.95) |
| T score: vitality | 0.79 (9.45) | 0.92 (6.10) | 2.15 (8.44) | 2.56 (6.80) |
| Global Fatigue Index absolute change in score from baseline | ||||
| GFI score derived from MAF | –0.74 (11.1) | –0.26 (7.8) | –1.93 (10.1) | –2.31 (10.6) |
| EQ-5D summary changes from baseline | ||||
| EQ-5D VAS score | –1.3 (13.67) | –1.2 (12.62) | 2.7 (17.74) | 2.3 (12.33) |
| EQ-5D-5L index score | 0.02 (0.12) | 0.02 (0.14) | –0.01 (0.17) | –0.01 (0.14) |
Values are mean (SD).
GFI scores range from 1 (no fatigue) to 50 (severe fatigue).
Abbreviations: EQ-5D, Standardized Health Questionnaire (5L = 5-level); GFI, Global Fatigue Index; MAF, multidimensional assessment of fatigue; MR-HC, modified-release hydrocortisone; N, number of evaluable participants; NA, not available; SF-36, Medical Outcome Short Form Health Survey Form 36 (Subject Questionnaire); VAS, visual analog scale.
a Baseline is defined as start of study in the phase 3 study and pre–MR-HC initiation baseline in the safety extension study.
b A technical issue with the scoring of the bodily pain domain meant that these data are not available.
Safety
In this phase 3 study, no patients experienced adrenal crises in the MR-HC group compared with 3 (4.9%) in the standard group. A total of 299 adverse events (15 of therapeutic benefit) were reported by 96.7% of patients in the MR-HC group, and 224 adverse events (1 of therapeutic benefit) were reported by 78.7% of patients in the standard group. No serious adverse event was considered causally related to study intervention. Glucocorticoid stress dosing was reported by 26 patients (42.6%) in the MR-HC group and 36 patients (59.0%) in the standard group. Unexpected events considered to be of therapeutic benefit included resumption of regular menses in 5 patients (4 MR-HC, 1 standard), partner pregnancies of 2 patients in the MR-HC group with full-term deliveries; one of these patients had a history of testicular adrenal rest tissue with documented sperm count improvement (< 0.1 million/mL prior to MR-HC and 10.3 million/mL during MR-HC treatment).
In the extension study there were 4 patients with adrenal crises. A total of 780 adverse events (29 of therapeutic benefit) were reported by 87 participants (95.6%). Severe adverse events were reported for 14 participants (15.4%), of which one was considered related to MR-HC (hypokalemia). Glucocorticoid stress dosing was reported for 72 participants (79.1%). The most common (18 patients, 19.8%) reported events considered to be of therapeutic benefit were feeling more alert (11 patients) and improved menstrual cycle (4 patients). The partner of 2 patients on MR-HC became pregnant during the study and successfully delivered. Three patients became pregnant on MR-HC; one suffered an early miscarriage after transitioning to standard therapy.
Discussion
This is the first randomized, controlled trial of glucocorticoid treatment in patients with 21-OHD-CAH. Patients who received MR-HC had superior hormonal control during the morning and early afternoon compared to those receiving standard therapy, and this was sustained over 18 months of follow-up. Morning hormonal control is important in 21-OHD-CAH because failure to control the overnight increase in adrenal androgens results in excess glucocorticoid exposure and poor health outcomes (5). The trial failed its primary end point because the difference between the 2 groups in the morning did not translate into a difference over 24 hours at 24 weeks. The primary outcome was based on a phase 2 trial (19); however, the analysis was unhelpful in the phase 3 randomized trial because the SDS analysis overemphasized scores below the midpoint of the reference range, and the logarithmic transformation and use of a mean score over 24 hours obscured the impact of MR-HC in the morning and early afternoon. The raw data showed significant improvement of the clinically relevant end point of morning biochemical control, with reduced AUC and 17OHP amplitude in patients receiving MR-HC. The improvement in biochemical control was maintained at 18 months, with 80% displaying good control for 17OHP and 96% for androstenedione vs 52% and 45% at baseline, despite reduction of the hydrocortisone dose by 33%, to doses regularly used for adrenal replacement therapy.
MR-HC replicates the physiological overnight increase in cortisol, thereby preventing ACTH-driven excess production of adrenal androgens (19). MR-HC given twice daily replicates both the early morning increase in cortisol as well as daytime cortisol levels (22). There is some evidence that cortisol clearance may vary over 24 hours, possibly related to a circadian rhythm in cortisol-binding protein; however, the amplitude of this variation is small and does not appear clinically significant (23). Biochemical control was better on MR-HC compared to standard glucocorticoid at 4 weeks (ie, prior to dose titration) when patients were receiving MR-HC at an equivalent daily dose to baseline treatment. At 24 weeks, following dose titration, morning control was better on MR-HC than standard glucocorticoids, 91% in control vs 71%, respectively, with little fluctuation in 17OHP and no morning increase in the MR-HC–treated patients, similar to the profile of 17OHP in normal physiology. These findings are important because it is the high excursions in 17OHP that drive production of androstenedione and androgens that affect growth, puberty, and fertility.
Immediate-release hydrocortisone is the recommended first-line therapy in 21-OHD-CAH; prednisolone and dexamethasone are introduced when biochemical control cannot be achieved, but are associated with higher rates of adverse outcomes (15, 24, 25). Higher bedtime doses (reverse circadian regimen) are commonly used in an attempt to improve androgen control (11, 26, 27), with 84% at baseline in this study; however, evening glucocorticoid administration has the potential for adverse metabolic actions (28) and insomnia. The median hydrocortisone dose at 24 weeks was 15.8 and 17.0 mg/m2/day for MR-HC and the standard group, respectively, similar doses to those reported in cohort studies (15-18 mg/m2/day), in which biochemical control was worse than in this trial (10, 11, 29, 30). In the extension study, with local clinicians performing dose titrations, biochemical control was maintained at a reduced daily dose of 20 mg consistent with that recommended for replacement in adrenal insufficiency: 15 to 25 mg daily (31). On study entry the patients had their therapy optimized for 6 months, and yet were able to have a sustained dose reduction in the extension study. Optimal control of adrenal androgens is required for fertility in men and women with 21-OHD-CAH (32-34). Our findings demonstrate clinical value for improved morning hormonal control, with women restarting menstruation, improved sperm quality in a patient with testicular adrenal rest tissue, and 4 partner and 3 patient pregnancies on MR-HC.
There are few published data on the circadian variation of 17OHP in healthy individuals, but what there is demonstrate that in the normal individual 17OHP displays minor circadian variability within the reference range and varies by the phase of the menstrual cycle in females (35, 36). The reference range for 17OHP at the Mayo Clinic is less than 285 ng/dL during the luteal phase, which is the highest level seen in healthy individuals. Clinicians have recognized that in patients with CAH receiving standard therapy, it is not possible to control 17OHP in the morning even when raising the dose of glucocorticoid. This is shown in the present study in patients on standard treatment whose glucocorticoid dose was increased. To avoid overtreatment, guidelines have suggested not suppressing the 17OHP into the reference range, and 2 observational cohort studies of CAH defined an optimal range of 17OHP to be less than 1200 ng/dL, which approximates to 3 times the upper limit of the normal range (10, 11). Our phase 3 study shows that by treating with MR-HC, which provides a physiological increase in cortisol levels overnight, it is now possible to control the overnight increase in 17OHP so a relatively flat 17OHP profile over 24 hours is achieved, similar to that seen in healthy individuals. In the phase 3 study, we found that MR-HC at 30 mg per day controls many patients into the reference range and this dose is similar to that reported in all 4 CAH cohort studies in which dose is provided and control is generally less than 50% (10, 11, 29, 30). When the dose of MR-HC was reduced in the extension study to adrenal replacement levels, median dose 20 mg, the 17OHP remained in the optimal range in 80% of patients. This median dose is 20% to 40% less (5-10 mg/day less) than that reported in the cohort studies (10, 11, 29, 30). The “optimal” 17OHP level could be debated and practices vary among clinicians; however, there is agreement that lower glucocorticoid doses are beneficial and this was achieved in the extension study, with female patients reporting improvements in menstrual regularity and improvement in fertility in both sexes.
The evidence that an adrenal replacement dose of hydrocortisone has better health outcomes than the higher doses more commonly used in CAH comes from the literature examining hydrocortisone replacement therapy in adrenal insufficiency. In large retrospective studies of patients on glucocorticoids, a hydrocortisone equivalent dose greater than 20 mg per day was associated with an unfavorable metabolic profile (37), and a daily dose of hydrocortisone greater than 25 mg per day was associated with increased mortality (38). Similarly, higher replacement doses of hydrocortisone have been associated with lower QoL in patients with adrenal insufficiency (39), and lower psychological well-being (40). In a large longitudinal historical cohort study in pediatric patients, the dose of hydrocortisone in patients with CAH was negatively associated with final height, with each 1 mg/m2/day of hydrocortisone equating to a loss of 0.37 cm in height (41).
Currently there is no consensus on monitoring glucocorticoid therapy in CAH (10). Clinicians measure biomarkers before and after dosing (42), and aim for 17OHP levels above the reference range and androstenedione within the reference range for good control, with normal 17OHP indicating overtreatment and high androstenedione indicating undertreatment (14, 24). In 21-OHD-CAH, the physiological pathway to androstenedione biosynthesis from 17-hydroxypregenolone via DHEA (dehydroepiandrosterone) is downregulated (42, 43), and androstenedione biosynthesis primarily occurs through conversion of excess 17OHP. With MR-HC treatment, we observed near normalization of 17OHP and concurrently low androstenedione, despite a median daily MR-HC dose of 20 mg, suggesting that the low androstenedione is not explained by glucocorticoid-mediated suppression. Thus, you cannot keep downtitrating the glucocorticoid dose based on a low A4 because you will go below adrenal replacement doses and risk adrenal insufficiency. The mode of action of MR-HC flattens the 24-hour 17OHP profile, similar to that seen in healthy individuals, and provides the rationale for a monitoring and titration schedule whereby the morning 17OHP reflects the evening dose and the afternoon 17OHP the morning dose of MR-HC. Thus, in the extension study, patients have been titrated using a sample measured at 0900h and 1300h, which are times suitable for the clinic. Two samples are required and could potentially be provided remotely if suitable validated assays for saliva and blood spot are available. MR-HC provides a potential simplified paradigm for the treatment and monitoring of CAH with dosing at bedtime and waking, and hormonal measurements at 0900h to judge the nighttime dose and around midday to judge the morning dose.
The lack of significant differences in clinical and patient-reported outcomes between the 2 groups in the phase 3 study reflects the study’s short duration and similar daily glucocorticoid doses. QoL has been shown to improve when using a pump to deliver subcutaneous diurnal hydrocortisone infusion to 21-OHD-CAH patients with poor control and QoL at baseline (17); however, QoL in 21-OHD-CAH has variably been reported as either impaired or normal (44). Our patients were a diverse population at baseline with a QoL similar to the general population, which would make demonstrating improvement difficult.
More than one-third of patients had an infection during the study, consistent with the observation that patients with adrenal insufficiency have higher infection rates (45), and emphasizes the importance of teaching patients sick day rules. Mortality in patients with 21-OHD-CAH is increased and adrenal crisis is responsible for up to 42% of excess deaths (8, 9). The incidence of adrenal crisis in patients with adrenal insufficiency is estimated to be 5 to 10 adrenal crises/100 patient-years, with a mortality rate of 0.5/100 patient-years (20, 21, 46–48). Five of 122 patients had an adrenal crisis in the year before the study (3 randomly assigned to MR-HC), and during the randomized study, 3 patients in the standard group had an adrenal crisis but none did in the MR-HC group. In the extension study, 4 patients had an adrenal crisis with a frequency of 6.2 crises per 100 treatment-years, similar to population estimates (20, 48), and providing confidence that the safety profile of MR-HC does not differ from that of immediate-release hydrocortisone.
A strength of our phase 3 study was its international, multicenter, randomized design enabling us to study a large cohort in a rare disease; the extension study provided data on up to 2 years of treatment with MR-HC, a sound basis for efficacy and safety assessment. The limitations of the phase 3 study include its open-label design (mitigated by the blinded dose titration) and the complexity of the protocol and statistical analysis. Blinding was considered impractical because of the multiple dosing regimens and the difficulties replicating the bitterness of hydrocortisone in a placebo. It was challenging to admit patients for a 24-hour profile, which may have restricted recruitment and created a selection bias for better-controlled patients. The intensive monitoring and more aggressive dose uptitration than usually performed in clinical practice may explain the improved control in the standard group compared to previous observational studies of 21-OHD-CAH (10, 11), and the extension study demonstrated that MR-HC dose reduction could be achieved with more simplified monitoring.
In conclusion, we found that MR-HC improved morning and early afternoon biochemical control of 21-OHD-CAH over standard glucocorticoid therapy. This control was sustained for 18 months on hydrocortisone doses recommended for adrenal replacement therapy and lower than doses normally used in 21-OHD-CAH. MR-HC provides a well-tolerated and practical twice-daily treatment regimen for 21-OHD-CAH.
Acknowledgments
Financial Support: This work was supported by Diurnal Ltd UK, with further support from the Intramural Research Program of the National Institutes of Health (NIH).
Clinical Trial Information: EudraCT registration Nos. 2015-000711-40 and 2015-005448-32 (registered February 10, 2015); Clinicaltrials.gov registration Nos. NCT02716818 and NCT03062280 (registered March 22, 2016).
Author Contributions: The first and last authors vouch for the accuracy, completeness of the data, and analyses. All authors critically reviewed the manuscript, participated in the design, and analysis of the trial.
Glossary
Abbreviations
- 17OHP
17-hydroxyprogesterone
- 21-OHD
21-hydroxylase
- ACTH
adrenocorticotropin
- AUC
area under the curve
- CAH
congenital adrenal hyperplasia
- DEXA
dual-energy x-ray absorptiometry
- MR-HC
modified-release hydrocortisone
Additional Information
Disclosures: D.P.M. has received research funds from Diurnal Ltd through an NIH Cooperative Research and Development Agreement. A.M., W.A., A.B.P., A.H., A.J., J.N.P., C.P., A.P., A.R., N.R., N.S., and P.To. were study investigators. R.J.R. is a director; K.M. and J.P. are employees, and P.Tr. is a consultant of Diurnal Ltd.
Data Availability
Datasets generated during and analyzed during the current study are not publicly available but are available from the corresponding author on reasonable request.
References
- 1. Merke DP, Auchus RJ. Congenital adrenal hyperplasia due to 21-hydroxylase deficiency. N Engl J Med. 2020;383(13):1248-1261. [DOI] [PubMed] [Google Scholar]
- 2. Cutler GB Jr, Laue L. Congenital adrenal hyperplasia due to 21-hydroxylase deficiency. N Engl J Med. 1990;323(26):1806-1813. [DOI] [PubMed] [Google Scholar]
- 3. Rushworth RL, Torpy DJ, Falhammar H. Adrenal crisis. N Engl J Med. 2019;381(9):852-861. [DOI] [PubMed] [Google Scholar]
- 4. Merke DP, Bornstein SR. Congenital adrenal hyperplasia. Lancet. 2005;365(9477):2125-2136. [DOI] [PubMed] [Google Scholar]
- 5. Han TS, Walker BR, Arlt W, Ross RJ. Treatment and health outcomes in adults with congenital adrenal hyperplasia. Nat Rev Endocrinol. 2014;10(2):115-124. [DOI] [PubMed] [Google Scholar]
- 6. Tamhane S, Rodriguez-Gutierrez R, Iqbal AM, et al. Cardiovascular and metabolic outcomes in congenital adrenal hyperplasia: a systematic review and meta-analysis. J Clin Endocrinol Metab. 2018;103(11):4097-4103. [DOI] [PubMed] [Google Scholar]
- 7. Speiser PW, White PC. Congenital adrenal hyperplasia. N Engl J Med. 2003;349(8):776-788. [DOI] [PubMed] [Google Scholar]
- 8. Jenkins-Jones S, Parviainen L, Porter J, et al. Poor compliance and increased mortality, depression and healthcare costs in patients with congenital adrenal hyperplasia. Eur J Endocrinol. 2018;178(4):309-320. [DOI] [PubMed] [Google Scholar]
- 9. Falhammar H, Frisén L, Norrby C, et al. Increased mortality in patients with congenital adrenal hyperplasia due to 21-hydroxylase deficiency. J Clin Endocrinol Metab. 2014;99(12):E2715-E2721. [DOI] [PubMed] [Google Scholar]
- 10. Arlt W, Willis DS, Wild SH, et al. ; United Kingdom Congenital Adrenal Hyperplasia Adult Study Executive (CaHASE) . Health status of adults with congenital adrenal hyperplasia: a cohort study of 203 patients. J Clin Endocrinol Metab. 2010;95(11):5110-5121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Finkielstain GP, Kim MS, Sinaii N, et al. Clinical characteristics of a cohort of 244 patients with congenital adrenal hyperplasia. J Clin Endocrinol Metab. 2012;97(12):4429-4438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Cutler GB. Treatment of congenital adrenal hyperplasia. J Clin Endocrinol Metab. 1996;81:3185-3186. [DOI] [PubMed] [Google Scholar]
- 13. Debono M, Ghobadi C, Rostami-Hodjegan A, et al. Modified-release hydrocortisone to provide circadian cortisol profiles. J Clin Endocrinol Metab. 2009;94(5):1548-1554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Speiser PW, Arlt W, Auchus RJ, et al. Congenital adrenal hyperplasia due to steroid 21-hydroxylase deficiency: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2018;103(11):4043-4088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Whittle E, Falhammar H. Glucocorticoid regimens in the treatment of congenital adrenal hyperplasia: a systematic review and meta-analysis. J Endocr Soc. 2019;3(6):1227-1245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Merza Z, Rostami-Hodjegan A, Memmott A, et al. Circadian hydrocortisone infusions in patients with adrenal insufficiency and congenital adrenal hyperplasia. Clin Endocrinol (Oxf). 2006;65(1):45-50. [DOI] [PubMed] [Google Scholar]
- 17. Nella AA, Mallappa A, Perritt AF, et al. A phase 2 study of continuous subcutaneous hydrocortisone infusion in adults with congenital adrenal hyperplasia. J Clin Endocrinol Metab. 2016;101(12):4690-4698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Whitaker M, Debono M, Huatan H, Merke D, Arlt W, Ross RJ. An oral multiparticulate, modified-release, hydrocortisone replacement therapy that provides physiological cortisol exposure. Clin Endocrinol (Oxf). 2014;80(4):554-561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Mallappa A, Sinaii N, Kumar P, et al. A phase 2 study of Chronocort, a modified-release formulation of hydrocortisone, in the treatment of adults with classic congenital adrenal hyperplasia. J Clin Endocrinol Metab. 2015;100(3):1137-1145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. El-Maouche D, Hargreaves CJ, Sinaii N, Mallappa A, Veeraraghavan P, Merke DP. Longitudinal assessment of illnesses, stress dosing and illness sequelae in patients with congenital adrenal hyperplasia. J Clin Endocrinol Metab 2018;103(6):2336-2345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Allolio B. Extensive expertise in endocrinology. Adrenal crisis. Eur J Endocrinol. 2015;172(3):R115-R124. [DOI] [PubMed] [Google Scholar]
- 22. Porter J, Blair J, Ross RJ. Is physiological glucocorticoid replacement important in children? Arch Dis Child. 2017;102(2):199-205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Melin J, Hartung N, Parra-Guillen ZP, Whitaker MJ, Ross RJ, Kloft C. The circadian rhythm of corticosteroid-binding globulin has little impact on cortisol exposure after hydrocortisone dosing. Clin Endocrinol (Oxf). 2019;91(1):33-40. [DOI] [PubMed] [Google Scholar]
- 24. Auchus RJ, Arlt W. Approach to the patient: the adult with congenital adrenal hyperplasia. J Clin Endocrinol Metab. 2013;98(7):2645-2655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Paizoni L, Auer MK, Schmidt H, Hübner A, Bidlingmaier M, Reisch N. Effect of androgen excess and glucocorticoid exposure on metabolic risk profiles in patients with congenital adrenal hyperplasia due to 21-hydroxylase deficiency. J Steroid Biochem Mol Biol. 2020;197:105540. [DOI] [PubMed] [Google Scholar]
- 26. Auchus RJ. Management of the adult with congenital adrenal hyperplasia. Int J Pediatr Endocrinol. 2010;2010:614107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Pijnenburg-Kleizen KJ, Thomas CMG, Otten BJ, Roeleveld N, Claahsen-van der Grinten HL. Long-term follow-up of children with classic congenital adrenal hyperplasia: suggestions for age dependent treatment in childhood and puberty. J Pediatr Endocrinol Metab. 2019;32(10):1055-1063. [DOI] [PubMed] [Google Scholar]
- 28. Plat L, Leproult R, L’Hermite-Baleriaux M, et al. Metabolic effects of short-term elevations of plasma cortisol are more pronounced in the evening than in the morning. J Clin Endocrinol Metab. 1999;84(9):3082-3092. [DOI] [PubMed] [Google Scholar]
- 29. Chakhtoura Z, Bachelot A, Samara-Boustani D, et al. ; Centre des Maladies Endocriniennes Rares de la Croissance and Association Surrénales . Impact of total cumulative glucocorticoid dose on bone mineral density in patients with 21-hydroxylase deficiency. Eur J Endocrinol. 2008;158(6):879-887. [DOI] [PubMed] [Google Scholar]
- 30. Schnaider-Rezek GS, Lemos-Marini SHV, Baptista MTM, et al. Metabolic evaluation of young women with congenital adrenal hyperplasia. Arq Bras Endocrinol Metabol. 2011;55(8): 646-652. [DOI] [PubMed] [Google Scholar]
- 31. Bornstein SR, Allolio B, Arlt W, et al. Diagnosis and treatment of primary adrenal insufficiency: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2016;101(2): 364-389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Merke DP. Approach to the adult with congenital adrenal hyperplasia due to 21-hydroxylase deficiency. J Clin Endocrinol Metab. 2008;93(3):653-660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Casteràs A, De Silva P, Rumsby G, Conway GS. Reassessing fecundity in women with classical congenital adrenal hyperplasia (CAH): normal pregnancy rate but reduced fertility rate. Clin Endocrinol (Oxf). 2009;70(6):833-837. [DOI] [PubMed] [Google Scholar]
- 34. Bouvattier C, Esterle L, Renoult-Pierre P, et al. Clinical outcome, hormonal status, gonadotrope axis, and testicular function in 219 adult men born with classic 21-hydroxylase deficiency. A French national survey. J Clin Endocrinol Metab. 2015;100(6):2303-2313. [DOI] [PubMed] [Google Scholar]
- 35. Ghizzoni L, Bernasconi S, Virdis R, et al. Dynamics of 24-hour pulsatile cortisol, 17-hydroxyprogesterone, and androstenedione release in prepubertal patients with nonclassic 21-hydroxylase deficiency and normal prepubertal children. Metabolism. 1994;43(3):372-377. [DOI] [PubMed] [Google Scholar]
- 36. Fanelli F, Gambineri A, Belluomo I, et al. Androgen profiling by liquid chromatography-tandem mass spectrometry (LC-MS/MS) in healthy normal-weight ovulatory and anovulatory late adolescent and young women. J Clin Endocrinol Metab. 2013;98(7):3058-3067. [DOI] [PubMed] [Google Scholar]
- 37. Filipsson H, Monson JP, Koltowska-Häggström M, Mattsson A, Johannsson G. The impact of glucocorticoid replacement regimens on metabolic outcome and comorbidity in hypopituitary patients. J Clin Endocrinol Metab. 2006;91(10): 3954-3961. [DOI] [PubMed] [Google Scholar]
- 38. Sherlock M, Reulen RC, Alonso AA, et al. ACTH deficiency, higher doses of hydrocortisone replacement, and radiotherapy are independent predictors of mortality in patients with acromegaly. J Clin Endocrinol Metab. 2009;94(11):4216-4223. [DOI] [PubMed] [Google Scholar]
- 39. Bleicken B, Hahner S, Loeffler M, et al. Influence of hydrocortisone dosage scheme on health-related quality of life in patients with adrenal insufficiency. Clin Endocrinol (Oxf). 2010;72(3):297-304. [DOI] [PubMed] [Google Scholar]
- 40. Falhammar H, Butwicka A, Landén M, et al. Increased psychiatric morbidity in men with congenital adrenal hyperplasia due to 21-hydroxylase deficiency. J Clin Endocrinol Metab. 2014;99(3):E554-E560. [DOI] [PubMed] [Google Scholar]
- 41. Sarafoglou K, Addo OY, Turcotte L, et al. Impact of hydrocortisone on adult height in congenital adrenal hyperplasia—the Minnesota cohort. J Pediatr. 2014;164(5): 1141-1146.e1. [DOI] [PubMed] [Google Scholar]
- 42. Debono M, Mallappa A, Gounden V, et al. Hormonal circadian rhythms in patients with congenital adrenal hyperplasia: identifying optimal monitoring times and novel disease biomarkers. Eur J Endocrinol. 2015;173(6):727-737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Storbeck KH, Schiffer L, Baranowski ES, et al. Steroid metabolome analysis in disorders of adrenal steroid biosynthesis and metabolism. Endocr Rev. 2019;40(6):1605-1625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Han TS, Krone N, Willis DS, et al. ; United Kingdom Congenital adrenal Hyperplasia Adult Study Executive (CaHASE) . Quality of life in adults with congenital adrenal hyperplasia relates to glucocorticoid treatment, adiposity and insulin resistance: United Kingdom Congenital adrenal Hyperplasia Adult Study Executive (CaHASE). Eur J Endocrinol. 2013;168(6):887-893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Tresoldi AS, Sumilo D, Perrins M, et al. Increased infection risk in Addison’s disease and congenital adrenal hyperplasia. J Clin Endocrinol Metab. 2020;105(2):418-429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Rushworth RL, Torpy DJ, Stratakis CA, Falhammar H. Adrenal crises in children: perspectives and research directions. Horm Res Paediatr. 2018;89(5):341-351. [DOI] [PubMed] [Google Scholar]
- 47. Eyal O, Levin Y, Oren A, et al. Adrenal crises in children with adrenal insufficiency: epidemiology and risk factors. Eur J Pediatr. 2019;178(5):731-738. [DOI] [PubMed] [Google Scholar]
- 48. Reisch N, Willige M, Kohn D, et al. Frequency and causes of adrenal crises over lifetime in patients with 21-hydroxylase deficiency. Eur J Endocrinol. 2012;167(1):35-42. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Datasets generated during and analyzed during the current study are not publicly available but are available from the corresponding author on reasonable request.