Abstract
Autosomal Dominant Tubulointerstitial Disease (ADTKD) is a dominantly inherited progressive non-glomerular disease. Several factors, such as a non-specific clinical presentation and relative rarity, impede the phenotyping of ADTKD into clinically relevant subtypes, and impair the appropriate implementation of genetic testing. The study by Olinger et al describe the largest multicenter ADTKD cohort, which is likely to become a key resource. The authors also provide a new clinical tool that could guide diagnosis and genetic testing.
Autosomal dominant tubulointerstitial kidney disease (ADTKD) is an inherited disease entity manifesting as a progressive loss of kidney function without an explainable cause, particularly the absence of significant glomerular pathology. The latter can be defined clinically (bland urine sediment, absent or mild proteinuria) or on a pathology assessment of a renal biopsy predominantly showing interstitial fibrosis and tubular atrophy.1 Because of important breakthroughs in identifying major genes involved and key advances in understanding its cellular pathogenesis, ADTKD has received increased attention over the last few years, which led to recent standardization of its nomenclature (ADTKD-GENE involved or ADTKD-NOS, not otherwise specified).2 Despite being considered rare, emerging data suggest that ADTKD is likely more prevalent than previously thought.1 In fact, the clinical spectrum of ADTKD and its link to the genetic mutations are still not fully characterized. This uncertainty can hinder the ability to screen and pursue the appropriate venues in establishing an accurate diagnosis. Since ADTKD commonly affects multiple family members across generations, the medical and psycho-social burdens on entire families can be daunting.1 Therefore, it is important to accelerate the efforts to correctly detect and classify ADTKD and understand its pathogenesis.
ADTKD is caused by mutations in at least 5 genes identified to date: UMOD, MUC1, HNF1b, REN and SEC61A1.1 Based on previous data from small cohorts, mutations in UMOD and MUC1 are the most common subtypes of ADTKD.2 UMOD encodes uromodulin (Tamm-Horsfall protein), a protein uniquely produced in the kidney by cells of the thick ascending limbs (TAL) and early distal tubules. Uromodulin is the most abundant protein secreted in the urine through apical release into the urinary space and has important roles in maintaining homeostasis of the distal nephron and enhancing protection from urinary infections and kidney stones.3 Uromodulin is also released basolaterally into the kidney interstitium and circulation, where it has protective roles against inflammation following kidney injury, regulation of kidney phagocytes and mitigation of renal and systemic oxidative stress.3, 4 The MUC1 gene encodes mucin-1 protein, which is expressed at the apical surface of epithelial cells of many organs.5 Mucin-1 is thought to be involved in cellular signaling and protection of luminal surfaces. In the kidney, it is expressed in cells of the distal nephron, partially overlapping with the distribution of uromodulin expression.1, 5
Most ADTKD-UMOD gene mutations are missense, causing changes in protein folding, leading to the retention of the mutant protein in the endoplasmic reticulum (ER). These events lead to ER stress and upregulation of compensatory pathways such as the unfolded protein response (UPR). The sustained ER stress response eventually causes cellular injury to TAL cells and likely leads to an interstitial inflammatory response.6 Affected patients have reduced levels of urinary uromodulin and show accumulation of uromodulin aggregates intracellularly in the ER.1 Frameshift mutations in ADTKD-MUC1 lead to the formation of a neo-protein.5 The latter accumulates intra-cellularly in vesicles of the early secretory pathway, leading also to activation of UPR.5 However, it is not yet clear whether ER stress is also a cardinal feature of ADTKD-MUC1.
Despite an overlap in clinical presentations, histological findings and apparent similarities in underlying pathogenic mechanisms, the various subtypes of ADTKD tend to have divergent phenotypes and prognosis.1 Distinguishing subtypes of ADTKD is challenging due to the lack of practical tools for screening and prioritizing genetic testing. This difficulty could have direct negative implications on clinical management and care. Part of the knowledge gap is due to the low prevalence of disease, making clinicians rely on data from small cohorts.1 Therefore, there is a need to create an international registry and form a large longitudinal cohort of ADTKD patients that have undergone comprehensive clinical phenotyping and genetic screening. Such a cohort would be adequately powered to provide much-needed data linking genetic mutations to clinical course and outcomes.
In this issue of Kidney International, Olinger et al 7 present a welcome international effort, responding to the stated need by creating the International ADTKD cohort which includes 726 patients from 585 families. This cohort combines the US ADTKD and the Belgo-Swiss Registries. These patients were included based on the criteria defined by the KDIGO consensus,2 and underwent screening for UMOD and MUC1 mutations sequentially. The prevalence of ADTKD-UMOD and ATDKD-MUC1 in this cohort were 37.1% and 21.0 %, respectively. This cohort is the largest for ADTKD and will have a tremendous impact on the field, becoming an important resource for clinicians and patients. Because of its large sample size, important insights on the clinical course, based on ADTKD subtype, can be obtained. Indeed, this study confirms that patients with ADTKD-UMOD and ADTKD-MUC1 have distinct clinical features and outcomes.7 Kidney disease appears to be more severe in ADTKD-MUC1 compared to ADTKD-UMOD in terms of onset of end stage kidney disease and renal survival. Conversely, gout is more prevalent and earlier in onset in UMOD vs. MUC1 mutations. In addition, the findings suggest that the kidney phenotype of ADTKD is more severe in UMOD and MUC1 mutations compared to ADTKD-NOS cases.
This study also uncovers important insights into the pathobiology of ADTKD.7 Although uromodulin and mucin-1 share pathophysiological features of toxic mutant protein accumulation and activation of UPR in TAL cells,5, 6 MUC1 mutations appear not to cause ER stress and do not alter the trafficking of uromodulin itself as ADTKD-MUC1 patients do not have reduced levels of urine uromodulin. These observations suggest that each subtype of ADTKD activates distinct downstream pathogenic cellular and molecular pathways, which could explain the divergence in the clinical features and outcomes. Consequently, this work provides a clear rationale why it is essential to distinguish between ADTKD subtypes.
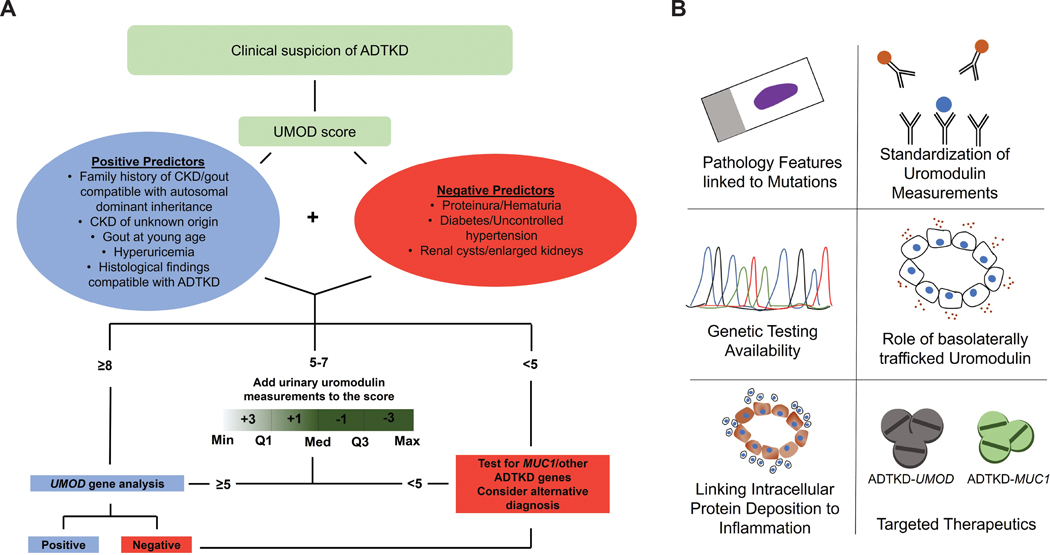
A key contribution of this work is providing a tool for screening and guidance for clinical and genetic testing to distinguish between major ADTKD subtypes.7 The devised clinical UMOD-score was based on discriminative clinical, biochemical, histological and imaging characteristics (Figure 1A) which were discovered in the Belgo-Swiss Registry and validated in the US Registry within this international cohort. The UMOD-score has a high negative predictive value to exclude ATDKD-UMOD (score < 5) and a high positive predictive value for the presence of UMOD mutations when the score is > 7. However, in the range 5 −7, where a large proportion of ADTKD patients may reside, the UMOD-score in itself has reduced positive predictive value (PPV). This was addressed by incorporating urine uromodulin levels/adjusted to glomerular filtration rate (GFR) which improved the overall receiver operating area under the curve from 0.69 to 0.89, and enhanced PPV to 84.2%. The authors also suggest a diagnostic algorithm,7 summarized in Figure 1A.
Figure 1: Summary of the approach proposed by Olinger et al and important areas of study to consider in ADTKD.
A) Schematic of a clinical workflow proposed by the authors to orient the diagnostic approach and prioritize genetic testing decisions in patients with a high suspicion of ADTKD using the clinical UMOD score, with the addition of urinary uromodulin measurements when necessary (Abbreviations: Min, minimum; Q, quartile(s); Med, median; Max, maximum).
B) Proposed topics that require additional investigations in the area of ADTKD
The authors are commended on the rigor and approach used in this study. This work is one of the first to provide a clear indication for the use of uromodulin measurements in patients. This study, along with others that show potential uses of measuring uromodulin levels in the urine or serum,3 should compel the medical community to prioritize resources to standardize uromodulin measurements and establish the reference ranges in the urine and blood. The utility of the proposed UMOD-score will next have to be vetted by additional studies and pragmatic use in clinical settings.
There are several remaining key issues that require investigation to advance the diagnostic, prognostic and potential therapeutic approaches in ADTKD (Figure 1B). As the authors point out,7 the access to clinical grade genetic testing, especially for MUC1 mutation, is limited, and likely to remain a barrier, particularly for patients with reduced access to healthcare. Better characterization of the histopathology of kidney biopsies in ADTKD and link to genetic findings is still underdeveloped. Current advances in digital pathology and artificial intelligence tools can provide an opportunity to link gene mutation to histological features. A low hanging fruit could also be the standardization and implementation of uromodulin staining in kidney biopsy to enable the diagnostic potential of kidney tissue for UMOD mutations.
In addition to continued efforts in understanding the link between the toxic accumulation of mutant protein and the inflammatory phenotype in the interstitium,6 it may be beneficial to explore few novel areas (Figure 1B). Insights are needed on the role of the basolaterally released uromodulin in ADTKD, the secretion of which may not be altered by UMOD mutations.8 Measurements of serum uromodulin levels in ADTKD-UMOD could be a first step.8 The key role of a gain of toxic function of mutant uromodulin and mucin-1 is well established,5, 6 but a contributory role for loss of function of the normal protein cannot be entirely excluded. For example, uromodulin knockout mice do develop hyperuricemia and an abnormal tubular response with aging.3 These mice have also a maladaptive response to stress states such as kidney injury.3 Finally, progress into therapeutic venues is still at an early stage, despite promising results of anti-inflammatory therapy in mouse models mimicking UMOD mutations,9 and successful therapy with a small molecule (BRD4780) in a transgenic mouse model with human MUC1 mutation and in ADTKD-MUC1 patients-derived kidney organoids.5
In conclusion, this landmark study will become an important resource for clinicians and patients with ADTKD.7 The work by Olinger et al delivers important clinical tools that could be used for screening and to prioritize genetic testing. The study also provides important insights into the pathobiology of ADTKD and underscores the continued need for investigating its pathogenesis.
Acknowledgments
T.M.E-A is supported by funding from the National Institute of Diabetes and Digestive and Kidney Diseases (R01DK111651) and a VA Merit Award. KL is supported by a Ben Lipps Fellowship Award from the American Society of Nephrology
The authors acknowledge Dr. Radmila Micanovic for review of the manuscript.
Footnotes
Disclosures
The authors declare no competing interests
References
- 1.Devuyst O, Olinger E, Weber S, et al. Autosomal dominant tubulointerstitial kidney disease. Nat Rev Dis Primers 2019; 5: 60. [DOI] [PubMed] [Google Scholar]
- 2.Eckardt KU, Alper SL, Antignac C, et al. Autosomal dominant tubulointerstitial kidney disease: diagnosis, classification, and management--A KDIGO consensus report. Kidney Int 2015; 88: 676–683. [DOI] [PubMed] [Google Scholar]
- 3.Micanovic R, LaFavers K, Garimella PS, et al. Uromodulin (Tamm-Horsfall protein): guardian of urinary and systemic homeostasis. Nephrol Dial Transplant 2020; 35: 33–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.LaFavers KA, Macedo E, Garimella PS, et al. Circulating uromodulin inhibits systemic oxidative stress by inactivating the TRPM2 channel. Sci Transl Med 2019; 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dvela-Levitt M, Kost-Alimova M, Emani M, et al. Small Molecule Targets TMED9 and Promotes Lysosomal Degradation to Reverse Proteinopathy. Cell 2019; 178: 521–535 e523. [DOI] [PubMed] [Google Scholar]
- 6.Trudu M, Schaeffer C, Riba M, et al. Early involvement of cellular stress and inflammatory signals in the pathogenesis of tubulointerstitial kidney disease due to UMOD mutations. Sci Rep 2017; 7: 7383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Olinger E, Hofmann P, Kidd K, et al. Clinical and Genetic Spectra of Autosomal Dominant Tubulointerstitial Kidney Disease due to Mutations in UMOD and MUC1. Kidney Int 2020. [DOI] [PubMed] [Google Scholar]
- 8.Jennings P, Aydin S, Kotanko P, et al. Membrane targeting and secretion of mutant uromodulin in familial juvenile hyperuricemic nephropathy. J Am Soc Nephrol 2007; 18: 264–273. [DOI] [PubMed] [Google Scholar]
- 9.Johnson BG, Dang LT, Marsh G, et al. Uromodulin p.Cys147Trp mutation drives kidney disease by activating ER stress and apoptosis. J Clin Invest 2017; 127: 3954–3969. [DOI] [PMC free article] [PubMed] [Google Scholar]