Abstract
Context
Adrenal tumors in noncancer patients are common.
Objective
Evaluate performance of 18F-fluorodeoxyglucose positron emission tomography computed tomography (18F-FDG-PET/CT) in distinguishing between benign and malignant adrenal tumors.
Design
Retrospective chart review 2010-2019.
Setting
Academic institution.
Patients
One hundred and seventeen noncancer patients, defined as having no history of cancer or with cancer in remission for ≥5 years, completed 18F-FDG-PET/CT to evaluate adrenal masses, with pathologic diagnoses or imaging follow-up (≥12 months).
Intervention
18F-FDG-PET/CT of 117 indeterminate adrenal masses.
Main Outcome Measures
Receiver operator characteristic curve of the ratios of adrenal lesion standardized uptake value (SUV)max to liver SUVmean and of adrenal lesion SUVmax to aortic arch blood pool SUVmean were constructed.
Results
Seventy benign and 47 malignant masses (35 adrenocortical carcinomas [ACCs], 12 adrenal metastases) were identified. Malignant masses had higher median liver SUV and blood pool SUV ratios than benign masses (6.2 and 7.4 vs 1.4 and 2.0, P < .001). Median liver and blood pool SUV ratios of ACC (6.1 and 7.3, respectively) and metastases (6.7 and 7.7, respectively) were higher than those of than adenomas (1.4 and 2.2, P < .05 for all comparisons). Optimal liver SUV ratio to discern between benign and malignant masses was 2.5, yielding 85% sensitivity, 90% specificity, and 7 false negative results (including 3 ACCs). Optimal blood pool SUV ratio was 3.4, yielding 83% sensitivity, 90% specificity, and 8 false negative results (including 4 ACCs).
Conclusion
When used in conjunction with other clinical assessments, 18F-FDG-PET/CT can be a valuable tool in evaluating adrenal masses in noncancer patients.
Diagnosis of incidental adrenal nodules has become increasingly common with the widespread availability and accessibility of cross-sectional imaging, yet definitive determination of biological behavior (benign vs malignant) often remains a challenge. Adrenal nodules have an estimated prevalence of 2% to 10% (1-4), of which the vast majority are benign nonfunctioning adrenocortical adenomas (ACAs) that do not require intervention. However, it is important to characterize the biochemical and pathologic features of adrenal lesions, as the presence of hormone production or malignancy strongly impacts therapy. Hormone-producing tumors require surgical and/or medical management. Preoperative concern for malignant adrenocortical tumors can guide surgical management and prompt open, rather than laparoscopic, surgical removal (5-7). Therefore, determining the underlying nature of the adrenal tumor is critical to management.
Cross-sectional imaging features on computed tomography (CT) and magnetic resonance imaging (MRI) can help differentiate benign from malignant masses to guide management. Imaging characteristics of homogeneous appearance and low Hounsfield units on unenhanced CT images reliably identify benign ACAs; a recent study indicates a threshold of 20 HU on unenhanced CT images can increase specificity for detecting adrenocortical carcinomas (ACCs) while maintaining sensitivity (8). High loss of signal intensity on out-of-phase vs in-phase MRI images (9-11) is indicative of a benign lipid rich adenoma, while high washout on contrast-enhanced CT images (10, 12-16) is consistent with a lipid poor benign adenoma. When cross-sectional imaging is equivocal, however, 18F-fluorodeoxyglucose positron emission tomography computed tomography (18F-FDG-PET/CT) can be a valuable tool for further characterization. A number of studies have evaluated the use of 18F-FDG-PET in cancer patients and found high sensitivity and specificity in differentiating benign from malignant adrenal masses. While the available data suggest that 18F-FDG-PET can be useful in cancer patients (17), only a limited number of studies have evaluated the use of 18F-FDG-PET in noncancer patients.
This study focuses on the performance of 18F-FDG-PET/CT completed specifically for the evaluation of incidental indeterminate adrenal masses in noncancer patients, for whom there was ultimately pathological diagnoses or stability on follow-up imaging. Due to our institution being a referral center for adrenal tumors, particularly for masses suspicious for ACC, our comprehensive cohort that includes many uncommon and malignant lesions gives a unique perspective on the practical applications of 18F-FDG-PET/CT imaging in differentiating between malignant and benign lesions.
Materials and Methods
Patient Identification and Inclusion
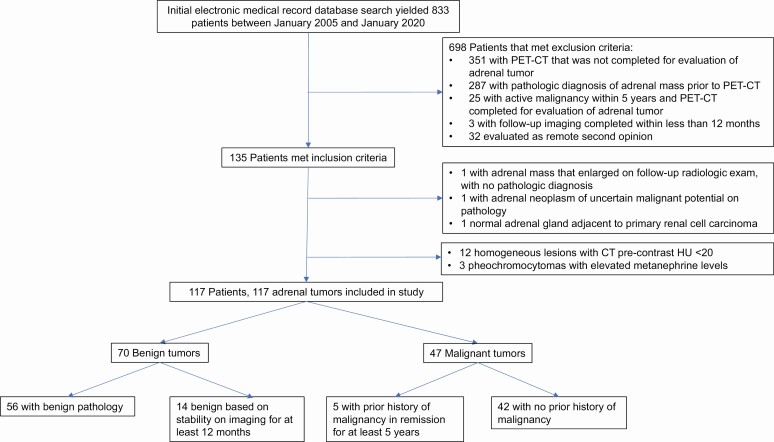
Retrospective chart review was completed on 833 patients who have received care for dedicated evaluation of adrenal lesions at University of Michigan between January 2010 and January 2020. These patients were first identified using a natural language processing algorithm Electronic Medical Record Search Engine (18). This cohort was then further narrowed down to patients who underwent 18F-FDG-PET/CT as part of the evaluation and management of 1 or more adrenal nodules to assess the risk of malignancy. Lesions that were homogeneous with precontrast HU <20 on CT were excluded (all benign or stable on follow-up). Lesions with catecholamine excess consistent with pheochromocytomas were also excluded. Patients with an adrenal mass were included if 18F-FDG-PET/CT imaging and either pathologic diagnosis or radiologic follow-up for at least 12 months were available. Patients were excluded if the pathologic diagnosis was known prior to 18F-FDG-PET/CT imaging, the 18F-FDG-PET/CT was not completed as part of workup for the specific evaluation of an adrenal mass, the patient had known active malignancy within 5 years prior to the diagnosis of the adrenal mass, or the patient had been evaluated only as a remote second opinion. The final cohort included 117 patients (Fig. 1). Relevant data were abstracted from their electronic medical records.
Figure 1.
Patient and tumor identification.
Tumor Diagnoses and Classification
Diagnoses were based on final pathology reports or stability on imaging for at least 12 months indicating a benign lesion. We analyzed only 1 lesion in each patient. For patients with bilateral lesions, we included the dominant adrenal lesion or the lesion with the highest standardized uptake value (SUVmax). For patients with multiple pathologies in a single lesion (eg, ACA with incidental myelolipoma) or with components of other histology in a single lesion (eg, myelolipomatous changes in an ACA), the lesion was categorized by their dominant component. Only lesions with pathologic diagnosis of ACAs were categorized as such.
Hormone Assessment
Hormone assessments were made based on laboratory results as well as clinical documentation. A lesion was classified as nonfunctional if all laboratory studies were within normal range, and the clinical history reported the lesion was nonfunctional. A lesion was classified as hormone producing if the clinical history specifically documented hormone production, or if the laboratory values met any of the following thresholds: aldosterone >15 ng/dL with suppressed plasma renin activity or renin mass, cortisol level of > 5.0 μg/dL following a 1 mg dexamethasone suppression test, 24-hour urinary free cortisol greater than the upper limit of normal, salivary cortisol greater than the upper limit of normal, androgen levels (testosterone or dehydroepiandrosterone sulfate), greater than the upper limit of normal, or plasma or urine metanephrines greater than the upper limit of normal. Mild autonomous cortisol secretion was defined as a morning cortisol level of 1.8 to 5 μg/dL following a 1 mg dexamethasone suppression test. When available, ACTH levels were included in the assessment to exclude presence of ACTH-dependent cortisol production.
Imaging Methods
An adrenal radiologist (E.M.C.) reviewed all cross-sectional imaging. Analysis of CT precontrast Hounsfield units, CT washout, and MRI signal dropout was only completed for homogeneous lesions. A nuclear medicine physician (A.M.A.) reviewed the 18F-FDG-PET/CT images for all adrenal lesion, liver, and blood pool SUV measurements. The final diagnoses and the 18F-FDG-PET calculated adrenal lesion SUVmax to liver SUVmean ratios (henceforth abbreviated to liver SUV ratio) were used to construct the receiver operator characteristic (ROC) curve. A similar ROC curve was constructed for the adrenal lesion SUVmax to blood pool SUVmean ratios (abbreviated to blood pool ratio).
The determination of 18F-FDG-PET positive versus 18F-FDG-PET negative lesions was made objectively: those with calculated liver SUV ratios of greater than 2.5 (as determined by ROC analysis, see below) were reported as 18F-FDG-PET positive. The performance of 18F-FDG-PET/CT was evaluated in 3 ways: by the liver SUV ratio threshold, by the blood pool SUV ratio threshold, and the 18F-FDG-PET/CT initial clinical report (referred to as “Visual Inspection”).
Sensitivity was calculated as the ratio of the number of malignant lesions with positive 18F-FDG-PET/CT results to the total number of malignant lesions. Similarly, specificity was calculated as the ratio of the number of benign lesions with positive 18F-FDG-PET/CT results, to the total number of benign lesions.
Statistical Methods
For analysis of imaging characteristics with continuous values, the Wilcoxon rank-sum test was performed. For comparison of the percentages of lesions having particular imaging characteristics, statistical significance was evaluated using the chi-squared test or Fisher’s exact test, for groups with n greater than 5 or less than 5, respectively. Statistical analyses were completed using R (R Foundation, Vienna, Austria).
Results
Patient and Tumor Characteristics
Demographic data of patients with benign and malignant masses were similar: mean age at the time of 18F-FDG-PET/CT was in the sixth decade, with the majority of patients being female and Caucasian (Table 1). Four patients had a diagnosis of hereditary tumor syndromes (Birt–Hogg–Dubé syndrome, hereditary paraganglioma syndrome, multiple endocrine neoplasia type 1, and familial adenomatous polyposis); the individuals with Birt–Hogg–Dubé syndrome and hereditary paraganglioma syndrome were diagnosed after the evaluation of their adrenal lesions. The number of patients with a history of prior malignancy was low overall, 4 patients (6%) with benign tumors and 5 patients (11%) with malignant tumors. However, the proportion of patients with prior history of malignancy was higher in the subgroup of patients with adrenal metastases (25%, 3 patients), which were all metastases from prior malignancy. Two patients in our cohort had history of renal cell carcinoma (RCC) and were ultimately diagnosed with RCC metastases to the adrenal gland after 7 and 21 years in remission.
Table 1.
Patient and tumor characteristics
| Characteristic | All benign tumors (n = 70) | Adrenocortical adenoma (n = 36) | Other benign tumors on pathology (n = 20) | Benign tumors on radiologic follow-up (n = 14) | All malignant tumors (n = 47) | Adrenocortical carcinoma (n = 35) | Adrenal metastases (n = 12) |
|---|---|---|---|---|---|---|---|
| Patient demographics | |||||||
| Age (years) | 55 ± 14 | 55 ± 14 | 50 ± 14 | 62 ± 11 | 55 ± 16 | 51 ± 16 | 66 ± 7 |
| Male sex (%) | 23 (33%) | 8 (22%) | 12 (60%) | 3 (21%) | 19 (40%) | 14 (40%) | 5 (42%) |
| Caucasian race (%) | 64 (91%) | 32 (89%) | 19 (95%) | 13 (93%) | 40 (85%) | 29 (83%) | 11 (92%) |
| Clinical history | |||||||
| History of hereditary tumor syndrome | 3 (4%) | 1 (3%) | 1 (5%) | 0 (0%) | 1 (3%) | 1 (3%) | 0 (0%) |
| Prior history of malignancy | 4 (6%) | 2 (6%) | 3 (15%) | 0 (0%) | 5 (11%) | 2 (6%) | 3 (25%) |
| Hormone production | |||||||
| Nonfunctional | 49 (70%) | 19 (53%) | 18 (90%) | 12 (86%) | 24 (51%) | 14 (40%) | 10 (83%) |
| Aldosterone production | 1 (1%) | 1 (3%) | 0 (0%) | 0 (0%) | 1 (2%) | 0 (0%) | 1 (8%) |
| Cortisol production only, excluding mild autonomous cortisol secretion | 5 (7%) | 5 (14%) | 0 (0%) | 0 (0%) | 12 (26%) | 12 (34%) | 0 (0%) |
| Androgen production only | 1 (1%) | 1 (3%) | 0 (0%) | 0 (0%) | 4 (9%) | 4 (11%) | 0 (0%) |
| Cortisol and androgen production | 1 (1%) | 1 (3%) | 0 (0%) | 0 (0%) | 4 (9%) | 4 (11%) | 0 (0%) |
| Other multiple hormone production | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 1 (2%) | 1 (3%) | 0 (0%) |
| Mild autonomous cortisol secretion | 13 (19%) | 9 (25%) | 2 (10%) | 2 (14%) | 1 (2%) | 0 (0%) | 1 (8%) |
Most tumors, 47 (70%) benign and 24 (51%) malignant, were nonfunctional. Notably, 13 (19%) benign tumors and 1 (2%) malignant tumor had biochemical evidence of mild autonomous cortisol secretion. However, this group likely includes patients with other causes of mild hypercortisolism as well (eg, pseudo-Cushing’s states). For example, a pheochromocytoma that did not secrete metanephrines did have biochemical testing results of mild autonomous cortisol secretion. With the exception of 1 ACA, a black adenoma producing cortisol and androgens, all other tumors with multiple hormone production were ACCs.
We identified a total of 70 benign masses and 47 malignant masses (Table 2). All pathologic diagnoses refer to the dominant adrenal lesion. Pathologically confirmed ACA (n = 36) was the most common benign pathology. Heterogeneity precluding calculation of washout percentage on cross-sectional imaging leading to an indeterminate result was most often due to hemorrhage, degenerative changes or myelolipomatous components. The majority of malignant masses were ACCs (n = 35) and the remainder were metastases to the adrenal gland (n = 12).
Table 2.
Tumor diagnoses
| Diagnosis | Number | Tumor largest dimension (median, range, in cm) |
|---|---|---|
| Benign tumors | 70 | 4.3 (0.7-17.9) |
| Adrenocortical adenoma by pathology | 36 | 4.6 (0.7-9.7) |
| Nonfunctional | 19 | 4.6 (0.7-9.7) |
| Mild autonomous cortisol secretion | 9 | 4.6 (2.7-5.3) |
| Hormone overproduction | 8 | 4.7 (2.9-9.5) |
| Benign based on stability on radiology | 14 | 3.2 (1.1-6.0) |
| Ganglioneuroma | 9 | 4.2 (3.4-6.5) |
| Hematoma | 3 | 7.7 (7.1-10.6) |
| Pheochromocytoma | 2 | 4.4 (4.2-10.3) |
| Hemangioma | 2 | 11.5 (5.1-17.9) |
| Myelolipoma | 1 | 7.9 |
| Normal adrenal gland | 1 | 6.9 |
| Langerhans histiocytosis | 1 | 3.9 |
| Leiomyoma | 1 | 8.3 |
| Malignant tumors | 47 | 8.6 (2.2-19.3) |
| Adrenocortical carcinoma | 35 | 10.5 (2.2-19.3) |
| Lung cancer metastasis | 6 | 8.0 (3.6-11.1) |
| Renal cell carcinoma metastasis | 2 | 4.0 (3.9-4.1) |
| Breast carcinoma metastasis | 1 | 6.1 |
| Unknown primary metastasis | 1 | 4.4 |
| Hepatocellular carcinoma metastasis | 1 | 4.6 |
| Lymphoma | 1 | 4.6 |
Seven patients in our final cohort had bilateral adrenal lesions. Diagnoses of the unilateral dominant lesions were as follows: 3 patients had benign lesions based on imaging stability, and 4 had diagnoses made based on pathology, which included 2 ACCs, 1 lung cancer metastasis, and 1 RCC metastasis.
Cross-sectional Anatomic Imaging Characteristics
All homogeneous lesions <20 HU either had a benign pathology or were stable on follow-up and were excluded from final analysis. Malignant masses compared with benign masses were larger (median size 8.6 cm with range 2.2 cm to 19.3 cm vs median size 4.5 cm with range 0.7 cm to 17.9 cm, P < .001), more likely to have cystic or necrotic features (74% vs 50%, P < .001), and less likely to have well-defined margins (74% vs 96%, P < .001) and macroscopic fat (4% vs 23%, P = .006) (Table 3).
Table 3.
Imaging characteristics of benign versus malignant tumors
| Imaging characteristic | Benign tumors (n = 70) | Malignant tumors (n = 47) | P value |
|---|---|---|---|
| Largest dimension, cm, median (range) | 4.5 (0.7-17.9) | 8.6 (2.2-19.3) | <.001 |
| Well-defined margins (%) | 67/70 (96%) | 35/47 (74%) | .001 |
| Homogeneous (%) | 10/70 (14%) | 6/47 (13%) | 1.000 |
| CT washout >60% (%) | 1/4 (25%) | 4/4 (100%) | .143 |
| Calcifications (%) | 27/70 (39%) | 12/47 (26%) | .205 |
| Cyst / necrosis (%) | 35/70 (50%) | 35/47 (74%) | .014 |
| Macroscopic fat (%) | 16/70 (23%) | 2/47 (4%) | .008 |
| Adrenal lesion SUVmax, median (range) | 3.7 (1.6-28.6) | 13.3 (2.3-70.8) | <.001 |
| Liver SUV ratio, median (range) | 1.4 (0.6-18.1) | 6.2 (0.9-30.0) | <.001 |
| PET-CT positive based on liver SUV ratio >2.5 (%) | 7/70 (10%) | 40/47 (85%) | <.001 |
| Blood pool SUV ratio, median (range) | 2.0 (1.2-21.2) | 7.4 (1.2-39.1) | <.001 |
| Blood pool SUV ratio >3.4 (%) | 7/70 (10%) | 39/47 (83%) | <.001 |
Liver SUV ratio, adrenal lesion SUVmax to liver SUVmean ratio. Blood pool ratio, adrenal lesion SUVmax to blood pool SUVmean ratio
Next, we compared characteristics of ACAs to ACCs and adrenal metastases. ACCs and metastases were larger (median size 10.5 cm with range 2.2-19.3 cm, and median size 4.6 cm with range 3.6-11.1 cm, respectively) and more likely to have features of malignancy as described above (Table 4).
Table 4.
Imaging characteristics of adrenocortical adenomas versus adrenocortical carcinomas and metastases
| Imaging characteristic | Adrenocortical adenoma (n = 36) | Adrenocortical carcinoma (n = 35) | P value | Adrenal metastases (n = 12) | P value |
|---|---|---|---|---|---|
| Largest dimension (cm) | 4.6 (0.7- 9.7) | 1.5 (2.2-19.3) | <.001 | 4.6 (3.6-11.1) | .156 |
| Well-defined margins (%) | 36/36 (100%) | 25/35 (71%) | <.001 | 10/12 (83%) | .059 |
| Homogeneous (%) | 5/36 (14%) | 4/35 (11%) | 1.000 | 2/12 (17%) | 1.000 |
| CT washout >60% (%) | 1/3 (33%) | 3/3 (100%) | .400 | 0/2 (0%) | 1.000 |
| Calcifications (%) | 11/36 (31%) | 12/35 (34%) | .934 | 0/12 (0%) | .044 |
| Cyst/necrosis (%) | 14/36 (39%) | 27/35 (77%) | .003 | 8/12 (67%) | .111 |
| Macroscopic fat (%) | 10/36 (28%) | 1/35 (3%) | .006 | 1/12 (8%) | .248 |
| Adrenal lesion SUVmax, median (range) | 4.0 (1.6-28.6) | 13 (3.9-7.9) | <.001 | 13.2 (2.3-51.5) | .021 |
| Liver SUV ratio, median (range) | 1.4 (1.0-18.1) | 6.1 (1.3-3.1) | <.001 | 6.7 (.9-2.3) | .007 |
| PET-CT positive based on liver SUV ratio >2.5 (%) | 10/36 (14%) | 32/35 (91%) | <.001 | 8/12 (67%) | .001 |
| Blood pool SUV ratio, median (range) | 2.2 (1.2–21.2) | 7.3 (1.5-39.1) | <.001 | 7.7 (1.2-27.4) | .013 |
| Blood pool SUV ratio >3.4 (%) | 5/36 (14%) | 31/35 (89%) | <.001 | 8/12 (67%) | .001 |
Last, we compared nonfunctioning ACAs versus those with mild autonomous cortisol production or frank hormone overproduction, but no significant differences were found; the small size of these cohorts likely precluded the identification of any differences (Table 5).
Table 5.
Imaging characteristics of nonfunctioning adrenocortical adenomas versus those with mild autonomous cortisol secretion and hormone overproduction
| Imaging characteristic | Nonfunctioning adenomas (n = 19) | Adenomas with mild autonomous cortisol secretion (n = 9) | P value | Adenomas with hormone overproduction (n = 8) | P value |
|---|---|---|---|---|---|
| Largest dimension (cm) | 4.6 (0.7-9.7) | 4.6 (2.7-5.3) | .671 | 4.7 (2.9-9.5) | 0.262 |
| Well-defined margins (%) | 19/19 (100%) | 9/9 (100%) | 1.000 | 8/8 (100%) | 1.000 |
| Homogeneous (%) | 4/19 (21%) | 1/9 (11%) | 1.000 | 0/8 (0%) | .286 |
| CT washout >60% (%) | 1/3 (33%) | NAa | NAa | NAb | NAb |
| Calcifications (%) | 6/19 (32%) | 2/9 (22%) | 1.000 | 3/8 (38%) | 1.000 |
| Cyst/necrosis (%) | 6/19 (32%) | 5/9 (56%) | .409 | 3/8 (38%) | 1.000 |
| Macroscopic fat (%) | 5/19 (26%) | 3/9 (33%) | 1.000 | 2/8 (25%) | 1.000 |
| Adrenal lesion SUVmax, median (range) | 3.8 (1.6-28.6) | 4.0 (2.6-7.2) | .559 | 4.6 (2.7-6.0) | .542 |
| Liver SUV ratio, median (range) | 1.4 (1.0-18.1) | 1.4 (1.0-2.4) | .689 | 1.8 (1.2-2.3) | .375 |
| PET-CT positive based on liver SUV ratio >2.5 (%) | 5/19 (26%) | 0/9 (0%) | .144 | 0/8 (0%) | .280 |
| Blood pool SUV ratio, median (range) | 2.1 (1.2-21.2) | 1.8 (1.3-3.2) | .819 | 2.5 (1.6-3.3) | .416 |
| Blood pool SUV ratio >3.4 (%) | 5/19 (26%) | 0/9 (0%) | .144 | 0/8 (0%) | 0.280 |
a NA, no homogeneous lesions with washout.
b NA, no homogeneous lesions.
18F-FDG-PET/CT Imaging Characteristics
There were no standardized criteria for the use of 18F-FDG-PET/CT. Providers used their own clinical judgment in ordering 18F-FDG-PET/CTs. The performance of 18F-FDG-PET/CT based on visual inspection (clinical report) and SUV ratios were strikingly different (Table 6). Visual inspection yielded 98% sensitivity, 43% specificity, 61% positive predictive value, and 96% negative predictive value. Of the total 117 18F-FDG-PET/CT reports, 17 (15%) stated the results were “indeterminate,” so these assessments were not included in the performance analysis of visual inspection, but undoubtedly decrease the utility of visual inspection results. The ultimate diagnoses of patients with indeterminate 18F-FDG-PET/CT reports were all benign: adenomas (n = 9), ganglioneuromas (n = 3), stable on imaging follow-up (n = 5).
Table 6.
18F-FDG-PET/CT performance
| Performance | Visual inspection (n = 100) | Adrenal SUVmax to liver SUVmean ratio > 2.5 (n = 117) | Adrenal SUVmax to blood pool SUVmean ratio > 3.4 (n = 117) |
|---|---|---|---|
| Sensitivity | 98% | 85% | 83% |
| Specificity | 43% | 90% | 90% |
| Positive predictive value | 61% | 85% | 89% |
| Negative predictive value | 96% | 90% | 85% |
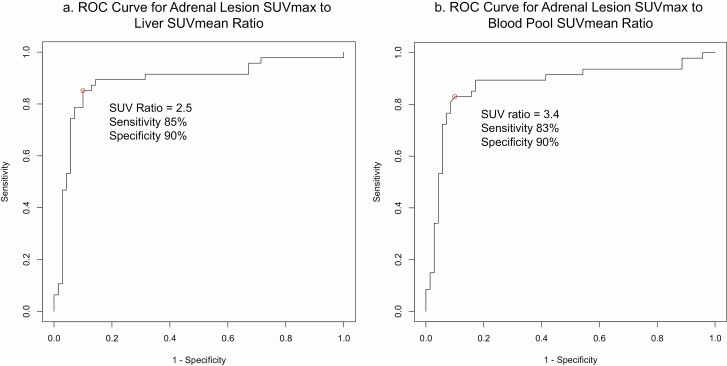
The receiver operating characteristic (ROC) curve of the 18F-FDG-PET adrenal lesion SUVmax to liver SUVmean ratio (abbreviated to liver SUV ratio) of all 117 patients was constructed, which identified the optimal liver SUV ratio cutoff of 2.5, corresponding to 85% sensitivity, 90% specificity, 85% positive predictive value, and 90% negative predictive value (Fig. 2A). There were no additional practical liver SUV ratio thresholds that improved sensitivity or specificity to a clinically meaningful degree.
Figure 2.
(A) ROC curve for adrenal lesion SUVmax to liver SUVmean ratio. (B) ROC curve for adrenal lesion SUVmax to blood pool SUVmean ratio.
The ROC curve of the 18F-FDG-PET adrenal lesion SUVmax to blood pool SUVmean ratio (abbreviated to blood pool SUV ratio) of all 117 patients was also constructed (Fig. 2B), which identified the optimal blood pool SUV ratio cutoff of 3.4 with similar performance to that of the liver SUV ratio cutoff of 2.5: 83% sensitivity, 90% specificity, 89% positive predictive value, and 85% negative predictive value (Table 6). Application of the blood pool SUV ratio threshold of 3.4 resulted in 1 additional false negative result (ACC).
As the question of malignancy most often arises for masses <6 cm, we conducted a separate analysis restricted to 67 tumors. The ROC curve for liver SUV ratio identified the same optimal threshold point of 2.5, with similar specificity of 92% and negative predictive value of 91%, but lower sensitivity of 67% and positive predictive value of 71%. Likewise, the ROC curve for blood pool SUV ratio found optimal threshold point of 3.4, with similar specificity of 92% and negative predictive value of 91%, but lower sensitivity of 67% and positive predictive value of 71%.
Compared with benign masses, malignant masses had higher median adrenal lesion SUVmax (median 3.7 with range 1.6-28.6 vs median 13.3 with range 2.3-70.8, P < .001), liver SUV ratios (median 1.4 with range 0.6-18.1 vs median 6.2 with range 0.9-30.0, P < .001), blood pool SUV ratios (median 2.0 with range 1.2-21.2 vs median 7.4 with range 1.2-39.1, P < .001), percentage of 18F-FDG-PET/CT positive diagnoses based on the cutoff of liver SUV ratio greater than 2.5 (10% vs 85%, P < .001), and percentage of blood pool ratio greater than 3.4 (10% vs 83%, P < .001). The vast majority of benign masses had liver SUV ratios below 2.5, while malignant masses had liver SUV ratios of a much broader distribution overall (Fig. 3). Our cohort also included a large group of ganglioneuromas (n = 9), which were all benign on 18F-FDG-PET with a median adrenal lesion SUVmax of 3.5 (range 2.7-5.9) and median liver SUV ratio of 1.5 (range 1.1-2.4).
Figure 3.
Dot plot of adrenal lesion SUVmax to liver SUVmean ratio.
Similarly, ACCs and metastases had higher median adrenal lesion SUVmax (median 13.0 with range 3.9-70.9 and median 13.2 with range 2.3-51.5, respectively), liver SUV ratios (median 6.1 with range 1.3-30.1 and median 6.7 with range 0.9-20.3), blood pool SUV ratios (median 7.3 with range 1.5-39.1 and median 7.7 with range 1.2-27.4), 18F-FDG-PET positive diagnoses based on liver SUV ratios (91% and 67%), and percentage of blood pool SUV ratios greater than 3.4 (89% and 67%), compared with ACAs (median adrenal lesion SUVmax 4.0 with range 1.6-28.6, median liver SUV ratio 1.4 with range 1.0-18.1, median blood pool SUV ratio 2.2 with range 1.2-21.2, 14% 18F-FDG-PET positive, and 14% with blood pool SUV ratio >3.4, P < .05 for all measurements). However, no significant differences in these 18F-FDG-PET/CT characteristics were observed among ACAs based on degree of hormone production.
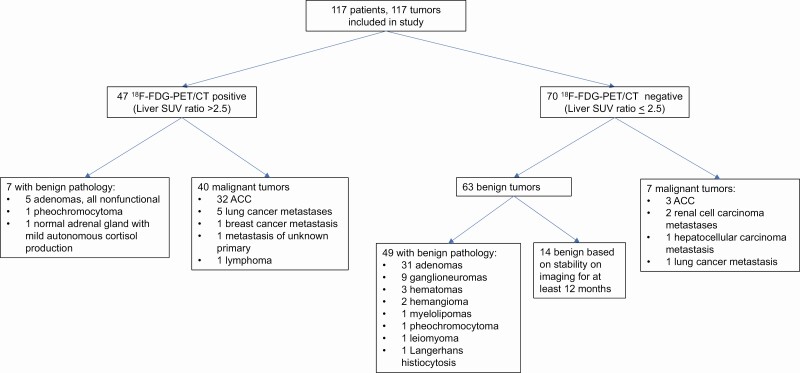
While benign lesions generally had lower adrenal lesion SUVmax and SUV ratios and were therefore 18F-FDG-PET/CT negative as defined by the SUV ratio cutoff of 2.5, there were notable exceptions (Fig. 4). Seven benign lesions were found to have liver SUV ratio higher than 2.5, with the highest liver SUV ratios observed in nonfunctional oncocytic ACAs and a pheochromocytoma. Conversely, 7 malignant tumors had liver SUV ratios of less than 2.5 and were classified as 18F-FDG-PET/CT negative, which included 3 (9%) ACCs (4 ACCs [11%] with blood pool SUV ratio <3.4) and 4 metastases. The lower liver SUV ratios may be explained by the low-grade stage 2 pathology of 1 ACC, and substantial regions of necrosis in 2 other ACCs. The remaining lesions were 2 RCC metastases, 1 hepatocellular carcinoma metastasis, and a metastasis from a sarcomatoid lung cancer. For RCC and hepatocellular carcinomas, 18F-FDG-PET/CT is known to have limited utility (19, 20).
Figure 4.
18F-FDG-PET/CT results and tumor diagnoses.
Discussion
Evaluation of adrenal masses requires biochemical evaluation to determine potential hormone excess, and imaging to determine likelihood of malignancy. Differentiating between benign and malignant adrenal tumors is key to clinical decision making. Studies evaluating cross-sectional imaging methods (CT and MRI) have identified a number of characteristics that are associated with benign pathology, which include Hounsfield units of <20 on unenhanced CT images, relative washout >40% or absolute washout >60% on contrast-enhanced CT images (10, 12-16), and >20% loss of signal intensity on MRI (9-11). However, by definition, these imaging methods can only evaluate homogeneous lesions and at least 12% of adrenal tumors remain indeterminate (21). In this study, we show the value of applying 18F-FDG-PET/CT in such indeterminate lesions.
Prior studies have examined the use of 18F-FDG-PET/CT to evaluate indeterminate adrenal tumors, mostly in cancer patients and found that elevated adrenal to liver SUV ratio thresholds of 1.0 to 3.1 on 18F-FDG-PET, alone or in conjunction with elevated CT Hounsfield units, yield high sensitivity (83-100%) and specificity (84-100%) in diagnosing malignant adrenal lesions (22-28). Several retrospective studies did not report the cancer history of their study populations, but showed similar results regarding 18F-FDG-PET SUV in malignant compared with benign masses (29, 30). A large systematic review and meta-analysis of 1391 adrenal lesions in cancer and noncancer patients found 18F-FDG-PET/CT to have overall high sensitivity (97%) and specificity (91%), with specificity even higher (98%) in the subset of noncancer patients than cancer patients (17); however, a more recent and selective systematic review examining various imaging modalities in nonfunctioning masses found that existing 18F-FDG-PET/CT data were too heterogeneous to draw conclusions about its utility (31).
Data suggest that sensitivity and specificity of 18F-FDG-PET/CT are different in cancer versus noncancer patients (17), but there are relatively few studies of applying 18F-FDG-PET/CT in noncancer patients. One retrospective study demonstrated 100% sensitivity and specificity in differentiating between malignant and benign masses in noncancer patients; however, the study cohort was small, only including 3 ACCs, and hormone secreting lesions were excluded (32). In a larger prospective study of 109 noncancer patients, 9 benign hormone-secreting ACAs were found to have elevated adrenal lesion SUVmax compared with nonfunctional masses (33). However only 2 malignant masses (ACC) were included.
Three prospective surgical studies have employed 18F-FDG-PET/CT in noncancer patients. Two of these studies determined that 18F-FDG-PET/CT can help decide whether surgery is needed: 1 found that high 18F-FDG uptake was indicative of malignancy and suggested that surgery could be avoided in the absence of 18F-FDG uptake (34), and the other found that adrenal lesion SUVmax to liver SUVmax ratio of >1.5 helped to discern between malignant masses with a sensitivity of 86.7% and specificity of 86.1% (35). The third study, which included only patients who underwent surgery, found that adrenal lesion SUVmax to liver SUVmax ratio of >1.45 detected ACCs (n = 22) with 100% sensitivity (36).
In this retrospective study, we evaluated the practical application of using 18F-FDG-PET/CT specifically to evaluate indeterminate adrenal masses in noncancer patients. Compared with prior retrospective and prospective studies in noncancer patients, the cohort in our current study included a larger number of patients overall, with a definitive diagnosis and a higher proportion of uncommon masses (eg, ganglioneuromas, metastases of unknown primary). Most importantly, we included a large number of malignant masses, particularly ACCs, which had been a shortcoming of prior studies. While the retrospective nature of the study is a limitation, it did also allow for the inclusion of diverse types of lesions aggregated over a decade, which would otherwise be very challenging to achieve in prospective studies.
Similar to prior studies, we found that malignant masses had wider ranges of and higher values of adrenal lesion SUVmax and liver SUV ratios than benign masses. Unlike a few of the prior studies that found higher SUV values in hormone-producing ACAs (33, 37, 38), we did not observe significant differences in SUV ratios in functional vs nonfunctional ACAs. The optimal liver SUV ratio in this study was 2.5 (89% specificity, 86% sensitivity, 81% positive predictive value, and 92% negative predictive value). It is notable that visual inspection yielded high sensitivity (98%) and negative predictive value (97%), but with the trade-off of a low specificity (46%), low positive predictive value (57%), and a significant number of 18F-FDG-PET/CT reports stated the imaging results were “indeterminate” (15%). In other words, many nuclear medicine physicians appear hesitant and have a high threshold to report lesions as benign.
In addition to validating the previously described criterion of liver background comparison, we also analyzed a second quantitative criterion for 18F-FDG-PET/CT interpretation for adrenal lesions compared with aortic arch blood pool activity as a measure of background metabolic activity. Under circumstances in which the metabolic activity of the liver may be heterogeneous (39) and influenced by underlying liver disease such as steatosis or cirrhosis (40, 41), defining a second criterion for analysis of functional metabolic images can be very useful for clinical interpretation. We assessed the use of adrenal lesion SUVmax to blood pool SUVmean and found that its performance is similar to that of the more often used adrenal lesion SUVmax to liver SUVmean.
18F-FDG-PET performance characteristics remained the same or were slightly worse in a subgroup of lesions <6 cm in size, suggesting that 18F-FDG-PET is not more helpful in smaller adrenal tumors where decision for surgery is less straightforward.
Using the cut-off determined by ROC curve analysis, 10% of benign lesions were 18F-FDG-PET positive and 15% of malignant lesions were 18F-FDG-PET negative. Most strikingly, 3 ACCs (9% of all ACCs) were 18F-FDG-PET negative (11% negative using blood pool SUV ratio), which had only been reported in case reports (42). Conversely, 14% of pathologically proven adrenal adenomas were 18F-FDG-PET positive.
These findings show the importance of integrating all clinical information, including prior history and additional cross-sectional imaging. For example, RCC is known to have a predilection for adrenal metastasis and can recur even years after initial treatment. Two of the 18F-FDG-PET negative ACCs were large and heterogeneous masses with significant areas of necrosis areas and therefore remained clinically suspicious for malignancy despite 18F-FDG-PET negativity. Additionally, other features, such as concurrent production of androgens and glucocorticoids raise the suspicion for malignancy regardless of imaging findings.
In summary, we find that, in our cohort of diverse adrenal pathologies, 18F-FDG-PET/CT can be valuable in the practical assessment of the underlying nature of adrenal masses, with objective adrenal lesion SUVmax to liver SUVmean ratio of 2.5 and blood pool SUVmean ratio of 3.4 providing the highest performance in differentiating malignant from benign masses and should be noted in clinical 18F-FDG-PET/CT reports. 18F-FDG-PET/CT can further increase the diagnostic accuracy, particularly for indeterminate, large, unusual adrenal masses and lesions with a higher pretest probability of malignancy. However, 18F-FDG-PET/CT must not be used as a single differentiating tool, but rather contributes to the overall clinical assessment.
Acknowledgments
Financial Support: X.H. is supported by grant T32DK07245 from the National Institutes of Diabetes and Digestive and Kidney Diseases.
Glossary
Abbreviations
- 18F-FDG-PET/CT
18F-fluorodeoxyglucose positron emission tomography computed tomography
- ACC
adrenocortical carcinoma
- CT
computed tomography
- MRI
magnetic resonance imaging
- RCC
renal cell carcinoma
- ROC
receiver operating characteristic
- SUV
standardized uptake value
Additional Information
Disclosures: The authors have nothing to disclose.
Data Availability
The datasets generated during and/or analyzed during the current study are not publicly available but are available from the corresponding author on reasonable request.
References
- 1. Barzon L, Sonino N, Fallo F, Palu G, Boscaro M. Prevalence and natural history of adrenal incidentalomas. Eur J Endocrinol. 2003;149(4):273-285. [DOI] [PubMed] [Google Scholar]
- 2. Mansmann G, Lau J, Balk E, Rothberg M, Miyachi Y, Bornstein SR. The clinically inapparent adrenal mass: update in diagnosis and management. Endocr Rev. 2004;25(2):309-340. [DOI] [PubMed] [Google Scholar]
- 3. Bovio S, Cataldi A, Reimondo G, et al. Prevalence of adrenal incidentaloma in a contemporary computerized tomography series. J Endocrinol Invest. 2006;29(4):298-302. [DOI] [PubMed] [Google Scholar]
- 4. Grumbach MM, Biller BMK, Braunstein GD, et al. Management of the clinically inapparent adrenal mass (Incidentaloma). Ann Intern Med. 2003;138(5):424-429. [DOI] [PubMed] [Google Scholar]
- 5. Cooper AB, Habra MA, Grubbs EG, et al. Does laparoscopic adrenalectomy jeopardize oncologic outcomes for patients with adrenocortical carcinoma? Surg Endosc. 2013;27(11):4026-4032. [DOI] [PubMed] [Google Scholar]
- 6. Miller BS, Ammori JB, Gauger PG, Broome JT, Hammer GD, Doherty GM. Laparoscopic resection is inappropriate in patients with known or suspected adrenocortical carcinoma. World J Surg. 2010;34(6):1380-1385. [DOI] [PubMed] [Google Scholar]
- 7. Miller BS, Gauger PG, Hammer GD, Doherty GM. Resection of adrenocortical carcinoma is less complete and local recurrence occurs sooner and more often after laparoscopic adrenalectomy than after open adrenalectomy. Surgery. 2012;152(6):1150-1157. [DOI] [PubMed] [Google Scholar]
- 8. Bancos I, Taylor AE, Chortis V, et al. ; ENSAT EURINE-ACT Investigators . Urine steroid metabolomics for the differential diagnosis of adrenal incidentalomas in the EURINE-ACT study: a prospective test validation study. Lancet Diabetes Endocrinol. 2020;8(9):773-781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Korobkin M, Giordano TJ, Brodeur FJ, et al. Adrenal adenomas: relationship between histologic lipid and CT and MR findings. Radiology. 1996;200(3):743-747. [DOI] [PubMed] [Google Scholar]
- 10. Ilias I, Sahdev A, Reznek RH, Grossman AB, Pacak K. The optimal imaging of adrenal tumours: a comparison of different methods. Endocr Relat Cancer. 2007;14(3):587-599. [DOI] [PubMed] [Google Scholar]
- 11. Haider MA, Ghai S, Jhaveri K, Lockwood G. Chemical shift MR imaging of hyperattenuating (>10 HU) adrenal masses: does it still have a role? Radiology. 2004;231(3):711-716. [DOI] [PubMed] [Google Scholar]
- 12. Caoili EM, Korobkin M, Francis IR, et al. Adrenal masses: characterization with combined unenhanced and delayed enhanced CT. Radiology. 2002;222(3):629-633. [DOI] [PubMed] [Google Scholar]
- 13. Caoili EM, Korobkin M, Francis IR, Cohan RH, Dunnick NR. Delayed enhanced CT of lipid-poor adrenal adenomas. AJR Am J Roentgenol. 2000;175(5):1411-1415. [DOI] [PubMed] [Google Scholar]
- 14. Zhang HM, Perrier ND, Grubbs EG, et al. CT features and quantification of the characteristics of adrenocortical carcinomas on unenhanced and contrast-enhanced studies. Clin Radiol. 2012;67(1):38-46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Peña CS, Boland GW, Hahn PF, Lee MJ, Mueller PR. Characterization of indeterminate (lipid-poor) adrenal masses: use of washout characteristics at contrast-enhanced CT. Radiology. 2000;217(3):798-802. [DOI] [PubMed] [Google Scholar]
- 16. Boland GW, Lee MJ, Gazelle GS, Halpern EF, McNicholas MM, Mueller PR. Characterization of adrenal masses using unenhanced CT: an analysis of the CT literature. AJR Am J Roentgenol. 1998;171(1):201-204. [DOI] [PubMed] [Google Scholar]
- 17. Boland GW, Dwamena BA, Jagtiani Sangwaiya M, et al. Characterization of adrenal masses by using FDG PET: a systematic review and meta-analysis of diagnostic test performance. Radiology. 2011;259(1):117-126. [DOI] [PubMed] [Google Scholar]
- 18. Hanauer DA, Mei Q, Law J, Khanna R, Zheng K. Supporting information retrieval from electronic health records: a report of University of Michigan’s nine-year experience in developing and using the Electronic Medical Record Search Engine (EMERSE). J Biomed Inform. 2015;55:290-300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Liu Y. The place of FDG PET/CT in renal cell carcinoma: value and limitations. Front Oncol. 2016;6:201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Lin Y, Jeng L-B, Wang H-Y, Tsai S-C, Lin W-Y, Kao C-H. Clinical value of 18F-FDG PET/CT in detecting adrenal metastasis in patients with hepatocellular carcinoma. Technol Cancer Res Treat. 2014;14(5):593-599. [DOI] [PubMed] [Google Scholar]
- 21. Song JH, Chaudhry FS, Mayo-Smith WW. The incidental adrenal mass on CT: prevalence of adrenal disease in 1,049 consecutive adrenal masses in patients with no known malignancy. AJR Am J Roentgenol. 2008;190(5):1163-1168. [DOI] [PubMed] [Google Scholar]
- 22. Delivanis DA, Bancos I, Atwell TD, et al. Diagnostic performance of unenhanced computed tomography and 18 F-fluorodeoxyglucose positron emission tomography in indeterminate adrenal tumours. Clin Endocrinol (Oxf). 2018;88(1):30-36. [DOI] [PubMed] [Google Scholar]
- 23. Brady MJ, Thomas J, Wong TZ, Franklin KM, Ho LM, Paulson EK. Adrenal nodules at FDG PET/CT in patients known to have or suspected of having lung cancer: a proposal for an efficient diagnostic algorithm. Radiology. 2009;250(2):523-530. [DOI] [PubMed] [Google Scholar]
- 24. Caoili EM, Korobkin M, Brown RK, Mackie G, Shulkin BL. Differentiating adrenal adenomas from nonadenomas using (18)F-FDG PET/CT: quantitative and qualitative evaluation. Acad Radiol. 2007;14(4):468-475. [DOI] [PubMed] [Google Scholar]
- 25. Metser U, Miller E, Lerman H, Lievshitz G, Avital S, Even-Sapir E. 18F-FDG PET/CT in the evaluation of adrenal masses. J Nucl Med. 2006;47(1):32-37. [PubMed] [Google Scholar]
- 26. Refaat R, Elghazaly H. Employing 18F-FDG PET/CT for distinguishing benign from metastatic adrenal masses. Egypt J Radiol Nucl Med. 2017;48(4):1065-1071. [Google Scholar]
- 27. Vikram R, Yeung HD, Macapinlac HA, Iyer RB. Utility of PET/CT in differentiating benign from malignant adrenal nodules in patients with cancer. AJR Am J Roentgenol. 2008;191(5):1545-1551. [DOI] [PubMed] [Google Scholar]
- 28. Blake MA, Slattery JM, Kalra MK, et al. Adrenal lesions: characterization with fused PET/CT image in patients with proved or suspected malignancy–initial experience. Radiology. 2006;238(3):970-977. [DOI] [PubMed] [Google Scholar]
- 29. Launay N, Silvera S, Tenenbaum F, et al. Value of 18-F-FDG PET/CT and CT in the diagnosis of indeterminate adrenal masses. Int J Endocrinol. 2015;2015:213875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Paladino NC, Guérin C, Lowery A, et al. Characterization of adrenocortical tumors by 18F-FDG PET/CT: does steroid hormone hypersecretion status modify the uptake pattern? Surg Oncol. 2018;27(2):231-235. [DOI] [PubMed] [Google Scholar]
- 31. Dinnes J, Bancos I, Ferrante di Ruffano L, et al. MANAGEMENT OF ENDOCRINE DISEASE: Imaging for the diagnosis of malignancy in incidentally discovered adrenal masses: a systematic review and meta-analysis. Eur J Endocrinol. 2016;175(2):R51-R64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Tessonnier L, Sebag F, Palazzo FF, et al. Does 18F-FDG PET/CT add diagnostic accuracy in incidentally identified non-secreting adrenal tumours? Eur J Nucl Med Mol Imaging. 2008;35(11):2018-2025. [DOI] [PubMed] [Google Scholar]
- 33. Akkuş G, Güney IB, Ok F, et al. Diagnostic efficacy of 18F-FDG PET/CT in patients with adrenal incidentaloma. Endocr Connect. 2019;8(7):838-845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Ansquer C, Scigliano S, Mirallié E, et al. 18F-FDG PET/CT in the characterization and surgical decision concerning adrenal masses: a prospective multicentre evaluation. Eur J Nucl Med Mol Imaging. 2010;37(9):1669-1678. [DOI] [PubMed] [Google Scholar]
- 35. Guerin C, Pattou F, Brunaud L, et al. Performance of 18F-FDG PET/CT in the characterization of adrenal masses in noncancer patients: a prospective study. J Clin Endocrinol Metab. 2017;102(7):2465-2472. [DOI] [PubMed] [Google Scholar]
- 36. Groussin L, Bonardel G, Silvéra S, et al. 18F-Fluorodeoxyglucose positron emission tomography for the diagnosis of adrenocortical tumors: a prospective study in 77 operated patients. J Clin Endocrinol Metab. 2009;94(5):1713-1722. [DOI] [PubMed] [Google Scholar]
- 37. Moog S, Houy S, Chevalier E, et al. 18F-FDOPA PET/CT uptake parameters correlate with catecholamine secretion in human pheochromocytomas. Neuroendocrinology. 2018;107(3):228-236. [DOI] [PubMed] [Google Scholar]
- 38. Patel D, Gara SK, Ellis RJ, et al. FDG PET/CT scan and functional adrenal tumors: a pilot study for lateralization. World J Surg. 2016;40(3):683-689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Liu G, Hu Y, Zhao Y, Yu H, Hu P, Shi H. Variations of the liver standardized uptake value in relation to background blood metabolism: An 2-[18F]Fluoro-2-deoxy-D-glucose positron emission tomography/computed tomography study in a large population from China. Medicine (Baltimore). 2018;97(19):e0699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Keramida G, Potts J, Bush J, Verma S, Dizdarevic S, Peters AM. Accumulation of 18F-FDG in the liver in hepatic steatosis. Am J Roentgenol. 2014;203(3):643-648. [DOI] [PubMed] [Google Scholar]
- 41. Verloh N, Einspieler I, Utpatel K, et al. In vivo confirmation of altered hepatic glucose metabolism in patients with liver fibrosis/cirrhosis by 18F-FDG PET/CT. EJNMMI Res. 2018;8(1):98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Ghander C, Tissier F, Tenenbaum F, et al. A concomitant false-negative 18F-FDG PET imaging in an adrenocortical carcinoma and a high uptake in a corresponding liver metastasis. J Clin Endocrinol Metab. 2012;97(4):1096-1097. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets generated during and/or analyzed during the current study are not publicly available but are available from the corresponding author on reasonable request.